- Department of Infectious Diseases, Weifang People’s Hospital, Weifang, Shandong, China
Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related deaths worldwide. Recent advances in immunotherapies, targeted therapies, and combination treatments have significantly improved outcomes for many patients with HCC. This review summarizes key findings from the 2024 ASCO Annual Meeting, focusing on emerging therapies, including immune checkpoint inhibitors (ICIs), CAR-T cell therapies, oncolytic viruses, and locoregional treatments like transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC). ICIs, particularly when combined with other agents, have shown promising efficacy, though challenges such as immune-related adverse events and resistance mechanisms remain. CAR-T cell therapies and oncolytic viruses offer novel therapeutic avenues for advanced HCC, but their long-term efficacy in solid tumors is still under investigation. Locoregional therapies, especially in combination with systemic treatments, continue to play a critical role in managing unresectable HCC and improving conversion rates to surgical resection. Additionally, the potential of biomarkers, such as hypoxia scores and CTNNB1 mutations, is being explored to better personalize treatment and predict patient responses. These biomarkers could pave the way for more targeted and effective therapeutic strategies. Overall, the recent studies presented at the ASCO meeting highlight progress in HCC treatment, underscoring the importance of continued innovation. Future research should focus on overcoming resistance mechanisms, optimizing combination therapies, and integrating biomarker-driven approaches to improve patient outcomes and enhance personalized treatment strategies.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, making up 75% of cases (1). It is the sixth most common cancer and the fourth leading cause of cancer deaths globally (2). Despite advances in the early detection and treatment of HCC, the prognosis for many patients remains poor, largely due to late diagnosis and the aggressive nature of the disease (3). Current standard-of-care treatments, including surgical resection, liver transplantation, and locoregional therapies such as transarterial chemoembolization (TACE) and radiofrequency ablation (RFA), are primarily effective in patients with early-stage or localized tumors (4, 5). Current standard-of-care treatments, including surgical resection, liver transplantation, and locoregional therapies such as transarterial chemoembolization (TACE) and radiofrequency ablation (RFA), are primarily effective in patients with early-stage or localized tumors (6, 7).
A significant limitation of existing therapies is the development of resistance, which is frequently driven by mechanisms such as epithelial-mesenchymal transition (EMT) and cancer cell plasticity. These processes not only promote metastasis but also enhance tumor cells’ ability to evade therapeutic interventions, leading to therapy resistance and poor patient outcomes (8). Emerging evidence also suggests that nanoparticles, particularly those functionalized with peptides, offer new opportunities to enhance drug delivery and target tumor cells more effectively, potentially overcoming some of the limitations seen with traditional therapeutic agents (9).
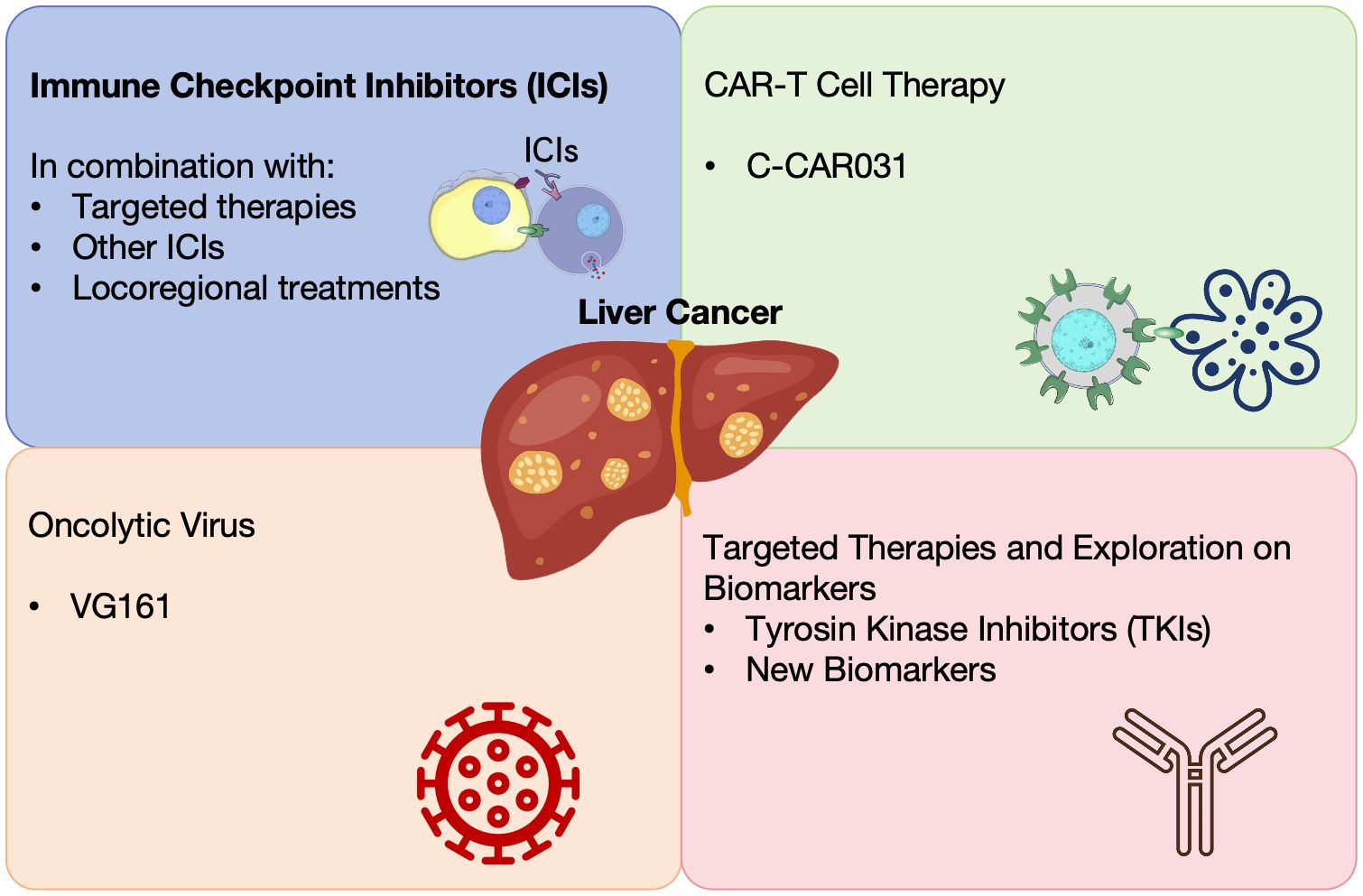
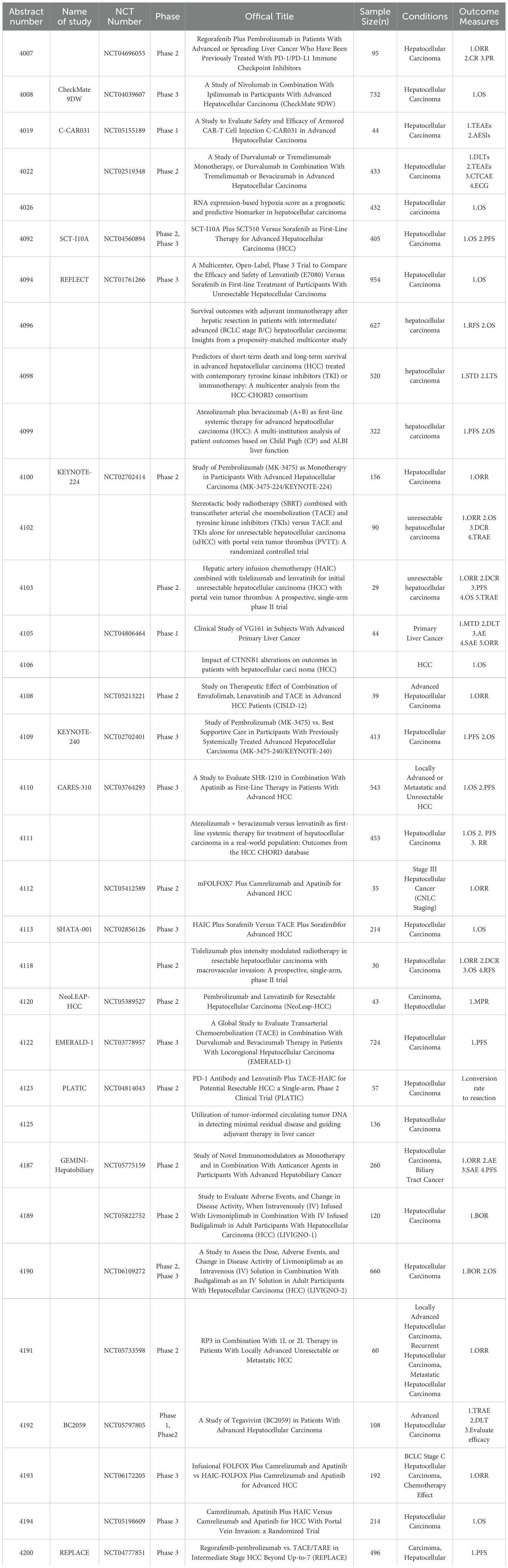
The landscape of HCC treatment has been revolutionized by the advent of immune checkpoint inhibitors (ICIs), targeted therapies, and novel combinations (10) (Figure 1). Different strategies including targeting the tumor-infiltrating neutrophils and using oncolytic viruses have been explored to enhance the efficacy of liver cancer immunotherapy (11, 12). These advancements offer new hope for improved survival and quality of life for patients with advanced HCC. These offer hope for better survival and quality of life in advanced HCC. We summarized 34 clinical studies on liver cancer from the 2024 ASCO meeting, highlighting progress in treatments like ICIs, locoregional therapies, CAR-T cell therapies, and more (Table 1).
ICIs
mDurvalumab, an anti-PD-L1 antibody, has been explored in various combinations for the treatment of unresectable HCC. A phase 2 trial (NCT02519348) evaluated durvalumab monotherapy and combinations with tremelimumab and bevacizumab (13). The study found that both the STRIDE regimen (Single Tremelimumab Regular Interval Durvalumab) and durvalumab plus bevacizumab (D+B) demonstrated higher objective response rates (ORR) compared to durvalumab alone. Notably, the STRIDE regimen exhibited significant immune modulation effects, indicating potential complementary actions when combined with durvalumab and bevacizumab. Another multi-institutional analysis focused on atezolizumab and bevacizumab (A+B) as a first-line systemic therapy for advanced HCC (14). Results showed that median overall survival (OS) was significantly better in patients with Child-Pugh A liver function compared to those with poorer liver function. Additionally, the albumin-bilirubin (ALBI) grade was found to be an effective predictor of patient outcomes, underscoring the importance of liver function scores in treatment planning. A real-world study from the HCC CHORD database compared atezolizumab plus bevacizumab (AB) versus lenvatinib (LEN) as first-line systemic therapy for HCC. AB was associated with superior OS compared to LEN (19.7 vs. 14.4 months) but had similar PFS and RR (15).
In the additional follow-up of the KEYNOTE-224 study investigating the efficacy of pembrolizumab in patients with advanced HCC previously treated with sorafenib, pembrolizumab exhibited durable responses with ORRs of 18.3% and 17.6% in sorafenib-treated and treatment-naive cohorts, respectively, with a manageable safety profile (16). A multicenter study found adjuvant immunotherapy improved recurrence-free and overall survival in intermediate/advanced HCC patients post-hepatic resection, compared to non-immunotherapy groups, highlighting benefits for high-risk patients (17). Moreover, in the NeoLEAP-HCC trial, a single-arm, multi-center, phase II study, pembrolizumab combined with lenvatinib was evaluated as a perioperative treatment for resectable HCC (18). The combination demonstrated promising anti-tumor efficacy, with 37.8% of patients achieving a major pathological response (MPR) and an acceptable safety profile, showing potential in reducing recurrence rates post-surgery. Tislelizumab, another PD-1 inhibitor, was studied in combination with intensity-modulated radiotherapy (IMRT) in a single-arm phase II trial for patients with resectable HCC and macrovascular invasion (MVI) (19). This study reported an ORR of 30.0% and a significant pathological response in 66.7% of patients who underwent surgery, indicating the combination could be effective and tolerable as perioperative therapy.
Meanwhile, a phase II trial investigated livmoniplimab (anti-GARP-TGF-b1) combined with budigalimab (anti-PD-1) in advanced HCC patients who progressed on first-line therapy. Early results showed a 42% ORR, indicating promising clinical activity and manageable safety (20). Additionally, a phase 1/2 study evaluated tegavivint, a TBL1 inhibitor, in advanced HCC patients with beta-catenin activating mutations, aiming to characterize the safety, pharmacokinetics/pharmacodynamics (PK/PD), and preliminary antitumor activity of tegavivint, offering a novel targeted approach for HCC with specific genetic profiles (21). The GEMINI-Hepatobiliary phase II trial evaluated novel immuno-oncology regimens for advanced hepatobiliary cancers, including HCC (22). This study explored the efficacy of volrustomig (anti-PD-1/CTLA-4) or rilvegostomig (anti-PD-1/TIGIT) in combination with standard anticancer agents. The trial aims to provide new insights into improving outcomes for advanced hepatobiliary cancers through innovative IO-based treatments.
A phase II/III study is investigating the combination of livmoniplimab and budigalimab in patients with locally advanced or metastatic HCC. The study aims to determine the optimal dose and evaluate the efficacy and safety of this novel combination, potentially offering a new therapeutic option for advanced HCC (23). Another randomized, open-label, phase III trial is comparing apatinib and camrelizumab with or without HAIC for HCC with portal vein tumor thrombus (PVTT). This study aims to determine the efficacy and safety of adding to the combination of apatinib and camrelizumab, potentially offering an enhanced treatment strategy for patients with high-risk HCC (24). A phase III trial (NCT05198609) is evaluating the efficacy and safety of apatinib and camrelizumab plus intravenous FOLFOX or HAIC with FOLFOX for advanced HCC. This trial seeks to compare the efficacy of these regimens, aiming to optimize treatment for advanced HCC (25).
A phase III study of SCT-I10A, an anti-PD-1 antibody, combined with a bevacizumab biosimilar versus sorafenib in advanced HCC demonstrated that SCT-I10A plus SCT510 showed significantly longer median OS (22.1 vs. 14.2 months) and PFS (7.1 vs. 2.9 months) compared to sorafenib (26). The combination also had a higher ORR with an acceptable safety profile. A phase II trial (NCT04039607) is assessing nivolumab and ipilimumab versus Lenvatinib or sorafenib as first-line treatment for unresectable HCC. Nivolumab plus ipilimumab showed a significant OS benefit, higher ORR, and durable responses, suggesting it as a potential new first-line standard of care for unresectable HCC (27). Lastly, the final OS analysis of the study CARES-310 showed that Camrelizumab plus rivoceranib had a median OS of 23.8 months compared to 15.2 months for sorafenib, demonstrating superior efficacy (28). An international phase II study evaluated regorafenib and pembrolizumab in advanced HCC previously treated with ICIs (29). The study reported modest activity, with an ORR of 5.9% and a median PFS of 2.8 months, suggesting benefits for selected patients who progressed on prior ICI treatment.
ICIs have shown improvements in OS and PFS, particularly when combined with agents like bevacizumab. However, patient response remains variable, with factors such as liver function and biomarkers like PD-L1 expression influencing outcomes. irAEs are a notable concern, often requiring intensive management. Additionally, resistance mechanisms may limit long-term efficacy. Future strategies should focus on identifying reliable biomarkers to guide patient selection and combining ICIs with other therapies to mitigate resistance and improve outcomes.
Locoregional treatment-related therapies
Locoregional treatments including TACE and HAIC have been playing an important role in HCC treatment. Additionally, using locoregional treatment to activate antitumor immunity has been a useful way to enhance the efficacy of ICIs. The phase III EMERALD-1 study investigated durvalumab with or without bevacizumab combined with TACE in unresectable HCC. The combination significantly improved PFS compared to placebo plus TACE, supporting its potential as a new standard of care for embolization-eligible HCC (30). A phase II study investigated the combination of envafolimab, an anti-PD-L1 antibody, with lenvatinib and TACE in initially unresectable HCC (31). The combination demonstrated promising survival outcomes, with a median PFS of 8.78 months and a manageable safety profile, indicating its potential as a conversion therapy for surgical resection. A single-arm, phase II trial (PLATIC) evaluated sintilimab, lenvatinib, and TACE-HAIC as conversion therapy for initially unresectable HCC. The study reported a 77.2% conversion to resection rate, with an ORR of 77.2% (mRECIST) and 42.1% (RECIST 1.1). The mPFS was 14.3 months. Grade 3/4 TRAEs occurred in 64.9% of patients (32).
A phase II trial assessed HAIC with tislelizumab and lenvatinib in unresectable HCC. The study showed efficacy, with a median PFS of 15 months and a high conversion to resection rate, highlighting the potential for downstaging advanced HCC (33). A phase III trial compared sorafenib plus HAIC with sorafenib plus TACE in advanced HCC (34). Results showed HAIC with sorafenib significantly improved OS and PFS compared to TACE with sorafenib, especially benefiting patients with high fatty acid degradation (FAD) activity. Additionally, a phase III trial (REPLACE) is comparing the efficacy and safety of regorafenib and pembrolizumab versus locoregional therapy (TACE/TARE) for intermediate-stage HCC beyond the up-to-7 criteria, addressing the need for effective systemic therapy in intermediate-stage HCC (35). A phase II trial evaluated venous infusion chemotherapy (VIC) combined with apatinib and camrelizumab for CNLC stage III HCC. The study reported a confirmed ORR of 60.0%, a disease control rate of 97.1%, and a manageable safety profile, indicating significant anti-tumor effects (36). Another study compared stereotactic body radiotherapy (SBRT) combined with TACE and tyrosine kinase inhibitors (TKIs) versus TACE and TKIs alone in HCC patients with PVTT. Results showed that the SBRT+TACE+TKIs group had significantly better progression-free survival (PFS) and overall survival (OS), suggesting improved efficacy without additional safety concerns (37).
Locoregional treatments like TACE and HAIC, when combined with ICIs or TKIs, have improved tumor control and, in some cases, allowed resection of previously unresectable tumors. However, their efficacy is closely tied to liver function, and recurrence rates remain high. Procedural variability and differences in embolization techniques contribute to inconsistent outcomes. Standardization of treatment protocols and optimization of combinations with systemic therapies will be crucial in improving long-term results.
CAR-T cell therapies
C-CAR031, a GPC3-specific TGFbRIIDN armored autologous CAR-T therapy, was evaluated in a phase I study for advanced HCC (38). The treatment exhibited a manageable safety profile, with an ORR of 50% and a median PFS of 4.27 months. These preliminary results indicate promising efficacy for CAR-T cell therapy in heavily treated advanced HCC patients, offering a new potential treatment modality. However, challenges persist in solid tumor settings. Tumor microenvironment factors, such as immunosuppression, limit CAR-T cell persistence and efficacy. Furthermore, managing toxicities like CRS and neurotoxicity is critical. Although the high cost and complexity of CAR-T production are obstacles, further refinement in CAR-T engineering could enhance their role in treating advanced HCC.
Novel oncolytic virus therapies
VG161, an oncolytic virus expressing IL12, IL15, and PD-L1 blocking peptide, was evaluated in a phase I trial for HCC patients refractory to two prior lines of therapy (39). The study demonstrated a 17.14% ORR and a 60.00% disease control rate (DCR), with a median OS of 9.40 months. These results suggest that VG161 has the potential to benefit heavily pre-treated HCC patients, offering a new therapeutic approach. A multicenter phase II trial assessed the combination of oncolytic immunotherapy RP2 with atezolizumab and bevacizumab in advanced HCC. The study aims to evaluate the safety and efficacy of this combination as a second-line treatment, with primary endpoints including ORR and progression-free survival (40). Oncolytic viruses offer a novel approach with potential in patients who have failed other treatments. However, response rates have been modest, and improvements in delivery mechanisms and immune activation are needed. Combining oncolytic viruses with ICIs or other systemic therapies may boost their efficacy, but further large-scale trials are necessary to validate these combinations.
Exploration of biomarkers for targeted therapies
Biomarkers hold significant potential for personalizing cancer treatment. However, significant challenges remain in integrating biomarker-driven approaches into routine clinical practice, particularly in HCC, where heterogeneity in both tumor biology and liver function complicates treatment decisions. Examples from other cancers provide valuable insights into the potential application of biomarker-driven therapies in HCC. For instance, in non-small cell lung cancer (NSCLC), the presence of EGFR mutations or ALK rearrangements has successfully guided the use of targeted therapies, dramatically improving outcomes (41, 42). Similarly, in melanoma, the identification of BRAF V600E mutations has led to the development of targeted therapies like vemurafenib, which have significantly extended survival in patients with this specific mutation (43, 44). These examples demonstrate the profound impact of biomarker-driven treatment on improving patient outcomes when the right molecular target is identified.
An analysis of RNA expression-based hypoxia scores (HS) identified it as a significant prognostic biomarker in HCC (45). The study revealed that tumors with high hypoxia scores (HS-high) had worse OS but were more likely to respond to immunotherapy. In contrast, tumors with low hypoxia scores (HS-low) showed better OS and responded more favorably to sorafenib. These findings suggest that the hypoxia score could guide personalized treatment strategies for HCC, enabling more tailored and effective approaches.
A multicenter analysis from the HCC-CHORD consortium examined predictors of short-term death (STD) and long-term survival (LTS) in advanced HCC patients treated with TKIs or immunotherapy (46). The study identified several key predictors: higher ALBI grade, microvascular invasion (MVI), and distant metastases were linked to increased short-term death risk, while lower ALBI grade and absence of MVI were associated with better long-term survival.
An exploratory analysis from the KEYNOTE-240 trial found that CTNNB1 mutations did not significantly impact pembrolizumab outcomes in advanced HCC (47). However, another investigation into CTNNB1 mutations in HCC patients revealed that lower CTNNB1 expression was associated with improved overall survival (OS), particularly in patients treated with ICIs or tyrosine kinase inhibitors (TKI), highlighting the potential of CTNNB1 expression as a biomarker for treatment selection (48). A post-hoc analysis from the REFLECT trial investigated ctDNA mutations in HCC patients treated with lenvatinib or sorafenib. Common mutations included TERT, TP53, and CTNNB1, with TP53 mutations linked to overall survival. This highlights the need for further research on these mutations’ impact on treatment outcomes (49). Another study showed that tumor-informed ctDNA is a reliable biomarker for detecting minimal residual disease (MRD) and guiding adjuvant therapy in HCC, enhancing postoperative management precision (50).
Targeted therapies, particularly TKIs, have shown efficacy but are limited by the development of resistance, often due to tumor heterogeneity. The use of biomarkers, such as hypoxia scores and ctDNA, may help personalize therapy and optimize outcomes. Combination strategies with locoregional treatments or immunotherapies may improve efficacy, but further research is needed to refine these approaches and address resistance mechanisms.
Conclusion
The therapeutic landscape of HCC continues to evolve, with significant advancements across various treatment modalities. ICIs have demonstrated notable improvements in survival outcomes, especially in combination therapies, though variability in patient responses and immune-related adverse events (irAEs) remain key challenges. Locoregional treatments like TACE and HAIC play a crucial role, particularly in combination with systemic therapies. CAR-T therapies, while promising, face significant hurdles in the solid tumor microenvironment and require further refinement in delivery and toxicity management. Oncolytic viruses offer potential as novel therapies, though their response rates remain modest, and targeted therapies, particularly TKIs, are hindered by the development of resistance. Future efforts should focus on optimizing combination therapies, improving patient selection with biomarker-driven approaches, and addressing the challenges of treatment resistance and adverse effects to maximize the benefits of these innovations for HCC patients.
Author contributions
HY: Writing – original draft, Writing – review & editing. YL: Formal analysis, Writing – review & editing. NZ: Formal analysis, Writing – review & editing. FT: Data curation, Writing – review & editing. GY: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
ChatGPT 4.0 was used for the correction of grammar and wording for this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
3. Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2023) 20:203–22. doi: 10.1038/s41575-022-00704-9
4. Chakraborty E, Sarkar D. Emerging therapies for hepatocellular carcinoma (HCC). Cancers (Basel). (2022) 14:2798. doi: 10.3390/cancers14112798
5. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
6. Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, et al. Management of hepatocellular carcinoma: A review. JAMA Surg. (2023) 158:410–20. doi: 10.1001/jamasurg.2022.7989
7. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. (2022) 19:151–72. doi: 10.1038/s41571-021-00573-2
8. Ashrafizadeh M, Dai J, Torabian P, Nabavi N, Aref AR, Aljabali AAA, et al. Circular RNAs in EMT-driven metastasis regulation: modulation of cancer cell plasticity, tumorigenesis and therapy resistance. Cell Mol Life Sci. (2024) 81:214. doi: 10.1007/s00018-024-05236-w
9. Dai J, Ashrafizadeh M, Aref AR, Sethi G, Ertas YN. Peptide-functionalized, -assembled and -loaded nanoparticles in cancer therapy. Drug Discovery Today. (2024) 29:103981. doi: 10.1016/j.drudis.2024.103981
10. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
11. Feng M, Wang F, Liu X, Hao T, Zhang N, Deng M, et al. Neutrophils as key regulators of tumor immunity that restrict immune checkpoint blockade in liver cancer. Cancer Biol Med. (2023) 20:421–37. doi: 10.20892/j.issn.2095-3941.2023.0019
12. Yang B, Chen K, Liu X, Liu W, Ma Y, Tian X, et al. Advance in tumor immunotherapy: establishing a new paradigm for oncological treatment. Trans Surg Oncol. (2023) 1. Available online at: https://translsuronco.org/index.php/tso/article/view/8247.
13. Kelley RK, Lee YS, Conway J, Kurland JF, Negro A, McCoon P. T cell receptor and immune gene expression pharmacodynamics for durvalumab monotherapy and in combination with tremelimumab or bevacizumab in unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. (2024) 42:4022. doi: 10.1200/JCO.2024.42.16_suppl.4022
14. Storandt MH, Zemla TJ, Patell K, Naleid N, Gile J, Tran NH, et al. Atezolizumab plus bevacizumab (A+B) as first-line systemic therapy for advanced hepatocellular carcinoma (HCC): A multi-institution analysis of patient outcomes based on Child Pugh (CP) and ALBI liver function. J Clin Oncol. (2024) 42:4099. doi: 10.1200/JCO.2024.42.16_suppl.4099
15. Freeman M, Krishnan T, Lee CL, Dibajnia P, Ramjeesingh R, Vasconcelos JPS, et al. Atezolizumab + bevacizumab versus lenvatinib as first-line systemic therapy for treatment of hepatocellular carcinoma in a real-world population: Outcomes from the HCC CHORD database. J Clin Oncol. (2024) 42:4111. doi: 10.1200/JCO.2024.42.16_suppl.4111
16. Finn RS, Kudo M, Borbath I, Edeline J, Cattan S, Vlierberghe HV, et al. Pembrolizumab (pembro) in patients (pts) with sorafenib-treated (cohort 1) and treatment (tx)-naive (cohort 2) advanced hepatocellular carcinoma (aHCC) after additional follow-up in the phase 2 KEYNOTE-224 study. J Clin Oncol. (2024) 42:4100. doi: 10.1200/JCO.2024.42.16_suppl.4100
17. Yang T, Wang M-D, Xu X, Xu J-H, Fan Z-Q, Diao Y-K, et al. Survival outcomes with adjuvant immunotherapy after hepatic resection in patients with intermediate/advanced (BCLC stage B/C) hepatocellular carcinoma: Insights from a propensity-matched multicenter study. J Clin Oncol. (2024) 42:4096. doi: 10.1200/JCO.2024.42.16_suppl.4096
18. Sun H-C, Zhu X-D, Wang K, Xue H, Yang N, Yang Y-C, et al. Perioperative pembrolizumab and lenvatinib for resectable hepatocellular carcinoma: A single-arm, multi-center, phase II trial (NeoLEAP-HCC). J Clin Oncol. (2024) 42:4120. doi: 10.1200/JCO.2024.42.16_suppl.4120
19. Pan H, Zhou L, Cheng Z, Yang C, Zhang J, Shen N, et al. Tislelizumab plus intensity modulated radiotherapy in resectable hepatocellular carcinoma with macrovascular invasion: A prospective, single-arm, phase II trial. J Clin Oncol. (2024) 42:4118. doi: 10.1200/JCO.2024.42.16_suppl.4118
20. Abou-Alfa GK, Chiu C-F, Piscaglia F, Sangro B, Henkel A, Fang H, et al. Phase 2 study of livmoniplimab in combination with budigalimab in patients with hepatocellular carcinoma. J Clin Oncol. (2024) 42:TPS4189–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4189
21. Li D, Chen EX, Hu ZI, Hsieh D, Hwang JJ, Feun LG, et al. A phase 1/2 study of the TBL1 inhibitor, tegavivint (BC2059), in patients (pts) with advanced hepatocellular carcinoma (aHCC) with β-catenin activating mutations. J Clin Oncol. (2024) 42:TPS4192–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4192
22. Zhou J, Cheng A-L, Ikeda M, Lim HY, Akce M, Qin S, et al. GEMINI-Hepatobiliary: A phase 2 study of novel first-line immuno-oncology-based treatments in patients with advanced hepatobiliary cancers. J Clin Oncol. (2024) 42:TPS4187–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4187
23. Abou-Alfa GK, Bouattour M, Cheng A-L, Dayyani F, Khalil D, Li D, et al. Phase 2/3 study of livmoniplimab in combination with budigalimab in patients with locally advanced or metastatic hepatocellular carcinoma. J Clin Oncol. (2024) 42:TPS4190–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4190
24. He M, Du Z, Lai Z, Kan A, Shi M. Apatinib and camrelizumab plus hepatic arterial infusion with oxaliplatin and 5-fluorouracil vs. apatinib and camrelizumab as the first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A randomized multi-center clinical trial. J Clin Oncol. (2024) 42:TPS4194–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4194
25. Yunxiuxiu X, Peng L, Chen Y, Chen T, Wang J, Yang Y-P, et al. Apatinib and camrelizumab plus Intravenous FOLFOX or hepatic arterial infusion chemotherapy with FOLFOX for advanced HCC: A multicenter, prospective, randomized phase III trial. J Clin Oncol. (2024) 42:TPS4193–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4193
26. Xu J, Zhang Y, Wang G, Chen W, Zhuang L, Gu S, et al. SCT-I10A combined with a bevacizumab biosimilar (SCT510) versus sorafenib in the first-line treatment of advanced hepatocellular carcinoma: A randomized phase 3 trial. J Clin Oncol. (2024) 42:4092. doi: 10.1200/JCO.2024.42.16_suppl.4092
27. Galle PR, Decaens T, Kudo M, Qin S, Fonseca L, Sangro B, et al. Nivolumab (NIVO) plus ipilimumab (IPI) vs lenvatinib (LEN) or sorafenib (SOR) as first-line treatment for unresectable hepatocellular carcinoma (uHCC): First results from CheckMate 9DW. J Clin Oncol. (2024) 42:LBA4008–LBA. doi: 10.1200/JCO.2024.42.17_suppl.LBA4008
28. Vogel A, Chan SL, Ren Z, Bai Y, Gu S, Lin X, et al. Camrelizumab plus rivoceranib vs sorafenib as first-line therapy for unresectable hepatocellular carcinoma (uHCC): Final overall survival analysis of the phase 3 CARES-310 study. J Clin Oncol. (2024) 42:4110. doi: 10.1200/JCO.2024.42.16_suppl.4110
29. El-Khoueiry AB, Kim T-Y, Blanc J-F, Rosmorduc O, Decaens T, Mathurin P, et al. International, open-label phase 2 study of regorafenib plus pembrolizumab in patients with advanced hepatocellular carcinoma (HCC) previously treated with immune checkpoint inhibitors (ICI). J Clin Oncol. (2024) 42:4007. doi: 10.1200/JCO.2024.42.16_suppl.4007
30. Chan SL, Sangro B, Kudo M, Erinjeri JP, Qin S, Ren Z, et al. Safety analysis by treatment periods from EMERALD-1: A phase 3, randomized, placebo-controlled study of transarterial chemoembolization with durvalumab with/without bevacizumab in participants with embolization-eligible unresectable hepatocellular carcinoma. J Clin Oncol. (2024) 42:4122. doi: 10.1200/JCO.2024.42.16_suppl.4122
31. Bai X, Chen Y, Zhang J, Hu W, Li X, Shen Y, et al. Efficacy and safety of envafolimab plus lenvatinib combined with TACE in initially unresectable hepatocellular carcinoma: An open-label, single-arm, phase II study. J Clin Oncol. (2024) 42:4108. doi: 10.1200/JCO.2024.42.16_suppl.4108
32. Yuan Y, Qiu J, Huang Z, He W, Yuan Y, Wang C, et al. PD-1 inhibitor (sintilimab) and lenvatinib plus TACE-HAIC as conversion therapy for initially unresectable HCC: A single-arm, phase 2 clinical trial (PLATIC). J Clin Oncol. (2024) 42:4123. doi: 10.1200/JCO.2024.42.16_suppl.4123
33. Pan L, Sun R, He Q, Zhou Y, Li J, Gou Y, et al. Hepatic artery infusion chemotherapy (HAIC) combined with tislelizumab and lenvatinib for initial unresectable hepatocellular carcinoma (HCC) with portal vein tumor thrombus: A prospective, single-arm phase II trial. J Clin Oncol. (2024) 42:4103. doi: 10.1200/JCO.2024.42.16_suppl.4103
34. Lai Z, Kan A, He M, Du Z, Shi M. Sorafenib plus hepatic arterial infusion of oxaliplatin and fluorouracil vs sorafenib plus transarterial chemoembolization for advanced hepatocellular carcinoma: A biomolecular exploratory, randomized, phase III trial (SHATA-001). J Clin Oncol. (2024) 42:4113. doi: 10.1200/JCO.2024.42.16_suppl.4113
35. Galle PR, Kudo M, Llovet JM, Cheng A-L, Lencioni R, Oviedo Y, et al. REPLACE: A phase III, randomized, open-label trial to evaluate the safety and efficacy of regorafenib and pembrolizumab versus locoregional therapy (LRT) with transarterial chemoembolization (TACE) or transarterial radioembolization (TARE), for the first-line treatment of intermediate-stage hepatocellular carcinoma (HCC) with beyond up-to-7 criteria. J Clin Oncol. (2024) 42:TPS4200–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4200
36. Peng L, Chen T, Chen Y, Yunxiuxiu X, Wang J, Chen J, et al. Updated results from venous infusion chemotherapy (VIC) plus apatinib and camrelizumab for hepatocellular carcinoma (HCC) in CNLC stage III: A prospective, single-arm, phase II trial. J Clin Oncol. (2024) 42:4112. doi: 10.1200/JCO.2024.42.16_suppl.4112
37. Duan J, Zhou J, Liu C, Jiang H, Zhou J, Xie K, et al. Stereotactic body radiotherapy (SBRT) combined with transcatheter arterial chemoembolization (TACE) and tyrosine kinase inhibitors (TKIs) versus TACE and TKIs alone for unresectable hepatocellular carcinoma (uHCC) with portal vein tumor thrombus (PVTT): A randomized controlled trial. J Clin Oncol. (2024) 42:4102. doi: 10.1200/JCO.2024.42.16_suppl.4102
38. Zhang Q, Fu Q, Cao W, Wang H, Xu X, Huang J, et al. Phase I study of C-CAR031, a GPC3-specific TGFβRIIDN armored autologous CAR-T, in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. (2024) 42:4019. doi: 10.1200/JCO.2024.42.16_suppl.4019
39. Shen Y, Liang X, Jin X, Tan Q, Zhao R, Wei G, et al. Clinical outcomes from a phase I clinical trial of a novel oncolytic virus VG161 in patients with hepatocellular carcinoma (HCC) refractory after 2 prior lines of therapy including checkpoint inhibitors (CPI). J Clin Oncol. (2024) 42:4105. doi: 10.1200/JCO.2024.42.16_suppl.4105
40. Kim K, Saeed A, Sohal D, Edeline J, Heo J, Bhansali A, et al. An open-label, multicenter study investigating RP2 oncolytic immunotherapy in combination with second-line systemic atezolizumab combined with bevacizumab in patients with locally advanced unresectable or metastatic hepatocellular carcinoma. J Clin Oncol. (2024) 42:TPS4191–TPS. doi: 10.1200/JCO.2024.42.16_suppl.TPS4191
41. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. (2004) 304:1497–500. doi: 10.1126/science.1099314
42. Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol Cancer. (2018) 17:52. doi: 10.1186/s12943-018-0810-4
43. Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. (2009) 41:544–52. doi: 10.1038/ng.356
44. Puzanov I, Ribas A, Robert C, Schachter J, Nyakas M, Daud A, et al. Association of BRAF V600E/K mutation status and prior BRAF/MEK inhibition with pembrolizumab outcomes in advanced melanoma: pooled analysis of 3 clinical trials. JAMA Oncol. (2020) 6:1256–64. doi: 10.1001/jamaoncol.2020.2288
45. Connor A, Krause HB, Price BA, Elliott A, Khushman M, Lou E, et al. RNA expression-based hypoxia score as a prognostic and predictive biomarker in hepatocellular carcinoma. J Clin Oncol. (2024) 42:4026. doi: 10.1200/JCO.2024.42.16_suppl.4026
46. Dibajnia P, Freeman M, Krishnan T, LEE CL, Ramjeesingh R, Vasconcelos JPS, et al. Predictors of short-term death and long-term survival in advanced hepatocellular carcinoma (HCC) treated with contemporary tyrosine kinase inhibitors (TKI) or immunotherapy: A multicenter analysis from the HCC-CHORD consortium. J Clin Oncol. (2024) 42:4098. doi: 10.1200/JCO.2024.42.16_suppl.4098
47. Kudo M, Cheng A-L, Merle P, Ryoo B-Y, Decaens T, Cicin I, et al. Association between beta-catenin (CTNNB1) mutations and clinical outcomes of pembrolizumab in advanced hepatocellular carcinoma (aHCC): Exploratory analyses from KEYNOTE-240. J Clin Oncol. (2024) 42:4109. doi: 10.1200/JCO.2024.42.16_suppl.4109
48. Pai J, Baca Y, Ganguly R, Akce M, Algaze S, Chow LD, et al. Impact of CTNNB1 alterations on outcomes in patients with hepatocellular carcinoma (HCC). J Clin Oncol. (2024) 42:4106. doi: 10.1200/JCO.2024.42.16_suppl.4106
49. Evans TRJ, Kudo M, Cheng A-L, Wyrwicz LS, Ngan RK, Blanc J-F, et al. ctDNA analysis of patients (pts) with unresectable hepatocellular carcinoma (uHCC) treated with lenvatinib (LEN) or sorafenib (SOR) as 1L therapy. J Clin Oncol. (2024) 42:4094. doi: 10.1200/JCO.2024.42.16_suppl.4094
Keywords: hepatocellular carcinoma, 2024 ASCO meeting, immunotherapy, targeted therapy, combinational therapy
Citation: Yang H, Liu Y, Zhang N, Tao F and Yin G (2024) Therapeutic advances in hepatocellular carcinoma: an update from the 2024 ASCO annual meeting. Front. Oncol. 14:1453412. doi: 10.3389/fonc.2024.1453412
Received: 23 June 2024; Accepted: 09 October 2024;
Published: 25 October 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Hongwei Cheng, University of Macau, ChinaJin Zhang, University of Mississippi Medical Center, United States
Jin Chen, Fujian Medical University, China
Yang Ke, Kunming Medical University, China
Copyright © 2024 Yang, Liu, Zhang, Tao and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaozheng Yin, eWluZ2FvemhlbmdAb3V0bG9vay5jb20=
 Hongyuan Yang
Hongyuan Yang Gaozheng Yin
Gaozheng Yin