- 1Department of Thyroid and Breast Surgery, Binzhou Medical University Hospital, Binzhou, China
- 2Department of Oral and Maxillofacial Surgery, Binzhou Medical University Hospital, Binzhou, China
- 3Department of Oncology, Binzhou Medical University Hospital, Binzhou, China
Purpose: Parotid gland metastases originating from breast origin are extremely rare, with their clinical presentation, therapeutic approaches, and prognostic indicators remaining to be elucidate.
Methods: A comprehensive retrospective review was conducted, analyzing the clinical characteristics and prognostic factors of 57 patients diagnosed with parotid metastasis of breast cancer in the existing literature. Notably, our study included two unique cases of patients who developed contralateral and ipsilateral parotid metastases, occurring 5 years and 32 years respectively after primary surgery. This analysis aimed to provide a deeper understanding of the disease presentation and identify potential prognostic indicators.
Results: The primary clinical manifestation presented in breast cancer patients with parotid metastases was painless masses in the parotid glands, synchronously or metachronously occurred with primary breast tumors. The predominant pathological subtype among these patients was invasive ductal carcinoma. Out of the 57 patients studied, 24 (42.1%) exhibited metastases solely in the ipsilateral parotid gland, while 18 cases (31.6%) involved either the contralateral or bilateral parotid gland. Patients may solely exhibit metastasis in the parotid gland, or they may present with concurrent multiple metastases in other organs. Patients who suffered from parotid metastases, either merely or accompanied with bone-only metastasis, exhibited significantly longer overall survival (OS) rates compared to those who had concomitant metastases in other organs (1.23 ± 0.26 years vs 4.46 ± 0.77 years, P=0.046). While no statistically significant differences in OS were observed among patients presenting with metastases in the ipsilateral, contralateral, or bilateral parotid glands, a notable variance could be discerned from the Kaplan-Meier curve analysis. Additionally, no significant difference in survival was exhibited between patients with different interval of progression from primary breast sites to initial diagnosis of parotid metastases (uDF), nor for patients who were treated with surgery or palliative therapy.
Conclusion: Parotid metastasis, a rare and distinctive form of breast cancer metastasis, demands particular scrutiny in patients exhibiting metastasis to multiple organs or contralateral or bilateral parotid glands.
1 Introduction
The parotid gland is an unusual site for metastatic disease and when metastasis occurs, it commonly originates from head and neck primaries. Malignant melanoma and squamous cell cancer are the two most common tumors which metastasize to the parotid gland (1). Parotid gland metastases are rarely from any source other than the head and neck (2), due to differences in the number of lymph nodes, anatomical relationships, and their drainage (3). Metastasis of breast cancer to the parotid gland is an exceedingly rare occurrence, which can manifest synchronously or metachronously, even years after the initial diagnosis, irrespective of primary disease stage and appropriate primary treatment. In 1975, Katz described a metastatic adenocarcinoma from the left breast to the right parotid gland (4). Since then, only occasional studies have reported this specific metastasis. Although the morbidity is low, the mortality rate is quite high. Two previous studies have retrospectively described parotid metastasis of breast cancer and reviewed the literature, however, the literature included is insufficient, and the incidence, clinical presentation, management and prognosis of the disease are not adequately described (5, 6).
In this article, we presented two patients with a history of an invasive ductal carcinoma who developed contralateral and ipsilateral parotid metastases 5 and 32 years after surgery, respectively, as a first step. Subsequently, a retrospective review of 57 patients diagnosed with parotid metastasis of breast cancer in the literature was conducted to describe the presentation, management strategies and investigate the prognostic factors. To our knowledge, this is the first comprehensive analysis regarding to the clinical features and prognostic factors of parotid gland metastasis from breast cancer.
2 Materials and methods
Firstly, we initially presented 2 cases of parotid gland metastasis of breast cancer patients in our hospital. Secondly, a comprehensive literature search of the PubMed and Medline databases with key words including “breast cancer”,”breast carcinoma” AND “parotid” to identify studies of parotid metastasis from breast cancer published from 1975 to 2023 was performed, including articles provided in literature. A total of 42 articles and 57 patients were included. Detailed information about these patients were retrieved from these articles. Thirdly, the descriptive analyses of clinical characteristics and statistical analyses of prognostic factors associated with parotid metastasis of breast cancer were conducted.
2.1 Statistical analysis
Data were analyzed using the SPSS software (for Windows, version 22.0; IBM Corp., Armonk, NY, USA). Kaplan–Meier and log-rank tests were used to estimate the overall survival (OS). Two-tailed P values <0.05 denoted statistically significant differences.
3 Results
3.1 Case 1
A 58-year-old female patient presented with the concern of a painless lump in the right parotid region, accompanied by discomfort on the corresponding side of her face in April 2022. Upon clinical examination, a firm yet painless mass was detected in the right parotid region, exhibiting no signs of inflammation or facial nerve paralysis.
The computed tomography (CT) scan with contrast revealed a distinct, heterogeneous, round enhancement in the superficial lobe of the parotid gland, measuring approximately 1.5cm x 1.8cm in size. Notably, the bone of the right mandibular ramus appeared thickened, whereas the adjacent soft tissue appeared unremarkable (see Figure 1A). Furthermore, the neck, submandibular, and submental regions exhibited bilateral cervical lymph nodes, all measuring below the centimeter range. Upon further questioning, the patient revealed a past diagnosis of infiltrating ductal carcinoma (IDC) in the left breast, occurring approximately five years ago. The clinical staging at that time was designated as T2N1M0. She underwent a comprehensive treatment strategy including mastectomy and axillary lymph node dissection, adjuvant chemotherapy and loco-regional radiotherapy followed by a five-year course of hormone therapy to ensure optimal recovery and reduce the risk of recurrence.
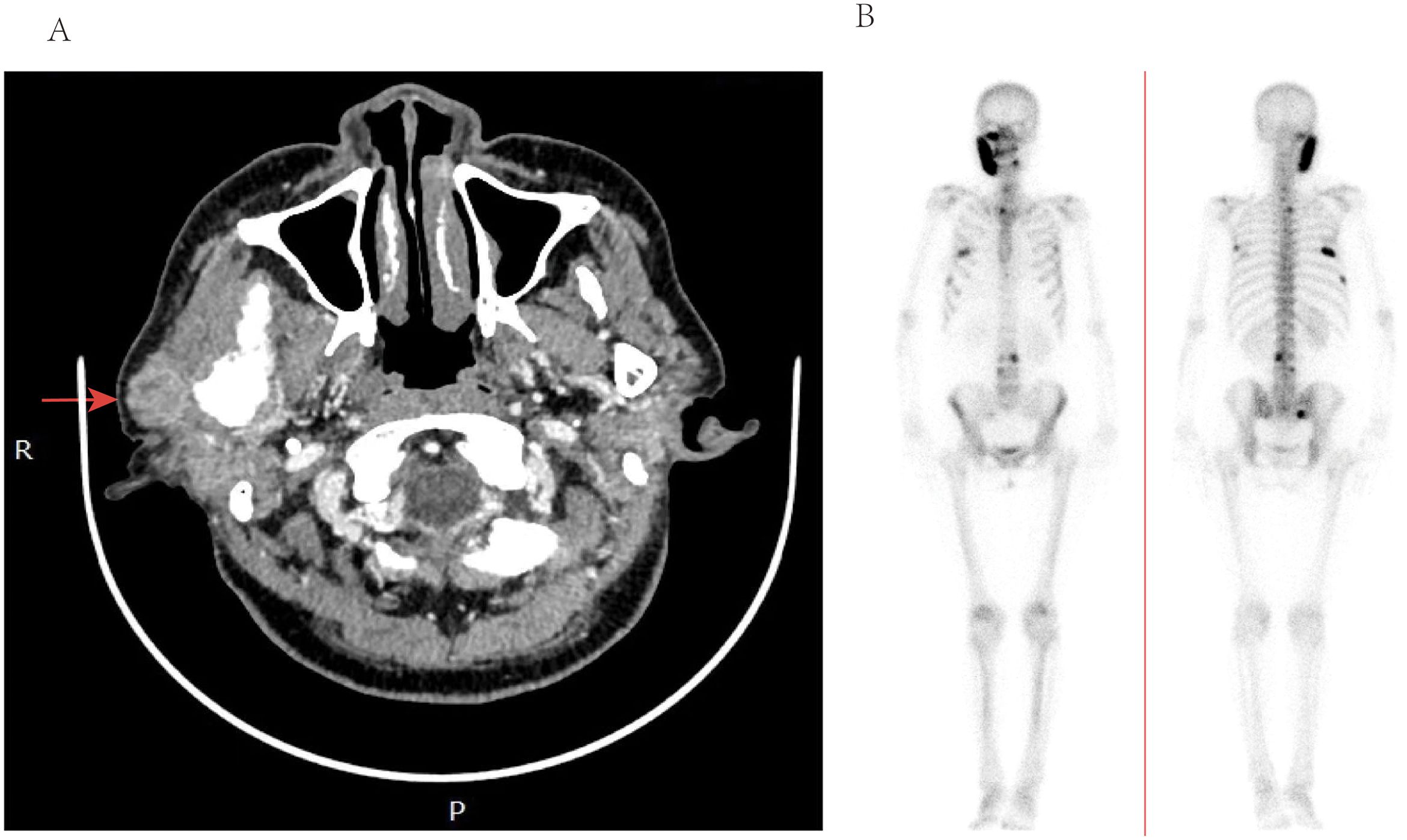
Figure 1. (A) Computed tomography of the parotid with contrast—axial view, demonstrating heterogenous enhancement and enlargement of the right parotid glands. (B) ECT imaging, demonstrating marked multiple lesions with increased uptake of imaging agents in ribs and vertebrae.
The patient underwent resection of the mass in the superficial lobe of the right parotid gland, and intraoperative pathological examination subsequently revealed the presence of metastatic breast cancer cells. Additionally, a biopsy conducted on the left mandible did not demonstrate any evidence of malignant tumor metastasis, indicating that the cancer had not spread to this region. No local biopsy or radiotherapy was performed on the subcm nodes in bilateral cervical with a consideration of non-metastases.
Subsequently, a superficial parotidectomy was executed. Immunohistochemical analysis revealed a positive expression of estrogen receptor (ER), progesterone receptor (PR), GATA-3, and human epidermal growth factor receptor 2 (HER-2) with a high score of 3+. Figure 2 depicted the HE staining and GATA-3 staining images of this particular patient. Additionally, the monoclonal antibody Ki-67 index indicated a proliferative rate of 30%. Furthermore, emission computed tomography (ECT) scans confirmed the presence of multiple metastases in the ribs and vertebrae, as depicted in Figure 1B. An extensive treatment regime was carefully devised to ensure optimal care for the patient. Subsequently, the patient underwent several rounds of adjuvant chemotherapy, which was complemented by targeted therapy employing trastuzumab and pertuzumab. Radiotherapy was administered as the next phase of treatment, followed by hormone therapy to further consolidate the therapeutic gains. Over the past two years, the patient experienced a notable remission from the disease, but unfortunately, there has been a recent localized recurrence in the right sternocleidomastoid muscle.
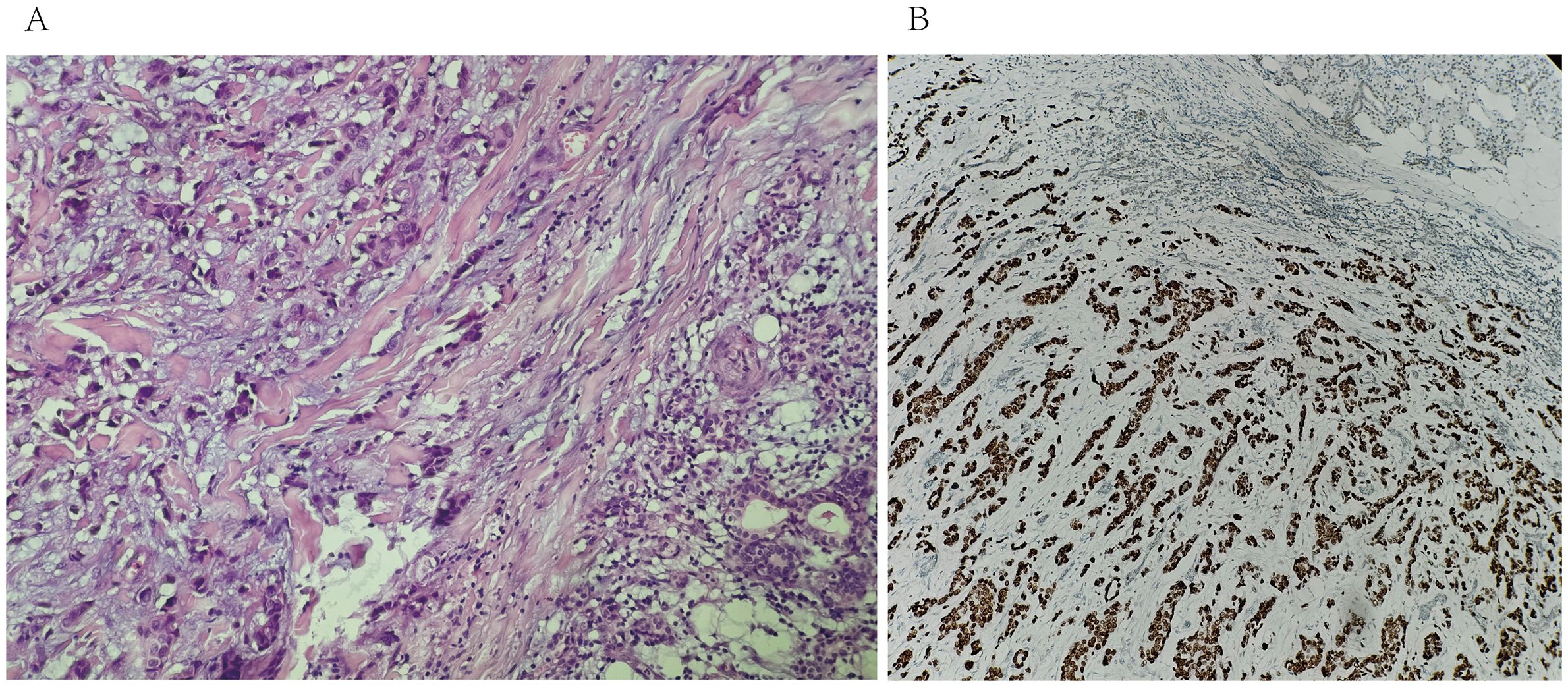
Figure 2. (A) biopsy of the right parotid mass with Hematoxylin & Eosin staining (20X) shows tumoral cells in the salivary gland. (B) immunohistochemistry for GATA-3 shows positive staining of tumor cells (20X).
3.2 Case 2
In March 2023, a 71-year-old female patient presented with a one-month history of a painless lump behind her right ear. A parotid CT scan with contrast enhancement confirmed the existence of a 0.7-centimeter, clearly defined nodular mass (Figure 3A). Notably, this patient had undergone surgery for right breast cancer approximately 32 years prior to her current presentation. Additionally, a chest CT scan revealed abnormalities in multiple vertebrae and ribs, suggestive of bone involvement.
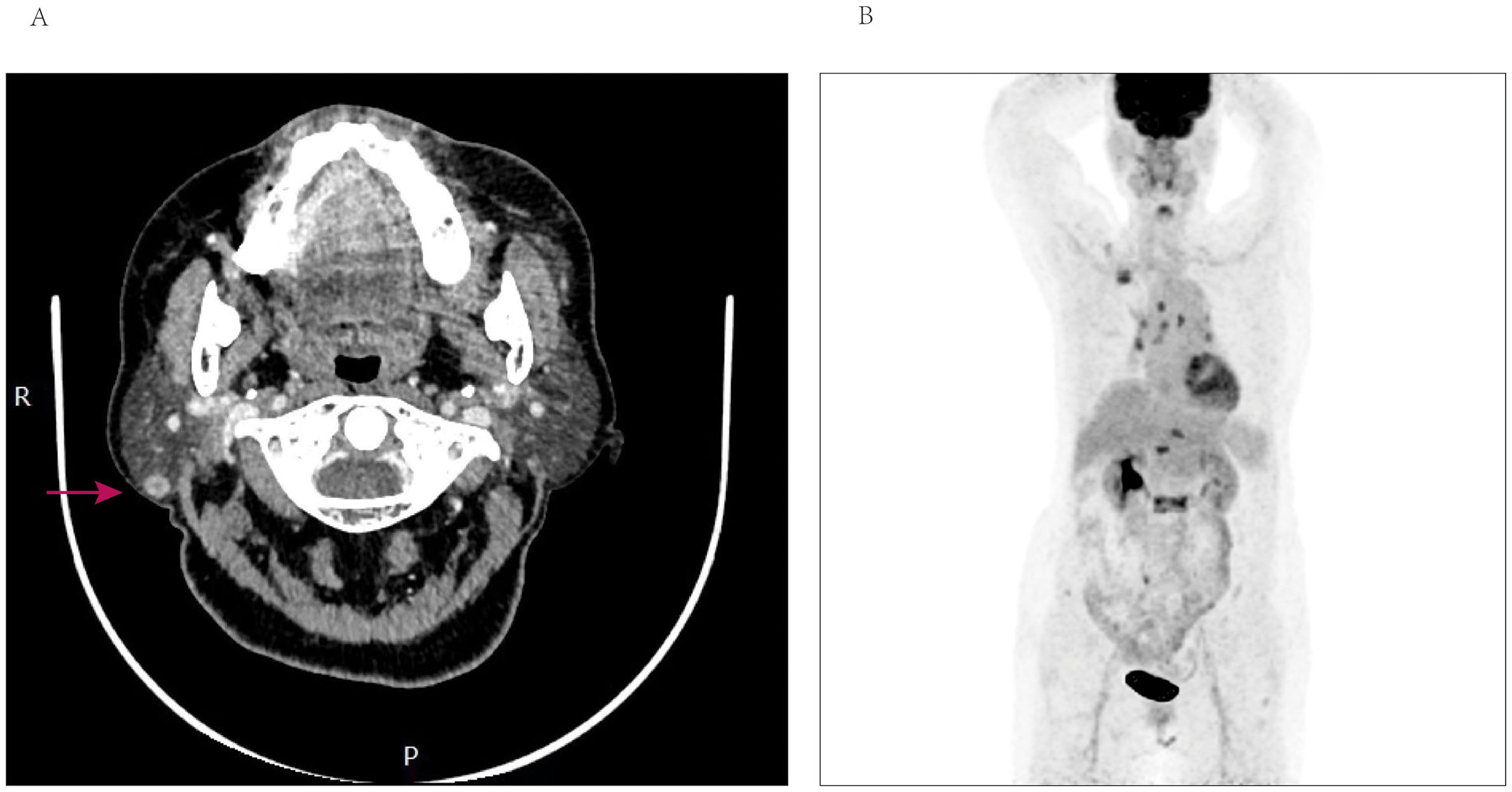
Figure 3. (A) Computed tomography of the parotid with contrast—axial view, demonstrating well-demarcated enhancement nodular of the right parotid gland. (B) PET-CT scan reveals accumulation of SUV more than 8 in multiple vertebrae, ribs, the right ilium, and lymph nodes.
Taking into account the patient’s medical history and the observed findings, the possibility of metastases was seriously considered. Consequently, a core needle biopsy was promptly conducted in the parotid region. Pathological analysis indicated the presence of metastatic breast cancer, while immunohistochemical studies revealed positive expression of estrogen receptor (ER), GATA-3 and negative expression of progesterone receptor (PR). Additionally, the Ki-67 index was determined to be 30%, indicating a moderate proliferative activity. HER-2 staining was recorded as 2+, but FISH testing yielded negative results. Figure 4 depicted the HE staining and GATA-3 staining images of this patient. The results of positron-emission tomography CT (PET-CT) revealed multiple osteoblastic bone changes in the skull base, multiple vertebrae, the right first rib, the left sixth rib, the right ilium. The patient exhibited a significant accumulation of standardized uptake value (SUV max) in the L2 vertebrae, reaching 9.3, and a similar uptake of 8.8 in the right parotid gland. Furthermore, the PET/CT scan revealed multiple metastases in the lymph nodes, including those located in the right supraclavicular region, mediastinal area, and bilateral hilar regions (Figure 3B). Subsequently, the patient underwent hormone therapy with a combination of palbociclib and fulvestrant. Notably, the patient has maintained clinical stability up to the present time, and there has been no detection or confirmation of recurrence at any other sites.

Figure 4. Core needle biopsy of the right parotid mass with Hematoxylin & Eosin staining (20X) shows tumoral cells in the salivary gland. (B) immunohistochemistry for GATA-3 shows positive staining of tumor cells (20X).
3.3 Clinical manifestations and prognostic indicators of 57 patients
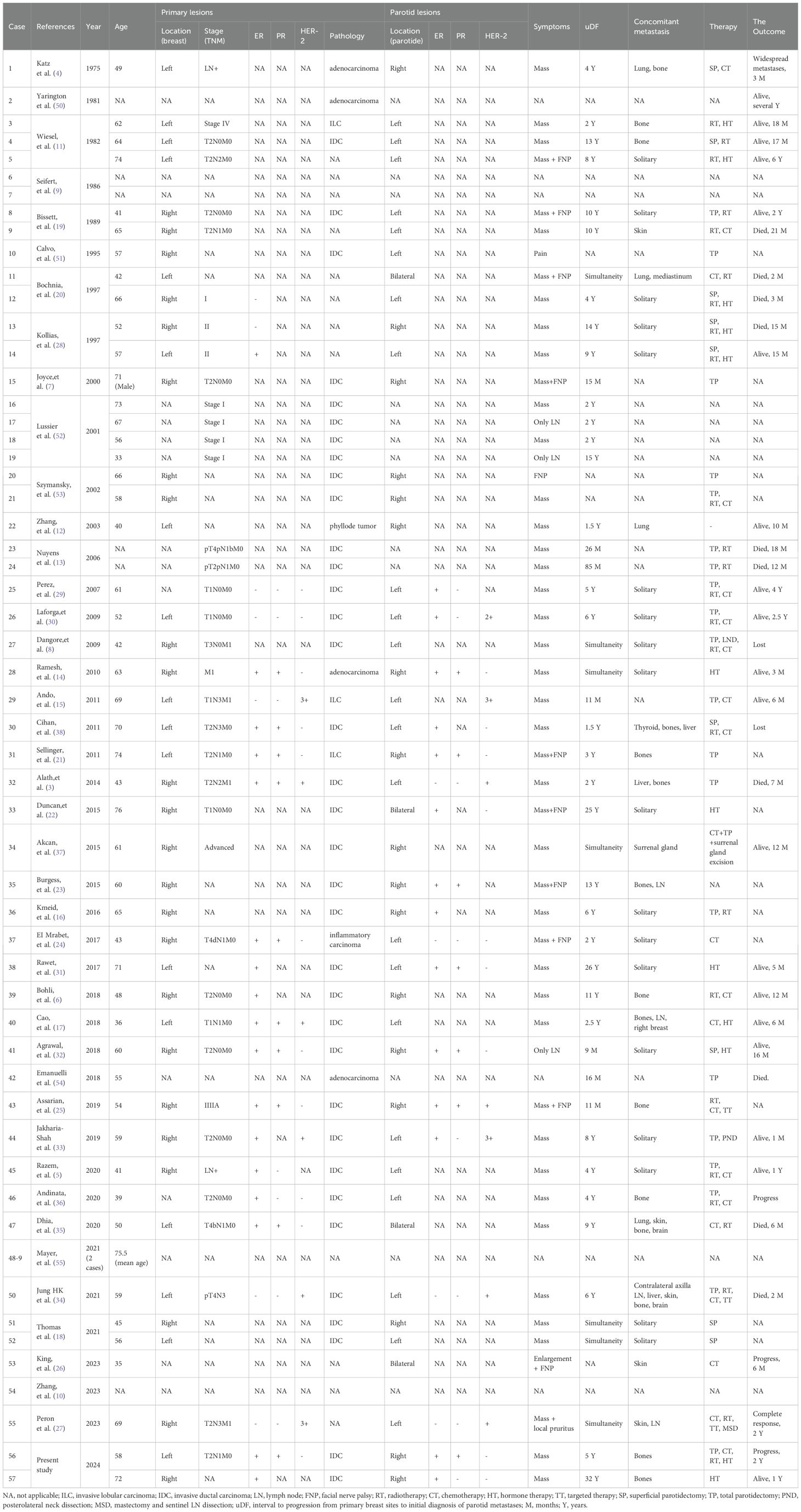
The detailed characteristics of the primary tumor, including age, pathology, localization, staging, and molecular subtypes, as well as the specifics of parotid lesions, their molecular subtypes, symptoms exhibited, the presence of concomitant metastases, the interval to progression from the primary breast cancer sites to the initial diagnosis of parotid metastases, the treatment modalities employed for the metastases, and the ultimate outcomes for the cohort of 57 patients are comprehensively outlined in Table 1.
Between 1975 and 2023, there have been 57 worldwide reported instances of parotid metastasis originating from breast cancer, with one notable case involving a parotid metastasis of malignant phyllodes tumor of the breast. Notably, the majority of these patients have been female, with the exception of a single male case (7). The age range of these patients has spanned from 33 to 76 years, with an average age of 57 years.
Of the 34 patients who were reported with definite presentation, the primary clinical presentation was a painless parotid gland mass. Additionally, 11 patients exhibited symptoms of facial nerve paralysis, while 1 patient complained of facial skin pruritus and another patient experienced parotid pain. Furthermore, 3 patients were diagnosed with intraparotid lymph node metastasis. Lastly, the clinical manifestations of 7 patients remained unclear.
Out of the 57 patients presenting with parotid metastasis of breast cancer, 40.4% (or 23 patients) exhibited involvement in the left parotid gland, while 29.8% (or 17 patients) demonstrated involvement in the right parotid gland. Additionally, 7.0% (or 4 patients) exhibited bilateral parotid involvement, and the involvement status for 13 patients remains unknown.
In 24 patients (42.1%), breast cancer had metastasized to the ipsilateral parotid gland, while in 4 patients (7.0%) it had metastasized to bilateral parotid glands. Additionally, 14 patients (24.6%) had metastases in the contralateral parotid gland. Of these contralateral cases, 4 patients with left breast cancer exhibited metastases in the right parotid gland, whereas 10 patients with right breast cancer displayed metastases in the left parotid gland. However, for 15 patients, information regarding the side of parotid metastasis was unavailable.
The predominant pathological subtype of primary breast cancer was invasive ductal carcinoma (IDC) in 35 patients (61.4%), followed by invasive lobular carcinoma (ILC) in 3 patients, adenocarcinoma in 4 patients, inflammatory breast cancer in 1 patient, malignant phyllode tumor in 1 patient, and unknown in 13 patients. Molecular subtyping of the primary tumor was available for only 19 patients, and in 5 patients, the metastatic lesion in the parotid gland exhibited differences from the primary breast cancer.
Immunohistochemical results were available for a total of 24 patients. In primary breast cancer, the ER status was positive among 17 patients, accounting for 70.8% of the total, while it was negative in 7 patients, representing 29.2% of the cases; PR was positive in 10 patients (58.8%) and negative in 7 patients (41.2%). HER-2 was positive in 6 patients (35.3%) and negative in 11 patients (64.7%). In the parotid metastasis of breast cancer, ER status was positive in 14 patients (82.4%) and negative in 3 patients (17.6%); PR was positive and negative in 7 patients, respectively. HER-2 was positive in 7 patients (43.8%) and negative in 9 patients (56.2%). There were 4 patients with ER/PR-, only 1 case was unifocal metastasis of parotid, and the other 3 cases were presented with concomitant metastasis in other organs. Among the patients, four individuals initially exhibited ER+ status in the primary breast cancer, yet subsequently developed ER-negative status in the metastatic parotid gland. Two patients exhibited a transition in PR status from positive to negative, while only a single patient displayed a shift in HER-2 status, transforming from negative to positive.
The metastatic status of 18 patients was unavailable. Notably, 18 patients (46.2%) exhibited merely metastasis in the parotid gland, whereas 21 patients (53.8%) presented with concurrent metastases in other organs. Among these patients with multiple metastases, 15 individuals demonstrated bone metastases. Additionally, the other metastatic sites, in descending order of frequency, encompassed the skin, lung, cervical lymph nodes, liver, mediastinum, brain, thyroid, and adrenal gland.
Seven cases exhibited concurrent occurrences of primary breast cancer and parotid metastases, while fifty cases displayed metachronous manifestations. The time elapsed between the initial diagnosis of primary breast cancer and the subsequent detection of parotid metastases was designated as uDF. Notably, the uDF was unavailable in ten patients, whereas the median uDF for the remaining patients stood at four years, ranging from a minimum of nine months to a maximum of thirty-two years.
Of the 46 patients for whom treatment strategies were known, 21 cases underwent total parotidectomy (TP), 9 cases underwent superficial parotidectomy (SP), 21 cases underwent chemotherapy, and 25 patients received radiotherapy. Of these patients who underwent surgery, 8 cases had parotid gland metastases solitary, 4 cases were concomitant with bone metastases, and 5 cases presented concomitant metastases in multiple organs. Therefore, surgeons have made patient selection based on the patients’ disease state prior to the surgery. Ultimately, of the patients who underwent surgery, 10 were alive, 7 patients succumbed to the disease and 2 patients exhibited disease progression. Thus, surgery doesn’t seem to improve the survival.
Based on the survival data outlined in the available literature, it was discovered that the survival status was unknown for 21 patients, while 2 patients were lost to follow-up. Twenty patients (accounting for 58.8% of the total) were confirmed to be alive, whereas 14 patients (41.2%) had either succumbed to their illness or experienced disease progression. Furthermore, there were significant disparities in the characteristics of these two patient cohorts.
Of the patients who were still alive, the median uDF was 5.5 years, ranging from 0 to 32 years. Similarly, the median survival time among these patients was 15 months, with a range extending from 1 month to 6 years. Fourteen patients, representing 70% of the total, exhibited metastasis to the ipsilateral parotid gland. Five patients, accounting for 25%, showed metastasis to the contralateral parotid gland, while the metastatic status of one patient remained unknown. Ten patients, or 50% of the total, exclusively harbored parotid gland metastasis. Eight patients, constituting 40%, presented with metastasis to other organs, including five patients with bone metastasis alone. The metastatic status of two patients, however, were unknown.
Among those who died or progressed, 10 patients succumbed to the illness, while another 4 developed progression or widespread metastases. The median uDF was 5.0 years, ranging from 0 to 14 years, with a median time to either death or disease progression of 6 months(2 months-2 years). Among the patients, contralateral or bilateral parotid metastases were detected in 8 cases (accounting for 57.1% of the total), ipsilateral parotid metastases in 2 cases, and unknown in 4 cases. Only 2 patients (14.3%) presented with parotid metastases exclusively, whereas 9 patients (64.3%) exhibited metastases in other organs as well, and the status of 3 patients was undetermined.
A subsequent analysis was conducted to identify the prognostic factors associated with parotid metastasis in breast cancer patients. The cumulative survival curve, presented in Figure 3, provides a comprehensive overview of the survival outcomes for 57 patients with this condition. Notably, patients who exhibited parotid metastases alone or combined with bone metastases exhibited significantly longer overall survival compared to those with concurrent metastases in other organs (1.23 ± 0.26 years vs 4.46 ± 0.77 years, P=0.046). The Kaplan-Meier curve, depicted in Figure 5A, further illustrates these survival patterns. Patients with metastases to the contralateral or bilateral parotid gland exhibited a mean OS of 1.29 ± 0.28 years, whereas those without such metastases had a significantly longer mean OS of 4.85 ± 0.76 years (P=0.101) (Figure 5B). Although statistical significance was not reached, a notable difference in OS between the two groups was observable from the Kaplan–Meier curve (Figure 5C). Furthermore, neither the presence of different uDFs nor the type of treatment received (surgical or palliative) had a significant impact on survival rates. The detailed results are summarized in Table 2.
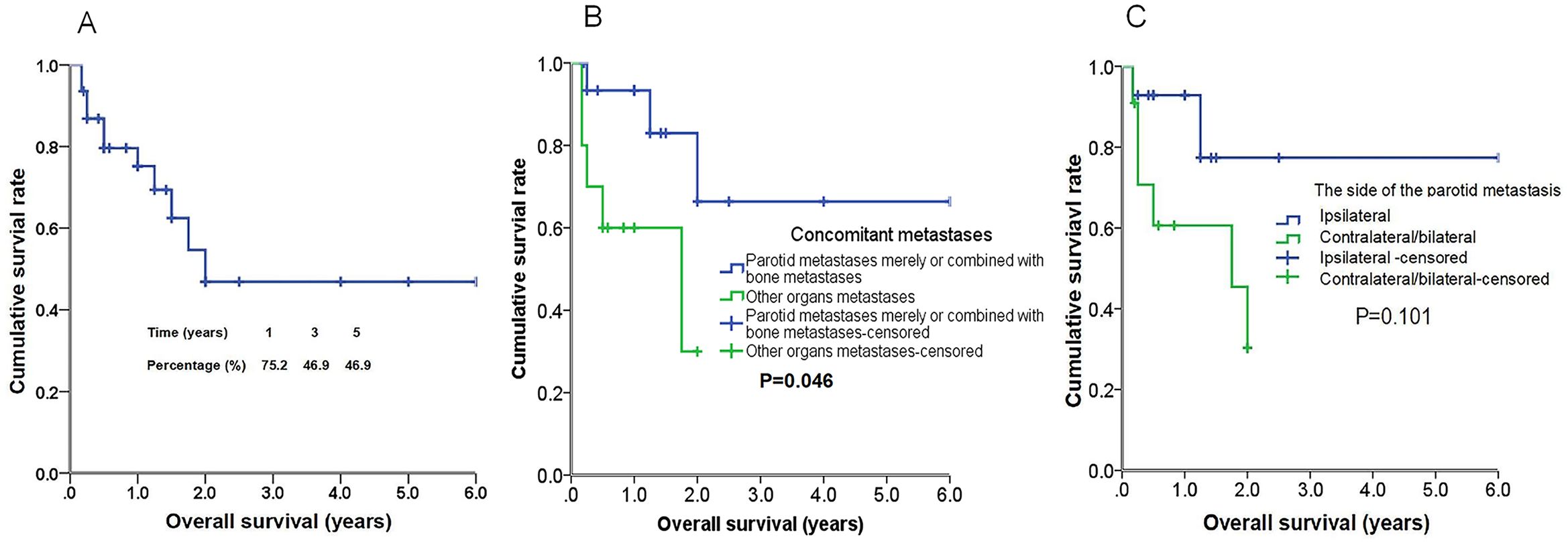
Figure 5. Kaplan-Meier curves of overall survival (OS) of 57 patients with parotid metastasis of breast cancer (A). Kaplan–Meier curves of OS in patients with parotid metastases merely or combined with bone metastases versus patients with other organs metastases (B) for OS, and the differences between groups were significant (P<0.05, log-rank test). Kaplan–Meier curves of OS in patients with ipsilateral parotid metastases versus contralateral or bilateral parotid metastases (C) for OS, and the differences between groups were not significant (P > 0.05, log-rank test).
4 Discussion
The most common sites for breast cancer metastases include the lungs, liver, bone, adrenals and brain with rare cases disseminated to head and neck (8). Although rarely encountered, metastatic disease to the parotid glands was occasionally reported in the medical literature as isolated case reports or small series. Siefert et al. (9) conducted a retrospective analysis on 10,944 patients harboring parotid gland tumors, revealing that only 75 of these cases were metastatic from primary cancers in other organs. Notably, just two of these metastatic instances originated from breast cancer. In a more recent study, Zhang et al. (10) analyzed data from 6274 consecutive patients with stage IV breast cancer who had developed unusual metastases. Surprisingly, only a single case involved the parotid gland. Given the rarity of this particular metastasis, there remains a significant knowledge gap in understanding its etiology and pathophysiology.
The clinical presentation of parotid metastases were mostly painless masses (4, 8, 11–18), solitary or multifocal, some patients associated with facial nerve paralysis (7, 11, 19–25), and a few patients had special manifestations. King et al. (26) documented an occurrence of unique patient presenting with synchronous bilateral facial nerve paralysis secondary to metastatic breast carcinoma to the bilateral parotid glands, while the CT scan of neck demonstrated heterogenous enhancement and enlargement of the bilateral parotid glands, rather than the presence of distinct masses. Additionally, a patient was reported with have complained of parotid mass accompanied by local pruritus (27). The diversity of symptoms observed in these cases has been hypothesized to be associated with the malignant nature of the underlying condition (16).
Breast cancer can present with parotid gland metastasis merely (5, 8, 11, 14, 16, 18, 24, 28–33), or it can presents with synchronous multiple metastases of other organs (12, 27, 34–37), including bone, lung, mediastinum, brain, lymph node, adrenal gland, etc. Bones are particularly susceptible to metastatic involvement. In addition, metastasis in other rare sites can also be noted. Cao et al. (17) and Jung HK et al. (34) described instances where breast cancer metastasized to the parotid gland, accompanied by concurrent metastasis to the contralateral breast and contralateral axillary lymph nodes, respectively. Cihan et al. (38) reported a patient who exhibited synchronous metastasis to both the parotid and thyroid glands originating from breast cancer.
Breast cancer metastases not only to the ipsilateral parotid gland, but also to the contralateral and bilateral parotid glands. Of the 44 patients with known sides of parotid gland metastases, 40.9% (18 out of 44) exhibited either contralateral or bilateral metastases. Katz et al. (4) firstly reported a metastatic adenocarcinomarcinoma from the left breast to the right parotid gland in 1975. Since then, a mere 13 additional instances have been reported where the breast cancer has involved the contralateral parotid gland. Notably, only four cases have been documented with bilateral parotid metastases (20, 22, 26, 35). Nevertheless, due to the rarity of this metastatic variation, the precise metastatic pathway remains elusive.
No matter the patients with breast cancer metastasis to the ipsilateral parotid, or to the contralateral or bilateral parotid, non-involvement of axillary nodes was identified, indicating that hematogenous dissemination appears to be the predominant mode of spread, rather than direct lymphatic metastasis. This assertion is corroborated by prior research, which has reported similar frequencies of metastases to the ipsilateral and contralateral parotid glands from breast cancer (15, 16, 33). Additionally, the occurrence of metastases in the contralateral and bilateral parotid glands may serve as predictors of a poor prognosis.
Invasive ductal carcinoma is the most commonly reported pathological subtype of primary breast cancer in the extant literature, as evidenced by the majority of studies (5, 8, 17, 18, 25, 33–36, 39), which means that the most likely culprit of parotid mets is actually the most common form of breast cancer. It is of utmost importance for head/neck surgeons to maintain a high index of suspicion for parotid gland metastasis stemming from breast cancer in patients presenting with a parotid gland tumor and a prior history of invasive ductal carcinoma of the breast. Additionally, metastases arising from other types of breast cancer, including invasive lobular carcinoma, inflammatory carcinoma, and even malignant phyllodes tumor, have also been described (12, 15, 21, 24). While primary malignancies of the parotid glands can exhibit sex hormone receptors, the majority tend to express androgen receptors, with only a minority expressing oestrogen receptors (40). Similarities between salivary ductal carcinoma of the parotid gland and metastatic breast carcinoma, make it difficult to distinguish metastasis from primary carcinoma. During the process of breast cancer metastasis to the parotid gland, the immunohistochemistry profiles of the patient changed, resulting in different molecular subtype from primary site (3, 24, 25, 30). Previous studies have reported divergent expression of hormonal receptors between primary tumors and metastatic sites (41). Fine needle aspiration cytology remains the gold standard for accurately distinguishing between primary and secondary tumors, with an accuracy rate of 85% (16, 33). This technique plays a crucial role in the diagnosis and management of patients with breast cancer metastasis to the parotid gland.
A differential diagnosis is salivary duct carcinoma (SDC), which is with morphological resemblance to IDC of the breast. The main discrepancy between IDC and SDC in the immunohistochemical profiles was a distinctly different absence in ER-α, PR and HER-2 (42). Positive reactivity to ER-α, PR or both and negative HER-2 favors a diagnosis of IDC while ER-α, PR negative, HER-2 positive tumors are more likely SDC. However, for patients present with unifocal lesion in parotid and ER/PR negative, accurate identification of the primary tumor is particularly difficult in morphology. SDC has a male predilection and mostly occurs in elderly patients in clinical, which is a distinctly different with IDC. The differential diagnosis should be made based on the patient’s medical history, sex, age, and immunohistochemistry.
The interval between the diagnosis of primary breast cancer and the development of distant metastases is quite variable. In fact, distant metastases can occur up to several decades from the original diagnosis (43, 44). A latest study (10), the researchers discovered that the median duration between the initial diagnosis of primary breast cancer and the detection of unusual metastatic occurrences was approximately 121.3 months. The median uDF for the entire cohort of 57 patients was 4 years, with a remarkable outlier of one patient who exhibited a protracted uDF period extending as long as 32 years. The preponderance of metastatic cells often remains in a quiescent state for extended durations, referred to as metastatic dormancy. However, these cells can subsequently become activated and proliferate, ultimately leading to the manifestation of pronounced metastases (45–47). Given that 5-year freedom from recurrence does not guarantee long-term disease-free survival, the sustained surveillance of these patients is of paramount importance.
In cases where the metastasis is confined to the parotid gland, the management remains palliative. However, an appropriate parotidectomy with negative margins and preservation of the facial nerve should be carefully considered to alleviate clinical symptoms and contain local disease progression (15, 46).
Consequent to their rarity, no consensus was achieved on treatment protocol in the metastatic cancer scenario involving the parotid gland. For instances of single parotid gland metastasis originating from breast cancer, previous studies have primarily suggested surgeries such as superficial or total parotidectomy, with preservation of facial nerves to achieve negative margin in previous studies (6, 15, 16). In the case involving multiple-site metastases, the recommended approach often involves systemic therapy, encompassing chemotherapy, hormonal therapy, and possibly a combination with radiation therapy (17). Despite the proposed treatments, parotid surgery dose not seem to improve survival, patients with metastatic involvement of the parotid gland have poor prognosis, with the 5-year survival rate of only 10% (16, 17). Of the 30 patients undergoing surgery for parotid metastatic breast cancer, 12 patients were found to have metastasis solely to the parotid gland or in combination with bone metastases. Despite careful patient selection by medical professionals prior to surgery, surgical intervention did not seem to significantly enhance survival rates. Although the management of a parotid metastasis is palliative, in cases where the metastasis is merely to the parotid gland, it is advisable to consider an appropriate parotidectomy that ensures negative margins and preservation of the facial nerve, to relieve clinical symptoms and control local progression (16, 48). It is crucial to tailor the management approach based on the individual patient’s condition and the progression of their disease.
Survival rates can be influenced by several factors, including the clinical presentation of the disease, the duration between the initial diagnosis of breast cancer and the emergence of distant metastases, as well as the specific location of recurrence. Previous research underscores the notion that patients with metachronous solitary parotid metastases and a prolonged disease-free survival period are regarded as having favorable prognostic indicators (49). Additionally, those individuals who exhibit a longer uDF and present with non-visceral metastatic deposits are generally predisposed to more favorable outcomes. Patients who present with parotid-only metastases or those combined with bone-only metastases tend to enjoy a significantly more favorable prognosis. While the available survival data in the literature remains limited, our findings reveal that these subsets of patients fare much better in terms of survival compared to those who suffer from metastases in other organs. This observation is further corroborated by prior studies, which indicate that individuals who solely develop rare metastasis or bone-only metastasis enjoy a significantly superior prognosis compared to those who also have common visceral metastasis (10).
Furthermore, among patients who survived, the uDF was extended compared to those who either succumbed to the disease or experienced disease progression(5.5 years vs 5.0 years), and 70% (14 out of 20) displayed metastases to the ipsilateral parotid gland. Of the patients who died or experienced disease progression, a notable 57.1% (8 out of 14) exhibited metastasis in the contralateral or bilateral parotid glands, while only two individuals exhibited metastasis in the ipsilateral parotid gland. Despite the statistical analysis failing to uncover a statistically significant difference in survival rates between patients with ipsilateral and contralateral or bilateral parotid metastases, the Kaplan-Meir curve revealed a marked divergence between the two groups. The survival rates of patients who were alive were grossly underestimated due to inconsistencies in the censoring dates of survival data reported in the literature. However, the survival outcomes of patients who succumbed to the disease or experienced disease progression were unambiguous. Consequently, by obtaining comprehensive survival data for these alive patients, we anticipate that additional factors influencing the prognosis of patients with parotid metastasis from breast cancer may be uncovered.
As a retrospective study, limitations can’t be neglected. Firstly, the data were derived from literature review, which led to an inevitable heterogeneity; Secondly, the grouping is not detailed enough due to the sample size limitation; thirdly, the censoring dates of survival were different in the literature, resulting in inaccurate survival time. Nevertheless, the clinical characteristics and prognosis predictors of parotid gland metastases from breast cancer in present study, remains valid.
5 Conclusion
In conclusion, parotid gland metastases from breast origin are extremely rare. Despite its low incidence, its unique presentation, biological behavior, and prognostic factors can be found from the literature review. Parotid gland metastases can occur synchronously or metachronously, involve ipsilateral, contralateral or bilateral parotid glands, or coexist with concomitant metastasis in multiple organs. Notably, patients with parotid-only metastases or those with combined bone-only metastases, as well as those with ipsilateral parotid metastases, tend to have more favorable prognostic outcomes. Consequently, it is imperative to exercise greater vigilance in monitoring patients who exhibit metastasis in other organs, as well as those with contralateral or bilateral parotid metastasis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Medical Research Ethics Committee of Binzhou Medical University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FG: Writing – original draft, Conceptualization, Investigation. HF: Writing – original draft, Formal analysis. YW: Writing – original draft, Investigation. YH: Investigation, Writing – original draft, Methodology. XW: Supervision, Writing – original draft. YZ: Project administration, Writing – original draft. JJ: Writing – review & editing, Supervision. ZJ: Writing – review & editing, Validation, Visualization. GZ: Writing – review & editing, Funding acquisition, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 81902702), Natural Science Foundation of Shandong Province (No.ZR2023MH115 and ZR2017LH072), National Key Research and Development Project (No. 2018YFC0114705), The Special Funds for The Qilu Health and Health Leading Talents Cultivation Project (XW). This study was also funded by The 69th batch of China Postdoctoral Science Foundation (NO.2021M692576), the Projects of medical and health technology development program in Shandong province (NO.202104010995, 202304011426) and Projects of Binzhou Technology Development Program (BY2020KJ27).
Acknowledgments
We thanks for our two patients for their consent for publication of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ILC, invasive lobular carcinoma; IDC, invasive ductal carcinoma; SDC, salivary duct carcinoma; LN, lymph node; FNP, facial nerve palsy; RT, radiotherapy; CT, chemotherapy; HT, hormone therapy; TT, targeted therapy; SP, superficial parotidectomy; TP, total parotidectomy; PND, posterolateral neck dissection; MSD, mastectomy and sentinel LN dissection; uDF, interval to progression from primary breast sites to initial diagnosis of parotid metastases; OS, overall survival; M, months; Y, years.
References
1. Wang H, Hoda RS, Faquin W, Rossi ED, Hotchandani N, Sun T, et al. FNA biopsy of secondary nonlymphomatous Malignancies in salivary glands: A multi-institutional study of 184 cases. Cancer CYTOPATHOL. (2017) 125:91–103. doi: 10.1002/cncy.21798
2. Cain AJ, Goodlad J, Denholm SW. Metachronous bilateral submandibular gland metastases from carcinoma of the breast. J LARYNGOL Otol. (2001) 115:683–4. doi: 10.1258/0022215011908649
3. Alath P, Kapila K, Hussein S, Amanguno H, Hebbar HG, George SS, et al. Parotid gland metastasis of breast cancer diagnosed on fine needle aspiration cytology: case report and review of literature. CYTOPATHOLOGY. (2014) 25:346–8. doi: 10.1111/cyt.12108
4. Katz AD. Unusual lesions of the parotid gland. J Surg Oncol. (1975) 7:219–35. doi: 10.1002/jso.2930070308
5. Razem B, Slimani F. Parotid metastasis of breast cancer–A case report. Clin Case Rep. (2020) 10:1394. doi: 10.37421/jccr.2020.10.1394
6. Bohli M, Tebra S, Jaffel H, Bouaouina N. Parotid gland metastasis from breast origin. J Clin Case Rep. (2018) 8:1139. doi: 10.4172/2165-7920.10001139
7. Joyce MR, Awad ZT, Saleem T, Salmo EN, Gormley M, Given HF. The parotid gland: an unusual site of metastasis from carcinoma of breast. Ir J Med Sci. (2000) 169:230. doi: 10.1007/BF03167708
8. Dangore-Khasbage SB, Degwekar SS, Bhowate RR, Bhake A. Metastatic involvement of parotid from carcinoma of the breast–a case report. Oral Maxillofac Surg. (2009) 13:49–53. doi: 10.1007/s10006-009-0146-8
9. Seifert G, Hennings K, Caselitz J. Metastatic tumors to the parotid and submandibular glands–analysis and differential diagnosis of 108 cases. Pathol Res Pract. (1986) 181:684–92. doi: 10.1016/S0344-0338(86)80044-9
10. Zhang Y, Sun C, Yau V, Chen S, Yang Q, Chen W, et al. Clinical features and prognosis of uncommon metastatic breast cancer: A retrospective analysis of 82 cases. Technol Cancer Res Treat. (2023) 22:15330338231184990. doi: 10.1177/15330338231184990
11. Wiesel JM, Weshler Z, Sherman Y, Gay I. Parotid gland metastatic carcinoma of breast origin. J Surg Oncol. (1982) 20:227–30. doi: 10.1002/jso.2930200408
12. Zhang JZ, Gu M. Malignant phyllodes tumor of the breast metastatic to the parotid gland diagnosed by fine needle aspiration biopsy. A Case Rep Acta Cytol. (2003) 47:253–8. doi: 10.1159/000326512
13. Nuyens M, Schupbach J, Stauffer E, Zbaren P. Metastatic disease to the parotid gland. Otolaryngol Head Neck Surg. (2006) 135:844–8. doi: 10.1016/j.otohns.2006.05.010
14. Ramesh RS, Manjunath S, Ustad TH, Pais S, Shivakumar K. Breast cancer presenting as parotid tumour - first reported case in literature. Indian J Surg Oncol. (2010) 1:76–7. doi: 10.1007/s13193-010-0015-9
15. Ando K, Masumoto N, Sakamoto M, Teraoka K, Suzuki T, Kurihara T, et al. Parotid gland metastasis of breast cancer: case report and review of the literature. Breast Care (Basel). (2011) 6:471–3. doi: 10.1159/000335222
16. Kmeid M, Kamar FG, Nasser S, Moukarzel N. Metachronous, single metastasis to the parotid, from primary breast cancer: A case report and review of the literature. Case Rep Oncol Med. (2016) 2016:3965283–5. doi: 10.1155/2016/3965283
17. Cao XS, Cong BB, Yu ZY. Parotid gland metastasis from carcinoma of the breast detected by PET/CT: Case report and review. Med (Baltimore). (2018) 97:e10616. doi: 10.1097/MD.0000000000010616
18. Thomas S, Bhake A. Cytodiagnosis of unusual metastases in parotid gland. J Oral Maxillofac Pathol. (2021) 25:171–6. doi: 10.4103/jomfp.JOMFP_267_20
19. Bissett D, Bessell EM, Bradley PJ, Morgan DA, McKenzie CG. Parotid metastases from carcinoma of the breast. Clin Radiol. (1989) 40:309–10. doi: 10.1016/s0009-9260(89)80218-1
20. Bochnia M JMJM. Bilateral parotid gland metastases with oncocytosis. Med Sci Monit. (1997) 3:CS578–580.
21. Sellinger M, Neubauer K, William M, Hemmerlein B, Friedrich M, Salehin D. Contralateral metastasis of parotid gland in advanced breast cancer with peripheral facial paralysis. Arch GYNECOL OBSTET. (2011) 284:1557–60. doi: 10.1007/s00404-011-1989-4
22. Duncan M, Monteiro M, Quante M. Bilateral parotid gland metastases from carcinoma of the breast that presented 25 years after initial treatment. Br J Oral Maxillofac Surg. (2015) 53:94–6. doi: 10.1016/j.bjoms.2014.09.023
23. Burgess CA, Foden NM, Winter SC. A lesion in the parotid gland. JAMA Otolaryngol Head Neck Surg. (2015) 141:845–6. doi: 10.1001/jamaoto.2015.1412
24. El M'rabet F, Kanab R, Ameuraoui T, Sidibe F, Azegrare M, Arifi S, et al. Métastase parotidienne dun cancer: A propos dun cas literature. Pan Afr Med J. (2017) 27:8876. doi: 10.11604/pamj.2017.27.79.8876
25. Assarian A, Mashoori N, Soleimani V. Parotid gland: an unusual site for breast cancer metastasis: A case report and review of literature. Arch Breast Cancer. (2019) 6:42–6. doi: 10.32768/abc.2019642-46
26. King J, Virani FR, Thomas R, Squires L. Spontaneous bilateral facial paralysis secondary to metastatic breast cancer. Ear Nose Throat J. (2023) 102:NP56–9. doi: 10.1177/0145561320982693
27. Peron V, Miyasaki PM, Martins ME, Andre DRB, Teixeira LV. Oligometastatic breast cancer to parotid gland with complete response. Breast Dis. (2023) 42:67–71. doi: 10.3233/BD-210049
28. Kollias J, Gill PG. Superficial parotidectomy for parotid metastases from breast cancer. Breast. (1997) 6:108–9. doi: 10.1016/S0960-9776(97)90182-X
29. Perez-Fidalgo JA, Chirivella I, Laforga J, Colio JM, Blanes MD, Baydal R, et al. Parotid gland metastasis of a breast cancer. Clin Transl Oncol. (2007) 9:264–5. doi: 10.1007/s12094-007-0051-2
30. Laforga JB, Gasent JM. Mammary invasive duct carcinoma metastatic to parotid gland: report of a case diagnosed by fine-needle aspiration. Diagn CYTOPATHOL. (2009) 37:154–8. doi: 10.1002/dc.21010
31. Rawet T, Jegannathen A, Soumian S. Parotid gland: an unusual site of breast cancer metastasis. BMJ Case Rep. (2017) 2017:brc221842. doi: 10.1136/bcr-2017-221842
32. Agrawal CR, Dutta K, Doval DC, Pasricha S, Gupta M. Breast cancer recurrence presenting as solitary intraparotid nodal metastasis detected by 18F-fluorodeoxyglucose positron emission tomography: A very unusual occurrence. Indian J Nucl Med. (2018) 33:233–6. doi: 10.4103/ijnm.IJNM_17_18
33. Jakharia-Shah A, Wheatley H, Beesley M. Reminder of an important clinical lesson: breast cancer metastasis to the parotid gland. BMJ Case Rep. (2019) 12(10):e226494. doi: 10.1136/bcr-2018-226494
34. Jung HK, Lim YJ, Kim W. Breast cancer metastasis to the parotid: A case report with imaging findings. Am J Case Rep. (2021) 22:e934311. doi: 10.12659/AJCR.934311
35. Dhia SB, Belaid I, Stita W, Hochlaf M, Ezzairi F, Ahmed SB. Bilateral parotid gland metastasis from a breast invasive ductal carcinoma. J Cancer Res Ther. (2020) 16:672–4. doi: 10.4103/jcrt.JCRT_1047_17
36. Andinata B, Irani D, Karsiyah A. Parotid gland metastasis from breast cancer: A case report. Indonesian J Cancer. (2020) 14:97–100. doi: 10.33371/ijoc.v14i3.723
37. Akcan F, Tatar E, Saylam G, Korkmaz H. Management of breast cancer metastasis to parotid gland. CANCER-AM Cancer Soc. (2015) 5:100–30. doi: 10.16899/ctd.58937
38. Cihan YB, Deniz K, Yilmaz MS. Synchronous parotid and thyroid gland metastases from breast cancer. Breast Care (Basel). (2011) 6:133–5. doi: 10.1159/000327508
39. Agarwal R, Rana D, Gupta L, Singh M, Jain S, Rathi AK. Mucinous breast carcinoma metastatic to parotid gland: Report of a case diagnosed by fine needle aspiration. CYTOPATHOLOGY. (2019) 30:128–30. doi: 10.1111/cyt.12645
40. Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of Malignant salivary gland tumors. Am J Clin Pathol. (2003) 119:801–6. doi: 10.1309/RVTP-1G0Q-727W-JUQD
41. Clarke R, Brunner N. Acquired estrogen independence and antiestrogen resistance in breast cancer: estrogen receptor driven phenotypes? Trends Endocrinol Metab. (1996) 7:291–301. doi: 10.1016/s1043-2760(96)00127-0
42. Jalaly JB, Sanati S, Chernock RD, Dibe DG, El-Mofty SK. Salivary duct carcinoma and invasive ductal carcinoma of the breast: A comparative immunohistochemical study. Head Neck Pathol. (2018) 12:488–92. doi: 10.1007/s12105-017-0882-2
43. Mamby CC, Love RR, Heaney E. Metastatic breast cancer 39 years after primary treatment. Wis Med J. (1993) 92:567–9.
44. Dal Lago D, Villa G, Miguoli R, Annoni G, Vergani C. An unusual case of breast cancer relapse after 30 years of disease-free survival. AGE AGEING. (1998) 27:649–50. doi: 10.1093/ageing/27.5.649
45. Demicheli R, Abbattista A, Miceli R, Valagussa P, Bonadonna G. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Cancer Res Treat. (1996) 41:177–85. doi: 10.1007/BF01807163
46. Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev CANCER. (2007) 7:834–46. doi: 10.1038/nrc2256
47. Aron M, Kapila K, Verma K. Role of fine-needle aspiration cytology in the diagnosis of secondary tumors of the thyroid–twenty years' experience. Diagn CYTOPATHOL. (2006) 34:240–5. doi: 10.1002/dc.20329
48. Franzen AM, Gunzel T, Lieder A. Parotid gland metastases of distant primary tumours: A diagnostic challenge. AURIS NASUS LARYNX. (2016) 43:187–91. doi: 10.1016/j.anl.2015.09.010
49. Spreafico R, Nicoletti G, Ferrario F, Scanziani R, Grasso M. Parotid metastasis from renal cell carcinoma: a case report and review of the literature. Acta Otorhinolaryngol Ital. (2008) 28:266–8.
50. Yarington CJ. Metastatic Malignant disease to the parotid gland. LARYNGOSCOPE. (1981) 91:517–9. doi: 10.1288/00005537-198104000-00004
51. Calvo BE, Rodriguez GA, Munoz HA, Soria CP. Metastasis of breast carcinoma in parotid. A case report and review of the literature. Acta Otorrinolaringol Esp. (1995) 46:391–3.
52. Lussier C, Klijanienko J, Vielh P. Fine-needle aspiration of metastatic nonlymphomatous tumors to the major salivary glands: a clinicopathologic study of 40 cases cytologically diagnosed and histologically correlated. CANCER-AM Cancer Soc. (2000) 90:350–6. doi: 10.1002/1097-0142(20001225)90:6<>1.0.CO;2-Z
53. Szymanski M, Siwiec H, Olszanski W, Golabek W. Parotid metastases from breast cancer. Wiad Lek. (2002) 55:494–7.
54. Emanuelli E, Ciorba A, Borsetto D, Cazzador D, Sarcognato S, Marino F, et al. Metastasis to parotid gland from non Head and Neck tumors. J BUON. (2018) 23:163–6.
Keywords: breast cancer, parotid, concomitant metastases, prognosis, overall survival
Citation: Guo F, Fu H, Wang Y, Hua Y, Wang X, Zhang Y, Jian J, Jia Z and Zhang G (2024) Clinical features and prognosis of parotid metastasis of breast cancer: retrospective analysis of 57 cases. Front. Oncol. 14:1442713. doi: 10.3389/fonc.2024.1442713
Received: 02 June 2024; Accepted: 13 August 2024;
Published: 02 September 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Christopher Fundakowski, Thomas Jefferson University, United StatesDaniel Zaccarini, Upstate Medical University, United States
Copyright © 2024 Guo, Fu, Wang, Hua, Wang, Zhang, Jian, Jia and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinbo Jian, jianjinbo08408@163.com; Zhongming Jia, 752447802@qq.com; Guoqiang Zhang, zhanggq1982@163.com
†These authors have contributed equally to this work and share first authorship
 Fengli Guo
Fengli Guo