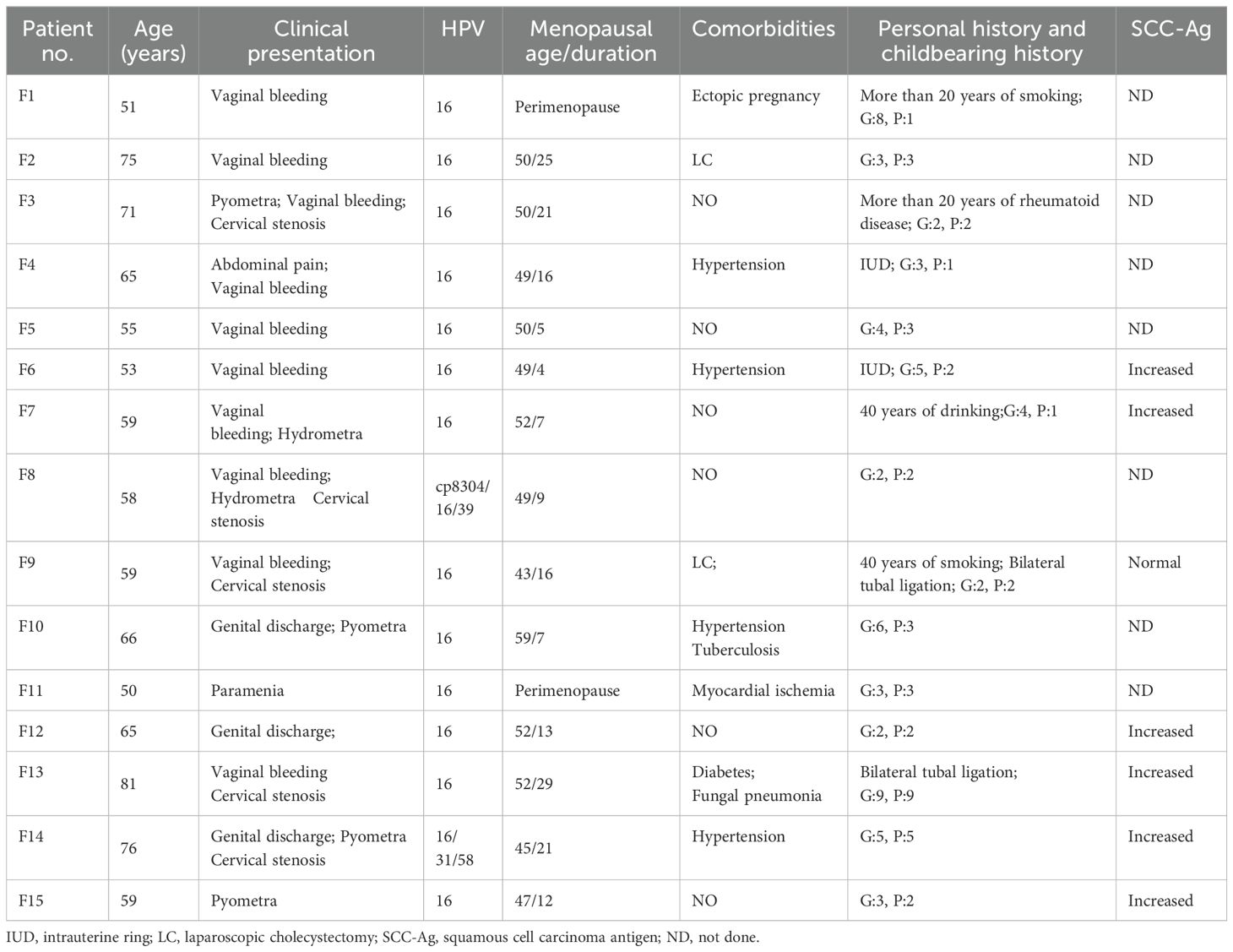
- 1Department of Pathology, Guangyuan Central Hospital, Guangyuan, China
- 2Department of Pathology, Guangyuan City Hospital of Traditional Chinese Medicine, Guangyuan, China
Background: Cervical squamous cell carcinoma (SCC) is the most common type of cervical carcinoma. Usually, the cancer metastasizes through lymphatic or hematogenous dissemination. However, it is uncommon for a superficial spreading of cervical cancer to reach the endometrium, fallopian tubes, and the ovaries.
Objectives: In the present study, we report 15 cases of superficial spreading SCC and discuss the possible mechanism involved.
Methods: We collected 15 samples diagnosed by histopathology after surgery. Immunostaining, which included P16, P63, CD138, CD34, D2-40, and Ki-67, were performed for all samples.
Results: All patients were postmenopausal or perimenopausal women. The commonest clinical presentation was vaginal bleeding in 66.67%. All patients were infected with HPV 16. The endometrium was replaced by high-grade squamous intraepithelial lesion (HSIL), which involved the endometrial gland, even squeezing into the myometrium and forming SCC. Bilateral fallopian tubes and ovaries involvement was in 1/15. A total of 10/15 (66.67%) of the women had disease of stage 1B or less. All SCCs were moderately or poorly differentiated. Immunohistochemistry revealed that the tumor cells were positive for P63 and P16, with a high Ki-67 labeling index. There was CD138 positive expression in varying degrees, which was strongly and diffusely expressed in 6/15 (40.00%).
Conclusion: Superficial spread of cervical cancer towards the endometrium is a rare but cognizable phenomenon, and a guideline for the management of these cases has not been established. Our present findings suggest that multiple factors may interact with each other simultaneously, contributing to this rare disease.
Introduction
Cervical cancer is the fourth most common cancer among women globally (1). Normally, it extends downward into the vagina or, laterally, invading the parametrial tissue, and it also metastasizes through the lymphatic glands or by blood dissemination. Few cases with superficial spread to the uterine endometrium, fallopian tubes, and ovaries have been observed. Cervical SCC, spreading superficially to the inner surface of the uterus and replacing the endometrium with carcinoma cells, is called superficial spreading SCC (2). It is not included in the 2020 (fifth edition) World Health Organization (WHO) Classification of Female Genital Tract Tumors or the 2018 FIGO (International Federation of Gynecology and Obstetrics) cervical cancer staging system. It is undetermined if this kind of superficial spread changes the stage, management, and prognosis.
Notably, superficial spreading SCC should be distinguished from cervical squamous cell carcinoma directly invading the uterine wall and primary endometrial SCC (PESCC). The diagnostic criteria for PESCC were established by Fluhmann (3) in 1953 and included the following: (a) no evidence of coexisting endometrial adenocarcinoma or primary cervical SCC, (b) no connection between the endometrial tumor and squamous epithelium of the cervix, or (c) no connection between any existing cervical in situ carcinoma and independent endometrial neoplasm.
In the literature, only a few dozen cases have been reported. In the present study, we reported 15 cases of superficial spreading SCC of the cervix involving the endometrium and discussed the possible mechanism of this unusual spreading form of cervical SCC.
Materials and methods
Ethical approval was obtained from the Guangyuan Central Hospital Ethics Review Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. In this retrospective study, we collected 15 samples in the Department of Pathology of Guangyuan Central Hospital and Guangyuan City Hospital of Traditional Chinese Medicine from January 2020 to December 2023. All patients underwent surgery.
The pathology specimens in all cases were fixed in 10% neutral formalin. The samples were embedded in paraffin blocks, cut at 4-μm thickness, and stained with standard hematoxylin and eosin (H&E). Immunostaining was performed according to the manufacturer’s instructions, and the tested antibodies included P16, P63, CD138, CD34, D2-40, and Ki-67 (Zhongshan, Beijing, China).
All histopathological slides from each patient were reviewed, and superficial spreading SCC was confirmed by two professional and experienced pathologists with consistent diagnosis independently and in duplicate. Focal LVSI is defined by the presence of a single focus around the tumor, substantial LVSI as multifocal or diffuse arrangement of LVSI, or the presence of tumor cells in four or more lymphovascular spaces (4). The prognosis of the patients was defined as survival period in months after surgery and histological diagnosis.
Statistical analysis
Statistical analysis and data management were conducted using SPSS, version 20 (SPSS, Chicago, IL, USA). Categorical data were expressed as absolute or relative frequencies, and continuous data were expressed as mean ± SD.
Results
The study involved 15 patients. The age of all patients was over 50 years (age, 62.86 ± 9.52 years; range, 50–81 years). Except for patient F1 and F11, who were perimenopausal women, all other patients were postmenopausal women (menopausal age, 49.77± 3.85 years; menopausal duration, 14.23 ± 7.96 years). Most of the women were 50–60 years (53.33%). There was a wide spectrum of clinical presentations, with the most common clinical presentation being vaginal bleeding (10/15, 66.67%), followed by cervical stenosis (5/15, 33.33%), pyometra (4/15, 26.67%), genital discharge (3/15, 20.00%), hydrometra (2/15, 13.33%), paramenia (1/15, 6.67%), and abdominal pain (1/15, 6.67%). One woman may have more than one complaint. All patients were infected with HPV 16. Increased squamous cell carcinoma antigen (SCC-Ag) patients were 6/7 (85.71%). Comorbidities and personal history are in shown Table 1. Seven of the 15 (46.67%) patients had history of miscarriage, including spontaneous and induced abortion. All patients had two or more children. There were five leiomyoma of the uterus and two adenomyosis.
Under microscopic examination, the cervix showed high-grade squamous intraepithelial lesion (HSIL) and/or SCC with chronic inflammation. These atypical squamous cells exhibited high nuclear to cytoplasmic ratio, rounded nuclei with distinct nucleoli, and high mitotic activity (Figure 1A). The commonest histological subtype of cervical lesion was SCC with HSIL in 12/15 (80.00%) cases, SCC without HSIL in 2/15 (13.33%), and one case of HSIL with microinvasive SCC (6.67%).
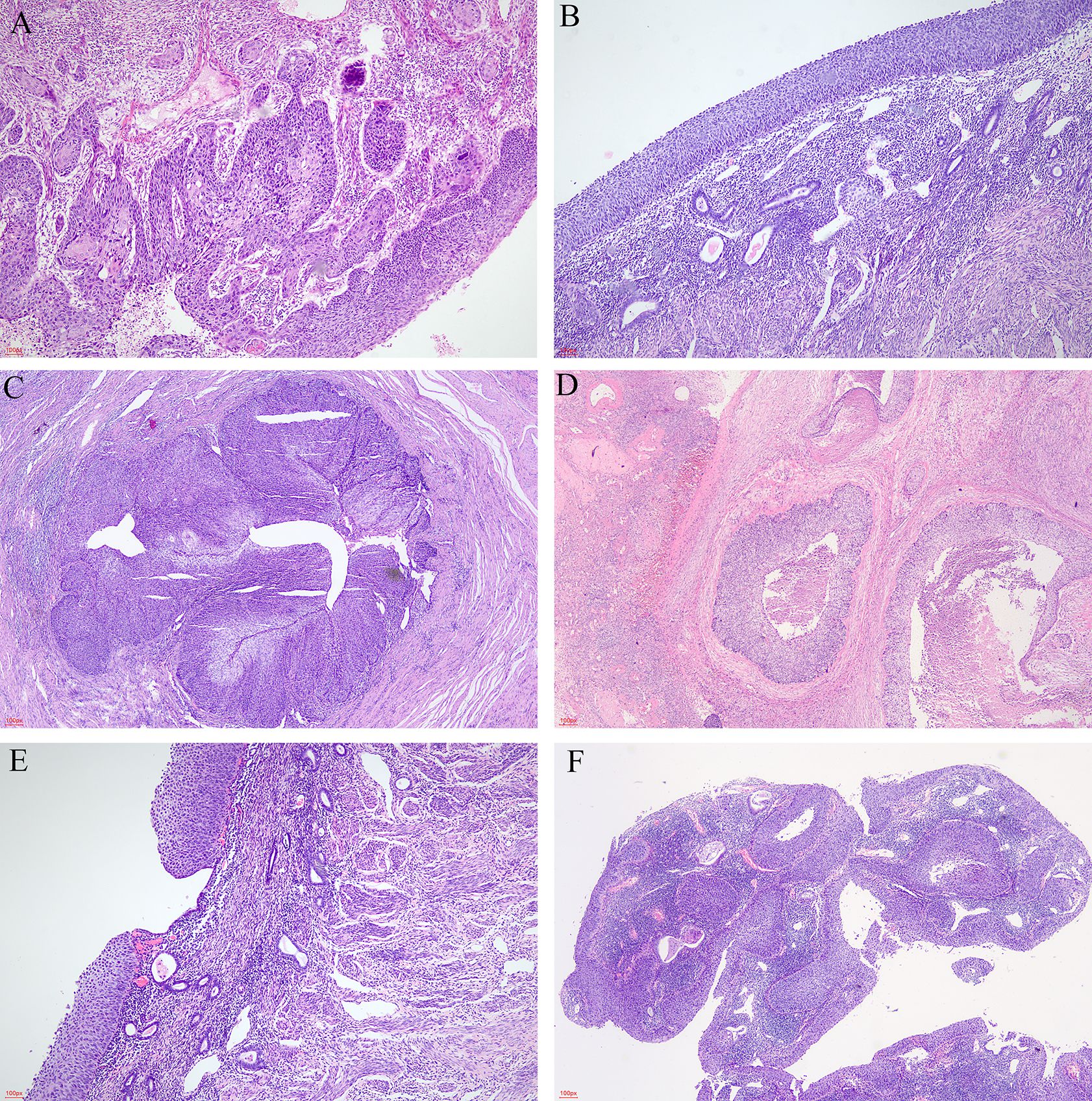
Figure 1. The microscopic appearance of lesions. (A) Cervical lesion (HE, ×40). (B) Superficial spreading atypical squamous cells in the endometrium (HE, ×40). (C) Superficial spreading SCC in bilateral fallopian tubes (HE, ×20). (D) Superficial spreading SCC in bilateral ovaries (HE, ×20). (E) Intermittent or skipping superficial spreading in endometrium (HE, ×40). (F) Endometrial polyps covered by HSIL (HE, ×20).
The endometrium was also replaced by HSIL (Figure 1B), which involved the endometrial gland, even squeezing into the myometrium and forming SCC. This endometrial lesion was directly contiguous to the cervical lesion. The extensional lesion of the 15 superficial spreading SCC cases are as follows: endometrial HSIL was in 6/15 (40.00%), endometrial HSIL with microinvasive in 3/15 (20.00%), and endometrial HSIL with SCC in 6/15 (40.00%). Vaginal HSIL was 2/15 (F7 and F10), bilateral fallopian tubes and ovaries involvement was in 1/15 (F12) (Figures 1C, D), and pelvic lymph nodes metastasis was in 1/15 (F1). An interesting finding was that F7 had SCC and HSIL, which had intermittent or skipping superficial spreading in the endometrium (Figure 1E). The patient F8 underwent laparoscopic hysterectomy and bilateral adnexectomy. By macroscopic examination, we found that the endometrium was slightly thicker with an adherent polyp-shaped lump extending to the right uterine cornua. The pathology results of F8 showed SCC and HSIL within the cervix, microinvasive and HSIL in the endometrium, and endometrial polyps covered by HSIL (Figure 1F). Lymph-vascular space invasion (LVSI) was also discovered in 11/15. Focal LVSIs were 5/15. Substantial LVSIs were 6/15.
The patients were classified by FIGO 2018 staging system as follows: 6.67% (1/15) patients with stage IA1, 40.00% (6/15) patients with stage IB1, 6.67% (1/15) with stage IB2, 13.33% (2/15) with stage IB3, 20.00% (3/15) with stage IIA1, 6.67% (1/15) with stage IIB, and 6.67% (1/15) with stage IIIC1. A total 10/15 (66.67%) of the women had disease of stage 1B or less. Thus, despite the lower stage in the cervix, the tumor extended cephalad, indicating an inherent propensity for cephalad extension rather than infiltration as is the usual case.
Of the SCC tumors, 3/14 (21.43%) had poorly differentiated tumors, and 11/14 (78.57%) had moderately differentiated tumors. Majority of the patients were treated with radiotherapy or chemotherapy after surgery, including five with chemoradiotherapy and six with chemotherapy.
The results of immunohistochemical staining revealed that carcinoma cells, whether in the cervix, endometrium, ovaries, or fallopian tubes, were diffusely positive for P63 and p16. The Ki-67 labeling index was 40%–90%. CD34 and D2–40 staining verified carcinoma invasion in the cervical stromal lymph vascular space. There was positive expression in varying degrees of CD138 in the cervix (Figure 2A) and extended lesion (Figures 2B, C). CD138 was strongly and diffusely expressed in 6/15 (40.00%).
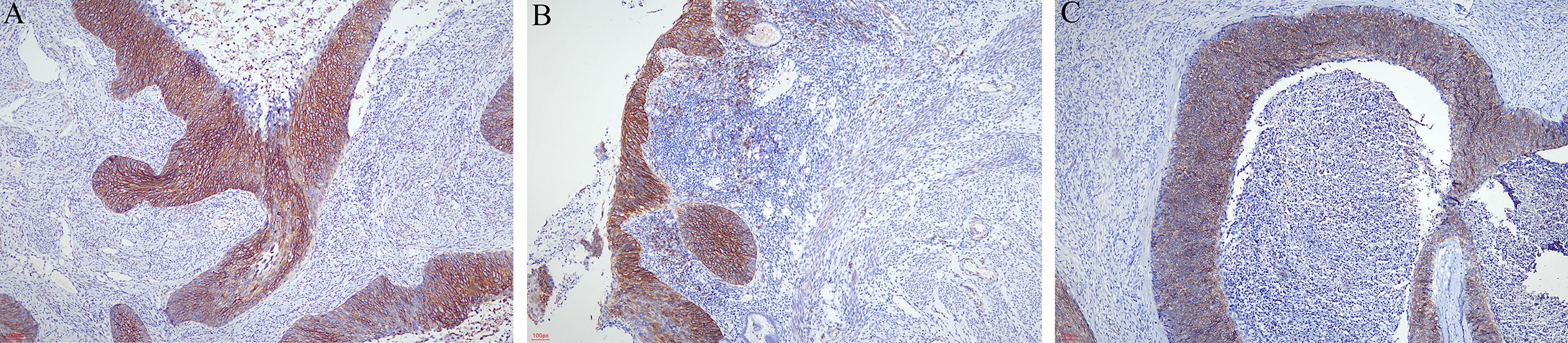
Figure 2. Immunohistochemical staining of CD138. (A) CD138 was strongly and diffusely expressed in the cervix (×40). (B) CD138 was strongly expressed in the neoplastic squamous cells spreading in the endometrium (×40). (C) Superficial spreading SCC in the ovary also expressed CD138 (×40).
Survival data were available for all cases (Table 2). The median duration of follow-up was 13 months (range, 6–49 months). All patients were alive after surgery with no evidence of clinical recurrence or metastasis.
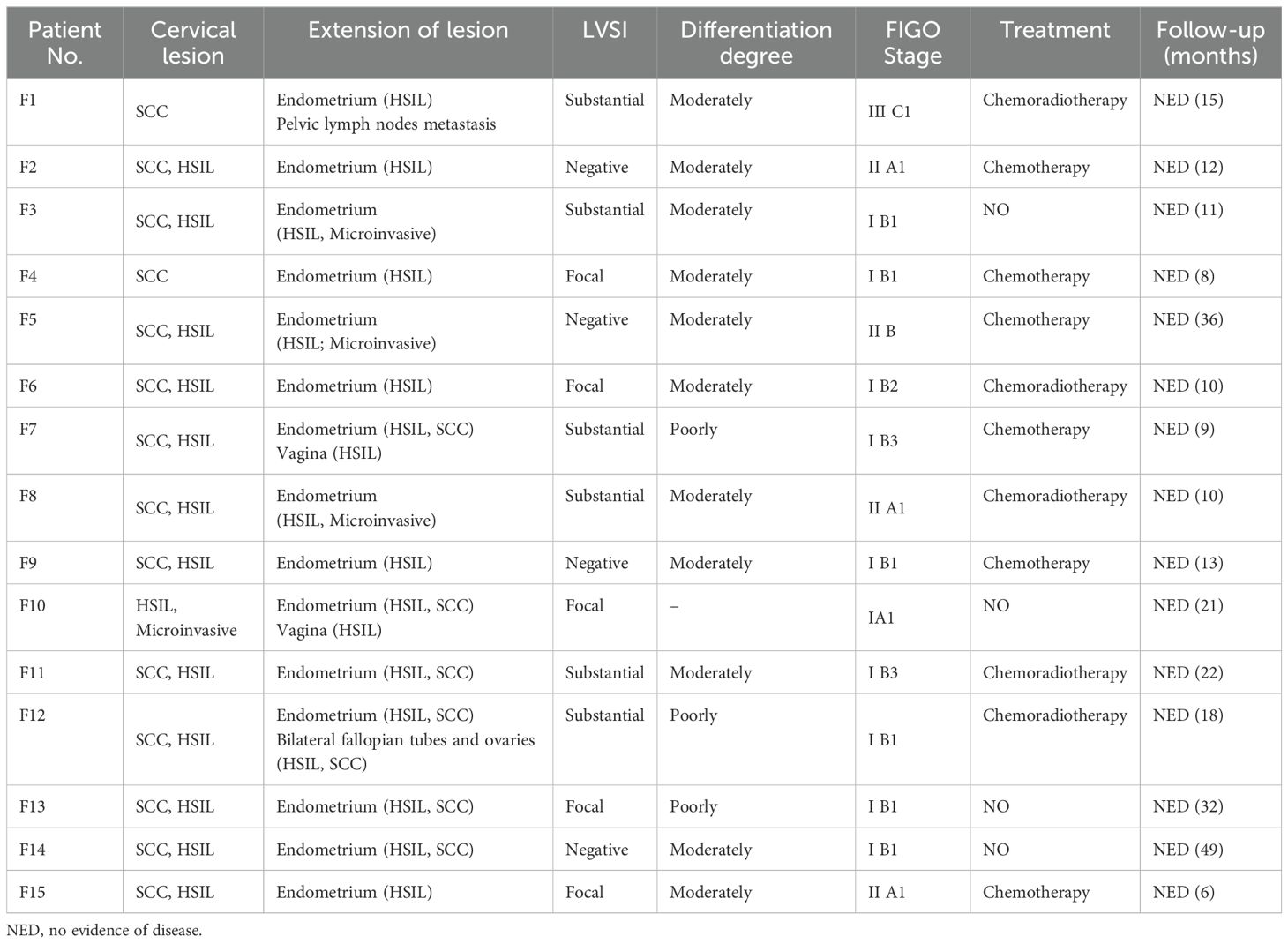
Table 2. Superficial spreading SCC of uterine cervix involving the endometrium and other extended lesion.
Discussion
In 1900, Cullen first discovered a case of cervical SCC that spread to the entire endometrium; since then, only few cases have been reported, with an occurrence of 0.7% (5). Among these rare cases, the endometrium is the most common site for metastasis. Various types of cervical cancer can superficially spread to the endometrium, fallopian tube, and ovaries. Among these types of cervical cancer, squamous cell carcinoma (91.11%) has the highest frequencies (6).
Since the 1960s, scholars have discussed the potential risk factors that may contribute to upward metastasis, such as long-term estrogen usage, vitamin A deficiency, HPV infection, senile endometrium, pyometra, and radiotherapy (5); among them, some have persisted until today, namely, advanced age, cervical stenosis, and pyometra (7). The most common clinical presentation is vaginal bleeding and pyometra in the previous literature (8).
In the previous report, the age of all patients was over 50 (2), and all were menopausal or post-menopausal (7). The most common clinical presentation was genital bleeding (2), which is similar to our result. Cervical stenosis was proposed to be a reason for endometrial rather than the lateral extension (9). However, our analysis indicates that among 15 cases with information about the cervical lesion, only five (33.33%) women had stenosis; hence, this may not be the only reason for the superficial spread of the tumor. In postmenopausal women, cervical stenosis is easily formed after cervical treatment due to the loss of periodic abscission, and it encloses the uterine cavity and accelerates the pyometra. Anne Chao et al. (10) also reported a case with fatal pyometra in a 60-year-old patient whose pathology also revealed cervical HSIL that progressed into SCC in the endometrium. In this study, 4/15 (26.67%) patients had pyometra.
CD 138 is a key cell surface adhesion molecule. It is a cell surface heparin sulfate proteoglycan that is responsible for cell–cell and cell–extracellular matrix interaction. It has been proposed that this may be one possible reason for the superficial spread of this tumor rather than infiltration due to retained CD138 expression in these cells (2). Expression of CD138 may participate in superficial spread by cell–cell interactions. Two authors reported that the superficial spreading cells were strongly positive for CD138 in superficial carcinoma cells in both the cervix and endometrium (2, 11). In our study, CD138 was positively expressed in varying degrees, whereas it was strongly expressed in 40.00%. The report is not entirely consistent with our conclusion, which may be related to the rare sample and limited information about CD138.
As the most common malignant tumor in the female reproductive system, cervical SCC (>90–95%) is HPV associated in the large majority of cases. In one of the studies reviewed, all the samples analyzed were HPV 16 positive (10). Consistent with our case, all samples were positive for HPV 16. This suggests that persistent HR-HPV infection is a key factor in the development of superficial spreading cervical SCC.
Another proposed mechanism is the transformation of endometrial cells to squamous rather than actual spread. P16 over expression and HPV linkage have been proposed to be responsible for transforming the reserve cells in the endometrium misconceived as the superficial spread (12). Of the patients with endometrial cancer, 90% have abnormal vaginal bleeding as the first main symptom, most frequently during the postmenopausal period. Due to a new diagnostic classification centered on molecular and immunohistochemical indicators, the risk classification is much more accurate. On the basis of the outcomes of the Cancer Genome Atlas, and the ProMisE (Proactive Molecular Risk Classifier for EC), endometrial cancer is divided into four subgroups: POLEmut, p53 wild type (low copy number—CNL—or nonspecific molecular profile—NSMP), p53 null/missense mutations (high copy number), and mismatch repair deficient (MMRd) (13). But in another study, Kushima et al. (14) studied the loss of heterozygosity markers in the endometrial cells in five women indicating that a monoclonal origin of endometrial and cervical tumors supported spread from the cervix rather than endometrial cell transformation.
It has been reported that the bilateral tube and ovary were directly spread from HPV-associated superficial uterine cervical squamous carcinoma through the endometrium and the fallopian tube and lymph-vascular space invasion at the same (15). The mechanism may involve either LVSI moving to the ovarian hilum or ovary surface involvement of the endometrium and fallopian tubes, similar to double-hit ways hand in hand. LVSIs are known risk factors for recurrence in cervical cancer and are also predictive of lymph node metastasis. In our findings, all SCCs were moderately or poorly differentiated; we suggest that it may be related to superficial spread of cervical SCC.
It is currently difficult to draw any conclusion regarding optimal mechanism. Therefore, it is reasonable to infer that these factors often interact with each other simultaneously, contributing to this rare disease.
Deel et al. (16) postulate that in women with the localized cervical disease, the superficial spread to the endometrium and adnexa may not be associated with an inferior prognosis. Our patient was alive and well at 6 months after surgery. Perhaps due to the short follow-up time of our cases, the relationship between superficial spreading SCC and prognosis cannot be confirmed at present. To date, too few cases of superficial spreading SCC of the cervix have been reported to establish a conclusion regarding their treatment and prognosis. Perez et al. proposed that endometrial involvement of cervical SCC may be an indicator of poor prognosis and is usually diagnosed after hysterectomy; early detection is of extreme significance (17). It is wise to evaluate the endometrium thoroughly in every case of cervical cancer to offer the best management modalities to the patient. If untreated, this superficial spread may lead to the recurrence of the tumor. A careful evaluation and therapeutic strategy must be adopted for optimal disease-free outcomes.
Superficial spread of cervical cancer towards the endometrium is a rare but cognizable phenomenon, and a guideline for the management of these cases has not been established. In addition, the FIGO staging system has no descriptions for such a condition. Superficial spread of cervical cancer is a distinct entity. Our present findings suggest that multiple factors may interact with each other simultaneously, contributing to this rare disease. There are insufficient data to compare superficial spreading SCC of the cervix with other types of cervical cancer. More clinical cases are needed to identify additional prognostic factors and inform clinical practice guidelines on the management of this disease.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Guangyuan Central Hospital Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XJ: Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. ZH: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing. ZC: Data curation, Investigation, Validation, Writing – review & editing. BW: Data curation, Investigation, Supervision, Validation, Writing – review & editing. TC: Data curation, Investigation, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Ishida M, Okabe H. Superficial spreading squamous cell carcinoma of the uterine cervix involving the endometrium: Report of two cases with emphasis on the likely molecular mechanism. Oncol Lett. (2013) 5:31–4. doi: 10.3892/ol.2012.953
3. Fluhmann CF. The histogenesis of squamous cell metaplasia of the cervix and endometrium. Surg Gynecol Obstet. (1953) 97:45–58.
4. Peters EEM, León-Castillo A, Smit V, Boennelycke M, Hogdall E, Hogdall C, et al. Defining substantial lymphovascular space invasion in endometrial cancer. Int J Gynecol Pathol. (2022) 41:220–6. doi: 10.1097/pgp.0000000000000806
5. Kanbour AI, Stock RJ. Squamous cell carcinoma in situ of the endometrium and fallopian tube as superficial extension of invasive cervical carcinoma. Cancer. (1978) 42:570–80. doi: 10.1002/1097-0142(197808)42:2<570::AID-CNCR2820420225>3.0.CO;2-N
6. Bagde MN, Bagde NKD, Hussain N, Thangaraju P. A review and case report of enigmatic superficial endometrial spread of cancer of the uterine cervix: Need for vigilance in the primary care setting. J Family Med Prim Care. (2021) 10:3505–10. doi: 10.4103/jfmpc.jfmpc_39_21
7. Wang W, Zhou F. Cervical HSIL involving the endometrium and adenomyosis: A case report and literature review. J Coll Physicians Surg Pak. (2021) 31:337–9. doi: 10.29271/jcpsp.2021.03.337
8. Martín-Vallejo J, Laforga JB, Molina-Bellido P, Clemente-Pérez PA. Superficial spreading cervical squamous cell carcinoma in situ involving the endometrium: a case report and review of the literature. J Med Case Rep. (2022) 16:196. doi: 10.1186/s13256-022-03433-4
9. Marwah N, Garg M, Singh S, Sethi D, Sen R. Unusual form of squamous cell carcinoma of the cervix extending in situ into the endometrium: Three case reports and review of literature. Int J Appl Basic Med Res. (2012) 2:139–41. doi: 10.4103/2229-516x.106359
10. Chao A, Wang AM, Wang TH, Wu TI, Chao AS. An atypical and fatal case of pyometra accompanied by the superficial spread of squamous cell carcinoma of the endometrium and the fallopian tubes. Taiwan J Obstet Gynecol. (2013) 52:440–2. doi: 10.1016/j.tjog.2013.05.004
11. Muthusamy RK, Mehta SS. Squamous cell carcinoma in situ of the cervix with superficial intraepithelial extension to the endometrium of lower uterine segment: A rare presentation. Indian J Med Paediatr Oncol. (2017) 38:88–9. doi: 10.4103/0971-5851.203509
12. Sood N, Sinha KS. Superficial Spreading Squamous Cell Carcinoma Endometrium andIcthyosis Uteri with CINIII with p16 Expression: Report of 2 Unusual Cases. J Krishna Institute Med Sci University. (2017) 6:126 – 31.
13. D’Oria O, Giannini A, Besharat AR, Caserta D. Management of endometrial cancer: molecular identikit and tailored therapeutic approach. CEOG. (2023) 50:210. doi: 10.31083/j.ceog5010210
14. Kushima M, Fujii H, Murakami K, Ota H, Matsumoto T, Motoyama T, et al. Simultaneous squamous cell carcinomas of the uterine cervix and upper genital tract: loss of heterozygosity analysis demonstrates clonal neoplasms of cervical origin. Int J Gynecol Pathol. (2001) 20:353–8. doi: 10.1097/00004347-200110000-00007
15. Zhang Y, Zhang X, Wang H, Shen D. Stage IA1 HPV-associated cervical squamous cell carcinoma metastasizing to ovary by special pathway: a case report and literature review. J Ovarian Res. (2022) 15:21. doi: 10.1186/s13048-022-00949-7
16. Deel CD, Allen RA, Holman LL, Zuna RE. Adenocarcinoma of the cervix involving the fallopian tube mucosa: report of a case. Diagn Pathol. (2016) 11:77. doi: 10.1186/s13000-016-0529-8
Keywords: superficial spreading, squamous cell carcinoma, HSIL, cervix, endometrium
Citation: Jiang X, Han Z, Chun Z, Wen B and Chen T (2024) An unusual pattern of endometrial involvement: superficial spreading squamous cell carcinoma of the cervix. Front. Oncol. 14:1456297. doi: 10.3389/fonc.2024.1456297
Received: 28 June 2024; Accepted: 11 September 2024;
Published: 01 October 2024.
Edited by:
Chengquan Zhao, University of Pittsburgh, United StatesReviewed by:
Indranil Chakrabarti, Kalyani (AIIMS Kalyani), IndiaOttavia D’Oria, Sapienza University of Rome, Italy
Copyright © 2024 Jiang, Han, Chun, Wen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhirong Han, MzMwNjg5MzMwQHFxLmNvbQ==
 Xiaolin Jiang1
Xiaolin Jiang1 Zhirong Han
Zhirong Han