- 1Department of Gynecology and Obstetrics, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Rehabilitation Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Introduction: In order to comprehensive understanding of the characteristics of high-risk HPV-negative cervical cancer and aiming to provide certain evidence in the diagnosis and treatment of this kind of disease, the clinical and pathological characteristics of high-risk HPV-negative (hr-HPV-negative) cervical cancer was evaluated through systematic review and meta-analysis.
Methods: We employed a systematic search strategy incorporating both MeSH terms and free words across multiple databases including PubMed, Embase, Cochrane Library, Web of Science, CNKI and Wanfang. The strategy was specifically designed to identify articles investigating risk factors or clinical characteristics of hr-HPV-negative cervical cancer. Our database search spanned from their inception until October 2023. The literature selection and data collection processes were guided by predetermined inclusion and exclusion criteria. Each included research was independently evaluated by two reviewers using the Newcastle-Ottawa Scale (NOS) and a meta-analysis was conducted utilizing RevMan5.4.
Results: In total, nine studies published between 2013 and 2020 were included. The risk of lymph node metastasis was notably higher in hr-HPV-negative cervical cancer patients compared to hr-HPV-positive counterparts [RR=1.99, 95%CI (1.46–2.72), P<0.0001]. It was found that clinical staging of Ib2 or higher grade was significantly increased in hr-HPV-negative cervical cancer patients, as reported by seven studies [RR=1.42, 95%CI (1.15–1.77), P=0.0001]. Non-squamous carcinoma incidence, reported in seven studies, was significantly higher in hr-HPV-negative patients [RR=2.56, 95%CI (1.63–4.01), P=0.001]. Adenocarcinoma incidence was also significantly higher in hr-HPV-negative cervical cancer patients [RR=2.36, 95%CI (1.44–3.89), P=0.005].
Conclusions: Cervical cancer patients not infected by hr-HPV exhibited increased rates of lymph node metastasis, adenocarcinoma, and non-squamous carcinoma. And they were often diagnosed at more advanced stages. These variables suggest that hr-HPV-negative cervical cancer patients presented with a more severe health status at the time of their initial cancer diagnosis.
1 Introduction
Cervical cancer, a commonly occurring gynecological malignant tumour, is typified by a high incidence and mortality rate (1). Extant data suggest that approximately 90% of cervical cancer cases can be ascribed to human papillomavirus (HPV) infection, predominantly high-risk HPV (hr-HPV) infection (2). Indeed, hr-HPV infection is a crucial factor in the initiation and progression of precancerous cervical lesions (3). Screening for hr-HPV has been proven to be an effective method in circumventing the invasive form of cervical cancer. Although hr-HPV is recognized as a necessary but insufficient prerequisite for cervical cancer, HPV screening has demonstrated superior capacity to detect persistent high-grade lesions at an early stage and provide a more substantial reduction in risk over an extended period, compared to cytology (4, 5). However, in younger women, HPV screening may lead to an overdiagnosis of cervical intraepithelial neoplasia (CIN II) (6).
Presently, studies on the relationship between hr-HPV infection and cervical cancer have gained significant attention. However, it is important to note that not all patients with cervical cancer are hr-HPV-positive. Clinical practice revealed the existence of hr-HPV-negative patients. This subgroup may face a delayed diagnosis due to their negative hr-HPV status, which can result in advanced-stage detection or poor prognosis (7, 8).
Cervical cancer continues to have a significant incidence, despite global efforts in HPV vaccination campaigns (9). Considering China’s vast population, even a relatively low percentage of hr-HPV-negative cervical cancer patients amounts to a significant number of individuals. Thus, to minimize the risk of missed diagnoses and to improve the prognosis and quality of life for these patients, it is imperative to give due consideration to hr-HPV-negative cervical lesions in clinical practice. So we need to fully understand the characteristics of the hr-HPV-negative cervical cancer.
Although numerous independent studies have identified risk factors associated with hr-HPV-negative cervical cancer, few meta-analyses have been conducted on these studies. Hence, this study employs a meta-analytical approach to systematically analyze the clinical and pathological characteristics of patients with hr-HPV-negative cervical cancer. The ultimate objective is to generate innovative insights that can inform clinical practice in cervical cancer treatment and enhance patient outcomes.
2 Methods
2.1 Search strategy
A computerized literature search was executed on databases including PubMed, Embase, Cochrane Library Web of Science, CNKI and Wanfang to identify studies concerning risk factors or clinical characteristics of hr-HPV-negative cervical cancer. The search strategy entailed an amalgamation of topic keywords and unrestricted terms. Every entry within these databases was retrieved, dating back to their inception until October 2023. The core search terms included: cervical cancer, cervical lesions, high-risk human papillomavirus, high-risk HPV, hr-HPV, negative, characteristics, risk factors.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
Inclusion criteria included: (1) Studies designed such as case-control, retrospective, and cohort studies; (2) Patients diagnosed with cervical cancer by histopathological examination; (3) Subjects screened for HPV and definitively diagnosed as hr-HPV-negative; (4) Papers discussing the epidemiology, prevention, diagnosis and treatment of hr-HPV-negative cervical cancer; (5) Literature providing full text and complete data; (6) Patients not in the pregnancy period; (7) Patients without autoimmune disease; (8) Patients without a history of hysterectomy and cervical surgery.
2.2.2 Exclusion criteria
Exclusion criteria comprised: (1) Duplicate publications; (2) Foundational literature or reviews; (3) Literature where full-text could not be obtained; (4) Literature with unclear outcome indicators; (5) Literature encompassing abstracts, case reports, and meeting minutes; (6) Literature providing incomplete or inaccurate clinical data.
2.3 Data extraction and quality assessment
Upon completion of the literature search, Endnote 20 was utilized to review and eliminate articles including repeated publications and irrelevant researches. Two independent reviewers meticulously scrutinized and cross-verified the selected literature based on the stipulated inclusion and exclusion criteria. Following a preliminary screening of titles and abstracts, the full content of potentially relevant papers were thoroughly read to finalize inclusion. In case of disagreements between the two reviewers, a third reviewer was consulted. Data extracted from the included literature comprised: (1) Title, author, year of publication, total sample size, hr-HPV-negative sample size, and hr-HPV-positive sample size; (2) Research characteristics such as the age of study subjects and relevant study factors. The quality of the selected publications was evaluated using the Newcastle Ottawa Scale (NOS) by the two independent reviewers. Criteria for evaluation included case selection, case representativeness, control selection, comparability of case and control groups, exposure analyses and follow-up integrity. Papers scoring 1 to 3 points were deemed poor quality, 4 to 6 points medium quality, and 7 to 9 points high quality. In case of subjective selection bias, two reviewers required to discuss, or a third reviewer was to be consulted.
2.4 Statistical analysis
The meta-analysis was performed using RevMan5.4 software. For count data, the risk ratio (RR) was used as the effect index. The corresponding 95% confidence interval (CI) was then calculated and expressed via a forest plot. The degree of heterogeneity among the articles was ascertained using I2 and Q statistics. The dichotomous outcomes were analyzed using the fixed-effects model. Random-effects model was used when the heterogeneity was greater than 50%. I² > 50.0% or P < 0.10 was considered to be significant heterogeneity. Then sensitivity analyses were conducted to assess the robustness of the pooled conclusions by using the fixed effects model. In our research, we calculated the fixed-effects model and random-effects model at the meantime in all the forest map. P < 0.05 indicated statistical significance.
3 Results
3.1 literature search results
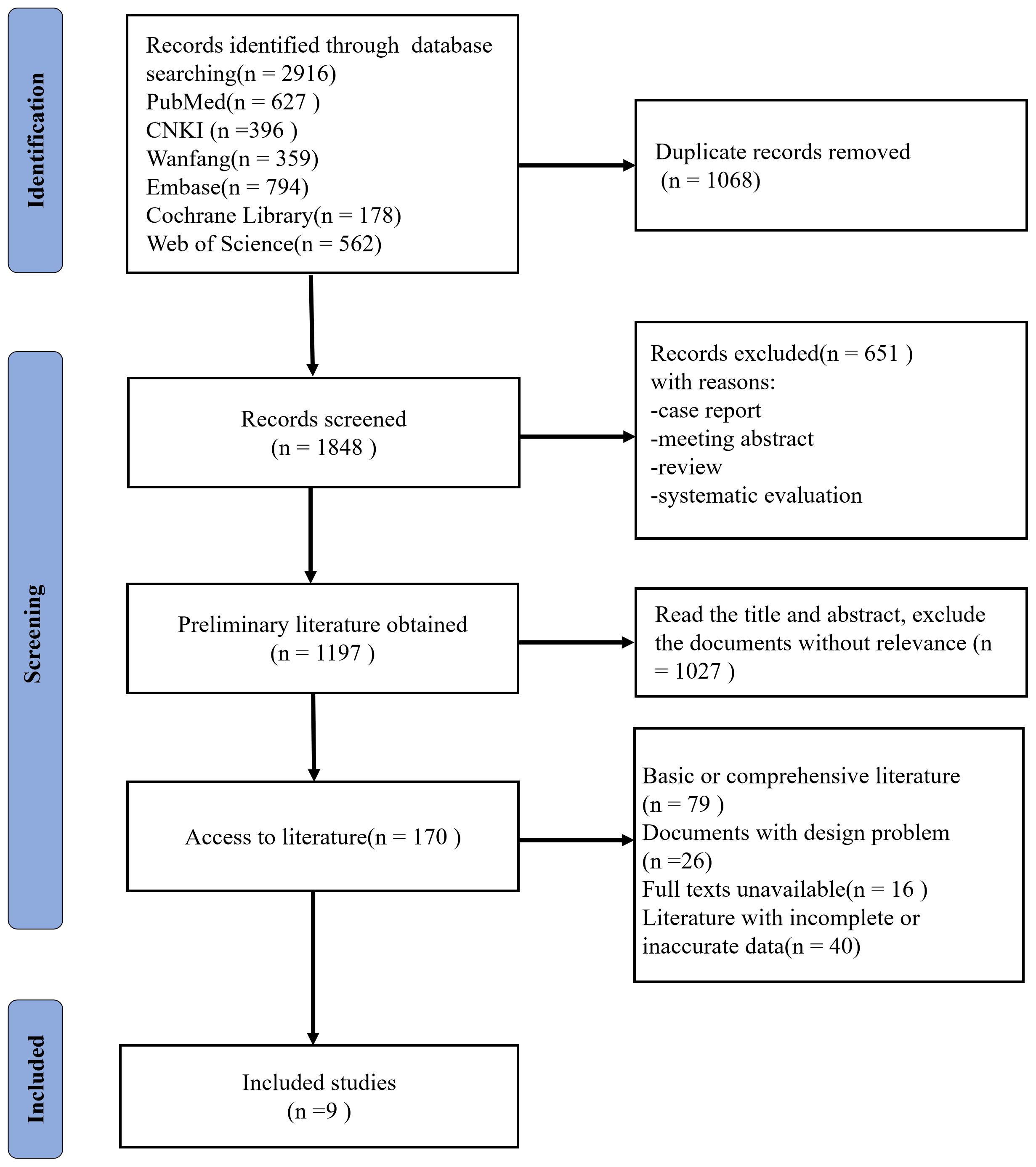
Initially, 2916 records were retrieved, which reduced to 1848 following the removal of 1068 duplications. 651 records were excluded with reasons of case report, meeting abstract, systematic evaluations or review. Upon perusing the titles and abstracts, 1027 articles that lacked apparent relevance were discarded, resulting in a total of 170 articles. Further scrutiny led to the exclusion of 79 foundational class articles or reviews, 26 papers with unresolvable design issues, 16 records with full texts unavailable, 40 documents with incomplete or inaccurate clinical data. Ultimately, nine articles were deemed suitable for inclusion (10–18). Figure 1 illustrates the steps involved in the literature review.
3.2 Basic characteristics and quality assessment of included literature
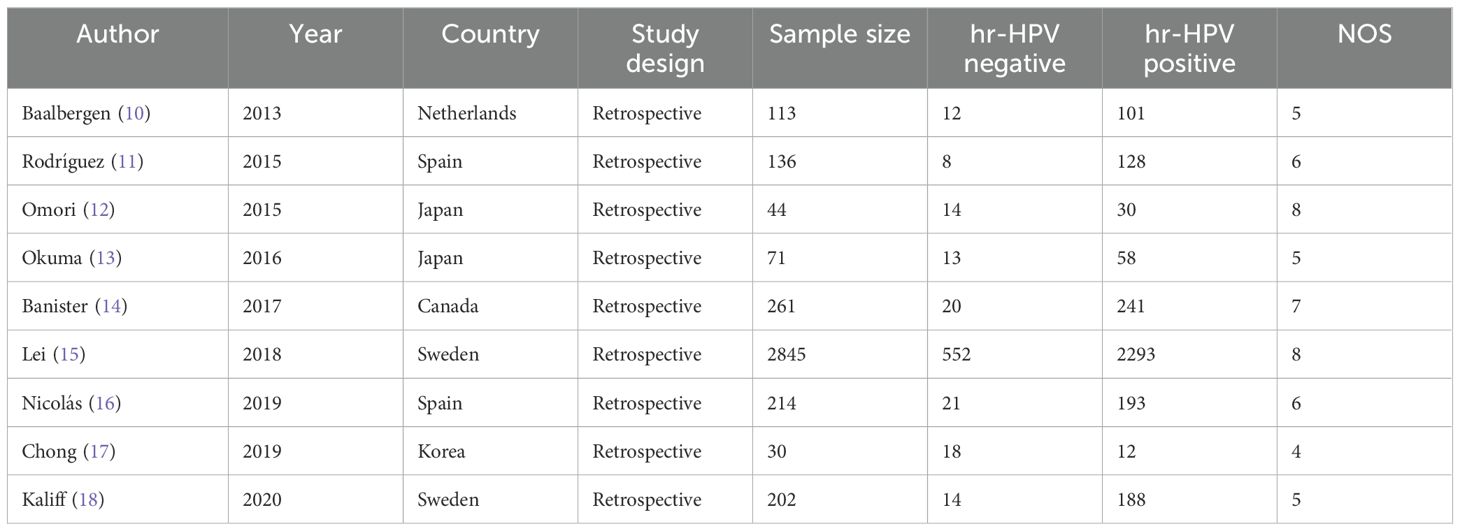
The analysis encompassed nine articles published between 2013 and 2020, with a collective total of 3916 patients, 672 hr-HPV-negative and 3244 hr-HPV-positive. The Newcastle Ottawa Scale (NOS) was employed to gauge the quality of the included articles. Table 1 provides a summary of the included studies and an evaluation of their respective quality.
3.3 Results of meta-analysis
3.3.1 Meta-analysis of positive lymph node metastasis
Five separate studies assessed positive lymph node metastasis as a potential outcome indicator. Figure 2 indicates a substantial divergence in the positive rate of lymph node metastasis between hr-HPV-negative and hr-HPV-positive cervical cancer patients [RR=1.99, 95%CI (1.46-2.72), P<0.0001]. This statistically significant discrepancy serves as evidence.
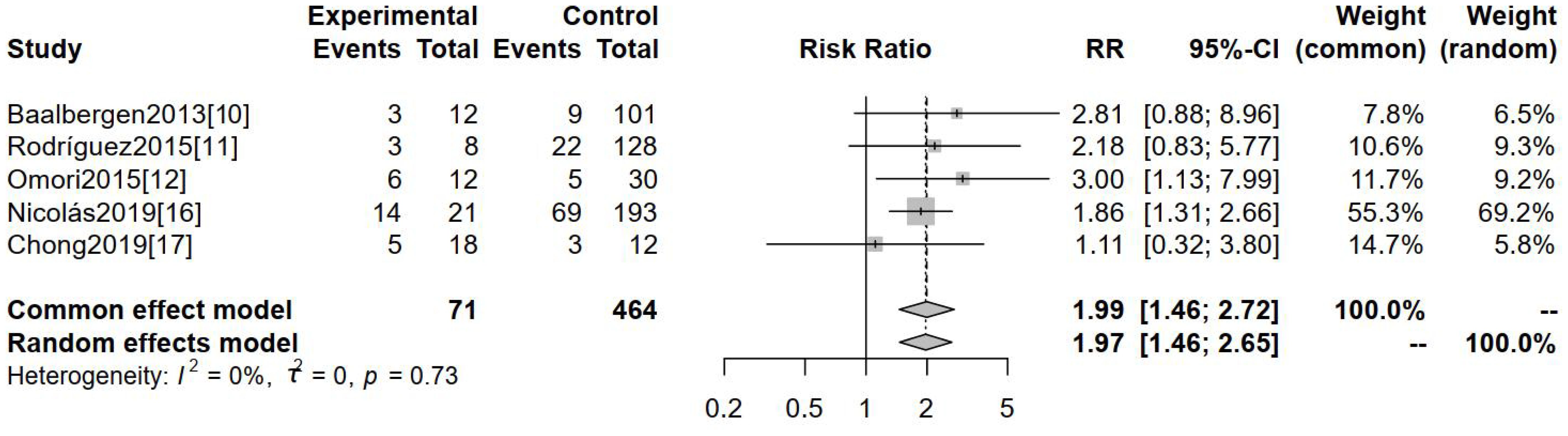
Figure 2. Comparison of positive lymph node metastases between hr-HPV-negative and -positive cervical cancer.
3.3.2 Meta-analysis of clinical staging
Seven studies deployed clinical staging ≥Ib2 as an outcome measure. Clinical stages in studies included were all based on FIGO 2009. Figure 3, featuring the forest map analysis, reveals a significant difference in clinical staging ≥Ib2 between hr-HPV-negative and hr-HPV-positive cervical cancer patients [RR=1.42, 95%CI (1.15-1.77), P=0.0001].
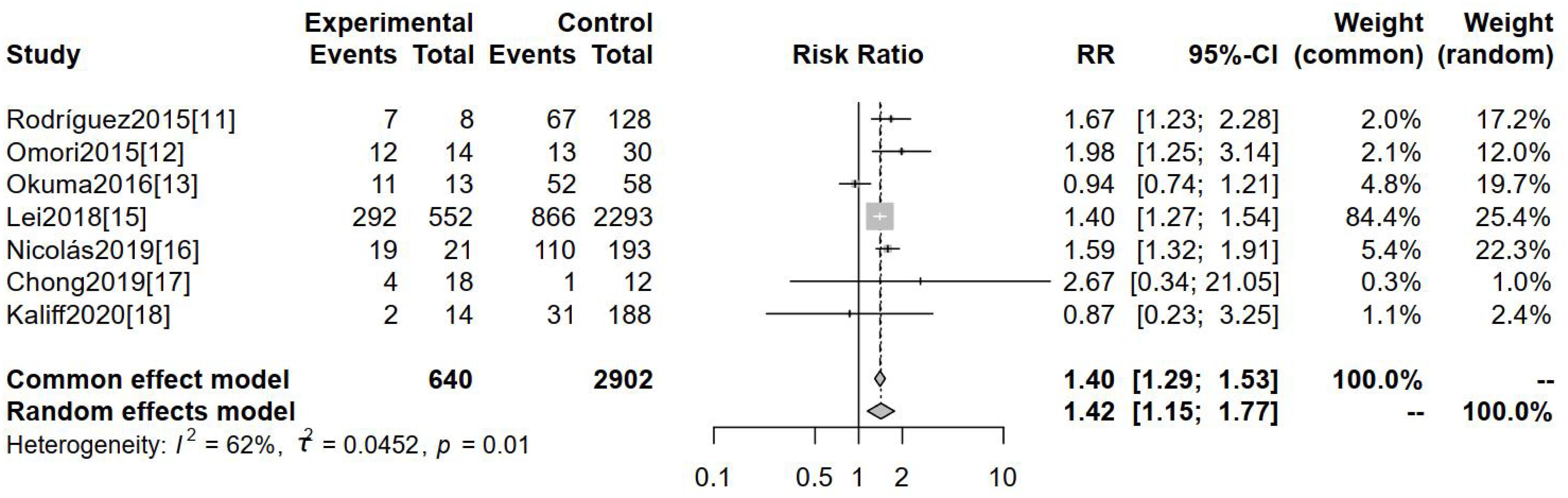
Figure 3. Comparison of the clinical staging ≥Ib2 of cervical cancer in patients with hr- HPV-negative and -positive.
3.3.3 Meta-analysis of incidence of non-squamous carcinoma
The incidence of non-squamous carcinoma was evaluated as an outcome indicator across seven studies. Figure 4 demonstrates a statistically significant difference in the incidence of non-squamous carcinoma between these two groups [RR=2.56, 95%CI (1.63-4.01), P=0.001].
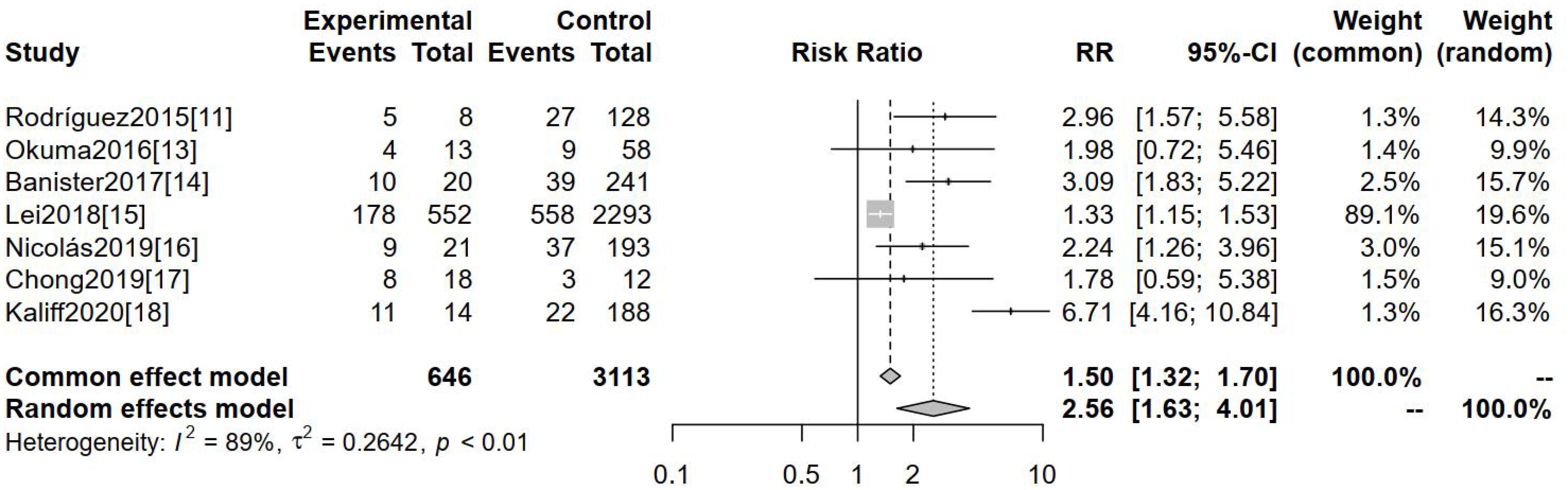
Figure 4. Comparison of the incidence of non-squamous carcinoma in hr-HPV-negative and -positive cervical cancers.
3.3.4 Meta-analysis of incidence of adenocarcinoma
Seven studies investigated the incidence of adenocarcinoma as an outcome indicator. The forest map results, presented in Figure 5, indicate a significantly higher incidence of adenocarcinoma in hr-HPV-negative cervical cancer patients compared to those testing hr-HPV-positive [RR=2.36, 95%CI (1.44-3.89), P=0.005].

Figure 5. Comparison of the incidence of adenocarcinoma in hr-HPV-negative and -positive cervical cancer cases.
3.4 Publication bias analysis
The funnel plots showed that there was no obvious risk of literature publication bias in the meta-analysis of positive lymph node metastasis and clinical staging ≥Ib2, while the risk was exisit in the incidence of non-squamous carcinoma and adenocarcinoma(Figure 6). The risk of bias could have arisen from various factors including the limited sample size of the studies, subpar quality of the sourced papers, among other influences.
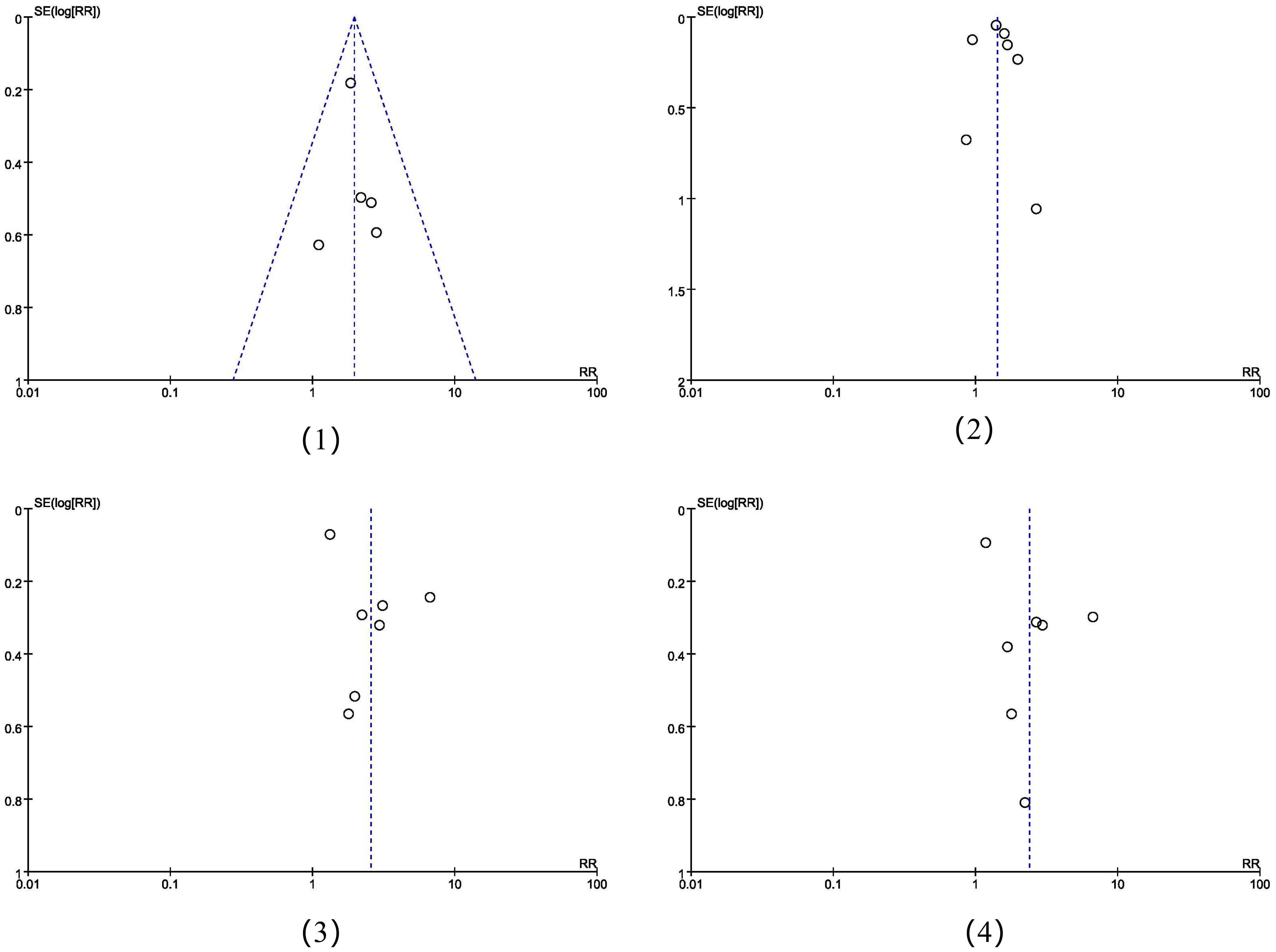
Figure 6. The funnel plots of the four meta-analysis placed in sequential order: (1) positive lymph node metastasis, (2) clinical staging ≥Ib2, (3) the incidence of non-squamous carcinoma, (4) the incidence of adenocarcinoma.
4 Discussion
The global incidence of high-risk HPV infections and cervical cancer poses a significant threat to the health and quality of life of women, engendering profound psychological stress and economic burdens. HPV vaccines have shown potential to decrease the prevalence of hr-HPV infections and associated cervical lesions (19). Nonetheless, not all instances of cervical cancer are initiated and progressed through HPV infection, approximately 5.5-11.0% of cervical cancer pathology has been proven to be HPV-negative upon detection (20, 21).
Evidence posits a robust correlation between hr-HPV infection and cervical squamous carcinoma, whereas a linkage between hr-HPV infection and cervical adenocarcinoma has been discerned but has yet to achieve statistical significance (22). Certain studies have suggested that the formalin fixation of tissues can instigate alterations in the high-frequency sequences of genes, potentially resulting in false negatives, thereby reducing the detection rate of PCR (23). However, researches also found that some rare pathological types of cervical cancer with poor prognosis were truly HPV-negative (24, 25).
As such, it is of paramount importance to administer thorough care to individuals presenting with clinically hr-HPV-negative cervical lesions. In consideration of the above, the current study employs a meta-analytical approach to explore the clinical features, pathological characteristics, and associated factors in patients with hr-HPV-negative cervical cancer. The aim is to generate innovative concepts for clinical guidance in the treatment of cervical cancer, ultimately enhancing the prognosis for individuals diagnosed with the condition.
The study encompassed a total of nine publications, predominantly spanning from 2013 to 2020. The findings demonstrated that patients with hr-HPV-negative cervical cancer manifested a risk of lymph node metastasis 1.99 times greater than that in patients with hr-HPV-positive cervical cancer [RR=1.99, 95%CI (1.46-2.72), P<0.0001]. This could be attributed to the fact that certain cervical cancers, not instigated by the HPV virus, do not demonstrate typical progression or manifestation (26). By the time such illnesses are detected, they have likely advanced, thus posing a significant risk of lymph node metastasis.
In relation to clinical staging, individuals with hr-HPV-negative cervical cancer were 1.42 times more likely to be staged at greater than Ib2 compared to their hr-HPV-positive counterparts [RR=1.42, 95%CI (1.15-1.77), P=0.001]. The early detection of hr-HPV-negative cervical cancer is challenging, often leading to late-stage diagnosis, possibly bypassing the optimal window for surgical intervention (27). This late discovery often results in a poor prognosis and reduced survival rates.
With respect to non-squamous cancer, patients with hr-HPV-negative cervical cancer were found to have a 2.56 times greater incidence rate compared to patients with hr-HPV-positive cervical cancer [RR=2.56, 95%CI (1.63–4.01), P=0.001]. However, gastric adenocarcinoma and other pathological types remain relatively rare among hr-HPV-negative cervical adenocarcinomas. Current preventative measures and treatment options for hr-HPV are predominantly effective against squamous carcinoma and squamous epithelial lesions, which are primarily driven by HPV. Cervical gastric adenocarcinoma represents a separate entity with a clinically aggressive demeanor (28, 29). The risk of adenocarcinoma was 2.36 times higher in patients with hr-HPV-negative cervical cancer compared to those with hr-HPV-positive cervical adenocarcinoma [RR=2.36, 95%CI (1.44–3.89), P=0.005]. This could be attributed to the fact that hr-HPV infection may not contribute to the etiology of adenocarcinoma and other uncommon pathological types of cervical cancer (30).
A review found that the HPV positive rate for the period 1990-1999, 2000-2005 and 2006-2010 was 85.9%, 87.9% and 92.9%, respectively (31). The decrease in HPV negative cancers is likely to reflect improvements in the HPV detection methods (32). It also shows that the hr-HPV negative cervical cancers may contain false negative cases. Sampling errors including inadequate cellularity, obscuring blood, inflammation and cytolysis may lead to false negative. In addition, samples stored for more than 30 years have a significantly lower HPV detection rate (24). Banister et al. found that there is a subset of tumors, which no longer express HPV E6/E7 oncogenes (HPV-inactive). These HPV-inactive tumors have a global decrease in DNA methylation and an increased WNT/β-catenin and Sonic Hedgehog signaling, which may decrease the HPV positive detection rate (14). And a negative test result may occur if the test does not detect a specific rare hr-HPV type. More advanced detection methods will improve the accuracy of the detection, for instance, a study initiated by Cancer Genome Atlas Research Network used next-generation sequencing to characterize primary cervical cancers, found that only 5% of cervical cancers were HPV-negative (33). HPV vaccination and HPV screening can not prevent or detect the hr-HPV negative cervical cancers (32). Therefore, how to detect and prevent hr-HPV negative cervical cancer will become an important study.
Despite yielding some clinically relevant findings that could contribute to evidence-based therapeutic counseling and treatment, this study also has its limitations: The limited number of articles included may impact the comprehensiveness of the meta-analysis. Potential biases might have arisen since all the included papers were retrospective studies. Due to the detection technique, there might be false negative hr-HPV diagnoses, adding the risk of bias. In the future, we will strive to incorporate high quality and multi-center research results into our work.
5 Conclusion
In conclusion, patients with hr-HPV-negative cervical cancer are typically diagnosed at later stages compared to those with hr-HPV-positive cancer. Furthermore, the incidence of adenocarcinoma, non-squamous malignancies, and lymph node metastases were found to be higher among hr-HPV-negative cervical cancer patients, indicating a more advanced disease stage at diagnosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JL: Conceptualization, Data curation, Writing – original draft. FG: Formal analysis, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. (2007) 7:11–22. doi: 10.1038/nrc2050
3. Kusakabe M, Taguchi A, Sone K, Mori M, Osuga Y. Carcinogenesis and management of human papillomavirus-associated cervical cancer. Int J Clin Oncol. (2023) 28:965–74. doi: 10.1007/s10147-023-02337-7
4. Clavel C, Masure M, Bory JP, Putaud I, Mangeonjean C, Lorenzato M, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7932 women. Br J Cancer. (2001) 84:1616–23. doi: 10.1054/bjoc.2001.1845
5. Gottschlich A, Gondara L, Smith LW, Cook D, Martin RE, Lee M, et al. Human papillomavirus-based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. (2022) 151:897–905. doi: 10.1002/ijc.34039
6. Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. (2010) 11:249–57. doi: 10.1016/S1470-2045(09)70360-2
7. Stolnicu S, Hoang L, Chiu D, Hanko-Bauer O, Terinte C, Pesci A, et al. Clinical outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the international endocervical adenocarcinoma criteria and classification (IECC). Am J Surg Pathol. (2019) 43:466–74. doi: 10.1097/PAS.0000000000001224
8. Petry KU, Liebrich C, Luyten A, Zander M, Iftner T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. (2017) 4:85–9. doi: 10.1016/j.pvr.2017.10.003
9. Restaino S, Pellecchia G, Arcieri M, Bogani G, Taliento C, Greco P, et al. Management for cervical cancer patients: A comparison of the guidelines from the international scientific societies (ESGO-NCCN-ASCO-AIOM-FIGO-BGCS-SEOM-ESMO-JSGO). Cancers. (2024) 16:2541. doi: 10.3390/cancers16142541
10. Baalbergen A, Smedts F, Ewing P, Snijders PJ, Meijer CJ, Helmerhorst TJ. HPV-type has no impact on survival of patients with adenocarcinoma of the uterine cervix. Gynecol Oncol. (2013) 128:530–4. doi: 10.1016/j.ygyno.2012.12.013
11. Rodríguez-Carunchio L, Soveral I, Steenbergen RD, Torné A, Martinez S, Fusté P, et al. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG. (2015) 122:119–27. doi: 10.1111/1471-0528.13071
12. Omori M, Hashi A, Kondo T, Katoh R, Hirata S. Dysregulation of CDK inhibitors and p53 in HPV-negative endocervical adenocarcinoma. Int J Gynecol Pathol. (2015) 34:196–203. doi: 10.1097/PGP.0000000000000121
13. Okuma K, Yamashita H, Yokoyama T, Nakagawa K, Kawana K. Undetected human papillomavirus DNA and uterine cervical carcinoma: Association with cancer recurrence. Strahlenther Onkol. (2016) 192:55–62. doi: 10.1007/s00066-015-0909-0
14. Banister CE, Liu C, Pirisi L, Creek KE, Buckhaults PJ. Identification and characterization of HPV-independent cervical cancers. Oncotarget. (2017) 8:13375–86. doi: 10.18632/oncotarget.14533
15. Lei J, Ploner A, Lagheden C, Eklund C, Nordqvist Kleppe S, Andrae B, et al. High-risk human papillomavirus status and prognosis in invasive cervical cancer: A nationwide cohort study. PloS Med. (2018) 15:e1002666. doi: 10.1371/journal
16. Nicolás I, Marimon L, Barnadas E, Saco A, Rodríguez-Carunchio L, Fusté P, et al. HPV-negative tumors of the uterine cervix. Mod Pathol. (2019) 32:1189–96. doi: 10.1038/s41379-019-0249-1
17. Chong GO, Han HS, Park JY, Lee SD, Lee YH, Lee HJ, et al. Prevalence, survival outcomes, and clinicopathologic factors associated with negative high risk human papillomavirus in surgical specimens of cervical cancer with pretreatment negative DNA genotype test. Int J Gynecol Cancer. (2019) 29:10–6. doi: 10.1136/ijgc-2018-000003
18. Kaliff M, Karlsson MG, Sorbe B, Bohr Mordhorst L, Helenius G, Lillsunde-Larsson G. HPV-negative tumors in a swedish cohort of cervical cancer. Int J Gynecol Pathol. (2020) 39:279–88. doi: 10.1097/PGP.0000000000000612
19. Yousefi Z, Aria H, Ghaedrahmati F, Bakhtiari T, Azizi M, Bastan R, et al. An update on human papilloma virus vaccines: history, types, protection, and efficacy. Front Immunol. (2022) 12:805695. doi: 10.3389/fimmu.2021.805695
20. Castle PE. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. (2015) 123:566. doi: 10.1002/cncy.21572
21. Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. (2012) 131:2349–59. doi: 10.1002/ijc.27485
22. Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. (2006) 98:303–15. doi: 10.1093/jnci/djj067
23. Xing B, Guo J, Sheng Y, Wu G, Zhao Y. Human papillomavirus-negative cervical cancer: A comprehensive review. Front Oncol. (2021) 10:606335. doi: 10.3389/fonc.2020.606335
24. Pirog EC, Lloveras B, Molijn A, Tous S, Guimerà N, Alejo M, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. (2014) 27:1559–67. doi: 10.1038/modpathol.2014.55
25. Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, et al. International endocervical adenocarcinoma criteria and classification (IECC): A new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. (2018) 42:214–26. doi: 10.1097/PAS.0000000000000986
26. Giannella L, Di Giuseppe J, Delli Carpini G, Grelloni C, Fichera M, Sartini G, et al. HPV-negative adenocarcinomas of the uterine cervix: from molecular characterization to clinical implications. Int J Mol Sci. (2022) 23:15022. doi: 10.3390/ijms232315022
27. Iacobone AD, Bottari F, Guerrieri ME, Vidal Urbinati AM, Ghioni M, Spolti N, et al. The potential impact of high-risk human papillomavirus-negative cervical intraepithelial neoplasia 2+ on primary human papillomavirus screening. Am J Clin Pathol. (2022) 157:130–5. doi: 10.1093/ajcp/aqab103
28. Pirog EC, Park KJ, Kiyokawa T, Zhang X, Chen W, Jenkins D, et al. Gastric-type adenocarcinoma of the cervix: tumor with wide range of histologic appearances. Adv Anat Pathol. (2019) 26:1–12. doi: 10.1097/PAP.0000000000000216
29. Ehmann S, Sassine D, Straubhar AM, Praiss AM, Aghajanian C, Alektiar KM, et al. Gastric-type adenocarcinoma of the cervix: Clinical outcomes and genomic drivers. Gynecol Oncol. (2022) 167:458–66. doi: 10.1016/j.ygyno.2022.10.003
30. Park KJ. Cervical adenocarcinoma: integration of HPV status, pattern of invasion, morphology and molecular markers into classification. Histopathology. (2020) 76:112–27. doi: 10.1111/his.13995
31. Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. (2011) 128:927–35. doi: 10.1002/ijc.25396
32. Tjalma W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis Obgyn. (2018) 10:107–13.
Keywords: high-risk human papillomavirus-negative cervical cancer, hr-HPV-negative cervical cancer, characteristics, systematic review and meta-analysis, cervical cancer
Citation: Lou J and Guo F (2024) The characteristics of high-risk HPV-negative cervical cancer: a systematic review and meta-analysis. Front. Oncol. 14:1452834. doi: 10.3389/fonc.2024.1452834
Received: 21 June 2024; Accepted: 25 September 2024;
Published: 09 October 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Martina Arcieri, Ospedale Santa Maria della Misericordia di Udine, ItalyLeabaneng Tawe, Botswana-UPenn Partnership (BUP), Botswana
Copyright © 2024 Lou and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Guo, Z2ZqaWFueGlhb2RhbkBodXN0LmVkdS5jbg==
 Jicheng Lou
Jicheng Lou Feng Guo
Feng Guo