- 1Department of Radiation Oncology, King Hussein Cancer Center, Amman, Jordan
- 2Department of Medical Oncology, King Hussein Cancer Center, Amman, Jordan
- 3Department of Biostatistics, King Hussein Cancer Center, Amman, Jordan
- 4Department of Internal Medicine, Ibn Al-Hytham Hospital, Amman, Jordan
- 5Department of Surgical Oncology, King Hussein Cancer Center, Amman, Jordan
- 6Department of Special Surgery, Jordan, University of Science and Technology, Irbid, Jordan
- 7Department of Nuclear Medicine, King Hussein Cancer Center, Amman, Jordan
- 8Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, ON, Canada
Purpose: To evaluate clinical outcomes and prognostic factors in non-metastatic oral cavity squamous cell carcinoma (OCSCC) patients who underwent surgery with or without adjuvant therapy.
Methods: From 2007 and 2018, 116 patients were analyzed. The primary endpoint was overall survival (OS), and secondary endpoints were disease-free survival (DFS), local failure (LF), regional failure (RF), and distant metastases (DM). Kaplan-Meier method and log-rank test assessed survival outcomes, while Cox proportional hazard tests analyzed prognostic factors.
Results: Median patient age was 53 years, most were smokers (93.5%) and males (62.9%). Predominant subsite was the oral tongue (58.6%). Treatment included surgery alone (16.4%), adjuvant radiotherapy (46.6%), or adjuvant concurrent chemoradiotherapy (CCRT) (37%). The median follow-up time was 45.9 months. There were significant differences between groups in terms of gender (P=0.028) and RT dose (P=0.01). The 3-year OS, DFS, LF, RF and DM for the entire cohort were 60.9%, 55.1%, 20.11%, 8.43%, and 17.13%, respectively. Surgery alone yielded higher 3-year OS (81.4%) than adjuvant RT (70%) or adjuvant CCRT (41.4%), (p=0.012). Adjuvant CCRT correlated with higher LF compared to adjuvant RT and surgery alone groups (p=0.029). Lymphovascular invasion (LVI) impacted OS (HR=2.034, p=0.0498) and DM (HR=3.380, p=0.0132), while higher tumor grade increased DM likelihood (HR=8.477, p=0.0379).
Conclusions: This study reports OCSCC patient outcomes in Jordan across different treatment modalities. Adjuvant CCRT correlated with higher LF rates, and LVI impacted OS and DM, aligning with existing OCSCC treatment literature.
Introduction
Oral cavity cancer stands as one of the most common malignancies globally ranking among the top 10 incident cancers (1). The most common histology is squamous cell carcinoma (SCC). Tobacco and alcohol use are the main etiologic factors (2). Other risk factors include poor oral hygiene, betel nut chewing and immune suppression (3). Incidence of oral cavity squamous cell carcinoma (OCSCC) in the Arab countries is variable. In Syria, the incidence is 0.5/100,000 while it reaches up to 10/100,000 in the Southern parts of Saudi Arabia (4). In the Middle East and North Africa (MENA), newly diagnosed cases of OCSCC represents 1.5% of all malignancies (5). The crude incidence and mortality rates of these cancers are expected to double by 2030 (5). In Jordan, 97 new cases of OCSCC were reported in 2020, with 47 deaths due to the disease (6). The incidence is approximately twofold in males compared to females, possibly due to more frequent use of tobacco and alcohol among males (6).
The primary intervention for resectable OCSCC is surgery followed by risk-adapted postoperative radiotherapy (PORT) with or without concurrent chemotherapy (7, 8). Patients with higher risk of locoregional failure require PORT, such as those with pT3-4, pathologic nodal involvement (pN+), close resection margin, and other pathologic features e.g., high histologic grade, peri-neural invasion (PNI), lymphovascular space invasion (LVI), depth of invasion, and pENE (9). A combined analysis of the RTOG 9501 and EORTC 2291 phase III randomized controlled trials (10) showed that patients with pathologic extranodal extension (pENE) and/or involved resection margin(s) benefited from the addition of concurrent chemotherapy to postoperative RT.
This retrospective study aims to report the clinical outcomes associated with OCSCC and assess the potential prognostic factors in patients with non-metastatic OCSCC who underwent surgery with or without adjuvant RT or CCRT, within a single institution in Jordan.
Materials and methods
Study population
This is a retrospective review of OCSCC patients treated at King Hussein Cancer Center (KHCC) from January 2007 to October 2018. After institutional review board (IRB) approval (IRB No 22 KHCC 150), we included OCSCC patients above 18 years who underwent curative-intent surgical resection +/- postoperative RT. Cancer stage was reviewed according to the American Joint Committee on Cancer (AJCC8th edition) (11). Patients who had prior radiation to the head and neck (HN) area, radiation outside KHCC, received radiation dose less than 50Gy, or had poor Eastern Cooperative Oncology Group (ECOG) performance status >2 were excluded.
Diagnostic approach
The multidisciplinary pre-treatment assessment and staging process included a thorough medical history, comprehensive physical examination, imaging evaluation with HN MRI scans, as well as PET/CT and/or chest CT scans. Before initiating postoperative RT, specialized teams of dentistry, nutritionists, and speech pathologists conducted pre-RT evaluations for each patient.
Treatment approach
Patients with OCSCC are treated primarily with upfront surgical resection of the primary tumor with neck dissection. This is followed by adjuvant RT if the patient was considered at-risk of locoregional recurrence (e.g., close resection margin[s, pT3-4, pN2–3, involved node at levels IV-V, or presence of combined risk features e.g., PNI, LVI, high histologic grade, and pN1). If the patient had positive resection margin(s) and/or pENE, then they were considered to receive adjuvant platinum based CCRT (if deemed clinically suitable).
RT, ideally started within six weeks of surgery, and was delivered using 3‐dimensional conformal radiotherapy (3D‐CRT, until 2012) or intensity‐modulated radiation therapy (IMRT, after 2012) techniques (12). RT was given at a dose of 60Gy in 30 fractions if standard-risk, 66Gy in 33 fractions if high risk without residual/recurrent gross disease, and 70Gy in 35 fractions if early locoregional recurrent gross disease (i.e., prior to the planned postoperative RT). For patients who received adjuvant CCRT, the concurrent radiosensitizing chemotherapy was high‐dose cisplatin (100 mg/m2 given on days 1, 22, and 43) or low‐dose cisplatin (40 mg/m2 weekly during RT). For patients with contraindication to cisplatin, weekly carboplatin (area under the curve (AUC =1.5) was used instead of cisplatin.
Post-treatment evaluation and follow-up
Post-treatment imaging included HN MRI scans and PET/CT, conducted 10–12 weeks post-RT completion. Subsequent follow-up appointments occurred every three months during the initial two years, then every four months in the third year, every six months in the fourth and fifth years, and annually thereafter.
Statistical methods
The primary endpoint was OS. Secondary endpoints were disease-free survival (DFS), local failure (LF), regional failure (RF), and distant metastases (DM). The OS and DFS were analyzed using the Kaplan-Meier method and compared using log-rank test. LF, RF, and DM rates were calculated using the cumulative incidence method, with death as a competing risk. Outcomes were calculated from the date of diagnosis to the first event. Multivariable analysis (MVA) using Cox proportional hazards regression was used to identify predictors of OS, DFS and DM. All reported p values were 2-sided, with a statistical significance level of p < 0.05. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC), and the figures were generated using GraphPad PRISM 7.
Results
Patient, tumor, and treatment characteristics
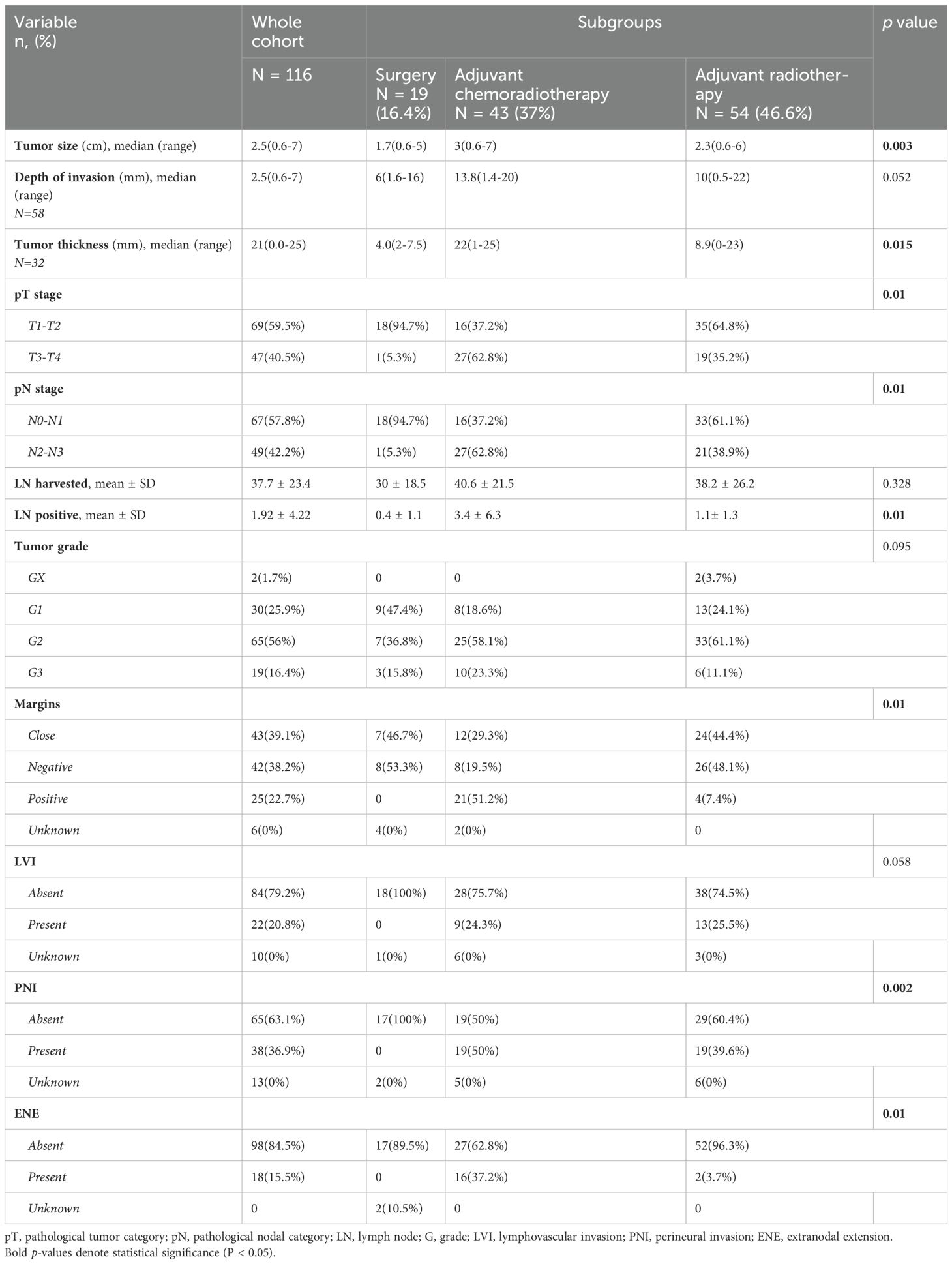
Patient, tumor, and treatment characteristics are summarized in Table 1. A total of 116 patients with OCSCC were included in our study, with a median age of 53 years. Median follow-up time for the whole cohort was 46 (1.2-144 months), 73 (62.9%) were male, 101 (93.5%) were smokers, while only 4 (3.9%) drank alcohol. The most common subsite of disease was the oral tongue 68 (58.6%), followed by the buccal mucosa 17 (14.7%).
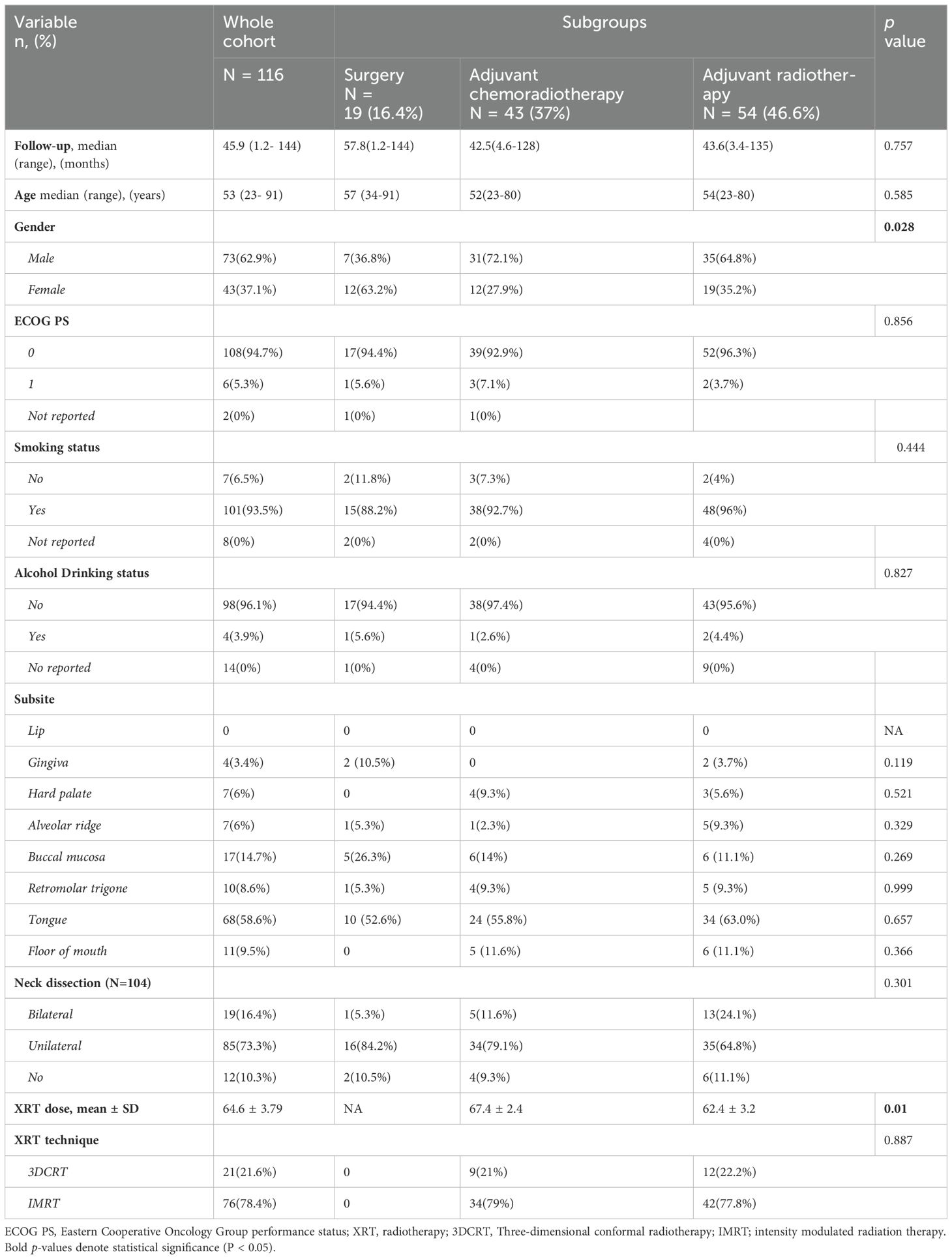
Table 1. Demographic, clinical, and treatment characteristics of patients with oral cavity squamous cell carcinoma.
Regarding treatment, 19 (16.4%) were treated with surgery alone, 54 (46.6%) received adjuvant RT, and 43 (37%) received adjuvant CCRT. 19 patients (16.4%) had bilateral neck dissection, 85 (73.3%) underwent unilateral neck dissection, while 12 patients (10.3%) managed with resection of the primary cancer without neck dissection. In those who received RT, the median dose was 66 Gy (range 58-74 Gy), and most patients were treated with IMRT (n=76,78.4%). There were significant differences between the treatment groups in terms of gender (p = 0.028), see Table 1. Pathological characteristics are summarized in Table 2.
Survival outcomes
For the entire cohort, the 3‐year OS and DFS were 60.9% (95% CI, 50.9%-70.4%) and 55.1% (95% CI, 45.3%-64.7%), respectively (Figure 1). The 3-year OS for the surgery alone cohort was 81.4% (95% CI, 59.4%-96%), for the surgery followed by adjuvant RT cohort was 70% (95% CI, 55.5%-87.2%), and for the surgery followed by adjuvant CCRT cohort was 41.4% (95% CI, 26.2%-57.6%), (p=0.01). The 3-year DFS was 70.6% for the surgery alone cohort (95% CI, 47.5%-89.2%), 68.1% (95% CI, 54%,80.6%) for the surgery followed by adjuvant RT cohort (95% CI, 54%-80.6%), and 33.8% for the surgery followed by adjuvant CCRT cohort (95% CI, 19.9%-49.3%), (p=0.03).
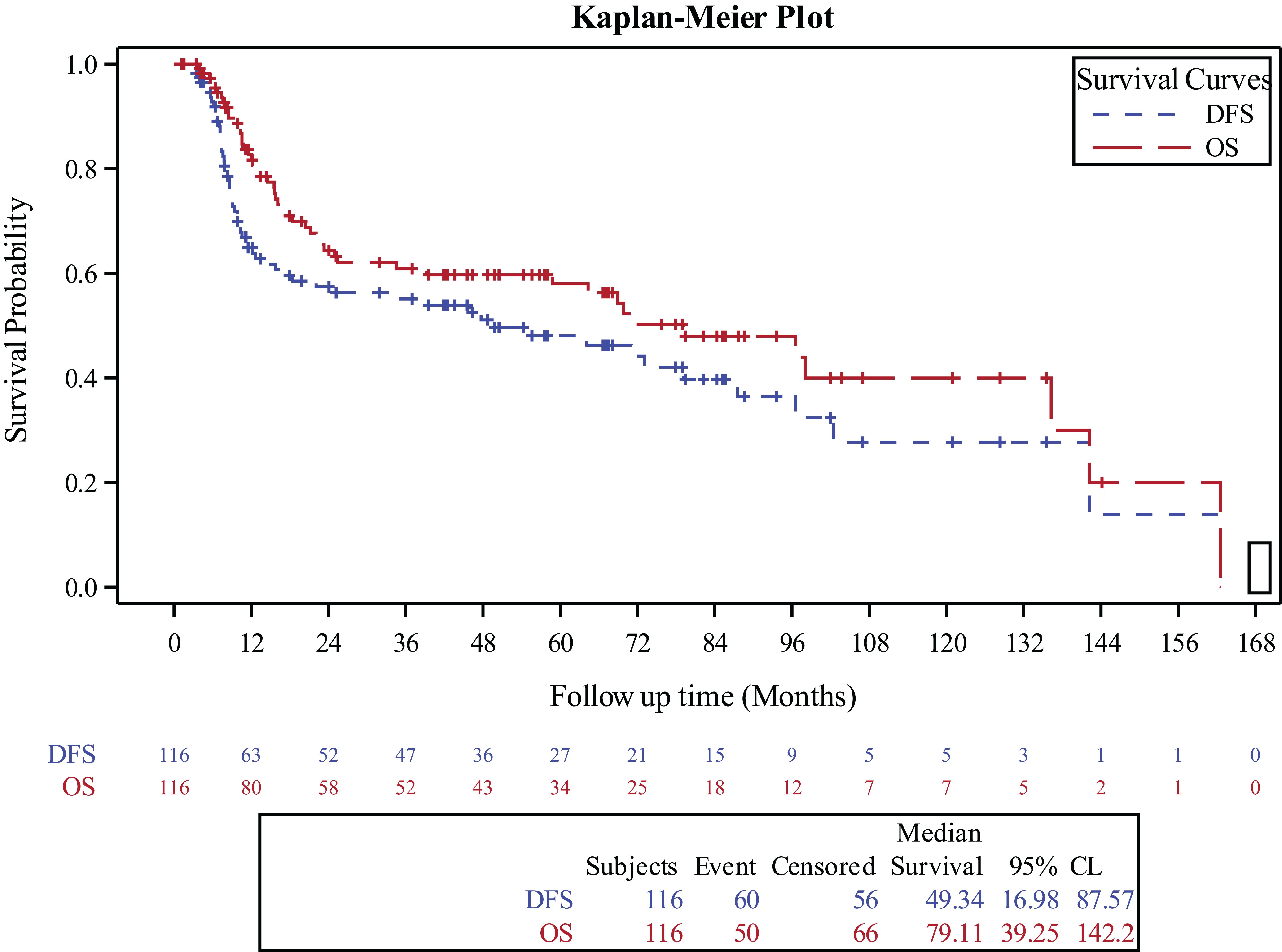
Figure 1. Kaplan-Meier curve for overall survival and disease free survival in the entire study population.
Tumor control outcomes
For the entire cohort, the 3-year LF, RF and DM cumulative incidence rates were 20.11% (95% CI, 12.3%–29.4%), 8.43% (95% CI, 3.9% –15.2%), and 17.13% (95% CI, 10.2% – 25.6%), respectively, as shown in Figure 2.
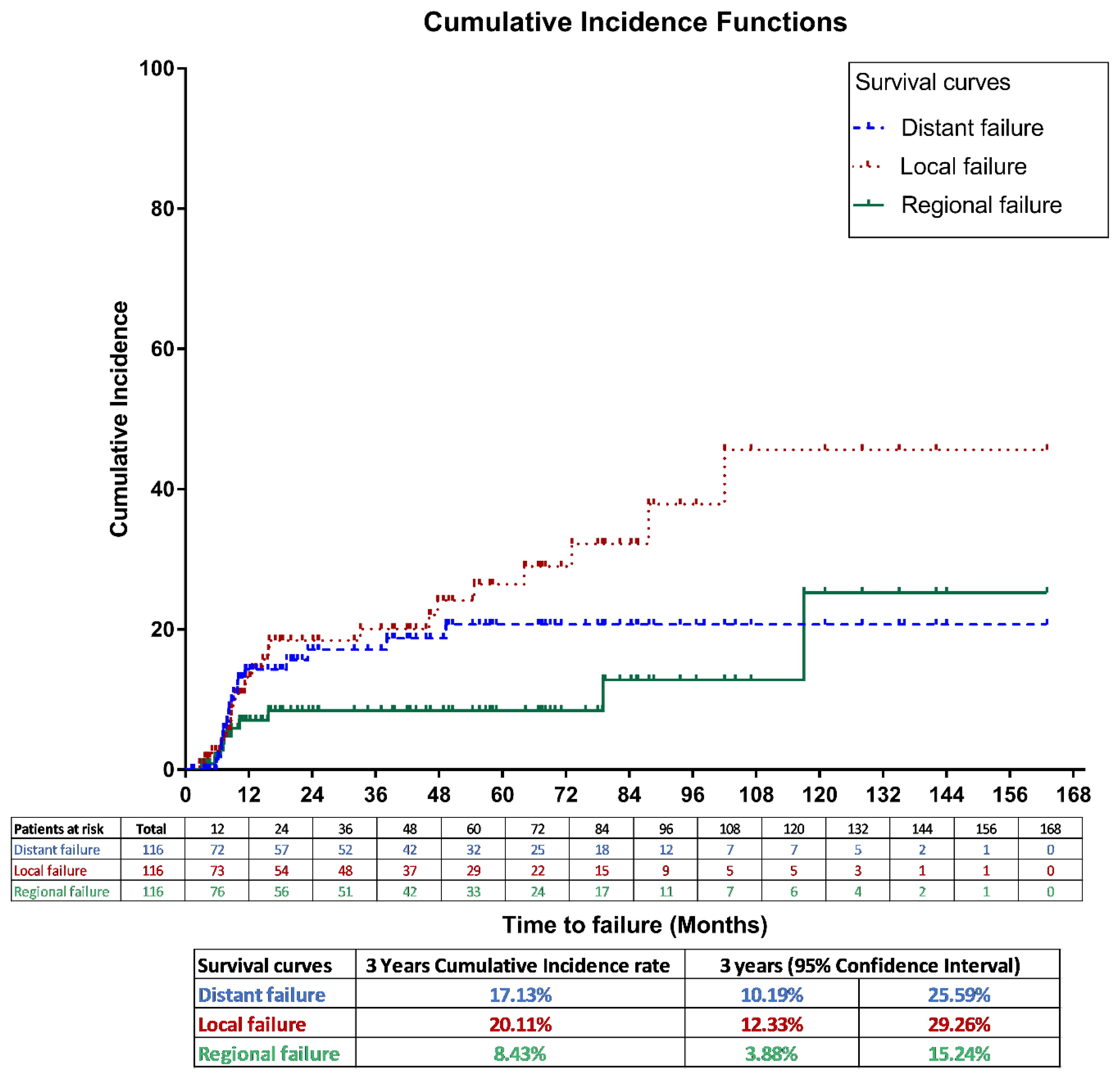
Figure 2. Cumulative incidence method for local, regional and distant failures in the entire study population.
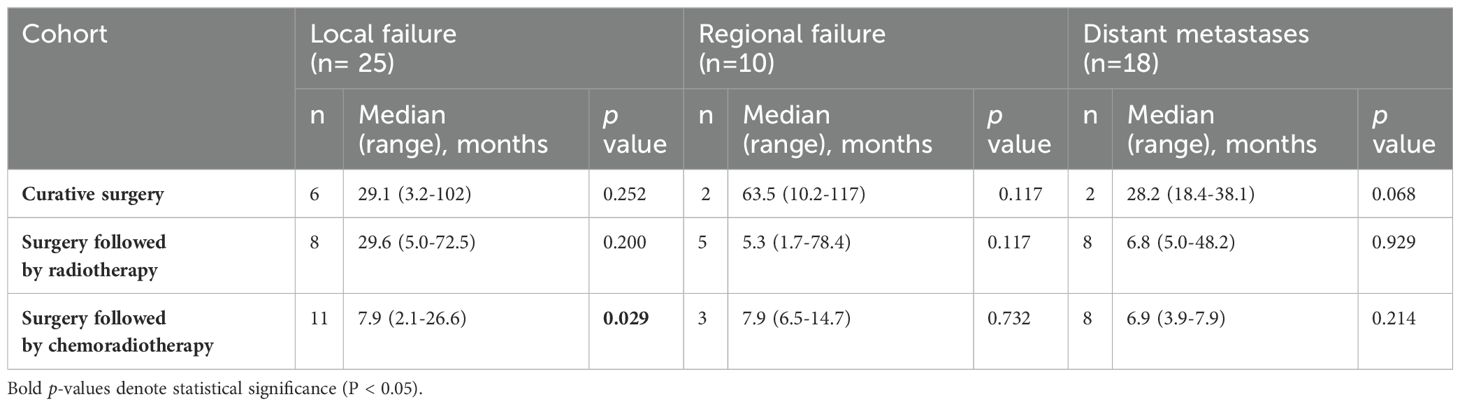
Patterns of failure are shown in Table 3. For patients who underwent curative surgery alone, LF was observed in 6 cases after a median duration of 29 months post treatment (range 3-102).Among patients in this cohort who developed LF, half (n=3) were managed with salvage surgery followed by adjuvant RT, while the other half were managed with salvage surgery followed by adjuvant CCRT. RF occurred in 2 cases after a median duration of 10-and 117 months post-surgery, whilee DM were observed in 2 cases with a median duration of 18 and -38 months post-surgery. Among the two patients in this cohort who developed RF both had previously undergone unilateral neck dissection. One developed recurrence at bilateral level III cervical nodes, and was managed by salvage surgery followed by adjuvant CCRT, while the other developed recurrence at the ipsilateral previously dissected level II cervical nodes, and was managed by salvage surgery followed by adjuvant RT.
In the cohort who received surgery followed by adjuvant RT, LF was reported in 8 cases with a median duration of 29.6 months post treatment (range 5.0-72.5), RF in 5 cases with a median duration of 5.3 months (range 1.7-78.4), and DM in 8 cases with a median duration of 6.8 months (range 5.0-48.2). Patients in this cohort who developed LF were managed with either salvage surgery followed by adjuvant re-RT (n=3), salvage surgery followed by adjuvant CCRT (n=2), re-RT (n=1), or CCRT (n=1). Among the patients in this cohort who developed RF, one had previously undergone unilateral neck dissection, and recurrence occurred in the contralateral undissected neck at level IV. Meanwhile, two had undergone bilateral neck dissection, developed recurrences at level II and III, and were treated with salvage surgery followed by adjuvant RT. Two others had had no neck dissection done in the past, developed recurrence at level II, and were managed in one instance by CCRT, and in the other by palliative chemotherapy, as this patient had developed distant metastases as well.
Notably, the surgery followed by CCRT cohort demonstrated a statistically significant association with LF, as evidenced by 11 cases and a median duration of 7.9 months (range 2.1-26.6). For RF (3 cases, median 7.9 months, range 6.5-14.7) and DM (8 cases, median 6.9 months, range 3.9-7.9), no statistically significant associations were observed. Among patients in this cohort who developed LF, four were managed with chemotherapy alone, three were managed with re-RT, and one was managed with salvage surgery followed by adjuvant CCRT. Among the three patients in this cohort who developed RF, all three had previously undergone unilateral neck dissection. Two developed RR in the contralateral previously undissected neck at levels IV and VIII, while the other patient developed RR in the ipsilateral previously dissected neck at level I. One was managed by re-RT, one was managed by salvage surgery followed by adjuvant re-RT, and one was managed by salvage surgery followed by adjuvant CCRT. Finally, among patients who developed DM without LF or RF, six were found in the cohort treated with surgery followed by adjuvant RT, and five were found in the cohort treated with surgery followed by adjuvant CRT. Five of these patients’ DM were managed by RT, while three were managed by palliative chemotherapy.
Predictors of survival
Table 4 describes the multivariate analysis of prognostic factors for OS, DFS and DM. In the comparison of treatment cohorts, patients who underwent surgery followed by CCRT did not show a significant difference in OS compared to those who had surgery alone (HR=3.135, 95% CI 0.643-15.278, p=0.157). Similarly, the combination of surgery followed by adjuvant RT did not demonstrate a significant impact on OS compared to surgery alone (HR=1.819, 95% CI 0.400-8.273, p=0.438). The corresponding results for DFS were also not statistically significant.

Table 4. Multivariable analysis of prognostic factors for overall survival, distant metastases free survival and disease-free survival.
Examining the impact of tumor stage (pT stage group), patients with T3 + T4 stages did not exhibit a significantly different OS compared to those with T1 + T2 stages (HR=1.136, 95% CI 0.539-2.395, p=0.736). The corresponding results for DFS were also not statistically significant. However, the tumor grade group G2 + G3 showed a trend towards worse OS compared to G1 (HR=2.386, 95% CI 0.994-5.727, p=0.051). This difference was more pronounced in DM, where the hazard ratio was 8.477 (95% CI 1.127-63.776, p=0.037).
The presence of LVI emerged as a significant prognostic factor regarding both OS (HR=2.034, 95% CI 1.001-4.136, p=0.049) and DM (HR=3.380, 95% CI 1.291-8.850, p=0.013). Margins status (negative vs. close, positive vs. close) did not significantly affect OS. However, the comparison of positive margins to close margins showed a trend towards worse OS for those with positive margins (HR=2.111, 95% CI 0.863-5.166, p=0.101). Similarly, the use of RT did not yield a statistically significant impact on OS, with a hazard ratio of 2.968 (95% CI 0.683-12.899, p=0.146).
Discussion
This study from Jordan shows the oncologic outcomes of patients with OCSSC who were treated with curative surgery, surgery followed by RT, or surgery followed by CCRT at KHCC. This study demonstrated a 3‐year OS and DFS of 60.9% and 55.1%, respectively for the entire cohort. The presence of LVI and tumor grade group were both found to be significantly associated with DM, while only the presence of LVI was found to be associated with OS. The patients who received surgery followed by CCRT were significantly more likely to develop LF. Although treatment of OCSCC needs specialized care with advanced surgical and radiation oncology techniques, our outcomes from a developing country are consistent with the outcomes of treating OCSCC in developed countries.
116 cases of OCSCC were included in our study. 59.5% of them had T1-T2 disease, while 40.5% had T3-T4 disease. Of the former group, 64.8% required adjuvant RT, while 37.2% required adjuvant CCRT. These percentages are higher than those reported in a UK study (13), where only 19.1% of T-T2 cases required adjuvant RT and 2.6% required adjuvant CCRT. However, most of the reported cases in that study were N0, unlike ours. The most common subsites were the oral tongue (58.6%), followed by the buccal mucosa (14.7%), the floor of the mouth (9.5%) and the retromolar trigone (8.6%). These results are similar to those of two other studies in the UAE, in which the most common subsites were also the oral tongue and the buccal mucosa (14, 15). However, another study covering the epidemiology of oral cancer in Arab countries reported the most common sites to be the oral tongue and the lips (4). This is in contrast to our study, in which no squamous cell carcinomas of the lip were reported. This is due to the different classification of tumors at our institution, where lip cancers are classified as skin cancers, rather than cancers of the oral cavity anatomic subsite which was proven to be associated with tumor size and prognosis (16). One study in the UAE showed better prognosis for cancers of the tongue and buccal mucosa (14); meanwhile, another study found cancers of the oral tongue to be associated with worse OS, and higher rates of LF and DM (16).
Tobacco and alcohol consumption are widely recognized as primary risk factor for OCSSC, whereas in our region, the significance of alcohol is obscured by social and religious constraints (5). Notably, 93.5% of the patients in our study were smokers. This is similar to the results of other countries in the region, namely Egypt, Iraq and Libya, where more than half of patients afflicted with OCSSC were smokers (4). Smoking, both of cigarettes and hookah (water-pipe), is highly prevalent in Jordan – 57.4% of males and 12.7% of females are current smokers, amounting to 2.3 million smokers across the country, and leading to 3.2 thousand annual deaths attributable to smoking (17). Even more worrisome is the prevalence of smoking among the youth of Jordan. According to the World Health Organization’s (WHO) Global Youth Tobacco Survey (18), 23.2% of students aged 13-15 in Jordan are current tobacco smokers, with another 18.2% describing themselves as having never smoked, but are susceptible to tobacco use in the future. A case-control study conducted in Jordan found that hookah smoking especially was an independent risk factor associated with development of oral cancer at a younger age, and that hookah smokers were significantly younger when diagnosed with oral cancer (19).
Our study demonstrated a 3‐year OS of 60.9%. This is comparable to a Portuguese study, in which 3-year OS was 58.6% (20). A Chinese study done on OCSSC patients under the age of 45, however, demonstrated a 3-year OS rate of 77%; this is consistent with other published series showing a relatively better survival among younger patients (21). Multiple factors are known to influence OS, including notably the invasion of tumor cells into surrounding tissue, whether it be blood or lymphatic vessels. In our study, the presence of LVI was significantly associated with worse OS (HR = 2.034, p = 0.049). These findings align with outcomes from other studies that have shown LVI as an independent adverse prognostic factor, linked to worse OS (22, 23). Additionally, while pT stage, histologic grade and margin status were not found to be significantly associated with OS in this study, other studies conducted elsewhere have confirmed the significance of the association (20, 24–26). DFS at 3 years in our study was found to be 55.1%, similar to the forementioned Portuguese study in which 3-year DFS was 55.4% (20). Neither treatment cohort nor pT stage were found to be associated with DFS in our study. Meanwhile, other studies have previously found that advanced tumor stage, in addition to other factors such as PNI, node-positive disease and positive margin status, were associated with worse DFS (20, 21, 25, 26).
Regarding DM, both advanced tumor grade (HR = 8.477, p = 0.037) and the presence of LVI (HR =3.38, p = 0.013) were found to be significantly associated with higher potential for DM. The presence of LVI in particular indicates that tumor cells have entered the vascular compartment, heralding the potential for metastases (27). These results are consistent with those of other studies, which found risk factors for the development of DM to include advanced tumor grade and LVI, in addition to other factors such as tumor thickness, pENE, and lymph node metastases to levels IV and V (28–30).
The primary contributors to mortality related to OSCC are local and regional recurrences, with the 5-year survival rate declining from 92% in patients without recurrence to 30% in those experiencing recurrence (31, 32). In our study, 25 patients (21.5%) developed LF, 10 patients (8.6%) developed RF and 18 patients (15.5%) developed DM. In comparison, studies from India, Qatar and China demonstrated LF rates of 2.72%, 11.7%, and 9.7% respectively (26, 32, 33). Additionally, in the Indian and Chinese studies, and in a study from Taiwan, RF rates were found to be 4.7%, 12.5%, and 32.7% respectively (26, 31, 32). Hence, while RF rates at our center appear to be similar to those found in other countries, LF rates appear to be higher. This may be attributable to the high prevalence of poor pathologic characteristics found in our patients, such moderate to poor differentiation of tumors (73.7%), close or positive margins (61.8%), and T3-T4 stage tumors (47%). Additionally, in our study, the cohort of patients treated by surgery followed by CCRT was significantly more likely to develop LF. According to available literature, the 5-year survival rates for these patients with locally advanced disease range from 11% to 64%. Relapse occurs in about one-third of patients, with locoregional recurrence being the most frequent pattern of failure (34). This may be attributable to the poor tumor characteristics seen in this high-risk group, such as positive postsurgical margins and ENE, which are known to be risk factors for reduced OS and LF (35).
Our study has a number of limitations. Firstly, the study’s retrospective nature may introduce selection bias. Additionally, as this is a single-institution study, the generalizability of findings to a broader population may be somewhat limited. Thirdly, patients with lip OCSSC were not included as an OCSCC in this study, as they are classified in our institution as skin cancers. Furthermore, we did not collect treatment related toxicity in this study.
Conclusions
We present the clinical outcomes of a cohort of patients with OCSCC treated with surgical resection alone, surgery followed by postoperative RT, or surgery followed by CCRT in Jordan. Those who received adjuvant CCRT were more likely to have LF, and LVI was associated with worse OS and DM. These outcomes are concordant with the known literature about the treatment of OCSCC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by King Hussein Cancer center review board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This is a retrospective study of patients with oral cavity cancer. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because This is a retrospective data of oral cavity cancer.
Author contributions
SA: Data curation, Methodology, Writing – original draft, Writing – review & editing. TH: Writing – review & editing. LW: Writing – review & editing, Conceptualization, Data curation. AA: Data curation, Writing – review & editing, Methodology. MB: Data curation, Writing – review & editing. MQ: Data curation, Writing – review & editing. MM: Data curation, Writing – review & editing. FA: Data curation, Writing – review & editing. RA: Writing – review & editing. AT: Writing – review & editing, Formal analysis. TH: Writing – review & editing, Data curation. MA: Writing – review & editing. OS: Writing – review & editing. WA: Writing – review & editing. AA: Writing – original draft. HG: Methodology, Writing – review & editing. AH: Methodology, Writing – review & editing, Validation. IM: Methodology, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. (1988) 48:3282–7.
3. Paré A, Joly A. Oral cancer: Risk factors and management. Presse Medicale (Paris France: 1983). (2017) 46:320–30. doi: 10.1016/j.lpm.2017.01.004
4. Al-Jaber A, Al-Nasser L, El-Metwally A. Epidemiology of oral cancer in Arab countries. Saudi Med J. (2016) 37:249–55. doi: 10.15537/smj.2016.3.11388
5. Kujan O, Farah CS, Johnson NW. Oral and oropharyngeal cancer in the Middle East and North Africa: Incidence, mortality, trends, and gaps in public databases as presented to the Global Oral Cancer Forum. Trans Res Oral Oncol. (2017) 2:2057178X17698480. doi: 10.1177/2057178x17698480
6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
7. Nandini DB, Rao RS, Hosmani J, Khan S, Patil S, Awan KH. Novel therapies in the management of oral cancer: An update. Disease-a-month: DM. (2020) 66:101036. doi: 10.1016/j.disamonth.2020.101036
8. Mohamad I, Glaun M, Prabhash K, Busheri A, Lai SY, Noronha V, et al. Current treatment strategies and risk stratification for oral carcinoma. Am Soc Clin Oncol Educ Book. (2023) 43:3–11. doi: 10.1200/EDBK_389810
9. Shah JP, Cendon RA, Farr HW, Strong EW. Carcinoma of the oral cavity. factors affecting treatment failure at the primary site and neck. Am J Surg. (1976) 132:504–7. doi: 10.1016/0002-9610(76)90328-7
10. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (22931) and RTOG (9501). Head Neck. (2005) 27:843–50. doi: 10.1002/hed.20279
11. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8ed. Chicago, USA: Springer Nature, Springer International Publishing AG (2017). p. XVII, 1032.
12. Mohamad I, Salem A, Abu Hejleh T, Wadi-Ramahi S, Qandeel M, El Khatib S, et al. Peer-assisted learning: intensity-modulated radiotherapy transition in developing countries. Clin Oncol (Royal Coll Radiologists (Great Britain)). (2017) 29:689–95. doi: 10.1016/j.clon.2017.06.002
13. Jerjes W, Upile T, Petrie A, Riskalla A, Hamdoon Z, Vourvachis M, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol. (2010) 2:9. doi: 10.1186/1758-3284-2-9
14. Al-Rawi NH, Hachim IY, Hachim MY, Salmeh A, Uthman AT, Marei H. Anatomical landscape of oral squamous cell carcinoma: A single cancer center study in UAE. Heliyon. (2023) 9:e15884. doi: 10.1016/j.heliyon.2023.e15884
15. Anis R, Gaballah K. Oral cancer in the UAE: a multicenter, retrospective study. Libyan J Med. (2013) 8:21782. doi: 10.3402/ljm.v8i0.21782
16. Zelefsky MJ, Harrison LB, Fass DE, Armstrong J, Spiro RH, Shah JP, et al. Postoperative radiotherapy for oral cavity cancers: impact of anatomic subsite on treatment outcome. Head Neck. (1990) 12:470–5. doi: 10.1002/hed.2880120604
17. World Health O. WHO global report on trends in prevalence of tobacco use 2000–2025. 4th ed. Geneva: World Health Organization (2021).
18. Organization WH. Global Youth Tobacco Survey, Vol. 2014. Geneva, Switzerland: World Heath Organization (WHO) (2014).
19. Al-Amad SH, Awad MA, Nimri O. Oral cancer in young Jordanians: potential association with frequency of narghile smoking. Oral Surg Oral Med Oral Pathol Oral Radiol. (2014) 118:560–5. doi: 10.1016/j.oooo.2014.08.002
20. Monteiro LS, Amaral JB, Vizcaíno JR, Lopes CA, Torres FOA. clinical-pathological and survival study of oral squamous cell carcinomas from a population of the North of Portugal. Med Oral Patol Oral y Cirugia Bucal. (2014) 19:e120–126. doi: 10.4317/medoral.19090
21. Fan Y, Zheng L, Mao M-H, Huang M-W, Liu S-M, Zhang J, et al. Survival analysis of oral squamous cell carcinoma in a subgroup of young patients. Asian Pacif J Cancer Prevent: APJCP. (2014) 15:8887–91. doi: 10.7314/APJCP.2014.15.20.8887
22. Huang S, Zhu Y, Cai H, Zhang Y, Hou J. Impact of lymphovascular invasion in oral squamous cell carcinoma: A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. (2021) 131:319–328.e311. doi: 10.1016/j.oooo.2020.10.026
23. Huang Q, Huang Y, Chen C, Zhang Y, Zhou J, Xie C, et al. Prognostic impact of lymphovascular and perineural invasion in squamous cell carcinoma of the tongue. Sci Rep. (2023) 13:3828. doi: 10.1038/s41598-023-30939-8
24. Bjerkli I-H, Jetlund O, Karevold G, Karlsdóttir Á, Jaatun E, Uhlin-Hansen L, et al. Characteristics and prognosis of primary treatment-naïve oral cavity squamous cell carcinoma in Norway, a descriptive retrospective study. PloS One. (2020) 15:e0227738. doi: 10.1371/journal.pone.0227738
25. Choi YS, Kim MG, Lee JH, Park JY, Choi SW. Analysis of prognostic factors through survival rate analysis of oral squamous cell carcinoma patients treated at the National Cancer Center: 20 years of experience. J Korean Assoc Oral Maxillofac Surgeons. (2022) 48:284–91. doi: 10.5125/jkaoms.2022.48.5.284
26. Suresh GM, Koppad R, Prakash BV, Sabitha KS, Dhara PS. Prognostic indicators of oral squamous cell carcinoma. Ann Maxillofac Surg. (2019) 9:364–70. doi: 10.4103/ams.ams_253_18
27. Batsakis JG. Invasion of the microcirculation in head and neck cancer. Ann Otol Rhinol Laryngol. (1984) 93:646–7. doi: 10.1177/000348948409300621
28. Tomioka H, Yamagata Y, Oikawa Y, Ohsako T, Kugimoto T, Kuroshima T, et al. Risk factors for distant metastasis in locoregionally controlled oral squamous cell carcinoma: a retrospective study. Sci Rep. (2021) 11:5213. doi: 10.1038/s41598-021-84704-w
29. Wilson HL, Beuerlein WL, Laurini JA, Tummala N, Russell GB, Waltonen JD. Prognostic factors in oral squamous cell carcinoma treated with primary bone resection. Wake For J Sci Med. (2021) 6.
30. Ting KC, Lee TL, Li WY, Chang CF, Chu PY, Wang YF, et al. Perineural invasion/lymphovascular invasion double positive predicts distant metastasis and poor survival in T3-4 oral squamous cell carcinoma. Sci Rep. (2021) 11:19770. doi: 10.1038/s41598-021-99280-2
31. Wang B, Zhang S, Yue K, Wang XD. The recurrence and survival of oral squamous cell carcinoma: a report of 275 cases. Chin J Cancer. (2013) 32:614–8. doi: 10.5732/cjc.012.10219
32. Liu CH, Chen HJ, Wang PC, Chen HS, Chang YL. Patterns of recurrence and second primary tumors in oral squamous cell carcinoma treated with surgery alone. Kaohsiung J Med Sci. (2013) 29:554–9. doi: 10.1016/j.kjms.2013.03.001
33. Elaiwy O, El Ansari W, AlKhalil M, Ammar A. Epidemiology and pathology of oral squamous cell carcinoma in a multi-ethnic population: Retrospective study of 154 cases over 7 years in Qatar. Ann Med Surg (2012). (2020) 60:195–200. doi: 10.1016/j.amsu.2020.10.029
34. Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clinics North America. (2015) 24:491–508. doi: 10.1016/j.soc.2015.03.006
Keywords: oral cavity squamous cell carcinoma, surgery, adjuvant therapy, radiotherapy, chemoradiotherapy, clinical outcomes, prognostic factors
Citation: Abu Taha S, Abu Hejleh T, Wahbeh L, Alzibdeh A, Berawi M, Qambar M, Mukahal M, Abuhijla F, Abu-Hijlih R, Taqash A, Hussein T, Alrousan M, Saraireh OA, Al-Gargaz W, Al-Ibraheem A, Ghatasheh H, Hosni A and Mohamad I (2024) Clinical outcomes and impact of prognostic factors in resectable oral cavity squamous cell carcinoma. Front. Oncol. 14:1443367. doi: 10.3389/fonc.2024.1443367
Received: 03 June 2024; Accepted: 25 September 2024;
Published: 11 October 2024.
Edited by:
Waseem Jerjes, Imperial College London, United KingdomReviewed by:
Rong-Hui Xia, Shanghai Jiao Tong University, ChinaNikolas Kalavrezos, University College Hospital (UCH), United Kingdom
Copyright © 2024 Abu Taha, Abu Hejleh, Wahbeh, Alzibdeh, Berawi, Qambar, Mukahal, Abuhijla, Abu-Hijlih, Taqash, Hussein, Alrousan, Saraireh, Al-Gargaz, Al-Ibraheem, Ghatasheh, Hosni and Mohamad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Issa Mohamad, aW1vaGFtYWRAa2hjYy5qbw==
†ORCID: Issa Mohamad, orcid.org/0000-0003-0153-9131
 Shatha Abu Taha
Shatha Abu Taha Taher Abu Hejleh
Taher Abu Hejleh Lina Wahbeh
Lina Wahbeh Abdulla Alzibdeh1
Abdulla Alzibdeh1 Fawzi Abuhijla
Fawzi Abuhijla Ramiz Abu-Hijlih
Ramiz Abu-Hijlih Ayat Taqash
Ayat Taqash Medyan Alrousan
Medyan Alrousan Akram Al-Ibraheem
Akram Al-Ibraheem Issa Mohamad
Issa Mohamad