- 1Division of Medical Oncology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan
- 2Division of Medical Pharmacology, Department of Pharmacology, Showa University School of Medicine, Tokyo, Japan
- 3Department of Clinical Immuno-Oncology, Clinical Research Institute of Clinical Pharmacology and Therapeutics, Showa University, Tokyo, Japan
- 4Department of Clinical Diagnostic Oncology, Clinical Research Institute of Clinical Pharmacology and Therapeutics, Showa University, Tokyo, Japan
- 5Showa University Hospital Esophageal Cancer Center, Esophageal Surgery, Tokyo, Japan
- 6Pharmacological Research Center, Showa University, Tokyo, Japan
Introduction: Immune checkpoint inhibitors (ICIs) have emerged as a promising treatment option for esophageal cancer (EC). Although ICIs enable long-term survival in some patients, the efficacy of ICIs varies widely among patients. Therefore, predictive biomarkers are necessary for identifying patients who are most likely to benefit from ICIs to improve the efficacy of the treatment. We retrospectively analyzed the outcomes of combination therapy, including nivolumab plus ipilimumab or chemotherapy plus anti-programmed cell death 1 (PD-1) antibodies in our institute to identify biomarkers.
Methods: Twenty-seven patients received nivolumab plus ipilimumab, and thirty-six patients received chemotherapy plus anti-PD-1 antibodies were included in this study. We analyzed patient characteristics, efficacy, and safety. Multivariable analysis of biomarkers evaluated the correlation among overall survival (OS), progression-free survival (PFS), and the following variables: body mass index, performance status, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein level, and albumin level before treatment.
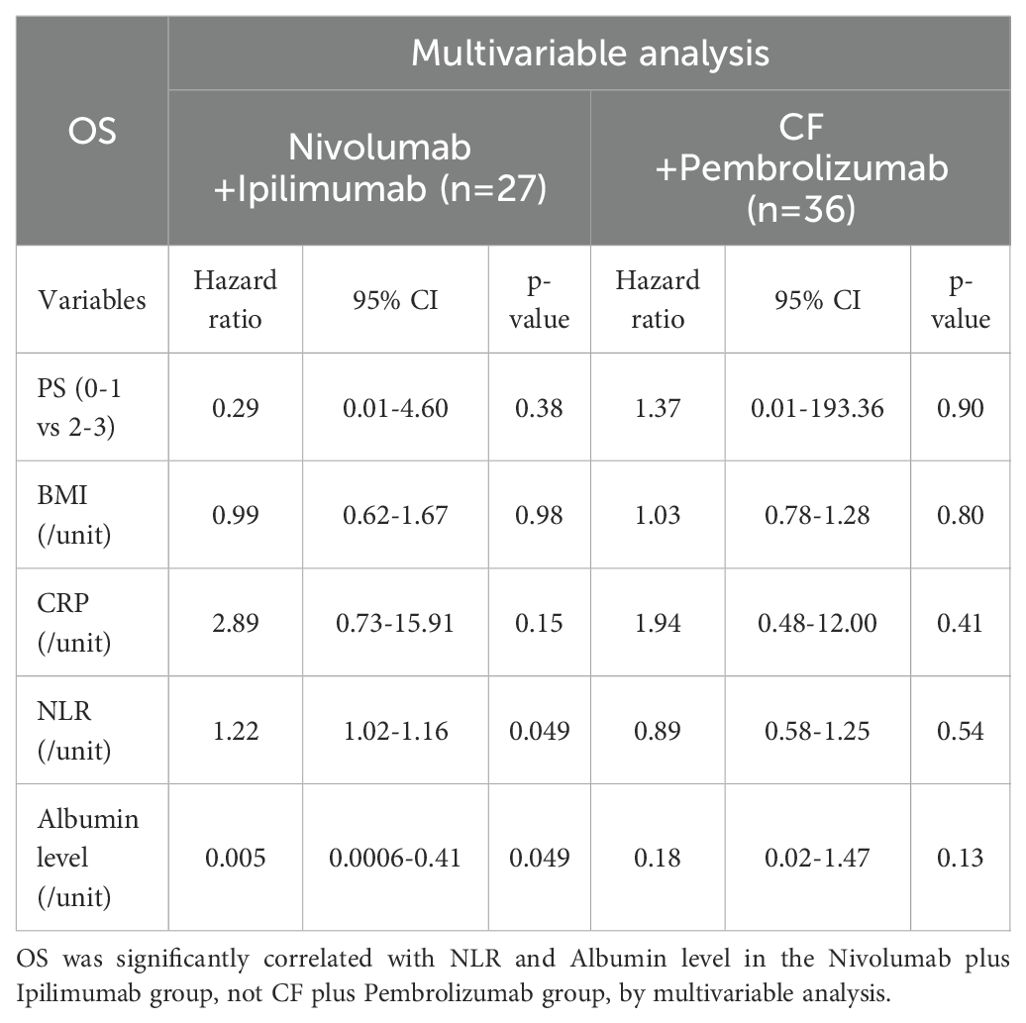
Results: In multivariable analysis, albumin level was significantly correlated with PFS in the cisplatin plus 5-fluorouracil (CF) plus pembrolizumab group. NLR and albumin level were significantly correlated with OS in the nivolumab plus ipilimumab group. Other variables, including PS, BMI, and CRP did not correlate with any of the outcomes.
Conclusions: High NLR in EC patients prior to treatment was significantly less effective for ICIs. In chemotherapy combined with ICIs, NLR before the treatment was not associated with treatment efficacy, suggesting combination chemotherapy may be beneficial for EC patients with high NLR. NLR may be an indicator of immunocompetence in anti-tumor immunity and a convenient predictive biomarker for selecting appropriate treatments including ICIs.
Introduction
Esophageal cancer (EC) is one of the most fatal malignancies globally, with limited therapeutic options and a poor prognosis. In last decade, immune checkpoint inhibitors (ICIs) have emerged as a promising treatment option for various kinds of malignancies, including EC. ICIs targeting programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), have shown promise in improving prognosis in EC patients. Nivolumab plus ipilimumab, or chemotherapy plus anti-PD-1 antibodies, is approved as a first-line treatment for EC (1, 2). However, there are no established criteria to select a first-line treatment. Although ICIs enable long-term survival in some patients, the efficacy of ICIs varies widely among patients. Reliable biomarkers are necessary for identifying patients who are most probable to benefit from ICIs in order to improve the efficacy of the treatment. The role of biomarkers such as PD-L1 expression, microsatellite instability (MSI), and tumor mutation burden (TMB) has been limited in predicting the efficacy of ICIs (3–7). As a convenient predictive biomarker, neutrophil-lymphocyte ratio (NLR) has gained attention as a predictive biomarker for the efficacy of ICIs. Higher NLR prior to the treatment has been consistently associated with poor overall survival (OS) and progression-free survival (PFS) in patients treated with ICIs across various cancer types, including EC (8–18). The underlying mechanisms involve the role of neutrophils in the tumor microenvironment (TME). Neutrophils can promote tumor progression and suppress T cell-mediated anti-tumor responses, which may explain the association between higher NLR and poor therapeutic outcomes (19–21). These findings suggest that NLR, a cost-effective and accessible biomarker, may play an important role in selecting appropriate first-line treatment, including ICIs. We retrospectively analyzed the patients’ baseline characteristics, efficacy, and safety of first-line treatment for EC in clinical practice and analyzed the predictive biomarker to identify the patients who benefit from ICIs. In addition, the immune system is impaired with age due to the phenomenon of immunosenescence, which includes a decrease in naïve T cells, an increase in memory T cells, reduced immune responsiveness, and inflammation caused by senescent T cells. To evaluate whether NLR and treatment efficacy are affected by immunosenescence, we conducted a stratified analysis of NLR and the best overall response (BOR) between patients under 65 years and those over 65 years in each treatment group (22–27).
Materials and methods
Patients who received ICIs for pathologically confirmed EC between February 2020 and June 2023 were included in this study. Twenty-seven patients were administered nivolumab plus ipilimumab, and thirty-six patients were administered cisplatin plus 5-fluorouracil (CF) plus pembrolizumab. We conducted a retrospective analysis to evaluate the clinical effectiveness, safety, and clinical laboratory data of the patients. The data collection focused on routine medical care, involving patient characteristics such as age, sex, ECOG performance status (PS), body mass index (BMI), stage, and histology. Laboratory findings, imaging studies, and assessments of safety and efficacy were also included. Biomarker analysis involved ECOG PS, BMI, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), and albumin level prior to the treatment. Safety evaluation was based on the presence and severity of immune-related adverse events (irAEs) according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Efficacy was measured according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver 1.1, which measured the overall response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS).
The studies involving human participants were reviewed and approved by the Ethics Committee of Showa University School of Medicine (approval No. 22-168-A). Informed consent was obtained through an opt-out method on the website since this is a retrospective study using only medical records. We ensured a proper opportunity to refuse the use of medical records.
Statistical analysis for biomarkers
Efficacy was assessed according to OS and PFS. Variables included in the multivariable analysis were PS (0-1 vs. 2-3), BMI, NLR, CRP, and albumin level, all measured before treatment. Multivariable analyses were conducted using the Cox proportional hazards model for both OS and PFS. Survival time analysis was performed by plotting survival curves using the Kaplan–Meier method, which were compared using the log-rank test. Two-sided p-values less than 0.05 were considered statistically significant. Statistical analyses were conducted using JMP ® Pro 15(SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics of the patients
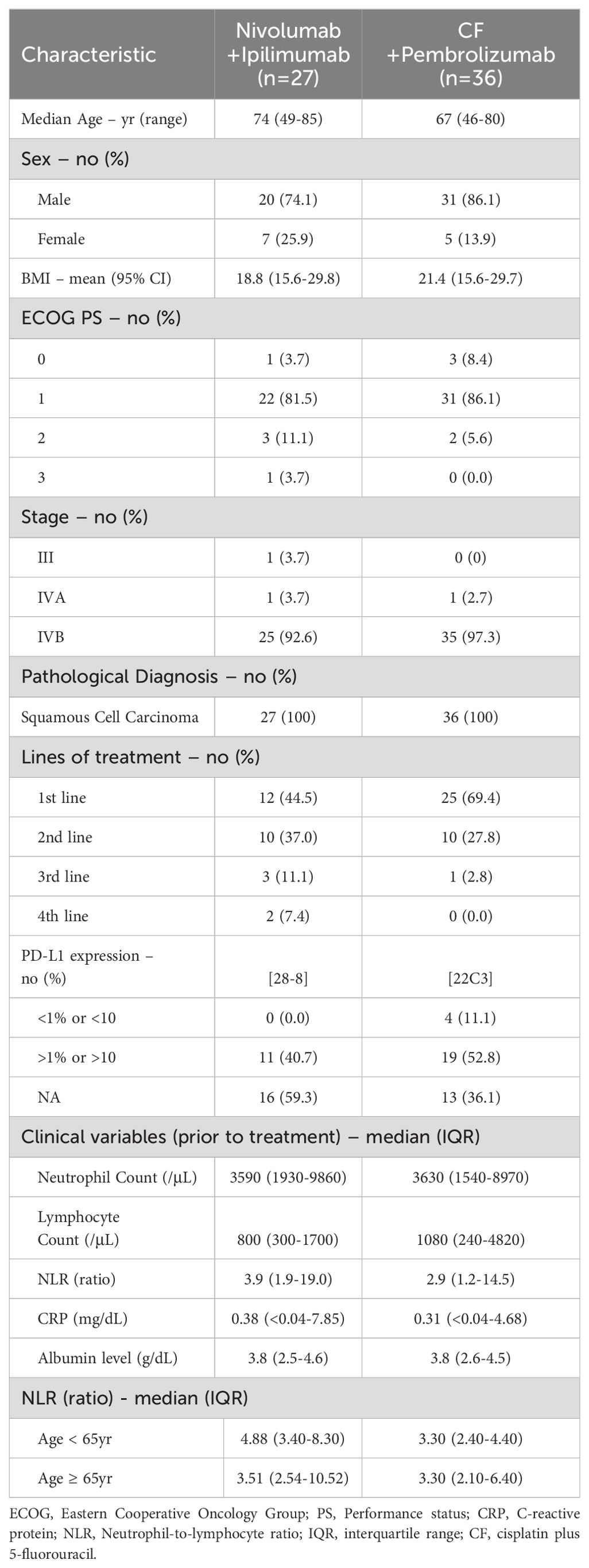
The demographic and clinical features of the patients are showed in Table 1. The median patient age was 69 years (range 46–85 years), 80.6% were men, and 90.5% of the cases were ECOG PS 0-1, whereas 7.9% and 1.6% of the patients had PS 2 and 3, respectively. Stage IVB occurred in 95.2% of the cases. All patients were diagnosed with squamous cell carcinoma of the esophagus. As for the treatment, 58.7% were first-line treatment, while 31.7% were second-line treatment. The reasons for administering the treatment as a second-line treatment were approximately equally divided: half of the cases involved patients who were deemed unresectable after neoadjuvant chemotherapy, and the other half involved patients who initiated treatment following chemoradiotherapy. PD-L1 expression was examined in approximately 54.0% of patients. There were no notable findings in pretreatment BMI, neutrophil count, lymphocyte count, NLR, CRP, or albumin levels. NLR was not higher in patients over 65 years than in those under 65 years. Conversely, in the nivolumab plus ipilimumab group, NLR was higher in patients under 65 years than in those over 65 years.
Response to treatment and safety with nivolumab plus ipilimumab
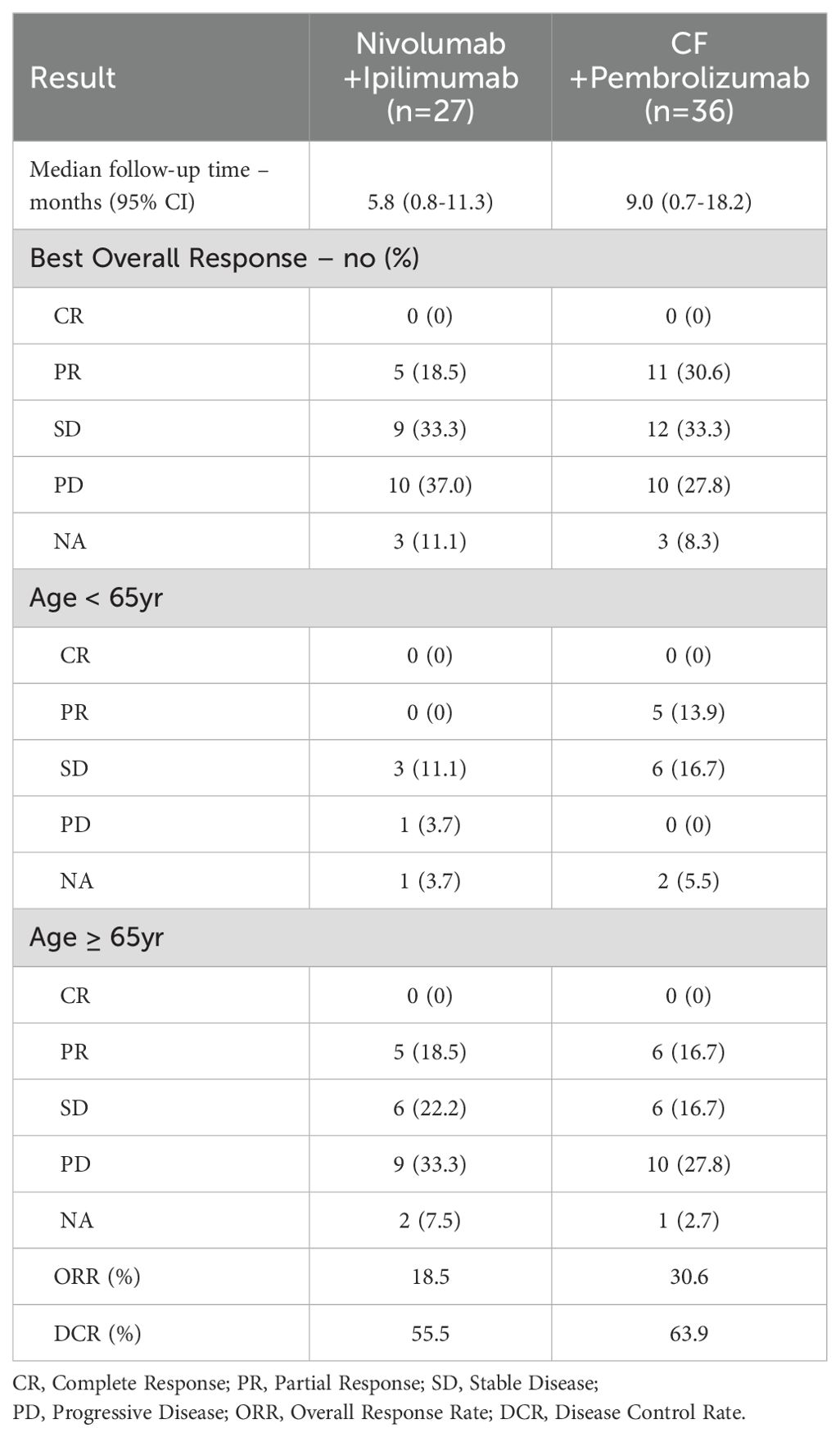
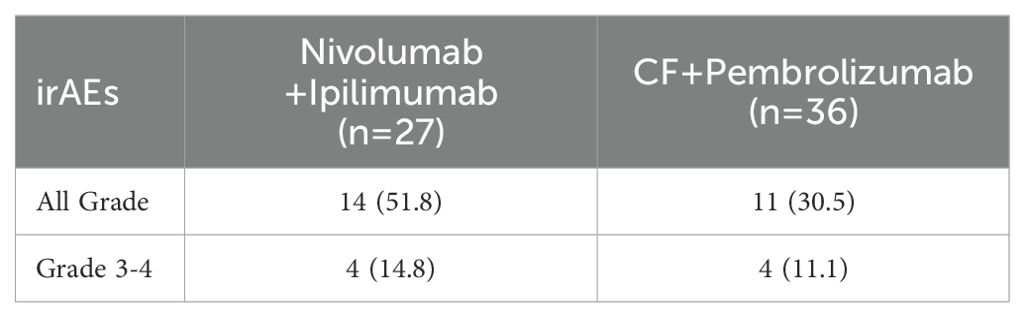
The median PFS was 5.5 months (95% confidence interval [CI]: 2.6–NA; Figure 1A), and the median OS was not reached (Figure 1B) for patients treated with nivolumab plus ipilimumab. The best overall response (BOR) was 18.5% for partial response (PR), 33.3% for stable disease (SD), and 37.0% for progressive disease (PD), respectively. The ORR was 18.5% and the DCR was 55.5% (Table 2A). There were five cases of PR in patients over 65 years. IrAEs were observed in 51.8% of cases of all grades and 14.8% in grades 3–4 with nivolumab plus ipilimumab (Table 2B).
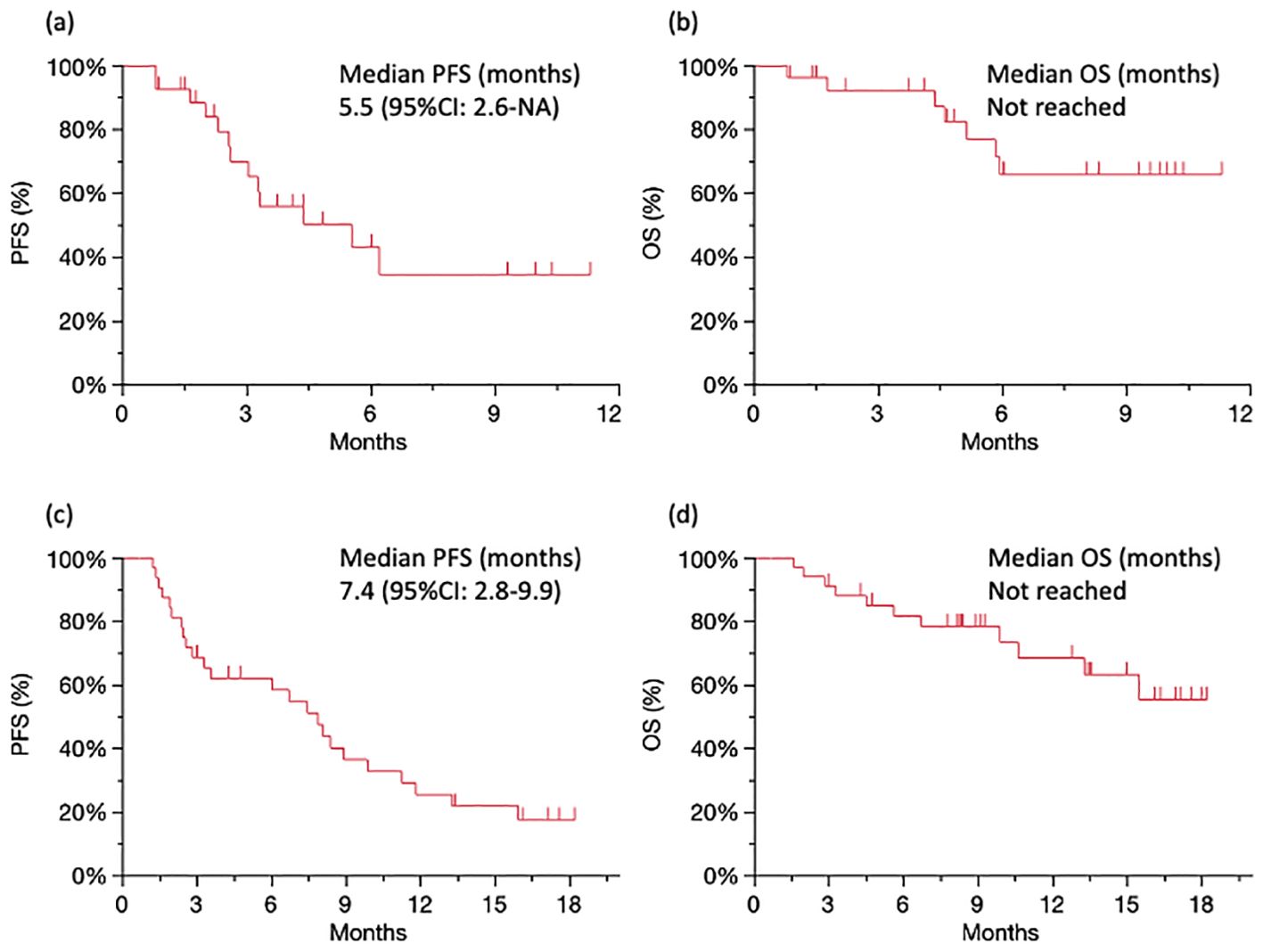
Figure 1. Survival analysis Kaplan–Meier curves of PFS (A) and OS (B) for patients treated with nivolumab plus ipilimumab. Kaplan–Meier curves of PFS (C) and OS (D) for patients treated with CF plus pembrolizumab. PFS, progression-free survival; OS, overall survival.
Response to treatment and safety with chemotherapy plus pembrolizumab
The median PFS was 7.4 months (95% CI: 2.8–9.9; Figure 1C), and the median OS was not reached (Figure 1D) for patients treated with CF plus pembrolizumab. The BOR was 30.6% for PR, 33.3% for SD, and 27.8% for PD, respectively. The ORR was 30.6% and the DCR was 63.9% (Table 2A). There were no cases of PD in 13 patients under 65 years. IrAEs were observed in 30.5% of cases of all grades and 11.1% in grades 3–4 with CF plus pembrolizumab (Table 2B).
Multivariable analysis of biomarkers
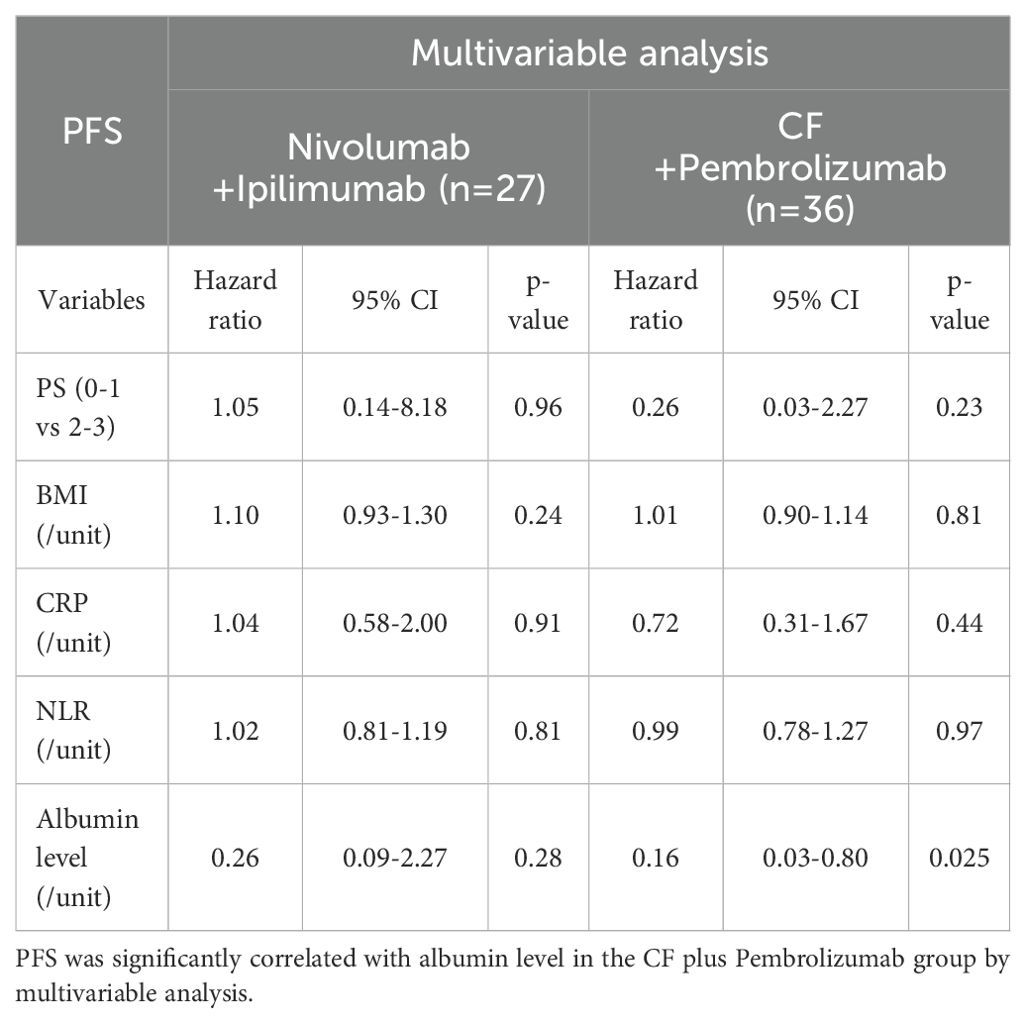
Multivariable analysis was performed to determine predictive biomarkers from routine clinical data. Albumin level, not NLR, was significantly correlated with PFS in the CF plus pembrolizumab group (Table 3A). NLR and albumin level were significantly correlated with OS in the nivolumab plus ipilimumab group (Table 3B). Other variables, including PS, BMI, and CRP did not correlate with any of the outcomes.
Discussion
Predictive biomarker analysis was demonstrated that NLR before the treatment was significantly correlated with OS in the nivolumab plus ipilimumab group. Conversely, NLR was not significantly correlated with efficacy in the CF plus pembrolizumab group. We focused on NLR because it has been reported to be associated with immunological response.
As for biomarkers, the function of PD-L1 expression, MSI, and TMB as biomarkers in predicting the efficacy of ICIs is uncertain, because ICIs are ineffective for some patients with these biomarkers (3–7). Gut microbiota, cytokine signatures, and tertiary lymphoid structure (TLS) have all been proposed as biomarkers, but they have not yet been authorized in clinical practice (28–34).
In aspects of NLR as a predictive biomarker, previous reports have shown that a high NLR prior to treatment correlates with poor clinical efficacy in patients with various solid tumors, such as pancreatic, lung, and colorectal tumors, treated with chemotherapy (35–37). Patients with higher pretreatment NLR also demonstrated poor clinical response to ICIs in various cancer types, including EC, gastric cancer (GC), non-small cell lung cancer (NSCLC), malignant melanoma, urothelial carcinoma, renal cell carcinoma, respectively (8–18). A retrospective cohort study of 1714 patients with 16 different cancer types also showed that higher NLR is significantly associated with poor overall survival (OS) and progression-free survival (PFS) with ICIs treatment (38). However, some reports have demonstrated no correlation between NLR and clinical efficacy; thus, these results are still controversial (39). Caution is required in its evaluation and utilization of NLR, since the cutoff values vary, and its availability as a biomarker varies by cancer type and treatment (40, 41). The underlying mechanisms responsible for the association between higher NLR and poor therapeutic outcomes involves the role of neutrophils in the tumor microenvironment. Neutrophils can promote tumor progression and suppress T cell-mediated anti-tumor responses (19–21).
NLR is typically elevated, reflecting chronic stress and inflammation. NLR tends to increase with aging in many vertebrates, including humans (42). Conversely, the bigmouth buffalo (Ictiobus cyprinellus), a long-living fish, has been reported to show a negative correlation between age and NLR (43). This finding suggests that a low NLR may be indicative of good health conditions, characterized by low levels of stress and inflammation. In aspects of cancer, neutrophils are elevated by immunosuppressive mediators, primarily IL-6 derived from tumors. Tumor-derived IL-6 has been shown to be significantly associated with tumor size, stage, and proliferative activity as measured by Ki-67; as a result, it will probably increase with the disease progresses (44, 45). IL-6 stimulates the bone marrow and increase the number of neutrophils. Additionally, tumor-derived IL-8 stimulates neutrophil migration to the tumor sites (46). Neutrophils secrete matrix metalloproteinase-8 (MMP-8), vascular endothelial growth factor (VEGF), and neutrophil elastase, which enhance tumor growth, angiogenesis, and suppression of T cells activity in the TME (19–21). Thus, neutrophils are considered to promote tumor progression and are referred to as tumor-associated neutrophils (TANs) (47). It is remarkable that TANs suppress activity of T cells in TME (48, 49). In contrast, the lymphocyte count is a key factor in antitumor immunity. Thus, low NLR which implies a lymphocyte-dominant condition may be an indicator of high immunocompetence in anti-tumor immunity. Furthermore, a correlation has been reported between the therapeutic efficacy of ICIs and the presence of TLS in tumor tissue, and a positive correlation has been demonstrated between low NLR and high TLS expression (50, 51). On the other hand, favorable ICI responses have been demonstrated with high intra-tumoral CD8-to-neutrophil ratios, while low ratios predicted ICI treatment failures and poor progression-free survival (52). These findings suggest that a neutrophil-dominant conditions, including high NLR, are in favor of tumors.
Conversely, NLR was not significantly correlated with efficacy in the CF plus pembrolizumab group, although ICIs were included in the treatment. The underlying mechanisms may involve chemotherapy-induced bone marrow suppression. Chemotherapy-induced bone marrow suppression mainly reduces neutrophil counts. These findings suggest that chemotherapy may reduce the detrimental effects of neutrophils on anti-tumor immunity, such as some suppression of T-cell mediated anti-tumor responses in the TME. It is suggested that the combination of chemotherapy may create TME favorable to the effect of ICIs and might be beneficial for EC patients with high NLR.
A limitation of this study is that it was a retrospective analysis conducted at a single institute. Another limitation is that the observation period was not long enough, and the number of patients involved in this study was relatively small. It is necessary to confirm these findings in a prospective study.
The main purpose of this manuscript was to describe NLR as a predictive biomarker. Further analysis will be performed to evaluate the difference between pre-treatment and post-treatment NLR values in patients treated with nivolumab plus Ipilimumab vs patients treated with chemotherapy plus pembrolizumab and to evaluate if these differences are statistically significant and if they correlate with the disease response. In addition, data regarding the utility of post-treatment NLR as a biomarker, including not only BOR but also analysis of OS and PFS will be presented in a new manuscript.
Conclusion
High NLR in patients with EC prior to the treatment may be less effective for ICIs. In chemotherapy combined with ICIs, NLR at pre-treatment was not associated with clinical efficacy, suggesting that combination chemotherapy may be beneficial for EC patients with high NLR before the treatment. NLR might be an indicator of immunocompetence in antitumor immunity and a convenient predictive biomarker for selecting appropriate treatment including ICIs in daily practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Showa University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Informed consent was obtained through an opt-out method on the website since this is a retrospective study using only medical records. We ensured a proper opportunity to refuse the use of medical records.
Author contributions
YH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YKu: Supervision, Validation, Writing – review & editing. EM: Validation, Writing – review & editing. RS: Validation, Writing – review & editing. ToT: Validation, Writing – review & editing. NI: Validation, Writing – review & editing. TI: Validation, Writing – review & editing. RO: Validation, Writing – review & editing. MS: Validation, Writing – review & editing. HA: Validation, Writing – review & editing. AH: Validation, Writing – review & editing. SW: Validation, Writing – review & editing. TY: Validation, Writing – review & editing. TA: Validation, Writing – review & editing. SG: Validation, Writing – review & editing. KO: Supervision, Validation, Writing – review & editing. MM: Supervision, Validation, Writing – review & editing. YKi: Supervision, Validation, Writing – review & editing. KY: Supervision, Validation, Writing – review & editing. TaT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Professor Eisuke Inoue, Showa University Research Administration Center, Showa University, for advice on statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. (2022) 386:449–62. doi: 10.1056/NEJMoa2111380
2. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
3. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. (2014) 515:563–7. doi: 10.1038/nature14011
4. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
5. Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. (2022) 33:929–38. doi: 10.1016/j.annonc.2022.05.519
6. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. (2017) 377:2500–1. doi: 10.1056/NEJMc1713444
7. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. (2020) 21:1353–65. doi: 10.1016/S1470-2045(20)30445-9
8. Man Q, Li P, Fan J, Yang S, Xing C, Bai Y, et al. The prognostic role of pre-treatment neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in esophageal squamous cell carcinoma treated with concurrent chemoradiotherapy. BMC Cancer. (2024) 24:464. doi: 10.1186/s12885-024-12242-5
9. Gao Y, Zhang Z, Li Y, Chen S, Lu J, Wu L, et al. Pretreatment neutrophil-to-lymphocyte ratio as a prognostic biomarker in unresectable or metastatic esophageal cancer patients with anti-PD-1 therapy. Front Oncol. (2022) 12:834564. doi: 10.3389/fonc.2022.834564
10. Ruan DY, Chen YX, Wei XL, Wang YN, Wang ZX, Wu HX, et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol Rep (Oxf). (2021) 9:560–70. doi: 10.1093/gastro/goab032
11. Booka E, Kikuchi H, Haneda R, Soneda W, Kawata S, Murakami T, et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Res. (2022) 42:2977–87. doi: 10.21873/anticanres.15781
12. Alessi JV, Ricciuti B, Alden SL, Bertram AA, Lin JJ, Sakhi M, et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J Immunother Cancer. (2021) 9:e003536. doi: 10.1136/jitc-2021-003536
13. Ishihara M, Ochiai R, Haruyama T, Sakamoto T, Tanzawa S, Honda T, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts treatment efficacy and prognosis of cytotoxic anticancer drugs, molecular targeted drugs, and immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10:221–32. doi: 10.21037/tlcr
14. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
15. Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. (2018) 6:74. doi: 10.1186/s40425-018-0383-1
16. Nassar AH, Mouw KW, Jegede O, Shinagare AB, Kim J, Liu CJ, et al. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br J Cancer. (2020) 122:555–63. doi: 10.1038/s41416-019-0686-0
17. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. (2016) 27:732–8. doi: 10.1093/annonc/mdw016
18. Chen X, Meng F, Jiang R. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front Oncol. (2021) 11:746976. doi: 10.3389/fonc.2021.746976
19. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
20. Hwang M, Canzoniero JV, Rosner S, Zhang G, White JR, Belcaid Z, et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer. (2022) 10:e004688. doi: 10.1136/jitc-2022-004688
21. Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. (2020) 26:688–92. doi: 10.1038/s41591-020-0856-x
22. Ferrando-Martinez S, Ruiz-Mateos E, Hernandez A, Gutierrez E, Rodriguez-Mendez Mdel M, Ordonez A, et al. Age-related deregulation of naive T cell homeostasis in elderly humans. Age (Dordr). (2011) 33:197–207. doi: 10.1007/s11357-010-9170-8
23. Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. (2008) 8:512–22. doi: 10.1038/nri2318
24. Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. (2009) 30:306–12. doi: 10.1016/j.it.2009.03.013
25. Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. (2016) 37:866–76. doi: 10.1016/j.it.2016.09.002
26. Mirza N, Duque MA, Dominguez AL, Schrum AG, Dong H, Lustgarten J. B7-H1 expression on old CD8+ T cells negatively regulates the activation of immune responses in aged animals. J Immunol. (2010) 184:5466–74. doi: 10.4049/jimmunol.0903561
27. Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, et al. Human CD8(+) EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. (2018) 17:e12675. doi: 10.1111/acel.12675
28. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
29. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
30. Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. (2022) 28:704–12. doi: 10.1038/s41591-022-01694-6
31. Zhao C, Wu L, Liang D, Chen H, Ji S, Zhang G, et al. Identification of immune checkpoint and cytokine signatures associated with the response to immune checkpoint blockade in gastrointestinal cancers. Cancer Immunol Immunother. (2021) 70:2669–79. doi: 10.1007/s00262-021-02878-8
32. Wei F, Azuma K, Nakahara Y, Saito H, Matsuo N, Tagami T, et al. Machine learning for prediction of immunotherapeutic outcome in non-small-cell lung cancer based on circulating cytokine signatures. J Immunother Cancer. (2023) 11:e006788. doi: 10.1136/jitc-2023-006788
33. Hayashi Y, Makino T, Sato E, Ohshima K, Nogi Y, Kanemura T, et al. Density and maturity of peritumoral tertiary lymphoid structures in oesophageal squamous cell carcinoma predicts patient survival and response to immune checkpoint inhibitors. Br J Cancer. (2023) 128:2175–85. doi: 10.1038/s41416-023-02235-9
34. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8
35. Cedrés S, Torrejon D, Martínez A, Martinez P, Navarro A, Zamora E, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. (2012) 14:864–9. doi: 10.1007/s12094-012-0872-5
36. Iwai N, Okuda T, Sakagami J, Harada T, Ohara T, Taniguchi M, et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep. (2020) 10:18758. doi: 10.1038/s41598-020-75745-8
37. Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. (2011) 104:1288–95. doi: 10.1038/bjc.2011.100
38. Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. (2021) 12:729. doi: 10.1038/s41467-021-20935-9
39. Nishiyama N, Hirobe M, Kikushima T, Matsuki M, Takahashi A, Yanase M, et al. The neutrophil-lymphocyte ratio has a role in predicting the effectiveness of nivolumab in Japanese patients with metastatic renal cell carcinoma: a multi-institutional retrospective study. BMC Urol. (2020) 20:110. doi: 10.1186/s12894-020-00679-2
40. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. (2020) 18:360. doi: 10.1186/s12916-020-01817-1
41. Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. (2019) 9:19673. doi: 10.1038/s41598-019-56218-z
42. Li J, Chen Q, Luo X, Hong J, Pan K, Lin X, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal. (2015) 29:437–43. doi: 10.1002/jcla.21791
43. Sauer DJ, Heidinger BJ, Kittilson JD, Lackmann AR, Clark ME. No evidence of physiological declines with age in an extremely long-lived fish. Sci Rep. (2021) 11:9065. doi: 10.1038/s41598-021-88626-5
44. Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. (1999) 85:2526–31. doi: 10.1002/(ISSN)1097-0142
45. Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. (2009) 12:95–100. doi: 10.1007/s10120-009-0509-8
46. De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. (2004) 10:4895–900. doi: 10.1158/1078-0432.CCR-03-0760
47. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. (2011) 11:519–31. doi: 10.1038/nri3024
48. Michaeli J, Shaul ME, Mishalian I, Hovav AH, Levy L, Zolotriov L, et al. Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFalpha and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology. (2017) 6:e1356965. doi: 10.1080/2162402X.2017.1356965
49. Emmons TR, Giridharan T, Singel KL, Khan ANH, Ricciuti J, Howard K, et al. Mechanisms driving neutrophil-induced T-cell immunoparalysis in ovarian cancer. Cancer Immunol Res. (2021) 9:790–810. doi: 10.1158/2326-6066.CIR-20-0922
50. Komura K, Tokushige S, Ishida M, Hirosuna K, Yamazaki S, Nishimura K, et al. Tertiary lymphoid structure and neutrophil-lymphocyte ratio coordinately predict outcome of pembrolizumab. Cancer Sci. (2023) 114:4622–31. doi: 10.1111/cas.15976
51. Fukuhara M, Muto S, Inomata S, Yamaguchi H, Mine H, Takagi H, et al. The clinical significance of tertiary lymphoid structure and its relationship with peripheral blood characteristics in patients with surgically resected non-small cell lung cancer: a single-center, retrospective study. Cancer Immunol Immunother. (2022) 71:1129–37. doi: 10.1007/s00262-021-03067-3
Keywords: immune checkpoint inhibitors, immunotherapy, neutrophil-to-lymphocyte ratio, predictive biomarker, esophageal cancer
Citation: Hirasawa Y, Kubota Y, Mura E, Suzuki R, Tsurui T, Iriguchi N, Ishiguro T, Ohkuma R, Shimokawa M, Ariizumi H, Horiike A, Wada S, Yamashita T, Ariyoshi T, Goto S, Otsuka K, Murakami M, Kiuchi Y, Yoshimura K and Tsunoda T (2024) Chemotherapy combined with immune checkpoint inhibitors may overcome the detrimental effect of high neutrophil-to-lymphocyte ratio prior to treatment in esophageal cancer patients. Front. Oncol. 14:1449941. doi: 10.3389/fonc.2024.1449941
Received: 16 June 2024; Accepted: 16 September 2024;
Published: 11 October 2024.
Edited by:
Kwong Tsang, Precision Biologics, Inc., United StatesReviewed by:
Massimo Fantini, Precision Biologics, Inc., United StatesCarol Nieroda, Mercy Medical Center, United States
Copyright © 2024 Hirasawa, Kubota, Mura, Suzuki, Tsurui, Iriguchi, Ishiguro, Ohkuma, Shimokawa, Ariizumi, Horiike, Wada, Yamashita, Ariyoshi, Goto, Otsuka, Murakami, Kiuchi, Yoshimura and Tsunoda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takuya Tsunoda, dHRzdW5vZGFAbWVkLnNob3dhLXUuYWMuanA=
 Yuya Hirasawa
Yuya Hirasawa Yutaro Kubota1
Yutaro Kubota1 Risako Suzuki
Risako Suzuki Ryotaro Ohkuma
Ryotaro Ohkuma Masahiro Shimokawa
Masahiro Shimokawa Atsushi Horiike
Atsushi Horiike Satoshi Wada
Satoshi Wada Takeshi Yamashita
Takeshi Yamashita Yuji Kiuchi
Yuji Kiuchi Kiyoshi Yoshimura
Kiyoshi Yoshimura Takuya Tsunoda
Takuya Tsunoda