- 1Department of Gynecology, Jinhua Municipal Central Hospital, Jinhua, Zhejiang, China
- 2Department of Hepatopancreatobiliary Surgery, Jinhua Municipal Central Hospital, Jinhua, Zhejiang, China
Background: Melanotic schwannoma (MS), a rare variant of peripheral nerve sheath tumor, is especially infrequent when originating from the peritoneum. Its definitive diagnosis relies on postoperative histopathological examination and immunohistochemical analysis, while preoperative diagnosis is difficult.
Case presentation: In the present study, we reported a rare case of giant MS in the retroperitoneum, which was previously misdiagnosed before surgery. However, intraoperative exploration revealed it was retroperitoneal tumor. The tumor had invaded the abdominal aorta and bilateral common illiac artery walls. A surgical resection was subsequently executed, and postoperative histopathological examination confirmed it as a MS.
Conclusion: The incidence of peritoneal MS is extremely rare, and surgical resection remains the preferred treatment modality. Given the absence of established guidelines for postoperative adjuvant therapy, long-term follow-up becomes imperative to accumulate valuable clinical expertise.
Introduction
MS, an exceedingly rare nerve sheath tumor, was first described and reported by Miler in 1932 as a malignant melanoma originating from the thoracic sympathetic ganglion (1). The malignant potential of MS remains uncertain, comprising 1% of all nerve sheath tumors. To date, around 300 cases have been reported in diverse anatomical locations, particularly affecting the cervical and thoracic spine. Notably, one of the rare location for MS is the retroperitoneum, where only nine cases have been reported. This study presents a rare case of retroperitoneal MS, which is a challenge in preoperative diagnosis and the potential for misdiagnosis. The case report offers valuable insights from a clinical perspective.
Case presentation
The consent for publication was obtained from the patient and approved by Jinhua Central Hospital Medical Ethics Review Committee (2023No.202). In March 2021, a 47-year-old female patient was admitted to our hospital due to intermittent abdominal pain for a week. She has a height of 150 cm and a weight of 52 kg, resulting in a BMI of 22.507. The laboratory parameters is shown in Supplementary Table 1. A computed tomography (CT) scan revealed the presence of an abdominal mass (Figure 1A). The magnetic resonance imaging (MRI) revealed a large cystic and solid lesion in the pelvic and abdominal cavity, internally segmented by multiple septa, with approximate dimensions of 11.7cm x 8.7cm x 19.1cm (Figure 1B). The T1-weighted image (T1WI) showed heterogeneous slightly high signal intensity, accompanied by patchy areas of low signal intensity (Figure 1C). Meanwhile, the T2-weighted image (T2WI) exhibited heterogeneous slightly high signal intensity, with patchy low signal areas (Figure 1D). Diffusion-weighted imaging(DWI) revealed patchy areas of high signal intensity (Figure 1E), while the apparent diffusion coefficient(ADC) demonstrated regions of low signal intensity (Figure 1F). Post-contrast images showed a thickened and segmented wall with enhanced cystic regions, indicating a significant cystic-solid lesion in the pelvic and abdominal cavity originating from the right ovary. This is suggestive of a hemorrhagic cystadenoma. Based on these findings, a laparoscopic exploratory surgery was conducted, revealing a large, heterogeneous abdominal mass with a blackish-grey coloration. The mass, which had a soft consistency, exhibited significant fixation. Internal hemorrhage was observed within the cyst, and its size and extent of the mass were consistent with preoperative imaging findings. The mass extended from the abdominal aorta to the pelvis, with its upper portion reaching up to the posterior duodenal wall at the twelfth level. Complete timeline of the events is shown in Supplementary Figure 1.
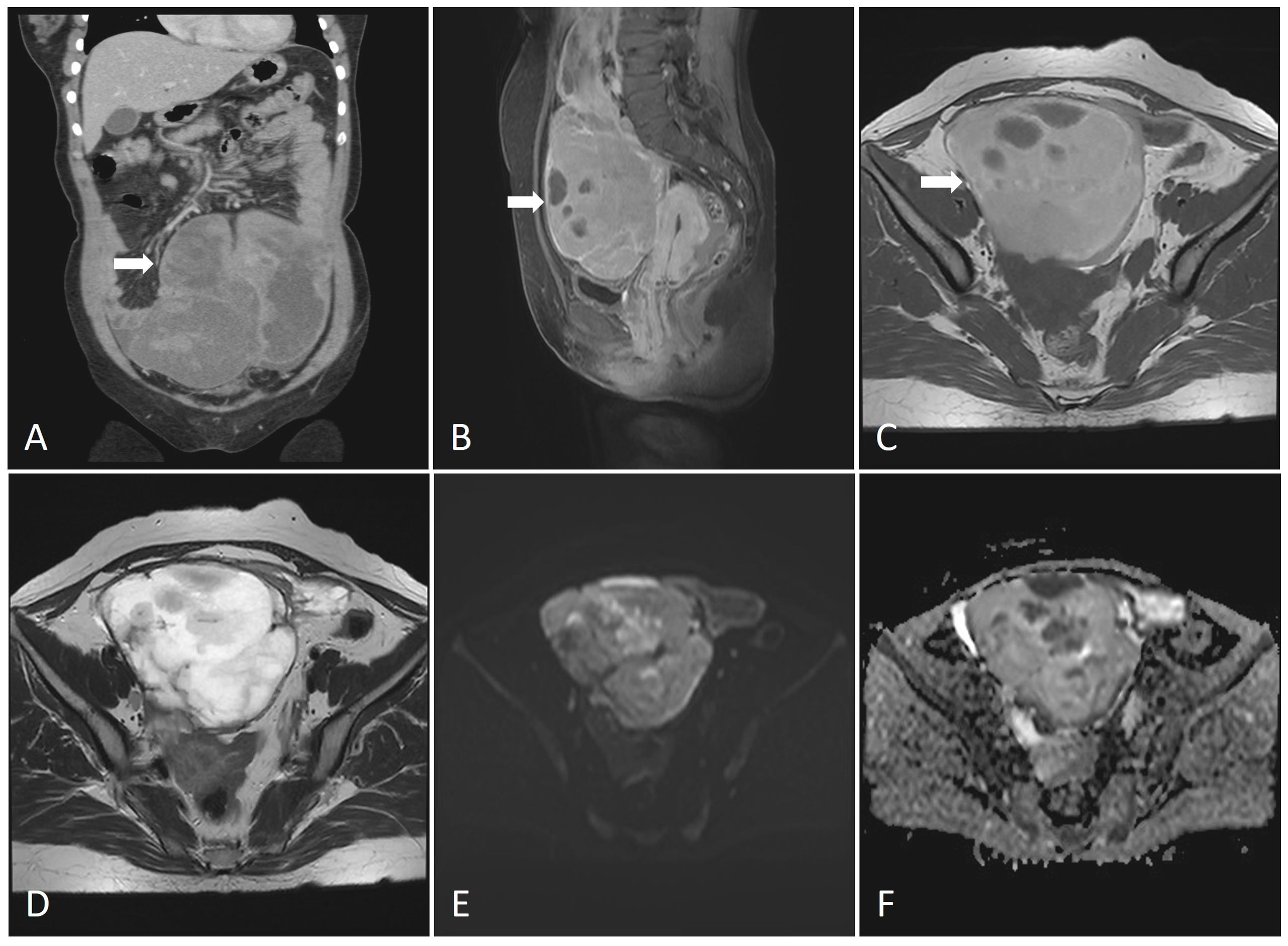
Figure 1. (A) CT imaging of the lesions (coronal plane).The abdominal mass was huge with inhomogeneous density and uneven enhancement. (B) MRI imaging of the lesions (sagittal plane). The MRI revealed a large cystic and solid lesion in the pelvic and abdominal cavity, internally segmented by multiple septa, with approximate dimensions of 11.7cm x 8.7cm x 19.1cm. (C) T1-weighted image (T1WI). The T1-weighted image (T1WI) showed heterogeneous slightly high signal intensity, accompanied by patchy areas of low signal intensity. (D) T2-weighted image (T2WI). The T2-weighted image (T2WI) exhibited heterogeneous slightly high signal intensity, with patchy low signal areas. (E) Diffusion-weighted imaging (DWI). Diffusion-weighted imaging (DWI) revealed patchy areas of high signal intensity. (F) Apparent diffusion coefficient (ADC). The apparent diffusion coefficient (ADC) demonstrated regions of low signal intensity.
Considering the presence of the tumor, an open abdominal surgery was necessary for the treatment, thus requiring a conversion to an open procedure. In collaboration with the hepatobiliary surgeon, a comprehensive investigation of the suspected duodenal tumor, which appeared to have a broad base, was conducted to ensure complete excision. Utmost caution was taken to prevent any inadvertent injury to both the inferior mesenteric artery and superior mesenteric vein. The rapid intraoperative pathology findings suggested the possibility of extra-gastric intestinal mesothelioma (clear cell sarcoma, pending confirmation), with the tissue type to be further validated through routine histopathological examination and immunohistochemical staining.
During the operation, further dissection of the retroperitoneal space surrounding the abdominal aorta revealed tumor infiltration into the walls of the abdominal aorta and bilateral iliac arteries. Subsequently, lymphatic tissue encompassing the abdominal aorta, iliac arteries, and sacral region was resected along with excision of the greater omentum. To avoid catastrophic risks, the tumor capsule adhering to the surface of the abdominal aorta and bilateral iliac arteries was not resected (Figure 2). The surgery has been completed, and a retroperitoneal drainage tube was inserted. The postoperative recovery went smoothly, and the surgical wounds healed well. The patient was discharged from the hospital 7 days after the surgery. Postoperative pathology revealed a MS(retroperitoneal mass, retroperitoneal lymphoid tissue, omentum). The reticular fiber staining analysis was positive. Immunohistochemical analysis results supported the diagnosis of MS (Figure 3). For example, HMB45, Melan-A, SOX10, S-100, and CD117 showed positive results. Ki67 expression was detected at 2% positivity. Desmin staining showed negative results. Follow-up examination is recommended based on these findings. After consultation with the Pathology Department of Fudan University Shanghai Cancer Center, it was still considered as MS. Molecular testing showed no definite mutation in exon 15 of the BRAF gene. Regular follow-up was conducted for 3 years after surgery, and there is no obvious recurrence and metastasis on MRI and PET-CT monitoring until now.
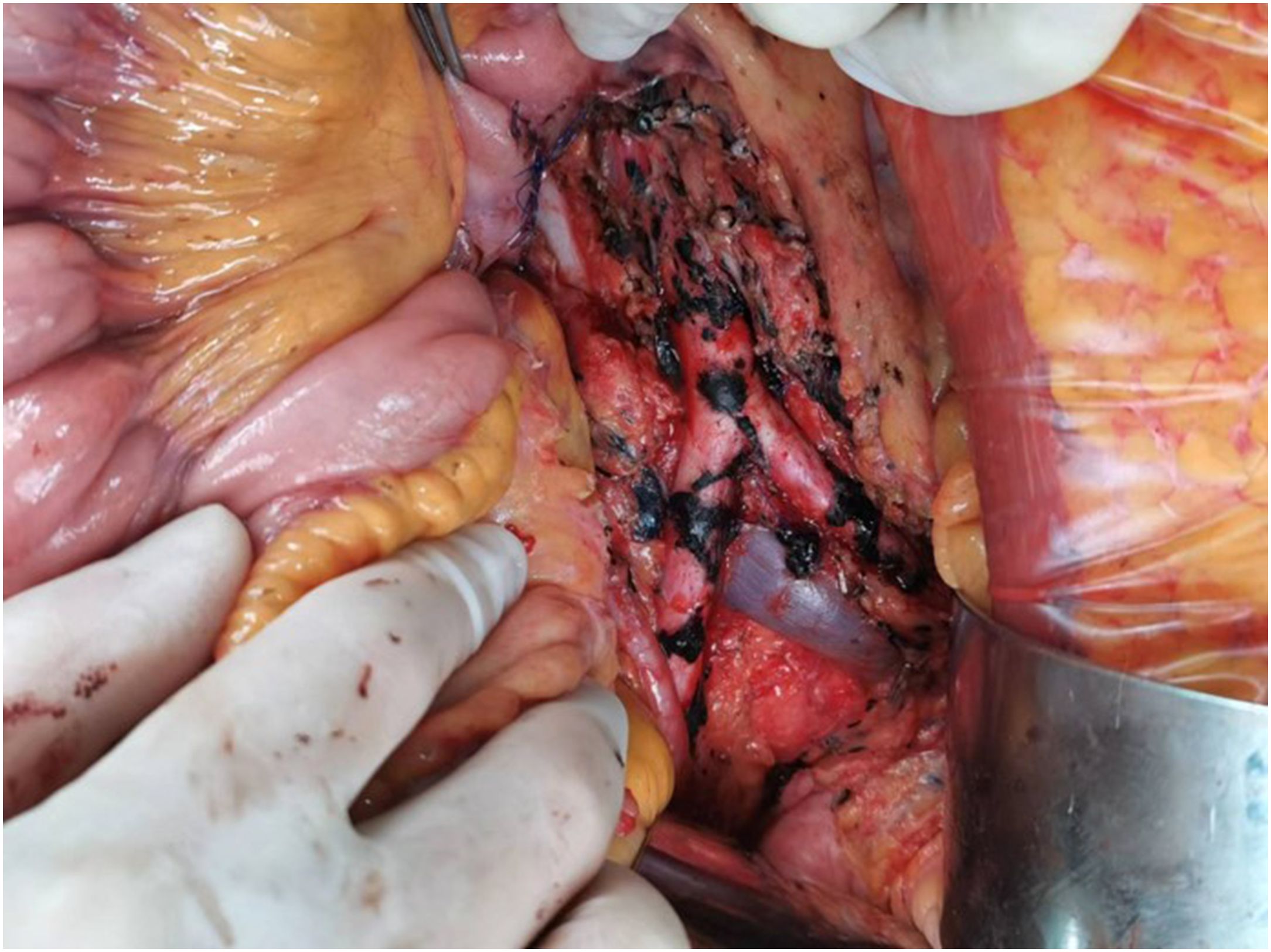
Figure 2. Postoperative photograph of the resected tumor. Lymphatic tissue encompassing the abdominal aorta, iliac arteries, and sacral region was resected along with excision of the greater omentum. To avoid catastrophic risks, the tumor capsule adhering to the surface of the abdominal aorta and bilateral iliac arteries was not resected.
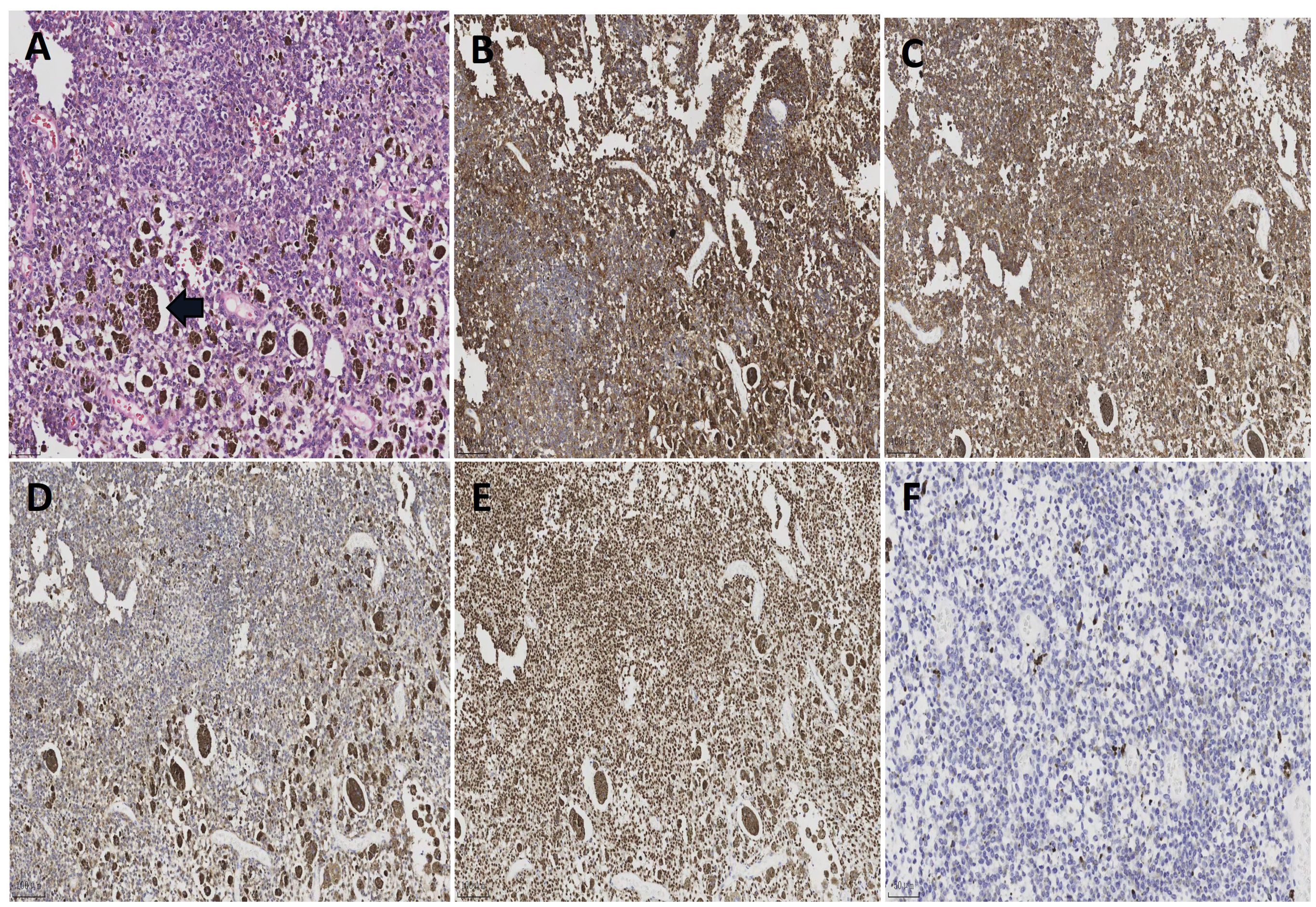
Figure 3. (A) Histological sections of patient’s tumor [H&E; ×200]. The tumor cells are oval in shape, with obvious nucleoli and significant melanin granule deposition. (B) Positive immunoactivity for MelanA (×100). (C) Positive immunoactivity for HMB45 (×100). (D) Positive immunoactivity for S-100 (×100). (E) Positive immunoactivity for SOX10 (×100). (F) Ki67 immunostain with a proliferative index of 2% (×200).
Discussion
MS was first described by Millar in 1932 as malignant melanotic tumor of ganglion cells and subsequently termed as MS by Fu et al., in 1975 (1, 2). MS was previously classified as a benign tumor in the 2013 WHO classification, but in 2020 WHO classification, the term “MS” was revised to “malignant melanotic nerve sheath tumor (MMNST)” due to its malignant properties (3). Until now, the MMNST pathogenesis remains unclear. There are two different types of MS: the sporadic and the psammomatous melanotic schwannomas of Carney complex (4–9). Among them, up to 50% of these tumors are related to Carney complex, an autosomal dominant genetic syndrome characterized by spotty pigmentation of the skin, heart, breast, and uterine myxomas, primary pigmented nodular adrenocortical disease with Cushing’s syndrome, growth hormone-producing pituitary adenoma, testis/ovaries Sertoli cell tumors, thyroid adenomas, and breast adenomas (10, 11). In this case, the MS is located in the retroperitoneum, which is a rare site for this type of tumor. However, there is no indication of genetic inheritance or Carney syndrome, and no genetic mutations were found. The clinical symptoms of MS are non-specific, making preoperative diagnosis difficult. Preoperative diagnosis is based on MRI, which showed MS exhibit high signal intensity on T1WI and low signal intensity on T2WI compared to the non-schwannomas types which showed low signal intensity on T1 and high signal intensity on T2 sequences (12–15). The definitive diagnosis relies on pathological examination. MMNST typically expresses strong S100 and melanocyte markers such as SOX10, HMB45, Melan-A, and tyrosinase (16). The mitotic rate is the only histological feature predictive of clinical outcome. Study showed that a mitotic rate of more than 2/10 HPF would be associated with metastases (P=0.008) (17). MS are usually solitary, partially circumscribed or encapsulated, and heavily pigmented (18). Their diameters range from 0.5 cm to 25 cm, but most of them are over 5 cm (19).
MS exhibited potentially malignant biological properties, with the possibility of local recurrence and distant metastasis. Its prognosis is unpredictable. Torres-Mora et al. (16) reported MMNST local recurrence and metastatic rates was 35% and 44%, respectively (with an average follow-up of 55 months), and 73% of metastases occurred within four years. Metastases are commonly found in the lung and pleura. Currently, surgery is the primary treatment for MS. Due to the rarity of this tumor, there are no formal guidelines for adjuvant therapies, including radiotherapy and chemotherapy, and their use remains controversial (20, 21). However, it was reported in the literature that postoperative radiotherapy was used as an adjunctive treatment to reduce the possibility of local recurrence. A recent report emphasized the importance of adjuvant radiotherapy, in which the surgical exploration did not include sufficient surgical margins and hence 60 gray of adjuvant radiotherapy was taken. After 24 months follow-up, the patient showed no signs of recurrence or metastasis (22). In terms of chemotherapy, only Italian et al. (23) reported in 2011 that the application of chemotherapeutic agents could slow down the growth of tumor. Although the therapeutic efficacy of radiotherapy and chemotherapy has not been definitively confirmed, if it was proven, these treatments are recommended because of the aggressive potential of the lesions (24, 25). Other treatments such as targeted therapy and immune checkpoint therapy are currently emerging as promising options for tumor treatment (26). Thus, the use of immune checkpoint inhibitors in MMNST are limited to three reported cases. However, they provide some evidence suggesting that these inhibitors can offer symptomatic improvement and clinical response in some PD-L1-positive MMNST patients (18).
The pathophysiology and prognosis of MMNST require further data for a comprehensive understanding, as well as to improve survival rates. Surgical intervention should aim to achieve complete resection of MMNST to prevent the potential recurrence or transformation into more aggressive malignancies. In this case, the postoperative cytomorphological and immunohistochemical staining results indicated a low risk of malignancy, while the PET-CT assessment conducted two months after surgery revealed inactive metabolism in residual tissue. Consequently, adjuvant treatment was not administered to the patient who was advised regular follow-up and observation instead. Over a three-year period, the patient underwent annual PET-CT scans without any signs of recurrence or metastasis. However, there remains a residual tumor capsule on the surface of the blood vessels requiring long-term follow-up observation. If necessary, treatments such as chemotherapy, radiation therapy, or surgery should be considered.
Conclusion
Retroperitoneal melanotic schwannoma is extremely rare, and currently, there are no reliable biomarkers for monitoring. Preoperative diagnosis is difficult and requires pathological and immunohistochemical confirmation. Surgery is the corerstone of MMNST treatment. There are no standard treatment guidelines for postoperative management. Therefore, we recommended thorough surgical resection, while taking care to protect of surrounding anatomical structures during the operation, as an effective treatment to avoid local tumor recurrence and preserve function. Furthermore, close long-term postoperative follow-up is necessary to accumulate diagnostic and treatment experience for better management of such cases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Jinhua Central Hospital Medical Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PC: Investigation, Formal analysis, Methodology, Writing – original draft. JC: Investigation, Methodology, Writing – review & editing. LZ: Investigation, Writing – review & editing, Conceptualization, Resources, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We wish to acknowledge our patient and thank her for providing consent for publication of this case report and the associated images.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1448112/full#supplementary-material
Abbreviations
ADC, apparent diffusion coefficient; CT, computed tomography; DWI, diffusion-weighted imaging; MMNST, malignant melanotic nerve sheath tumor; MRI, magnetic resonance imaging; MS, melanotic schwannoma; PET-CT, positron emission tomography/computed tomography; T1WI, T1-weighted image; T2WI, T2-weighted image.
References
1. Millar W. A Malignant melanotic tumour of ganglion cells arising from a thoracic sympathetic ganglion. J Pathol Bacteriol. (1932) 35:351–7. doi: 10.1002/path.1700350305
2. Fu YS, Kaye GI, Lattes R. Primary Malignant melanocytic tumors of the sympathetic ganglia, with an ultrastructural study of one. Cancer-Am Cancer Soc. (1975) 36:2029–41. doi: 10.1002/cncr.2820360917
3. Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol. (2021) 28:44–58. doi: 10.1097/PAP.0000000000000284
4. Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol. (1990) 14:206–22. doi: 10.1097/00000478-199003000-00002
5. Goldstein MM, Casey M, Carney JA, Basson CT. Molecular genetic diagnosis of the familial myxoma syndrome (Carney complex). Am J Med Genet. (1999) 86:62–5. doi: 10.1002/(ISSN)1096-8628
6. Léger F, Vital C, Rivel J, Benjelloun B, Galli FS, Guerin J. Psammomatous melanotic schwannoma of a spinal nerve root. Relationship Carney complex Pathol Res Pract. (1996) 192:1142–6. doi: 10.1016/S0344-0338(96)80034-3
7. Ludvíková M, Michal M, Marek J, Syrucek M. Psammomatous melanotic schwannoma. Cesk Patol. (1997) 33:141–5.
8. Martin-Reay DG, Shattuck MC, Guthrie FW. Psammomatous melanotic schwannoma: an additional component of Carney’s complex. Report of a case. Am J Clin Pathol. (1991) 95:484–9. doi: 10.1093/ajcp/95.4.484
9. Prieto-Rodríguez M, Camañas-Sanz A, Bas T, Cortes B, Vera-Sempere FJ. Psammomatous melanotic schwannoma localized in the mediastinum: diagnosis by fine-needle aspiration cytology. Diagn Cytopathol. (1998) 19:298–302. doi: 10.1002/(ISSN)1097-0339
10. Keskin E, Ekmekci S, Oztekin O, Diniz G. Melanotic Schwannomas are rarely seen pigmented tumors with unpredictable prognosis and challenging diagnosis. Case Rep Pathol. (2017) 2017):1807879. doi: 10.1155/2017/1807879
11. Choi SE, Cha YJ, Kim J, Cha H, Seo J, Kuh SU, et al. A rare case of aggressive melanotic schwannoma occurred in spinal nerve of a 59-year-old male. J Pathol Transl Med. (2017) 51:505–8. doi: 10.4132/jptm.2017.01.04
12. Aprile I, Scott CA, Cervesato D, Beltrami CA, Meo A, Fabris G. Two rare lumbar tumours with unusual MRI characteristics. Neuroradiology. (2000) 42:458–61. doi: 10.1007/s002340000306
13. Goasguen O, Boucher E, Pouit B, Soulard R, Charpentier ML, Pernot P. Melanotic schwannoma, a tumor with a unpredictable prognosis: case report and review of the literature. Neurochirurgie. (2003) 49:31–8.
14. Höllinger P, Godoy N, Sturzenegger M. Magnetic resonance imaging findings in isolated spinal psammomatous melanotic schwannoma. J Neurol. (1999) 246:1100–2. doi: 10.1007/s004150050522
15. Riffaud L, Morandi X, Massengo S, Carsin-Nicol B, Heresbach N, Guegan Y. MRI of intramedullary spinal schwannomas: case report and review of the literature. Neuroradiology. (2000) 42:275–9. doi: 10.1007/s002340050885
16. Torres-Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL. Malignant melanotic schwannian tumor: a clinicopathologic, immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of “melanotic schwannoma. Am J Surg Pathol. (2014) 38:94–105. doi: 10.1097/PAS.0b013e3182a0a150
17. Bajpai J, Kapoor A, Jalali R, Gounder MM. Checkpoint inhibitors and radiotherapy in refractory Malignant melanocytic schwannoma with Carney complex: first evidence of efficacy. BMJ Case Rep. (2021) 14:e240296. doi: 10.1136/bcr-2020-240296
18. Terry M, Wakeman K, Williams BJ, Miller DM, Sak M, Abdullaev Z, et al. Malignant melanotic nerve sheath tumor with PRKAR1A, KMT2C, and GNAQ mutations. Free Neuropathol. (2022) 3:3–21. doi: 10.17879/freeneuropathology-2022-3864
19. Alexiev BA, Chou PM, Jennings LJ. Pathology of melanotic schwannoma. Arch Pathol Lab Med. (2018) 142:1517–23. doi: 10.5858/arpa.2017-0162-RA
20. Siordia J, Golden T. Current discoveries and management of psammomatous melanotic schwannoma. J Cancer Tumor Int. (2016) 3:1–7. doi: 10.9734/jcti/2016/23786
21. Mennemeyer RP, Hallman KO, Hammar SP, Raisis JE, Tytus JS, Bockus D. Melanotic schwannoma. Clinical and ultrastructural studies of three cases with evidence of intracellular melanin synthesis. Am J Surg Pathol. (1979) 3:3–10. doi: 10.1097/00000478-197902000-00001
22. Gulati HK, Joshi AR, Anand M, Deshmukh SD. Non psammomatous melanocytic schwannoma presenting as a subcutaneous nodule: A rare presentation of a rare lesion. Asian J Neurosurg. (2016) 11:317–8. doi: 10.4103/1793-5482.148789
23. Italiano A, Kind M, Stoeckle E, Jones N, Coindre JM, Bui B. Temsirolimus in advanced leiomyosarcomas: patterns of response and correlation with the activation of the mammalian target of rapamycin pathwayn. Anticancer Drugs. (2011) 22:463–7. doi: 10.1097/CAD.0b013e3283442074
24. Liessi G, Barbazza R, Sartori F, Sabbadin P, Scapinello A. CT and MR imaging of melanocytic schwannomas; report of three cases. Eur J Radiol. (1990) 11:138–42. doi: 10.1016/0720-048x(90)90163-6
25. Buhl R, Barth H, Hugo HH, Mautner VF, Mehdorn HM. Intracranial and spinal melanotic schwannoma in the same patient. J Neurooncol. (2004) 68:249–54. doi: 10.1023/b:neon.0000033491.23654.6c
Keywords: melanotic schwannoma(MS), retroperitoneal schwannoma, malignant melanotic nerve sheath tumor(MMNST), rare giant tumor, surgical resection
Citation: Chen P, Cheng J and Zhang L (2024) Rare giant retroperitoneal melanotic schwannoma: a case report and literature review. Front. Oncol. 14:1448112. doi: 10.3389/fonc.2024.1448112
Received: 12 June 2024; Accepted: 14 August 2024;
Published: 29 August 2024.
Edited by:
Jiang Chen, Zhejiang University, ChinaReviewed by:
Zhenhua Li, Harvard Medical School, United StatesYi Mu, ClinChoice Inc, United States
Kunsheng Li, Nanjing Drum Tower Hospital, China
Copyright © 2024 Chen, Cheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhang, ZmJ5ZmsxMjNAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Pan Chen1†
Pan Chen1† Lin Zhang
Lin Zhang