- 1Department of Oncology, ASUFC University Hospital, Udine, Italy
- 2Department of Medical Oncology, San Giovanni di Dio Hospital, Crotone, Italy
- 3Immunopathology and Cancer Biomarkers/Bio-Proteomics Facility, Centro di Riferimento Oncologico, IRCCS, Aviano, Italy
- 4Department of Medical Oncology, Centro di Riferimento Oncologico di Aviano (CRO) IRCCS, Aviano, Italy
- 5Pathology Unit, IRCCS CRO National Cancer Institute, Aviano, Italy
- 6Department of Medical, Surgical and Health Sciences, University of Trieste, Trieste, Italy
- 7Department of Oncology, University and General Hospital, Udine, Italy
- 8Department of Oncology, San Bortolo General Hospital, Vicenza, Italy
- 9Division of Thoracic Oncology, European Institute of Oncology (IEO) IRCCS, Milano, Italy
- 10Department of Biochemistry and Molecular Pharmacology, IRCCS-Istituto di Ricerche Farmacologiche “Mario Negri”, Milan, Italy
- 11Departiment of Medicine, University of Udine, Udine, Italy
Background: Gastric cancer is a heterogeneous collection of tumors characterized by low survival rates. All-trans retinoic acid (retinoic-acid) is a clinically useful therapeutic agent belonging to the chemical family of retinoids, which consists of both natural and synthetic derivatives of vitamin-A. Retinoids are essential components of the normal diet and they regulate different physiological processes. From a therapeutic point of view, retinoic-acid is the first example of clinically useful differentiating agent. Indeed, the differentiating properties of this compound have promoted the use of retinoic-acid as a standard of care in Acute-Promyelocytic-Leukemia, a rare form of acute myeloid leukemia. In this study, we determine the RNA expression of the six isoforms of Retinoic-Acid-Receptors (RARα/RARβ/RARγ/RXRα/RXRβ/RXRγ) in view of their potential use as gastric cancer progression markers and/or therapeutic targets. In addition, we evaluate associations between the expression of these receptors and a simplified molecular classification of stomach tumors as well as the clinical characteristics of the cohort of patients analyzed. Finally, we define the prognostic value of the various Retinoic-Acid-Receptors in gastric cancer.
Methods: In this single institution and retrospective RAR-GASTRIC study, we consider 55 consecutive gastric cancer patients. We extract total RNA from the pathological specimens and we perform a NanoString Assay using a customized panel of genes. This allows us to determine the expression levels of the RAR and RXR mRNAs as well as other transcripts of interest.
Results: Our data demonstrate ubiquitous expression of the RAR and RXR mRNAs in gastric cancers. High levels of RARα, RARβ, RXRα and RXRβ show a significant association with stage IV tumors, “de novo” metastatic disease, microsatellite-stable-status, epithelial-to-mesenchymal-transition, as well as PIK3CA and TP53 expression. Finally, we observe a worse overall-survival in gastric cancer patients characterized by high RARα/RARβ/RARγ/RXRβ mRNA levels.
Conclusions: In gastric cancer, high expression levels of RARα/RARβ/RARγ/RXRβ transcripts are associated with poor clinical and molecular characteristics as well as with reduced overall-survival. Our data are consistent with the idea that RARα, RARβ, RARγ and RXRβ represent potential prognostic markers and therapeutic targets of gastric cancer.
Introduction
Gastric cancer is a heterogeneous type of tumor and it represents the 5th most common cause of cancer worldwide as well as the 3rd cause of cancer related death (https://www.iarc.who.int/news-events/latest-world-cancer-statistics-globocan-2012-estimated-cancer-incidence-mortality-and-prevalence-worldwide-in-2012/). Despite many improvements in the field of medical oncology, the survival rate of the various forms of gastric cancer remains low even following completion of cornerstone clinical trials (1, 2). Thus, an effective and personalized treatment of gastric cancer requires the development of novel therapeutic strategies based on a refinement of the available molecular classifications of stomach tumors. The scientific literature presents with two distinct molecular classifications of gastric cancer, which rely on the information available in the TCGA (The-Cancer-Genome-Atlas) (3) and ACRG (Asian-Cancer-Research-Group) websites (4). From these two classifications, some molecular subgroups have emerged as potentially responsive to specific treatments: MicroSatellite-Instability [MSI] and Epstein-Barr-Virus-[EBV]-positive tumors to immunotherapy; Chromosomal-Instability [CIN] tumors to targeted therapy (5). Although, these two classifications are of little interest in the clinical practice, because of the low reproducibility and elevated costs of the methodologies necessary to generate them. Although, these two classifications are of little interest in the clinical practice, because of the low reproducibility and elevated costs of the methodologies necessary to generate them, they may acquire interest in the coming era of precision oncology. Indeed, routine exome sequencing and Molecular Tumor Boards (MTBs) are likely to play an increasingly important role in optimizing treatment selection. In addition, routine exome sequencing and MTBs are expected to improve outcomes by reviewing and interpreting molecular-profiling data and matching patients with appropriate molecularly targeted therapies.
All-trans retinoic acid (ATRA) is a clinically useful therapeutic agent belonging to the chemical family of retinoids, which consists of both natural and synthetic derivatives of vitamin-A (retinol). Retinoids are essential components of the normal diet and they regulate different physiological processes, such as the embryonal development, the reproduction and maturation of cells found in regenerative tissues and the process of vision (6, 7). From a therapeutic point of view, ATRA is the first example of clinically useful differentiating agent. Indeed, the differentiating properties of this compound have promoted the use of ATRA as a standard of care in Acute-Promyelocytic-Leukemia, a rare form of acute myeloid leukemia (8, 9). In addition, ATRA is used in the clinical management of neuroblastoma (10). The exceptional clinical results obtained in Acute-Promyelocytic-Leukemia and neuroblastoma have raised interest in the use of ATRA for the prevention of pre-cancerous lesions and the treatment of solid tumors. With respect to this, it is important to mention that a number of available studies demonstrates the efficacy of ATRA in preventing and treating solid tumors such as leukoplakia, actinic keratosis and cervical dysplasia (11–13), while pre-clinical studies support the use of ATRA in the treatment of breast cancer (14–17). Finally, a number of pre-clinical and clinical studies provide insights into the therapeutic potential of ATRA in the context of gastric cancer (18–20).
Retinoid receptors, which are ligand-dependent transcription factors belonging to the family of nuclear steroid receptors (14, 21, 22), are the major mediators of the biological and pharmacological effects exerted by ATRA. Two classes of retinoid receptors are known, Retinoic-Acid-Receptors (RARα, RARβ, RARγ) and Retinoid-X-Receptors (RXRα, RXRβ, RXRγ), which act under the form of heterodimeric RAR/RXR complexes. In these complexes, RARs function as the ligand-binding moiety (14). RAR/RXR complexes are involved in the modulation of gene-transcription and regulate the expression of numerous target genes. ATRA binds and activates all the RAR/RXR isoforms with similar affinity and efficiency. In addition, selective RAR/RXR synthetic agonists, such as AM580 (RARα agonist), CD2314 (RARβ agonist), CD4317 (RARγ agonist) and Bexarotene (RXR agonist), as well as antagonists, like BMS195614 (RARα antagonist), CD2665 (RARβ/γ antagonist) and LG100754 (RXR antagonist), are available (14).
In this single-institution/retrospective clinical and translational study (RAR-GASTRIC), we define the expression of RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ in stomach tumors with the use of archival tissue samples obtained from gastric cancer patients. We investigate the associations between each RAR/RXR isotype and the clinical/biological characteristics of gastric cancer. In addition, we provide information regarding the associations between the RAR/RXR receptors and a simplified molecular classification derived from the TCGA (The-Cancer-Genome-Atlas) as well as the ACRG (Asian-Cancer-Research-Group) databases. Finally, we define the potential prognostic impact of RARs and RXRs on the overall-survival of gastric cancer patients. The work supports the idea that RARα, RARβ, RARγ and RXRβ represent potential prognostic markers and actionable therapeutic targets in the context of the personalized treatment of gastric cancer.
Materials and methods
Study design
RAR-GASTRIC is a single-institution/retrospective clinical and translational study. In RAR-GASTRIC, we retrospectively collected and analyzed all the consecutive gastric cancer patients with archival tissue specimens available in the Pathology Unit of the IRCCS CRO National Cancer Institute of Aviano with confirmed diagnoses (from January 1, 2010 to March 30, 2019). We retrieved the data from electronic and paper-based chart reviews according to strict privacy standards. All the patients included in the study signed an anonymous GE.CO (GESTIONE.CONTROLLO) consent for the use of clinical data in the context of clinical research, epidemiology, training and disease-induction/-progression studies. The Departmental Review Board and the internal Ethics Committee (Protocol number CRO-2019-74) approved the RAR-GASTRIC study. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Departmental Review Board and by the Ethics Committee of CRO Oncology Reference Center of Aviano, Italy (Protocol number CRO-2019-74). All included patients have signed a consent for the use of clinical data with the purpose of retrospective analyses aimed at clinical research. Informed consent was obtained from all subjects involved in the study.
Objectives of the study
The primary objective of the RAR-GASTRIC study was to elucidate the expression and distribution of RARs and RXRs in the general population of gastric cancer patients. The secondary objectives of the study were: 1) to determine associations between the expression of the various RAR/RXR mRNAs and clinical factors, such as stage, anatomical site and simplified molecular classifiers, which are available in the TCGA and ACRG datasets; 2) to characterize the prognostic impact of RARs/RXRs expression on overall-survival.
Sample preparation and RNA-expression analysis
Paraffin embedded tissues (core biopsies and gastrectomy samples) were sliced and reviewed by an expert pathologist. We extracted total RNA from the primary tumor samples obtained from biopsies or after surgery at diagnosis, using the RNeasy DSPE FFPE kit (Qiagen) and we assessed RNA quality using the High Sensitivity RNA ScreenTape kit (Agilent) and the 2200 TapeStation system (Agilent). We performed RNA quantification with the Qubit™ RNA HS assay (Invitrogen) using the Qubit fluorometer 2.0. (Invitrogen). For each sample, we hybridized RNA (250 ng) with a customized panel of gene-probes (see below). We performed RNA detection and scanning using the NanoString nCounter Analysis System (NanoString Technologies) according to the manufacturer’s instructions. The background level of each tumor sample was obtained by subtraction of the Mean ± 2SD of the counts of the negative control probes included in the assay. We normalized the data using the geometric mean of the positive controls and the housekeeping genes (B2M, GAPDH, HPRT1 and RPL19) included in the custom panel. We performed data analyses using the nSolver 4.0 software (NanoString Technologies). We employed the Log2 transformed mRNA expression values for the analyses. The genes represented in our customized panel were: CDH1 (Cadherin-1), CDKN1A (Cyclin-Dependent-Kinase-Inhibitor-1A), EBER1 (Epstein–Barr-Virus–Encoded-small-RNA1), MDM2 (MDM2-Proto-Oncogene), MLH1 (MutL-Homolog-1), MSH2 (MutS-Homolog-2), MSH6 (MutS-Homolog-6), PIK3CA (Phosphatidylinositol-4,5-Bisphosphate-3-Kinase-Catalytic-Subunit-Alpha), PMS2 (PMS1-Homolog-2, Mismatch-Repair-System-Component), RARα (Retinoic-Acid-Receptor-Alpha), RARβ (Retinoic-Acid-Receptor-Beta), RARγ (Retinoic-Acid-Receptor-Gamma), RXRα (Retinoid-X-Receptor-Alpha), RXRβ (Retinoid-X-Receptor-Beta), RXRγ (Retinoid-X-Receptor-Gamma), TP53 (Tumor-Protein-P53) and ZEB1 (Zinc-Finger-E-Box-Binding Homeobox1). The normalization genes were: B2M (Beta-2-Microglobulin), GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase), HPRT1 (Hypoxanthine-Phosphoribosyltransferase-1) and RPL19 (Ribosomal-Protein-L19).
Associations between overall-survival and gastric cancer patients’ clinical characteristics
We collected the data on sex, age, histology type, primary tumor site, stage at diagnosis, primitive-tumor resection, date of diagnosis of the metastatic disease, types and lines of treatment received (Supplementary Table S1). For the calculation of the overall-survival values, we used the time between the start of the treatment and death for any cause. For the calculation of the overall-survival-after-metastatic-disease values, we calculated the time between diagnosis of the metastatic disease and death for any cause. Given the observational nature of the study, we did not calculate any formal sample size. We summarized the patients’ clinical-pathological characteristics via descriptive analysis. We defined categorical variables on the basis of the frequency distribution, while we described continuous variables using the Mean ± SD, the Median of the first and third quartile (Q1-Q3) and the minimal as well as maximal values. We performed Chi-square or Fisher’s exact test, as appropriate, and Kruskal-Wallis tests to compare the distributions of the categorical and continuous variables, respectively. We explored the potential effects of the various RARs and RXRs on overall-survival and overall-survival-after-metastatic-disease with the use of COX proportional hazard models, which we adjusted for the demographic and clinical prognostic characteristics of the patients. The results of these analyses were expressed as Hazard-Ratios (HRs) and 95% Confidence-Intervals (95%-CIs). We estimated the survival curves according to the Kaplan-Meier method and we compared them using the log-rank test. For bilateral tests, statistical significance was set at p<0.05. We performed subgroup analyses according to the clinical-pathological features and the molecular classifications. We performed statistical analyses using STATA (StataCorp. [2015] Stata Statistical Software: Release 14.2. College Station, TX: StataCorp LP).
Results
The cohort of gastric cancer patients: demographic characteristics
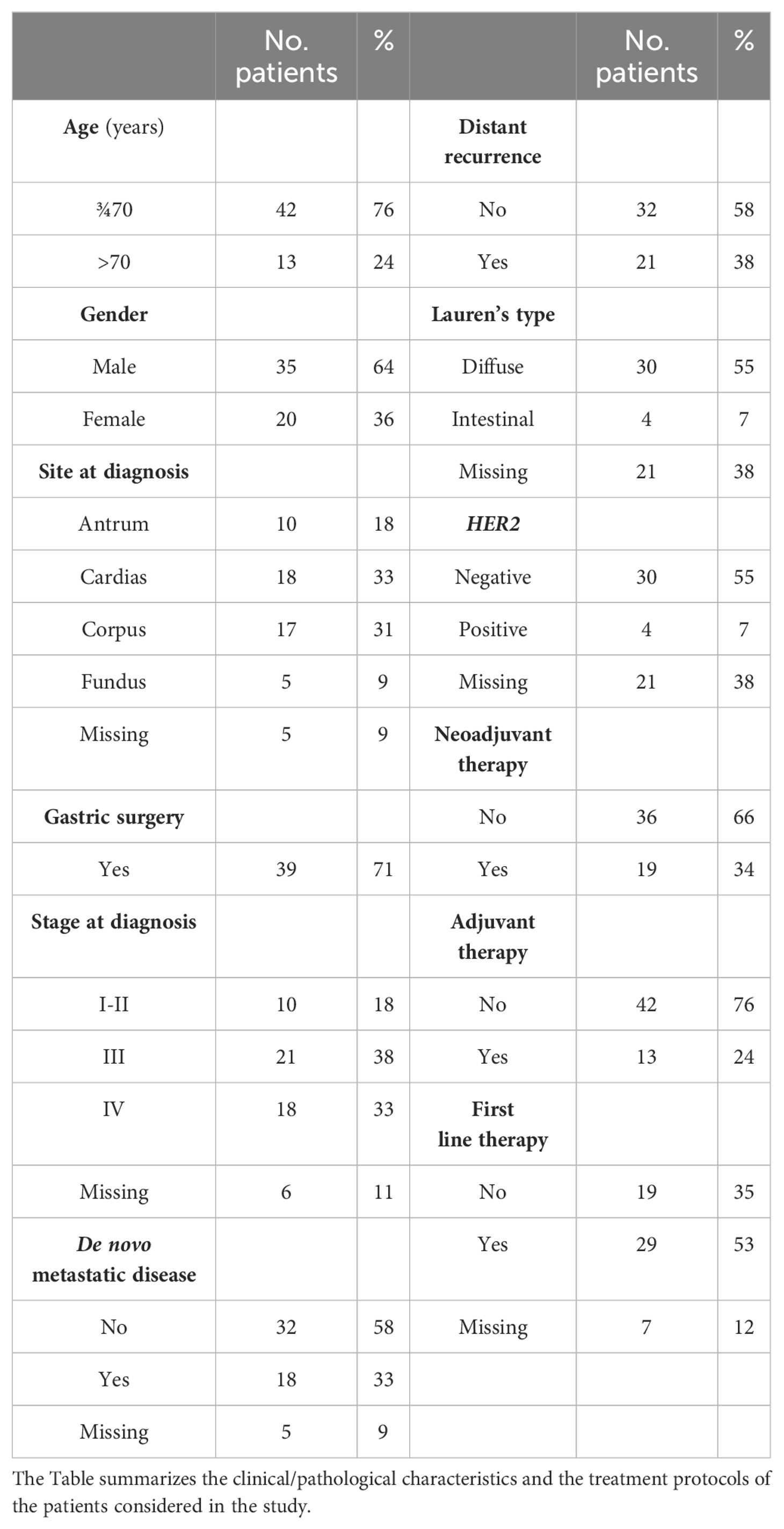
The total population of gastric cancer patients considered in the RAR-GASTRIC study consisted of 55 individuals (Table 1; Supplementary Table S1). To simplify the analysis of our data, we set a threshold age value of 70 to divide our population into two groups. In fact, this age is currently considered the standard threshold value above which aged Italian individuals are separated from the remainder of the Italian population. Forty-two patients (76%) were aged below 70 years, while 13 patients (24%) were aged above 70 years. Thirty-five patients (64%) were males, while 20 patients (36%) were females. Approximately one third of the primary stomach tumors localized into the cardias (33%) or in the corpus (31%), while the remaining cases localized into the antrum (18%) and fundus (9%), respectively. Thirty-nine patients (71%) underwent gastrectomy. At diagnosis, 18% of the cases showed early-stage tumors (stage-I/stage-II). In contrast, 38% and 33% of the patients suffered from locally advanced (stage-III) and advanced (stage-IV) tumors, respectively. Significantly, 18 cases (33%) suffered from de novo metastatic gastric cancer. Finally, 21 of the 39 individuals (38%), who underwent gastrectomy, experienced a relapse.
As for the pathological characteristics (Table 1), the prevalent type of gastric cancer observed in our cohort of patients for whom data were available, was the Lauren’s Diffuse type (30 individuals = 55%). By converse, only a small fraction of patients (4 individuals; 7%) showed a Lauren’s Intestinal type of gastric cancer. In addition, a HER2-positive phenotype was evident in 18% of the gastric cancer cases. Finally, 25% of the tumor specimens presented with an MSI (MicroSatellite-Instability) status, due to a defective MMR (DNA-Mismatch-Repair) process, as indicated by the gene expression data (see Figure 1, lowest panel).
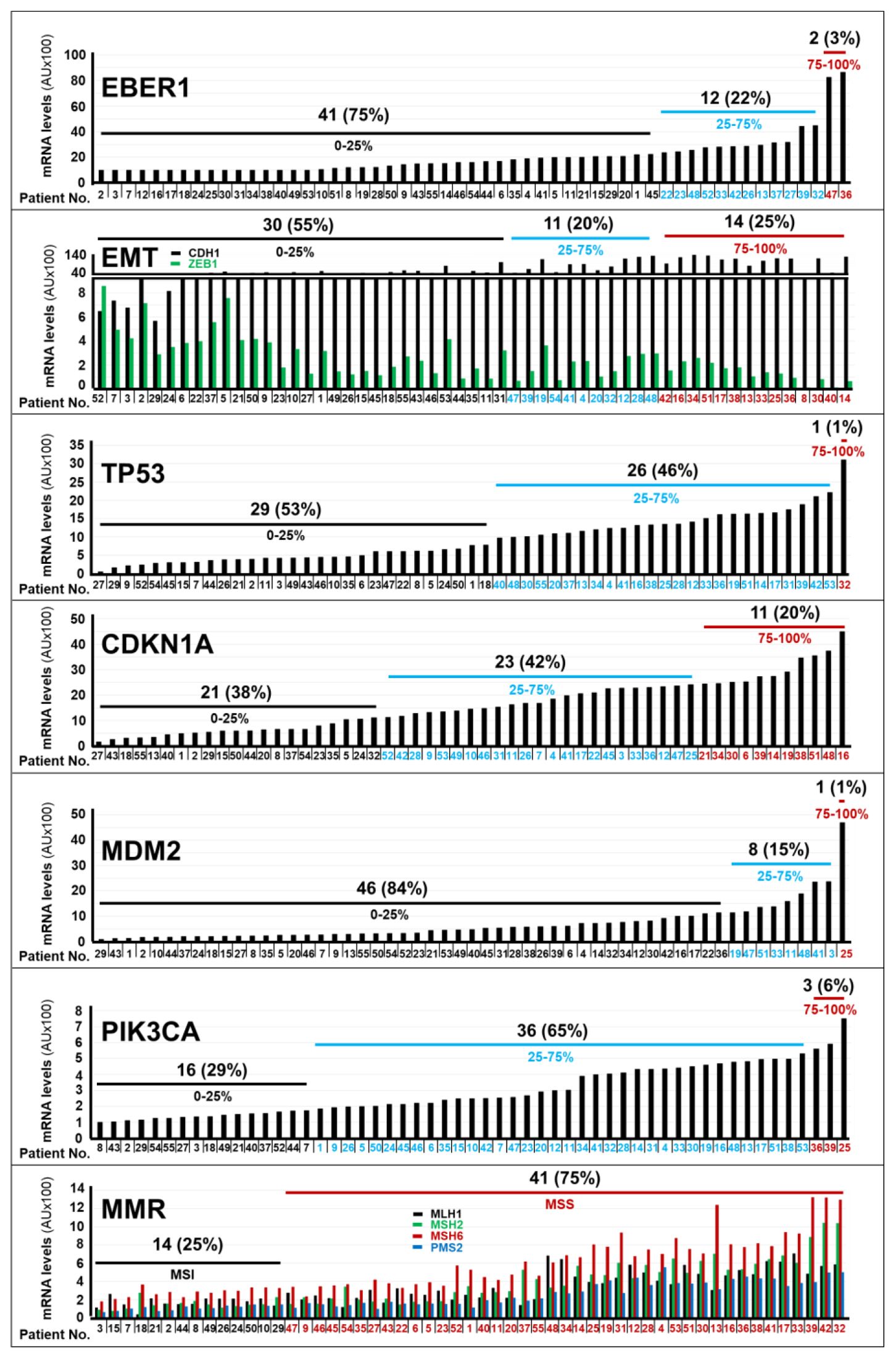
Figure 1. Expression levels of the mRNAs coding for different marker proteins in gastric cancer specimens. The bar-graphs illustrate the expression levels of the indicated mRNAs in the tumor samples of each cohort patient, which were determined with the use of the NanoString nCounter Analysis System. The genes considered are involved in EBV (Epstein-Barr-Virus) positivity (EBER1), in EMT (Epithelial to Mesenchymal Transition; CDH1/ZEB1), in the control of the TP53 pathway (TP53/CDKN1A/MDM2), in the molecular landscape of gastric cancer (PIK3CA) and in the control of the MMR (Mutation-Mismatch-Repair; MLH1/MSH2/MSH6/PMS2) process. The number and percentage of patients (black values) presenting with the indicated expression quartiles (black blue and red values) of the various mRNAs considered. *We determined the normalized expression-levels (see Materials and Methods) of the indicated mRNAs in tumor tissues and we calculated the expression-level percentiles. We divided the calculated expression-level percentiles in tertiles, as indicated. Normalization mRNAs = B2M (Beta-2-Microglobulin); GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase); HPRT1 (Hypoxanthine-Phosphoribosyltransferase-1); RPL19 (Ribosomal-Protein-L19). Acronyms of the mRNAs considered: CDH1, Cadherin-1; ZEB1, Zinc-Finger-E-Box-Binding Homeobox1; TP53 = Tumor-Protein-P53, CDKN1A, Cyclin-Dependent-Kinase-Inhibitor-1A; EBER1, Epstein–Barr-Virus–Encoded-small-RNA1; MDM2, MDM2-Proto-Oncogene; MLH1, MutL-Homolog-1; MSH2, MutS-Homolog-2; MSH6, MutS-Homolog-6; PIK3CA, Phosphatidylinositol-4,5-Bisphosphate-3-Kinase-Catalytic-Subunit-Alpha; PMS2, PMS1-Homolog-2, Mismatch-Repair-System-Component; MSI, MicroSatellite-Instability; MSS, MicroSatellite-Stability.
In terms of treatment (Table 1), approximately one third (18/55; 33%) and one quarter (13/55; 24%) of the patients were subjected to neo-adjuvant and adjuvant therapy, respectively. Data on first-line treatment were available for 48 patients and they indicated that 29 of the cases (53%) underwent this type of therapeutic strategy. Further details on the specific treatment(s) applied to each patient are available in Supplementary Table S1.
Molecular characteristics and expression levels of the Retinoic Acid Receptors in gastric cancer
We approximated the RAR-GASTRIC set of tumor specimens to the molecular classification of gastric cancer available in the TCGA (The-Cancer-Genome-Atlas) and ACRG (Asian-Cancer-Research-Group) datasets (3, 4), by determining the RNA-expression levels of the 17 probes under study, which were contained in our customized panel of genes (Nanostring technology, see MATERIALS AND METHODS). The tumor tissues of only 2 patients (3%) showed high levels of the EBER1 mRNA, a transcript coding for an EBV (Epstein-Barr-Virus) marker (Figure 1). This indicated that EBV-negative stomach tumors affected the vast majority of our patients. In addition, gastric cancer samples from 14 patients (25%) presented with a transcriptomic profile (low levels of CDH1; high levels of ZEB1) consistent with the presence of an EMT (Epithelial-to-Mesenchymal-Transition) phenotype (Figure 1). Only one patient expressed high levels of the TP53 mRNA (Figure 1). With respect to the TP53 pathway, it is interesting to notice that we found high expression levels of the CDKN1A mRNA in the tumor samples of 11 patients (20%) and large amounts of the MDM2 (p53-binding protein) mRNA in a single patient (Figure 1). This information is relevant, as the CDKN1A and MDM2 genes code for proteins involved in the control of p53 levels and activity. As for other genes of interest in the molecular landscape of gastric cancer, the neoplastic tissues of 3 patients (6%) were characterized by high levels of the PIK3CA mRNA. Finally, the expression profiles of genes involved in the Mutation-Mismatch-Repair (MMR) system (MLH1/MSH2/MSH6/PMS2) indicated that the vast majority of gastric cancer samples (41; 75%) showed a Micro-Satellite-Stability (MSS) phenotype. By converse, only a minority (14; 25%) of stomach tumors presented with a Micro-Satellite-Instability (MSI) phenotype (Figure 1).
In our cohort of 55 patients, we determined the relative expression levels of the mRNAs coding for the RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ receptors, with the use of our Nanostring data, (Figure 2). To perform this analysis, we measured the “Absolute-Amount” ranges of each receptor mRNA in our panel of tumor samples. The relative expression levels of the RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ mRNAs were classified in 3 groups: “High” (75-100% maximal value), “Intermediate” (25-75% maximal value) and “Low” (0-25% maximal value). Following this type of analysis, 5%, 53% and 42% of the tumor samples showed high, intermediate and low expression levels of RARα, respectively. High levels of RARβ mRNA were evident in 14% of the specimens. By converse, 22% of the tumors presented with intermediate levels of the RARβ mRNA and we observed low levels of the transcript in 64% of the neoplastic tissues. In general, RARγ expression was deficient, as 93% of the patients presented with tumors characterized by low relative levels of this receptor isoform. A minority of cases (9%) expressed high levels of the RXRα transcript, while a large proportion of tumors showed intermediate (44%) or low (47%) levels of this mRNA. Finally, we observed intermediate amounts of the RXRβ mRNA in most of our samples (55%), whereas the RXRγ transcript showed a tendency towards low expression levels (66% of the cases). At present the molecular mechanisms underlying the selective upregulation of certain RAR/RXR isotypes in different subgroups of gastric cancer are unknown and further studies will be necessary to clarify the point.
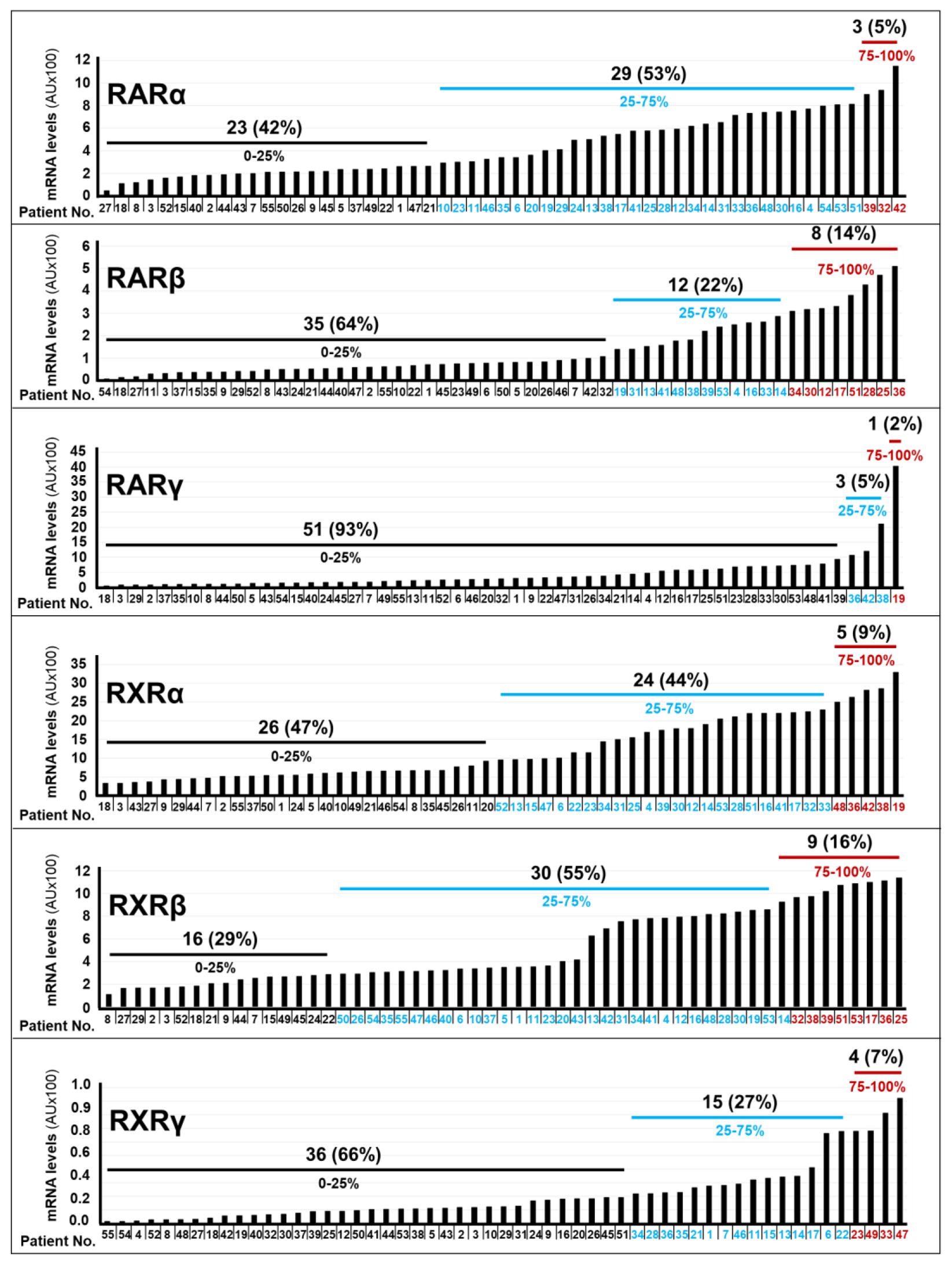
Figure 2. RAR/RXR mRNA levels in gastric cancer specimens. The bar-graphs illustrate the expression levels of the indicated RAR/RXR mRNAs in the tumor samples of each cohort patient, which were determined with the use of the NanoString nCounter Analysis System. The number and percentage of patients (black values) presenting with the indicated quartile (black blue and red values) of RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ mRNA levels.
RARs/RXRs associations with gastric cancer clinical and molecular characteristics
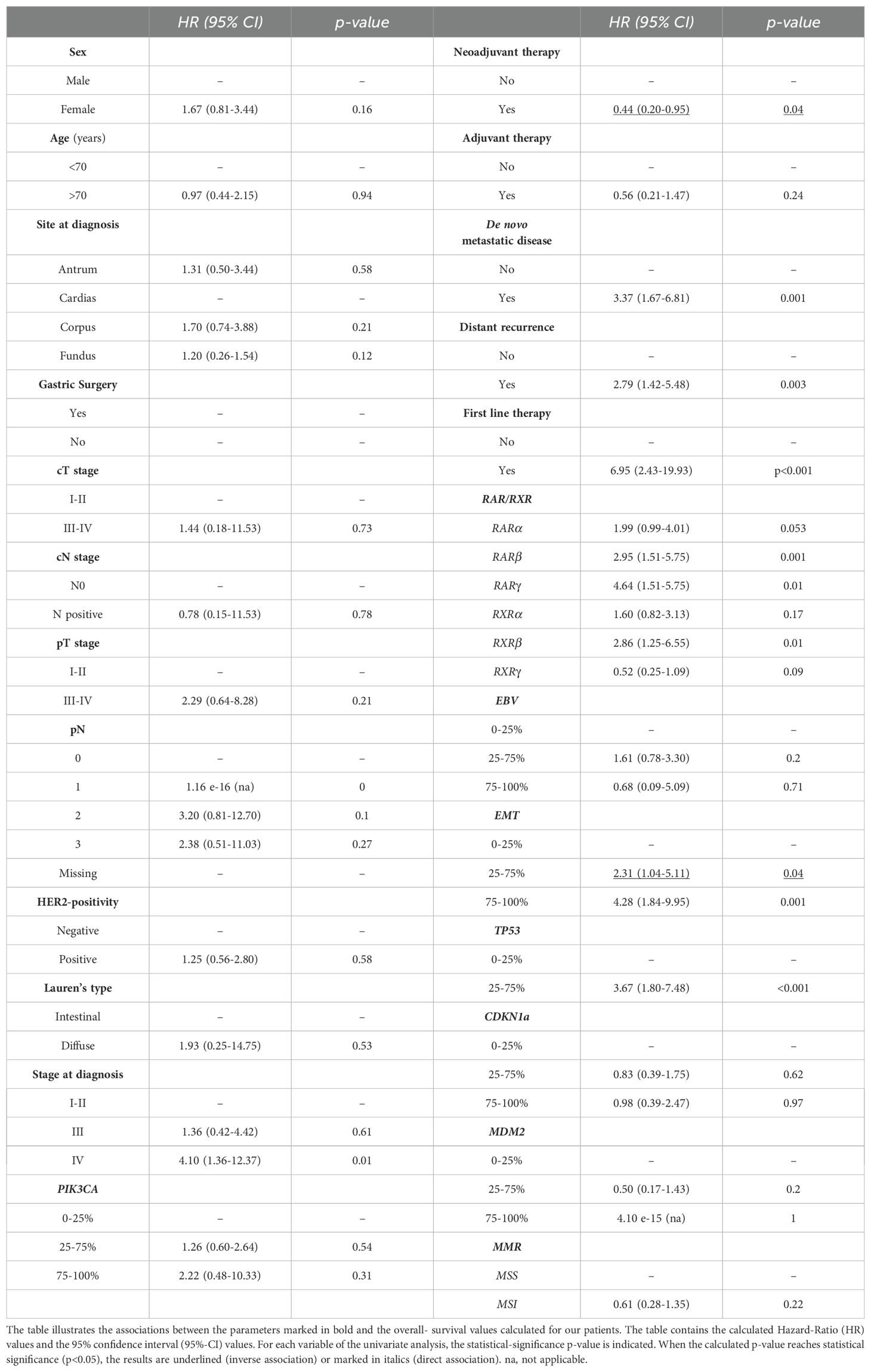
We investigated whether the expression levels of the various RAR/RXR isotypes (Figures 3, 4) showed any statistical association with the clinical/molecular features of gastric cancer, which are listed in Table 1. As indicated in Figure 3, high levels of the RARα and RARβ mRNAs correlated with: stage-IV gastric cancer (RARα, p= 0.004; RARβ, p=0.004), de novo metastatic disease (RARα, p=0.007; RARβ, p<0.001), MSS (RARα, p<0.001; RARβ, p<0.001), EMT (RARα, p<0.001; RARβ, p<0.001) as well as high levels of the PIK3CA (RARα, p<0.001; RARβ, p<0.001), CDKN1A (RARα, p=0.015; RARβ, p<0.001) and TP53 (RARα, p= 0.015; RARβ, p<0.001) mRNAs. By converse, high expression levels of the RARγ transcript presented significant associations only with EMT (p=0.04) and the content of TP53 mRNA (p= 0.03).
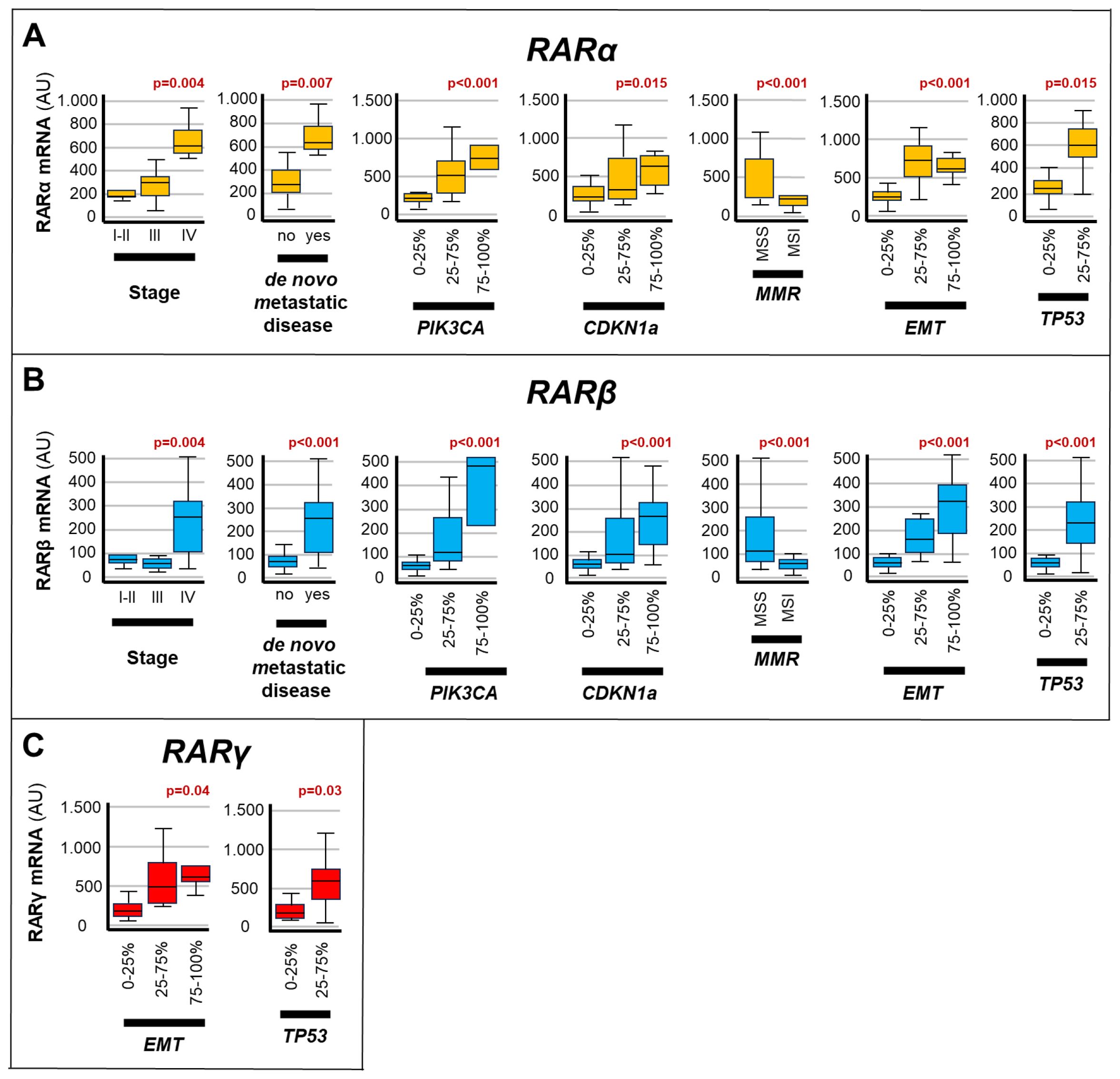
Figure 3. Associations between RARs levels and the clinical/molecular characteristics of gastric cancers. The box plots show the relative levels of the RARα (A), RARβ (B) and RARγ (C) mRNAs in the indicated subgroups of gastric cancers. The relative levels of the 3 transcripts are grouped into expression quartiles, as indicated. The statistical-significance p-values of the correlations between the calculated RARα/RARβ/RARγ mRNA levels and the indicated clinical/molecular characteristics of the gastric cancer samples are shown in red above the corresponding box plots. Each panel illustrates only the p-values of the comparisons reaching statistical significance. PIK3CA, Phosphatidylinositol-4,5-Bisphosphate-3-Kinase-Catalytic-Subunit-Alpha; CDKN1A, Cyclin-Dependent-Kinase-Inhibitor-1A; EMT, Epithelial-to-Mesenchymal-Transition; MMR, DNA-Mismatch-Repair; MSI, MicroSatellite-Instability; MSS, MicroSatellite-Stability; TP53, Tumor-Protein-P53.
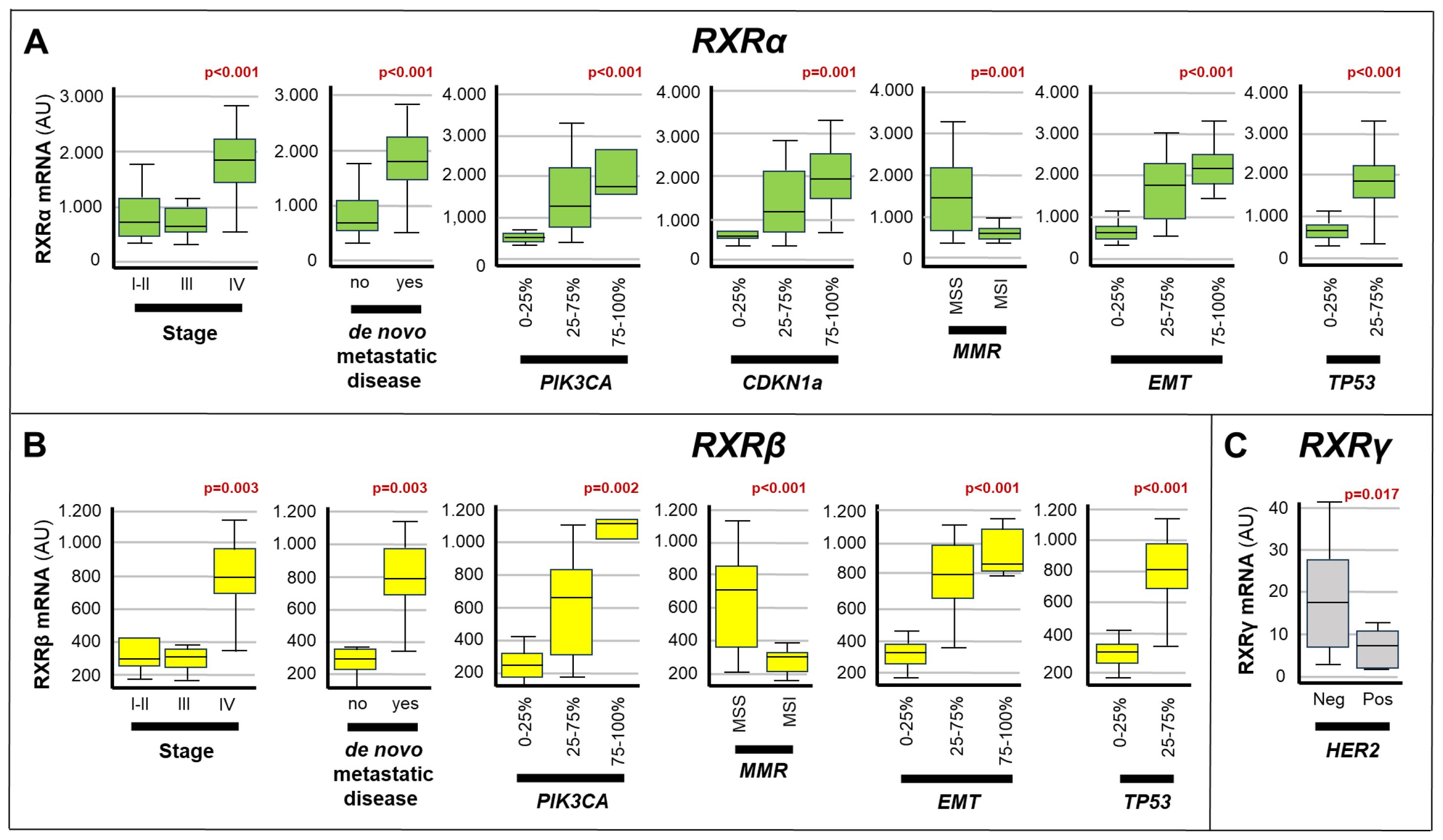
Figure 4. Associations between the expression of RXRs and the clinical/molecular characteristics of gastric cancers. The box plots show the relative levels of the RXRα (A), RXRβ (B) and RXRγ (C) mRNAs in the indicated subgroups of gastric cancers. The statistical-significance p-values of the correlations between the calculated RXRα/RXRβ/RXRγ mRNA levels and the indicated clinical/molecular characteristics of the gastric cancer samples are shown in red above the corresponding box plots. Each panel illustrates only the p-values of the comparisons reaching statistical significance. PIK3CA, Phosphatidylinositol-4,5-Bisphosphate-3-Kinase-Catalytic-Subunit-Alpha; CDKN1A, Cyclin-Dependent-Kinase-Inhibitor-1A; EMT, Epithelial-to-Mesenchymal-Transition; MMR, DNA-Mismatch-Repair; MSI, MicroSatellite-Instability; MSS, MicroSatellite-Stability; TP53, Tumor-Protein-P53.
As for the RXR transcripts (Figure 4), high expression levels of the RXRα mRNA showed significant correlations with: stage-IV disease (p<0.001), de novo metastatic disease (p<0.001), MSS status (p=0.001), EMT (p<0.001) as well as the amounts of the TP53 (p< 0.001), CDKN1A (p=0.001) and PIK3CA (p<0.001) transcripts. In addition, the RXRβ mRNA moved in association with stage-IV disease (p=0.003), de novo metastatic disease (p=0.003), MSS status (p< 0.001), EMT (p<0.001) and high levels of TP53 (p<0.001) as well as PIK3CA (p=0.002). Finally, high levels of the RXRγ transcript presented a significant correlation only with the HER2-negative status of gastric cancer (p=0.017).
Associations of gastric cancer clinical and pathological factors with overall-survival
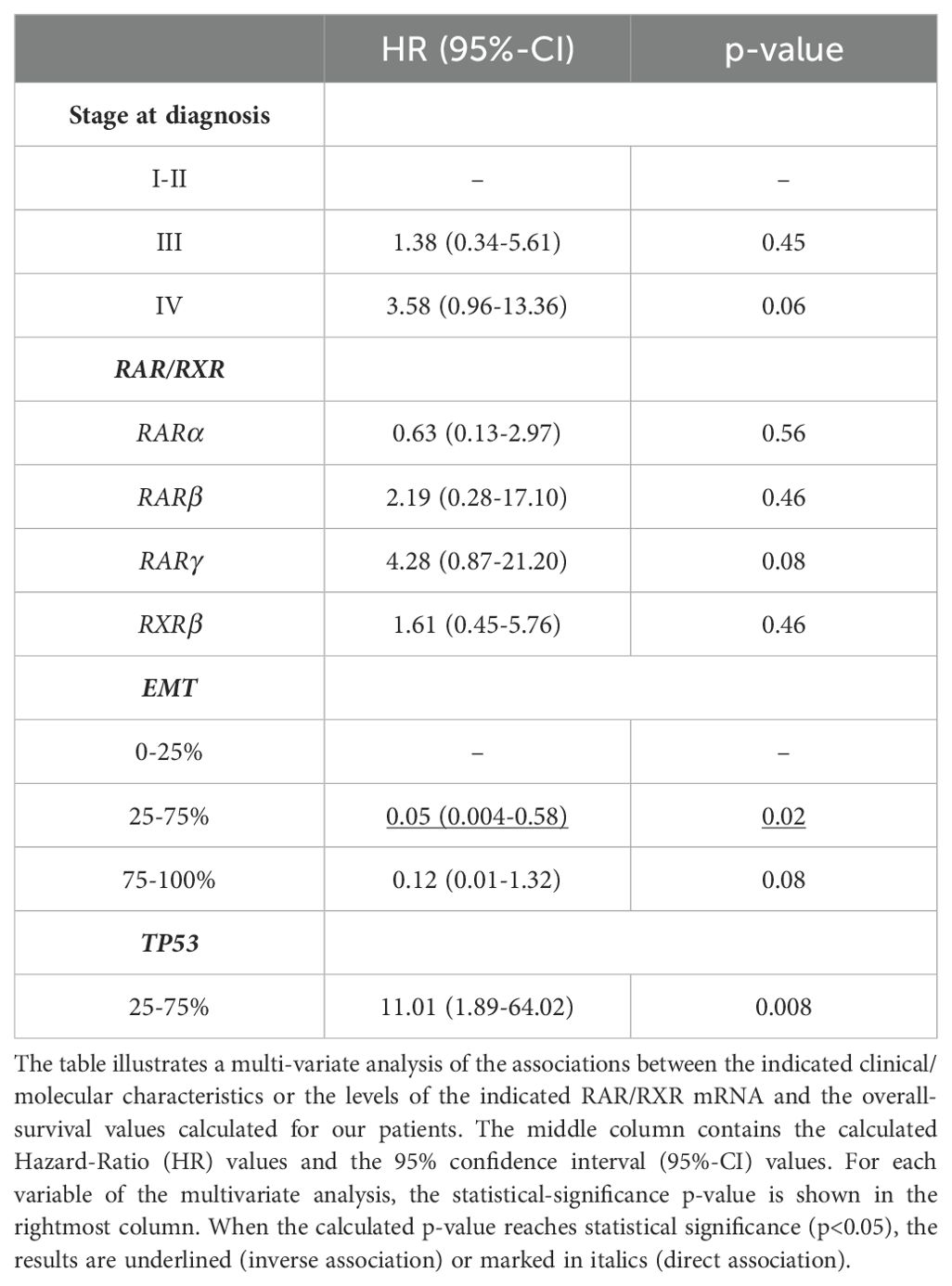
Based on a median follow-up of 50.8 months [Inter-Quartile-range (IQr) 22.1-93.6], the median overall-survival of our gastric cancer patients reached a value of 18.3 months (IQr 13.9-62.2), when this parameter was calculated from the diagnosis. If survival analysis was limited to the 37 patients experiencing a metastatic disease, a median overall-survival of 10.52 months (IQr 7.1-18.3) was calculated. Following univariate COX-regression analysis (Table 2), the main clinical/pathological factors showing significant correlations with a low overall-survival value were: de novo metastatic disease (p=0.001); distant recurrence (p=0.003); first-line therapy (p<0.001); stage-IV disease (p=0.01); high levels of the mRNAs involved in the EMT process (CDH1 and ZEB1) (p=0.001) and high levels of TP53 (p<0.001). In contrast, neo-adjuvant therapy and intermediate levels of the mRNAs involved in EMT (25%-75%) showed significant associations with a high overall-survival value. A last and relevant result of our analyses refers to the fact that neo-adjuvant therapy was also identified as a favorable prognostic factor (HR=0.44; 95%CI 0.20-0.95; p=0.04), being directly associated with high overall-survival (Table 2). Notably, the negative prognostic significance of TP53 expression (p=0.008) and stage-IV disease (p=0.06, close to statistical significance) were substantially confirmed following multi-variate analysis (Table 3). The same type of multivariate analysis ratified the observation that intermediate expression levels of the mRNAs involved in EMT appeared as a favorable prognostic factor (p=0.02) (Table 3).
Associations between retinoic acid receptors mRNA expression levels and overall-survival in gastric cancer
In a last set of analyses, we determined possible associations between the expression levels of the various RAR/RXR mRNAs in our gastric cancer samples and overall-survival, using the same univariate COX-regression approach described in the previous chapter. The results obtained demonstrated that RXRα and RXRγ levels did not show any significant association with overall-survival (Table 3). In contrast, patients with high RARα expression levels were characterized by a significantly lower overall-survival relative to what was observed in patients endowed with small amounts of the RARα mRNA (16 months vs. 33.9 months; p= 0.05) (Table 3, Figure 5A). The same detrimental effect on overall-survival was observed in patients with high RARβ (p= 0.001) levels (Table 3, Figure 5B), high RARγ (p=0.007) levels (Table 3, Figure 5C) and high RXRβ (p= 0.013) levels (Table 3, Figure 5D). In the case of patients with high and low levels of RARβ expression, median overall-survival values of 11.31 months (IQr = 8.8-18-6) and 33.9 months (IQr = 16.0-98.4) were calculated. By the same token, patients with high levels of RARγ expression were associated with a poorer median overall-survival value (10.9 months; IQr = 8.8-11.3) than the low-expression counterparts (18.64 months; IQr = 14.2-74.5). The median overall-survival of RXRβ over-expressing tumors was 16.0 months (95%-CI = 10.1-35.0), as compared to 98.4 months in tumors showing low levels of the receptor (95%-CI = 16.7-NR) (Figure 5). Taken together, our data support the idea that the tumor levels of RARα, RARβ, RARγ and RXRβ mRNAs are potential determinants of a low overall-survival rate in gastric cancer.
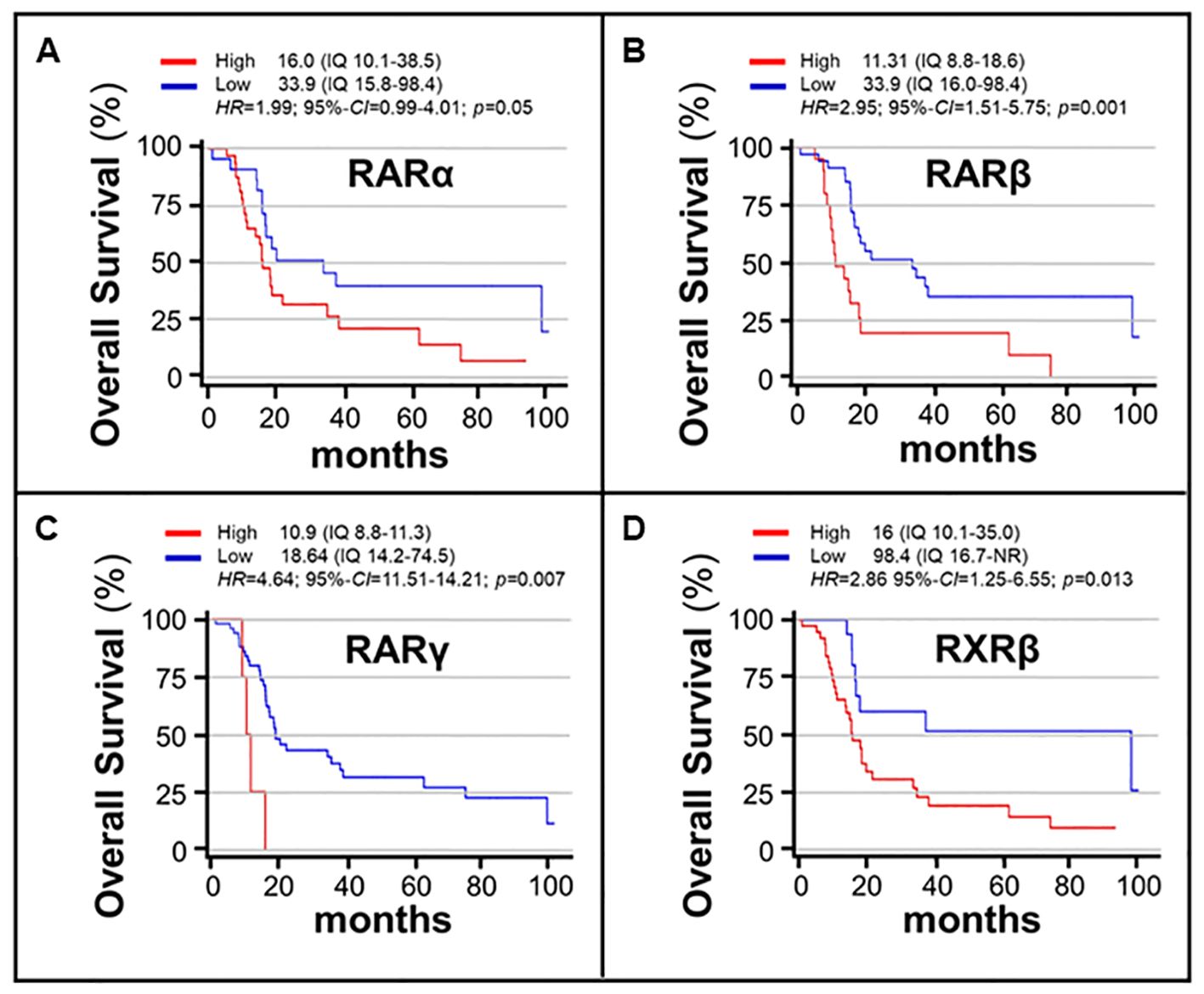
Figure 5. Associations between the overall-survival of gastric cancer patients and the expression levels of RARα/RARβ/RARγ/RXRβ mRNAs. The associations of the RARα (A), RARβ (B), RARγ (C) and RXRβ (D) mRNA expression levels and overall-survival values are illustrated by the curves. The expression levels of the various RAR and RXR isoforms are gathered binomially using the indicated expression ranges (IQ). The red curves illustrate the overall-survival rates of patients whose gastric cancer tumors are characterized by high levels of the indicated Retinoic-Acid-Receptor. The blue curves illustrate the overall-survival rates of patients whose gastric cancer tumors are characterized by low levels of the indicated Retinoic-Acid-Receptor. The associations were explored with the use of the COX proportional Hazard-Ratios (HRs). The analysis results are expressed as HRs and 95% Confidence-Intervals (95%-CIs). Survival curves are estimated according to the Kaplan-Meier method and compared using the log-rank test. Statistical significance is set at p<0.05 for bilateral tests.
Discussion
ATRA is a vitamin-A metabolite, which is involved in cell and tissue maturation, particularly during embryonic development. The scientific literature supports the idea that pharmacologically active dosages of ATRA may be beneficial in the treatment and prevention of different types of solid tumors, including gastric cancer (11–20).
As already discussed in the Results section, the pharmacological activity of ATRA is mediated by the retinoic acid receptors, RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ, which act under the form of RAR/RXR heterodimers. The development of ATRA-based therapeutic strategies in gastric cancer requires an initial assessment of the levels and relevance of RARs/RXRs in this heterogeneous group of tumors. The present work is a retrospective archive-based translational study, whose major aim was to define the levels of the mRNAs encoding retinoic acid receptors in gastric cancer. The specific aims of the RAR-GASTRIC study were: a) to assess the RARs/RXRs mRNA levels using archival gastric cancer specimens; b) to define potential associations between the expression levels of specific RARs/RXRs and gastric cancer clinical features; c) to establish whether specific retinoic acid receptors were over-represented in any molecular subtype of gastric cancer, using our TCGA/ACRG-like classification; d) to assess the impact of RARs and RXRs on the overall-survival of gastric cancer patients.
Our cohort consisted of 55 consecutive gastric cancer patients characterized by the presence of an archival histology specimen (biopsy or gastrectomy) which was collected during the time-period 2010-2019. The RAR-GASTRIC series contained a majority of male subjects (64%), and most of our patients (76%) fell under the age of 70 years. Interestingly, 66% of the patients presented with an initial diagnosis of localized disease. The overall-survival determined in our gastric cancer patients is in line with the data reported in clinical trials and the oncology literature (23), indicating that our cohort of patients was representative of the real-world population. In our cohort, we observed that 3% of the stomach tumors were EBV-positive, 25% of them were EMT-like cancers and 1% of the cases were TP53-positive. In addition, 20% and 25% of our gastric cancer cases classified as HER2-positive and MSI tumors, respectively. With respect to this last aspect, the scientific literature reports that HER2-positive and MSI tumors represent 15-30% and 10-30% of all stomach tumors, respectively (24).
The RAR-GASTRIC tumors were grouped according to our simplified version of the TCGA/ACRG-like classification of gastric cancer. At present, we cannot compare our analyses with a gold-standard validation procedure. In fact, the TCGA/ACRG molecular classification of gastric cancer lacks implementation in the clinical practice due to technical difficulties, costs, and the absence of direct therapeutic implications. Nevertheless, the prevalence of the molecular sub-groups in our cohort of patients and the TCGA/ACRG datasets is substantially overlapping in terms of MSI and HER2 phenotypes, though slightly different in terms of EBV, EMT and TP53 phenotypes.
As for the expression of the RAR and RXR transcripts, we confirmed that all these mRNAs were detectable in the gastric cancer samples, which we analyzed. In our tumor samples, we determined large amounts of the RARα, RARβ, RXRα and RXRβ transcripts. By converse, our data indicated that gastric cancer samples tended to express low levels of the RARγ and RXRγ mRNAs. The high levels of RARα, RARβ and RXRα determined in our tumor tissues associated with unfavorable clinical factors such as relapsed and “de novo” metastatic disease. This last observation supports the idea that advanced and high-stage stomach tumors contain large amounts of RARα, RARβ, RXRα and RXRβ. The determination of possible associations with the gastric cancer molecular features, which we examined, are substantially in line with the concept that RARα/RARβ/RXRα/RXRβ levels correlate with MSS, EMT and TP53 expression in a positive manner. Thus, our data indicate that expression of these four retinoic acid receptors is linked to stomach tumors characterized by an aggressive molecular phenotype and poor overall-survival.
In the RAR-GASTRIC study, we also focused on possible associations between the RAR/RXR mRNA levels and the expression of the two transcripts encoding PIK3CA/CDKN1A. The PIK3CA-gene is of interest because about 15% of the stomach tumors present with a mutation observed predominantly in the EBV-positive molecular subgroup (4, 5, 25). Indeed, PIK3CA is a key determinant of various intra-cellular signaling pathways, as it is involved in processes such as cell proliferation and survival. In addition, mutations of the PIK3CA gene are potential pharmacological targets of aromatase-inhibitor resistant breast cancers (26, 27). Finally, there is pre-clinical evidence of an interplay between ATRA and PIK3CA in the control of cell proliferation (28, 29). As for the CDKN1A-gene, the encoded protein is an inhibitor of CDK2, a cyclin dependent kinase involved in the control of cell cycle. Moreover, the TP53 tumor suppressor controls CDKN1A expression (https://www.ncbi.nlm.nih.gov/gene/1026). Finally, TP53, PIK3CA and CDKN1A are part of an integrated pathway controlling p53 functional activity, which identifies p53-active and -inactive tumors in a simple manner (5). Studies available in the scientific literature indicate that ATRA targets CDK2, causing an arrest of breast cancer cell proliferation (30). Other studies link ATRA to the CDK-mediated cell proliferation processes (31, 32). Interestingly, we demonstrated that the levels of RARα, RARβ and RXRα show a significant association with TP53, PIK3CA and CDKN1A expression. In addition, we observed that RXRβ expression correlated with TP53 and PIK3C expression levels. Finally, we established associations of the RARα, RARβ, RARγ and RXRβ levels with a detrimental effect on the overall-survival of gastric cancer patients.
Conclusions
The results of the RAR-GASTRIC study demonstrate that gastric cancer specimens contain significant amounts of RARα/RARβ/RXRα/RXRβ mRNAs. By converse, the levels of RARγ and RXRγ tend to be low. Based on our results, it is reasonable to assume that high expression levels of RARα, RARβ, RXRα and RXRβ have a negative impact on gastric cancer patients’ overall-survival. In fact, high levels of RARα, RARβ, RXRα and RXRβ are associated with unfavorable prognostic clinical and pathological characteristics of gastric cancer, such as stage-IV and “de novo” metastatic disease. In addition, there are gastric cancer molecular subtypes (MSS, EMT-like and possibly p53-inactive cancers), which are enriched in specific types of retinoic acid receptors. The relevance of our study resides in the originality of the concept and the attempt to link the evaluation of ATRA receptors expression, not only to clinical characteristics but also to a reproducible as well as simplified molecular classification of gastric cancer based on the use of a limited number of molecular-subtypes hallmarks.
Clearly, further studies are necessary to add robustness to the clinical/biological associations that we determined. Nevertheless, our results indicate that specific retinoic acid receptors represent potential gastric cancer prognostic markers. In addition, the data contained in this study have significant implications in the context of the stratified/personalized treatment of gastric cancer. In fact, they support the idea that certain retinoic acid receptors represent targets for the development of innovative therapeutic strategies based on ATRA or available RAR/RXR agonists/antagonists. Finally, association studies on RAR/RXR expression levels and treatment response are necessary and must be conducted on a larger cohort of patients.
Data availability statement
Original datasets are available in a publicly accessible repository: The original contributions presented in the study are publicly available. This data can be found here: https://github.com/silvioken/RAR-Gastric/blob/main/Repository.xlsx.
Ethics statement
The studies involving humans were approved by Departmental Review Board and by the Ethics Committee of CRO Oncology Reference Center of Aviano, Italy (Protocol number CRO-2019-74). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SG: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft, Writing – review & editing. DB: Data curation, Formal analysis, Methodology, Writing – review & editing. VD: Data curation, Methodology, Resources, Validation, Writing – review & editing. GB: Data curation, Visualization, Writing – review & editing. GM: Methodology, Visualization, Writing – review & editing. VC: Data curation, Methodology, Visualization, Writing – review & editing. GA: Methodology, Visualization, Writing – review & editing. CC: Data curation, Visualization, Writing – review & editing. SB: Data curation, Visualization, Writing – review & editing. EG: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. FP: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We would like to thank the Associazione Italiana per la Ricerca contro il Cancro (AIRC) for the Investigation Grant (ID22963) to EG which allowed completion of some of the analyses performed in the study. This work was supported by the Italian Ministry of Health-Ricerca Corrente.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1453934/full#supplementary-material
Supplementary Table 1 | Overview of the treatments for each patient.
References
1. Bang Y-J, Cutsem EV, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
2. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
3. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. (2014) 513:202–9. doi: 10.1038/nature13480
4. Cristescu R, Lee J, Nebozhyn M, Kim K-M, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. (2015) 21:449–56. doi: 10.1038/nm.3850
5. Garattini SK, Basile D, Cattaneo M, Fanotto V, Ongaro E, Bonotto M, et al. Molecular classifications of gastric cancers: Novel insights and possible future applications. World J Gastrointest Oncol. (2017) 9:194–208. doi: 10.4251/wjgo.v9.i5.194
6. Marshall H, Morrison A, Studer M, Pöpperl H, Krumlauf R. Retinoids and hox genes. FASEB J. (1996) 10:969–78. doi: 10.1096/fasebj.10.9.8801179
7. Kumar S, Dollé P, Ghyselinck NB, Duester G. Endogenous retinoic acid signaling is required for maintenance and regeneration of cornea. Exp Eye Res. (2017) 154:190–5. doi: 10.1016/j.exer.2016.11.009
8. Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. (2001) 1:181–93. doi: 10.1038/35106036
9. Lo-Coco F, Hasan SK, Montesinos P, Sanz MA. Biology and management of therapy-related acute promyelocytic leukemia. Curr Opin Oncol. (2013) 25:695–700. doi: 10.1097/CCO.0000000000000013
10. Makimoto A, Fujisaki H, Matsumoto K, Takahashi Y, Cho Y, Morikawa Y, et al. Retinoid therapy for neuroblastoma: historical overview, regulatory challenges, and prospects. Cancers (Basel). (2024) 16:544–59. doi: 10.3390/cancers16030544
11. Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, Kerr AR, et al. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev. (2016) 7:CD001829. doi: 10.1002/14651858.CD001829.pub4
12. Puviani M, Milani M. Treatment of grade II and III actinic keratosis lesions with a film-forming medical device containing sunscreen/piroxicam 0.8% and a retinoic acid/glycolic gel: A pilot trial. Dermatol Ther (Heidelb). (2018) 8:399–404. doi: 10.1007/s13555-018-0244-3
13. Meyskens FL Jr, Surwit E, Moon TE, Childers JM, Davis JR, Dorr RT, et al. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: a randomized trial. J Natl Cancer Inst. (1994) 86:539–43. doi: 10.1093/jnci/86.7.539
14. Garattini E, Bolis M, Garattini SK, Fratelli M, Centritto F, Paroni G, et al. Retinoids and breast cancer: from basic studies to the clinic and back again. Cancer Treat Rev. (2014) 40:739–49. doi: 10.1016/j.ctrv.2014.01.001
15. Bolis M, Paroni G, Fratelli M, Vallerga A, Zanetti A, Kurosaki M, et al. All-trans retinoic acid stimulates viral mimicry, interferon responses and antigen presentation in breast-cancer cells. Cancers (Basel). (2020) 12:1169–91. doi: 10.3390/cancers12051169
16. Paroni G, Zanetti A, Barzago M, Kurosaki M, Guarrera L, Fratelli M, et al. Retinoic acid sensitivity of triple-negative breast-cancer cells characterized by constitutive activation of the NOTCH1 pathway: the role of RARβ. Cancers (Basel). (2020) 12:3027–49. doi: 10.3390/cancers12103027
17. Centritto F, Paroni G, Bolis M, Garattini SK, Kurosaki M, Barzago MM, et al. Cellular and molecular determinants of all-trans retinoic acid sensitivity in breast cancer: Luminal phenotype and RARα expression. EMBO Mol Med. (2015) 7:950–72. doi: 10.15252/emmm.201404670
18. Nguyen PH, Giraud J, Staedel C, Chambonnier L, Dubus P, Chevret E, et al. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene. (2016) 35:5619–28. doi: 10.1038/onc.2016.87
19. Jin J, Li X, Xing L, Chang Y, Wu L, Jin Z, et al. Addition of all-trans-retinoic acid to omeprazole and sucralfate therapy improves the prognosis of gastric dysplasia. J Int Med Res. (2015) 43:204–16. doi: 10.1177/0300060514559791
20. Nguyen PH, Giraud J, Chambonnier L, Dubus P, Wittkop L, Belleannée G, et al. Characterization of biomarkers of tumorigenic and chemoresistant cancer stem cells in human gastric carcinoma. Clin Cancer Res. (2017) 23:1586–97. doi: 10.1158/1078-0432.CCR-15-2157
21. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. (1996) 10:940–54. doi: 10.1096/fasebj.10.9.8801176
22. Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. (2009) 7:e002. doi: 10.1621/nrs.07002
23. Osumi H, Takahari D, Chin K, Ogura M, Ichimura T, Wakatsuki T, et al. Modified FOLFOX6 as a first-line treatment for patients with advanced gastric cancer with massive ascites or inadequate oral intake. Onco Targets Ther. (2018) 11:8301–7. doi: 10.2147/OTT.S184665
24. Bermúdez A, Arranz-Salas I, Mercado S, López-Villodres JA, González V, Ríus F, et al. Her2-positive and microsatellite instability status in gastric cancer-clinicopathological implications. Diagnostics. (2021) 11:944–60. doi: 10.3390/diagnostics11060944
25. Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. (2016) 7:32925–32. doi: 10.18632/oncotarget.9076
26. Janku F, Wheler JJ, Naing A, Stepanek VM, Falchook GS, Fu S, et al. PIK3CA mutations in advanced cancers: characteristics and outcomes. Oncotarget. (2012) 3:1566–75. doi: 10.18632/oncotarget.716
27. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. (2019) 380:1929–40. doi: 10.1056/NEJMoa1813904
28. Yu M, Ge C, Zeng W, Mi Y, Zhang C. Retinoic acid promotes proliferation of chicken primordial germ cells via activation of PI3K/Akt-mediated NF-κB signalling cascade. Cell Biol Int. (2012) 36:705–12. doi: 10.1042/CBI20110542
29. Sun B, Wang Y, Sun J, Zhang C, Xia R, Xu S, et al. Establishment of patient-derived xenograft models of adenoid cystic carcinoma to assess pre-clinical efficacy of combination therapy of a PI3K inhibitor and retinoic acid. Am J Cancer Res. (2021) 11:773–92.
30. Teixeira C, Pratt MA. CDK2 is a target for retinoic acid-mediated growth inhibition in MCF-7 human breast cancer cells. Mol Endocrinol. (1997) 11:1191–202. doi: 10.1210/mend.11.9.9977
31. Jung H-Y, Park S-H, Yoo YD, Kim JS, Kim YH. CDK2/4 regulate retinoic acid-induced G1 arrest in hepatocellular carcinoma cells. Hepatol Res. (2005) 31:143–52. doi: 10.1016/j.hepres.2004.12.006
32. Zhang R, Banik NL, Ray SK. Combination of all-trans retinoic acid and interferon-gamma upregulated p27(kip1) and down regulated CDK2 to cause cell cycle arrest leading to differentiation and apoptosis in human glioblastoma LN18 (PTEN-proficient) and U87MG (PTEN-deficient) cells. Cancer Chemother Pharmacol. (2008) 62:407–16. doi: 10.1007/s00280-007-0619-0
Keywords: retrospective-clinical-study, gastric cancer, retinoic-acid-receptors, prognosticbiomarkers, therapeutic-targets
Citation: Garattini SK, Basile D, De Re V, Brisotto G, Miolo G, Canzonieri V, Aprile G, Corvaja C, Buriolla S, Garattini E and Puglisi F (2024) The potential of retinoic acid receptors as prognostic biomarkers and therapeutic targets in gastric cancer. Front. Oncol. 14:1453934. doi: 10.3389/fonc.2024.1453934
Received: 24 June 2024; Accepted: 06 August 2024;
Published: 11 September 2024.
Edited by:
Ewa Teresa Marcinkowska, University of Wrocław, PolandReviewed by:
Vera Mugoni, University of Turin, ItalyMeike Kaehler, University Hospital Schleswig-Holstein, Germany
Copyright © 2024 Garattini, Basile, De Re, Brisotto, Miolo, Canzonieri, Aprile, Corvaja, Buriolla, Garattini and Puglisi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvio Ken Garattini, c2lsdmlva2VuLmdhcmF0dGluaUBhc3VmYy5zYW5pdGEuZnZnLml0; Enrico Garattini, ZW5yaWNvLmdhcmF0dGluaUBtYXJpb25lZ3JpLml0
 Silvio Ken Garattini1*
Silvio Ken Garattini1* Debora Basile
Debora Basile Valli’ De Re
Valli’ De Re Giulia Brisotto
Giulia Brisotto Gianmaria Miolo
Gianmaria Miolo Vincenzo Canzonieri
Vincenzo Canzonieri Giuseppe Aprile
Giuseppe Aprile Enrico Garattini
Enrico Garattini Fabio Puglisi
Fabio Puglisi