- 1Department of Reproductive Medicine Center, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Pathology, The First People’s Hospital of Kunshan, Suzhou, China
- 3Department of Urology, The First Affiliated Hospital of Soochow University, Suzhou, China
Objective: To assess CD276 expression and explore its relationship with the clinicopathological characteristics and prognosis of patients with bladder cancer.
Methods: In total, RNA-sequencing data and clinical profiles of 436 bladder cancer cases from The Cancer Genome Atlas (TCGA) were assessed using the University of California Santa Cruz Xena (UCSC) platform. We compared the CD276 levels in cancerous and adjacent normal tissues and used the R software for statistical association with the clinical stage, grade, and survival (the overall survival, disease-specific survival, and progression-free survival). A single-gene GSEA analysis on TCGA-BLCA data was performed to explore potential pathways through which CD276 might influence bladder cancer. Additionally, CD276 expression was analyzed by comparing data from 9 cancerous tissues and 3 adjacent normal tissues in the GEO dataset GSE7476. Furthermore, we analyzed 133 cancerous bladder and adjacent tissue samples from the Soochow University Hospital, collected between January 1, 2016, and September 30, 2022, to assess the CD276 protein expression using immunohistochemistry. We examined the relationship between tumor CD276 levels and clinical outcomes and prognosis of bladder cancer.
Results: Bioinformatic analysis revealed elevated CD276 expression in tumors compared to that in adjacent tissues (p<0.05), correlating with poor survival. GSEA revealed that CD276 was significantly involved in extracellular matrix-related pathways. Immunohistochemistry confirmed CD276 overexpression in tumor tissues, with higher levels linked to advanced pathological grades and worse prognosis.
Conclusion: CD276 is markedly upregulated in bladder cancer and associated with severe pathological features, advanced disease, potential for metastasis, and diminished survival rates. It may promote bladder cancer development and progression by influencing extracellular matrix-related-related pathways, making it a viable diagnostic and prognostic biomarker for bladder cancer.
1 Introduction
Bladder cancer (BLCA) is among the most prevalent malignancies of the urinary tract worldwide, with approximately 386,000 new cases and 150,000 deaths per year (1–3). Incidence of urinary system cancers has increased, with BLCA being the fifth leading cause of cancer-related deaths in China. Urothelial carcinoma constitutes more than 95% of all BLCA cases, of which non-muscle invasive BLCA (NMIBC) represents 75-80% of all BLCA cases, known for its tendency to recur and progress, especially when limited to the mucosa (Ta) or submucosa (T1) (4, 5). Remarkably, in recent decades, China has experienced a swift increase in both the incidence and mortality rates of BLCA (6). Despite advances in treatment, the outlook for patients remains grim (7). Moreover, BLCA is more prevalent in urban areas than in rural settings (8).
Recent advances have revealed potential targets for the early detection, treatment, and prognostic evaluation of cancer. One such target in the B7 superfamily is the B7-Homologue 3 molecule (B7-H3, also known as the cluster of differentiation 276 [CD276]). This immunoglobulin family protein, discovered in 2001 by Andrei et al., plays an important role in modulating T-cell-driven immune responses and is a member of the B7/CD28 clan of the immune costimulatory molecules. CD276 shares 20-27% amino acid similarity with its B7 kin and exists in two isoforms: 2IgB7-H3 and 4IgB7-H3 (9, 10). Research has established that CD276 can markedly dampen T cell activity, positioning it as a prototypical immune checkpoint molecule (11, 12). Various tumor cells, vascular endothelial cells within tumors, and immune infiltrates such as dendritic cells and macrophages exhibit widespread and aberrant expression of the inhibitory molecule CD276. In contrast, its expression in the normal tissues is reported to be negligible (13, 14). Programmed cell death ligand 1 (PD-L1, also known as B7-H1) was initially identified in antigen-presenting cells, where its binding to the T-cell PD-1 receptor transmits a signal that curbs the immune response. Subsequently, this inhibitory mechanism was also observed in malignant cells; by expressing PD-L1, they interacted with T cell PD-1 to impair the immune response to tumors, enabling immune escape (15). Similarly, CD276 exerts a PD-L1-like suppressive effect in T cells (16).
Despite the undiscovered and unconfirmed receptors for human CD276, its precise immune and biological roles and mechanisms of action remain largely unknown. Nonetheless, research indicates that CD276, functioning as a “membrane molecule,” might facilitate “reverse signaling” by relaying signals to the cells in which it is expressed (17, 18).
CD276 generally serves as an activator of T cells. However, it can also act as a T cell inhibitor, potentially facilitating cancer cell invasiveness and proliferation (13–18). Additionally, it plays a role in cell-to-cell adhesion and interacts with the extracellular matrix (ECM) (19). In this study, we thoroughly examined CD276 expression to investigate its association with the prognosis and immune infiltration in BLCA, providing novel insights into potential diagnostic and therapeutic strategies for bladder urothelial carcinoma.
2 Materials and methods
2.1 Bioinformatics data description
2.1.1 TCGA sample data collection
Using the University of California Santa Cruz (UCSC) Xena platform (https://xena.ucsc.edu/), we acquired RNA sequencing expression profiles and pertinent clinical data for BLCA from The Cancer Genome Atlas (TCGA) database. We determined CD276 levels in bladder tumors and assessed their associations with patient survival outcomes, specifically considering the overall survival (OS), disease-specific survival (DSS), and progression-free survival (PFS). The dataset included clinical information on 408 individuals with BLCA with 19 adjacent non-tumor tissue samples and 436 patients with BLCA.
2.1.2 Association analysis between CD276 and clinic-pathological features of bladder cancer
The CD276 expression profiles in bladder tumor samples were integrated with the corresponding clinical data using the R language and the ‘ggpubr’ package (Table 1). This analysis investigated whether CD276 expression levels varied according to patient characteristics, such as sex, age, pathological grade, and tumor stage. For the sex-based analysis, the cohort was classified into 317 male and 119 female patients. Age was categorized using a median cutoff of 69 years, creating an older group comprising 209 patients and a younger group comprising 227 patients. With respect to the postoperative pathological grade, patients were grouped into high-grade urothelial carcinoma (413 cases) and low-grade urothelial carcinoma (23 cases). Tumor staging considered the depth of tumor invasion, occurrence of lymph node metastasis, and presence of distant metastasis, leading to a segregation of patients into either early stage (stage I+II, 140 cases with neither lymph node nor distant metastasis) or advanced stage (stage III+IV, 296 cases with lymph node metastasis or distant metastasis). This stratification facilitated targeted exploration of CD276 expression across different stages of tumor progression.
2.1.3 Expression of CD276 in bladder cancer and its prognostic analysis
The patients were stratified into high- and low-expression groups based on whether their CD276 expression levels were above or below the median. We applied the R language and employed the ‘survival, ‘surveyor,’ ‘survminer’ and ‘lima’ packages to depict the survival outcomes of these patients. This enabled the analysis of the association between CD276 expression and the key survival indicators OS, DSS, and PFS.
2.1.4 GEO sample data collection
The dataset GSE7476 was obtained from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) by applying specific inclusion and exclusion criteria: (1) only studies on human tissues were included; (2) only BLCA studies were considered; (3) studies without a control group were excluded; and (4) only mRNA-level arrays were included, while dual-channel microarray studies were excluded. Differentially expressed genes (DEGs) between BLCA and normal samples were identified and analyzed using R language software. DEGs were defined as samples with corrected p-values <0.05 and log fold changes (FC) >1. The GSE7476 dataset contained 9 bladder cancer tissues and 3 adjacent normal tissues. Differences between the Tumor Group and the Normal Group were assessed using the Wilcoxon rank-sum test.
2.1.5 Single-cell gene set enrichment analysis of CD276
Using data from TCGA-BLCA, we screened for differentially expressed genes between ccRCC and control samples with the DESeq2 package in R. Pearson association analysis identified 2,951 genes with an absolute association coefficient of CD276 >1 and a P-value <0.05. These genes were considered associated genes. They underwent enrichment analysis, with the top 10 related pathways visualized. We then employed the gene set enrichment analysis (GSEA) package in R to identify and visualize highly associated pathways.
2.2 Clinical data description
2.2.1 Clinical sample collection
This study was a retrospective analysis involving 133 BLCA patients from the First Affiliated Hospital of Soochow University, evaluated between January 1, 2016, to September 30, 2022, with follow-up extending to close to 2023. This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (approval no. 283, 2024). Written informed consent was obtained from all participants before they enrolled in the study. This study adhered to the principles of the 2013 Declaration of Helsinki.
2.2.2 Inclusion criteria
The inclusion criteria for the enrolled patients were as follows: (1) pathologically confirmed diagnosis of urothelial carcinoma; (2) complete clinical and follow-up data with willingness to participate in the study; (3) no concurrent malignant tumors or immunological diseases, including acquired immune deficiency syndrome; and (4) absence of infectious diseases prior to surgery and no prior exposure to radiation, chemotherapy, or other adjuvant treatments.
2.2.3 Immunohistochemistry
Tissue samples were collected from 133 patients with BLCA, along with healthy tissues from neighboring areas, ensuring a minimum two-centimeter distance from the tumor border and confirming the absence of cancer cells post-surgery. These samples were used for IHC staining as described in our previous study (20). Briefly, IHC involves fixing tissue sections and treating them to expose antigens. Sections were then incubated with primary antibodies specific to the target proteins, followed by incubation with secondary antibodies conjugated with a detectable marker. After washing the sections, the presence of the target antigen was visualized using a colorimetric reaction or fluorescence and the tissue was counterstained for contrast before examination under a microscope. We utilized Aperio ImageScope software for the quantitative analysis of IHC staining.
CD276 was identified as yellow to brown granular staining of the cytoplasm at 100x magnification. To gauge positivity, ten random fields at 200x magnification were inspected, using a scoring system that combined the proportion of stained cells and staining intensity. Positivity percentages were scored as follows: less than 5% scored zero, 5–30% scored one, 31–60% two, and > 60% scored, three. Similarly, the intensity was scored from zero (no color) to three (dark brown). Multiplying these scores provided the final assessment; scores below two indicated negative results, while two or more were positive (21).
2.2.4 Relationship between CD276 expression and survival of BLCA patients
The postoperative survival data for a cohort of 133 patients with BLCA were meticulously examined, with the follow-up extended until 2023. In this study, we investigated the association between CD276 expression and patient outcomes, specifically PFS and OS.
2.3 Statistical analysis
The gene expression data were normalized using log transformations. Data visualization was performed using the R software (version 4.2.1) along with auxiliary packages incorporating tools such as stats, limma, ggpubr, survival, survey, survminer, surveillance, ggplot2, DESeq2, and edgeR. Normal distributions are delineated by mean ± standard deviation (SD) and analyzed using t-tests. Alternatively, non-normally distributed data were expressed as medians (interquartile range, IQR) and examined using the Wilcoxon rank-sum test. The chi-squared test was used for categorical data assessment. Kaplan-Meier analysis facilitated the creation of survival curves, while the log-rank test was used for survival data comparison. Statistical significance was set at p < 0.05.
3 Results
3.1 The expression of CD276 is elevated in bladder cancer tissues in TCGA database
In this study, we assessed 408 BLCA specimens and 19 noncancerous tissue samples from the TCGA-BLCA dataset. As depicted in Figure 1, the results demonstrated that the median CD276 expression level in cancerous tissues was markedly elevated at 4.703, whereas in normal bladder tissues, the median expression was notably lower at 3.594. This pronounced discrepancy clearly indicates that CD276 is overexpressed in BLCA tissues compared with adjacent non-tumor tissues. Statistical analysis confirmed a significant difference in the CD276 expression between malignant bladder tissues and adjacent healthy tissues (p<0.001, Figure 1).
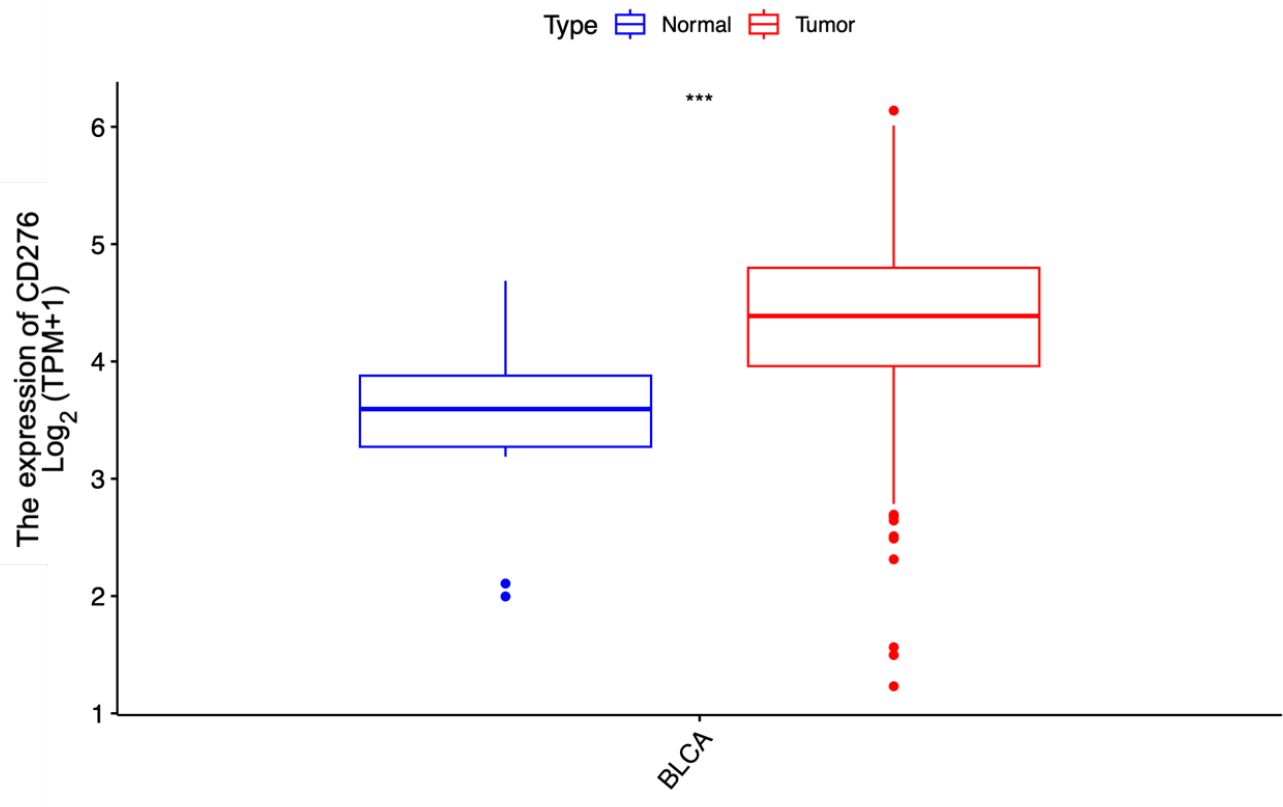
Figure 1. Expression of CD276 in bladder cancer tissues and para-cancer tissues in the TCGA database and the difference between the two groups. A significant difference was noted in the expression level of CD276 between 408 tumor tissues and 19 para-cancer tissues (p<0.001). CD276, cluster of differentiation 276; TCGA, The Cancer Genome Atlas, TPM, Transcripts Per Million; ***, p<0.001.
3.2 Association analysis between CD276 and gender, age, pathological grading, and staging of bladder tumors (436 cases) in TCGA database
Integrating CD276 expression data with clinical characteristics of BLCA patients from the (TCGA) database, we examined the variability of CD276 expression across various demographic and pathological variables. Our analysis of sex-specific expression revealed that CD276 levels remained remarkably consistent across sexes, showing no statistical significance in this disparity (Figure 2A). In contrast, further investigation into age, pathological grades, and tumor stages highlighted a clear pattern of differential CD276 expression; CD276 levels were notably higher in older patients, suggesting a potential age-related increase in expression (Figure 2B, p<0.05). Examining the pathological grades, we discovered a pronounced elevation of CD276 in high-grade urothelial carcinoma compared to its lower expression in its low-grade counterparts, with this variation being statistically significant (Figure 2C, p<0.001). Additionally, staging analysis revealed that CD276 expression intensified in the middle-to-late stages, especially in cases with lymph node involvement or distant metastasis. In contrast, early stage and non-metastatic tumors manifested lower CD276 levels, with the difference between advanced and early-stage patients being significant (Figure 2D, p<0.01). These findings indicate that high CD276 expression is significantly associated with advanced age, the presence of distant metastasis, and advanced disease stages, signifying deeper muscle invasion and a less favorable prognosis in patients with BLCA.

Figure 2. Expression and relationship of CD276 with different clinical characteristics in bladder cancer in TCGA database. (A)CD276 Expression levels in bladder tumor patients of different genders. (B) Expression level of CD276 in bladder tumors of different age groups (p<0.05). (C) Expression level of CD276 in bladder tumor tissues with different pathological grades (p<0.001). (D) Expression level of CD276 in bladder tumor tissues of different staging groups (p<0.01). CD276, cluster of differentiation 276; TCGA, The Cancer Genome Atlas; BLCA, bladder cancer, TPM, Transcripts Per Million.
3.3 Survival analysis of BLCA patients between high expression and low expression of CD276 in TCGA database
Our survival analyses indicated that elevated CD276 expression associated with the OS, DSS, and PFS among patients with BLCA (Figure 3). Specifically, we observed that increased levels of CD276 portend a diminished OS, with the category expressing higher CD276 levels, revealing significantly lower survival rates compared to the low-expression cohort (Figure 3A, p=0.006). A similar trend was noted for both DSS and PFS, where the high CD276 expression group exhibited markedly lower survival rates for DSS (Figure 3B, p=0.008) and PFS (Figure 3C, p=0.038). Based on these findings, we inferred a strong association between high CD276 expression and reduced OS and suboptimal prognostic outcomes in patients with BLCA.
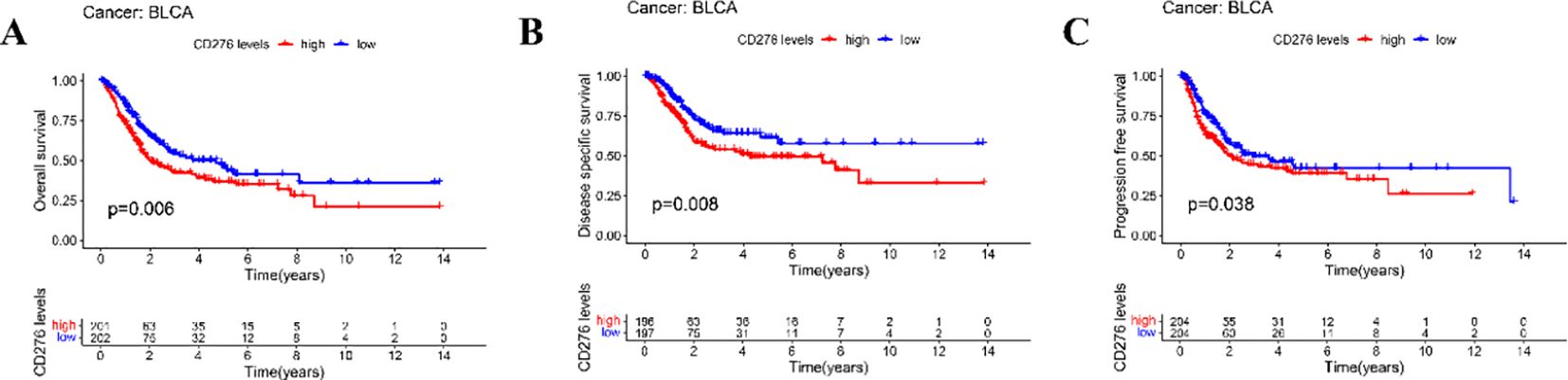
Figure 3. Relationship between the expression of CD276 in bladder cancer and survival of patients in TCGA database. (A) Association between the expression of CD276 and OS in bladder cancer patients(p=0.006). (B) Association between CD276 expression and DSS in bladder cancer patients (p=0.008). (C) Association between the expression of CD276 and PFS in bladder cancer patients(p=0.038). CD276, cluster of differentiation 276; TCGA, The Cancer Genome Atlas; OS, overall survival; DSS, disease-specific survival; PFS, progression-free survival; BLCA, bladder cancer.
3.4 The expression of CD276 is elevated in bladder cancer tissues in GEO database
We also extracted CD276 expression data for 9 bladder cancer tissues and 3 adjacent normal tissues from the GEO dataset GSE7476. Our analysis further confirmed that CD276 expression was significantly higher in bladder cancer tissues compared to adjacent normal tissues (7.769 ± 0.276 vs. 7.303 ± 0.161, p=0.022, Figure 4).
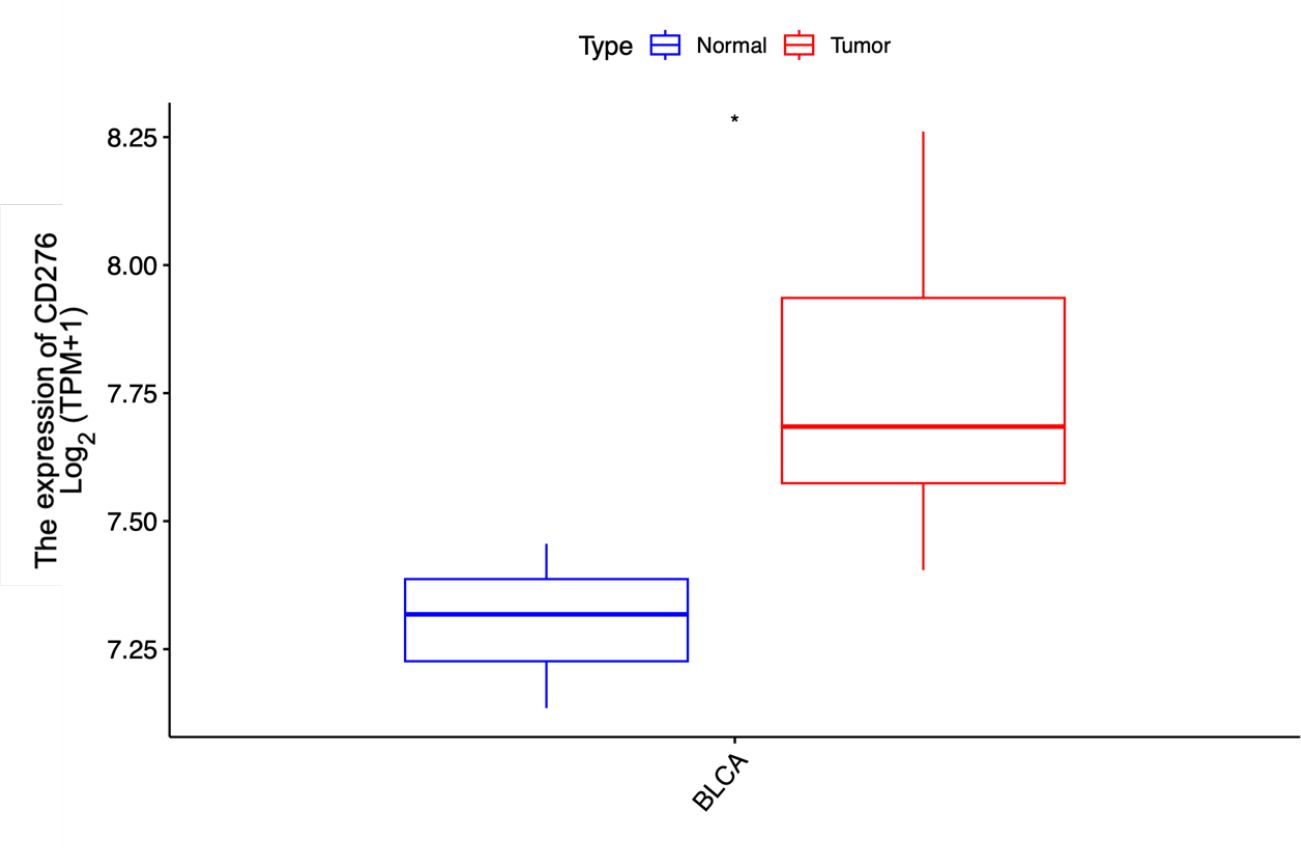
Figure 4. Expression of CD276 in bladder cancer tissues and para-cancer tissues in the GEO database and the difference between the two groups. The expression level of CD276 in 9 bladder tumor tissues was higher than that in 3 para-cancer tissues. CD276, cluster of differentiation 276; GEO, Gene Expression Omnibus, TPM, Transcripts Per Million; *, p<0.05.
3.5 Gene set enrichment analysis for pathways related to CD276 in patients with bladder cancer
To elucidate the regulatory mechanism of CD276 in bladder cancer, GSEA and pathway visualization were conducted based on the differentially expressed genes between BLCA tissues and control samples (Figure 5A). GSEA analysis revealed that high CD276 expression was significantly enriched in core matrisome, matrisome, extracellular matrix organization, and ECM glycoproteins pathways (Figures 5B–E).

Figure 5. The high CD276 expression group in patients with bladder cancer based on GSEA. (A) The top 10 pathways enriched in the high CD276 expression data set; (B–E) The high CD276 expression data set was enriched in core matrisome, matrisome, extracellular matrix organization, and ECM glycoproteins pathways. CD276, cluster of differentiation 276; GESA, gene set enrichment analysis.
3.6 The relationship between the expression of CD276 in bladder cancer and clinic-pathological characteristics in clinical data
Upon dissecting the interplay between CD276 expression levels and a range of clinical attributes in 133 BLCA specimens, we found no major disparities in CD276 expression based on sex, age, body mass index (BMI), history of hypertension (HBP), diabetes mellitus (DM), hyperlipidemia, tumor diameter, or the presence of multiple tumors (Table 2, p>0.05). However, a significant divergence was observed when the CD276 expression was compared across different pathological grades. Specifically, high-grade urothelial carcinoma showed elevated CD276 expression, reinforcing the connection between high CD276 levels and more aggressive cancer grades (Table 2, p<0.05). By delving deeper into the relationship between CD276 expression and postoperative tumor staging, we discovered an increasing trend in CD276 levels in relation to advancing tumor stages. Statistical analyses confirmed that these differences in expression were discernible across different stages, indicating a broader implication of CD276 in the progression of BLCA (Table 2, p<0.05).

Table 2. Association between expression of CD276 and clinical features of bladder urothelial cell carcinoma.
3.7 The expression of CD276 in cancer and para-cancer tissues in clinical data
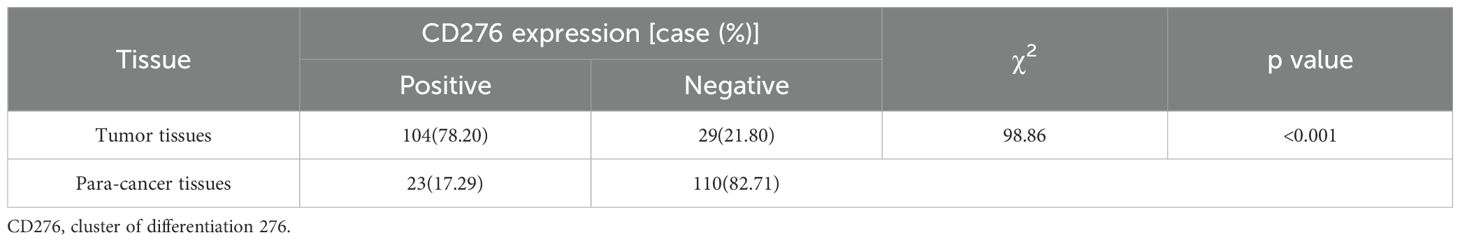
IHC analysis was conducted on a cohort of 133 paired samples comprising BLCA tissues and their adjacent non-tumor counterparts, as depicted in Figure 6. IHC evaluation revealed that the prevalence of CD276 expression was substantially elevated in the cancerous cohort (78.20%) compared with that in the non-cancerous cohort (17.29%). These findings underscore a markedly increased expression of CD276 in cancerous tissues relative to neighboring non-cancerous tissues, with the difference being statistically significant (χ²=98.86, p<0.001, as shown in Table 3).
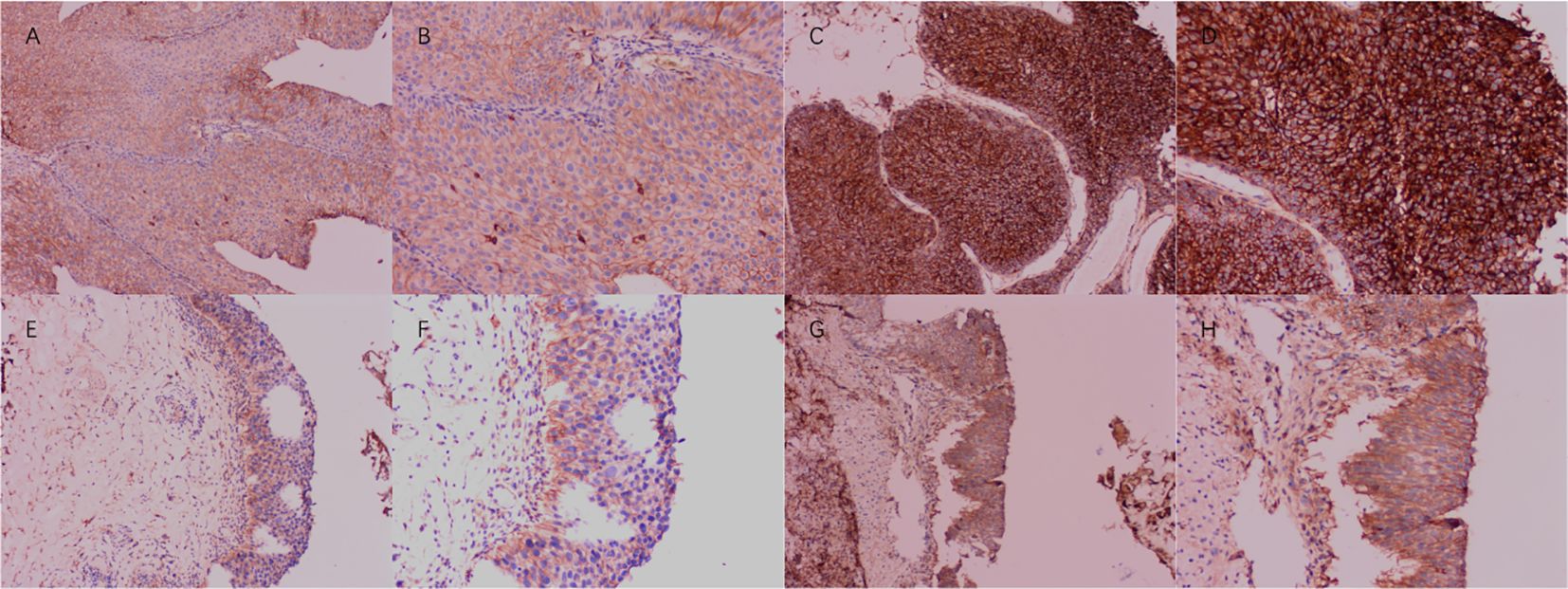
Figure 6. Expression of CD276 in bladder cancer and para-cancer tissues. (A) Negative expression of CD276 in bladder cancer (100x). (B) Negative expression of CD276 in bladder cancer (200x). (C) Positive expression of CD276 in bladder cancer (100x). (D) Positive expression of CD276 in bladder cancer (200x). (E) Negative expression of CD276 in para-cancer tissues (100x). (F) Negative expression of CD276 in para-cancer tissues (200x). (G) Positive expression of CD276 in para-cancer tissues (100x). (H) Positive expression of CD276 in para-cancer tissues (200x). CD276, cluster of differentiation 276.
3.8 Relationship between CD276 expression and survival of BLCA patients in clinical data
Examination of postoperative follow-up data from 133 patients with BLCA revealed an association between CD276 expression and survival. An analysis focusing on the association between CD276 levels and PFS revealed that elevated CD276 expression was associated with diminished PFS (Figure 7A, p<0.001, hazard ratio [HR]=0.43). Similarly, when assessing the impact of CD276 expression on the OS, a pattern was observed in which increased CD276 levels corresponded to reduced OS (Figure 7B, p<0.001, HR=0.35). Elevated CD276 expression is associated with patient survival outcomes in BLCA, with higher levels indicating a truncated survival span and a poorer prognostic outcome.
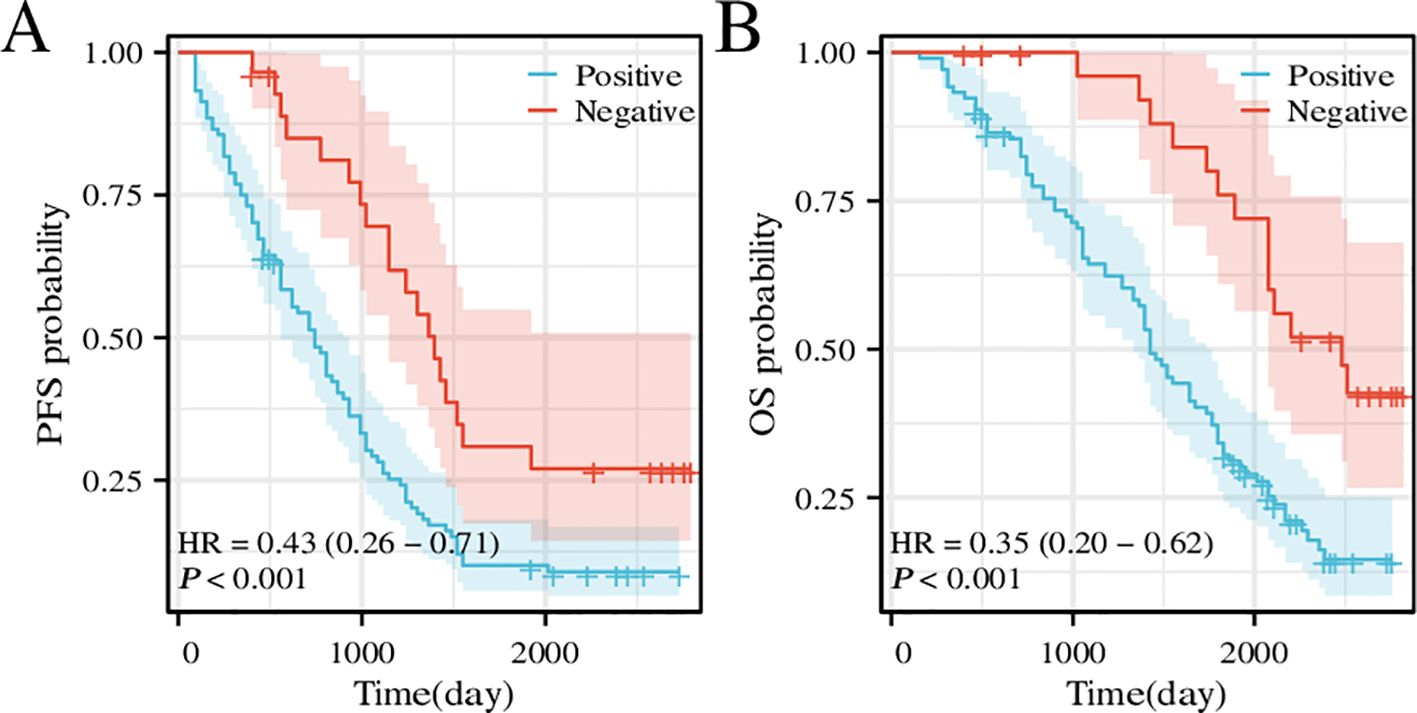
Figure 7. Survival curve of PFS and OS for patients with high or low CD276 expression in bladder cancer. (A) Survival curve of PFS for patients with high or low CD276 expression in bladder cancer. (B) Survival curve of OS for patients with high or low CD276 expression in bladder cancer. CD276, cluster of differentiation 276; PFS, progress-free survival; OS, overall survival; HR, hazard ratio.
4 Discussion
BLCA is the most common urinary tract malignancies in China, and postoperative chemotherapy is the primary strategy to combat this cancer. However, resistance to chemotherapy often results in treatment failure, posing a severe risk to patient survival. The introduction of immunosuppressive agents has significantly altered the treatment landscape for numerous solid cancers (22), although the effectiveness of such immunotherapies is restricted to selective tumor profiles (23). Among the various immune checkpoints that have been investigated, CD276 has attracted considerable attention. Blocking CD276 has shown promise for the treatment of various solid cancers as it is a protein prolific protein across tumor sites, affecting tumor cells, dendritic cells, and macrophages. Thus, its inhibition can have far-reaching effects. CD276 is an immunomodulatory protein that promotes cancer cell growth. It also inhibits immune cell-mediated tumor responses and promotes cancer cell differentiation (24). Regulatory T-cells (Tregs) induce interleukin-10 and CD276 expression in dendritic cells (DCs), conferring immunosuppressive properties. Upon Treg exposure, DCs upregulate CD276 and reduce major histocompatibility complex peptide complex presentation, thereby weakening T cell activation. Hence, CD276 is critical for the T cell-mediated suppression of DCs. However, the mechanism of action of CD276 is yet to be fully elucidated. Moreover, CD276 is implicated in pathways such as PI3K/AKT, NF-KB, Ras/Raf/MEK/MAPK, JAK2/STAT3, and glucose metabolism (24).
In recent years, substantial advancements have been made in the field of tumor immunotherapy, notably the triumphant integration of the B7 family molecule PD-L1 and its receptor PD-1 inhibitors into clinical practice, heralding new perspectives for cancer treatment. B7-H3 is a newly identified member of the B7 lineage and is frequently upregulated in a wide spectrum of human malignancies, where its elevated expression often parallels the progression, prognosis, or classification of tumors (25). Although the precise biological role and mechanisms underlying its contribution to immune evasion are the subjects of ongoing investigation, CD276’s pronounced expression in cancerous tissues and vasculature, including its prevalence in as many as 98% of renal cell carcinoma (RCC) blood vessels (26), underscores its potential as a distinctive and potent vessel-specific marker of cancer. Owing to its close association with the biological attributes of tumors, CD276 shows promise as an emergent biomarker target for cancer diagnostics, increasingly capturing the attention of the medical community.
This study investigated the association between CD276 expression within BLCA and patient prognosis, validating CD276 expression in these tumors through IHC assessment. Our research utilized RNA sequencing expression profiles and the corresponding clinical data for BLCA retrieved from TCGA database via the Xena platform. We scrutinized both the expression data and related clinical details. An initial review of CD276 expression in 408 BLCA specimens and 19 adjacent non-cancerous tissues revealed a markedly elevated level of CD276 in cancerous tissues (Figure 1, p<0.001). Data from another GEO dataset, GSE7476, further confirmed that CD276 expression is higher in bladder cancer tissues compared to adjacent normal tissues (Figure 4, p<0.05). Our subsequent analyses probed the relationship between CD276 expression in various samples and clinically relevant characteristics. We observed a uniform expression pattern of CD276 among patients with BLCA, irrespective of the sex. Nevertheless, enhanced levels of CD276 were more evident in older patients, those with high-grade urothelial carcinoma, and individuals exhibiting lymph node or distant metastases during advanced stages of the disease (Figure 2, p<0.05). This finding also supports the idea that elevated CD276 expression is indicative of advanced muscle invasion, distant metastasis, and unfavorable prognosis in individuals with BLCA. The association between high CD276 expression and OS was further corroborated by survival analysis, which identified a link between reduced survival odds in BLCA patients (Figure 3, p<0.05). We conducted a single-cell GSEA for CD276 to investigate its potential association with extracellular matrix-related pathways, including core matrisome, matrisome, extracellular matrix organization, and ECM glycoproteins pathways. The ECM is a component of all mammalian tissues, consisting predominantly of fibrous proteins like collagen, elastin and associated microfibrils, fibronectin, and laminins, embedded in a viscoelastic gel of anionic proteoglycan polymers. Beyond its structural role, the ECM performs various functions by influencing cell behaviors such as proliferation, adhesion, migration, and regulating cell differentiation and death as a crucial component of the cellular microenvironment (19, 27, 28). Bin Lim S et al. (27) reported an association between CD276 and the matrisome pathway in breast cancer. Additionally, Mei J et al. (28) found that CD276 expression had consistent associations with collagen signatures in bladder cancer, contributing to tumor metastasis and poor prognosis.
To reinforce our findings, we examined the association between CD276 expression levels and diverse clinical parameters in 133 BLCA samples obtained from the First Affiliated Hospital of Soochow University. Our analysis highlighted a more pronounced expression of CD276 in high-grade urothelial carcinoma, thereby upholding the established link between elevated CD276 levels and this aggressive cancer variant. Additionally, we observed a direct association between tumor stage advancement and increased CD276 expression (Table 2; p<0.05). Immunohistochemical assays revealed that CD276 was present in 78.20% of tumor specimens, as opposed to 17.29% of normal tissue samples. The CD276 expression is revealed to be significantly higher in tumor tissues compared to that in adjacent non-cancerous tissues (χ2 = 98.86, p<0.001, Table 3). This empirical evidence lends substantial weight to the initial conclusions.
In addition to CD276, which we have introduced, there are other urinary biomarkers for bladder cancer that have been used in clinical settings. For instance, the bladder tumor antigen (BTA) test has been utilized, but it shows a positive expression in only 54% of patients (29). Other reported gene expression measurements, such as CXCR2 and CDC2, are currently still in the experimental stages, including cell and animal studies (30, 31). These biomarkers have not yet been widely implemented in clinical practice, and their efficacy requires further validation through clinical trials. Beyond molecular biomarkers like CD276, recent studies have underscored the significance of the urinary microbiome in bladder cancer. Specifically, an analysis of patients undergoing transurethral resection of bladder tumors found an elevated presence of Porphyromonas and Porphyromonas somerae in morning urine samples from male bladder cancer patients, indicating their potential as specific predictive biomarkers (32). These findings support the hypothesis that a multifactorial diagnostic approach, combining molecular and microbiological biomarkers, could significantly improve the accuracy of bladder cancer diagnosis and prognosis.
Nevertheless, this study has certain limitations. Although our bioinformatics analysis was based on the TCGA and GEO databases, further validation of our findings through additional databases was still required. The clinical sample size was somewhat limited and originated from a single-center study. Consequently, the findings presented here should be treated with caution and further validated using larger-scale multicenter studies. Moving forward, we intend to delve deeper into the molecular mechanisms of action of CD276 in BLCA.
In conclusion, the findings of our study suggest that the CD276 expression is significantly associated with the prognostic outcomes of BLCA. Moreover, this expression associates with the disease pathological grade, stage, and occurrence of distant metastasis, demonstrating a robust predictive capacity for patient prognosis. Additionally, CD276 may promote the development and progression of bladder cancer through ECM-related pathways. These results further highlight CD276’s potential as an immunotherapeutic biomarker for BLCA.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QZ: Data curation, Formal analysis, Resources, Writing – original draft. JX: Formal analysis, Resources, Visualization, Writing – original draft. XC: Data curation, Formal analysis, Writing – original draft. JO: Funding acquisition, Project administration, Supervision, Writing – review & editing. CM: Methodology, Supervision, Writing – review & editing. ZZ: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by the Suzhou Science and Technology Project (Grant number: SLJ201906).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, et al. Bladder cancer. Lancet. (2016) 388:2796–810. doi: 10.1016/S0140-6736(16)30512-8
3. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: A review. JAMA. (2020) 324:1980–91. doi: 10.1001/jama.2020.17598
4. Deng-Xiong L, Qing-Xin Y, De-Chao F, Fa-Cai Z, Rui-Cheng W, Shi X, et al. Systemic immune-inflammation index (SII) during induction has higher predictive value than preoperative SII in non-muscle-invasive bladder cancer patients receiving intravesical bacillus calmette -guerin. Clin Genitourin Cancer. (2023) 21:e145–52. doi: 10.1016/j.clgc.2022.11.013
5. Jin YH, Zeng XT, Liu TZ, Bai ZM, Dou ZL, Ding DG, et al. Treatment and surveillance for non-muscle-invasive bladder cancer: a clinical practice guideline (2021 edition). Mil Med Res. (2022) 9:44. doi: 10.1186/s40779-022-00406-y
6. Pang C, Guan Y, Li H, Chen W, Zhu G. Urologic cancer in China. Jpn J Clin Oncol. (2016) 46:497–501. doi: 10.1093/jjco/hyw034
8. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
9. Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. (2001) 2:269–74. doi: 10.1038/85339
10. Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C, et al. Characterization of mouse and human B7-H3 genes. J Immunol. (2002) 168:6294–7. doi: 10.4049/jimmunol.168.12.6294
11. Guo C, Figueiredo I, Gurel B, Neeb A, Seed G, Crespo M, et al. B7-H3 as a therapeutic target in advanced prostate cancer. Eur Urol. (2023) 83:224–38. doi: 10.1016/j.eururo.2022.09.004
12. Malapelle U, Parente P, Pepe F, Di Micco MC, Russo A, Clemente C, et al. B7-H3/CD276 inhibitors: is there room for the treatment of metastatic non-small cell lung cancer? Int J Mol Sci. (2022) 23:16077. doi: 10.3390/ijms232416077
13. Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. (2003) 4:899–906. doi: 10.1038/ni967
14. Zhou WT, Jin WL. B7-H3/CD276: an emerging cancer immunotherapy. Front Immunol. (2021) 12:701006. doi: 10.3389/fimmu.2021.701006
15. Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. (2008) 14:5150–7. doi: 10.1158/1078-0432.CCR-08-0536
16. Saeednejad Zanjani L, Madjd Z, Axcrona U, Abolhasani M, Rasti A, Asgari M, et al. Cytoplasmic expression of B7-H3 and membranous EpCAM expression are associated with higher grade and survival outcomes in patients with clear cell renal cell carcinoma. Ann Diagn Pathol. (2020) 46:151483. doi: 10.1016/j.anndiagpath.2020.151483
17. Inamura K, Amori G, Yuasa T, Yamamoto S, Yonese J, Ishikawa Y. Relationship of B7-H3 expression in tumor cells and tumor vasculature with FOXP3+ regulatory T cells in renal cell carcinoma. Cancer Manag Res. (2019) 11:7021–30. doi: 10.2147/CMAR.S209205
18. Saeednejad Zanjani L, Vafaei S, Abolhasani M, Fattahi F, Madjd Z. Prognostic value of Talin-1 in renal cell carcinoma and its association with B7-H3. Cancer biomark. (2022) 35:269–92. doi: 10.3233/CBM-220018
19. Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. (2016) 22:3425–31. doi: 10.1158/1078-0432.CCR-15-2428
20. Zhang Z, Xu J, Song Z, Zhang J, Lin Y, Ouyang J, et al. Bioinformatic analysis and clinical case studies identify CD276 as a promising diagnostic biomarker for clear cell renal cell carcinoma. Cancer Control. (2024) 31:10732748241250181. doi: 10.1177/10732748241250181
21. Zhang Z, Zhou Q, Ouyang J, Pu J, Hou J, Zhang J. Expression and clinical significance of interleukin-9 in renal tumors. Transl Androl Urol. (2020) 9:2657–64. doi: 10.21037/tau-20-761
22. Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. (2017) 31:501–15.e8. doi: 10.1016/j.ccell.2017.03.005
23. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discovery. (2021) 11:838–57. doi: 10.1158/2159-8290.CD-20-1680
24. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. (2018) 50:1–11. doi: 10.1038/s12276-018-0191-1
25. Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. (2014) 134:2764–71. doi: 10.1002/ijc.28474
26. Qin X, Zhang H, Ye D, Dai B, Zhu Y, Shi G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets Ther. (2013) 6:1667–73. doi: 10.2147/OTT.S53565
27. Bin Lim S, Chua MLK, Yeong JPS, Tan SJ, Lim WT, Lim CT. Pan-cancer analysis connects tumor matrisome to immune response. NPJ Precis Oncol. (2019) 3:15. doi: 10.1038/s41698-019-0087-0
28. Mei J, Cai Y, Xu R, Li Q, Chu J, Luo Z, et al. Conserved immuno-collagenic subtypes predict response to immune checkpoint blockade. Cancer Commun (Lond). (2024) 44:554–75. doi: 10.1002/cac2.12538
29. Murphy WM, Rivera-Ramirez I, Medina CA, Wright NJ, Wajsman Z. The bladder tumor antigen (BTA) test compared to voided urine cytology in the detection of bladder neoplasms. J Urol. (1997) 158:2102–6. doi: 10.1016/S0022-5347(01)68167-9
30. Ismail NFB, Foth M, Yousef ARE, Cui N, Leach JDG, Jamieson T, et al. Loss of Cxcr2 in myeloid cells promotes tumour progression and T cell infiltration in invasive bladder cancer. Bladder Cancer. (2022) 8:277–90. doi: 10.3233/BLC-211645
31. Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. (2007) 6:3039–48. doi: 10.1158/1535-7163.MCT-07-0281
Keywords: CD276 expression, bladder cancer, prognosis, immunohistochemistry, bioinformatics database
Citation: Zhou Q, Xu J, Chen X, Ouyang J, Mao C and Zhang Z (2024) CD276 as a promising diagnostic and prognostic biomarker for bladder cancer through bioinformatics and clinical research. Front. Oncol. 14:1445526. doi: 10.3389/fonc.2024.1445526
Received: 07 June 2024; Accepted: 19 August 2024;
Published: 10 September 2024.
Edited by:
Ricardo Ribeiro, Universidade do Porto, PortugalReviewed by:
Savio Domenico Pandolfo, Federico II University Hospital, ItalyTianyi Zhou, University of Houston, United States
Copyright © 2024 Zhou, Xu, Chen, Ouyang, Mao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyu Zhang, YWJuZXJfNjY2QDEyNi5jb20=
†These authors have contributed equally to this work
 Qi Zhou
Qi Zhou Jianhao Xu2†
Jianhao Xu2† Zhiyu Zhang
Zhiyu Zhang