- 1Clinical Medical College of Hebei University, Affiliated Hospital of Hebei University, Baoding, China
- 2Thoracic Surgery Department, Affiliated Hospital of Hebei University, Baoding, China
- 3Thoracic Surgery Department, Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 4Basic Research Key Laboratory of General Surgery for Digital Medicine, Affiliated Hospital of Hebei University, Baoding, China
- 5Information center, Affiliated Hospital of Hebei University, Baoding, China
- 6Institute of Life Science and Green Development, Hebei University, Baoding, China
- 73D Image and 3D Printing Center, Affiliated Hospital of Hebei University, Baoding, China
- 8Department of Radiology, Affiliated Hospital of Hebei University, Baoding, China
Lung cancer remains a leading cause of cancer-related deaths globally, with its incidence steadily rising each year, representing a significant threat to human health. Early detection, diagnosis, and timely treatment play a crucial role in improving survival rates and reducing mortality. In recent years, significant and rapid advancements in artificial intelligence (AI) technology have found successful applications in various clinical areas, especially in the diagnosis and treatment of lung cancer. AI not only improves the efficiency and accuracy of physician diagnosis but also aids in patient treatment and management. This comprehensive review presents an overview of fundamental AI-related algorithms and highlights their clinical applications in lung nodule detection, lung cancer pathology classification, gene mutation prediction, treatment strategies, and prognosis. Additionally, the rapidly advancing field of AI-based three-dimensional (3D) reconstruction in lung cancer surgical resection is discussed. Lastly, the limitations of AI and future prospects are addressed.
1 Introduction
Lung cancer is a prevalent malignancy with high incidence and mortality rates worldwide. According to the latest statistics from GLOBOCAN (1), lung cancer ranks second in terms of incidence and first in terms of mortality among all malignant tumors. In 2020, there were over 2.2 million new cases of lung cancer globally, with approximately 1.8 million deaths. This disease poses a significant threat to human health and life. However, early-stage lung cancer is often challenging to detect based on symptoms, leading to the majority of patients being diagnosed at an advanced stage or with distant metastases, resulting in a five-year survival rate of only 15-16% (2). Consequently, the limitations in treatment options and prognosis assessment present challenges for clinicians. Early diagnosis and appropriate treatment are crucial factors in reducing lung cancer mortality.
With the continuous development of imaging and computer technology, X-ray computed tomography (CT) has been widely used in clinical practice, and population-based lung cancer screening has demonstrated high cost-effectiveness and significant value for early prediction in the community. Data from the National Lung Screening Trial (NLST) have shown that regular screening of high-risk individuals for lung cancer using low-dose CT imaging can detect more early-stage cancers and reduce lung cancer-related mortality (3).
In recent years, artificial intelligence (AI) technology has found extensive applications across various industries, especially in the healthcare sector (4). The combination of “AI + healthcare” has become a prominent trend, encompassing areas such as assisted diagnosis, risk prediction, treatment selection, and outcome evaluation (5, 6). By utilizing AI technology to analyze extensive medical data and train specialized AI models, it is possible to significantly enhance healthcare professionals’ diagnostic efficiency and accuracy. Moreover, AI can identify patterns and features that may be overlooked in manual diagnosis, thereby providing clinicians with more comprehensive and detailed diagnostic and treatment recommendations. This review aims to outline the advancements in the application of artificial intelligence in the field of lung cancer.
2 Overview of AI
AI is a branch of computer science that explores and develops theoretical methods for simulating, extending, and enhancing human intelligence, with the aim of automating tasks that are typically performed by humans (7). Since the 1950s, AI has undergone three major stages: symbolism (1950s to 1980s), the flourishing development of ML (1990s), and the significant success of DL in the early 21st century. Over time and with technological advancements, symbolic AI has gradually taken a backseat, making room for ML and DL as the core subfields in the field of AI. Table 1 lists the key terms associated with AI.
ML is one of the core technologies in the field of Artificial Intelligence, aiming to enable computers to learn from data, automatically identify and grasp patterns and regularities within the data, thereby achieving the capability of prediction and decision-making. ML is generally categorized into three types: supervised learning (SL), unsupervised learning (UL), and reinforcement learning (RL). SL algorithms use classified training data to create a prediction function that can generalize to classify unseen data correctly. UL differs in that no target variable exists. All variables are treated as inputs, and therefore unsupervised learning is used to find patterns in the data (8). RL is the process in which computers learn to complete tasks by learning from the outcomes of successes and failures. Common ML algorithms include support vector machines, random forests, decision trees, logistic regression, k-nearest neighbors, Bayesian networks, and clustering algorithms etc.
DL is a subfield of ML (Figure 1) that uses multi-layer artificial neural networks (ANN) to recognize patterns in data. Unlike traditional ML algorithms, DL algorithms can automatically learn features from raw data for pattern recognition. They learn abstract features through multiple layers of stacking, making models more powerful. The structure of DL models allows them to automatically learn and extract features from data, performing well on complex tasks and large datasets. During training, gradient descent and backpropagation are commonly used to adjust the model’s parameters, improving performance by reducing the difference between predicted and actual results. Common DL models include Convolutional Neural Networks (CNNs) and Recurrent Neural Networks (RNNs). CNNs are mainly used for image-related tasks, while RNNs are good at processing text and speech. These models are essential in DL and have many variations and combinations. They provide powerful tools for solving problems in areas like computer vision and natural language processing.
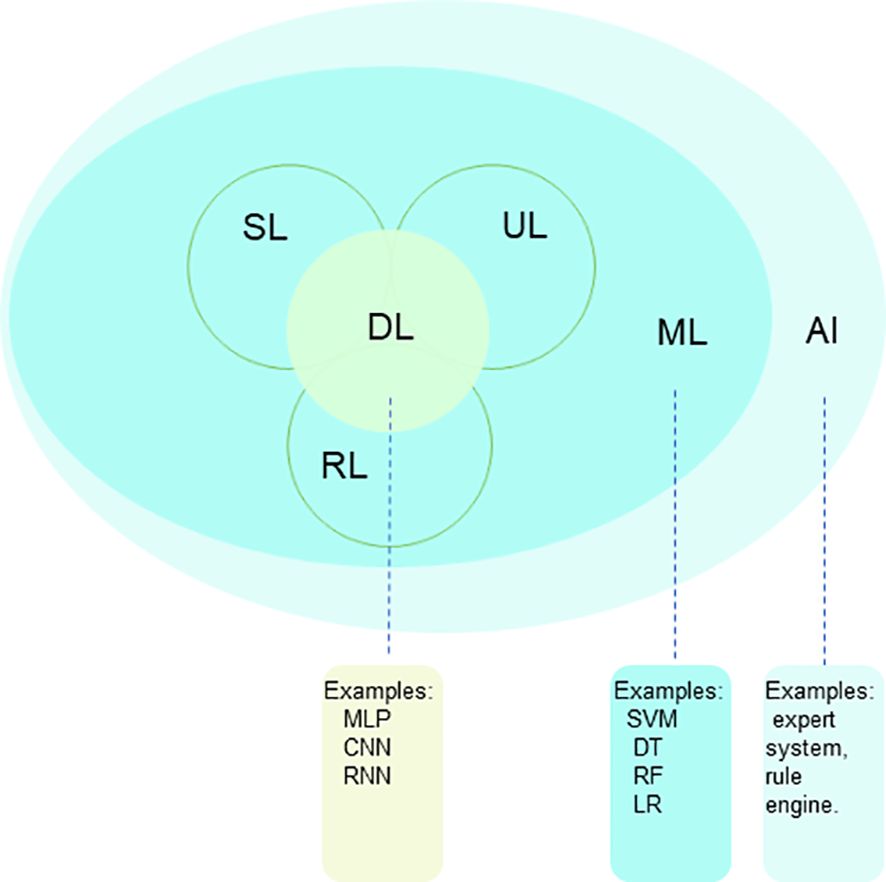
Figure 1. The relationship between AI, ML and DL, which are methodologies and technologies that enable computers to learn, analyze patterns, understand languages and images, and make predictions. AI, artificial intelligence; ML, machine learning; DL, deep learning; SL, supervised learning; UL, unsupervised learning; RL, reinforcement learning.
AI and radiomics are two closely related but distinct fields. AI aims to develop systems that simulate human intelligence and is widely applied in medical diagnostic support, medical imaging analysis, and more. Radiomics, on the other hand, focuses on extracting quantitative features from medical images and combining them with clinical data to predict disease and assess prognosis. AI is a broader field, while radiomics specifically concentrates on medical image analysis.
3 Application of AI in lung cancer diagnosis
3.1 Application of AI in lung nodule detection
Early-stage lung cancer is closely related to lung nodules. Lung nodules are typically detected through chest CT scans. On CT scans, a nodule appears as a rounded or irregular opacity, well or poorly defined, measuring up to 3 cm in diameter (9). Although most lung nodules are benign, some may develop into lung cancer. The National Lung Screening Trial has shown that lung cancer screening can reduce mortality rates among high-risk populations (3). Therefore, the increased use of lung cancer screening will inevitably lead to the discovery of many pulmonary nodules whose malignancy is uncertain. For incidentally detected nodules, the most commonly used guidelines are those of the Fleischner Society (10) and the British Thoracic Society (BTS) (11). The Fleischner Society guidelines are based on the mean diameter and type of the nodule, while the BTS guidelines recommend volumetric measurement of nodules rather than 2D measurements.
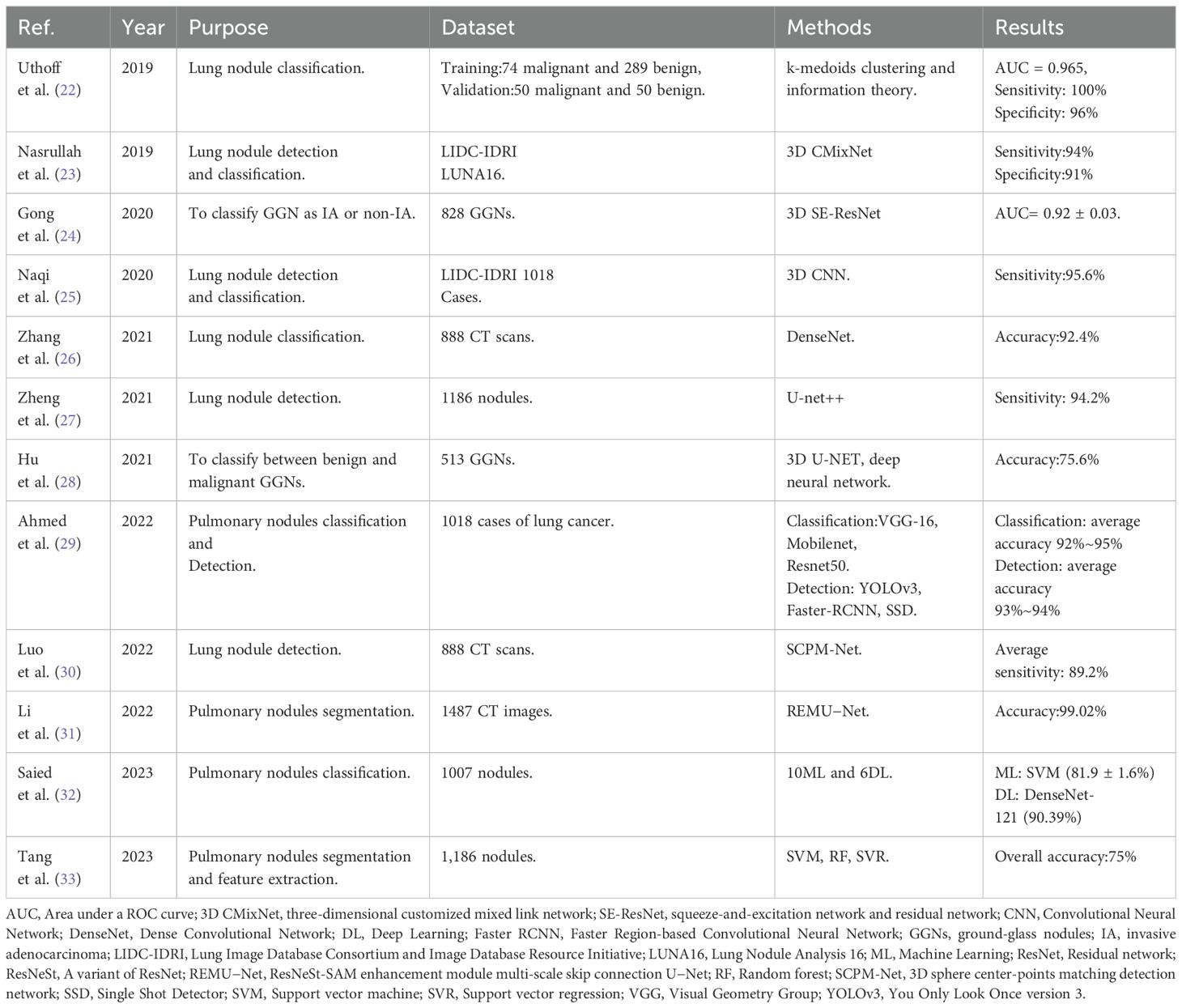
However, identifying lung nodules is not always easy, and radiologists’ sensitivity in detecting these nodules varies significantly, which can be influenced by various characteristics such as size, shape, location, density, and their relationship with adjacent structures (12). A large number of manual image readings can lead to missed diagnoses. Nowadays, there is growing interest in using AI to detect lung nodules. In lung cancer screening, AI can be applied not only for automatic detection, but also for patient selection and the reconstruction of low-dose CT scans (13). Kim et al. (14) have shown that an AI-based tool can improve the performance of radiologists and pulmonologists when estimating malignancy risk for indeterminate pulmonary nodules on chest CT scans. Moreover, application to predict benignity/malignity of the nodules are also available (15). However, AI-based tools require further research to determine their diagnostic benefits for clinicians in assessing indeterminate pulmonary nodules on chest CT scans (16).
Lung nodule CT images are multi-dimensional. 3D CNN can better utilize the 3D spatial information of the input, enabling tasks such as detection, segmentation, and classification of 3D objects, thereby improving detection accuracy. Khosravan et al. (17) proposed a novel DL-based method for lung nodule detection called S4ND. The whole detection pipeline is designed as a single 3D CNN with dense connections, trained in an end-to-end manner. This method uses a single feedforward pass of a single network to detect lung nodules without further processing, achieving a sensitivity of 95.2%. Subsequently, following detection, the focus shifts to precisely delineating or segmenting lung nodules from the adjacent pulmonary parenchyma. Segmentation entails separating nodules from other structures in the image, thus facilitating their comprehensive analysis and precise measurement of relevant characteristics. Manual segmentation is time-consuming and highly variable between observers. AI-based methods, including U-Net and other DL models, can replace this process to objectively quantify lung nodules and cancer, and extract radiomic features for tissue characterization (18). Ronneberg et al. (19) developed the U-Net, which is a CNN architecture, for biomedical segmentation tasks. U-Net can be used to perform fine pixel-level segmentation of detected lung nodules, accurately outlining the spatial extent of nodules, laying the foundation for subsequent quantitative analysis and growth assessment. Bhattacharyya et al. (20) employed a weighted bidirectional feature network to construct an improved U-NET architecture (DB-NET), which demonstrates excellent performance in lung nodule segmentation. Through this approach, they achieved better performance in segmenting ground-glass nodules, cavitary nodules, small nodules. Lastly, lung nodules are classified into distinct categories, including benign or malignant, solid or subsolid, and specific subtypes, based on their respective features. In order to improve the accuracy of classifying benign and malignant lung nodules, Guo et al. (21) proposed a 3D segmentation attention network integrating asymmetric convolution (SAACNet) classification model combined with a gradient boosting machine (GBM). The proposed nodule classification performance was evaluated on the LUNA16 dataset, which contains 888 CT scan images, achieving a classification accuracy of 95.18%, and the area under the curve (AUC) is 0.977. Figure 2 depicts the segmentation and classification of lung nodules.
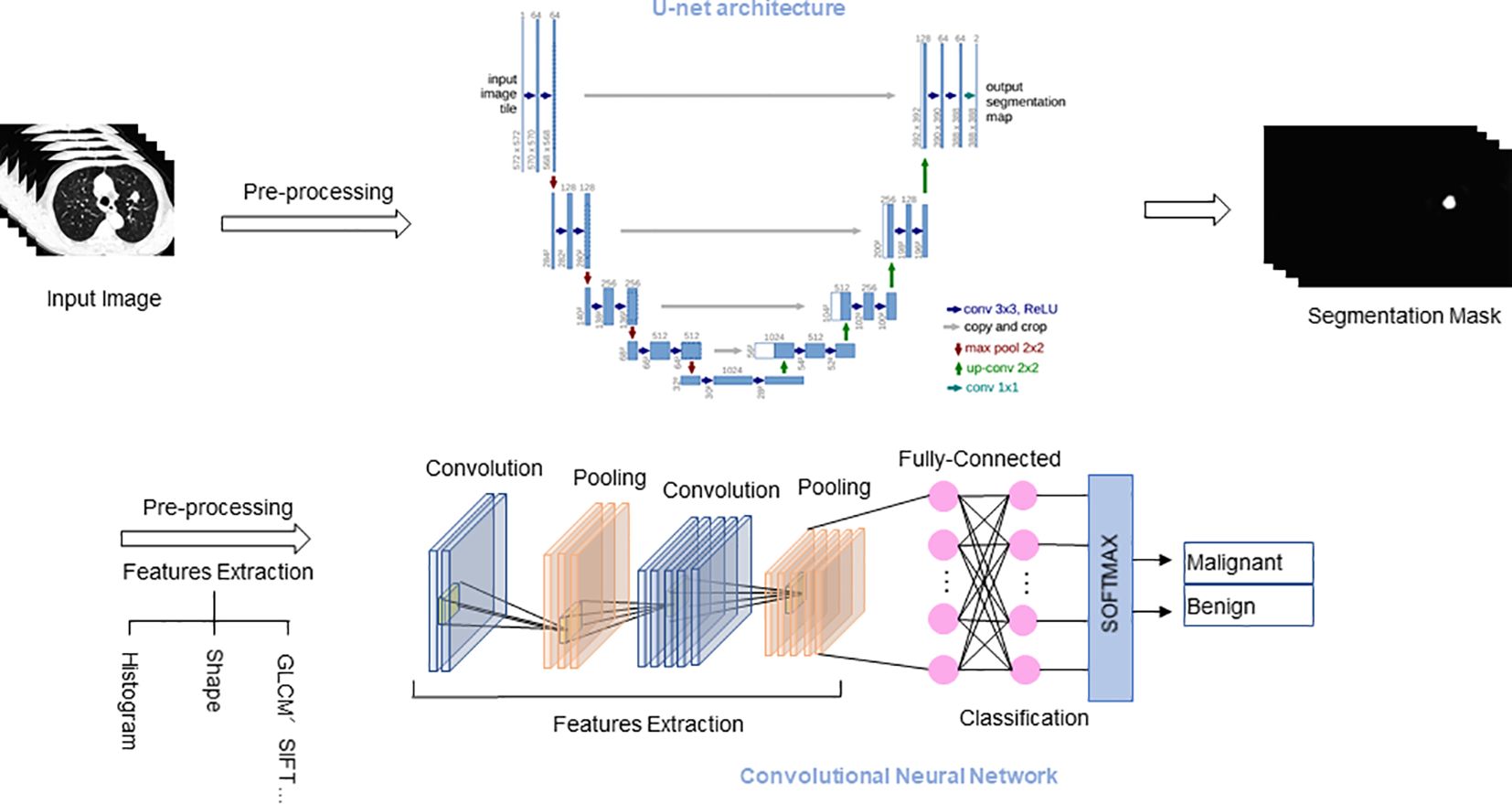
Figure 2. Segmentation and classification of lung nodules. U-Net architecture for segmentation tasks, and CNN architecture for classification tasks. GLCM, Gray-level co-occurrence matrix; SIFT, Scale-invariant feature transform.
Table 2 presents a summary of applications of AI in lung nodules. These AI methods each have their own advantages and disadvantages. k-medoids clustering is robust to noise and outliers but is computationally intensive and sensitive to initial selection. CNNs perform well in image processing but require large amounts of labeled data and high computational resources. 3D CNNs can handle three-dimensional medical images, capturing more spatial information, but have higher computational costs. DenseNet improves information flow through dense connections, but the complexity of the model increases. 3D U-NET is suitable for medical image segmentation but has longer training times.
3.2 Application of AI in lung cancer pathology diagnosis
Lung cancer is mainly classified into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), with NSCLC accounting for approximately 80%-85% of all lung cancers (34). NSCLCs are classified according to histopathological characteristics. Lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are the most common subtypes of NSCLC. Histopathologic confirmation remains the gold standard in clinical workflow for diagnosis (35), and due to significant differences between different subtypes, clinical treatments also vary greatly. Therefore, accurate classification of lung cancer subtypes is crucial. Pathologists examine tissue samples from lung biopsies and resections to detect and classify cells, identify tumor morphology and subtypes, and assess features that predict treatment response and prognosis (36). Manual slide reading is time-consuming and prone to fatigue, while the development of AI-based tools can assist pathologists and thoracic surgeons in improving clinical workflows and patient management.
It has been demonstrated that AI can accurately classify lung cancer subtypes and predict the prognosis of NSCLC patients. Yu et al. (37)used histopathology whole-slide images of LUAD and LUSC patients from The Cancer Genome Atlas (TCGA) to extract a large number of image features. They employed regularized ML methods to select the most important features to differentiate short-term survivors from long-term survivors. Their study results indicate that automatically extracted image features can predict the prognosis of lung cancer patients, thus contributing to precision oncology. This method can also be applied to histopathology images of other organs. Similarly, Coudray et al. (38)trained a deep CNN (Inception v3) with 1,634 randomly selected histopathological whole-slide images from the TCGA database to classify them as LUAD, LUSC, or normal lung tissue based on their morphological features. The results were consistent with the analysis of pathologists, with an average AUC of 0.97.
AI tools have also been applied to cytopathological samples obtained through fine-needle aspiration, assisting in the diagnosis of lung cancer and differentiation from other diseases by analyzing various samples such as bronchial secretions, sputum, bronchoalveolar lavage fluid, and needle aspiration. Compared to tissue biopsies, this examination is less invasive and is increasingly being used for lung cancer assessment and staging (39). Gonzalez et al. (40)studied cytological and biopsy specimens from 40 patients and trained a DL algorithm based on CNN to differentiate SCLC from large-cell neuroendocrine carcinoma based on morphological features. Although the dataset used was small, the DL models employed in the study accurately identified the majority of cases of both tumor types.
The spatial distributions of different types of cells could reveal a cancer cell’s growth pattern, its relationships with the tumor microenvironment and the immune response of the body. Wang et al. (41)developed a software tool for LUAD digital pathological image analysis aided by a CNN, ConvPath, which includes nuclei segmentation, CNN-based tumor cell, stromal cell, and lymphocyte classification, and extraction of tumor microenvironment-related features for lung cancer pathology images. Moreover, it has the capability to convert the pathology image into a “spatial map” of tumor cells, stromal cells and lymphocytes. This could greatly facilitate and empower comprehensive analysis of the spatial organization of cells, as well as their roles in tumor progression and metastasis. The overall classification accuracy was 92.9% and 90.1% in training and independent testing datasets, respectively. They also developed and validated a prognostic model, enabling personalized treatment planning for individual patients using readily available tissue images, which proves to be highly beneficial for pathologists and clinical practitioners.
3.3 Application of AI in predicting gene mutations in lung cancer
In recent years, researchers have utilized ML and DL techniques to analyze large-scale lung cancer genomic data, exploring gene variations associated with the occurrence and development of lung cancer, and predicting genes that may serve as driver mutations. LUAD is driven by a series of accumulated genetic changes known as driver mutations, such as mutations in epidermal growth factor receptor (EGFR), kirsten rat sarcoma viral oncogene homolog (KRAS), and anaplastic lymphoma kinase (ALK) fusion (42–44), which are potential therapeutic targets. Coudray et al. (38)downloaded gene mutation data from TCGA matched with patient samples and trained a CNN model to predict the 10 most common mutated genes in LUAD. The results showed that six of them (STK11, EGFR, FAT1, SETBP1, KRAS, TP53) could be predicted using pathological images, with AUC ranging from 0.733 to 0.856. In another study, Wang et al. (45)developed an innovative AI system known as the Fully Automated Artificial Intelligence System (FAIS) to predict EGFR gene mutations in lung cancer, as well as to assess the progression-free survival (PFS) of patients receiving EGFR-targeted therapy. By utilizing non-invasive CT images, the FAIS achieved promising predictive performance with AUC values ranging from 0.748 to 0.813 for EGFR gene mutations and demonstrated statistically significant associations with the PFS of patients (log-rank p<0.05). The FAIS efficiently achieved these predictions without requiring manual annotation, highlighting its potential in advancing lung cancer diagnostics and personalized therapy.
In addition to the common EGFR and KRAS gene mutations, ALK fusions are also observed in NSCLC. Song et al. (46)conducted a study in which they trained and validated a DL model using CT images and clinical pathological information to predicting the ALK fusion status in 937 NSCLC patients, achieving an AUC of 0.8046. Furthermore, the study predicted the prognosis of 91 patients undergoing ALK-TKI drug therapy, revealing that ALK-positive patients experienced longer PFS (16.8 vs. 7.5 months, P = 0.010).
Overall, the application of AI in predicting gene mutations in lung cancer demonstrates significant potential. Utilizing AI models can not only rapidly detect and identify gene mutations to formulate personalized treatment plans but also predict patients’ treatment responses and prognosis. These capabilities not only improve the effectiveness of diagnosis and treatment but also reduce the waste of medical resources. In the future, AI models are expected to discover new therapeutic targets and further enhance treatment precision by integrating multiple data sources.
4 Application of AI in lung cancer treatment
4.1 Application of AI-based 3D reconstruction in lung cancer
3D reconstruction technology, as part of AI-assisted surgery, has been increasingly used in clinical practice. By utilizing computer software to reconstruct 3D images from various imaging modalities such as CT, magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT), clearer and more intuitive 3D models of thoracic lesions and surrounding structures can be obtained, aiding in preoperative evaluations. This technology boosts the confidence of medical professionals and provides valuable assistance in formulating surgical plans and conducting surgical procedures. It has been reported that 20-30% of patients have variations in pulmonary vasculature (47). Therefore, the identification of anatomical variations is essential during preoperative planning or intraoperative navigation in lung cancer surgery. In a retrospective cohort study, thoracic surgeons achieved an accuracy rate of 85% in identifying anatomical variations using AI-assisted CT, with a median time of 2 (1–3) minutes (48). AI-driven reconstruction enables surgeons to achieve a high level of accuracy in identifying anatomical patterns within a short period, which has practical value in surgical planning for segmentectomy. CT-based pulmonary broncho-vascular 3D reconstruction facilitates precise anatomical pulmonary segmentectomy, making complex lung segmentectomy via a thoracoscopic approach safer and more convenient. This is especially advantageous for patients with deep nodules and vascular anatomical variations, where the benefits of 3D reconstruction are particularly evident.
Lung 3D reconstruction models can be created using semi-automatic tools such as Mimics, OsiriX, and 3DSlicer, which simulate anatomical structures, define lung segment divisions, and determine lesion locations. Intraoperative navigation using accurate 3D reconstruction models significantly reduces surgical time and improves surgical success rates (49). However, the high level of expertise required and the substantial time consumption may limit the widespread clinical application of these systems. AI technology can automatically learn from raw data and rapidly generate 3D models, enhancing clinical efficiency. Currently, AI-based 3D reconstruction software has been developed. Li et al. (50)constructed a fully automated 3D reconstruction system based on 3D CNN to assist thoracic surgery and to determine its accuracy, efficiency, and safety for clinical use. The AI system resulted in a significant reduction in operation time by 24.5 min for lobectomy (P < 0.001) and 20 min for segmentectomy (P = 0.007). Compared to manual reconstruction software (Mimics), the AI system reduced the model reconstruction time by 14.2 min (P < 0.001), and it also outperformed Mimics in model quality scores (P < 0.001).
Currently, AI-based 3D reconstruction systems mostly focus on the automatic reconstruction of pulmonary vessels and bronchi (48, 50, 51). There are no reported studies on automatic 3D reconstruction systems for tissue structure changes after neoadjuvant therapy, which could be one of the future development directions for AI in the field of lung cancer 3D reconstruction. In the future, with the continuous development of AI technology, various devices such as AI-guided robotic surgery systems, AI-assisted lung cancer biopsy and treatment robots, and automated lung cancer surgery robots may become a reality in this field. Figure 3 illustrates the application of AI in 3D reconstruction for lung cancer.
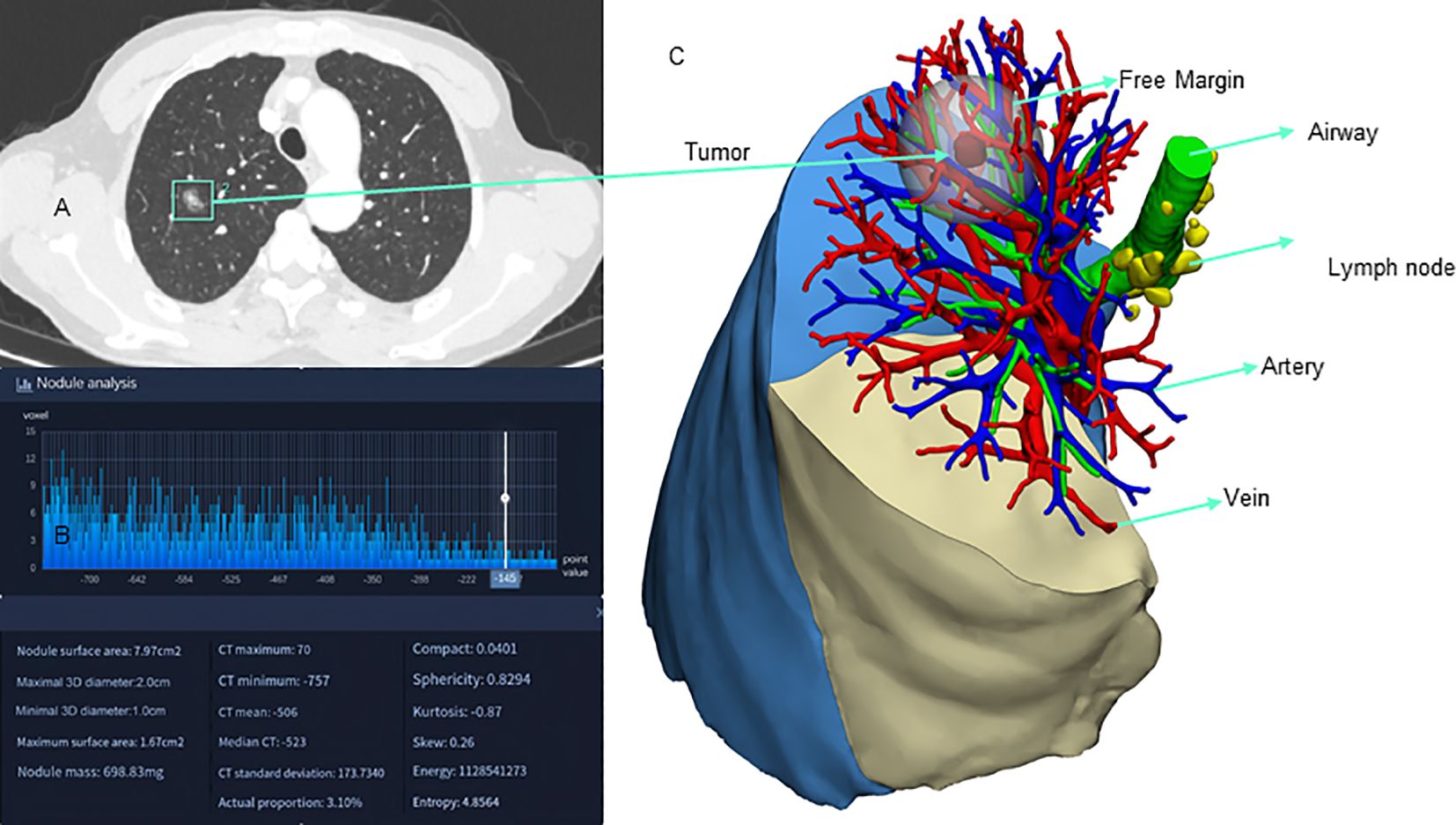
Figure 3. Application of artificial intelligence 3D reconstruction in lung cancer. (A) AI-assisted nodule localization; (B) AI-assisted nodule analysis; (C) AI-assisted 3D reconstruction.
4.2 AI-assisted preoperative assessment
Surgical resection, radiation therapy, and chemotherapy are common treatment approaches for lung cancer. In recent years, there have been significant advancements in molecular biology and genomics, leading to the widespread use of targeted therapy, immunotherapy, neoadjuvant therapy, and other treatment modalities in clinical practice. These advancements have resulted in improved patient prognosis to some extent. The selection of lung cancer treatment strategies generally relies on histopathological classification, immunohistochemical markers, and Tumor Node Metastasis (TNM) staging. However, despite having similar clinical characteristics, patients can exhibit significant individual differences in their response to the same treatment strategy. Therefore, early assessment and prediction of treatment efficacy for various treatment strategies are particularly crucial. AI-assisted preoperative assessment facilitates more accurate treatment decisions for doctors and provides patients with more personalized treatment plans.
The preoperative diagnosis of invasiveness is still difficult in the clinical setting since the pathological invasiveness of lung cancer is evaluable only after scrutinizing the pathological specimen. AI models can be utilized to achieve preoperative diagnosis of invasiveness. Onozato et al. (52)enrolled 873 patients who underwent lobectomy or segmentectomy for primary lung cancer and extracted the radiomic features from preoperative PET and CT images. They compared seven ML models and an ensemble model (ENS) combining PET and CT features. The results showed that all models achieved an AUC of ≥0.880 in predicting tumor invasiveness in the training set. In the test set, ENS showed the highest mean AUC of 0.880 and accuracy of 0.804.
In other studies, AI models demonstrated radiologist-level performance in predicting visceral pleural invasion (53) and identifying early-stage LUAD suitable for sublobar resection (54). Lv et al. (55)developed a deep learning model that achieved comparable performance to intraoperative frozen section analysis in determining tumor invasiveness. The proposed method may contribute to clinical decisions related to the extent of surgical resection. Due to the lack of risk stratification models for invasive adenocarcinoma, Zhou et al. (56)proposed an Ensemble Multi-View 3D Convolutional Neural Network (EMV-3D-CNN) model to study the risk stratification of lung adenocarcinoma. Their model outperformed senior physicians in risk stratification of invasive adenocarcinoma, achieving an accuracy of 77.6%. This provides detailed predictive histological information for the surgical treatment of pulmonary nodules.
4.3 AI-predicted immunotherapy efficacy
Immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1) or programmed death-ligand 1 (PD-L1) have become the mainstay of therapy for NSCLC patients without targeted treatment options, although only 20-40% of patients benefit from these new therapies (57). Immunotherapy has shown the potential to improve the prognosis of lung cancer patients, significantly prolonging PFS and overall survival (OS) in advanced NSCLC patients. Despite these promising results, the use of ICI remains constrained by its high costs and toxicity. Additionally, its clinical efficacy is limited to a small subset of patients, primarily assessed by monitoring the PD-L1 expression levels in tumor cells. The KEYNOTE-042 trial demonstrated a strong correlation between PD-L1 expression and treatment efficacy, with higher PD-L1 expression levels associated with greater benefits from immunotherapy in NSCLC patients (58).
Multiple AI techniques have been successfully applied to predict PD-L1 expression levels. Monaco et al. (59)constructed a tri-variate linear discriminant model based on ML algorithms, extracting metabolic parameter features from PET/CT images, achieving a sensitivity of 81% and a specificity of 82% in the test set. DL techniques can also be employed to identify the expression of PD-L1 in lung cancer tissues, with strong objectivity and repeatability of the results, eliminating human errors. Yang et al. combined radiomics with laboratory and clinical data to develop and validate a DL model for the identification of immunotherapy responders and non-responders. This model predicts the response of advanced NSCLC patients to anti-PD-1/PD-L1 drugs with an AUC of 0.80 (60).
AI systems have achieved promising results in predicting PD-L1 status. Clinical indicators and radiomics features play complementary roles in the prediction, providing accurate estimates for determining PD-L1 status (61). Combining various medical imaging and biological data, such as PET/CT, genomic data, and clinical information, through the comprehensive analysis of multimodal data, could lead to the identification of additional potential predictive factors, thereby enhancing the accuracy of the prediction model. These may represent potential directions for further development.
5 Prognosis
Compared to traditional prognostic prediction based on clinical features, AI-assisted prognostic prediction is more accurate and efficient (62). Trebeschi et al. (63) trained a neural network to identify morphological changes observed in retrospective chest CT scans of stage IV NSCLC patients during follow-up. They used a classifier to link the learned radiomic features with OS. The results showed significant performance in predicting 1-year OS from the time of image acquisition, with an average AUC of 0.69. The highest AUC occurred within the first 3-5 months of treatment, reaching 0.75. The AUC for predicting sustained clinical benefit (6-month PFS) was 0.67.
Conventional analysis of single-plex chromogenic immunohistochemistry (IHC) focused on quantitative but spatial analysis. AI algorithms can perform quantitative and spatial analysis of immune checkpoint expression and extract prognostic features from IHC images to assist the prediction of immune checkpoint features in survival and relapse. Guo et al. (64)utilized DL to analyze IHC pathology images and constructed a prognostic prediction model. U-net was applied to segment tumor cells and tumor-infiltrating lymphocytes, while ResNet was performed to extract prognostic features from IHC images. The results showed that the model achieved AUCs of 0.9 and 0.85 for predicting OS and relapse-free survival (RFS), respectively.
6 Limitations
Although AI has made significant progress in the field of lung cancer, it may still require some time to be fully integrated into clinical practice. Overall, the application of AI in lung cancer still faces certain limitations and challenges:
1. Database: AI models necessitate a substantial amount of data for training, and the quality of this data is crucial for the accuracy and reliability of the predictions. Nonetheless, certain data may suffer from issues such as missing information, biases, and noise, all of which can adversely affect the performance and predictive efficacy of the models. Furthermore, variations in data across different hospitals, including differences in equipment, techniques, and treatment protocols, can also influence the accuracy and reliability of the data. Through collaborative efforts to create standardized datasets and benchmarks, the variability in data quality can be reduced, thereby enhancing the robustness of AI applications.
2. Interpretability: The interpretability of AI algorithms remains a challenge. ML and DL techniques are widely applied in lung cancer research, but these models often function as black-box models, making it difficult to understand their decision-making process and underlying reasons. This makes it challenging for doctors to interpret the output results of the models, posing risks and challenges in practical applications. Fortunately, Interpretability of AI systems is a quickly growing field that has been highlighted by the radiology community as an important area of development, with much potential for the development of safe and intelligible AI technologies (65).
3. Generalization issues: Generalization refers to the ability of a model to learn from a given set of data and apply the learned model to other domains. Unlike natural images, medical images exhibit significant distribution differences when trained DL models are applied to datasets from different vendors. As a result, the generalizability of models becomes a major concern (66).
4. Ethical considerations and privacy: When utilizing patient data for predictions, ensuring data security, privacy, and proper regulation of data usage are essential. AI should be employed as an aid in medical diagnosis and not as a standalone diagnostic tool. Overreliance on AI for diagnosis poses significant safety risks. Furthermore, addressing potential biases in AI models is crucial, as they can lead to unfair treatment outcomes.
5. Legal Issues: The application of AI in healthcare also faces legal and liability challenges. The current ambiguity in legal handling of artificial intelligence will profoundly impact the development of autonomous AI. Advocates of artificial intelligence in radiology and healthcare need to lobby for legislative action to better clarify the liability risks of AI without hindering technological advancement (67).
Additionally, AI models require rigorous prospective validation studies to ensure their efficacy and safety in clinical settings. If not properly managed, AI could potentially exacerbate existing healthcare disparities.
7 Conclusion and outlook
AI technology plays a crucial role in various aspects of lung cancer, including diagnosis, classification, and treatment. It aids healthcare professionals in enhancing their work efficiency and accuracy, facilitating precise allocation of medical resources, and offering optimal treatment strategies for patients. This will significantly enhance the level of lung cancer diagnosis and treatment and improve patient survival rates.
In the future, the application of AI in the field of lung cancer is expected to become more extensive and profound. Firstly, with ongoing optimization of algorithms and an increase in training data, AI can assist healthcare professionals in more accurately detecting abnormalities in lung imaging examinations. Secondly, AI can provide personalized treatment recommendations by establishing precise personalized treatment plans based on patients’ genetic information, clinical characteristics, and treatment responses. Real-time monitoring of treatment effectiveness is another potential application. By combining sensor technology and monitoring data, AI can achieve real-time monitoring and adjustment of treatment effectiveness for lung cancer patients. AI-assisted surgery is also possible, where the combination of robotic surgery and virtual reality technology can assist surgeons in performing precise operations, reducing surgical risks and complications. Lastly, AI-assisted clinical decision-making can be achieved. By utilizing AI techniques, intelligent decision support can be provided during the patient’s diagnosis, treatment, and prognosis assessment processes, enabling personalized, accurate, and efficient clinical management.
In conclusion, the advancement of computer technology, along with the accumulation of medical knowledge and relevant datasets, will lead to an increasingly significant role of artificial intelligence in every aspect of lung cancer, ultimately enabling precise screening, diagnosis, and personalized therapy. While AI proves to be a valuable tool, it will not replace doctors but rather complement their expertise. The future of medical development lies in harnessing the synergies between AI and healthcare professionals to deliver more effective, patient-centered care and drive advancements in lung cancer management.
Author contributions
DY: Writing – original draft, Writing – review & editing. YM: Supervision, Writing – review & editing. CL: Supervision, Writing – review & editing. NZ: Writing – review & editing. QG: Supervision, Writing – review & editing. DZ: Writing – review & editing. SG: Data curation, Writing – review & editing. LL: Data curation, Writing – review & editing. JW: Data curation, Writing – review & editing. SL: Writing – review & editing. PL: Writing – review & editing. XB: Writing – review & editing. KZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Medical Science Foundation of Hebei University (2023X02).
Acknowledgments
We would like to give our sincere thanks to Dr. Shujie Cheng, and Jinghua Li for conception of our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Bade BC, Dela Cruz CS. Lung cancer 2020. Clin Chest Med. (2020) 41:1–24. doi: 10.1016/j.ccm.2019.10.001
3. The National Lung Screening Trial Research Team. Reduced lung-Cancer mortality with low-Dose computed tomographic screening. N Engl J Med. (2011) 365:395–409. doi: 10.1056/NEJMoa1102873
4. Bitkina OV, Park J, Kim HK. Application of artificial intelligence in medical technologies: A systematic review of main trends. Digit Health. (2023) 2:20552076231189331. doi: 10.1177/20552076231189331
5. Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. (2017) 2:230–43. doi: 10.1136/svn-2017-000101
6. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. (2019) 25:30–6. doi: 10.1038/s41591-018-0307-0
7. Artificial intelligence (AI). Definition, Examples, Types, Applications, Companies, & Facts (2023). Britannica. Available online at: https://www.britannica.com/technology/artificial-intelligence (Accessed May 5, 2023).
8. Jones MT. Artificial intelligence: a systems approach. Sudbury, Mass: Jones and Bartlett Publishers (2009). 498 p.
9. Bankier AA, MacMahon H, Colby T, Gevenois PA, Goo JM, Leung ANC, et al. Fleischner society: glossary of terms for thoracic imaging. Radiology. (2024) 310:e232558. doi: 10.1148/radiol.232558
10. Farjah F, Monsell SE, Smith-Bindman R, Gould MK, Banegas MP, Ramaprasan A, et al. Fleischner society guideline recommendations for incidentally detected pulmonary nodules and the probability of lung cancer. J Am Coll Radiol. (2022) 19:1226–35. doi: 10.1016/j.jacr.2022.06.018
11. Callister MEJ, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. [published correction appears in Thorax. (2015) 70(12):1188. doi: 10.1136/thoraxjnl-2015-207168
12. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the fleischner society 2017. Radiology. (2017) 284:228–43. doi: 10.1148/radiol.2017161659
13. Cellina M, Cacioppa LM, Cè M, Chiarpenello V, Costa M, Vincenzo Z, et al. Artificial intelligence in lung cancer screening: the future is now. Cancers. (2023) 15:4344. doi: 10.3390/cancers15174344
14. Kim RY, Oke JL, Pickup LC, Munden RF, Dotson TL, Bellinger CR, et al. Artificial intelligence tool for assessment of indeterminate pulmonary nodules detected with CT. Radiology. (2022) 304:683–91. doi: 10.1148/radiol.212182
15. Cellina M, Cè M, Irmici G, Ascenti V, Khenkina N, Toto-Brocchi M, et al. Artificial intelligence in lung cancer imaging: unfolding the future. Diagnostics. (2022) 12:2644. doi: 10.3390/diagnostics12112644
16. Yanagawa M. Artificial intelligence improves radiologist performance for predicting Malignancy at chest CT. Radiology. (2022) 304:692–3. doi: 10.1148/radiol.220571
17. Khosravan N, Bagci U. S4ND: Single-Shot Single-Scale Lung Nodule Detection (2018). Available online at: http://arxiv.org/abs/1805.02279 (Accessed May 12, 2023).
18. Cellina M, De Padova G, Caldarelli N, Libri D, Cè M, Martinenghi C, et al. Artificial intelligence in lung cancer imaging: from data to therapy. Crit Rev Oncog. (2024) 29:1–13. doi: 10.1615/CritRevOncog.2023050439
19. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation (2015). Available online at: http://arxiv.org/abs/1505.04597 (Accessed May 12, 2023).
20. Bhattacharyya D, Thirupathi Rao N, Joshua ESN, Hu Y-C. A bi-directional deep learning architecture for lung nodule semantic segmentation. Vis Comput. (2022) 1–17. doi: 10.1007/s00371-022-02657-1
21. Guo Z, Yang J, Zhao L, Yuan J, Yu H. 3D SAACNet with GBM for the classification of benign and Malignant lung nodules. Comput Biol Med. (2023) 153:106532. doi: 10.1016/j.compbiomed.2022.106532
22. Uthoff J, Stephens MJ, Newell JD, Hoffman EA, Larson J, Koehn N, et al. Machine learning approach for distinguishing Malignant and benign lung nodules utilizing standardized perinodular parenchymal features from CT. Med Phys. (2019) 46:3207–16. doi: 10.1002/mp.13592
23. Nasrullah N, Sang J, Alam MS, Mateen M, Cai B, Hu H. Automated lung nodule detection and classification using deep learning combined with multiple strategies. Sensors. (2019) 19:3722. doi: 10.3390/s19173722
24. Gong J, Liu J, Hao W, Nie S, Zheng B, Wang S, et al. A deep residual learning network for predicting lung adenocarcinoma manifesting as ground-glass nodule on CT images. Eur Radiol. (2020) 30:1847–55. doi: 10.1007/s00330-019-06533-w
25. Naqi SM, Sharif M, Jaffar A. Lung nodule detection and classification based on geometric fit in parametric form and deep learning. Neural Comput Appl. (2020) 32:4629–47. doi: 10.1007/s00521-018-3773-x
26. Zhang G, Lin L, Wang J. Lung nodule classification in CT images using 3D denseNet. J Phys Conf Ser. (2021) 1827:12155. doi: 10.1088/1742-6596/1827/1/012155
27. Zheng S, Cornelissen LJ, Cui X, Jing X, Veldhuis RNJ, Oudkerk M, et al. Deep convolutional neural networks for multiplanar lung nodule detection: Improvement in small nodule identification. Med Phys. (2021) 48:733–44. doi: 10.1002/mp.14648
28. Hu X, Gong J, Zhou W, Li H, Wang S, Wei M, et al. Computer-aided diagnosis of ground glass pulmonary nodule by fusing deep learning and radiomics features. Phys Med Biol. (2021) 66:065015. doi: 10.1088/1361-6560/abe735
29. Ahmed I, Chehri A, Jeon G, Piccialli F. Automated pulmonary nodule classification and detection using deep learning architectures. IEEE/ACM Trans Comput Biol Bioinform. (2023) 20(4):2445–56. doi: 10.1109/TCBB.2022.3192139
30. Luo X, Song T, Wang G, Chen J, Chen Y, Li K, et al. SCPM-Net: An anchor-free 3D lung nodule detection network using sphere representation and center points matching. Med Image Anal. (2022) 75:102287. doi: 10.1016/j.media.2021.102287
31. Li D, Yuan S, Yao G. Pulmonary nodule segmentation based on REMU-Net. Phys Eng Sci Med. (2022) 45:995–1004. doi: 10.1007/s13246-022-01157-9
32. Saied M, Raafat M, Yehia S, Khalil MM. Efficient pulmonary nodules classification using radiomics and different artificial intelligence strategies. Insights Imaging. (2023) 14:91. doi: 10.1186/s13244-023-01441-6
33. Tang T-W, Lin W-Y, Liang J-D, Li K-M. Artificial intelligence aided diagnosis of pulmonary nodules segmentation and feature extraction. Clin Radiol. (2023) 78:437–43. doi: 10.1016/j.crad.2023.03.002
34. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non–small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. (2008) 83(5):584–94. doi: 10.4065/83.5.584
35. Gurcan MN, Boucheron LE, Can A, Madabhushi A, Rajpoot NM, Yener B. Histopathological image analysis: A review. IEEE Rev BioMed Eng. (2009) 2:147–71. doi: 10.1109/RBME.2009.2034865
36. Davidson MR, Gazdar AF, Clarke BE. The pivotal role of pathology in the management of lung cancer. J Thorac Dis. (2013) 5(Suppl 5):S463–S478. doi: 10.3978/j.issn.2072-1439.2013.08.43
37. Yu K-H, Zhang C, Berry GJ, Altman RB, Ré C, Rubin DL, et al. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun. (2016) 7:12474. doi: 10.1038/ncomms12474
38. Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyö D, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med. (2018) 24:1559–67. doi: 10.1038/s41591-018-0177-5
39. Jain D, Roy-Chowdhuri S. Molecular pathology of lung cancer cytology specimens: A concise review. Arch Pathol Lab Med. (2018) 142:1127–33. doi: 10.5858/arpa.2017-0444-RA
40. Gonzalez D, Dietz RL, Pantanowitz L. Feasibility of a deep learning algorithm to distinguish large cell neuroendocrine from small cell lung carcinoma in cytology specimens. Cytopathology. (2020) 31:426–31. doi: 10.1111/cyt.12829
41. Wang S, Wang T, Yang L, Yang DM, Fujimoto J, Yi F, et al. ConvPath: A software tool for lung adenocarcinoma digital pathological image analysis aided by a convolutional neural network. EBioMedicine. (2019) 50:103–10. doi: 10.1016/j.ebiom.2019.10.033
42. Da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol Mech Dis. (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
43. Karachaliou N, Mayo C, Costa C, Magrí I, Gimenez-Capitan A, Molina-Vila MA, et al. KRAS mutations in lung cancer. Clin Lung Cancer. (2013) 14:205–14. doi: 10.1016/j.cllc.2012.09.007
44. Won JK, Keam B, Koh J, Cho HJ, Jeon YK, Kim TM, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. (2015) 26:348–54. doi: 10.1093/annonc/mdu530
45. Wang S, Yu H, Gan Y, Wu Z, Li E, Li X, et al. Mining whole-lung information by artificial intelligence for predicting EGFR genotype and targeted therapy response in lung cancer: a multicohort study. Lancet Digit Health. (2022) 4:e309–19. doi: 10.1016/S2589-7500(22)00024-3
46. Song Z, Liu T, Shi L, Yu Z, Shen Q, Xu M, et al. The deep learning model combining CT image and clinicopathological information for predicting ALK fusion status and response to ALK-TKI therapy in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging. (2021) 48:361–71. doi: 10.1007/s00259-020-04986-6
47. Smelt JLC, Suri T, Valencia O, Jahangiri M, Rhode K, Nair A, et al. Operative planning in thoracic surgery: A pilot study comparing imaging techniques and three-dimensional printing. Ann Thorac Surg. (2019) 107:401–6. doi: 10.1016/j.athoracsur.2018.08.052
48. Chen X, Wang Z, Qi Q, Zhang K, Sui X, Wang X, et al. A fully automated noncontrast CT 3-D reconstruction algorithm enabled accurate anatomical demonstration for lung segmentectomy. Thorac Cancer. (2022) 13:795–803. doi: 10.1111/1759-7714.14322
49. Wu Z, Huang Z, Qin Y, Jiao W. Progress in three-dimensional computed tomography reconstruction in anatomic pulmonary segmentectomy. Thorac Cancer. (2022) 13:1881–7. doi: 10.1111/1759-7714.14443
50. Li X, Zhang S, Luo X, Gao G, Luo X, Wang S, et al. Accuracy and efficiency of an artificial intelligence-based pulmonary broncho-vascular three-dimensional reconstruction system supporting thoracic surgery: retrospective and prospective validation study. eBioMedicine. (2023) 87:104422. doi: 10.1016/j.ebiom.2022.104422
51. Chen X, Xu H, Qi Q, Sun C, Jin J, Zhao H, et al. AI-based chest CT semantic segmentation algorithm enables semi-automated lung cancer surgery planning by recognizing anatomical variants of pulmonary vessels. Front Oncol. (2022) 12:1021084. doi: 10.3389/fonc.2022.1021084
52. Onozato Y, Iwata T, Uematsu Y, Shimizu D, Yamamoto T, Matsui Y, et al. Predicting pathological highly invasive lung cancer from preoperative [18F]FDG PET/CT with multiple machine learning models. Eur J Nucl Med Mol Imaging. (2023) 50:715–26. doi: 10.1007/s00259-022-06038-7
53. Choi H, Kim H, Hong W, Park J, Hwang EJ, Park CM, et al. Prediction of visceral pleural invasion in lung cancer on CT: deep learning model achieves a radiologist-level performance with adaptive sensitivity and specificity to clinical needs. Eur Radiol. (2021) 31:2866–76. doi: 10.1007/s00330-020-07431-2
54. Yoshiyasu N, Kojima F, Hayashi K, Bando T. Radiomics technology for identifying early-stage lung adenocarcinomas suitable for sublobar resection. J Thorac Cardiovasc Surg. (2021) 162:477–485.e1. doi: 10.1016/j.jtcvs.2020.05.009
55. Lv Y, Wei Y, Xu K, Zhang X, Hua R, Huang J, et al. 3D deep learning versus the current methods for predicting tumor invasiveness of lung adenocarcinoma based on high-resolution computed tomography images. Front Oncol. (2022) 12:995870. doi: 10.3389/fonc.2022.995870
56. Zhou J, Hu B, Feng W, Zhang Z, Fu X, Shao H, et al. An ensemble deep learning model for risk stratification of invasive lung adenocarcinoma using thin-slice CT. NPJ Digit Med. (2023) 6:119. doi: 10.1038/s41746-023-00866-z
57. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2021) 18:345–62. doi: 10.1038/s41571-021-00473-5
58. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
59. Monaco L, De Bernardi E, Bono F, Cortinovis D, Crivellaro C, Elisei F, et al. The “digital biopsy” in non-small cell lung cancer (NSCLC): a pilot study to predict the PD-L1 status from radiomics features of [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. (2022) 49:3401–11. doi: 10.1007/s00259-022-05783-z
60. Yang Y, Yang J, Shen L, Chen J, Xia L, Ni B, et al. A multi-omics-based serial deep learning approach to predict clinical outcomes of single-agent anti-PD-1/PD-L1 immunotherapy in advanced stage non-small-cell lung cancer. Am J Transl Res. (2021) 13(2):7430–56.
61. Wang C, Ma J, Shao J, Zhang S, Liu Z, Yu Y, et al. Predicting EGFR and PD-L1 status in NSCLC patients using multitask AI system based on CT images. Front Immunol. (2022) 13:813072. doi: 10.3389/fimmu.2022.813072
62. Hope A, Verduin M, Dilling TJ, Choudhury A, Fijten R, Wee L, et al. Artificial intelligence applications to improve the treatment of locally advanced non-small cell lung cancers. Cancers. (2021) 13:2382. doi: 10.3390/cancers13102382
63. Trebeschi S, Bodalal Z, Boellaard TN, Tareco Bucho TM, Drago SG, Kurilova I, et al. Prognostic value of deep learning-mediated treatment monitoring in lung cancer patients receiving immunotherapy. Front Oncol. (2021) 11:609054. doi: 10.3389/fonc.2021.609054
64. Guo H, Diao L, Zhou X, Chen J-N, Zhou Y, Fang Q, et al. Artificial intelligence-based analysis for immunohistochemistry staining of immune checkpoints to predict resected non-small cell lung cancer survival and relapse. Transl Lung Cancer Res. (2021) 10:2452–74. doi: 10.21037/tlcr-21-96
65. Reyes M, Meier R, Pereira S, Silva CA, Dahlweid F-M, Tengg-Kobligk HV, et al. On the interpretability of artificial intelligence in radiology: challenges and opportunities. Radiol Artif Intell. (2020) 2:e190043. doi: 10.1148/ryai.2020190043
66. Liang X, Nguyen D, Jiang SB. Generalizability issues with deep learning models in medicine and their potential solutions: illustrated with cone-beam computed tomography (CBCT) to computed tomography (CT) image conversion. Mach Learn Sci Technol. (2021) 2:015007. doi: 10.1088/2632-2153/abb214
Keywords: artificial intelligence, lung cancer, machine learning, deep learning, convolutional neural network
Citation: Yang D, Miao Y, Liu C, Zhang N, Zhang D, Guo Q, Gao S, Li L, Wang J, Liang S, Li P, Bai X and Zhang K (2024) Advances in artificial intelligence applications in the field of lung cancer. Front. Oncol. 14:1449068. doi: 10.3389/fonc.2024.1449068
Received: 14 June 2024; Accepted: 19 August 2024;
Published: 06 September 2024.
Edited by:
Michaela Cellina, ASST Fatebenefratelli Sacco, ItalyReviewed by:
David Naidich, New York University, United StatesYubao Guan, Fifth Affiliated Hospital of Guangzhou Medical University, China
Carlo Martinenghi, San Raffaele Hospital (IRCCS), Italy
Copyright © 2024 Yang, Miao, Liu, Zhang, Zhang, Guo, Gao, Li, Wang, Liang, Li, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuo Gao, MjQ1ODc2NTkwQHFxLmNvbQ==; Linqian Li, bGlsaW5xaWFuMTYzQDE2My5jb20=; Jianing Wang, amlhbmluZzAyMThAMTYzLmNvbQ==; Si Liang, bGlhbmdzaUBoYnUuZWR1LmNu; Peng Li, aGRmeWxpcGVuZ0AxMjYuY29t; Xuan Bai, MzA3ODgwODQ4QHFxLmNvbQ==; Ke Zhang, OTMzOTFAcXEuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Di Yang
Di Yang Yafei Miao1,2†
Yafei Miao1,2† Changjiang Liu
Changjiang Liu