- 1Centre for Cancer Biomarkers and Biotherapeutics, Barts Cancer Institute, Queen Mary University of London, London, United Kingdom
- 23rd Street Diagnostics, Cedars-Sinai, Los Angeles, CA, United States
- 3Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai, Los Angeles, CA, United States
- 4Department of Medicine, Cedars-Sinai, Los Angeles, CA, United States
- 5Barts Health, Royal London Hospital, London, United Kingdom
- 6Institute for Liver and Digestive Health, University College London, London, United Kingdom
- 7Department of Molecular and Clinical Cancer Medicine, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom
- 8Centre for Cancer Screening, Prevention and Early Detection, Wolfson Institute of Population Health, Queen Mary University of London, London, United Kingdom
- 9Department of Pediatrics and Pediatric Infectious Diseases, Institute of Child´s Health, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related death worldwide. Up to now, no specific screening or diagnostic tests are available for early PDAC detection. As a result, most patients are diagnosed with advanced or metastatic disease, which leads to a poor prognosis. In this study, we aimed to evaluate the diagnostic value of urinary CRP (uCRP) alone and in combination with our previously established urine biomarker panel (REG1B, LYVE1 and TFF1) for early detection of PDAC. A total of 534 urine samples from multiple centres were analysed: 93 from healthy individuals, 265 from patients with benign hepatobiliary diseases and 176 from PDAC patients. The uCRP and the urinary biomarker panel were assessed using commercial ELISA assays, while plasma CA19-9 and blood CRP (bCRP) were measured using Roche Cobas platform. Multiple logistic regression and nonparametric Kruskal–Wallis test were used for statistical analysis. An internal validation approach was applied, and the validated AUC estimators were reported to ensure accuracy. A significant difference was observed in the medians of uCRP between healthy and benign controls and PDAC sample groups (p < 0.001). uCRP levels were not dependent on gender and age, as well as cancer stage. When uCRP was combined with the urinary biomarker panel, it achieved AUCs of 0.878 (95% CI: 0.802-0.931), 0.798 (95% CI: 0.738-0.859) and 0.813 (95% CI: 0.758-0.869) in healthy vs PDAC, benign vs PDAC and healthy and benign vs PDAC sample groups, respectively. However, adding plasma CA19-9 to the urinary biomarker panel yielded a better performance, with AUCs of 0.978 (95% CI: 0.959-0.996), 0.911 (95% CI: 0.873-0.949) and 0.919 (95% CI: 0.883-0.955) in the healthy vs PDAC, benign vs PDAC and healthy and benign vs PDAC comparisons, respectively. In conclusion, we show that measuring CRP in urine is a feasible analytical method, and that uCRP could potentially be a promising biomarker in various diseases including other cancer types.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for approximately 90% of all pancreatic neoplasms (1). The majority of patients with this disease experience nonspecific symptoms and are diagnosed at an advanced stage, resulting in exceptionally poor prognosis with a five-year survival of patients with distant metastasis being only 3% (2). This dismal survival rates can be improved through early detection when the disease is still localised and amenable to surgical intervention (3, 4).
C-reactive protein (CRP) is a major acute-phase protein predominantly produced by the liver (5). It plays a vital role in the response to infection, inflammation and tissue injury where its concentration in blood increases, so it is a widely used systemic marker of severity for these conditions (5, 6). Elevated levels of blood CRP (bCRP) have been associated with an increased risk of cardiovascular disease (7) but also with increased risk of cancer in the general population (8), including non-sigmoid colon and lung cancers (9), breast, ovarian and liver cancers (10–12). The highest risk was seen in squamous cell lung cancer, where CRP levels were elevated up to 5 years before cancer diagnosis and risk of cancer rose steadily with increasing CRP levels (13).
bCRP has also been studied as a diagnostic biomarker for various types of cancer (14, 15). In PDAC, it was demonstrated that combination of bCRP and CA19-9, as well as several inflammatory cytokines can distinguish patients with PDAC from patients with chronic pancreatitis (CP) and healthy individuals (16–18). In addition, bCRP has been widely reported as a prognostic marker in a number of cancers, including PDAC (8, 19–26).
Despite CRP being one of the most commonly used biomarkers, it is typically only measured in blood samples, and there are scarce data available on measuring CRP in urine (uCRP). Chuang et al. showed that CRP is not a normal constituent of urine and is not a biomarker of local inflammation in the urinary tract (27); similarly, in two studies that measured uCRP in patients with lower urinary tract symptoms (LUTS) and children with urinary tract infections (UTI), uCRP was not found to be a specific biomarker for either condition (27, 28). In contrast, in studies by Andersson et al. (29) and Ashkenazi-Hoffnung et al. (30), urinary CRP was found to be expressed at higher levels in pediatric UTI and enabled effective differentiation of bacterial from viral urinary tract infections. Except for these reports, we could not find any additional publications on measuring CRP in urine.
We have previously described and validated a panel of urine biomarkers comprising LYVE1, REG1B and TFF1 which could detect resectable PDAC with over 90% accuracy (31, 32). We have also developed a PancRISK score for the easy interpretation of data from the panel (33). Recently, we have also demonstrated that this panel can detect PDAC up to two years prior to diagnosis (34). Here, similarly to what we have shown previously, CA19-9, a commonly used PDAC biomarker, improved the performance of the urinary panel. As CA19-9 is a blood-based biomarker, we are searching for additional urine biomarker(s) to replace it, in order to devise a wholly urine-based test for the early detection of PDAC.
It is well established that inflammation plays a crucial role in promoting tumour growth and progression (35, 36) and CRP is a widely utilised marker of ongoing inflammation. Interestingly, CRP was shown to be an independent predictor of the risk of developing type 2 diabetes (37) and it is elevated in patients with newly diagnosed diabetes mellitus (38), which are a known risk factor and an early clinical manifestation of PDAC, respectively (39). In the present study, we therefore explore the role of uCRP as a diagnostic biomarker for PDAC to determine whether it can be reliably measured in urine, and whether it can distinguish control groups from PDAC samples. Provided this is possible, we then aim to understand to what extent uCRP can improve the accuracy of our current urinary panel.
Methods
Clinical samples
This case-control study was performed using prospectively collected urine samples from Royal London Hospital, University College London and University of Liverpool in the UK and Cedars-Sinai in Los Angeles, USA, collected using common protocols. All the samples were collected from patients above the age of 18 who gave informed consent. The exclusion criteria were: current or prior treatment (chemotherapy, radiotherapy, surgical resection, biological therapy, and immunotherapy) for any malignancy other than basal cell carcinoma within 5 years of enrolment.
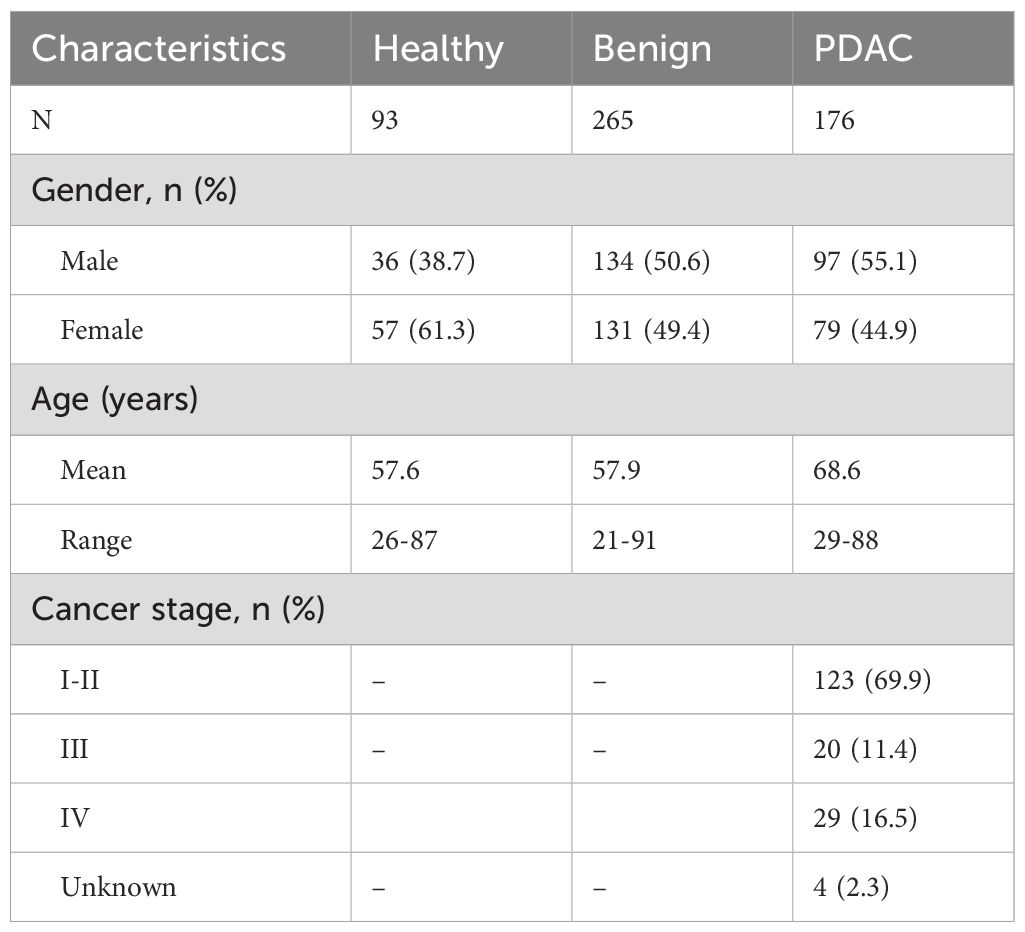
The study was approved by the Northeast - York Research Ethics Committee (18/NE/0070). In total, 534 urine samples were analysed: 93 samples forming the healthy control group were from people with no history of pancreatic conditions or malignancies at the time of collection, of these, 37 had family history of pancreatic cancer; 176 samples were collected from patients diagnosed with PDAC (123 stage I–II, 49 stage III–IV and 4 unknown), and 265 were from patients with benign hepatobiliary diseases (benign control group). This control group included patients presenting symptoms suggestive of pancreatic cancer, including, but not limited to, abdominal pain, back pain, nausea, vomiting, diarrhoea, constipation or new onset diabetes, as well as patients undergoing surgical interventions for suspected pancreatic cancer, such as cystic lesions of the pancreas. The benign sample group included: 97 samples with pancreatitis, of which 65 were chronic pancreatitis; further 54 samples were from patients with pancreatic cysts; 50 samples were from patients with biliary duct diseases such as cholecystitis and cholelithiasis; 20 samples represented various liver diseases and 44 other samples included samples collected from patients with gastritis and unspecified abdominal pain.
The demographic details of the samples are summarised in Table 1.
Sample preparation and analysis of CRP
Urinary CRP was measured using an ELISA kit from Immundiagnostik AG in Bensheim, Germany (Cat# K9710s; intra- and inter-assay coefficients of variation <7% and <8%, respectively; detection limit 1.015 ng/ml), according to the manufacturer’s protocol, with urine samples being diluted 1:5. The absorbance was measured with a microplate reader (FLUOstar® Omega, BMG LABTECH, Offenburg, Germany) at 450 nm. uCRP levels were calculated from the calibration curve. Each sample was analysed in duplicate to ensure accuracy, and the average value was used for calculations. Samples below the detection limit were taken as half of the detection limit (0. 508 ng/ml). Matched blood CRP values (measured by Roche Cobas platform, Roche Diagnostics, UK) close to the urine collection date were obtained from patients’ medical records.
Analysis of urinary biomarker panel and plasma CA19-9
Commercially available ELISA kits were used to measure the three biomarkers -TFF1, LYVE1 and REG1B- in urines as described elsewhere (31, 34). Briefly, TFF1 and LYVE1 were measured using R&D Systems (Cat# DY5237 and Cat# DY2089, respectively). Urine samples were diluted 1:10 and 1:75 for TFF1 and LYVE1, respectively. Urinary REG1B was measured with the ELISA pair set from Sino Biological Inc. (Cat# SEK11638) and urines were diluted 1:750. The substrate (TMB) and stop solution were from BioLegend (Cat# 421101 and 423001). The absorbance was determined with a microplate reader (FLUOstar® Omega, BMG LABTECH, Offenburg, Germany) at 450 nm. Plasma CA19-9 was measured using Roche platform (Cobas 601E [ECLIA] technology) according to routine protocols. The minimum detectable level of CA19-9 was 0.3 U/ml.
Statistical analysis
Descriptive statistics were calculated for baseline characteristics. Continuous variables were summarised as median and interquartile range (IQR) and categorical variables as frequency (percentage). Spearman correlation coefficient was used to examine the correlation of uCRP with the remaining biomarkers. Non-parametric Kruskal-Wallis test with Dunn’s correction was used to compare biomarker levels across the experimental groups.
All protein concentration data were standardised prior to the analysis. The biomarker panel was combined with age, CRP and CA19-9 in various combinations, which were analysed using receiver operating characteristics (ROC) curve to establish their ability to discriminate between PDAC and control specimens. Internal validation was performed by random splitting the whole dataset into the training and validation sets in a 1:1 ratio. Logistic regression was applied to both the training and validation sets: one group comprising of control and PDAC samples, the other comprising of benign and PDAC samples. The performance characteristics of the regression models were evaluated in the validation set and compared in terms of the area under the ROC curve (AUC). Confidence intervals (95% CIs) for AUCs were derived using 2,000 stratified bootstrap replicates.
Two-sided p-values were reported for all statistical tests; a p-value <0.05 was considered to be statistically significant. Statistical analysis was performed using IBM SPSS statistics version 28, GraphPad prism version 10 and R version 4.2.3 (https://cran.r-project.org).
Results
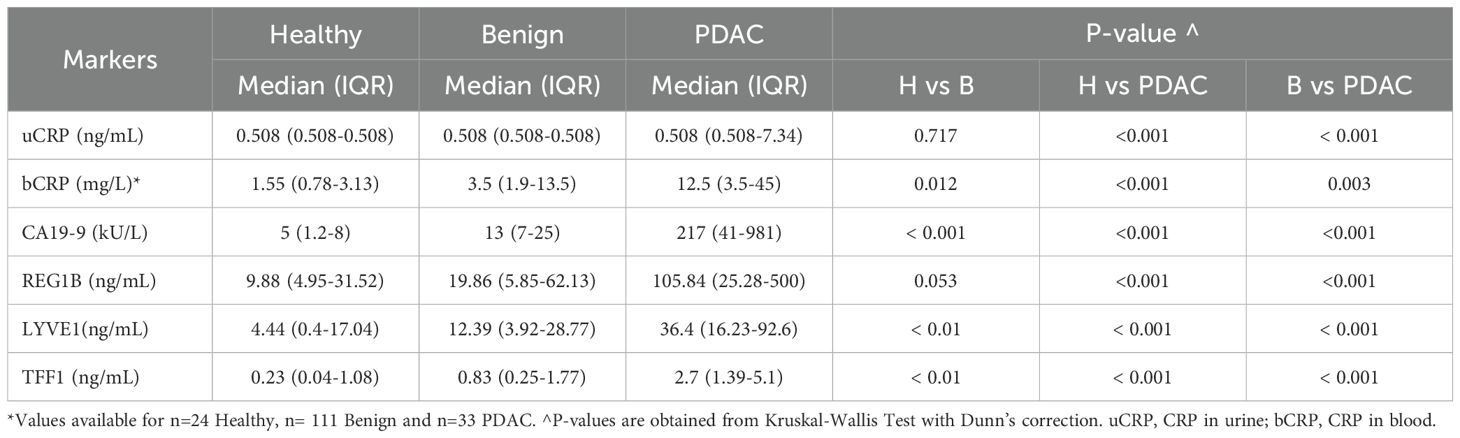
Table 1 presents the baseline characteristics of the study participants. Of the healthy and benign controls, only two (2.2%) and 21 participants (7.9%), respectively had uCRP levels above the detection limit, while this was the case in 73 PDAC patients (41.5%). When the cut-off value of 6ng/mL was used, the specificity (SP) of CRP was 99% and 94% (for healthy controls and healthy and benign controls combined, respectively) and sensitivity (SN) was 30%. The uCRP level was significantly higher (p < 0.001) in participants with PDAC than in both control groups (Figure 1A, Table 2). Values for bCRP were available only for a subset of samples, but the performance was very similar to uCRP (Figure 1B, Table 2). Of note, no significant difference was found in uCRP levels between males and females, and age did not show a significant correlation with uCRP in either the PDAC or control groups. As shown previously, CA19-9 and all three urinary biomarkers were significantly higher in PDAC compared to the control groups (Table 2). A significant but weak correlation (r = 0.353, p < 0.001) was found between uCRP and bCRP levels; uCRP also weakly correlated with CA19-9, LYVE1 and TFF1 (r = 0.302, r = 0.341 and r = 0.358, respectively, p < 0.001) (Supplementary Figure S1).
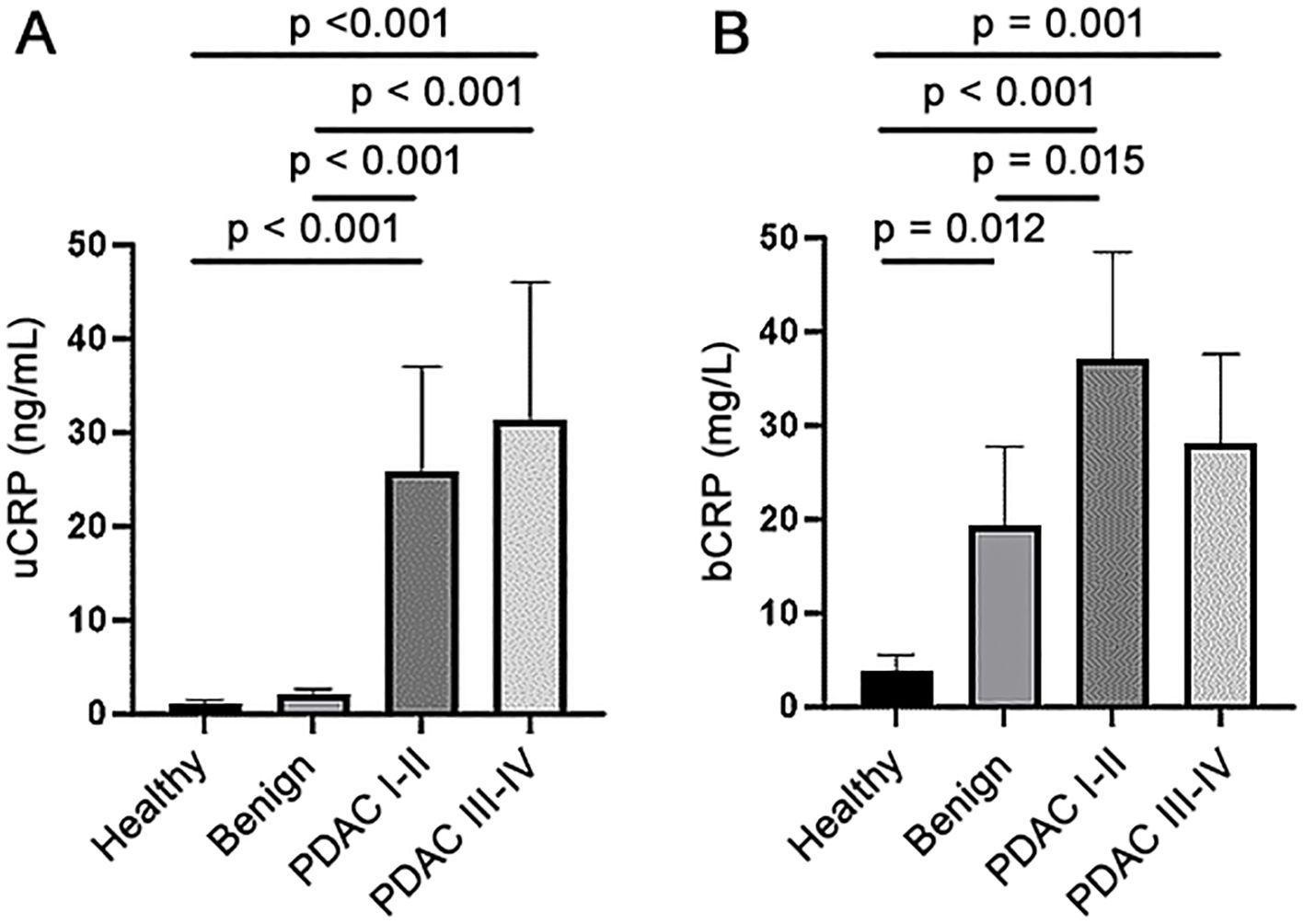
Figure 1. (A) CRP levels in urine (uCRP) and (B) blood (bCRP) in healthy, benign and early (stage I-II) and late stages (III-IV) PDAC samples.
The performance of uCRP alone and in combination with our urinary panel (REG1B, LYVE1 and TFF1 + age), and CA19-9 were assessed using the receiver operator characteristic (ROC) curve analysis (Figure 2). The uCRP alone resulted in AUCs of 0.679 (95% CI: 0.617-0.741) for healthy vs PDAC, 0.667 (95% CI: 0.610-0.724) for benign vs PDAC and 0.659 (95% CI: 0.602-0.717) for healthy + benign vs PDAC. When uCRP was combined with the urinary biomarker panel, it enhanced the performance with AUCs to 0.878 (95% CI: 0.802-0.931), 0.798 (95% CI: 0.738-0.859) and 0.813 (95% CI: 0.758-0.869) in healthy vs PDAC, benign vs PDAC and healthy + benign vs PDAC sample groups, respectively. However, the combination of plasma CA19-9 with the urinary panel yielded a superior performance, with AUCs of 0.978 (95% CI: 0.959-0.996), 0.911 (95% CI: 0.873-0.949) and 0.919 (95% CI: 0.883-0.955) in the healthy vs PDAC, benign vs PDAC and healthy + benign vs PDAC sample groups, respectively. Further addition of uCRP to plasma CA19-9 and the biomarker panel did not result in any further improvement (AUCs of 0.963 (95% CI: 0.926-0.1.00), 0.908 (95% CI: 0.869-0.947), 0.911 (95% CI: 0.874-0.948) for healthy vs PDAC, benign vs PDAC and healthy + benign vs PDAC sample groups, respectively).
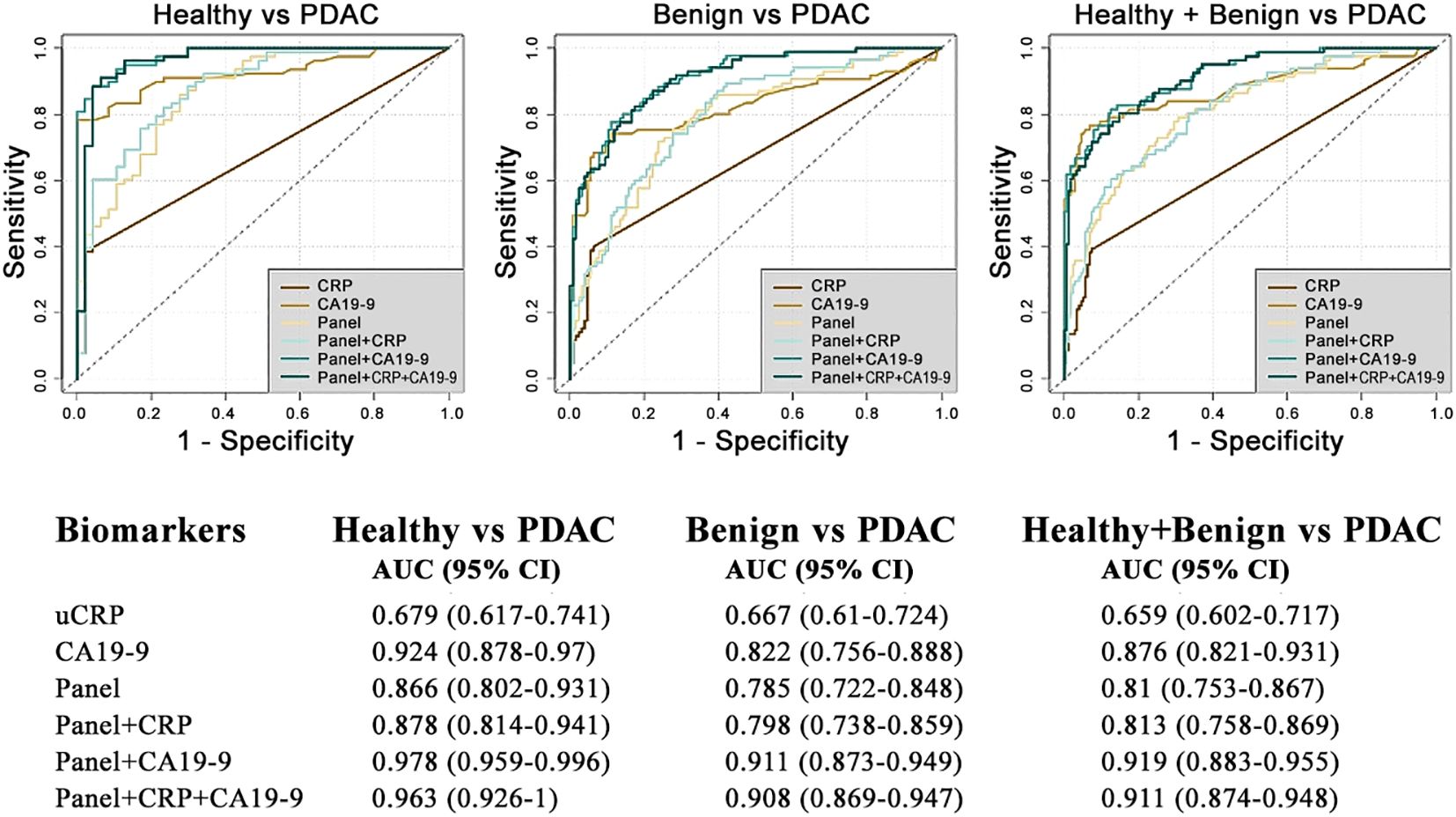
Figure 2. Diagnostic performance of biomarkers discriminating patients with PDAC from benign and healthy controls.
Discussion
Since its discovery in 1930 as a protein that reacts with C-polysaccharide on Streptococcus pneumoniae (40), elevated levels of CRP have been associated with both acute and chronic systemic inflammation. As inflammatory milieu can both favour cancer development and progression and can be induced by tissue injury during cancer growth and spread. Increased CRP levels have been associated with an increased risk of cancer as well as being a harbinger of poor prognosis for this disease (8, 9, 41).
The detailed mechanistic roles of CRP in cancer are still being unravelled. CRP exists in two forms: the circulating pentameric CRP (pCRP) and the monomeric isoform (mCRP). pCRP is highly soluble and rapidly dissociates into mCRP, which is less soluble and self-aggregates in tissues (14). mCRP binds to membrane lipids triggering complex intracellular signalling via phospholipase C, MAPK, ERK, Akt, STAT and NFkB, which are important effector pathways strongly associated with the intrinsic malignant characteristics of cancer cells (42). In addition, mCRP can also modulate inflammatory responses through stimulating platelets, leukocytes and complement system as a response to tumour growth (14).
The production of CRP in hepatocytes is regulated by cytokines, particularly by IL-6, which is secreted by macrophages, endothelial and other cells activated after local tissue injury, leading to an increase in pCRP concentrations in the bloodstream that is being measured (43).
In PDAC, combining bCRP with other biomarkers was shown to be able to differentiate PDAC from benign and control groups, thus aiding the early detection of PDAC: Zhang et al. showed that a panel of CRP with CA19-9, albumin and IL-8 had high diagnostic value for distinguishing PDAC and healthy controls with 96% SN at 90% SP in early stage disease, while CRP combined with CO2, CA19-9 and IL-6 discriminated between PDAC and benign cases with 75% SN at 90% SP (18). Furthermore, Lanki et al., showed that combining bCRP with CA19-9 and inflammatory cytokines CTACK, GRO-a, b-NGF may aid in distinguishing PDAC from CP, but the combination was not superior to CA19-9 alone (17). Ferri et al., tested CA19-9, CRP, IGF-1, CEA and albumin alone and in various combinations (16) and when a CRP cut-off value of 2.3 mg/L was used, SN of 76.6% and a SP of 55% for differentiating between PDAC and CP was established. Notably, significantly higher levels of CRP were seen in jaundiced PDAC patients, suggesting its potential role in distinguishing jaundiced PDAC from CP patients (16). Higher bCRP concentration in PDAC than in CP was also reported by Mroczko et al. (44). All of these studies measured CRP in blood, and with the exception of very few studies (27, 30, 45), hardly any attempt was made to measure CRP in urine. To the best of our knowledge, we could not find any reports on the use of uCRP as a potential diagnostic or prognostic marker for any cancer, including PDAC.
Our long-standing goal is to develop a non-invasive test for early detection of PDAC using urine samples. We chose urine because it is a less complex matrix than blood, with a lower dynamic range, which can be easily, repeatedly and completely non-invasively self-sampled (46, 47).
Limited information is available on CRP kinetics in humans. bCRP is pentameric, and its conversion to mCRP is rapid, with a lag of approximately 6-12 hours before the increase in CRP is seen in blood; clearance of bCRP is largely through renal filtration, as shown by monitoring of excretion of 125I-CRP in a study by Vigushin et al., where a similar rate of excretion was seen in both normal and diseased volunteers (48). While it is not yet clear which form of CRP is excreted into human urine, it has been shown that the binding of calcium and the chelation of proteolysis-resistant pCRP causes considerable protein compaction leading to a significant reduction of its Stoke’s radius (49), which could enable the filtration of this otherwise large (approximately 120kDa) protein through the glomerular barrier. Alternatively, as shown in mouse models, both pCRP and mCRP can be excreted in urine (50), so it is possible that the otherwise proteolysis-sensitive and poorly soluble mCRP is contained within microvesicles that are shed from activated cells during an inflammatory response.
Regardless of this, we found a weak but positive correlation of uCRP with bCRP, which was also shown in one of the rare studies that explored CRP in both urine and blood samples in patients with pneumonia (45). This suggests that completely non-invasive measuring of CRP using urine samples is a feasible option.
This study presents the first evaluation of uCRP in cancer, where the main aim was to establish whether uCRP can be used as a biomarker for early detection of PDAC. We show that uCRP exhibits a very high SP but a relatively low SN in distinguishing between healthy and benign controls from PDAC samples. Combining uCRP with our urine biomarker panel resulted in a slight increase of the AUC, particularly in healthy vs PDAC comparison, however, the addition of plasma CA19-9 to the panel led to a superior performance, which was not further improved by adding uCRP.
The main strength of our study is that we analysed uCRP levels along with our urinary panel biomarkers in control, benign, and PDAC samples from four different centres, including a large number (70%) of early stage PDACs. The significant limitation of our study was that matched bCRP values were obtained from clinical notes, rather than being re-measured.
In conclusion, we have demonstrated that measuring CRP in urine is a straightforward and feasible method. Consequently, this suggest that uCRP could potentially be a promising biomarker for detecting other diseases, including other cancer types, which warrants further confirmation in future studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Northeast - York Research Ethics Committee (18/NE/0070). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NA: Data curation, Methodology, Writing – original draft, Writing – review & editing. SD: Methodology, Writing – review & editing. EK: Methodology, Writing – review & editing. JT: Methodology, Writing – review & editing. NG: Methodology, Writing – review & editing. SP: Methodology, Writing – review & editing. PW: Methodology, Writing – review & editing. SP: Methodology, Writing – review & editing. BG: Methodology, Writing – review & editing. OB: Methodology, Writing – review & editing. TC: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We acknowledge the support from Cancer Research UK EDDCPJT/100022 award (NA) and Barts Charity grant 001522 (OB). SPP is supported by the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre.
Acknowledgments
We thank all the patients and sample donors that provided samples utilised in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1450326/full#supplementary-material
References
1. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: A review. JAMA. (2021) 326:851. doi: 10.1001/jama.2021.13027
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
3. Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. (2018) 15:333–48. doi: 10.1038/s41575-018-0005-x
4. Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, et al. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. (2020) 5:698–710. doi: 10.1016/S2468-1253(19)30416-9
5. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
6. Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. (2004) 279:48487–90. doi: 10.1074/jbc.R400025200
7. Zacho J, Tybjærg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. (2008) 359:1897–908. doi: 10.1056/NEJMoa0707402
8. Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. (2009) 27:2217–24. doi: 10.1200/JCO.2008.19.8440
9. Siemes C, Visser LE, Coebergh J-WW, Splinter TAW, Witteman JCM, Uitterlinden AG, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam study. J Clin Oncol. (2006) 24:5216–22. doi: 10.1200/JCO.2006.07.1381
10. Chen W, Wang J-B, Abnet CC, Dawsey SM, Fan J-H, Yin L-Y, et al. Association between C-reactive protein, incident liver cancer, and chronic liver disease mortality in the Linxian nutrition intervention trials: A nested case–control study. Cancer Epidemiol Biomarkers Prev. (2015) 24:386–92. doi: 10.1158/1055-9965.EPI-14-1038
11. Dossus L, Jimenez-Corona A, Romieu I, Boutron-Ruault M-C, Boutten A, Dupré T, et al. C-reactive protein and postmenopausal breast cancer risk: results from the E3N cohort study. Cancer Causes Control. (2014) 25:533–9. doi: 10.1007/s10552-014-0355-9
12. McSorley MA, Alberg AJ, Allen DS, Allen NE, Brinton LA, Dorgan JF, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gynecol. (2007) 109:933–41. doi: 10.1097/01.AOG.0000257126.68803.03
13. Chaturvedi AK, Caporaso NE, Katki HA, Wong H-L, Chatterjee N, Pine SR, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. (2010) 28:2719–26. doi: 10.1200/JCO.2009.27.0454
14. Hart PC, Rajab IM, Alebraheem M, Potempa LA. C-reactive protein and cancer—Diagnostic and therapeutic insights. Front Immunol. (2020) 11:595835. doi: 10.3389/fimmu.2020.595835
15. Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. (2011) 48:155–70. doi: 10.3109/10408363.2011.599831
16. Ferri MJ, Saez M, Figueras J, Fort E, Sabat M, López-Ben S, et al. Improved pancreatic adenocarcinoma diagnosis in jaundiced and non-jaundiced pancreatic adenocarcinoma patients through the combination of routine clinical markers associated to pancreatic adenocarcinoma pathophysiology. PloS One. (2016) 11:e0147214. doi: 10.1371/journal.pone.0147214
17. Lanki M, Mustonen H, Salmi M, Jalkanen S, Haglund C, Seppänen H. Serum cytokine profiles in patients with pancreatic cancer and chronic pancreatitis. Pancreatology. (2023) 23:657–62. doi: 10.1016/j.pan.2023.07.004
18. Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, et al. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer. (2014) 134:2646–55. doi: 10.1002/ijc.28584
19. O’Dowd C, McRae LA, McMillan DC, Kirk A, Milroy R. Elevated preoperative C-reactive protein predicts poor cancer specific survival in patients undergoing resection for non-small cell lung cancer. J Thorac Oncol. (2010) 5:988–92. doi: 10.1097/JTO.0b013e3181da78f9
20. Polterauer S, Grimm C, Tempfer C, Sliutz G, Speiser P, Reinthaller A, et al. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol Oncol. (2007) 107:114–7. doi: 10.1016/j.ygyno.2007.06.001
21. Schmid M, Schneitter A, Hinterberger S, Seeber J, Reinthaller A, Hefler L. Association of elevated C-reactive protein levels with an impaired prognosis in patients with surgically treated endometrial cancer. Obstet Gynecol. (2007) 110:1231–6. doi: 10.1097/01.AOG.0000292085.50987.f2
22. Chen J, Jing X, Deng X, Gao F, Wang X, Han D, et al. Prognostic value of serum C-reactive protein in pancreatic cancer: a meta-analysis. Int J Clin Exp Med. (2018) 11:11789–96.
23. Nurmi AM, Mustonen HK, Stenman U-H, Seppänen HE, Haglund CH. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci Rep. (2021) 11:781. doi: 10.1038/s41598-020-80778-0
24. Stevens L, Pathak S, Nunes QM, Pandanaboyana S, Macutkiewicz C, Smart N, et al. Prognostic significance of pre-operative C-reactive protein and the neutrophil–lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB. (2015) 17:285–91. doi: 10.1111/hpb.12355
25. Tani M, Iida H, Maehira H, Mori H, Miyake T, Kaida S. A high C-reactive protein level on postoperative day 7 is associated with poor survival of patients with pancreatic ductal adenocarcinoma after resection. Am Surg. (2022) 88:2024–9. doi: 10.1177/00031348211023406
26. Bonazzi VF, Aoude LG, Brosda S, Bradford JJ, Lonie JM, Loffler KA, et al. C-reactive protein is a prognostic biomarker in pancreatic ductal adenocarcinoma patients. Asia Pac J Clin Oncol. (2023) [A head of print]. doi: 10.1111/ajco.13993
27. Chuang Y-C, Tyagi V, Liu R-T, Chancellor MB, Tyagi P. Urine and serum C-reactive protein levels as potential biomarkers of lower urinary tract symptoms. Urol Sci. (2010) 21:132–6. doi: 10.1016/S1879-5226(10)60028-0
28. Nikibakhsh A, Mahmoodzadeh H, Hejazi S, Shfiei H, Gheibi S, Gazzavi A, et al. Evaluation of quantitative urinary CRP (C-reactive protein) level in children with urinary tract infection. J Pediatr Nephrol. (2013) 1:70–3. doi: 10.22037/jpn.v1i2.4344
29. Andersson L, Preda I, Hahn-Zoric M, Hanson LÅ, Jodal U, Sixt R, et al. Urinary proteins in children with urinary tract infection. Pediatr Nephrol. (2009) 24:1533–8. doi: 10.1007/s00467-009-1173-2
30. Ashkenazi-Hoffnung L, Livni G, Scheuerman O, Berger I, Eden E, Oved K, et al. Differential serum and urine CRP, IP-10, and TRAIL levels in pediatric urinary tract infection. Front Pediatr. (2021) 9:771118. doi: 10.3389/fped.2021.771118
31. Debernardi S, O’Brien H, Algahmdi AS, Malats N, Stewart GD, Plješa-Ercegovac M, et al. A combination of urinary biomarker panel and PancRISK score for earlier detection of pancreatic cancer: A case–control study. PloS Med. (2020) 17:e1003489. doi: 10.1371/journal.pmed.1003489
32. Radon TP, Massat NJ, Jones R, Alrawashdeh W, Dumartin L, Ennis D, et al. Identification of a three-biomarker panel in urine for early detection of pancreatic adenocarcinoma. Clin Cancer Res. (2015) 21:3512–21. doi: 10.1158/1078-0432.CCR-14-2467
33. Blyuss O, Zaikin A, Cherepanova V, Munblit D, Kiseleva EM, Prytomanova OM, et al. Development of PancRISK, a urine biomarker-based risk score for stratified screening of pancreatic cancer patients. Br J Cancer. (2020) 122:692–6. doi: 10.1038/s41416-019-0694-0
34. Debernardi S, Blyuss O, Rycyk D, Srivastava K, Jeon CY, Cai H, et al. Urine biomarkers enable pancreatic cancer detection up to 2 years before diagnosis. Int J Cancer. (2023) 152:769–80. doi: 10.1002/ijc.34287
35. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
36. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
37. Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GDO, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. (2002) 51:1596–600. doi: 10.2337/diabetes.51.5.1596
38. Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. (1999) 22:1978–83. doi: 10.2337/diacare.22.12.1978
39. Sah RP, Nagpal SJS, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. (2013) 10:423–33. doi: 10.1038/nrgastro.2013.49
40. Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. (1930) 52:561. doi: 10.1084/jem.52.4.561
41. Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. (2007) 61:824. doi: 10.1136/jech.2006.051292
42. Kim E-S, Kim SY, Moon A. C-reactive protein signaling pathways in tumor progression. Biomol Ther. (2023) 31:473. doi: 10.4062/biomolther.2023.132
43. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. (2003) 111:1805–12. doi: 10.1172/JCI200318921
44. Mroczko B, Groblewska M, Gryko M, Kędra B, Szmitkowski M. Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal. (2010) 24:256–61. doi: 10.1002/jcla.20395
45. Marimuthu S, Salunkhe V, Furmanek SP, Wolf LA. Association of urine levels of C-reactive protein with clinical outcomes in patients with pneumonia: A pilot study. Univ Louisville J Respir Infect. (2019) 3:2. doi: 10.18297/jri/
46. Balhara N, Devi M, Balda A, Phour M, Giri A. Urine; a new promising biological fluid to act as a non-invasive biomarker for different human diseases. URINE. (2023) 5:40–52. doi: 10.1016/j.urine.2023.06.001
47. Thomas S, Hao L, Ricke WA, Li L. Biomarker discovery in mass spectrometry-based urinary proteomics. Proteomics – Clin Appl. (2016) 10:358–70. doi: 10.1002/prca.201500102
48. Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. (1993) 91:1351–7. doi: 10.1172/JCI116336
49. Rajab IM, Hart PC, Potempa LA. How C-reactive protein structural isoforms with distinctive bioactivities affect disease progression. Front Immunol. (2020) 11:2126. doi: 10.3389/fimmu.2020.02126
Keywords: C-reactive protein, LYVE1, TFF1, REG1B, CA19-9, pancreatic cancer, urine
Citation: Ali N, Debernardi S, Kurotova E, Tajbakhsh J, Gupta NK, Pandol SJ, Wilson P, Pereira SP, Greenhalf B, Blyuss O and Crnogorac-Jurcevic T (2024) Evaluation of urinary C-reactive protein as an early detection biomarker for pancreatic ductal adenocarcinoma. Front. Oncol. 14:1450326. doi: 10.3389/fonc.2024.1450326
Received: 21 June 2024; Accepted: 19 August 2024;
Published: 06 September 2024.
Edited by:
Chun Wai Mai, UCSI University, MalaysiaReviewed by:
Md Ataur Rahman, University of Michigan, United StatesDmitry Aleksandrovich Zinovkin, Gomel State Medical University, Belarus
Copyright © 2024 Ali, Debernardi, Kurotova, Tajbakhsh, Gupta, Pandol, Wilson, Pereira, Greenhalf, Blyuss and Crnogorac-Jurcevic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tatjana Crnogorac-Jurcevic, dC5jLmp1cmNldmljQHFtdWwuYWMudWs=; Nurshad Ali, bnVyc2hhZC5hbGlAcW11bC5hYy51aw==
 Nurshad Ali
Nurshad Ali Silvana Debernardi
Silvana Debernardi Evelyn Kurotova1
Evelyn Kurotova1 Jian Tajbakhsh
Jian Tajbakhsh Stephen J. Pandol
Stephen J. Pandol Patrick Wilson
Patrick Wilson Oleg Blyuss
Oleg Blyuss