- Department of Breast Surgery, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
Purpose: Human epidermal growth factor receptor 2 (HER2) is vital for breast cancer prognosis. The aim of this study was to analyze the clinicopathological data of HER2-negative breast cancer patients receiving neoadjuvant chemotherapy and the associated factors affecting the pathological complete response rate (pCR) and prognosis.
Methods: Clinical data of 173 patients with primary HER2-negative breast cancer, who initially received neoadjuvant chemotherapy followed by surgical treatment at the Breast Surgery Department of Bethune Hospital in Shanxi Province from January 2012 to December 2022, were collected.
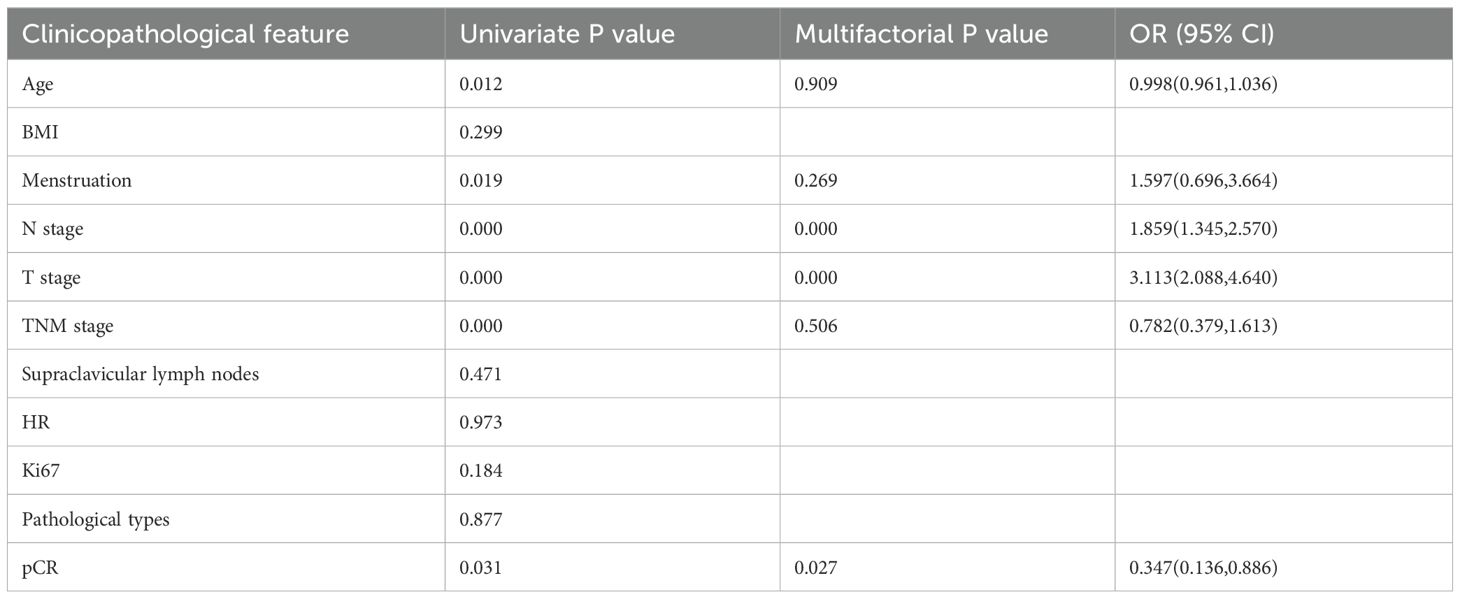
Results: Compared to HER2-0 patients, HER2-low patients had higher T staging (p = 0.008), higher Ki67 proliferation index (p < 0.001), lower N staging (p = 0.001), and lower pCR rate (p < 0.001). Univariate analysis revealed that T stage, TNM stage, HR status, HER2 status, and Ki67 are risk factors that affect the pCR rate in HER-2 negative. Multivariate analysis identified HR status as an independent predictor of pCR rate. Kaplan–Meier survival curves showed that menstrual status, N staging, T staging, TNM staging, and pCR status affected the prognosis of HER2-low breast cancer patients (p < 0.05).
Conclusion: HER2-low breast cancer exhibits distinct biological behaviors, suggesting personalized treatment approaches.
1 Introduction
Human epidermal growth factor receptor 2 (HER2) is a critical prognostic and predictive biomarker for breast cancer. All newly diagnosed patients with primary or metastatic breast cancer should be tested for HER2 protein levels by immunohistochemistry (IHC) and/or gene expression by in situ hybridization (ISH) to guide clinical treatment (1–3). Currently, HER2-positive breast cancer is defined when HER2 expression is 3+ or 2+ IHC score, and gene amplification is detected by ISH. Patients with HER2-positive tumors can be treated with HER2 pathway-blocking drugs that have significantly improved the clinical outcomes of HER2-positive breast cancer (4). Strikingly, 10–20% of breast cancer tumors are HER2-positive, and 80–90% are HER2-negative (5, 6). Patients with HER2-low status - defined as IHC scores 2+ and 1+ with negative ISH were considered to be HER2-negative. This group of patients was similar to HER2 non-expressing (HER2-0) patients, wherein none could benefit from the anti-HER2 therapy. The results of clinical trials of new antibody-drug conjugates (ADCs), such as T-DXd, suggested that the beneficiary population includes HER2-positive patients; also, the drugs show efficacy in HER2-low breast cancers (7, 8). These findings indicated that anti-HER2 therapy based on ADCs is efficacious in patients with low HER2 expression, further confirming that this group is not equivalent to the HER2-0 group and may have unique biological behaviors and therapeutic strategies.
HER2-low breast cancers account for approximately 45%–55% of all breast cancers (9). Owing to the large proportion of patients with HER2-low breast cancer, developing precision medicine strategies and improving survival in these patients is an urgent requisite that requires knowledge of the clinical characteristics and prognosis of these patients. Hitherto, only a few studies have focused on the clinicopathological characteristics of individuals with low HER2 expression and the relevance and prognosis of factors influencing the pathological complete response (pCR) with neoadjuvant chemotherapy. Thus, the present study aimed to analyze the clinicopathological data of HER2-0 breast cancer patients receiving neoadjuvant chemotherapy, further analyze the relevant factors affecting the pCR and prognosis of HER2-low breast cancer, and provide a basis for clinical research and the development of novel targeted drugs for precise treatment.
2 Information and methods
2.1 Clinical data
Clinical data of 173 patients with primary HER2-negative breast cancer treated surgically after initial neoadjuvant chemotherapy at the Breast Surgery Department of Bethune Hospital in Shanxi Province from January 2012 to December 2022 were collected. All patients had completed a dose-dense anthracycline sequential paclitaxel-based neoadjuvant chemotherapy regimen.
The inclusion criteria were as follows: ① women > 18-years-old; ② all of them underwent hollow-core needle puncture to identify breast cancer and confirm HER2-negative expression by IHC before chemotherapy; ③ met the indications of the Chinese Association Against Cancer (CAC) Breast Cancer Diagnostic and Treatment Guidelines and Criteria for neoadjuvant chemotherapy; ④ received neoadjuvant chemotherapy and underwent surgery after completing the established cycles of chemotherapy; ⑤ provided complete clinical data.
The exclusion criteria were as follows: (1) male; (2) inflammation, bilateral, lactation, and pregnancy breast cancer; (3) combined with other severe complications and malignant tumors; (4) intolerant of and; (5) chemotherapy while receiving other breast cancer-related treatments; (6) chemotherapy without surgical treatment; (7) distant metastases.
Relevant clinical data of patients, including age, menstrual status, body mass index (BMI), axillary and clavicular region lymph node status, T stage, N stage, TNM stage, hormone receptor (HR) status, Ki67, and tumor pathology type, were collected. The present study was approved by the institutional internal Ethics Review Board.
2.2 Treatment programs
Eight-cycle ddEC-ddP regimen: epirubicin 100 mg/m2, cyclophosphamide 600 mg/m2, one cycle every 14 days, four cycles followed by sequential four cycles of paclitaxel 175 mg/m2, one cycle every 14 days.
2.3 Immunohistochemical assessment
According to the guidelines of the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP)3, IHC-based HER2-negative is defined as HER2 0 or HER2 1+ or HER2 2+ and fluorescence in situ hybridization (FISH) negative. HER2-low indicates that the IHC result was 1+ or 2+, and the FISH result was negative (10). HER2 was accessed using HER2 antibody 4B5 (Roche, Basel, Switzerland) and ISH-techniques and classified according to the ASCO/CAP guidelines available during the different time periods (1–3). Estrogen receptor (ER) positivity and progesterone receptor (PR) positivity were standardized as ≥ 1% positive staining, whereas HR positivity was defined as ER or PR positivity.
2.4 Efficacy and survival analysis
The main objective of this study was to investigate the factors influencing the efficacy and prognosis of neoadjuvant chemotherapy in patients with HER2-low breast cancer. pCR was defined as the absence of invasive cancer residue in the breast and axillary lymph nodes. Only residual carcinoma in situ was classified as pCR. Disease-free survival (DFS) was defined as the time from surgery to all types of disease progression (including local recurrence, distant metastasis, neoplastic tumors, or death due to tumors). Follow-up time was calculated from the definitive pathological diagnosis to follow-up for up to five years.
2.5 Statistical methods
Statistical analyses were performed using R 4.3.2 and SPSS 25 software. Quantitative data that conformed to normal distribution were expressed as mean, whereas qualitative data were expressed as number of cases (percentage) [n (%)]. Univariate analyses were performed using t-test, chi-square test, Fisher’s exact test, univariate logistic regression model, and univariate Cox proportional regression model. Multivariate analyses used logistic and Cox proportional regression models. Survival curves were plotted using the Kaplan–Meier method. The test threshold was defined as a = 0.05.
3 Results
3.1 Comparison of clinicopathological features of differential HER2 protein expression
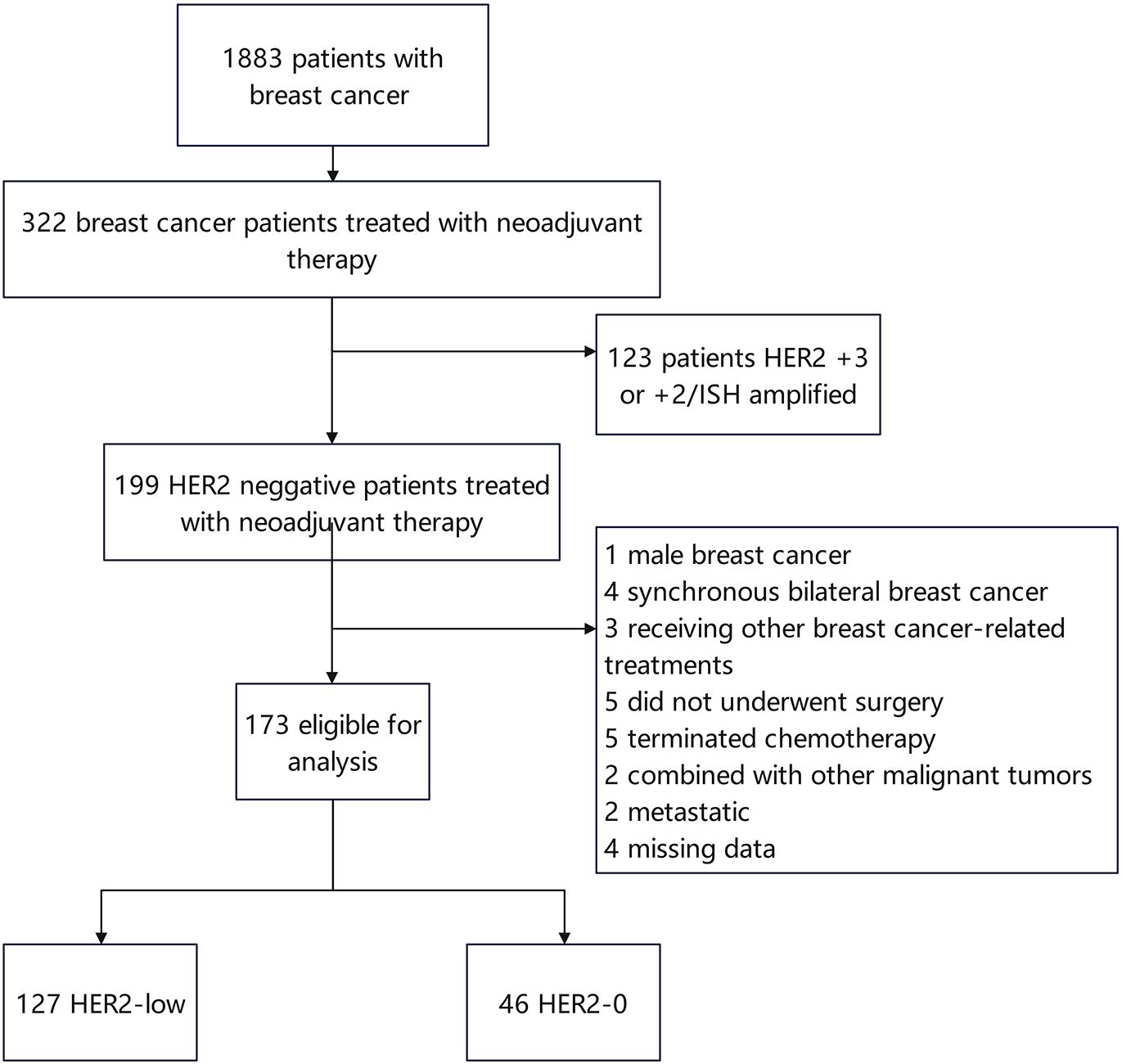
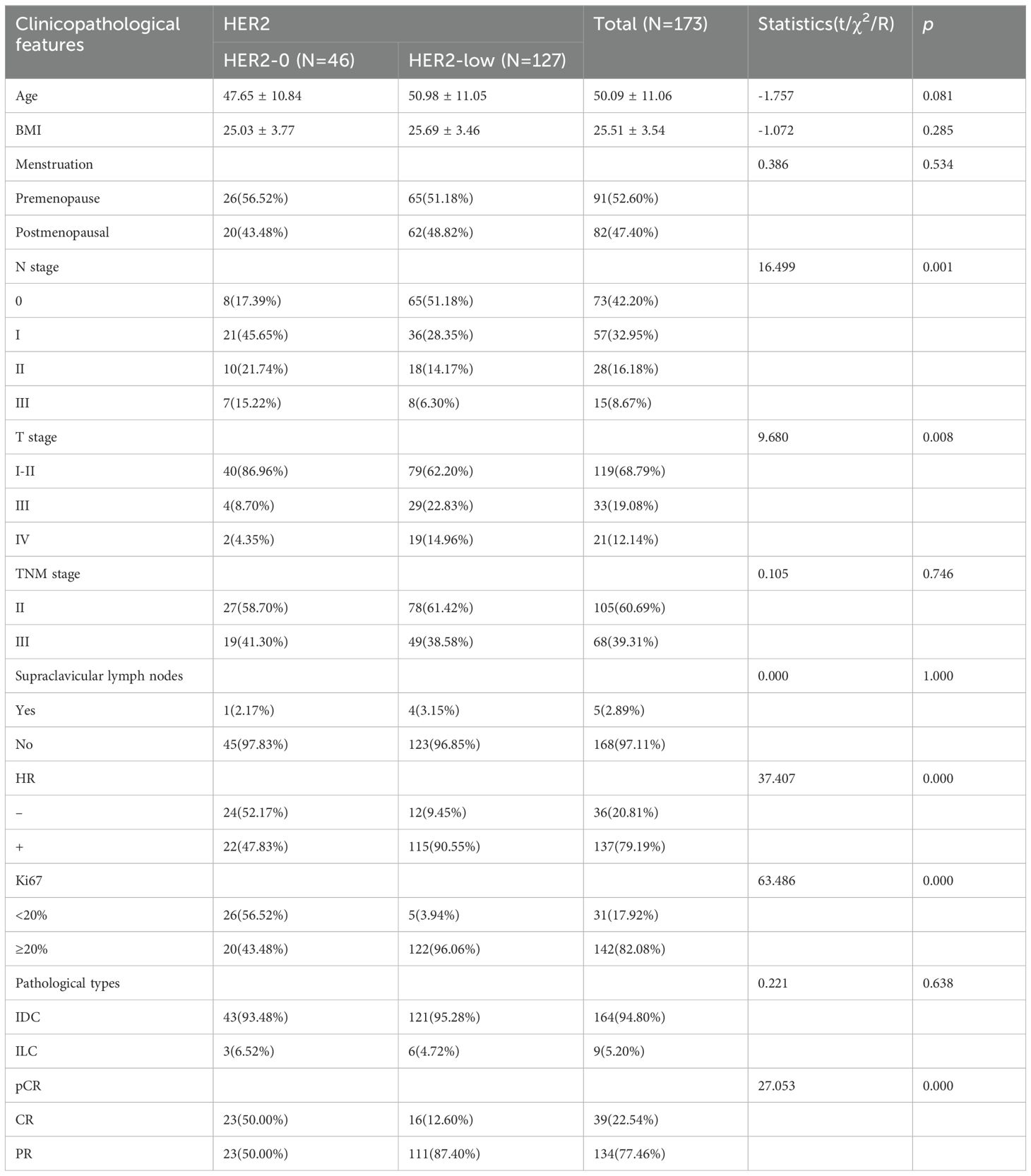
A total of 173 patients with HER2-negative breast cancer [including 127 (73.41%) patients with HER2-low and 46 (26.59%) patients with HER2-0] were recruited in this study (Figure 1). The median age of all patients was 50.09 years. 91/173 (52.60%) patients were in premenopausal status. Based on the IHC expression of HER2 (0, 1+, 2+), the patients were divided into HER2-0 and HER2-low to determine the differences in the clinicopathological features between the two subgroups. Compared to HER2-0 patients, HER2-low patients had higher T-stage (p = 0.008), higher Ki67 index (p < 0.001), lower N-stage (p = 0.001), and lower pCR rate (p < 0.001). The cohort comprised 79.19% luminal type (HR-positive) and 20.81% triple-negative breast cancer (TNBC) type (HR-negative) patients. A higher percentage of HR positivity was detected in the HER2-low group (p < 0.001) and was significantly frequent in the luminal type (83.9% vs. 16.1%) (Table 1).
3.2 Predictive factors for pCR
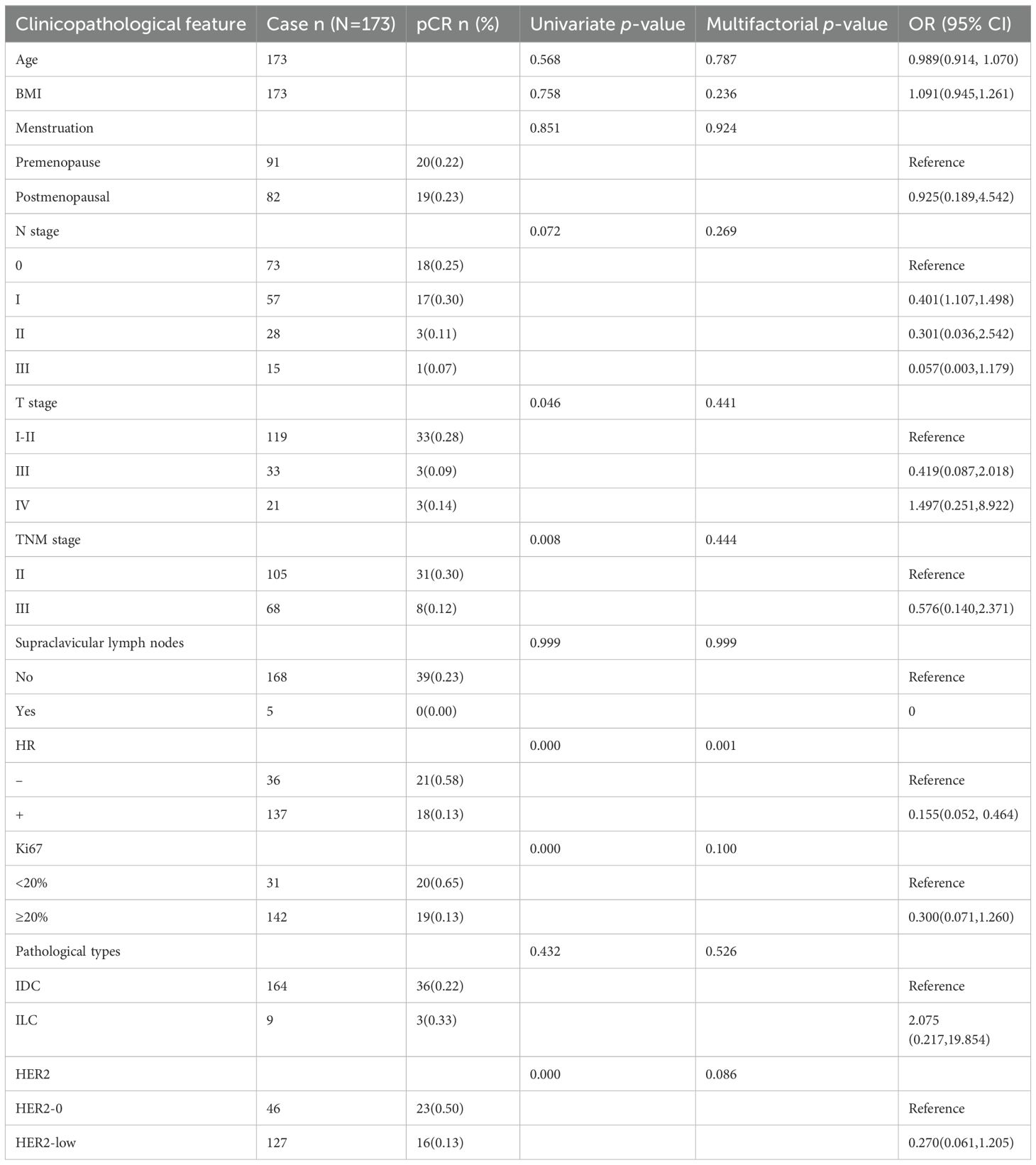
39/173 HER2-negative patients achieved pCR, with an overall rate of 22.54%. Univariate analysis identified T stage, TNM stage, HR status, HER2 status, and Ki67 as risk factors affecting the pCR rate. Moreover, patients with lower T stage, lower TNM stage, low Ki67, negative HR, and HER2-0 were likely to achieve pCR. Multifactorial analysis revealed that HR status has a significant effect on pCR and is an independent predictor of pCR rate: 13% in HR-positive (luminal type) patients compared to 58% in HR-negative (TNBC type) patients [odds ratio (OR) = 0.155, 95% confidence interval (95% CI): 0.052–0.464, p < 0.001]. The pCR rate of patients in the HER2-0 and HER2-low groups was 50% and 12.6%, respectively, indicating that the difference between the two groups was not statistically significant after multifactorial analysis (p = 0.086) (Table 2).
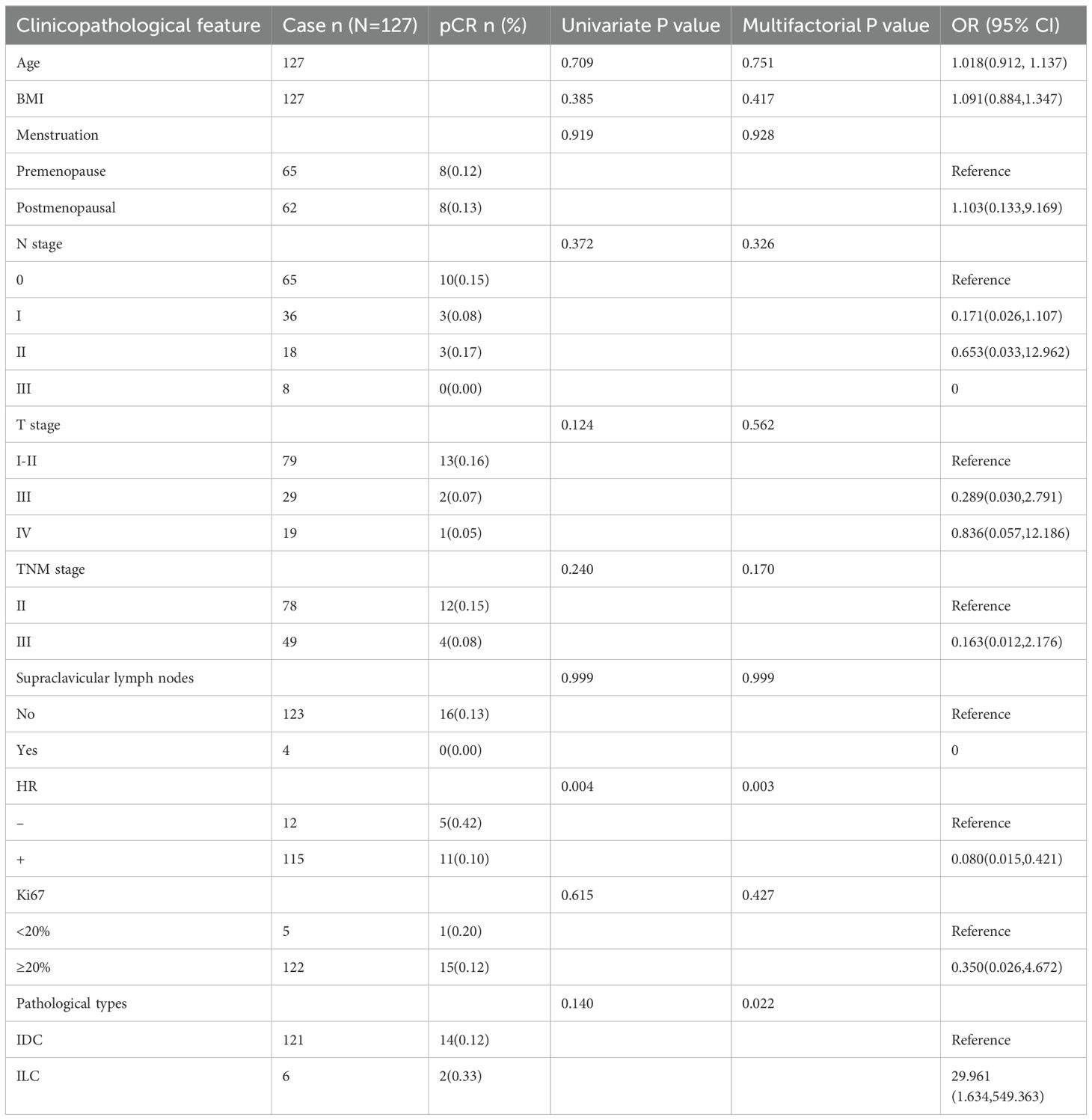
The analysis of factors influencing the pCR rate in the HER2-low subgroup revealed HR status as its independent predictor. The pCR rate was 42% in the HR-negative group and 10% in the HR-positive group (OR = 0.080, 95% CI: 0.015–0.421, p = 0.003; Table 3).
3.3 Survival analysis of low HER2 expression
68/173 patients had recurrence, metastasis, or were deceased; 6/68 were HER2-0, and the remaining 62 were HER2-low patients. The five-year DFS was 51.18% for HER2-low patients and 86.96% for patients in the HER2-0 group, and the difference was statistically significant (p < 0.001). Univariate Cox regression model analysis of overall survival in patients with low HER2 expression revealed that age, menstrual status, N stage, T stage, TNM stage, and pCR were factors that affected the prognosis of breast cancer patients (p < 0.05). Indicators with statistically significant results from univariate analysis were included in the Cox regression model for multivariate analysis. The results showed that N-stage, T-stage, and pCR were independent factors for the prognosis of patients with low HER2 expression breast cancers (p < 0.05) (Table 4).
Survival curves were plotted using the Kaplan–Meier method, and the results showed that menstrual status, N stage, T stage, TNM stage, and pCR factors affected the prognosis of HER2-low breast cancer (p < 0.05). The five-year DFS of premenopausal and postmenopausal patients was 58.5% and 43.5%, respectively (p = 0.017) (Figure 2A). The five-year DFS of the N0, N1, N2, and N3 groups was 64.6%, 36.1%, 38.9%, and 37.5%, respectively (p < 0.01) (Figure 2B). The five-year DFS of the T2, T3, and T4 groups was 65.8%, 34.5%, and 15.8%, respectively (p < 0.01) (Figure 2C). The five-year DFS reached 57.7% and 40.8% in TNM stages II and III, respectively (p < 0.01) (Figure 2D). Furthermore, the five-year DFS of patients with low HER2 expression who achieved pCR and those who were non-pCR after neoadjuvant therapy was 71.1% and 42.7%, respectively (p = 0.024) (Figure 2E).
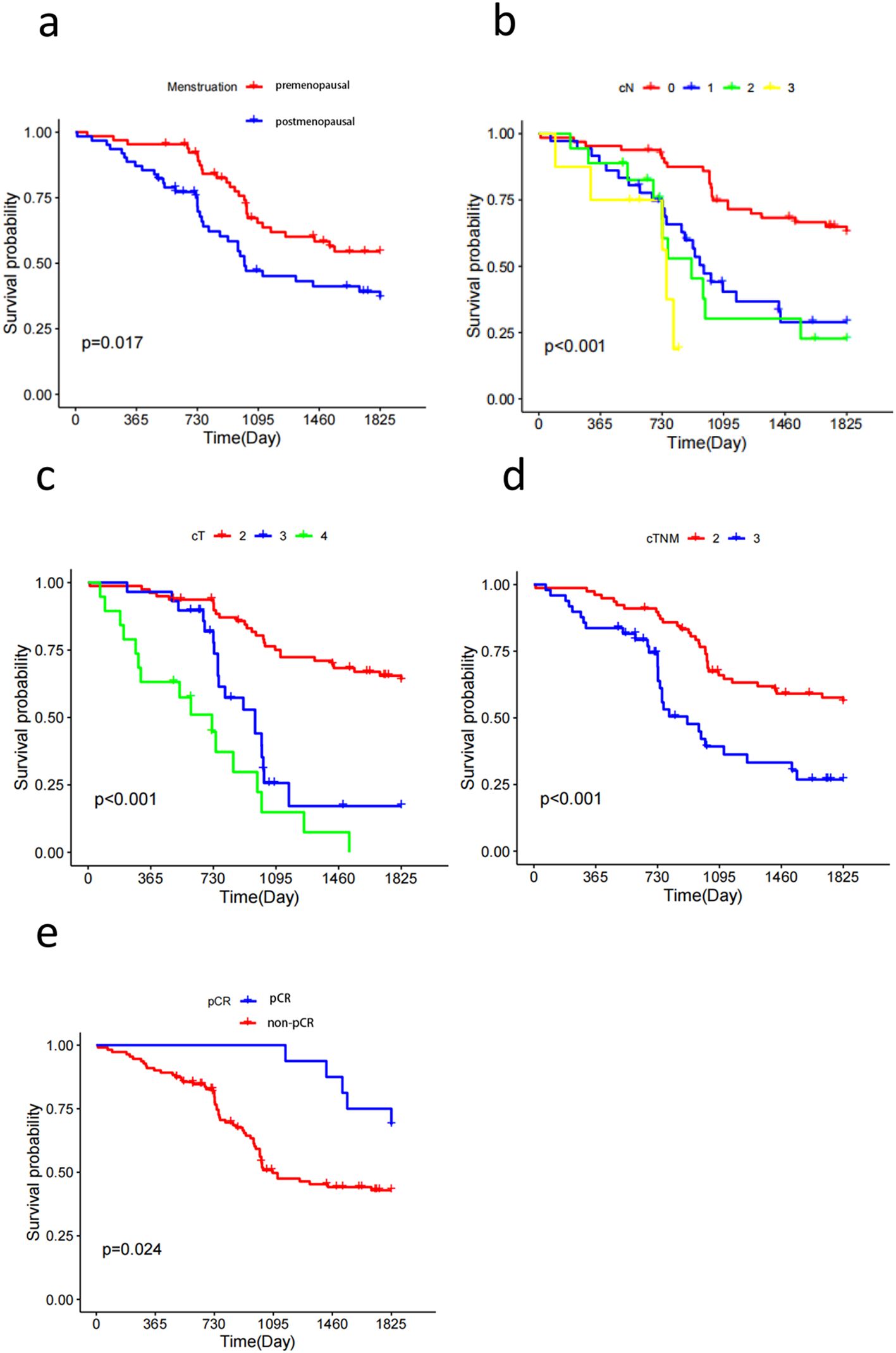
Figure 2. Kaplan-Meier survival curves show that Menstruation (A), cN (B), cT (C), cTNM (D), pCR (E) status are all factors affecting the prognosis of patients with Her2-low breast cancer.
4 Discussion
Recently, HER2 low-expressing breast cancer has gained increasing attention with the development of novel anti-HER2 targeted agents. HER2-low early-stage breast cancer appears to be a distinct biological entity. Further analysis revealed that factors such as menstrual status, N stage, T stage, TNM stage, and pCR affect the prognosis of patients with HER2-low breast cancer.
Previous studies have reported different clinical features of HER2-low breast cancer. Patients with low HER2 expression were often lymph node-positive. In addition, ductal histology revealed higher tumor grading and proliferation (11) characterized by the clinicopathological characteristics of breast cancers with varied levels of HER2 protein. Patients stratified according to HER2 status had similar age, menopausal status, and pathological stage distributions; however, statistical differences were noted in their histological types, histological grades, HR status, and Ki67 expression levels. Denkert et al. collected data of 2,310 patients with HER2-0 primary breast cancer from four prospective neoadjuvant clinical trials (12) and observed distinct clinical features of HER2-low breast cancer, such as significantly higher HR positivity, significantly lower Ki-67 expression, and lower pCR with neoadjuvant chemotherapy but longer overall survival. Another study (13) demonstrated that in patients with HR-positive breast cancer, HER2-low breast cancer was associated with fewer T4 tumors, high histologic grade, and negative lymphatic infiltration. In patients with TNBC, HER2-low was associated with a high lymph node ratio and positive lymphatic invasion. The current study showed that HER2-low patients had high T stage, high Ki67 index, low N stage, low pCR rate after neoadjuvant chemotherapy, high HR positivity, and high proportion of luminal type. Although the current findings were in line with previous studies, which concluded that HER2-low breast cancer has different clinicopathological features from HER2-0, there is no consensus on the specific manifestations.
The present study also analyzed the factors influencing the efficacy of neoadjuvant chemotherapy in HER2-negative patients. HR status was an independent predictor of pCR rate, whereas differential HER2 protein expression did not affect the pCR rate in multivariate analysis. In our study, we found that among all patients with HER2-negative breast cancer undergoing neoadjuvant treatment, HR-negative patients exhibited a higher pCR rate, which is consistent with previous research findings. For HR-positive, HER2-negative patients, chemotherapy can reduce the size of HR-positive tumors, aiding in optimizing surgical options. However, compared to histological types with higher proliferation rates, such as triple-negative breast cancer, it is more challenging for HR-positive breast cancer to achieve pathological complete response. On the other hand, univariate analysis revealed a pCR rate of 12.6% in HER2-low patients and 50% in HER2-0 patients (p < 0.001); however, the difference was not statistically significant after multivariate analysis (p = 0.086). Our study also suggested that HER2-low is less likely to achieve pCR in patients with HER2-0 breast cancer, consistent with previous findings (12, 14). Denkert et al. showed that HER2-low-positive tumors had a significantly lower pCR rate than HER2-0 tumors [321/1098 (29.2%) vs. 473/1212 (39.0%), p = 0.0002) (12). Another study found that HER2-low patients had significantly lower pCR rates with neoadjuvant chemotherapy than HER2-0 patients (15.9% vs. 37.5%, p = 0.042) (14). HER2-low tumors are frequently HR-positive (9), which was confirmed in our cohort with 79.19% of HR-positive tumors. This high proportion of HR-positive breast cancer explains our low pCR rate of 22.54%, which is lower than that expected for TNBC or HER2-positive tumors (15).
Finally, our study also analyzed the factors affecting the prognosis of HER2-low patients. Currently, the prognosis of HER2-overexpressing breast cancer is controversial. Denkert et al. concluded that patients with HER2-low-positive tumors had a significantly longer DFS than those with HER2-0 tumors [three-year rate: 83.4% (95% CI: 80.5–85.9) vs. 76.1% (95% CI: 72.9–79.0)] (12). Tarantino et al. (16) and other previous studies (17, 18) did not demonstrate marked differences in pCR rate or clinical outcomes between HER2-low and HER2-0 in multivariate analysis. Furthermore, we found that the five-year DFS was 51.18% in HER2-low patients and 86.96% in the HER2-0 group (p < 0.001). These conflicting results make distinguishing between HER2-low and HER2-0 difficult when using the traditional HER2 scoring system. The HER2 assay identified patients with HER2 overexpression tumors who benefited from trastuzumab; however, it has a limitation when evaluating the low levels of HER2 expression (19). This phenomenon could be ascribed to the wide variable incidence of HER2-low in retrospective studies: from 16.2% (20) to 64.4% (21). These findings suggested that precise diagnostic methods are essential to distinguish HER2-low breast cancer from HER2-0 breast cancer. The current study analyzed the factors affecting the prognosis of HER2-low patients; menstrual status, N-stage, T-stage, TNM-stage, and pCR affected the prognosis of patients with HER2-low breast cancer (p < 0.05). These results also provided a theoretical basis for evaluating the prognosis of HER2-low breast cancer patients after neoadjuvant chemotherapy.
Nevertheless, the present study has several limitations, such as the small sample size, its retrospective nature, absence of central pathological review and patient inclusion across a large time period, during which different guidelines for HER2 testing and interpretation were in use. It also provides some clues to the clinical and biological behaviors of the HER2-low breast cancer population that has a low pCR rate after neoadjuvant therapy and could benefit from new therapies. Currently, long-term follow-up of patients is limited; therefore, caution should be exercised while interpreting survival associations.
5 Conclusions
The current results have significant implications for future diagnostic concepts, a general understanding of the disease, and the development of new therapeutic strategies. The market launch of novel ADC drugs represented by T-DXd, especially the DESTINY-Breast 04 study results, has put forth a novel perspective on the diagnosis and treatment of HER2-low breast cancer. Both clinical and basic research have shown that HER2-low breast cancer exhibits distinct biological behaviors that may be associated with prognosis. This study also suggested that HER2-low breast cancer constitutes a significant proportion of the population and differs from HER2-0 in terms of clinicopathological features and prognostic characteristics, providing novel insights for individualized precision treatment of low HER2-expressing breast cancer patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Shanxi Bethune Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HZ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. YY: Conceptualization, Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Science Foundation of Shanxi Health Commission, grant number 2022049.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. (2006) 25:118–45. doi: 10.1200/JCO.2006.09.2775
2. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. (2013) 31:3997–4013. doi: 10.1200/jco.2013.50.9984
3. Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-college of American pathologists guideline update. J Clin Oncol. (2023) 41:3867–72. doi: 10.1200/jco.22.02864
4. Martínez-Sáez O, Prat A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol Pract. (2021) 17:594–604. doi: 10.1200/op.21.00172
5. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. (1987) 235:177–82. doi: 10.1126/science.3798106
6. Cronin KA, Harlan LC, Dodd KW, Abrams JS, Ballard-Barbash R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. (2010) 28:963–8. doi: 10.3109/07357907.2010.496759
7. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase ib study. J Clin Oncol. (2020) 38:1887–96. doi: 10.1200/jco.19.02318
8. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
9. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. (2020) 38:1951–62. doi: 10.1200/jco.19.02488
10. Xu B, Hu X, Feng J, Geng C, Jin F, Li H, et al. Chinese expert consensus on the clinical diagnosis and treatment of advanced breast cancer (2018). Cancer. (2020) 126 Suppl 16:3867–82. doi: 10.1002/cncr.32832
11. Eggemann H, Ignatov T, Burger E, Kantelhardt EJ, Fettke F, Thomssen C, et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer. (2015) 22:725–33. doi: 10.1530/erc-15-0335
12. Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. (2021) 22:1151–61. doi: 10.1016/s1470-2045(21)00301-6
13. Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. (2022) 24:22. doi: 10.1186/s13058-022-01519-x
14. Zhang G, Ren C, Li C, Wang Y, Chen B, Wen L, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. (2022) 20:142. doi: 10.1186/s12916-022-02346-9
15. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res. (2020) 26:2838–48. doi: 10.1158/1078-0432.ccr-19-3492
16. Tarantino P, Jin Q, Tayob N, Jeselsohn RM, Schnitt SJ, Vincuilla J, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. (2022) 8:1177–83. doi: 10.1001/jamaoncol.2022.2286
17. Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. (2021) 7:1. doi: 10.1038/s41523-020-00208-2
18. Hein A, Hartkopf AD, Emons J, Lux MP, Volz B, Taran FA, et al. Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. Eur J Cancer. (2021) 155:1–12. doi: 10.1016/j.ejca.2021.06.033
19. Moutafi M, Robbins CJ, Yaghoobi V, Fernandez AI, Martinez-Morilla S, Xirou V, et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest. (2022) 102:1101–8. doi: 10.1038/s41374-022-00804-9
20. Jacot W, Maran-Gonzalez A, Massol O, Sorbs C, Mollevi C, Guiu S, et al. Prognostic value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers (Basel). (2021) 13:6059. doi: 10.3390/cancers13236059
Keywords: breast cancer, low expression of human epidermal growth factor receptor 2, neoadjuvant chem-otherapy, pathological complete response, prognostic factors
Citation: Yao Y and Zhen H (2024) Efficacy and prognosis of neoadjuvant chemotherapy in HER2 low-expressing breast cancer: a retrospective single-center study. Front. Oncol. 14:1454726. doi: 10.3389/fonc.2024.1454726
Received: 05 July 2024; Accepted: 09 September 2024;
Published: 25 September 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Ami Patel, NewYork-Presbyterian, United StatesCarolin Müller, Saarland University Hospital, Germany
Copyright © 2024 Yao and Zhen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huifen Zhen, emhlbmh1aWZlbjE5ODIwNjE4QDE2My5jb20=
 Yarong Yao
Yarong Yao Huifen Zhen
Huifen Zhen