
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 29 July 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1446517
The management of chronic myeloid leukemia in the chronic phase (CML-CP) has witnessed significant advancements since the identification of a common chromosomal translocation anomaly involving chromosomes 9 and 22, which results in the formation of the Philadelphia chromosome driven by the BCR-ABL1 fusion protein. This discovery paved the way for the development of tyrosine kinase inhibitors (TKIs) that target the adenosine triphosphate (ATP) binding site of ABL1 through the BCR-ABL-1 fusion protein. Following the approval of Imatinib by the Food and Drug Administration (FDA) as the first TKI for CML treatment in 2001, the median overall survival (OS) for chronic phase CML (CML-CP) has significantly improved, approaching that of the general population. However, achieving this milestone crucially depends on reaching certain treatment response milestones. Since the introduction of imatinib, five additional TKIs have been approved for CML-CP treatment. Despite the availability of these treatments, many patients may experience treatment failure and require multiple lines of therapy due to factors such as the emergence of resistance, such as mutations in the ATP binding site of ABL, or intolerance to therapy. This review will primarily focus on exploring treatment options for patients who fail second-generation TKI therapy due to true resistance.
The primary objective of treating chronic phase chronic myeloid leukemia (CML-CP) is to prevent progression to more aggressive accelerated or blast phase CML, regardless of the tyrosine kinase inhibitor (TKI) line, enabling patients to achieve a life expectancy similar to the general population (1). Since the approval of the first TKI, imatinib, in 2000, the 10-year overall survival (OS) trajectory for CML-CP has increased from 20% to 80% (2). Presently, six TKIs are approved for CP-CML treatment: first-generation TKI imatinib; second-generation (2G) TKIs nilotinib, dasatinib, and bosutinib; and third-generation (3G) TKIs ponatinib and asciminib. For the majority of CP-CML patients, imatinib is recommended as the first-line (1L) therapy for long-term disease control (3). Imatinib is generally associated with fewer cardiovascular and arterio-occlusive events compared to 2G TKIs (4–6). However, due to various reasons, including disease-related factors or the pursuit of a higher and faster treatment-free remission (TFR), some patients may opt for a second-generation TKI as 1L treatment. Nevertheless, there is currently no evidence indicating a survival advantage of second-generation TKIs over imatinib (7–9).
In major multicenter, phase 3 clinical trials comparing imatinib to 2G TKIs in newly diagnosed CML-CP, such as dasatinib (DASISION), nilotinib (ENESTnd), and bosutinib (BFORE), higher rates of complete cytogenetic response (CCyR) and major molecular response (MMR) were observed with 2G TKIs in the 1L setting (4, 5, 10). Dasatinib demonstrated CCyR and MMR rates at 12 months of 77% and 46%, respectively, versus 66% (P=0.007) and 28% (P=0.0001) with imatinib. Nilotinib 400 mg showed a confirmed MMR rate at 12 months of 43% versus 22% (P=0.0001) with imatinib. Bosutinib exhibited CCyR and MMR rates at 12 months of 77% and 47%, respectively, versus 66% (P=0.0075) and 37% (P=0.0200) with imatinib. Cumulative 5-year MR4.5 rates were as follows: dasatinib 42% versus imatinib 33% (P=0.0251); nilotinib 52% versus imatinib 31% (P=0.0001); bosutinib 47.4% versus 36.6% (4–6).
Treatment failure may result from either primary resistance, defined as the inability to achieve target molecular responses within the specified duration, or secondary resistance, characterized by the loss of prior response. Intolerance is defined as recurrent grade ≥ 3 hematological toxicity or ≥ 2 nonhematological toxicity requiring discontinuation despite dose reduction (4). Discontinuation rates due to adverse events (AEs) were reported as follows: IRIS (7%), DASISION (16%), ENESTnd (12%) (nilotinib 300mg twice daily), BFORE (25%), PACE (21%), and ASCEMBL (5.8%) (4–6, 11, 12). Each TKI has unique toxicity profiles, so exercising caution when selecting an appropriate TKI can improve compliance and mitigate side effects.
After genuine resistance to 2G TKIs, a more potent therapy is needed. The choice should be based on disease-specific factors such as mutational profile, cytogenetics, risk profile, and adverse events of specific and prior TKI therapy. Current recommendations include switching to another 2G TKI or a 3G TKI, with plans for early allogeneic stem cell transplantation or enrollment in a clinical trial if treatment milestones or deep molecular responses (DMRs) are not achieved or maintained (13–16). However, data on precise clinical guidance post-2G TKI failure, whether used as 1L or second line (2L), are limited. This review will provide insights into clinical evidence and guidance, including new therapeutics in clinical trials, following 2G TKI failure due to genuine resistance.
Resistance to therapy most commonly arises from either novel mutations in the BCR::ABL1 gene, such as mutations in the kinase domain or overexpression/amplification of BCR::ABL1, disrupting TKI binding. Mutations account for resistance in approximately one-third of resistant CP patients. Resistance can also occur via non-BCR::ABL1 mechanisms, including SRC kinases or increased P-glycoprotein efflux pump activity, clonal evolution, reduced levels of human organic cation transporter (hoct1) leading to decreased TKI influx, or increased levels of the multi-drug exporter of the ATP binding cassette (17–21). Genetic aberrations in ASXL1 were found to be significantly higher in TKI-resistant patients treated with imatinib, raising concerns about possible preexisting ASXL1 mutations in the BCR::ABL1-positive leukemic clone impacting the clinical response to imatinib. However, further studies are needed to validate this correlation due to the limited sample size (22).
Mutations at diagnosis are rare but can emerge in patients due to noncompliance and may develop resistance to TKI therapy (23–27), or after multiple sequential TKI therapies, associated with decreased response and worse overall survival (18, 28). Mutations usually involve acquired point mutations in the BCR::ABL kinase domain (18, 28). Whole-genome sequencing with the identification of mutated genes such as ASXL1 and TP53 in CP-CML may hold prognostic and predictive significance, requiring further investigation in clinical management (29).
Sequential therapies with TKIs increase the vulnerability to the emergence of compound mutations, with two paired mutations occurring in 76% of cases, and triple (10.6%) and quadruple (1.5%) mutations within the same BCR::ABL1 allele. Unfortunately, these are usually insensitive even to third-generation TKIs (30–32). Ponatinib, a high-affinity pan BCR-ABL1 inhibitor, can suppress all single mutants in the BCR::ABL1 domain, including T315I. However, the emergence of compound mutations in a BCR::ABL1 allele, especially those involving T315I (e.g., Y253H/T315I, E2455V/T315I), may confer ponatinib resistance, even at a high dose of 45mg once a day (31, 33, 34). A clinical consideration is the relevant combination of asciminib and ponatinib, which appears effective in overcoming compound mutations involving T315I and reducing ponatinib toxicity (34).
Failure can be categorized as either true resistance or intolerance. However, the focus of this paper will be on true resistance to 2G TKIs. During therapy for CML-CP, there are specific recommendations regarding achieving target molecular responses at different time points (3, 6, 12 months) by measuring BCR::ABL transcript levels using real-time reverse transcriptase polymerase chain reaction (RT-PCR), as outlined in international standards (IS) (35, 36).
The 2013 European Leukemia Net (ELN) definition had different criteria for failure to first- and second-line TKIs, with less stringent instructions after failing second-line therapy. However, the 2020 ELN definition considers the presence of a mutation and failure to achieve a BCR::ABL1IS ≤1% or CCyR at 12 months as treatment failure, encompassing those receiving second-line TKIs. The ELN 2020 criteria are summarized in Table 1 (37).
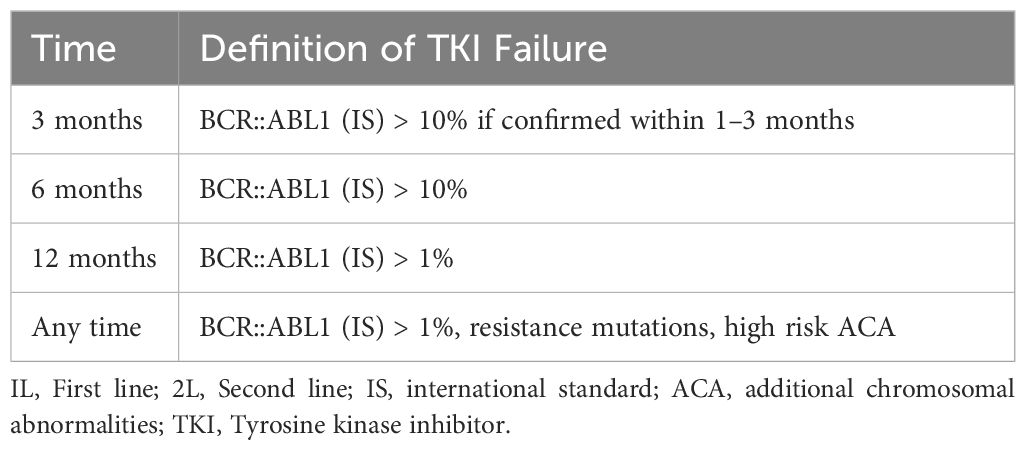
Table 1 ELN 2020 definition of failure to 1L and 2L treatment (37).
As generally acknowledged, second-generation TKIs achieve faster rates of CCyR at early time points compared to imatinib. Therefore, applying ELN 2020 criteria to the use of second-generation TKIs as initial therapy in CML-CP may not be optimal. Previously, studies by Jabbour et al. and more recently by Sasaki et al. have suggested that patients on 2G TKIs as frontline therapy had worse survival outcomes if an earlier switch to ponatinib or a novel TKI was not initiated when a 3-month BCR::ABL ≤10% and 6–12 month BCR::ABL1IS ≤1% were not achieved (38, 39). These guidelines establish treatment change targets to mitigate the risk of disease progression.
Treatment failure may result from either primary resistance, defined as the inability to achieve target molecular responses within the allocated duration (Table 1), or secondary resistance, characterized by the loss of prior response. The loss of CHR or CCyR necessitates a therapy switch, but the loss of MMR within the context of sustained CCyR allows for less precise interpretation (21, 40).
While imatinib is considered the safest option (41), it does not effectively inhibit several BCR::ABL mutations (42), with the exception of the gatekeeper mutation T315I, which is sensitive to ponatinib and asciminib (19, 21). By five years, 30–55% of patients treated with 2G TKIs achieve a 4.5 log reduction (MR4.5, BCR::ABL1IS <0.0032% IS), compared to approximately 30% treated with imatinib (4, 5). Although 2G and 3G TKIs have advantages over imatinib in achieving a faster and deeper response, there is currently no data confirming higher rates of cure (15, 37, 42).
Approximately 50% of patients with CML-CP treated with imatinib will switch therapy within five years, compared to 30–40% when treated with frontline 2G TKI. Among these, nearly 15%-25% change due to true resistance to imatinib associated with the T315I mutation, while only 5–7% are due to intolerance (4, 5, 43). At five years, the rates of resistance for nilotinib (ENESTend), dasatinib (DASISION), and bosutinib (BFORE) as first-line therapies are 23%, 26%, and 5.6%, respectively (4, 5, 44–46). The outcomes of 2G TKI as first-line therapy are outlined in Table 2.
A minority of patients are resistant to second-generation TKIs in the first-line setting and represent a population with a poor prognosis requiring a switch to alternative therapy. Failing a 2G TKI in the first-line setting is adverse compared to failing it in the second-line setting (21). Providers often choose 2G TKIs as first-line therapy as they provide higher rates of complete CHR, CCyR, and MMR and are more tolerable than high-dose imatinib (50–52).
Although there are no prospective trials and the patient numbers are low, the rates of CCyR after failure of imatinib and dasatinib were 27% and 20%, respectively, with nilotinib and bosutinib when used as third-line therapy, but were higher with sequential ponatinib at 54%. When patients failed imatinib and nilotinib, the CCyR rates were 25–26% with third-line therapy with dasatinib or bosutinib, whereas they were 67% when switched to ponatinib. Similar results were observed in patients who failed a 2G TKI and switched to an alternative 2G TKI, resulting in CCyR rates of 22–26%, compared to 60% with ponatinib, including T315I and non-T315I mutated patients (53, 54). Hence, an alternative 2G TKI has limited value after resistance to another 2G TKI in the absence of mutations, and few patients remained on treatment, indicating considerable failure across studies (55–60).
In a retrospective study of 62 patients with a median follow-up of 14 months, treated with fourth-line bosutinib post-failure to first-generation and remaining 2G TKIs, the probability of achieving and maintaining a CCyR and MMR was 25% and 24%, respectively. This number further decreased to 14% to achieve an MMR if patients were not in a CCyR at the time of starting bosutinib (61).
Recently, Kantarjian et al. demonstrated sustained high response and survival outcomes with ponatinib in patients resistant to 2G TKIs, regardless of T315I status, enrolled in the PACE (Ponatinib Philadelphia chromosome-positive acute lymphoblastic leukemia and CML Evaluation) and OPTIC (Optimizing Ponatinib Treatment in CP-CML) studies. The progression-free survival (PFS) and overall survival (OS) were 68% and 85% in the PACE study, and 80% and 91% in the OPTIC study (62).
Therefore, in the case of resistance to a 2G TKI due to a specific mutation, other 2G TKIs could be considered. For instance, following resistance to dasatinib, nilotinib, or bosutinib could be options depending on specific mutations, patient comorbidities, compliance, drug-drug interactions, and prior adverse effects. However, in accordance with ELN 2020 guidelines, an earlier use of ponatinib should be considered in all eligible patients without significant cardiovascular disease, as they are twice as likely to achieve a CCyR when treated with ponatinib than with another 2G TKI (62–65).
Multiple factors need to be assessed for resistance to a 2G TKI, but mutational analysis should be performed primarily, and discussions regarding finding a suitable donor should be initiated. Next-generation sequencing (NGS) is a more sensitive technique than Sanger sequencing and can detect low-level mutations and compound mutations. However, resistance to TKIs may not solely be due to low-level mutations and does not guide TKI selection unless it involves T315I, which necessitates ponatinib or a higher dose of asciminib (66). Detecting compound mutations, especially Y253H/T315I and E2455V/T315I, should prompt a search for a donor for allogeneic stem cell transplantation (30, 67, 68).
Cross tolerance is uncommon, and side effects usually change upon switching therapy, except for myelosuppression, which can persist across TKIs (69–71). However, patients who demonstrate failure to multiple TKIs and switch to an alternative 2G TKI may not experience high response rates, and if achieved, the response is not usually durable (53–55). Achieving a CCyR at three months is independently associated with event-free survival (EFS) and OS; hence, in patients who are not candidates for transplantation, maintaining a CCyR with different TKIs could be a therapeutic goal rather than aiming for MMR or a deeper response (72). However, following resistance to a first- or second-generation TKI, a reduced CCyR is observed.
The debate over the best strategy for initial therapy ranges from starting with a 2G TKI for a quicker and more profound response to switching to a 2G TKI after an inadequate response to imatinib. MMR is generally regarded as a surrogate for survival, and using 2G TKIs as initial therapy has not demonstrated improvements in overall survival (OS), progression-free survival (PFS), or treatment-free remission (TFR) (7, 62, 73–75).
While imatinib is commonly used worldwide as the first-line TKI, an increasing number of physicians are choosing 2G TKIs as first-line therapy to achieve a faster and deeper remission, with the aim of achieving TFR. However, TFR is only considered appropriate if patients achieve a MMR with sustained deep remission, typically defined as a 4-log decrease in BCR::ABL transcript levels from a standardized baseline, corresponding to a PCR <0.01% on the international scale (IS) (76, 77).
In a cohort of 113 patients, fewer than 10% achieved a CCyR at 3–6 months and eventually attained a major cytogenetic response (MCyR) at 12 months after receiving a 2G TKI (dasatinib/nilotinib) (78).
In patients with imatinib failure, the T315I mutation was reported in 10–27%, however, in the second-line setting, it was observed in 9–53% (43). Currently, FDA-approved treatment options for the T315I mutation include ponatinib, asciminib, omacetaxine (only approved in the USA), and allo-SCT (19, 33, 79). Olverembatinib (HQP1351) is in clinical trials and has shown activity against T315I.
Treatment with 2G TKIs after imatinib failure can result in high response rates and is a more effective option compared to higher doses of imatinib (800mg daily) in achieving higher CCyR and MMR (50, 51, 80, 81). Table 2 illustrates outcomes with second-line TKIs after imatinib resistance.
Ponatinib, a 3G TKI, is approved for patients with the T315I mutation or those resistant or intolerant to at least two TKIs in CML-CP (82, 83). In the 5-year follow-up of the pivotal PACE trial (Ponatinib Philadelphia chromosome-positive acute lymphoblastic leukemia and CML Evaluation), where a heavily pretreated cohort of patients resistant or intolerant to dasatinib or nilotinib, or with the T315I mutation, was enrolled, significant findings were observed (84, 85). Out of 267 evaluable patients with CML-CP and after a median follow-up of 56.8 months and median duration of treatment of 32.1 months, 144 (54%) achieved a CCyR, 108 (40%) achieved an MMR, and 64 (24%) achieved MR4.5. Of those who achieved an MCyR at 12 months and an MMR at any time, 82% and 59% of patients, respectively, maintained responses at 5 years. The median times to MCyR, CCyR, and MMR among those who achieved the response were 2.8, 2.9, and 5.5 months, respectively. The Kaplan-Meier estimated PFS and OS at 5 years were 53% and 73%, respectively (82).
To better determine the optimal dose of ponatinib while balancing potency and safety, the phase 2 open-label OPTIC study (Optimizing Ponatinib Treatment in CP-CML) was conducted (NCT02467270), where patients were randomized to receive either ponatinib at 45 mg daily (cohort A), 30 mg daily (cohort B), or 15 mg daily (cohort C). Preliminary analyses showed varying rates of achieving BCR::ABL1IS ≤ 1% (MMR) at 12 months across the cohorts (84, 85). At the recent 3-year follow-up update, MMR at 36 months was observed in different percentages across the cohorts (86). Adverse events occurred in varying percentages across the cohorts, with grade ≥3 adverse events reported in a smaller subset. Discontinuations due to treatment-emergent adverse events occurred in differing percentages across the cohorts, with a minimal number of deaths reported (12, 84, 86).
Recent data from the Belgian registry on 33 CML-CP patients previously treated with at least two TKIs showed promising results with ponatinib, albeit with some incidence of therapy discontinuation due to side effects (87). Similarly, real-life experience from Italy on treating patients with ponatinib demonstrated favorable responses but also highlighted therapy discontinuation rates due to resistance or intolerance (88).
In the US registry for CML-CP patients receiving ponatinib, various starting doses were observed, with preferences and recommendations outlined by ELN 2020 guidelines based on cardiovascular risk factors and resistance profiles (37, 89–91). The use of aspirin as primary thromboprophylaxis while on TKI remains uncertain (92, 93).
Asciminib, a pioneering selective allosteric BCR-ABL1 inhibitor, represents a distinct mechanism of action compared to currently available TKIs. FDA approval in October 2021 for third-line use or in patients harboring the T315I mutation underscores its significance (82, 83). By mimicking the myristoyl peptide, asciminib precisely targets the ABL Myristoyl Pocket (STAMP inhibitor), thereby restoring the inactive form of the kinase during the 9;22 translocation without affecting the ATP binding site. This unique mechanism grants asciminib activity against various ATP site resistance mutations, including the gatekeeper T315I mutation, catalytic site, and P-loop mutation (excluding F359) (19, 94, 95).
Asciminib’s efficacy was initially explored in the phase 1 CABL001X2101 trial, where it was assessed as monotherapy or in combination with nilotinib or dasatinib in CML-CP or CML-AP as third-line therapy or in the second line for T315I mutation. Results from the monotherapy cohort of 150 patients demonstrated promising outcomes, with significant proportions achieving MMRs at various time points (19). Subsequent updated results from 115 patients, after nearly four years of follow-up, revealed continued efficacy, with a considerable proportion maintaining MMRs and MR4s (96).
Common grade ≥3 adverse events included increased pancreatic enzymes, thrombocytopenia, hypertension, and neutropenia, while musculoskeletal pain, upper respiratory tract infection, and fatigue were frequent all-grade adverse events (96).
Furthermore, an expanded cohort of the phase 1 study evaluated asciminib in 52 T315I-positive CML-CP patients at an escalated dose of 200mg BID, showing notable MMR rates and a manageable safety profile (19, 97).
In a subgroup analysis of heavily pretreated patients, asciminib monotherapy demonstrated effectiveness in achieving MMRs, MR4s, and MR4.5s, with a favorable safety profile (96, 98).
These promising phase 1 results paved the way for the ASCEMBL study, a phase 3 trial comparing asciminib to bosutinib in CML-CP patients who had experienced lack of efficacy or intolerance to ≥2 prior TKIs. Results from this study highlighted the superior efficacy of asciminib over bosutinib, with higher MMR rates and fewer adverse events leading to treatment discontinuation (11, 99).
Real-world experience with asciminib across various countries has further supported its efficacy in the third-line setting, with significant proportions of patients achieving MMRs, even among those with prior ponatinib exposure (100–104).
Ponatinib and high-dose asciminib demonstrate comparable efficacy in the context of the T315I mutation, as observed in the OPTIC and ASCEMBL trials, where each TKI was assessed as a third-line option. Ponatinib stands out as the preferred choice for patients with the F359 mutation (which is resistant to asciminib) and those with BCR::ABL >10% IS. Notably, in the OPTIC study, patients in the 45mg ponatinib group with >10% BCR::ABL achieved a higher MMR rate at 3 years compared to those on asciminib at 24 weeks. However, the longer follow-up duration with OPTIC warrants consideration (11, 84, 85). Conversely, Asciminib boasts a favorable vascular or cardiovascular safety profile and may be favored over ponatinib in patients with T315I mutation and significant vascular disease. This preference might evolve with accumulating long-term asciminib data. Asciminib could also be preferred in patients intolerant to previous 1G or 2G TKIs but who have achieved molecular response, as demonstrated in the ASCEMBL trial (105).
Compound mutations pose resistance to both TKIs. Given the absence of a direct head-to-head clinical trial comparing the efficacy and safety of ponatinib and asciminib, the optimal therapy decision should be individualized based on patient comorbidities and clinical judgment.
Omacetaxine mepesuccinate is a protein translation inhibitor that does not target the BCR-ABL kinase domain but induces apoptosis in BCR::ABL1 positive cells by downregulating MCL-1. It is effective against the T315I mutation and has been FDA-approved and available in the USA since 2012 for patients resistant or intolerant to ≥ 2 TKIs, including those with the T315I mutation (106, 107).
In a study involving 76 heavily pretreated evaluable patients with CML-CP, omacetaxine was administered as induction therapy at 1.25mg/m² BID subcutaneously for up to 14 consecutive days every 28 days until hematological response, followed by maintenance at 1.25mg/m² BID for up to 7 days every 28-day cycle. The study reported that 53 patients (70%) achieved a CHR, 14 patients (18%) achieved an MCyR, and 7 patients (9%) achieved a CCyR. Additionally, a partial cytogenetic response (PCyR) was achieved in 3.9% of patients, and a MCyR was achieved in 18.4%. Among 40 patients who had received 2 prior TKIs, 31 (78%) achieved a CHR, 10 (25%) an MCyR, and 5 (13%) a CCyR. Among 36 patients who had received 3 prior TKIs, 22 (61%) achieved a CHR, 4 (11%) an MCyR, and 2 (6%) a CCyR. For the 22 patients with T315I mutations at baseline, 18 (82%) achieved a CHR, 5 (23%) an MCyR, and 3 (14%) a CCyR. In 35 patients with CML-AP, 14.3% achieved a major hematologic response, and 11.3% achieved a CHR with no evidence of leukemia in 2.9% (106, 108).
While omacetaxine is generally well-tolerated and suitable for long-term administration, it can cause prolonged and severe myelosuppression. Hematological side effects occurring in ≥ 5% of patients included bone marrow failure (11%), thrombocytopenia (11%), and febrile neutropenia (7%). Common non-hematological side effects were diarrhea (43%), nausea (38%), fatigue (30%), infections (26%), pyrexia (22%), headache (22%), asthenia (22%), and arthralgia (20%). The median OS for evaluable patients and for those who received more than three cycles were 40.3 and 49.3 months, respectively. The median PFS for evaluable patients and for those who received more than three cycles of therapy were 9.6 and 9.9 months, respectively. Due to its moderate efficacy, with an overall PFS of less than 10 months and an OS of under 4 years, omacetaxine is reserved for patients who are unable to use any of the available TKIs and those who are not candidates for allogenic stem cell transplantation (108).
Despite the wide array of therapeutic options for CML-CP, some patients remain resistant or intolerant to all available TKIs, creating a need for the development of new TKIs, particularly for third-line therapy and patients with T315I mutations.
One promising candidate is olverembatinib (HQP1351), a third-generation orally active BCR-ABL1 TKI effective in CML regardless of genotype. It completed phase I and II trials involving 101 patients (86 with CML-CP and 15 with CML-AP). The median time from diagnosis to the initiation of olverembatinib therapy was 6 years. Among the patients, 63% had the T315I mutation, and 83% had received ≥2 lines of TKI therapy. After a median follow-up of 30 months, the CHR, CCyR, and MMR rates for CML-CP patients were 97%, 62%, and 51%, respectively. For those with the T315I mutation, these rates were 100%, 84%, and 72%, respectively. At three years, the PFS was 96.3% for CML-CP patients and 71.4% for those with CML-AP. Dosing was administered every other day for 28 days, with cohorts receiving 1–60mg. Thrombocytopenia of any grade and grade 3/4 was reported in 75.2% and 49.5% of patients, respectively. The most common non-hematological side effects were grade 1/2 skin hyperpigmentation and hypertriglyceridemia (109–111).
In 2023, the American Society of Hematology updated the results of the phase 2 study, confirming olverembatinib’s efficacy in TKI-resistant CML-CP, including T315I, compared to the best available therapy (imatinib, nilotinib, and dasatinib) (112). Encouraging results led to olverembatinib’s approval in China in November 2021 for treating adult patients with TKI-resistant CML-CP and T315I-mutated CML-AP, and again in November 2023 for treating adult patients with CML-CP resistant to and/or intolerant of first- and second-generation TKIs (113).
Current treatment options in this specific setting are suboptimal. Prospective clinical trials of single-agent TKIs in the third-line setting are detailed in Table 3 and Figure 1. Additionally, several clinical trials are exploring combinations of TKIs with other agents targeting non-BCR-ABL-mediated CML leukemia stem cell (LSC) resistance. CML LSCs are not dependent on the kinase activity of BCR-ABL1 and are typically not eliminated by TKIs (117, 118). Figure 2 provides a schematic algorithm for managing CML-CP patients who fail 2G TKI therapy.
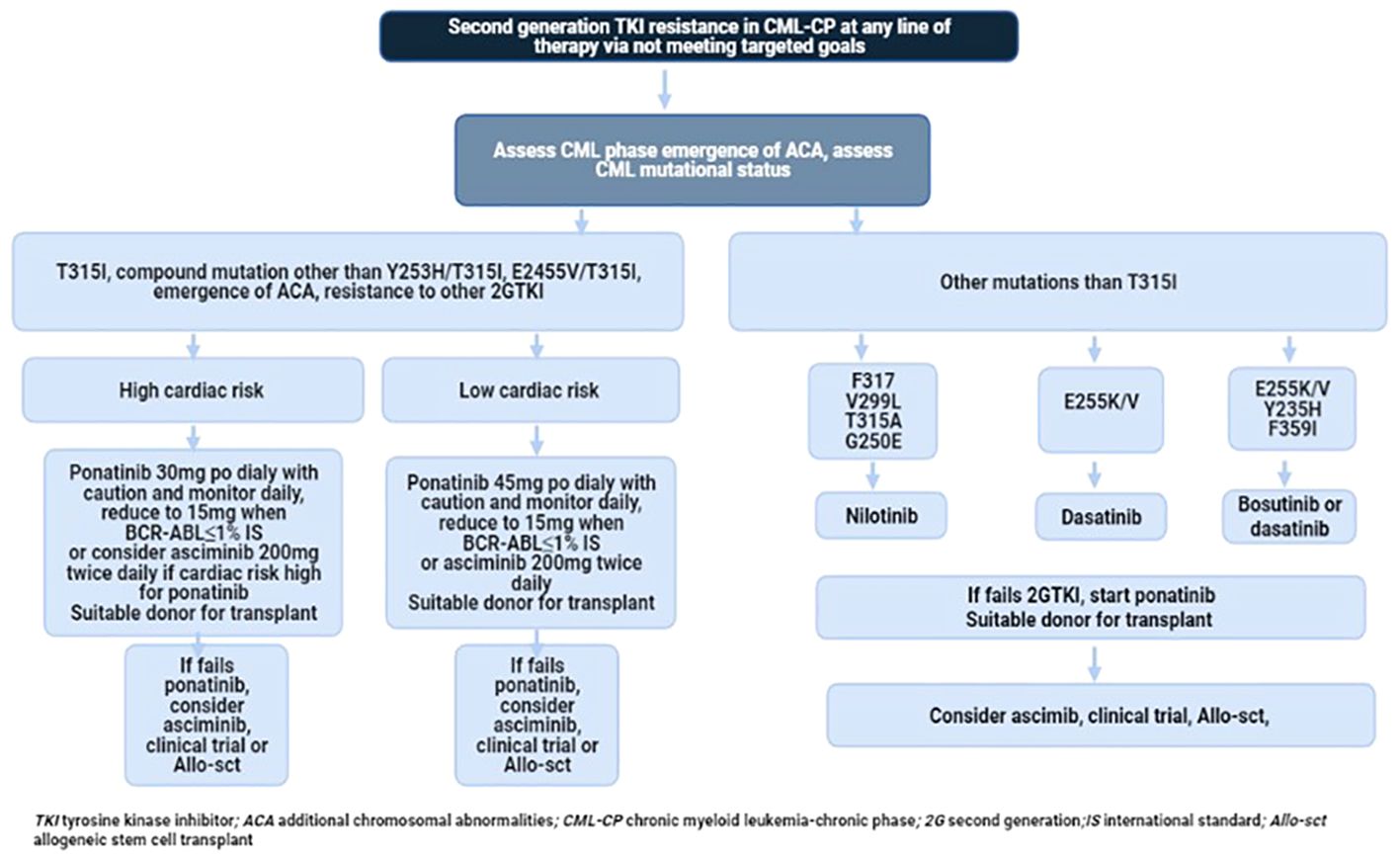
Figure 2 Recommended flow chart of management in patients with CP-CML who developed resistance to 2G TKI.
Attempting TFR in patients who exhibit resistance to 2G TKIs is not currently recommended, although it remains a widely desired goal that is premature to undertake at this stage.
Allo-SCT holds significant therapeutic implications as it represents a critical boundary between TKI treatment and transplantation. Before the era of TKIs like imatinib, allo-SCT was the only curative option and remains important today. Delaying transplant until all TKI options are exhausted is unfavorable, especially for patients with compound mutations or high risk of additional chromosomal aberrations (e.g., isochromosome 12, complex karyotype, trisomy 8, trisomy 19, monosomy 7, chromosome 3 abnormalities) (119).
Current ELN guidelines recommend considering allo-SCT for CML-CP patients resistant or intolerant to a second-line TKI or those with a T315I mutation (36, 37, 65). Further studies are needed to understand the implications of allo-SCT in the presence of somatic mutations such as ASXL1 or TP53 (22, 29).
The Center for International Blood and Marrow Research (CIBMTR) reported fewer than 300 transplants for CML-CP from 2014–2016. Compared to TKI therapy, allo-SCT achieves higher leukemia-free survival but is associated with nearly 20% transplant-related mortality at one year and decreased quality of life due to transplant complications like graft-versus-host disease. The five-year cumulative incidence of relapse (CIR) was 18%, with most relapses occurring in the first-year post-transplant, and the five-year overall survival (OS) was 68%. In 2020, CIBMTR reported fewer than 200 allotransplants for CML, mainly for accelerated and blast phases, while the European Bone Marrow Transplant (EBMT) registry reported nearly 400 transplants for CML, with almost half for CML-CP (120, 121). A recent Swedish study indicated three-year and five-year OS rates of approximately 85% and 96% for CML-CP, respectively, with a non-relapse mortality (NRM) of about 12% (122). Survival rates from various transplant registries are outlined in Table 4.
Advances in transplant techniques, including the use of matched related donors, preventing early relapses with donor lymphocyte infusion (DLI), stopping post-transplant immunosuppression, and treating with TKIs post-transplant, have improved three-year OS to above 85% and 15-year leukemia-free survival (LFS) to 80% (131–135). Although late relapses are rare, the risk of relapse continues indefinitely (135).
The impact of TKI use before and after transplant, as well as the number of TKIs used before transplant, on post-transplant OS remains unclear (134, 136). An EBMT score greater than 2 consistently shows an adverse impact on transplant outcomes and serves as a crucial tool for guiding transplant decisions (125, 133, 137). However, not all patients who fail TKI therapy are transplant candidates, especially older patients who may require reduced-intensity pre-transplant conditioning, which increases relapse risk (138). Therefore, the decision to proceed with a transplant in the chronic phase is complex, and early consideration for clinical trials should be optimized for patients not deemed transplant candidates.
Despite advancements and a wide range of treatment options for CML-CP, 30–50% of patients experience failure with frontline imatinib within five years (123). With newer therapies in development primarily targeting the ATP-competitive BCR-ABL1, there should be a greater focus on strategically sequencing the available TKIs to optimize response and minimize the emergence of mutations and resistance. Asciminib has shown promising results and can address some of these unmet needs. Additionally, other pathways, including JAK/STAT, mTOR, and immune signaling, are promising potential targets for CML. Unfortunately, some patients may be unable to receive a second-generation TKI, ponatinib, or undergo allo-HSCT and may also be ineligible for clinical trials. In such cases, interferon alpha could be a viable option.
BG: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. KC: Data curation, Validation, Writing – original draft, Writing – review & editing. AR: Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. (2016) 34:2851–7. doi: 10.1200/JCO.2015.66.2866
2. Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. (2011) 103:553–61. doi: 10.1093/jnci/djr060
3. Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. (2014) 32:415–23. doi: 10.1200/JCO.2013.49.9020
4. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. (2016) 34:2333–40. doi: 10.1200/JCO.2015.64.8899
5. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. (2016) 30:1044–54. doi: 10.1038/leu.2016.5
6. Brümmendorf TH, Cortes JE, Milojkovic D, Gambacorti-Passerini C, Clark RE, le Coutre P, et al. Bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: final results from the BFORE trial. Leukemia. (2022) 36:1825–33. doi: 10.1038/s41375-022-01589-y
7. Baccarani M, Abruzzese E, Accurso V, Albano F, Annunziata M, Barulli S, et al. Managing chronic myeloid leukemia for treatment-free remission: a proposal from the GIMEMA CML WP. Blood Adv. (2019) 3:4280–90. doi: 10.1182/bloodadvances.2019000865
8. Baccarani M, Gale RP. Why chronic myeloid leukaemia cannot be cured by tyrosine kinase-inhibitors. Leukemia. (2021) 35:2199–204. doi: 10.1038/s41375-021-01272-8
9. Deininger MW, Shah NP, Altman JK, Berman E, Bhatia R, Bhatnagar B, et al. Chronic myeloid leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:1385–415. doi: 10.6004/jnccn.2020.0047
10. Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. (2018) 36:231–7. doi: 10.1200/JCO.2017.74.7162
11. Réa D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. (2021) 138:2031–41. doi: 10.1182/blood.2020009984
12. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. (2018) 132:393–404. doi: 10.1182/blood-2016-09-739086
13. Baccarani M, Castagnetti F, Gugliotta G, Rosti G. A review of the European LeukemiaNet recommendations for the management of CML. Ann Hematol. (2015) 94 Suppl 2:S141–7. doi: 10.1007/s00277-015-2322-2
14. Fava C, Saglio G. The biology of CML supports second-generation TKIs as frontline treatment. Clin Adv Hematol Oncol. (2017) 15:302–7.
15. Saglio G, Jabbour E. First-line therapy for chronic phase CML: selecting the optimal BCR-ABL1-targeted TKI. Leuk Lymphoma. (2018) 59:1523–38. doi: 10.1080/10428194.2017.1379074
16. Lee SG, Lipton JH. Everything old is new again: the case for imatinib as frontline therapy in 2017. Clin Adv Hematol Oncol. (2017) 15:302–5.
17. Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. (2001) 293:876–80. doi: 10.1126/science.1062538
18. Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O'Brien S, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. (2007) 110:4005–11. doi: 10.1182/blood-2007-03-080838
19. Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. (2019) 381:2315–26. doi: 10.1056/NEJMoa1902328
20. Loscocco F, Visani G, Galimberti S, Curti A, Isidori A. BCR-ABL independent mechanisms of resistance in chronic myeloid leukemia. Front Oncol. (2019) 9:939. doi: 10.3389/fonc.2019.00939
21. Milojkovic D, Apperley J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. (2009) 15:7519–27. doi: 10.1158/1078-0432.CCR-09-1068
22. Machnicki MM, Pepek M, Solarska I, Niesiobedzka-Krezel J, Seferynska I, Gora Tybor J, et al. ASXL1 mutations detectable at diagnosis may predict response to imatinib in patients with chronic myeloid leukemia. Blood. (2019) 134:4148–8. doi: 10.1182/blood-2019-129834
23. Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. (2003) 102:276–83. doi: 10.1182/blood-2002-09-2896
24. Soverini S, Martinelli G, Rosti G, Bassi S, Amabile M, Poerio A, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. (2005) 23:4100–9. doi: 10.1200/JCO.2005.05.531
25. Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. (2006) 12:7374–9. doi: 10.1158/1078-0432.CCR-06-1516
26. Khorashad JS, de Lavallade H, Apperley JF, Milojkovic D, Reid AG, Bua M, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. (2008) 26:4806–13. doi: 10.1200/JCO.2008.16.9953
27. Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. (2008) 111:4022–8. doi: 10.1182/blood-2007-10-116475
28. Soverini S, Gnani A, Colarossi S, Castagnetti F, Abruzzese E, Paolini S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. (2009) 114:2168–71. doi: 10.1182/blood-2009-01-197186
29. Menezes J, Salgado RN, Acquadro F, Gómez-López G, Carralero MC, Barroso A, et al. ASXL1, TP53 and IKZF3 mutations are present in the chronic phase and blast crisis of chronic myeloid leukemia. Blood Cancer J. (2013) 3:e157. doi: 10.1038/bcj.2013.54
30. Soverini S, De Benedittis C, Machova Polakova K, Brouckova A, Horner D, Iacono M, et al. Unraveling the complexity of tyrosine kinase inhibitor-resistant populations by ultra-deep sequencing of the BCR-ABL kinase domain. Blood. (2013) 122:1634–48. doi: 10.1182/blood-2013-03-487728
31. Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. (2014) 26:428–42. doi: 10.1016/j.ccr.2014.07.006
32. Schmitt MW, Pritchard JR, Leighow SM, Aminov BI, Beppu L, Kim DS, et al. Single-molecule sequencing reveals patterns of preexisting drug resistance that suggest treatment strategies in philadelphia-positive leukemias. Clin Cancer Res. (2018) 24:5321–34. doi: 10.1158/1078-0432.CCR-18-0167
33. O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. (2009) 16:401–12. doi: 10.1016/j.ccr.2009.09.028
34. Eide CA, Zabriskie MS, Savage Stevens SL, Antelope O, Vellore NA, Than H, et al. Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell. (2019) 36:431–443.e5. doi: 10.1016/j.ccell.2019.08.004
35. Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. (2009) 27:6041–51. doi: 10.1200/JCO.2009.25.0779
36. Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. (2013) 122:872–84. doi: 10.1182/blood-2013-05-501569
37. Hochhaus A, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. (2020) 34:966–84. doi: 10.1038/s41375-020-0776-2
38. Jabbour E, et al. Front-line therapy with second-generation tyrosine kinase inhibitors in patients with early chronic phase chronic myeloid leukemia: what is the optimal response? J Clin Oncol. (2011) 29:4260–5. doi: 10.1200/JCO.2011.36.0693
39. Sasaki K, Kantarjian HM, Issa GC, Garcia-Manero G, Kadia TM, et al. Impact of molecular response at specific timepoints in patients with newly diagnosed chronic myeloid leukemia treated with second generation tyrosine kinase inhibitors. Blood. (2020) 136(Supplement 1):42–44. doi: 10.1182/blood-2020-143023
40. Hochhaus A, La Rosée P. Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistance. Leukemia. (2004) 18:1321–31. doi: 10.1038/sj.leu.2403426
41. O'Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. (2007) 110:2242–9. doi: 10.1182/blood-2007-03-066936
42. Jabbour E. Chronic myeloid leukemia: First-line drug of choice. Am J Hematol. (2016) 91:59–66. doi: 10.1002/ajh.24249
43. Soverini S, Branford S, Nicolini FE, Talpaz M, Deininger MW, Martinelli G, et al. Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leuk Res. (2014) 38:10–20. doi: 10.1016/j.leukres.2013.09.011
44. Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. (2011) 12:841–51. doi: 10.1016/S1470-2045(11)70201-7
45. Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. (2012) 119:1123–9. doi: 10.1182/blood-2011-08-376087
46. Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. (2021) 35:440–53. doi: 10.1038/s41375-020-01111-2
47. Shah NP, Guilhot F, Cortes JE, Schiffer CA, le Coutre P, Brümmendorf TH, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. (2014) 123:2317–24. doi: 10.1182/blood-2013-10-532341
48. Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. (2013) 27:107–12. doi: 10.1038/leu.2012.181
49. Brümmendorf TH, Cortes JE, Goh YT, Yilmaz M, Klisovic RB, Purcell S, et al. Bosutinib (BOS) for chronic phase (CP) chronic myeloid leukemia (CML) after imatinib (IMA) failure: ≥8-y update of a phase I/II study. J Clin Oncol. (2020) 38-:7549–9.
50. Kantarjian H, Pasquini R, Lévy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R). Cancer. (2009) 115:4136–47. doi: 10.1002/cncr.24504
51. Cortes JE, Kantarjian HM, Brümmendorf TH, Kim DW, Turkina AG, Shen ZX, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. (2011) 118:4567–76. doi: 10.1182/blood-2011-05-355594
52. Garcia-Gutierrez JV, Herrera P, Abalo LL. Impact of second-generation tyrosine kinase inhibitors as second line treatment for patients with chronic myeloid. Am Soc Hematol. (2011) 118:3780. doi: 10.1182/blood.V118.21.3780.3780
53. Lipton JH, Shah D, Tongbram V, Sidhu M, Huang H, McGarry LJ, et al. Comparative efficacy among 3rd line post-imatinib chronic phase-chronic myeloid leukemia (CP-CML) patients after failure of dasatinib or nilotinib tyrosine kinase inhibitors. Blood. (2014) 124:4551–1. doi: 10.1182/blood.V124.21.4551.4551
54. Lipton JH, Bryden P, Sidhu MK, Huang H, McGarry LJ, Lustgarten S, et al. Comparative efficacy of tyrosine kinase inhibitor treatments in the third-line setting, for chronic-phase chronic myelogenous leukemia after failure of second-generation tyrosine kinase inhibitors. Leuk Res. (2015) 39:58–64. doi: 10.1016/j.leukres.2014.10.005
55. Garg RJ, Kantarjian H, O'Brien S, Quintás-Cardama A, Faderl S, Estrov Z, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow-up. Blood. (2009) 114:4361–8. doi: 10.1182/blood-2009-05-221531
56. Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. (2010) 24:1299–301. doi: 10.1038/leu.2010.110
57. Ibrahim AR, Paliompeis C, Bua M, Milojkovic D, Szydlo R, Khorashad JS, et al. Efficacy of tyrosine kinase inhibitors (TKIs) as third-line therapy in patients with chronic myeloid leukemia in chronic phase who have failed 2 prior lines of TKI therapy. Blood. (2010) 116:5497–500. doi: 10.1182/blood-2010-06-291922
58. Cortes J, Quintas-Cardama A, Jabbour E, O'Brien S, Verstovsek S, Borthakur G, et al. The clinical significance of achieving different levels of cytogenetic response in patients with chronic phase chronic myeloid leukemia after failure to front-line therapy: is complete cytogenetic response the only desirable endpoint? Clin Lymphoma Myeloma Leuk. (2011) 11:421–6. doi: 10.1016/j.clml.2011.06.009
59. Ribeiro BF, Miranda ECM, de Albuquerque DulcinéiaM, Delamain MárciaT, Oliveira-Duarte G, Almeida MH, et al. Treatment with dasatinib or nilotinib in chronic myeloid leukemia patients who failed to respond to two previously administered tyrosine kinase inhibitors – a single center experience. Clinics. (2015) 70:550. doi: 10.6061/clinics/2015(08)04
60. Lomaia E, Zaritskey A, Shuvavev V, Martynkevich I, Forminykh M, Ovsyannikova E, et al. Efficacy of tyrosine kinase inhibitors in third line therapy in chronic phase chronic myeloid leukemia. Blood. (2015) 126:4051–1. doi: 10.1182/blood.V126.23.4051.4051
61. García-Gutiérrez V, Milojkovic D, Hernandez-Boluda JC, Claudiani S, Martin Mateos ML, Casado-Montero LF, et al. Safety and efficacy of bosutinib in fourth-line therapy of chronic myeloid leukemia patients. Ann Hematol. (2019) 98:321–30. doi: 10.1007/s00277-018-3507-2
62. Kantarjian HM, Jabbour E, Deininger M, Abruzzese E, Apperley J, Cortes J, et al. Ponatinib after failure of second-generation tyrosine kinase inhibitor in resistant chronic-phase chronic myeloid leukemia. Am J Hematol. (2022) 97:1419–26. doi: 10.1002/ajh.26686
63. García-Gutiérrez V, Hernández-Boluda JC. Tyrosine kinase inhibitors available for chronic myeloid leukemia: efficacy and safety. Front Oncol. (2019) 9:603. doi: 10.3389/fonc.2019.00603
64. Hochhaus A, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv41–51. doi: 10.1093/annonc/mdx219
65. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. (2020) 95:691–709. doi: 10.1002/ajh.25792
66. Machova Polakova K, Kulvait V, Benesova A, Linhartova J, Klamova H, Jaruskova M, et al. Next-generation deep sequencing improves detection of BCR-ABL1 kinase domain mutations emerging under tyrosine kinase inhibitor treatment of chronic myeloid leukemia patients in chronic phase. J Cancer Res Clin Oncol. (2015) 141:887–99. doi: 10.1007/s00432-014-1845-6
67. Baer C, Kern W, Koch S, Nadarajah N, Schindela S, Meggendorfer M, et al. Ultra-deep sequencing leads to earlier and more sensitive detection of the tyrosine kinase inhibitor resistance mutation T315I in chronic myeloid leukemia. Haematologica. (2016) 101:830–8. doi: 10.3324/haematol.2016.145888
68. Soverini S, Bavaro L, De Benedittis C, Martelli M, Iurlo A, Orofino N, et al. Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: the NEXT-in-CML study. Blood. (2020) 135:534–41. doi: 10.1182/blood.2019002969
69. Cortes JE, Hochhaus A, le Coutre PD, Rosti G, Pinilla-Ibarz J, Jabbour E, et al. Minimal cross-intolerance with nilotinib in patients with chronic myeloid leukemia in chronic or accelerated phase who are intolerant to imatinib. Blood. (2011) 117:5600–6. doi: 10.1182/blood-2010-11-318949
70. Khoury HJ, Goldberg SL, Mauro MJ, Stone RM, Deininger MW, Bradley-Garelik MB, et al. Cross-intolerance with dasatinib among imatinib-intolerant patients with chronic phase chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. (2016) 16:341–349.e1. doi: 10.1016/j.clml.2016.03.004
71. Kobayashi Y, Sakamaki H, Fujisawa S, Ando K, Yamamoto K, Okada M, et al. Lack of non-hematological cross intolerance of dasatinib to imatinib in imatinib-intolerant patients with Philadelphia chromosome positive chronic myeloid leukemia or acute lymphatic leukemia: a retrospective safety analysis. Int J Hematol. (2011) 93:745–9. doi: 10.1007/s12185-011-0864-1
72. Jabbour E, Kantarjian H, Ghanem H, O'Brien S, Quintas-Cardama A, Garcia-Manero G, et al. The achievement of a 3-month complete cytogenetic response to second-generation tyrosine kinase inhibitors predicts survival in patients with chronic phase chronic myeloid leukemia after imatinib failure. Clin Lymphoma Myeloma Leuk. (2013) 13:302–6. doi: 10.1016/j.clml.2012.12.005
73. Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. (2017) 31:2398–406. doi: 10.1038/leu.2017.253
74. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. (2017) 376:917–27. doi: 10.1056/NEJMoa1609324
75. Jain P, Kantarjian H, Nazha A, O'Brien S, Jabbour E, Romo CG, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. (2013) 121:4867–74. doi: 10.1182/blood-2013-03-490128
76. Etienne G, Faberes C, Bauduer F, Adiko D, Lifermann F, Dagada C, et al. Relevance of treatment-free remission recommendations in chronic phase chronic leukemia patients treated with frontline tyrosine kinase inhibitors. Cancer Med. (2021) 10:3635–45. doi: 10.1002/cam4.3921
77. Haddad FG, Sasaki K, Issa GC, Garcia-Manero G, Ravandi F, Kadia T, et al. Treatment-free remission in patients with chronic myeloid leukemia following the discontinuation of tyrosine kinase inhibitors. Am J Hematol. (2022) 97:856–64. doi: 10.1002/ajh.26550
78. Tam CS, Kantarjian H, Garcia-Manero G, Borthakur G, O'Brien S, Ravandi F, et al. Failure to achieve a major cytogenetic response by 12 months defines inadequate response in patients receiving nilotinib or dasatinib as second or subsequent line therapy for chronic myeloid leukemia. Blood. (2008) 112:516–8. doi: 10.1182/blood-2008-02-141580
79. Havrdova E, Hutchinson M, Kurukulasuriya NC, Raghupathi K, Sweetser MT, Dawson KT, et al. Oral BG-12 (dimethyl fumarate) for relapsing-remitting multiple sclerosis: a review of DEFINE and CONFIRM. Evaluation of: Gold R, Kappos L, Arnold D, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107. Expert Opin Pharmacother. (2013) 14:2145–56. doi: 10.1517/14656566.2013.826190
80. Vener C, Banzi R, Ambrogi F, Ferrero A, Saglio G, Pravettoni G, et al. First-line imatinib vs second- and third-generation TKIs for chronic-phase CML: a systematic review and meta-analysis. Blood Adv. (2020) 4:2723–35. doi: 10.1182/bloodadvances.2019001329
81. Lee SE, Choi SY, Kim SH, Jootar S, Kim HJ, Sohn SK, et al. Comparative analyses of nilotinib versus high-dose imatinib versus sustained standard-dose imatinib in patients with chronic phase chronic myeloid leukemia following suboptimal molecular response to first-line imatinib. Leuk Res. (2018) 70:100–5. doi: 10.1016/j.leukres.2018.06.002
82. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. (2013) 369:1783–96. doi: 10.1056/NEJMoa1306494
84. Cortes JE, Lomaia E, Turkina A, Moiraghi B, Sutton MU, Pavlovsky C. Interim analysis (IA) of OPTIC: a dose-ranging study of three ponatinib (PON) starting doses. J Clin Oncol. (2020) 38:7502. doi: 10.1200/JCO.2020.38.15_suppl.7502
85. Cortes J, Apperley J, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. (2021) 138:2042–50. doi: 10.1182/blood.2021012082
86. Cortes J, Deininger M, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, et al. Three-year update from the optic trial: A dose-optimization study of 3 starting doses of ponatinib. Blood. (2022) 140:1495–7. doi: 10.1182/blood-2022-157822
87. Devos T, Havelange V, Theunissen K, Meers S, Benghiat FS, Gadisseur A, et al. P699: REAL-LIFE OUTCOMES OF PONATINIB TREATMENT IN PATIENTS WITH CHRONIC MYELOID LEUKEMIA (CML) OR PHILADELPHIA CHROMOSOME-POSITIVE ACUTE LYMPHOBLASTIC LEUKEMIA (PH+ALL): 5-YEAR-DATA FROM A BELGIAN REGISTRY. HemaSphere. (2022) 6:594–5. doi: 10.1097/01.HS9.0000845680.44858.4f
88. Breccia M, Olimpieri PP, Celant S, Olimpieri O, Pane F, Iurlo A, et al. Management of chronic myeloid leukaemia patients treated with ponatinib in a real-life setting: A retrospective analysis from the monitoring registries of the Italian Medicines Agency (AIFA). Br J Haematol. (2022) 198:965–73. doi: 10.1111/bjh.18359
89. Müller MC, Cervantes F, Hjorth-Hansen H, Janssen JJWM, Milojkovic D, Rea D, et al. Ponatinib in chronic myeloid leukemia (CML): Consensus on patient treatment and management from a European expert panel. Crit Rev Oncol Hematol. (2017) 120:52–9. doi: 10.1016/j.critrevonc.2017.10.002
90. Saussele S, Haverkamp W, Lang F, Koschmieder S, Kiani A, Jentsch-Ullrich K, et al. Ponatinib in the treatment of chronic myeloid leukemia and philadelphia chromosome-positive acute leukemia: recommendations of a german expert consensus panel with focus on cardiovascular management. Acta Haematol. (2020) 143:217–31. doi: 10.1159/000501927
91. Mauro MJ, McGarry LJ, Lustgarden S, Huang H. Predictors of ponatinib therapy duration among real-world chronic phase chronic myeloid leukemia (CP-CML) patients in the US. Blood. (2016) 128:3081–1. doi: 10.1182/blood.V128.22.3081.3081
92. Heiblig M, Rea D, Chrétien ML, Charbonnier A, Rousselot P, Coiteux V, et al. Ponatinib evaluation and safety in real-life chronic myelogenous leukemia patients failing more than two tyrosine kinase inhibitors: the PEARL observational study. Exp Hematol. (2018) 67:41–8. doi: 10.1016/j.exphem.2018.08.006
93. Caocci G, Mulas O, Abruzzese E, Luciano L, Iurlo A, Attolico I, et al. Arterial occlusive events in chronic myeloid leukemia patients treated with ponatinib in the real-life practice are predicted by the Systematic Coronary Risk Evaluation (SCORE) chart. Hematol Oncol. (2019) 37:296–302. doi: 10.1002/hon.2606
94. Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. (2017) 543:733–7. doi: 10.1038/nature21702
95. Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. (2018) 61:8120–35. doi: 10.1021/acs.jmedchem.8b01040
96. Mauro MJ, Hughes TP, Kim DW, Rea D, Cortes JE, Hochhaus A, et al. Asciminib monotherapy in patients with CML-CP without BCR::ABL1 T315I mutations treated with at least two prior TKIs: 4-year phase 1 safety and efficacy results. Leukemia. (2023) 37:1048–59. doi: 10.1038/s41375-023-01860-w
97. Cortes JE, Hughes TP, Mauro MJ, Hochhaus A, Rea D, Goh YT, et al. Asciminib, a first-inclass STAMP inhibitor, provides durable molecular response in patients (pts) with chronic myeloid leukemia (CML) harboring the T315I mutation: primary efficacy and safety results from aphase 1 trial. Blood. (2020). doi: 10.1182/blood-2020-139677
98. Hughes T, Mauro M, Kim D, Cortes J, Rea D, Minami H. Asciminib in heavily pretreated patients with Ph+ CML-CP sensitive to TKI therapy. EHA 25 Library. (2020) 37:S170.
99. Hochhaus A, Réa D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, et al. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. (2023) 37:617–26. doi: 10.1038/s41375-023-01829-9
100. Khadadah FM, Cerquozzi S, Olney HJ, Fraga C, Dudebout J, Xenocostas A, et al. Canadian real-world experience of asciminib treatment in heavily pre-treated chronic myeloid leukemia (CML) patients who failed multiple lines of tyrosine kinase inhibitor (TKI) therapy. Leuk Res. (2023) 133:107374. doi: 10.1016/j.leukres.2023.107374
101. Luna A, Pérez-Lamas L, Boque C, Giraldo P, Xicoy B, Ruiz Nuño C, et al. Real-life analysis on safety and efficacy of asciminib for ponatinib pretreated patients with chronic myeloid leukemia. Ann Hematol. (2022) 101:2263–70. doi: 10.1007/s00277-022-04932-6
102. Kockerols CCB, Janssen JJWM, Blijlevens NMA, Klein SK, Van Hussen-Daenen LGM, Van Gorkom GGY, et al. Treatment patterns and clinical outcomes of asciminib in a real-world multiresistant chronic myeloid leukemia patient population. Haematologica. (2022) 108:240–4. doi: 10.3324/haematol.2022.281386
103. Innes AJ, Hayden C, Orovboni V, Rees D, Claudiani S, Fernando F, et al. Real-world experience of asciminib: factors associated with response. Blood. (2022) 140:6796–7. doi: 10.1182/blood-2022-165501
104. Garcia-Gutiérrez V, Luna A, Alonso-Dominguez JM, Estrada N, Boque C, Xicoy B, et al. Safety and efficacy of asciminib treatment in chronic myeloid leukemia patients in real-life clinical practice. Blood Cancer J. (2021) 11:16. doi: 10.1038/s41408-021-00420-8
105. Yeung DT, Shanmuganathan N, Hughes TP. Asciminib: a new therapeutic option in chronic-phase CML with treatment failure. Blood. (2022) 139:3474–9. doi: 10.1182/blood.2021014689
106. Gandhi V, Plunkett W, Cortes JE. Omacetaxine: a protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin Cancer Res. (2014) 20:1735–40. doi: 10.1158/1078-0432.CCR-13-1283
107. Damlaj M, Lipton JH, Assouline SE. A safety evaluation of omacetaxine mepesuccinate for the treatment of chronic myeloid leukemia. Expert Opin Drug Saf. (2016) 15:1279–86. doi: 10.1080/14740338.2016.1207760
108. Cortes JE, Kantarjian HM, Rea D, Wetzler M, Lipton JH, Akard L, et al. Final analysis of the efficacy and safety of omacetaxine mepesuccinate in patients with chronic- or accelerated-phase chronic myeloid leukemia: Results with 24 months of follow-up. Cancer. (2015) 121:1637–44. doi: 10.1002/cncr.29240
109. Jiang Q, Huang X, Chen Z, Niu Q, Men L, Wang H, et al. An updated safety and efficacy results of phase 1 study of HQP1351, a novel 3rd generation of BCR-ABL tyrosine kinase inhibitor (TKI), in patients with TKI resistant chronic myeloid leukemia. Blood. (2019) 134:493. doi: 10.1182/blood-2019-124295
110. Jiang Q, Huang X, Chen Z, Niu Q, Shi D, Li Z, et al. Novel BCR-ABL1 tyrosine kinase inhibitor (TKI) HQP1351 (olverembatinib) is efficacious and well tolerated in patients with T315I-mutated chronic myeloid leukemia (CML): results of pivotal (phase II) trials. Blood. (2020) 136:50–1. doi: 10.1182/blood-2020-142142
111. Qian J, Shi D, Li Z, Qin Y, Zhao T, Liu B, et al. Updated safety and efficacy results of phase 1 study of olverembatinib (HQP1351), a novel third-generation BCR-ABL tyrosine kinase inhibitor (TKI), in patients with TKI-resistant chronic myeloid leukemia (CML). Blood. (2021) 138:311–1. doi: 10.1182/blood-2021-153065
112. Jiang Q, Li Z, Zhang G, Hu Yu, Li W, Song Y, et al. Olverembatinib (HQP1351) demonstrates efficacy vs. best available therapy (BAT) in patients (pts) with tyrosine kinase inhibitor (TKI)-resistant chronic myeloid leukemia chronic-phase (CML-CP) in a registrational randomized phase 2 study. Blood. (2023) 142:869, 2023. doi: 10.1182/blood-2023-187740
113. Pharma A. Olverembatinib approved for new indications, allowing more patients with CML to benefit from the drug. (2023). Available at: https://www.ascentage.com/olverembatinib-approved-for-new-indicatation-allowing-more-patients-with-cml-to-benefit-from-the-drug/. (Accessed on April 15th, 2024)
114. ClinicalTrials.gov. Study to evaluate tolerability, safety, pharmacokinetics and preliminary efficacy of PF-114 for oral administration in adults with Ph+ chronic myeloidleukemia, which is resistant to the 2nd generation Bcr-Abl inhibitors or has T315I mutation in the BCR-ABL gene . Available online at: https://clinicaltrials.gov/ct2/show/NCT02885766 (Accessed May 2022).
115. Turkina A, Vinogradova O, Lomaia E, Shatokhina E, Shukhov O, ChelyshevaEagles J. PF-114 in patients failing prior tyrosine kinase-inhibitor therapy including BCR::ABL1T315I. Blood. (2021) 138(Supplement 1):1482. doi: 10.1182/blood-2021-150120
116. Cortes JE, Kim D, Nicolini FE, Saikia T, Charbonnier A, Apperley JF. Phase 1 trial of K0706, a novel oral BCR-ABL1 tyrosine kinase inhibitor (TKI): in patients with chronic myelogenous leukemia (CML) andPhildelphia positive acute lymphoblastic leukemia (Ph+ ALL) failing ≥ 3 prior TKI therapies: initial safety and efficacy. Blood. (2019) 134:4158. doi: 10.1182/blood-2019-129751
117. Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. (2012) 119:1501–10. doi: 10.1182/blood-2010-12-326843
118. Houshmand M, Simonetti G, Circosta P, Gaidano V, Cignetti A, Martinelli G, et al. Chronic myeloid leukemia stem cells. Leukemia. (2019) 33:1543–56. doi: 10.1038/s41375-019-0490-0
119. Hehlmann R, Voskanyan A, Lauseker M, Pfirrmann M, Kalmanti L, Rinaldetti S, et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. (2020) 34:2074–86. doi: 10.1038/s41375-020-0826-9
120. Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Impact of drug development on the use of stem cell transplantation: a report by the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. (2017) 52:191–6. doi: 10.1038/bmt.2016.258
121. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. (2017) 52:811–7. doi: 10.1038/bmt.2017.34
122. Lübking A, Dreimane A, Sandin F, Isaksson C, Märkevärn B, Brune M, et al. Allogeneic stem cell transplantation for chronic myeloid leukemia in the TKI era: population-based data from the Swedish CML registry. Bone Marrow Transplant. (2019) 54:1764–74. doi: 10.1038/s41409-019-0513-5
123. Arora M, Weisdorf DJ, Spellman SR, Haagenson MD, Klein JP, Hurley CK, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. (2009) 27:1644–52. doi: 10.1200/JCO.2008.18.7740
124. Radich JP, Gooley T, Bensinger W, Chauncey T, Clift R, Flowers M, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. (2003) 102:31–5. doi: 10.1182/blood-2002-08-2619
125. Gratwohl A, Pfirrmann M, Zander A, Kröger N, Beelen D, Novotny J, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. (2016) 30:562–9. doi: 10.1038/leu.2015.281
126. Bacher U, Klyuchnikov E, Zabelina T, Ottinger H, Beelen DW, Schrezenmeier H, et al. The changing scene of allogeneic stem cell transplantation for chronic myeloid leukemia–a report from the German Registry covering the period from 1998 to 2004. Ann Hematol. (2009) 88:1237–47. doi: 10.1007/s00277-009-0737-3
127. Ohashi K, Nagamura-Inoue T, Nagamura F, Tojo A, Miyamura K, Mori T, et al. Effect of graft sources on allogeneic hematopoietic stem cell transplantation outcome in adults with chronic myeloid leukemia in the era of tyrosine kinase inhibitors: a Japanese Society of Hematopoietic Cell Transplantation retrospective analysis. Int J Hematol. (2014) 100:296–306. doi: 10.1007/s12185-014-1632-9
128. Chaudhury S, Sparapani R, Hu ZH, Nishihori T, Abdel-Azim H, Malone A, et al. Outcomes of allogeneic hematopoietic cell transplantation in children and young adults with chronic myeloid leukemia: A CIBMTR cohort analysis. Biol Blood Marrow Transplant. (2016) 22:1056–64. doi: 10.1016/j.bbmt.2016.02.015
129. Lee SE, Choi SY, Kim SH, Jang EJ, Bang JH, Byeun JY, et al. Prognostic factors for outcomes of allogeneic stem cell transplantation in chronic phase chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Hematology. (2014) 19:63–72. doi: 10.1179/1607845413Y.0000000100
130. Koenecke C, Heim D, van Biezen A, Heuser M, Aljurf M, Kyrcz-Krzemien S, et al. Outcome of patients with chronic myeloid leukemia and a low-risk score: allogeneic hematopoietic stem cell transplantation in the era of targeted therapy. A report from the EBMT Chronic Malignancies Working Party. Bone Marrow Transplant. (2016) 51:1259–61. doi: 10.1038/bmt.2016.97
131. Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. (2010) 115:1880–5. doi: 10.1182/blood-2009-08-237115
132. Soverini S, Colarossi S, Gnani A, Castagnetti F, Rosti G, Bosi C, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Haematologica. (2007) 92:401–4. doi: 10.3324/haematol.10822
133. Oyekunle A, Zander AR, Binder M, Ayuk F, Zabelina T, Christopeit M, et al. Outcome of allogeneic SCT in patients with chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Ann Hematol. (2013) 92:487–96. doi: 10.1007/s00277-012-1650-8
134. Piekarska A, Gil L, Prejzner W, Wiśniewski P, Leszczyńska A, Gniot M, et al. Pretransplantation use of the second-generation tyrosine kinase inhibitors has no negative impact on the HCT outcome. Ann Hematol. (2015) 94:1891–7. doi: 10.1007/s00277-015-2457-1
135. Goldman JM, Majhail NS, Klein JP, Wang Z, Sobocinski KA, Arora M, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. (2010) 28:1888–95. doi: 10.1200/JCO.2009.26.7757
136. Kondo T, Nagamura-Inoue T, Tojo A, Nagamura F, Uchida N, Nakamae H, et al. Clinical impact of pretransplant use of multiple tyrosine kinase inhibitors on the outcome of allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia. Am J Hematol. (2017) 92:902–8. doi: 10.1002/ajh.24793
137. Rezvani K, Kanfer EJ, Marin D, Gabriel I, Rahemtulla A, Taylor A, et al. EBMT risk score predicts outcome of allogeneic hematopoietic stem cell transplantation in patients who have failed a previous transplantation procedure. Biol Blood Marrow Transplant. (2012) 18:235–40. doi: 10.1016/j.bbmt.2011.06.010
Keywords: chronic myeloid leukemia, second-generation tyrosine kinase inhibitor, CML, TKI, failure, therapeutic options
Citation: George B, Chan KH and Rios A (2024) Therapeutic options for chronic myeloid leukemia following the failure of second-generation tyrosine kinase inhibitor therapy. Front. Oncol. 14:1446517. doi: 10.3389/fonc.2024.1446517
Received: 10 June 2024; Accepted: 15 July 2024;
Published: 29 July 2024.
Edited by:
Marco Pizzi, University of Padua, ItalyCopyright © 2024 George, Chan and Rios. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binsah George, Qmluc2FoLnMuZ2VvcmdlQHV0aC50bWMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.