- 1Department of Nuclear Medicine, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 2Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
A 70-year-old woman underwent distal gastrectomy due to gastric adenocarcinoma in 2015. After 6 years, the follow-up CT revealed a suspicious mass in the right hilar of the lung mimicking mediastinal lymph nodes. Further dynamic PET/CT images showed a mass located in the right intermediate bronchus with increased FDG uptake and relatively high Ki value, which may imply the possibility of malignancy. However, the symmetrical mediastinal lymph nodes had intense FDG uptake but relatively low Ki value, suggesting benign lesions. The initial pathological result of the bronchoscopy biopsy was considered suspicious for metastatic gastric adenocarcinoma. However, it was then found consistent with middle-grade mucoepidermoid carcinoma, considered a second primary cancer without metastatic lymph nodes as confirmed by a surgical procedure (lower bilobectomy + hilar and mediastinal lymphadenectomy). 18F-FDG PET/CT has an important value in the follow-up of indeterminate findings for patients with a tumor history. Moreover, dynamic quantification parameters such as Ki may be additionally helpful in identifying malignancies in some equivocal situations.
Introduction
In recent decades, advances in cancer treatment and the aging of the population have led to more frequent survivors of cancer treatment. However, cancer survivors have at least 20% increased risk for secondary cancer compared to the general population (1). In patients with a prior cancer diagnosis, follow-up examinations are performed to rule out relapse and metastasis often over a period of several years. 18F-FDG PET/CT has an important role in diagnosis, staging, and restaging of a patient with a malignant tumor. In a large series of 1,912 patients, PET/CT detected additional FDG-avid primary malignant tumors in at least 1.2% of patients with cancer (2). Recently, dynamic PET/CT (dPET/CT) was developed to obtain absolute quantitative metabolic parameters (e.g., net influx rate Ki, FDG delivery rate K1, and phosphorylation rate k3) by continuously acquiring imaging data over a certain period of time (3). Compared to the standardized uptake value (SUV), a semi-quantitative metabolic parameter of traditional static PET/CT, such as absolute quantitative metabolic parameters have potential advantages in reflecting tumor characteristics and the differential diagnosis of benignity and malignancy (3, 4). Herein, we present a bronchial mass mimicking mediastinal lymph nodes on dynamic 18F-FDG PET/CT as a second primary cancer in a 70-year-old woman with a history of gastric carcinoma.
Case presentation
A 70-year-old woman underwent distal gastrectomy due to gastric adenocarcinoma in 2015. The patient had no complaints of specific discomfort during follow-up, and regular gastroscopy showed no evidence of local recurrence for 6 years after surgery. The blood serum levels of CEA, SCC, NSE, CA125, CYFRA21-1, and ProGRP were normal. Follow-up CT revealed a suspicious mass in the right hilar of the lung mimicking mediastinal lymph nodes. The images of contrast-enhanced CT and dPET/CT are shown in Figure 1. The contrast-enhanced CT revealed a markedly enhanced endobronchial mass (1.6 cm × 1.5 cm) adapting to the right intermediate bronchus. Further dPET/CT demonstrated the described right hilar mass with a moderately increased FDG uptake (SUVmax 3.9 and 3.8 for regular and delay scans, respectively) and a relatively high Ki value (0.029 mL/g/min). The symmetrical mediastinal lymph nodes had intense FDG uptake (SUVmax 12.3 and 17.4) but a relatively low Ki value (average Ki of 0.021 mL/g/min).
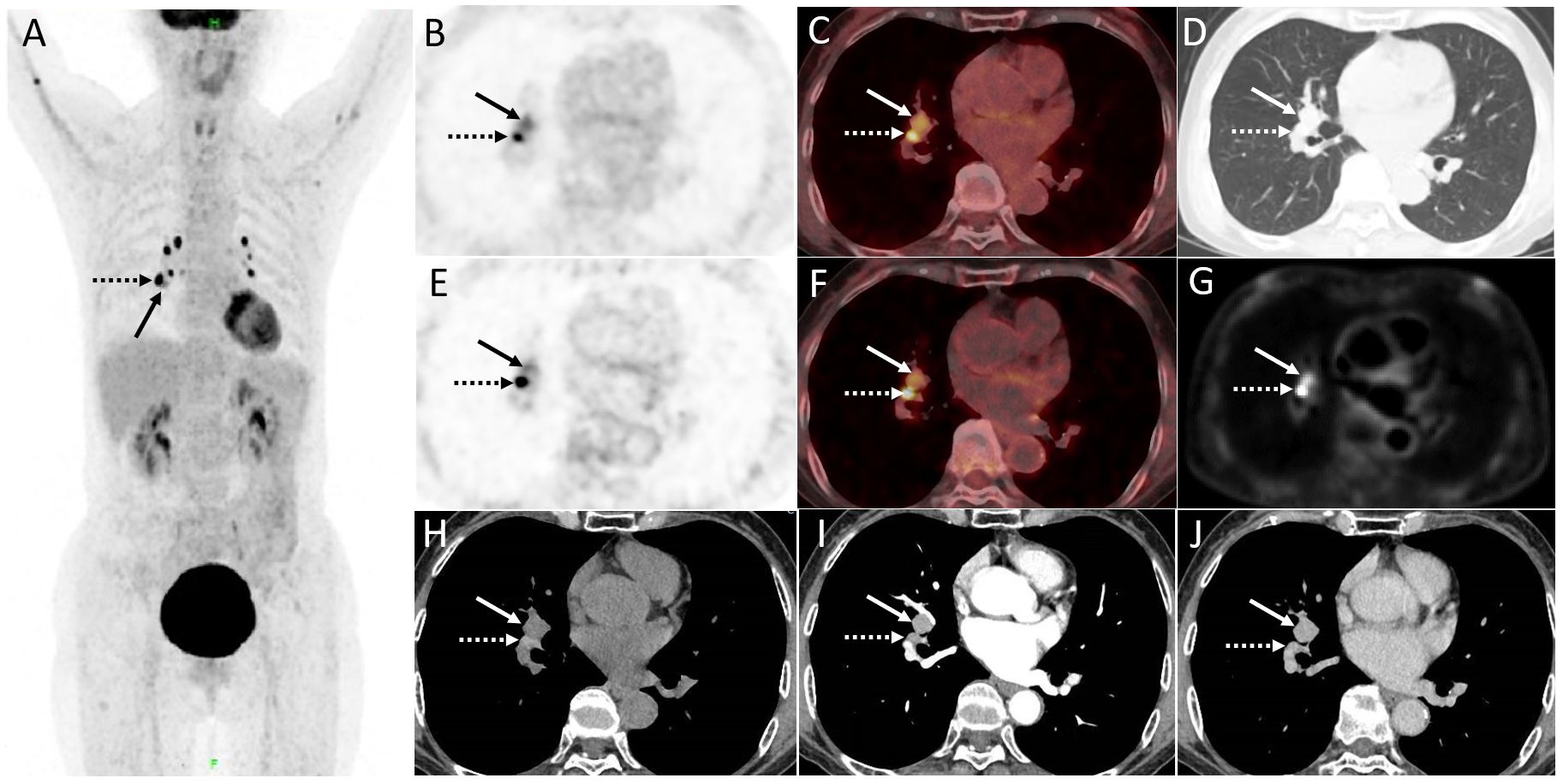
Figure 1. (A) The maximum intensity projection image (MIP) of PET/CT. The described right hilar mass (1.6 cm × 1.5 cm) displayed in (B, C) regular scan, (D) lung window, and (E, F) delay scan (arrow, SUVmax of 3.9 and 3.8, respectively) mimicking mediastinal lymph nodes with intense FDG uptake (dotted arrow, SUVmax 12.3 and 17.4. respectively). (G) Quantification analysis of dynamic parameters (Ki, net influx rate) shows that the mediastinal lymph nodes had a significantly high uptake of FDG but a relatively low Ki value (average Ki of 0.021 mL/g/min), while the Ki value for endobronchial mass (arrow) was 0.029 mL/g/min. (H–J) Contrast-enhanced CT revealed a markedly enhanced endobronchial mass adapting to the right intermediate bronchus.
The initial pathological result of preoperative bronchoscopy biopsy was considered suspicious for metastatic gastric adenocarcinoma and the EBUS biopsy results of mediastinal lymph nodes in regions 10R and 11L were negative. However, a second primary cancer cannot be ruled out for this single new lesion in an elderly carcinoma survivor, especially given the rarity of tracheal metastasis. Oligometastases or local malignancy may achieve long-term survival after surgery. In addition, the patient was in good condition and had a strong desire for thoracic surgery with the hope of a radical cure. Therefore, the patient then underwent a surgical procedure (lower bilobectomy + hilar and mediastinal lymphadenectomy) for an accurate diagnosis and staging. The pathological result was found consistent with middle-grade mucoepidermoid carcinoma as a second primary cancer without metastasis in 30 resected lymph nodes (Figure 2). The tumor was typically a mix of squamous cells, mucocytes, and intermediate cells. Immunohistochemically, the tumor cells were positive for PAS, CK7, and MUC5AC. Dual-color break-apart FISH analysis for the presence of MAML2 gene rearrangement was positive, which is reported as a diagnostic and prognostic index for PMEC (5). Furthermore, the patient was staged as pT1bN0, and no further treatment was deemed necessary. The regular follow-up during 2.5 years after surgery showed no signs of abnormality.
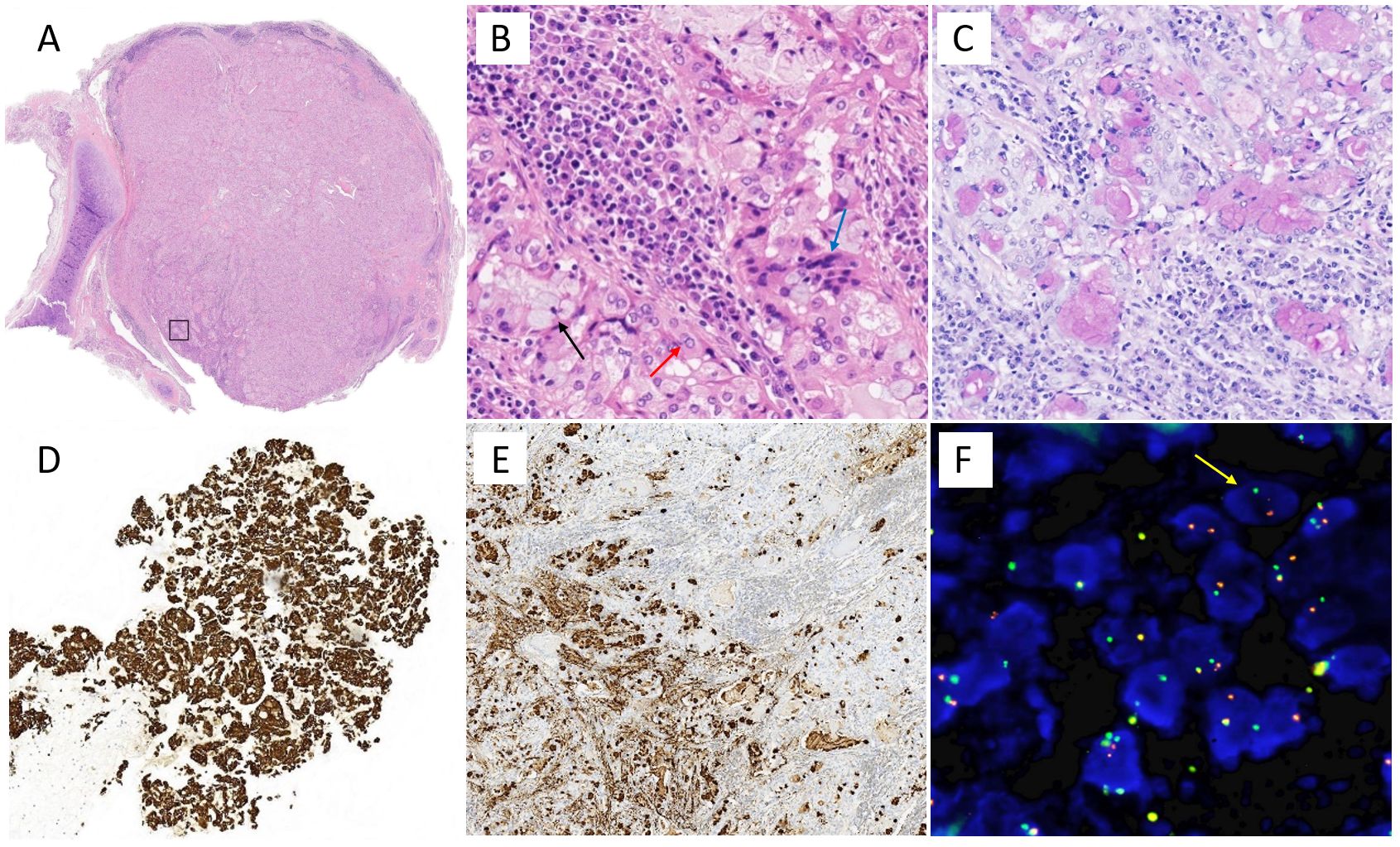
Figure 2. Pathological results of the patient. (A) Hematoxylin and eosin stain of ×10 and (B) ×400, namely, box area in (A). The tumor was typically a mix of squamous cells (red arrow), mucocytes (black arrow), and intermediate cells (blue arrow). Immunohistochemically, the tumor cells were positive for (C) PAS, (D) CK7, and (E) MUC5AC. (F) Dual-color break-apart FISH analysis for the presence of MAML2 gene rearrangement (yellow arrow), which is reported as a diagnostic and prognostic index for PMEC.
Discussion
For a patient with a previous cancer history, it can be difficult to establish the diagnosis of an additional primary, which is not uncommon nowadays. An unexpected bronchial mass found in a patient with known cancer was likely to be mistaken as mediastinal lymph nodes or tracheobronchial metastasis, which is rarely seen. Wumener et al. reported the value of dPET/CT in differentiating malignant and benign lymph nodes (6) and lung lesions (4) with the cutoff value of Ki as 0.022 mL/g/min and 0.025 mL/g/min, respectively. Despite the significant high uptake of FDG of mediastinal lymph nodes in this case, the bilateral symmetrical distribution and relatively low Ki value suggest benign lesions consistent with pathological results. Whereas the bronchial mass with moderately increased FDG uptake but a relatively high Ki value suggests its malignancy. On the other hand, tracheobronchial metastasis is rare and occasionally reported in cases with melanoma, breast, colorectal, and renal cancers (7).
Moreover, a single new lesion several years after a primary cancer diagnosis should alert clinicians to the possibility of a second primary tumor, especially in older adults (1). Approximately one-third of cancer survivors aged > 60 years have been diagnosed more than once with another cancer (8). Pulmonary mucoepidermoid carcinoma (PMEC) is extremely rare, with an estimated incidence of less than 1% of all primary lung neoplasms, and the majority of PMEC occurs in adults between 30 and 40 years of age (9). PMEC is classified as a salivary gland-type tumor originating from the submucosal glands of the tracheobronchial tree, which is difficult to diagnose by limited biopsy via bronchoscope (10). PMEC usually appears as oval or lobulated proximal airway masses with an occasionally punctate calcification (11), and shows different extents of FDG uptake, varying from 0 to 10.7 for low-grade and 2.9 to 23.4 for high-grade (10, 12–15), with a reported optimal cutoff of 6.5 (16). Several studies have reported that PMEC can present as multiple primary lung cancer (MPLC) with squamous or small cell lung cancer (17), or mixed lung cancer comprising mucoepidermoid carcinoma and conventional adenocarcinoma (18). However, to our knowledge, pulmonary mucoepidermoid carcinoma as a second primary cancer with a different origin from other tissues and organs has not been reported.
In conclusion, 18F-FDG PET/CT has an important value in the follow-up of indeterminate findings for patients with a tumor history. Moreover, dynamic quantification parameters such as Ki may be additionally helpful in identifying malignancies in some equivocal situations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SH: Conceptualization, Writing – original draft, Data curation. YZ: Data curation, Visualization, Writing – original draft. XW: Data curation, Funding acquisition, Writing – review & editing. YYL: Data curation, Validation, Writing – review & editing. YL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen (E010322003, SZ2020MS008), Shenzhen Clinical Research Center for Cancer and Shenzhen High-level Hospital Construction Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. (2017) 2:e000172. doi: 10.1136/esmoopen-2017-000172
2. Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary Malignancies with PET/CT. J Nucl Med: Off Publication Soc Nucl Med. (2005) 46:752–7.
3. Rahmim A, Lodge MA, Karakatsanis NA, Panin VY, Zhou Y, McMillan A, et al. Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging. (2019) 46:501–18. doi: 10.1007/s00259-018-4153-6
4. Wumener X, Zhang Y, Zang Z, Du F, Ye X, Zhang M, et al. The value of dynamic FDG PET/CT in the differential diagnosis of lung cancer and predicting EGFR mutations. BMC Pulmonary Med. (2024) 24:227. doi: 10.1186/s12890-024-02997-9
5. O'Neill ID. t(11,19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinoma. Oral Oncol. (2009) 45:2–9. doi: 10.1016/j.oraloncology.2008.03.012
6. Wumener X, Zhang Y, Wang Z, Zhang M, Zang Z, Huang B, et al. Dynamic FDG-PET imaging for differentiating metastatic from non-metastatic lymph nodes of lung cancer. Front Oncol. (2022) 12:1005924. doi: 10.3389/fonc.2022.1005924
7. Shepard JO, Flores EJ, Abbott GF. Imaging of the trachea. Ann Cardiothorac Surg. (2018) 7:197–209. doi: 10.21037/acs.2018.03.09
8. Soerjomataram I, Coebergh JW. Epidemiology of multiple primary cancers. Methods Mol Biol (Clifton NJ). (2009) 471:85–105. doi: 10.1007/978-1-59745-416-2_5
9. Kalhor N, Moran CA. Pulmonary mucoepidermoid carcinoma: diagnosis and treatment. Expert Rev Respir Med. (2018) 12:249–55. doi: 10.1080/17476348.2018.1428563
10. Zhou X, Zhang M, Yan X, Zhong Y, Li S, Liu J, et al. Challenges in diagnosis of pulmonary mucoepidermoid carcinoma: A case report. Med (Baltimore). (2019) 98:e17684. doi: 10.1097/MD.0000000000017684
11. Jeong SY, Lee KS, Han J, Kim BT, Kim TS, Shim YM, et al. Integrated PET/CT of salivary gland type carcinoma of the lung in 12 patients. Am J Roentgenol. (2007) 189:1407–13. doi: 10.2214/AJR.07.2652
12. Jindal T, Kumar A, Kumar R, Dutta R, Meena M. Role of positron emission tomography-computed tomography in bronchial mucoepidermoid carcinomas: a case series and review of the literature. J Med Case Rep. (2010) 4:277. doi: 10.1186/1752-1947-4-277
13. Krishnamurthy A, Ramshankar V, Majhi U. Role of fluorine-18-fluorodeoxyglucose positron emission tomography-computed tomography in management of pulmonary mucoepidermoid carcinomas and review of literature. Indian J Nucl Med. (2016) 31:128–30. doi: 10.4103/0972-3919.178264
14. Pedro PI, Canario D, Lopes M, Argyropoulou D. Rare cause of lung atelectasis in a young woman. BMJ Case Rep. (2019) 12:e227969. doi: 10.1136/bcr-2018-227969
15. Jiang L, Huang Y, Huang S, Li Q, Wang H. 18F-FDG PET/CT imaging of tracheal mucoepidermoid carcinoma. Clin Nucl Med. (2017) 42:874–5. doi: 10.1097/RLU.0000000000001835
16. Park B, Kim HK, Choi YS, Kim J, Zo JI, Choi JY, et al. Prediction of pathologic grade and prognosis in mucoepidermoid carcinoma of the lung using (1)(8)F-FDG PET/CT. Korean J Radiol. (2015) 16:929–35. doi: 10.3348/kjr.2015.16.4.929
17. Kawakami M, Tsujii E, Tokitsu K, Nakao K, Orino T, Tachibana S, et al. A case of synchronous double cancer in the same pulmonary lobe. Kyobu Geka Japanese J Thorac Surg. (1994) 47:1105–9.
Keywords: bronchial mass, dynamic PET/CT, FDG, mucoepidermoid carcinoma, second primary cancer
Citation: Huang S, Zhang Y, Wumener X, Lei Y and Liang Y (2024) Case report: Dynamic 18F-FDG PET/CT display of a bronchial mass as a second primary cancer mimicking mediastinal lymph node in a gastric carcinoma survivor. Front. Oncol. 14:1447843. doi: 10.3389/fonc.2024.1447843
Received: 12 June 2024; Accepted: 02 September 2024;
Published: 26 September 2024.
Edited by:
Antonio D’Andrilli, Sapienza University of Rome, ItalyReviewed by:
Luigi Ventura, Sheffield Teaching Hospital NHS Foundation Trust, United KingdomChunyin Zhang, Southwest Medical University, China
Copyright © 2024 Huang, Zhang, Wumener, Lei and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Liang, bGlhbmd5aW5nXzQ3M0AxNjMuY29t
 Shengyun Huang
Shengyun Huang Yarong Zhang1
Yarong Zhang1 Xieraili Wumener
Xieraili Wumener Yuanyuan Lei
Yuanyuan Lei