- 1Department of Nuclear Medicine, Jiangxi Provincial People’s Hospital, the First Affiliated Hospital of Nanchang Medical College, Nanchang, China
- 2Clinical Laboratory, Jiangxi Provincial Children’s Hospital, Children's Hospital Affiliated to Nanchang Medical College, Nanchang, China
- 3Cardio-Thoracic Surgery, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
Pulmonary enteric adenocarcinoma (PEAC), an uncommon variant of lung cancer, presents significant diagnostic challenges due to its overlapping characteristics with colorectal adenocarcinomas. We present a case of a 55-year-old non-smoking female patient diagnosed with PEAC. The patient’s initial symptoms included fever, cough, and sputum production, with air space consolidation on CT, leading to an initial diagnosis of pneumonia. Sputum culture after admission showed no growth of bacteria and fungi. Anti-inflammatory therapy was not ideal. Subsequent bronchoscopy with endobronchial ultrasound and biopsy confirmed the diagnosis of PEAC. Gastroscopy and colonoscopy yielded negative results, and a PET/CT scan revealed an FDG-avid lesion in the right middle lobe, with no other significant hypermetabolic gastrointestinal lesions, thereby excluding an extrapulmonary primary gastrointestinal malignancy. The patient was ultimately staged as PEAC (T4N1M0, stage IIIb). She declined anti-tumor therapy and experienced clinical deterioration during follow-up. This case report expands the radiological spectrum of PEAC, adds to the limited literature, and emphasizes the role of 18F-FDG PET/CT in diagnosing such diseases. It also underscores the importance of a multidisciplinary approach in the management of PEAC.
Introduction
Pulmonary enteric adenocarcinoma (PEAC) represents a rare subtype of non-small cell lung cancer (1), accounting for approximately 0.6% of all primary pulmonary adenocarcinomas (2). It is characterized by adenocarcinomas that are primarily lung-based yet share morphological and immunohistochemical features with colorectal adenocarcinomas, belonging to the histological variants of invasive pulmonary adenocarcinoma. PEAC exhibits several morphological and immunohistochemical features common to both lung cancer and colorectal adenocarcinoma, posing significant challenges in differential diagnosis for clinical pathologists (3). In PEAC, the enteric differentiation component exceeds 50% (4). To confirm the diagnosis of PEAC, colonoscopy and esophagogastroduodenoscopy should be performed to exclude primary gastrointestinal malignancies (5). The diagnosis and differential diagnosis require a careful integration of clinical and pathological data, including endoscopic findings, morphological characteristics of the lesion, and immunohistochemical expression profiles. Additionally, serum carbohydrate antigen 199 (CA199) levels may elevate in PEAC patients.
The 18F-FDG PET/CT imaging modality is a whole-body examination and provides a comprehensive assessment of both anatomical structures and metabolic activities. It is highly sensitive in identifying tumors and provides enhanced precision for oncological staging (6). To date, very few PET/CT studies of PEAC have been conducted. Here, we present a case of PEAC from China and review the relevant literature, attempting to enhance the understanding of this disorder and highlighting the role of 18F-FDG PET/CT in the diagnostic workup of PEAC, particularly in excluding pulmonary metastatic colorectal adenocarcinoma.
Case description
On March 29, 2024, a 55-year-old non-smoking female patient was admitted to our hospital with a 1-month history of fever, cough, and sputum production. She denied abdominal pain, vomiting, constipation, change in stool color, or any change in bowel habits. Her initial presentation occurred in late February 2024. At a local hospital; she complained of fever, cough, and expectoration, devoid of chills or hemoptysis. A chest CT scan was performed, which revealed bilateral pneumonia. She was commenced on a 1-week course of piperacillin and tazobactam, resulting in initial symptomatic improvement. A follow-up CT scan on March 5 showed resolution of the inflammation in the left lung; however, the lesion in the right lung had notably progressed. Thereafter, she was administered moxifloxacin for an additional week. Despite this, she experienced a recurrence of symptoms, including fever, cough, and expectoration, which were more intense than her initial presentation on March 26. In light of the exacerbation, she was referred to our hospital for further investigation and management. The patient’s past medical and family histories were unremarkable. The physical examination revealed no significant abnormalities, except for the presence of dry and moist rales upon lung auscultation.
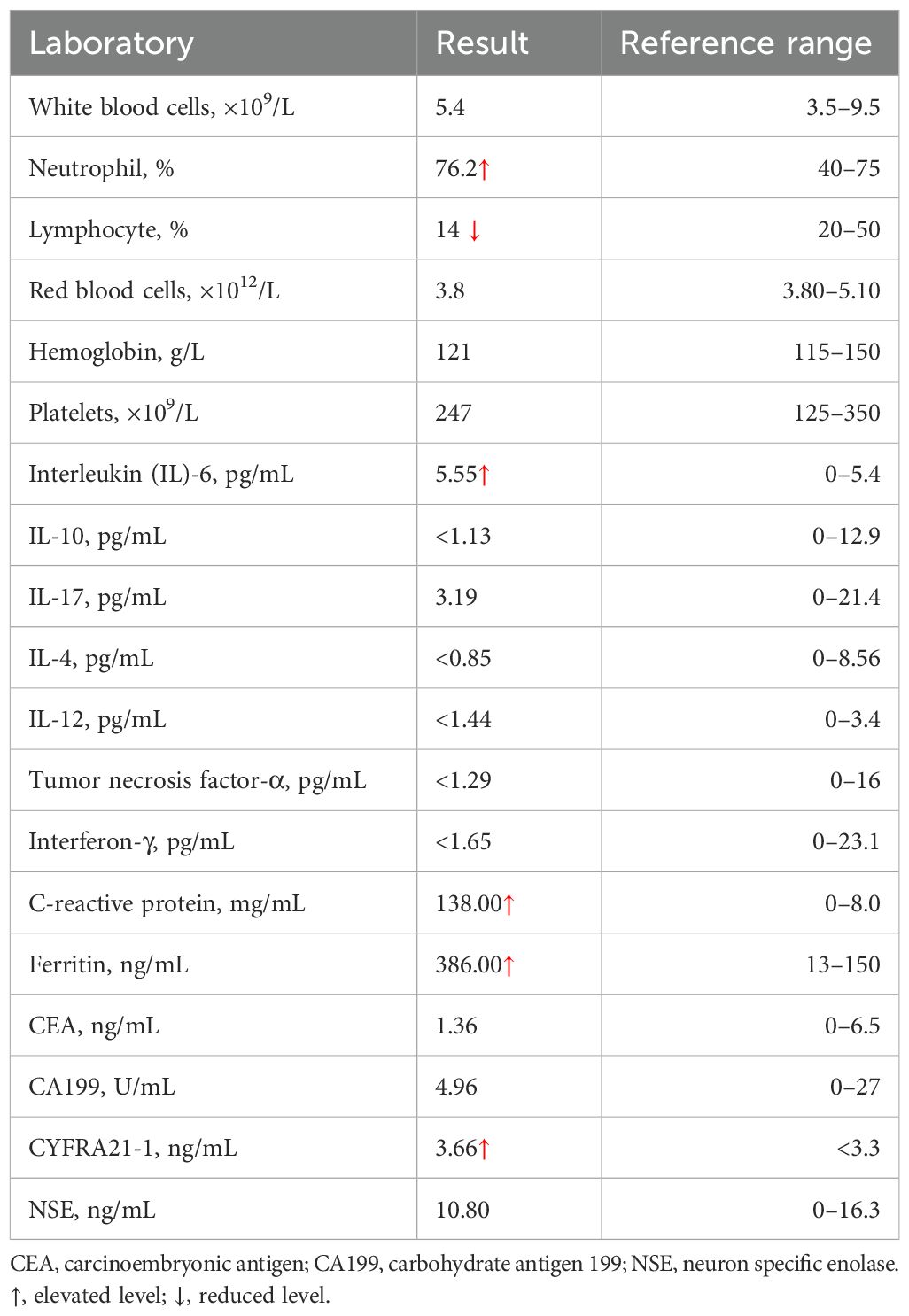
On admission, the patient’s vital signs were as follows: body temperature of 38.7°C, pulse rate of 91 beats per minute, respiratory rate of 20 breaths per minute, and blood pressure of 109/59 mmHg. Her weight was 57 kg. Laboratory investigations were conducted, and the findings are detailed in Table 1. Sputum culture after admission showed no growth of bacteria and fungi. A negative result was obtained for the TB interferon-gamma release assay (IGRA), and her liver and renal function tests were within normal ranges. A chest CT scan performed on March 31 revealed atelectasis of the right middle lobe with a honeycomb network appearance (Figure 1). On April 1, a bronchoscopy was conducted, which identified extrinsic compression of the right middle lobe bronchus. Furthermore, endobronchial ultrasound (EBUS) revealed a hypoechoic mass within the lateral segment of the right middle lobe, which was subsequently biopsied. Histopathological examination revealed that the lung parenchyma was replaced by adenoid tumors characterized by adenoid structures lined with columnar epithelium in over 50% of the specimens. Immunohistochemical staining showed CK (+), CK20 (+), CK7 (+), Ki-67 (+, approximately 40%), CDX-2 (−), Napsin A (−), P40 (−), Syn (−), and TTF-1 (−), suggesting a diagnosis of pulmonary enteric-type adenocarcinoma (PEAC) (Figure 2).
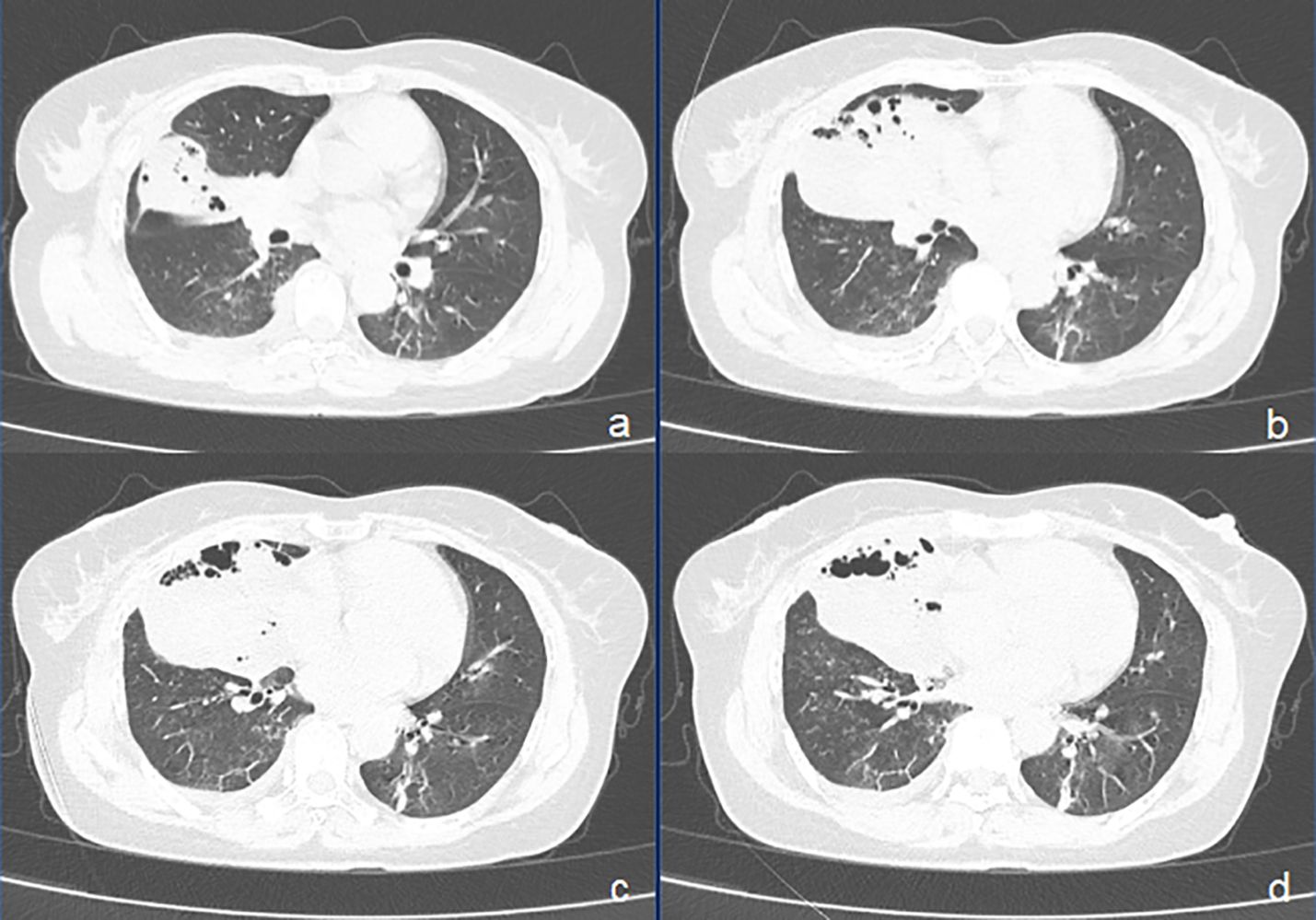
Figure 1. CT images of pulmonary enteric adenocarcinoma (PEAC) showing right middle lobe atelectasis with honeycomb and a small amount of fluid in the right pleural cavity. (A–D) axial CT (lung window).
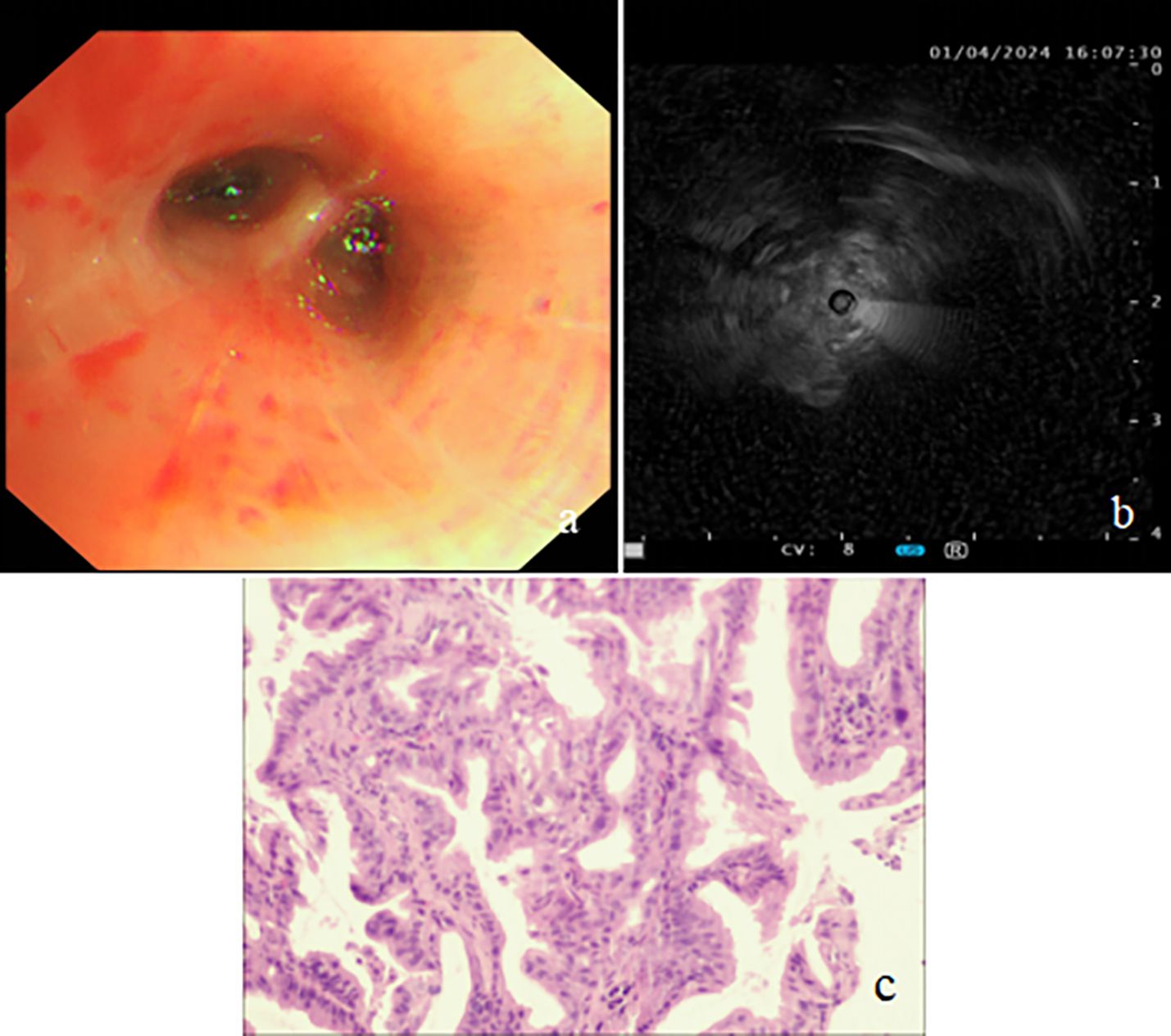
Figure 2. Bronchoscopy findings and biopsy pathology image. (A) Bronchoscopy revealing extrinsic compression of the right middle lobe bronchus. (B) Endobronchial ultrasound (EBUS) showing a hypoechoic mass in the lateral segment of the right middle lobe. (C) Biopsies showing the tumor with glandular architecture (H&E stain, power of magnification ×200).
To rule out the potential for lung metastasis from a gastrointestinal primary tumor, the patient underwent a comprehensive evaluation. On April 3, both gastroscopy and colonoscopy were performed, which did not detect any significant pathological findings. Subsequently, on April 4, an 18F-FDG PET/CT scan was conducted for further investigation, which identified an ill-defined FDG-avid lesion, measuring approximately 77 mm × 43 mm, with a maximum standardized uptake value (SUVmax) of 8.4, within the consolidative collapse of the right middle lobe. Additionally, an enlarged lymph node was identified in the right hilar region, measuring approximately 11 mm × 17 mm, with an SUVmax of 3.5. No other notable hypermetabolic lesions were observed within the gastrointestinal tract, suggesting that the primary tumor was located in the lung (Figure 3).
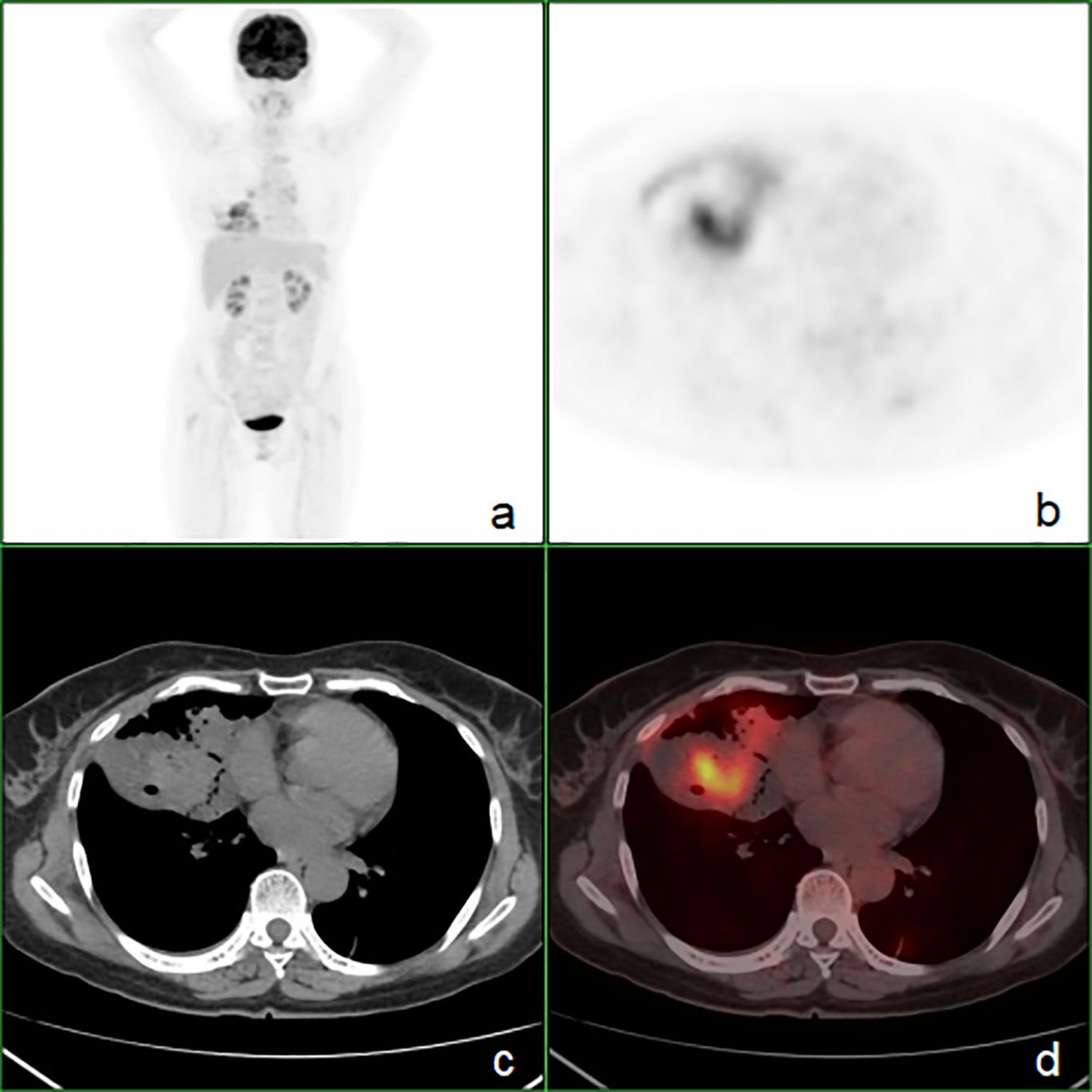
Figure 3. The whole-body 18F-FDG PET/CT showing heterogeneous FDG uptake (SUVmax = 8.4) within the right middle lobe and no evidence of hypermetabolic foci in the gastrointestinal tract. (A) PET maximum intensity projection map, (B) axial PET image, (C) axial CT image, (D) axial fusion image.
The patient’s final diagnosis was PEAC (T4N1M0, stage IIIb). Despite the recommendation for anti-tumor therapy, she declined treatment and was discharged on April 6 following the administration of symptomatic care only.
At the 2-month follow-up phone call after discharge, the patient reported a marked worsening of cough and sputum production, along with a 4-kg weight loss, and had not initiated any anti-tumor therapy. The timeline of clinical events from symptom onset to the last follow-up appointment is shown in Supplementary Figure S1.
Discussion
PEAC, first described by Tsao and Fraser in 1991 (7), is a rare primary lung adenocarcinoma with colorectal cancer-like histological and immunohistochemical features (3, 8–10). PEAC is a relatively rare condition, with fewer than 500 cases reported in the English literature, primarily as case reports or small series (1, 3, 4, 8–15). Diagnosis of PEAC necessitates distinguishing it from gastrointestinal adenocarcinoma metastasis (3, 9, 12, 16), as treatment approaches differ significantly. This case report details the clinical course, diagnostic workup, and follow-up of a PEAC patient and aims to increase understanding of the disorder.
PEAC predominantly affects the elderly, with a male predominance (4, 11, 17–19). The relationship between smoking and PEAC incidence remains controversial (11, 19, 20), warranting further study. Clinically, PEAC presents similarly to other primary lung adenocarcinomas, with respiratory symptoms such as cough, sputum, chest tightness, shortness of breath, and the absence of gastrointestinal symptoms (20). Our patient, a 55-year-old female non-smoker, initially presented with inflammatory symptoms. However, the progression of the right lung lesion after anti-inflammatory treatment raised suspicions of an alternative diagnosis.
Imaging of PEAC typically presents as nodules or masses (11, 18, 20), and some cases exhibit imaging findings similar to pneumonia (20). Bian et al. (20) analyzed chest CT data of 13 PEAC patients and 27 lung metastasis patients from colorectal cancer, finding no significant differences in lesion characteristics. The location type of PEAC predominantly exhibits characteristics of a peripheral nature. However, patients with PEAC did not present any ground-glass opacity (11). In our case, PEAC presented as atelectasis with honeycombing changes, a previously unreported finding, demonstrating the diversity of imaging findings.
In our case, bronchoscopy and EBUS play a crucial role in the visualization and biopsy of PEAC lesions, and biopsy via EBUS showed histopathological features of columnar epithelial adenomatous tumors with immunohistochemical characteristics consistent with PEAC. For the diagnosis and differentiation of PEAC, CK7, CDX2, CK20, and TTF-1 are important immunohistochemical markers (11, 14, 17, 18, 21, 22). However, it is crucial to highlight that the differentiation of PEAC from metastatic colorectal cancer should be based on comprehensive clinicopathological correlations, rather than solely on morphological, immunohistochemical, or molecular characteristics (3, 23). Our case’s immunohistochemical staining showed CK(+), CK20 (+), CK7(+), Ki-67(+, approximately 40%), CDX-2 (−), Napsin A (−), and TTF-1 (−).
Exclusion of primary gastrointestinal malignancies is essential, with gastroscopy and colonoscopy serving as vital diagnostic tools (3, 9, 10). PET/CT imaging offers a systemic and highly sensitive approach to tumor detection. It is pivotal in the diagnosis of lung malignancies. Our case, in conjunction with limited prior PEAC cases (15, 24–29) undergoing PET/CT, illustrates that PET/CT is instrumental in identifying malignant tumors, providing accurate staging, and effectively also ruling out primary gastrointestinal adenocarcinoma. This is confirmed through the integration of gastrointestinal endoscopy, including our case. Current data (15, 24, 25, 27, 29) indicate that the SUVmax for PEAC ranges from 2.6 to 12.7, exhibiting considerable variability. To rule out primary colorectal cancer on PET/CT, it is also necessary to differentiate it from physiological bowel FDG uptake. Physiological uptake typically appears diffuse or segmental, whereas the uptake of colorectal cancer is usually focal.
The non-specific clinical and radiological presentations of PEAC necessitate a high index of suspicion to avoid misdiagnosis. In our case, the lack of elevated tumor markers carcinoembryonic antigen (CEA) and CA199, along with symptoms and imaging, initially led to a misattribution to inflammation (11, 16). In fact, pneumonia might coexist with a tumor due to bronchial stenosis causing poor drainage, and the resolution of the left lung shadow on the CT scan after anti-inflammatory treatment also suggests concurrent inflammation and tumor presence.
The final diagnosis of PEAC (T4N1M0, stage IIIb) in this patient was based on tumor size, lymph node involvement, and the absence of distant metastasis. Surgical intervention is preferred for early-stage PEAC, while chemotherapy is the mainstay for advanced stages (30). Despite the generally poor prognosis, some patients achieve remission with radiotherapy and chemotherapy (25). Our patient was recommended anti-tumor therapy, but she declined, just opting for symptomatic care. Follow-up revealed clinical deterioration, highlighting the aggressive nature of PEAC.
In summary, this case report expands the radiological spectrum of PEAC, contributes to the limited literature, underscores the role of 18F-FDG PET/CT in diagnosis, and emphasizes the need for a multi-technique approach, providing valuable insights for future clinical management of this disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Z-HL: Conceptualization, Methodology, Project administration, Writing – review & editing. X-YL: Formal analysis, Writing – original draft. X-QL: Investigation, Writing – review & editing. A-FJ: Resources, Writing – review & editing. Q-YZ: Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1447453/full#supplementary-material
References
1. Liu Y, Lu T, Yuan M, Chen R, Lu J, Wang H, et al. Genomic and transcriptomic insights into the precision treatment of pulmonary enteric adenocarcinoma. Lung Cancer. (2023) 179:107169. doi: 10.1016/j.lungcan.2023.03.005
2. Xu X, Chen D, Wu X, Wang Q. A pulmonary enteric adenocarcinoma patient harboring a rare EGFR exon 19 P753S mutation: Case report and review. Front Oncol. (2022) 12:988625. doi: 10.3389/fonc.2022.988625
3. Jurmeister P, Schöler A, Arnold A, Klauschen F, Lenze D, Hummel M, et al. DNA methylation profiling reliably distinguishes pulmonary enteric adenocarcinoma from metastatic colorectal cancer. Mod Pathol. (2019) 32:855–65. doi: 10.1038/s41379-019-0207-y
4. Li H, Cao W. Pulmonary enteric adenocarcinoma: a literature review. J Thorac Dis. (2020) 12:3217–26. doi: 10.21037/jtd-19-4171
5. Zhang J, Xiang C, Han Y, Teng H, Li X, Shao J, et al. Differential diagnosis of pulmonary enteric adenocarcinoma and metastatic colorectal carcinoma with the assistance of next-generation sequencing and immunohistochemistry. J Cancer Res Clin Oncol. (2019) 145:269–79. doi: 10.1007/s00432-018-2788-0
6. Mariën H, Derveaux E, Vanhove K, Adriaensens P, Thomeer M, Mesotten L. Changes in metabolism as a diagnostic tool for lung cancer: systematic review. Metabolites. (2022) 12:545. doi: 10.3390/metabo12060545
7. Tsao MS, Fraser RS. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer. (1991) 68:1754–7. doi: 10.1002/1097-0142(19911015)68:8<1754::AID-CNCR2820680818>3.0.CO;2-E
8. Jurmeister P, Vollbrecht C, Behnke A, Frost N, Arnold A, Treue D, et al. Next generation sequencing of lung adenocarcinoma subtypes with intestinal differentiation reveals distinct molecular signatures associated with histomorphology and therapeutic options. Lung Cancer. (2019) 138:43–51. doi: 10.1016/j.lungcan.2019.10.005
9. Zuo Y, Zhong J, Bai H, Xu B, Wang Z, Li W, et al. Genomic and epigenomic profiles distinguish pulmonary enteric adenocarcinoma from lung metastatic colorectal cancer. EBioMedicine. (2022) 82:104165. doi: 10.1016/j.ebiom.2022.104165
10. Xie M, Chen D, Li Y, Liu X, Kuang D, Li X. Genetic mutation profiles and immune microenvironment analysis of pulmonary enteric adenocarcinoma. Diagn Pathol. (2022) 17:30. doi: 10.1186/s13000-022-01206-7
11. Zhao L, Huang S, Liu J, Zhao J, Li Q, Wang HQ. Clinicopathological, radiographic, and oncogenic features of primary pulmonary enteric adenocarcinoma in comparison with invasive adenocarcinoma in resection specimens. Med (Baltimore). (2017) 96:e8153. doi: 10.1097/MD.0000000000008153
12. Cui Y, Liang J, Kang X, Liu M, Zhang Q, Zhang H. Gefitinib, an effective treatment option for patients with pulmonary enteric adenocarcinoma harboring compound EGFR L858R and A871G mutation. Invest New Drugs. (2023) 41:787–90. doi: 10.1007/s10637-023-01401-3
13. Okada F, Takeda M, Fujii T, Uchiyama T, Sasaki S, Matsuoka M, et al. Clinicopathological and genetic analyses of pulmonary enteric adenocarcinoma. J Clin Pathol. (2024) 77:111–5. doi: 10.1136/jcp-2022-208583
14. Tu LF, Sheng LY, Zhou JY, Wang XF, Wang YH, Shen Q, et al. Diagnosis and treatment of primary pulmonary enteric adenocarcinoma: Report of Six cases. World J Clin Cases. (2021) 9:9236–43. doi: 10.12998/wjcc.v9.i30.9236
15. Gu L, Wang XZ, Wen W, Lin J, Chen XF, Lai GX, et al. Clinical analysis of 23 patients pathologically diagnosed with primary and secondary pulmonary enteric adenocarcinoma. Chin Med J (Engl). (2019) 132:1368–9. doi: 10.1097/CM9.0000000000000266
16. Smyth RJ, Thomas V, Fay J, Ryan R, Nicholson S, Morgan RK, et al. Tumour genome characterization of a rare case of pulmonary enteric adenocarcinoma and prior colon adenocarcinoma. J Pers Med. (2021) 11:768. doi: 10.3390/jpm11080768
17. Chen M, Liu P, Yan F, Xu S, Jiang Q, Pan J, et al. Distinctive features of immunostaining and mutational load in primary pulmonary enteric adenocarcinoma: implications for differential diagnosis and immunotherapy. J Transl Med. (2018) 16:81. doi: 10.1186/s12967-018-1449-z
18. Matsushima J, Yazawa T, Suzuki M, Takahashi Y, Ota S, Nakajima T, et al. Clinicopathological, immunohistochemical, and mutational analyses of pulmonary enteric adenocarcinoma: usefulness of SATB2 and β-catenin immunostaining for differentiation from metastatic colorectal carcinoma. Hum Pathol. (2017) 64:179–85. doi: 10.1016/j.humpath.2017.04.006
19. Palmirotta R, Lovero D, D'Oronzo S, Todisco A, Internò V, Mele F, et al. Pulmonary enteric adenocarcinoma: an overview. Expert Rev Mol Med. (2020) 22:e1. doi: 10.1017/erm.2020.2
20. Bian T, Zhao J, Feng J, Zhang Q, Qian L, Liu J, et al. Combination of cadherin-17 and SATB homeobox 2 serves as potential optimal makers for the differential diagnosis of pulmonary enteric adenocarcinoma and metastatic colorectal adenocarcinoma. Oncotarget. (2017) 8:63442–52. doi: 10.18632/oncotarget.18828
21. Nottegar A, Tabbò F, Luchini C, Brunelli M, Bria E, Veronese N, et al. Pulmonary adenocarcinoma with enteric differentiation: immunohistochemistry and molecular morphology. Appl Immunohistochem Mol Morphol. (2018) 26:383–7. doi: 10.1097/PAI.0000000000000440
22. Inamura K, Satoh Y, Okumura S, Nakagawa K, Tsuchiya E, Fukayama M, et al. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol. (2005) 29:660–5. doi: 10.1097/01.pas.0000160438.00652.8b
23. Wang Q, Zhang L, Li H, Liu L, Sun X, Liu H. Clinical features and prognosis of pulmonary enteric adenocarcinoma: A retrospective study in China and the SEER database. Front Oncol. (2023) 13:1099117. doi: 10.3389/fonc.2023.1099117
24. Xie L, Liu Z, Chen Y. Primary pulmonary enteric adenocarcinoma: rare imaging findings. Curr Med Imaging. (2024) 20:1–4. doi: 10.2174/0115734056272178231129103111
25. Nemoto Y, Kuroda K, Oyama R, Mori M, Shimajiri S, Tanaka F. Case report: Pathological complete response of pregnancy associated pulmonary enteric adenocarcinoma to chemoradiotherapy. Front Oncol. (2024) 14:1290757. doi: 10.3389/fonc.2024.1290757
26. Hu CH, Shi S, Dong W, Xiao L, Zang H, Wu F. Hyperprogressive disease after immunotherapy: A case report of pulmonary enteric adenocarcinoma. Front Oncol. (2022) 12:799549. doi: 10.3389/fonc.2022.799549
27. Handa Y, Kai Y, Ikeda T, Mukaida H, Egawa H, Kaneko M. Pulmonary enteric adenocarcinoma. Gen Thorac Cardiovasc Surg. (2016) 64:749–51. doi: 10.1007/s11748-015-0569-0
28. Hu Y, Wu D, Tian C, Wei Q, Bian Y. Diagnosis of multiple primary intestinal-type adenocarcinoma in the lung by 18F-FDG PET/CT. Clin Nucl Med. (2018) 43:693–4. doi: 10.1097/RLU.0000000000002171
29. Sun WW, Xu ZH, Wang CF, Wu F, Cao JM, Cui PJ, et al. Pulmonary enteric adenocarcinoma with pancreatic metastasis: A case report. Oncol Lett. (2017) 13:4651–6. doi: 10.3892/ol.2017.6060
Keywords: pulmonary enteric adenocarcinoma, 18F-fluordeoxyglucose, positron emission tomography/computed tomography, diagnosis, case report
Citation: Luo Z-H, Luo X-Y, Luo X-Q, Jin A-F and Zeng Q-Y (2024) Case report: 18F-FDG PET/CT in pulmonary enteric adenocarcinoma. Front. Oncol. 14:1447453. doi: 10.3389/fonc.2024.1447453
Received: 11 June 2024; Accepted: 23 September 2024;
Published: 14 October 2024.
Edited by:
Wanhe Wang, Northwestern Polytechnical University, ChinaReviewed by:
Julian Scherer, University Hospital Zürich, SwitzerlandZhehao Lyu, First Affiliated Hospital of Harbin Medical University, China
Copyright © 2024 Luo, Luo, Luo, Jin and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe-Huang Luo, bHpoNjM5MkBzaW5hLmNvbQ==
†ORCID: Zhe-Huang Luo, orcid.org/0000-0002-4630-9279
 Zhe-Huang Luo
Zhe-Huang Luo Xiao-Yan Luo2
Xiao-Yan Luo2 Ai-Fang Jin
Ai-Fang Jin