- Department of Surgery, First Affiliated Hospital of Xiamen University, Xiamen, China
Background: Pancreatic metastasis from renal cell carcinoma (PMRCC) is unusual and there is no consensus on its treatment. The present study aims to evaluate the clinical outcomes of surgical resection for PMRCC.
Methods: PubMed and Web of Science were searched for Eligible studies from January 1980 to January 2024. Individual-patient data were pooled.
Results: A total of 436 participants were identified. The morbidity and 90-day mortality were 38.1% and 3.4%, respectively. Post-pancreatectomy recurrence occurred in 44.1% of the patients. The overall median survival was 116 months, with a 3-, 5- and 10-year survival rate of 85.3%, 76.6%, and 46.5% respectively. On univariate analysis, repeat metastasectomy was associated with a significantly better prognosis (P =0.003).
Conclusion: These data suggest that surgical resection is a safe and effective therapeutic option for PMRCC. Repeat metastasectomy is positively suggested for recurrent disease provided all metastases can be removed curatively.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024525218.
Introduction
Renal cell carcinoma (RCC) is one of the most common urinary cancers, causing 179,368 deaths in 2020 worldwide (1), and half of these patients either had synchronous metastases at presentation or developed metachronous metastases after nephrectomy (2). RCC commonly spreads to the lung, bone, liver, and brain, and metastasis to the pancreas is rare, with the reported incidence of lower than 1% (3). Publications on surgical intervention of pancreatic metastases from RCC (PMRCC) are mostly in the form of case reports and small series (4–10), making it difficult to draw a definitive conclusion about their therapeutic outcomes. This study aims to evaluate the clinical value of pancreatectomy in the treatment of PMRCC based on a pooled analysis of individual-patient data derived from previous publications in the literature.
Methods
The present study was registered in PROSPERO (registry number:CRD42024525218) and conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (11). Two authors independently performed literature search, data extraction, and assessment of the methodological quality of the included studies. Any disagreement was resolved by discussion and consensus.
Search strategy
The electronic search was carried out using PubMed and Web of Science from January 1980 to January 2024. Search terms were: pancreatic metastasis, renal cell carcinoma, and resection. The references of all retrieved articles were screened manually for additional publications.
Inclusion and exclusion criteria
Included studies were case reports and case series that reported the clinical outcomes of PMRCC patients who underwent pancreatectomy with curative intent. Non-English-language articles, abstracts, reviews without original data, overlaps, and reports with uncertain follow-up data or without presenting individual data were excluded.
Data extraction
Data on patient demographics, presenting symptoms, intervals from nephrectomy to pancreatic metastasis or disease-free interval (DFI), surgical outcomes, pathological findings, and long-term survival were extracted. Late recurrence was defined as PMRCC that developed in patients with a 120-month DFI since the initial nephrectomy (12). Overall survival (OS) was calculated from the time of pancreatic metastasectomy to the time of death or last follow-up. Pancreatic fistulas were graded according to the International Study Group on Pancreatic Fistula (ISGPF) (13). Postoperative mortality was defined as death occurring within the 90-day postoperative course
The quality of the included studies was assessed using the Oxford Centre for Evidence-Based Medicine scoring system (14).
Statistical analysis
Descriptive statistics were applied for clinicopathologic characteristics parameters. Continuous variables are expressed as median with range, and categorical variables are expressed as frequencies and percentages. Missing values were not imputed. OS was generated by the Kaplan-Meier method and compared by the log-rank test. Prognostic factors affecting survival were identified using logistic regression models and presented as hazard ratio (HR) with 95% confidence intervals (CI). Statistics were performed via SPSS version 18.0 (SPSS Inc, Chicago, Illinois, USA). A 2-sided P < 0.05.
Results
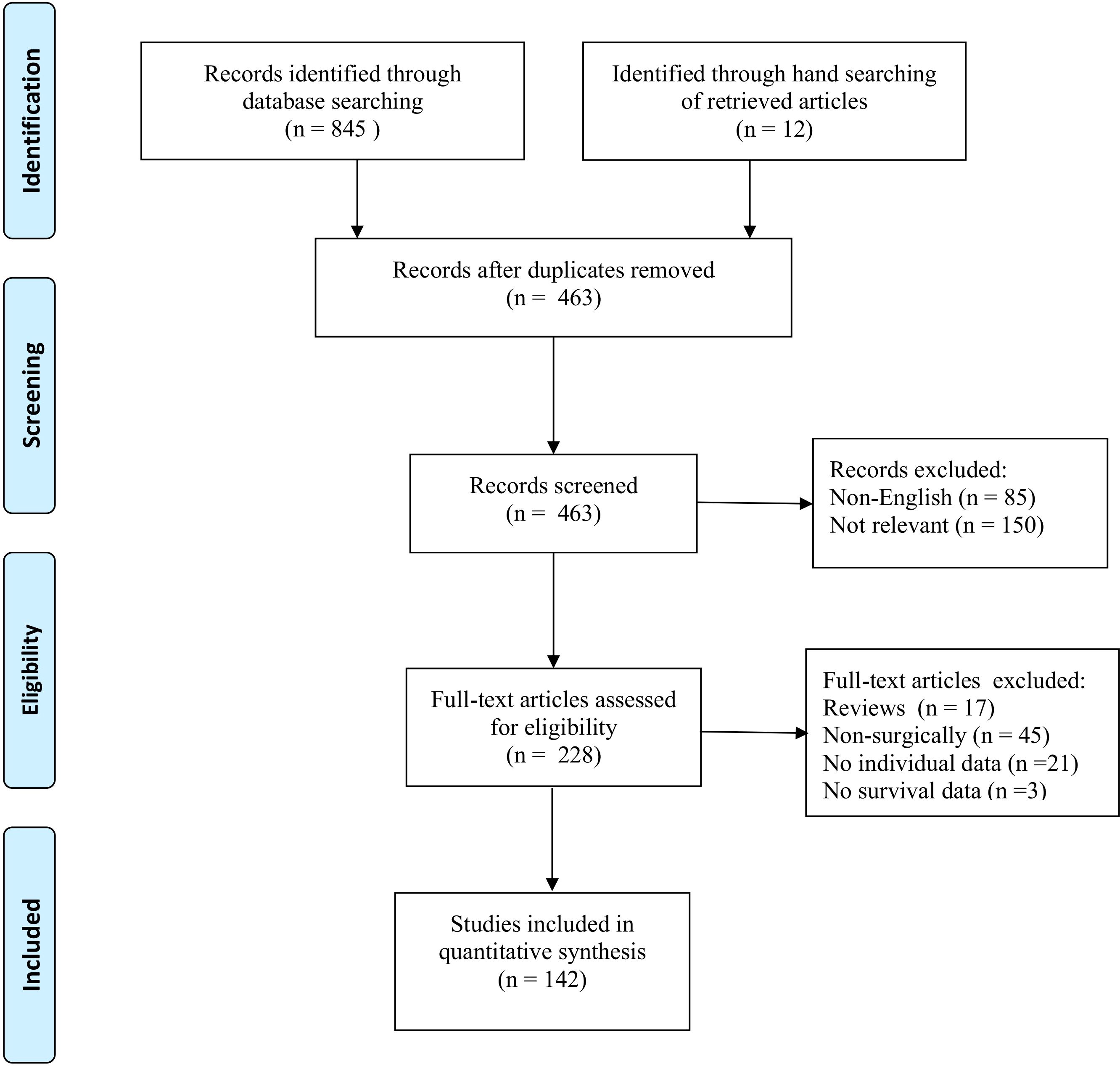
The systematic search identified 142 studies (Appendix A) eligible for inclusion, reporting on 436 patients who underwent pancreatectomy for PMRCC (Figure 1). All studies were retrospectively designed and therefore graded as low evidence of level 4.
Patient characteristics
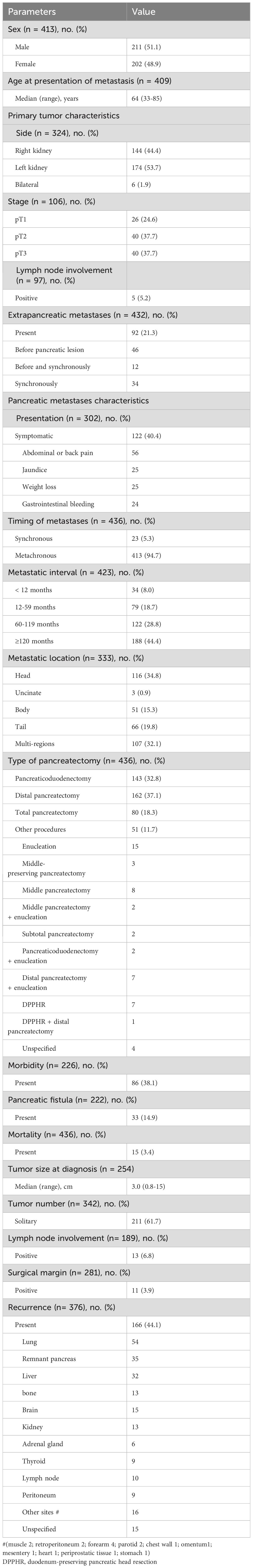
The median age at the time of pancreatic resection was 64 (range 33–88) years with a slight male predominance (51.1%) (Table 1). More than half of the subjects (59.6%) were asymptomatic at the time of diagnosis. The most common presenting symptom was abdominal or back pain (45.4%), followed by jaundice (21%), weight loss (21%), and gastrointestinal bleeding (20%). The primary RCC originated from the left kidney in 53.7% patients, right kidney in 44.4% patients, and both kidneys in 1.9% patients.
RCC metastases to the pancreas were synchronous in 5.3% patients and metachronous in 94.7% patients. The median DFI was 108 months (range 0-432) and 44.4% patients developed late recurrences. Lesions were localized at the pancreatic head in 34.8%, at the body in 15.3%, at the tail in 19.8%, and at the multi-pancreatic regions in 32.1% of patients.
Of the 432 patients with reported information, 92 (21.3%) had at least one extra-pancreatic metastatic lesion before and/or synchronous pancreatic metastasis involving the lung (31), thyroid (18), kidney (17), liver (13), adrenal gland (8), brain (7), lymph nodes (4), retroperitoneum (3), breast (2), ileum (2), parotid gland (2), peritoneum (2), chest wall (1), bone (1), falciform ligament (1), hip (1), arm (1), diaphragm(1), deltoid muscle (1), subcutaneous tissue (2), shoulder (1), spleen (1), trachea (1), tongue (1), scapula (1), abdominal wall (1), buttock (1), oral cavity (1), and hamstrings (1). Treatments were metastasectomy (81, 88.0%), radiation (2, 2.2%), systemic therapy (3, 3.3%), and unspecified (6, 6.5%).
Surgical outcomes
Of the 436 patients, 80 (18.3%) patients underwent total pancreatectomy, and the other 356 patients underwent partial pancreatectomy. The postoperative morbidity rate was 38.1%. Pancreatic fistula occurred in 33 (14.9%) of the 222 reported patients, and only two (6%) of them had grade C pancreatic fistula. Of the 436 patients, 15 patients (3.4%) died during the postoperative course due to multiorgan failure (3), malignant hyperpyrexia (1), heart failure (2), myocardial infarction (1), hemoperitoneum arising from splenic artery pseudoaneurysm rupture (1), liver failure (1), disease progression (1), pulmonary embolism (1), and unspecified reasons (4).
Histopathological examination revealed that the median tumor size was 3.0 (range 0.8-15) cm, and solitary metastases were identified in 61.7% patients. The overall incidence of nodal involvement was 6.8%, and rate of tumor-positive resection margins was 3.9%.
Long-term outcomes
After excluding perioperative mortality, long-term outcomes were assessed in 421 patients. Recurrent disease occurred in 166 (44.1%) of 376 patients with provided information. The most common recurrence site was the lung, followed by the remnant pancreas and liver. Of them, 49 patients underwent 62 repeat metastasectomies. Recurrence sites in the repeat surgery group were the pancreas (22), lung (10), thyroid (6), liver (5), kidney (4), forearm (4), adrenal gland (3), lymph node (2), bone (2), parotid (1), musculus obturatorius externus (2), and periprostatic tissue (1).
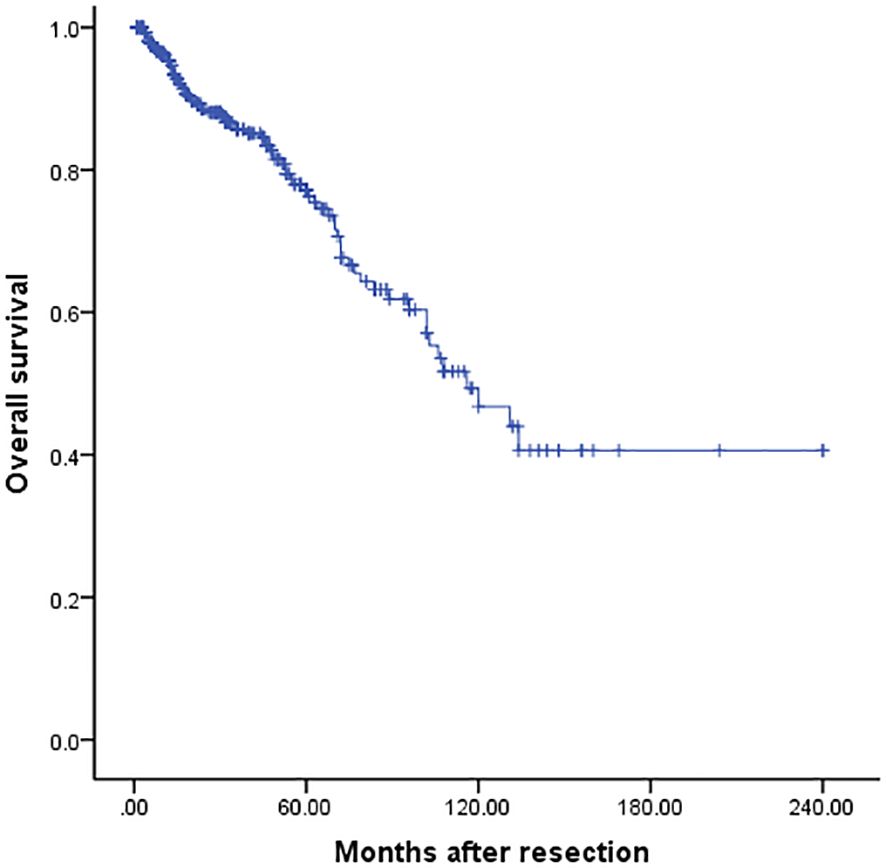
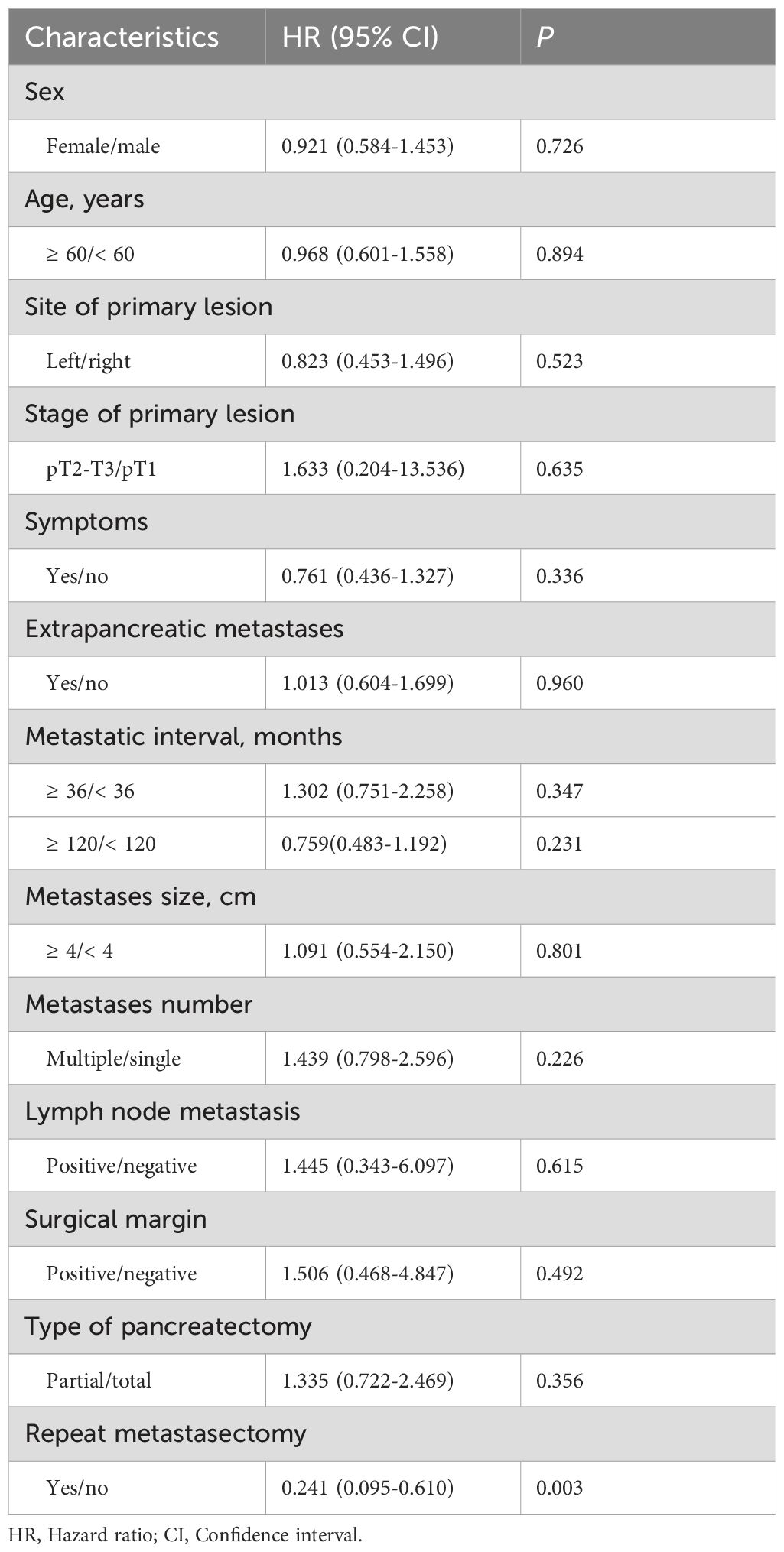
For the entire cohort, the median survival was 116 months, and the 3-, 5- and 10-year OS was 85.3%, 76.6%, and 46.5% respectively (Figure 2). Univariate analysis revealed that only repeat metastasectomy was associated with a significantly better prognosis (Table 2). Multivariable analysis was not performed because only one variable had a value of P < 0.05 in univariable analysis.
Discussion
To the best of our knowledge, this review of results in 436 patients constitutes the largest collective series on surgery for PMRCC yet reported. In comparison to a systematic review on this subject published in 2009 by Tanis et al. (40), a significant proportion (49.3%) of patients included in our report were reported in studies after 2010, indicating that our study is closer to the modern practice. The 5- and 10-year OS rates were 76.6% and 46.5% respectively, confirming the value of pancreatic resection for PMRCC in contributing to favorable long-term outcomes. There have been concerns regarding the safety of pancreatic resection, especially metachronous resection, where intra-abdominal adhesions may be present by the previous operation. However, the present study demonstrated that such operations can be undertaken safely with low mortality and acceptable morbidity, reflecting the improvements in surgical techniques and perioperative patient care in the field of pancreatic surgery over the last several decades.
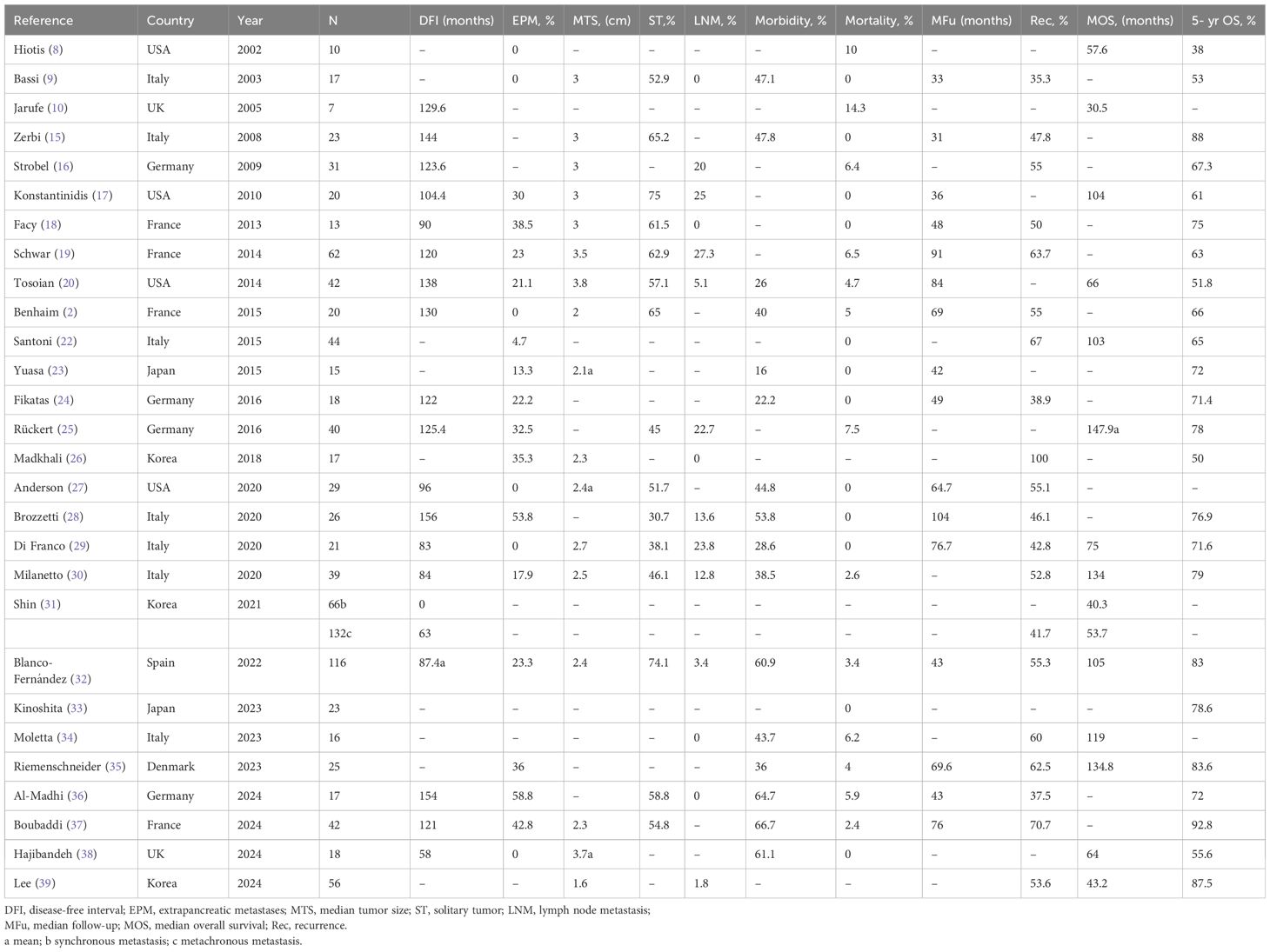
Table 3 summarizes the results of published series that were excluded from the analysis because individual patient data could not be extrapolated (8–10, 15–39). The median overall morbidity and mortality were 44.3% (range16–67%) and 2.4% (range 0–14.3%) respectively. During a median follow-up of 56.7 (range 31–104) months, 54.3% patients (range 35–100%) developed recurrences. The median survival since pancreatectomy was 75 (range 30.5–147.9) months with a 5-year OS rate of 71.8% (range 38–92.8%). The fact that these studies reported varied prognosis, probably due to the significant clinical heterogeneity of the study groups, rendering meta-analysis of survival outcomes inappropriately. Notable, most of these series contained very small number of patients, making them unable to provide specific data on prognostic factors of survival. In contrast, a pooled analysis of individual patient data from previous reports will increase the statistical power to address this issue.
About 21.3% of the patients reported in this study had additional extra-pancreatic lesions, and most of these lesions were resected. The survival time of these patients was not significantly different from that in the other patients (P = 0.960) (Table 2), which is consistent with the result reported by Tanis et al. (40). Therefore, it is reasonable to offer pancreatic surgery to patients with extra-pancreatic lesions as long as the metastases have been managed curatively.
As demonstrated in the current study, PMRCC presented a long median DFI of 108 months, and late recurrences were not an uncommon phenomenon. Thus, a lifelong surveillance is necessary for patients with a history of RCC. Some authors noted that a prolonged DFI (≥ 36 months) was associated with a better prognosis probably due to a less aggressive biological behavior of slow tumor growth (16), but this relationship was not validated in the current study.
Consistent with prior studies (30, 32), neither multiplicity nor size of PMRCC was found to confer a significant effect on patient survival. It seems that surgical treatment is indicated for all PMRCC cases provided it is technically feasible.
The extent of pancreatectomy remains a controversy in the treatment of PMRCC, especially in the setting of multifocal disease (9, 32, 37). Theoretically, total pancreatectomy has advantages of complete removal of occult micrometastases and is thought to reduce the risk of pancreatic recurrences. However, this extensive surgical resection may lead to major metabolic problems, culminating in the poor quality of life. It was found in this study that partial pancreatectomy did not impair survival outcomes, suggesting that such pancreas-sparing surgical procedures should be encouraged as an initial treatment for PMRCC. In order to achieve radicality, thorough exploration of the pancreatic parenchyma by palpation and/or ultrasound is required (18). Nevertheless, for diseases involving the entire pancreas, total pancreatectomy is indicated.
We found that lymph node involvement in PMRCC was rare and therefore had little prognostic significance, which is also supported by data from most previously published series (Table 3). As many surgeons were hesitant to perform routine lymphadenectomy for PMRCC in clinical practice, most patients (56.7%) presenting insufficient lymph node information were included in this pooled analysis. Nevertheless, from an oncological perspective, we believe that lymphadenectomy may provide staging information for personalized adjuvant therapy.
Nevertheless, it should be emphasized that recurrence after resection is common, clearly exceeding 40%. The problem arises in this condition as to whether a repeat metastasectomy could be indicated. However, little information exists about this issue to date. A striking finding in this study was that repeat metastasectomy confer substantial survival benefits, highlighting the necessity for surgical resection of relapse provided all metastases can be complete eradicated.
Although encouraging long-term survival outcomes have been seen after surgical management, the question of whether this aggressive approach is a preferable option for PMRCC remains in doubt in the light of recent advances in systemic therapy, including tyrosine kinase inhibitors (TKI) and immune checkpoint inhibitors (ICI). Santoni and colleagues concluded that surgical resection and TKI therapy could provide similar median OS (103 vs. 86 months; P = 0.201) (22). However, their study population was not large enough and there may be a bias in patient selection.
The main limitation of the present review is its retrospective design with inherited risk of information loss. Consequently, patients were not stratified by the Memorial Sloan-Kettering scoring system, and therefore no analysis regarding cancer-specific survival and disease-free survival could be carried out. Similarly, in the modern era of multidisciplinary strategies for metastatic RCC (41), we are unable to evaluate the role of pancreatic metastasectomy in conjunction with targeted and immune therapy, and therefore further investigation is required in future.
Conclusion
In summary, surgical resection is a safe and effective therapeutic option for PMRCC. In addition, recurrent disease should be treated by repeat metastasectomy provided all metastases can be removed curatively.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YZ: Conceptualization, Formal analysis, Funding acquisition, Resources, Writing – original draft. XW: Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing. SC: Software, Validation, Visualization, Writing – review & editing. SW: Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Foundation of Xiamen Science and Technology Bureau (3502Z20227263).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. You D, Lee C, Jeong IG, Song C, Lee JL, Hong B, et al. Impact of metastasectomy on prognosis in patients treated with targeted therapy for metastatic renal cell carcinoma. J Cancer Res Clin Oncol. (2016) 142:2331–8. doi: 10.1007/s00432-016-2217-1
3. Thomas AZ, Adibi M, Borregales LD, Wood CG, Karam JA. Role of metastasectomy in metastatic renal cell carcinoma. Curr Opin Urol. (2015) 25:381–9. doi: 10.1097/MOU.0000000000000196
4. Saxon A, Gottesman J, Doolas A. Bilateral hypernephroma with solitary pancreatic metastasis. J Surg Oncol. (1980) 13:317–22. doi: 10.1002/jso.2930130406
5. Audisio RA, La Monica G. Solitary pancreatic metastasis occurring 20 years after nephrectomy for carcinoma of the kidney. Tumori. (1985) 71:197–200. doi: 10.1177/030089168507100217
6. Simpson NS, Mulholland CK, Lioe TF, Spence RA. Late, solitary metastatic renal carcinoma in the pancreas. Ulster Med J. (1989) 58:198–9.
7. Temellini F, Bavosi M, Lamarra M, Quagliarini P, Giuliani F. Pancreatic metastasis 25 years after nephrectomy for renal cancer. Tumori. (1989) 75:503–4. doi: 10.1177/030089168907500522
8. Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. (2002) 9:675–9. doi: 10.1007/BF02574484
9. Bassi C, Butturini G, Falconi M, Sargenti M, Mantovani W, Pederzoli P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br J Surg. (2003) 90:555–9. doi: 10.1002/bjs.4072
10. Jarufe N, McMaster P, Mayer AD, Mirza DF, Buckels JA, Orug T, et al. Surgical treatment of metastases to the pancreas. Surgeon. (2005) 3:79–83. doi: 10.1016/s1479-666x(05)80066-6
11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
12. Miyao N, Naito S, Ozono S, Shinohara N, Masumori N, Igarashi T, et al. Late recurrence of renal cell carcinoma: retrospective and collaborative study of the Japanese Society of Renal Cancer. Urology. (2011) 77:379–84. doi: 10.1016/j.urology.2010.07.462
13. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014
14. Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol. (2008) 15:1765–74. doi: 10.1245/s10434-008-9848-7
15. Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. (2008) 15:1161–8. doi: 10.1245/s10434-007-9782-0
16. Strobel O, Hackert T, Hartwig W, Bergmann F, Hinz U, Wente MN, et al. Survival data justifies resection for pancreatic metastases. Ann Surg Oncol. (2009) 16:3340–9. doi: 10.1245/s10434-009-0682-3
17. Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, Fernandez-del Castillo C, et al. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. (2010) 211:749–53. doi: 10.1016/j.jamcollsurg.2010.08.017
18. Facy O, Angot C, Guiu B, Al Samman S, Matte A, Rat P, et al. Interest of intraoperative ultrasonography during pancreatectomy for metastatic renal cell carcinoma. Clin Res Hepatol Gastroenterol. (2013) 37:530–4. doi: 10.1016/j.clinre.2013.01.006
19. Schwarz L, Sauvanet A, Regenet N, Mabrut JY, Gigot JF, Housseau E, et al. Long-term survival after pancreatic resection for renal cell carcinoma metastasis. Ann Surg Oncol. (2014) 21:4007–13. doi: 10.1245/s10434-014-3821-4
20. Tosoian JJ, Cameron JL, Allaf ME, Hruban RH, Nahime CB, Pawlik TM, et al. Resection of isolated renal cell carcinoma metastases of the pancreas: outcomes from the Johns Hopkins Hospital. J Gastrointest Surg. (2014) 18:542–8. doi: 10.1007/s11605-013-2278-2
21. Benhaim R, Oussoultzoglou E, Saeedi Y, Mouracade P, Bachellier P, Lang H. Pancreatic metastasis from clear cell renal cell carcinoma: outcome of an aggressive approach. Urology. (2015) 85:135–40. doi: 10.1016/j.urology.2014.09.034
22. Santoni M, Conti A, Partelli S, Porta C, Sternberg CN, Procopio G, et al. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann Surg Oncol. (2015) 22:2094–100. doi: 10.1245/s10434-014-4256-7
23. Yuasa T, Inoshita N, Saiura A, Yamamoto S, Urakami S, Masuda H, et al. Clinical outcome of patients with pancreatic metastases from renal cell cancer. BMC Cancer. (2015) 15:46. doi: 10.1186/s12885-015-1050-2
24. Fikatas P, Klein F, Andreou A, Schmuck RB, Pratschke J, Bahra M. Long-term survival after surgical treatment of renal cell carcinoma metastasis within the pancreas. Anticancer Res. (2016) 36:4273–8.
25. Rückert F, Distler M, Ollmann D, Lietzmann A, Birgin E, Teoule P, et al. Retrospective analysis of survival after resection of pancreatic renal cell carcinoma metastases. Int J Surg. (2016) 26:64–8. doi: 10.1016/j.ijsu.2015.12.003
26. Madkhali AA, Shin SH, Song KB, Lee JH, Hwang DW, Park KM, et al. Pancreatectomy for a secondary metastasis to the pancreas: A single-institution experience. Med (Baltimore). (2018) 97:e12653. doi: 10.1097/MD.0000000000012653
27. Anderson B, Williams GA, Sanford DE, Liu J, Dageforde LA, Hammill CW, et al. A 22-year experience with pancreatic resection for metastatic renal cell carcinoma. HPB (Oxford). (2020) 22:312–7. doi: 10.1016/j.hpb.2019.05.019
28. Brozzetti S, Bini S, De Lio N, Lombardo C, Boggi U. Surgical-only treatment of pancreatic and extra-pancreatic metastases from renal cell carcinoma - quality of life and survival analysis. BMC Surg. (2020) 20:101. doi: 10.1186/s12893-020-00757-0
29. Di Franco G, Gianardi D, Palmeri M, Furbetta N, Guadagni S, Bianchini M, et al. Pancreatic resections for metastases: A twenty-year experience from a tertiary care center. Eur J Surg Oncol. (2020) 46:825–31. doi: 10.1016/j.ejso.2019.11.514
30. Milanetto AC, Morelli L, Di Franco G, David A, Campra D, De Paolis P, et al. A plea for surgery in pancreatic metastases from renal cell carcinoma: indications and outcome from a multicenter surgical experience. J Clin Med. (2020) 9:3278. doi: 10.3390/jcm9103278
31. Shin TJ, Song C, Jeong CW, Kwak C, Seo S, Kang M, et al. Metastatic renal cell carcinoma to the pancreas: Clinical features and treatment outcome. J Surg Oncol. (2021) 123:204–13. doi: 10.1002/jso.26251
32. Blanco-Fernández G, Fondevila-Campo C, Sanjuanbenito A, Fabregat-Prous J, Secanella-Medayo L, Rotellar-Sastre F, et al. Pancreatic metastases from renal cell carcinoma. Postoperative outcome after surgical treatment in a Spanish multicenter study (PANMEKID). Eur J Surg Oncol. (2022) 48:133–41. doi: 10.1016/j.ejso.2021.08.011
33. Kinoshita S, Yamashita YI, Kitano Y, Hayashi H, Sugimachi K, Nishizaki T, et al. Survival impact of pancreatic resection for metastases in the pancreas: A retrospective multi-center study. Surg Oncol. (2023) 48:101942. doi: 10.1016/j.suronc.2023.101942
34. Moletta L, Friziero A, Serafini S, Grillo V, Pierobon ES, Capovilla G, et al. Safety and efficacy of surgery for metastatic tumor to the pancreas: A single-center experience. J Clin Med. (2023) 12:1171. doi: 10.3390/jcm12031171
35. Riemenschneider KA, Farooqui W, Penninga L, Storkholm JH, Hansen CP. The results of surgery for renal cell carcinoma metastases of the pancreas. Scand J Gastroenterol. (2024) 59:354–60. doi: 10.1080/00365521.2023.2286911
36. Al-Madhi S, Acciuffi S, Meyer F, Dölling M, Beythien A, Andric M, et al. The pancreas as a target of metastasis from renal cell carcinoma: is surgery feasible and safe? A single-center experience in a high-volume and certified pancreatic surgery center in Germany. J Clin Med. (2024) 13:1921. doi: 10.3390/jcm13071921
37. Boubaddi M, Marichez A, Adam JP, Chiche L, Laurent C. Long-term outcomes after surgical resection of pancreatic metastases from renal Clear-cell carcinoma. Eur J Surg Oncol. (2024) 50:107960. doi: 10.1016/j.ejso.2024.107960
38. Hajibandeh S, Hajibandeh S, Romman S, Ghassemi N, Evans D, Laing RW, et al. Pancreatic resection for metastasis from renal cell carcinoma: A single institution experience and meta-analysis of survival outcomes. Pancreatology. (2024) 24:160–8. doi: 10.1016/j.pan.2023.11.014
39. Lee O, Yoon SK, Yoon SJ, Kim H, Han IW, Heo JS, et al. Pancreatic metastasectomy for secondary Malignancy of the pancreas: A single-institution experience. Anticancer Res. (2024) 44:703–10. doi: 10.21873/anticanres.16861
40. Tanis PJ, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. (2009) 96:579–92. doi: 10.1002/bjs.6606
Keywords: renal cell carcinoma, metastases, resection, pancreas, survival
Citation: Zhou Y, Wang X, Chen S and Wang S (2024) A pooled analysis of pancreatic resection for metastatic renal cell carcinoma. Front. Oncol. 14:1442256. doi: 10.3389/fonc.2024.1442256
Received: 01 June 2024; Accepted: 25 October 2024;
Published: 15 November 2024.
Edited by:
Giuseppe Zimmitti, Fondazione Poliambulanza Istituto Ospedaliero, ItalyReviewed by:
Giacomo Deiro, AOU Città della Salute e della Scienza, ItalyCosimo Sperti, University of Padua, Italy
Copyright © 2024 Zhou, Wang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanming Zhou, emhvdXltc3h5QHNpbmEuY24=
†These authors have contributed equally to this work
 Yanming Zhou
Yanming Zhou Xiao Wang
Xiao Wang Shi Chen
Shi Chen Shijie Wang
Shijie Wang