
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 November 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1442168
Purpose: We sought to develop and validate a nomogram for predicting extra-urinary recurrence (EUR) following radical nephroureterectomy (RNU) in patients with upper urinary tract urothelial carcinoma (UTUC).
Methods: Data from 556 UTUC patients post-RNU at the First Hospital of Jilin University were retrospectively analyzed. These patients were categorized into a training group (n=389) and a validation group (n=167). Variables significantly associated with prognosis were identified using univariate Cox regression and most minor absolute shrinkage and selection operator (LASSO) analysis. These independent predictors were incorporated into the nomogram to estimate extra-urinary recurrence-free survival (EURFS). Validation of the nomogram involved ROC curves, calibration plots, and decision curve analysis (DCA). Patients were stratified into two risk categories based on their nomogram scores to compare EURFS using the Kaplan-Meier method.
Results: Eight predictors were identified: T-stage, N-stage, tumor grade, local and nerve invasion, preoperative hemoglobin level, neutrophil-to-lymphocyte ratio (NLR), and creatinine, all proving to be independent predictors of EUR. A nomogram was created based on these eight factors, and using the ROC, calibration curves, and DCA, good prediction results were shown in both the training and validation groups. The training and validation groups also showed reliable predictive performance. In particular, there was a significant difference in survival between the high-risk and low-risk groups (P<0.0001). We have also built a network calculator that calculates patient survival time. The URL is https://haowu24.shinyapps.io/dynnomapp.
Conclusion: A nomogram for predicting distant metastases in UTUC patients was successfully developed, and its accuracy, reliability, and clinical value were demonstrated. This new tool helps to improve the clinical management of UTUC cases.
Upper urinary tract uroepithelial carcinoma (UTUC) is a relatively uncommon malignancy, comprising 7-8% of all urological tumors and 5% of all uroepithelial malignancies (1). Siegel et al. (2) observed a gender disparity in UTUC incidence, with a 2:1 male-to-female ratio. The established gold standard for UTUC management is radical nephroureterectomy, which includes nephroureterectomy combined with a cystectomy sleeve (3). Although RNU is the recommended treatment, high recurrence and metastasis rates remain a significant concern, especially in the advanced stages of UTUC.
UTUC is often detected at advanced stages and is associated with a dismal prognosis (4). Studies have demonstrated that even following radical nephroureterectomy, the rates of recurrence and metastasis for UTUC range between 22% and 66% (5, 6). Recurrences may occur locally, distally, in the bladder, or contralaterally, reflecting the aggressive nature of these tumors (7). The 5-year intravesical recurrence-free survival rate (IVRFS) is estimated to be between 46% and 54% (8). Moreover, postoperative complications include extravesical recurrences, which occur in about 27.3-33% of cases and involve lymph nodes, distal organs, and the original tumor site. These are referred to as extra-urinary recurrences (EUR) (9), and their 5-year survival rate for high-risk patients is notably lower at 36.2% (9). This highlights the aggressive nature of EUR and its distinct management challenges compared to intravesical recurrences.
The prognosis for UTUC patients is contingent upon factors across the preoperative, intraoperative, and postoperative stages. While most research focuses on intravesical recurrence risk following radical nephroureterectomy, factors such as gender, prior bladder cancer, chronic kidney disease, positive preoperative urine cytology, ureteral tumor location, tumor multifocality, and aggressive pathological staging (infiltrative pT) have been identified (10, 11). However, despite their clinical significance, there is a lack of research focusing on EUR-specific risk factors. Early identification of patients at high risk for EUR could greatly benefit treatment planning and monitoring.
Patients experiencing EUR tend to have poorer outcomes. Despite their importance, studies on EUR remain sparse (12), underscoring the need for enhanced predictive models to guide clinical decisions, including selecting more aggressive treatments and appropriate monitoring strategies (13). Unlike intravesical recurrence, EUR poses unique challenges in management and prognosis, further emphasizing the need for specialized risk stratification tools. In response, we conducted a retrospective analysis of demographic, pathological, and clinical data from 700 patients at the First Hospital of Jilin University. From this data, we developed and validated a nomogram model for EUR to identify and promptly address risk factors, improving patient management and outcomes. This nomogram aims to provide clinicians with a practical and reliable tool to predict EUR, helping tailor treatment strategies for high-risk patients.
We conducted a retrospective analysis of UTUC patients who underwent RNU from January 2015 to December 2022 at the First Hospital of Jilin University, China. The Institutional Research Ethics Committee approved this study, which adhered to the STROCSS guidelines (14). All participants provided informed consent. All procedures conformed to relevant regulations and the Declaration of Helsinki. Trained clinicians performed all diagnostic and assessment activities. Figure 1 shows a flow diagram of the study design.
Inclusion criteria were as follows: (1) patients diagnosed with primary and pathologically confirmed UTUC; (2) patients with comprehensive clinical records; (3) patients with unilateral disease; (4) patients who underwent RNU with a cystectomy sleeve. Exclusion criteria included: (1) patients with bilateral UTUC; (2) patients who did not receive RNU with a cystectomy sleeve; (3) patients with metastatic uroepithelial carcinoma; (4) patients lacking complete clinical data; (5) patients with congenital urological anomalies. None of the enrolled patients received neoadjuvant chemotherapy.
Patient data from the First Hospital of Jilin University were randomly assigned to training and validation cohorts in a 7:3 ratio. Follow-up occurred every three months for the first two years post-surgery and subsequently every six months up to 5 years. Evaluations included comprehensive histories, clinical examinations, serum and urine tests, physical examinations, urinary ultrasound, chest and abdominal CT scans, cystoscopic evaluations, and urine cytology. Additional imaging tests, such as magnetic resonance imaging (MRI), bone scans, or positron emission tomography-computed tomography (PET-CT), were performed based on clinical indications. This study did not categorize intravesical and contralateral upper urinary tract recurrences as extraendothelial recurrences. Overall survival (OS) was defined as the period from RNU to death from any cause. Extra-urothelial recurrence-free survival (EURFS) was measured from the surgery date to the occurrence of EUR. The neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) were also assessed.
Demographic and clinical variables, such as age, gender, body mass index (BMI), history of hypertension, diabetes mellitus, bladder cancer, tumor side, pathological stage (pT), lymph node status (pN0, pNx, or pN+), tumor grade, size, multifocality, margin status, lymphovascular invasion (LVI), urocytology, hydrocele, and hematological parameters including NLR, PLR, and LMR, were recorded or calculated from clinical records at the time of RNU.
In this study, to ensure the robustness and reliability of the model, variance inflation factor (VIF) was used to detect multicollinearity among the variables (Supplementary Table 1). All VIF values were within an acceptable range (below 3), indicating that there was no significant multicollinearity between the variables. This ensures that high correlations between variables do not compromise the model’s explanatory power and predictive accuracy.
All surgical specimens underwent standard pathological processing, and a genitourinary pathologist reviewed each section. Tumor staging adhered to the American Joint Committee on Cancer (AJCC) TNM classification (8th edition), and grading followed the 2016 World Health Organization (WHO) criteria. Tumor location referred to the primary tumor site. The presence of tumors or abnormal cells in preoperative urine samples was documented as positive urine cytology.
Continuous variables with normal distribution are reported as mean ± standard deviation, while those not normally distributed are presented as medians with interquartile ranges. Categorical variables are expressed as n (%). Statistical significance was assessed using two-tailed tests, with p values < 0.05 deemed significant. Variable selection was based on one-way COX regression analysis, most minor absolute shrinkage, and selection operator (LASSO) regularisation. To minimize overfitting, ten-fold cross-validation was utilized. LASSO coefficients for selected variables were nonzero at λmin, indicating potential independent risk factors. Independent risk factors were incorporated into graphs. EURFS at 1, 3, and 5 years was displayed using column-line plots. The model’s discriminative ability was evaluated with a consistency index (C-index), and calibration curves assessed model calibration.
Decision curve analysis (DCA) was calculated to evaluate the clinical utility of the nomogram model compared to predictions based solely on pathological tumor staging, assessing the accuracy of both the nomogram model and pathological T-staging. Patients’ EURFS was predicted using a nomogram, and a total risk score was calculated for each patient in the training and validation cohorts to classify them into high and low-risk groups. Differences in survival curves between these groups were analyzed in the training cohort and validated using Kaplan-Meier (K-M) analysis in the validation cohort. All assessment methods were applied in both the training and validation sets. P-values were two-tailed, and p < 0.05 was considered statistically significant. All statistical analyses and graphs were conducted using R software (version 4.2.2) and IBM SPSS Statistics (version 24).
Following the inclusion and exclusion criteria, 556 patients with pathologically confirmed UTUC were included. Of these, 189 (34.0%) experienced EUR. Patients were categorized into a no-recurrence group (n=367) and a recurrence group (n=189) based on the occurrence of EUR. The average age of all participants was 68.9 years, with 58.8% being female. Recurrence was associated with older age, lower hemoglobin levels, shorter follow-up periods, a higher choice of surgical approach for NLR, advanced pathological T-stages, regional lymph node status, tumor grade, infiltration degree, neurological invasion, and elevated creatinine levels in the recurrent group compared to the non-recurrent group, with a statistically significant difference (p < 0.05) (Table 1).
The patients were divided into a training set (n=389) and a validation set (n=167) in a 7:3 ratio. The mean follow-up duration was 43.9 months for the training set and 49.5 months for the validation set. Most variables were similar between the training and validation sets, except for the later onset of EUR in the validation set compared to the training set (49.5 months vs. 43.9 months, p<0.05). There was no statistical difference in the duration of follow-up between the two groups. The clinicopathological characteristics of the patients are detailed in Table 2.
We employed a one-way Cox regression with the LASSO model to identify significant predictors of EUR from the training cohort, identifying eight potential predictors with nonzero coefficients (Figure 2), as presented in Table 3: T stage, N stage, tumor grade, local invasion, neurological invasion, preoperative hemoglobin level, NLR, and creatinine. These factors were established as independent predictors of EUR.
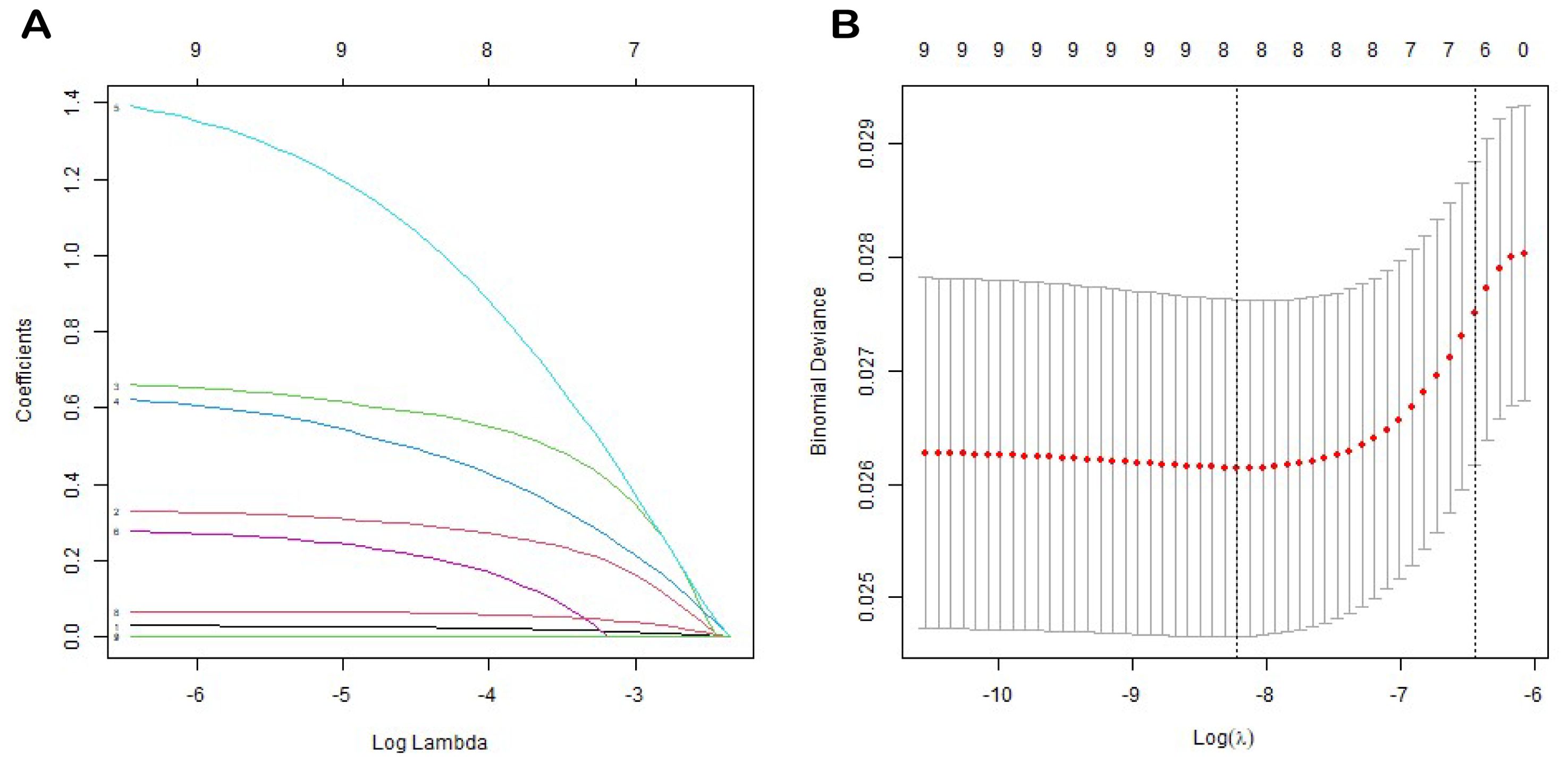
Figure 2. LASSO coefficient profiles of all variables predicting EURFS (A). Tenfold cross-validation for tuning parameter selection in the least LASSO model related to EURFS (B).
We constructed a nomogram to predict EURFS using these variables, illustrated in Figure 3. The total risk score was calculated for each patient in the training and validation cohorts. Patients were classified into high-risk and low-risk groups using the optimal risk score cut-off of 149 (Figure 4). K-M survival analysis revealed significantly higher EUR in the high-risk group (P for log-rank < 0.0001).
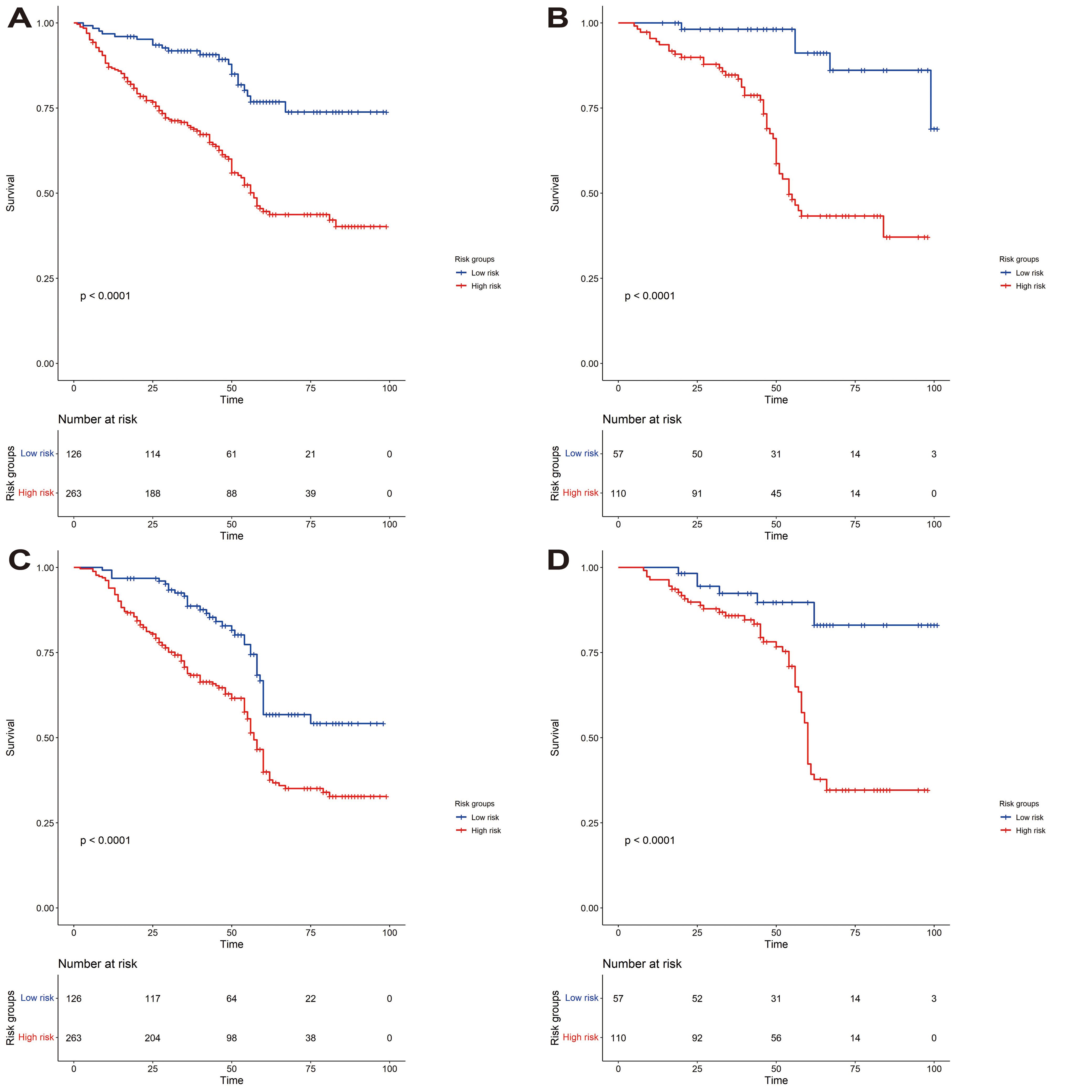
Figure 4. Kaplan-Meier curves for event-free survival analysis for EUR and OS. (A, B) Correspond to the Kaplan-Meier curves for the EUR training and validation sets. (C, D) are the Kaplan-Meier curves for the OS training and validation sets.
The model’s discriminative ability was evaluated using the c-index and calibration plots. The c-index was 0.661 for the training set and 0.757 for the validation set. Prediction performance in the training set yielded AUC values of 0.728, 0.687, and 0.662 for 1, 3, and 5 years respectively. In the validation set, AUC values were 0.84, 0.774, and 0.802 for the respective time points (Figure 5). Calibration plots for 1, 3, and 5 years demonstrated a good fit (Figure 6). Additionally, DCA analysis assessed the model’s predictive performance over these timeframes (Figure 7), showing high net benefit in both the training and validation sets, underscoring its predictive efficacy and clinical utility.
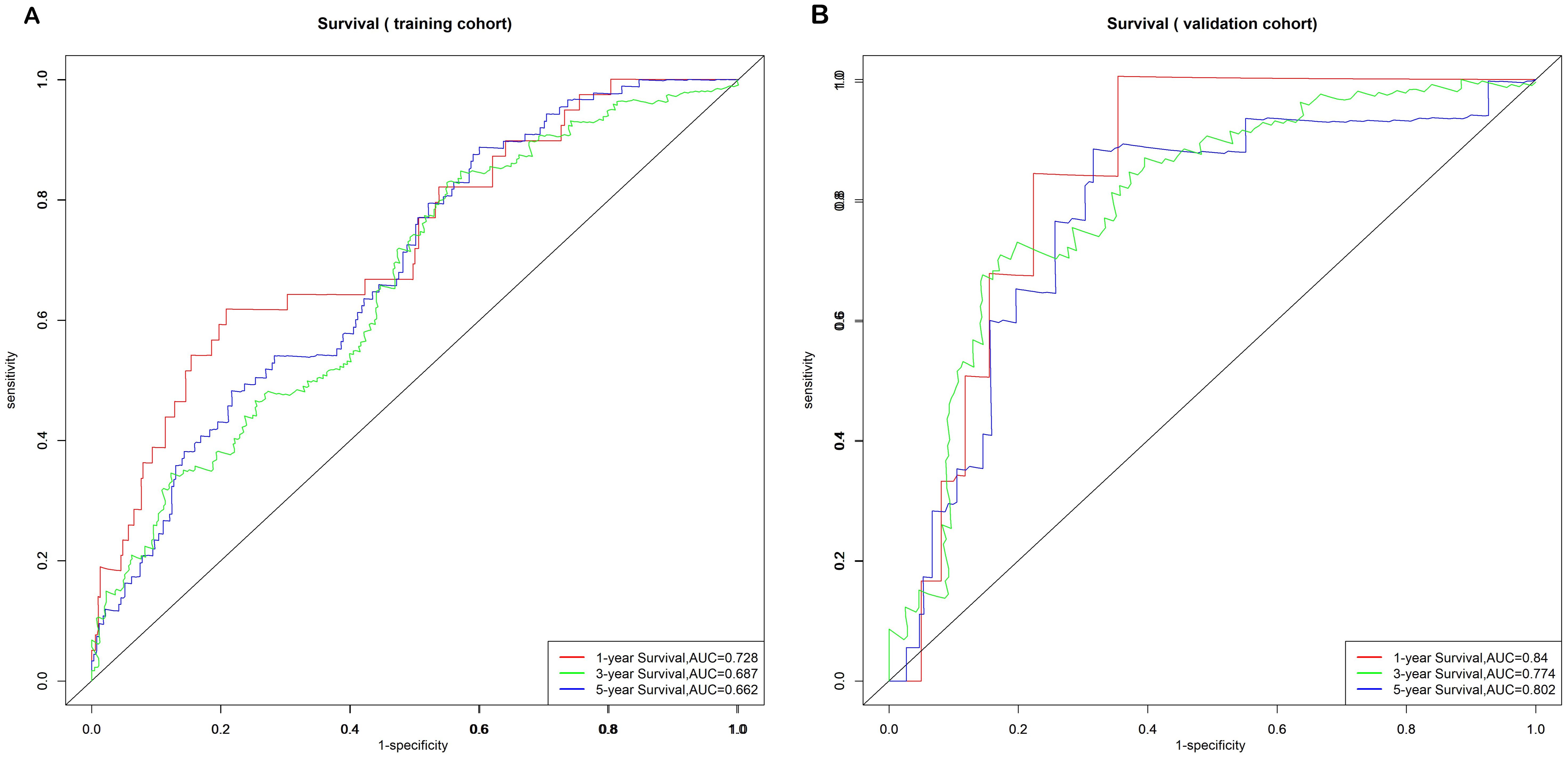
Figure 5. Nomogram ROC curves to predict 1-, 3-, and 5-year EUR in the training cohort (A) and validation cohort (B).

Figure 6. The calibration curves of the EUR nomogram at 1, 3, and 5 years in the training cohort (A, C, E) and the validation cohort (B, D, F).
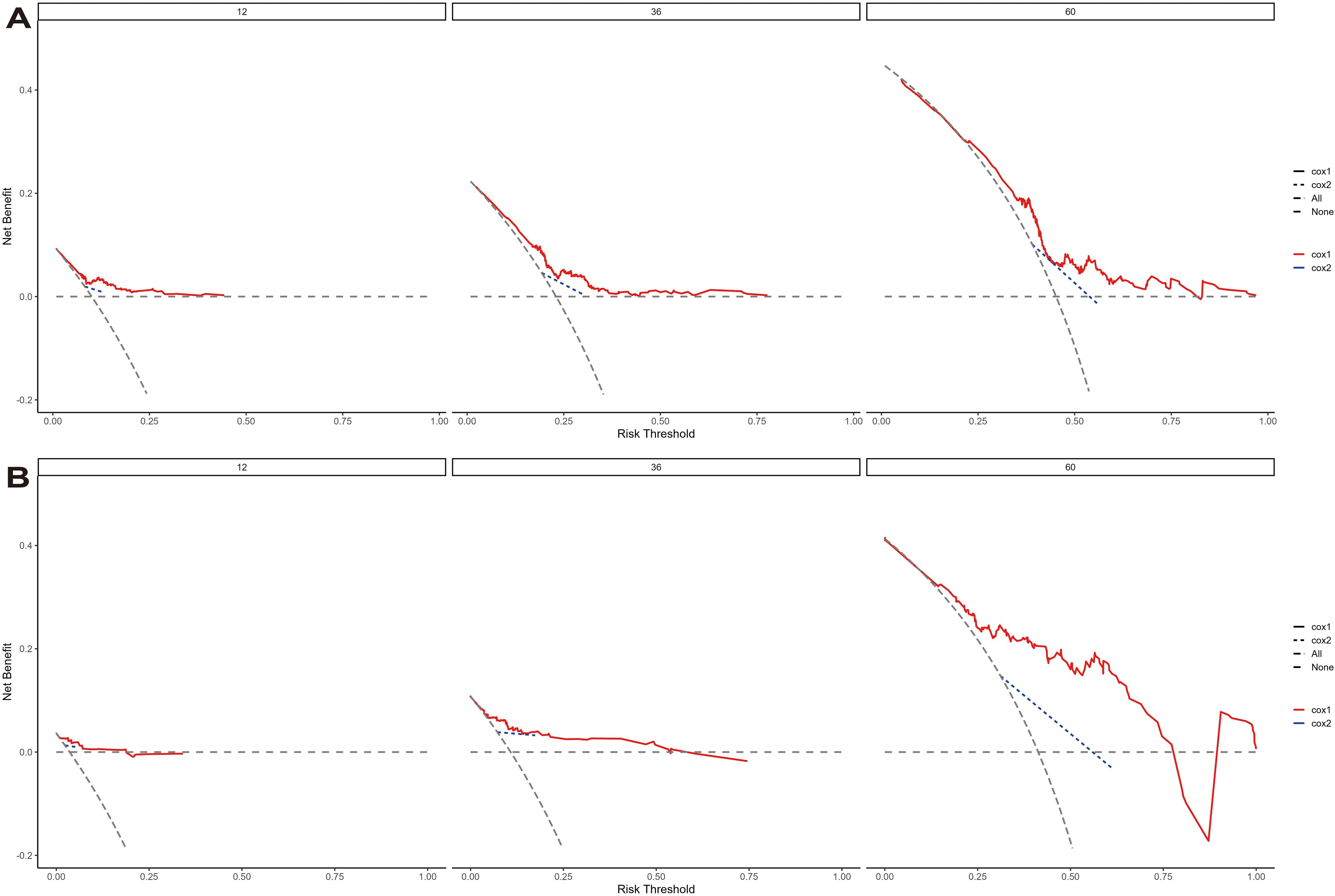
Figure 7. The decision curve analysis of EUR nomogram at 1, 3, and 5 years in the training cohort (A) and validation cohort (B). The y-axis measures the net benefit. The thick solid line represents the assumption that all patients have no EUR, and the thin solid line represents the assumption that all patients have EUR. The dotted line represents the risk nomogram.
DCA curves further highlighted the superior clinical utility of the nomogram over categorical T-staging in both the training and validation cohorts (Figure 7). We calculated risk scores for individuals in both cohorts using the nomogram model. Patients were divided into two groups based on threshold values derived from the training cohort’s risk points. K-M survival curve analysis was conducted to explore the correlation between clinical outcomes and risk group classifications.
To further improve the applicability of the nomogram model in clinical practice, we developed an online interactive calculator for predicting the risk of extraurinary tract recurrence (EUR) in patients with urothelial carcinoma (UTUC) of the upper tract after radical nephropeterectomy (RNU). The calculator allows users to enter the patient’s vital clinicopathological data, including T stage, N stage, tumor grade, preoperative hemoglobin level, neutrophil-to-lymphocyte ratio (NLR), and other variables, to calculate and display the probability of a patient developing EUR in the next 1, 3 and 5 years.
Using this network calculator, clinicians can quickly assess a patient’s risk of recurrence, leading to a more personalized follow-up plan and treatment strategy for high-risk patients. Compared to traditional manual risk calculations, this online tool is more intuitive and faster, helping to improve the efficiency of clinical decision-making.
The link to the web calculator is https://haowu24.shinyapps.io/dynnomapp.
UTUC is a relatively rare malignancy, representing 5% of all uroepithelial malignancies. It is often diagnosed late and generally has a poor prognosis (15). UTUC is increasingly recognized as a distinct entity, necessitating enhanced efforts from medical stakeholders to understand patient outcomes and their determinants better. Developing predictive models is crucial for patient selection and stratification in clinical trials, providing a valuable tool for physicians when discussing prognosis with patients. While the clinical outcomes of UTUC have been extensively studied, most research has concentrated on bladder recurrences (16); however, the 5-year survival rate for EUR mortality is lower than that for intravesical recurrence-free survival (IVRFS) (9), and studies on EUR are less common. In this study, utilizing numerous clinical samples from the First Hospital of Jilin University, we developed a nomogram to predict the prognosis of UTUC patients undergoing RNU.
T-stage, N-stage, and pathological grading are crucial determinants of postoperative EUR in UTUC. The T-stage is a critical indicator of tumor infiltration depth. Research indicates that patients with stages T3 and T4 face a significantly increased risk of postoperative recurrence, as higher T-stages are generally associated with deeper tumor infiltration and poorer outcomes (17, 18). Lymph node status (N staging) is another vital prognostic factor; the presence of lymph node metastases (N+) typically forecasts poorer postoperative survival and significantly impacts the risk of local and distant recurrences (17, 19). Moreover, the pathological grade of the tumor (e.g., G1, G2, G3) influences recurrence risk; high-grade tumors (e.g., G3) exhibit higher recurrence rates and worse prognosis compared to low-grade tumors (e.g., G1, G2) (18, 20).
UTUC is typically not diagnosed until a patient exhibits visual or microscopic hematuria, the most common symptom of this condition (21). Although it remains unclear whether macroscopic hematuria at diagnosis predicts a worse prognosis than microscopic hematuria, some studies provide partial insights (22). For instance, a retrospective analysis of 532 patients with pathologically confirmed UTUC revealed an approximately 80% increased risk of invasive disease (≥pT2) in patients presenting with gross hematuria (23); additionally, the study by Jakus et al. (24) identified a significant correlation between hematochezia and high-grade or stage ≥T2 UTUC. However, another study involving 179 patients with non-metastatic UTUC found no significant differences in OS or cancer-specific survival (CSS) between patients with or without gross hematuria (25). These findings are consistent with our research, which does not yet confirm that visible hematuria plays a critical role in predicting the prognosis for UTUC patients.
Nerve invasion (NI), characterized by the proliferation of tumor cells along nerve membranes or fibers, indicates a tumor’s invasiveness. A study on a cohort of 803 Chinese patients with non-metastatic UTUC undergoing RNU identified localized neuroinvasion as an independent predictor of poorer progression-free survival (PFS), CSS, and OS (26). This finding aligns with our results, suggesting that patients with NI may require more aggressive postoperative adjuvant therapy and closer follow-up.
Neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and monocyte/lymphocyte ratio (MLR) are widely utilized metrics that reflect systemic inflammation in patients. A meta-analysis encompassing 32 studies determined that an elevated pretreatment NLR independently predicted worse OS (HR=1.72, 95% CI=1.45-2.05), progression-free survival (PFS) (HR=1.68, 95% CI=1.44-1.96), and cancer-specific survival (CSS) (HR=1.64, 95% CI=1.39-1.93) (27). Another meta-analysis corroborated that increased preoperative NLR, PLR, and MLR were significantly linked with adverse outcomes in OS, CSS, disease-free survival/recurrence-free survival/metastasis-free survival (DFS/RFS/MFS), and PFS (28). These findings align with our results, highlighting the pivotal role of inflammation in cancer development and progression (29). Tumors exploit various inflammatory mediators to foster survival, proliferation, and favorable conditions for growth and metastasis (30), making these inflammatory markers valuable for prognostic evaluation.
Hemoglobin and creatinine levels are vital indicators of a patient’s nutritional status and renal function and are crucial for the prognostic assessment of UTUC patients. Low hemoglobin levels may indicate anemia and malnutrition, and preoperative anemia is strongly associated with decreased cancer-specific survival and extra-renal recurrence in UTUC patients post-surgery (13, 31, 32). Adequate renal function is crucial for patients undergoing nephrectomy for UTUC, as it affects postoperative quality of life and long-term survival. Elevated creatinine levels often suggest impaired renal function and a reduced glomerular filtration rate, typical in chronic kidney disease. Morizane et al. (32) found that higher preoperative serum creatinine levels were significantly linked with poorer postoperative CSS in UTUC patients.
Moreover, patients with chronic kidney disease experience higher rates of urothelial cancer-specific mortality (33), and chronic kidney disease is a risk factor for UTUC (34). The aggressiveness of UTUC increases with the severity of CKD (35). However, a meta-analysis involving 657 patients indicated that creatinine levels were not strongly correlated with CSS in UTUC patients (36). This suggests further investigation into the relationship between creatinine and postoperative survival in UTUC patients.
We developed an 8-parameter-based EUR prediction model demonstrating good discriminative ability across the training and external validation sets. The C-index for both predictive models exceeded 0.65, and in the time-dependent ROC analysis, the AUCs at 1, 3, and 5 years were also above 0.65. Furthermore, the calibration curves confirmed that the models were well-calibrated, indicating better net benefit and clinical applicability, as shown on the DCA curve. Patients were stratified into low and high-risk groups according to the nomogram total score, with the K-M method revealing a significant difference in EURFS between revealing groups. In conclusion, our models hold potential value in clinical settings and may assist in assessing the prognosis of UTUC patients following RNU.
While this study offers valuable insights into the under-studied population of patients with distant metastatic UTUC, it has limitations. As a single-center prospective study, its limited diversity may affect the external applicability of the prediction model. Additionally, hospital data constraints prevented the inclusion of genetic or molecular biomarkers. Despite a large sample size, the non-randomized, retrospective design lacks external validation, highlighting the need for more diverse, prospective studies. Future multicenter studies will enhance sample diversity and incorporate new clinical metrics to improve model performance. Despite these limitations, our nomogram performed well in both training and validation cohorts, confirming the reliability of our results.
We have developed a novel nomogram to predict the prognosis of UTUC patients post-RNU. This tool enables physicians to identify patients at high risk for EUR, thus providing robust support for clinical decision-making and fostering a more personalized treatment approach, ultimately enhancing treatment outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the Institutional Review Board of the First Hospital of Jilin University. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HW: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. DJ: Formal analysis, Funding acquisition, Methodology, Writing – original draft. XD: Data curation, Methodology, Writing – original draft. HC: Data curation, Methodology, Writing – original draft. FW: Resources, Validation, Visualization, Writing – original draft. TY: Resources, Validation, Visualization, Writing – original draft. TX: Investigation, Methodology, Software, Writing – original draft. LW: Data curation, Software, Validation, Writing – original draft. BG: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1442168/full#supplementary-material
1. Ryoo H, Kim J, Kim T, Kang M, Jeon HG, Jeong BC, et al. Effects of complete bladder cuff removal on oncological outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Cancer Res Treat. (2021) 53:795–802. doi: 10.4143/crt.2020.919
2. Mousavian A-H, Shafiee G, Sheidaei A, Balajam NZ, Ebrahimi M, Khatami F, et al. The 15-year national trends of urinary cancers incidence among Iranian men and women; 2005–2020. Int J Equity Health. (2024) 23:13. doi: 10.1186/s12939-023-02084-1
3. Wang C-S, Li C-C, Juan Y-S, Wu W-J, Lee H-Y. 5α-reductase inhibitors impact prognosis of urothelial carcinoma. BMC Cancer. (2020) 20. doi: 10.1186/s12885-020-07373-4
4. Blumendeller C, Boehme J, Frick M, Schulze M, Rinckleb A, Kyzirakos C, et al. Use of plasma ctDNA as a potential biomarker for longitudinal monitoring of a patient with metastatic high-risk upper tract urothelial carcinoma receiving pembrolizumab and personalized neoepitope-derived multipeptide vaccinations: a case report. J ImmunoTherapy Cancer. (2021) 9:e001406. doi: 10.1136/jitc-2020-001406
5. Cheng S, Zhong W, Xia K, Hong P, Lin R, Wang B, et al. Prognostic role of stromal tumor-infiltrating lymphocytes in locally advanced upper tract urothelial carcinoma: A retrospective multicenter study (TSU-02 study). OncoImmunology. (2021) 10:1861737. doi: 10.1080/2162402X.2020.1861737
6. Margulis V, Youssef RF, Karakiewicz PI, Lotan Y, Wood CG, Zigeuner R, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol. (2010) 184:453–8. doi: 10.1016/j.juro.2010.03.142
7. Pan S-Y, Huang C-P, Chen W-C. Synchronous/metachronous multiple primary Malignancies: review of associated risk factors. Diagnostics. (2022) 12:1940. doi: 10.3390/diagnostics12081940
8. Cutress ML, Stewart GD, Zakikhani P, Phipps S, Thomas BG, Tolley DA. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int. (2012) 110:614–28. doi: 10.1111/j.1464-410X.2012.11068.x
9. Luo Z, Jiao B, Yan Y, Liu Y, Chen H, Guan Y, et al. A novel nomogram for predicting extraurothelial recurrence in patients with upper urinary tract urothelial carcinoma after radical nephroureterectomy. J Cancer Res Clin Oncol. (2023) 149:14241–53. doi: 10.1007/s00432-023-05237-5
10. Seisen T, Granger B, Colin P, Léon P, Utard G, Renard-Penna R, et al. A systematic review and meta-analysis of clinicopathologic factors linked to intravesical recurrence after radical nephroureterectomy to treat upper tract urothelial carcinoma. Eur Urol. (2015) 67:1122–33. doi: 10.1016/j.eururo.2014.11.035
11. Lin M-Y, Li W-M, Huang C-N, Lee H-L, Niu S-W, Chen L-T, et al. Dialysis increases the risk of bladder recurrence in patients with upper tract urothelial cancer: A population-based study. Ann Surg Oncol. (2018) 25:1086–93. doi: 10.1245/s10434-017-6295-3
12. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. (2009) 115:1224–33. doi: 10.1002/cncr.24135
13. Milojevic B, Dzamic Z, Kajmakovic B, Durutovic O, Bumbasirevic U, Sipetic Grujicic S. Prognostic impact of preoperative anemia on urothelial and extraurothelial recurrence in patients with upper tract urothelial carcinoma. Clin Genitourinary Cancer. (2015) 13:485–91. doi: 10.1016/j.clgc.2015.03.007
14. Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G, et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg. (2019) 72:156–65. doi: 10.1016/j.ijsu.2019.11.002
15. Song B, Kim JK, Lee H, Lee S, Hong SK, Byun S-S, et al. Evaluation of histological variants of upper tract urothelial carcinoma as prognostic factor after radical nephroureterectomy. World J Urol. (2024) 42:225. doi: 10.1007/s00345-024-04878-6
16. Fan B, Teng Q, Sun M, Wang Y, Wang Y, Lin Z, et al. Assessment of therapeutic benefit and option strategy on intravesical instillation for preventing bladder cancer recurrence after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. J Oncol. (2022) 2022:1–19. doi: 10.1155/2022/1755368
17. Li X, Cui M, Gu X, Fang D, Li H, Qin S, et al. Pattern and risk factors of local recurrence after nephroureterectomy for upper tract urothelial carcinoma. World J Surg Oncol. (2020) 18:114. doi: 10.1186/s12957-020-01877-w
18. Liu J, Wu P, Lai S, Wang J, Hou H, Zhang Y. Prognostic models for upper urinary tract urothelial carcinoma patients after radical nephroureterectomy based on a novel systemic immune-inflammation score with machine learning. BMC Cancer. (2023) 23:574. doi: 10.1186/s12885-023-11058-z
19. Luo Z, Yan Y, Jiao B, Huang T, Liu Y, Chen H, et al. Prognostic value of the systemic immune-inflammation index in patients with upper tract urothelial carcinoma after radical nephroureterectomy. World J Surg Oncol. (2023) 21:337. doi: 10.1186/s12957-023-03225-0
20. Wang Q, Zhang T, Wu J, Wen J, Tao D, Wan T, et al. Prognosis and risk factors of patients with upper urinary tract urothelial carcinoma and postoperative recurrence of bladder cancer in central China. BMC Urol. (2019) 19:24. doi: 10.1186/s12894-019-0457-5
21. Cowan NC. CT urography for hematuria. Nat Rev Urol. (2012) 9:218–26. doi: 10.1038/nrurol.2012.32
22. Raman JD, Shariat SF, Karakiewicz PI, Lotan Y, Sagalowsky AI, Roscigno M, et al. Does preoperative symptom classification impact prognosis in patients with clinically localized upper-tract urothelial carcinoma managed by radical nephroureterectomy? Urol Oncol. (2011) 29:716–23. doi: 10.1016/j.urolonc.2009.11.007
23. Qi N, Zhang J, Chen Y, Wen R, Li H. Microscopic hematuria predicts lower stage in patients with upper tract urothelial carcinoma. Cancer Manag Res. (2018) 10:4929–33. doi: 10.2147/CMAR.S180606
24. Jakus D, Šolić I, Borovac JA, Šitum M. The influence of the initial clinical presentation of upper tract urothelial carcinoma on histopathological tumor features. Int Urol Nephrol. (2024) 56:1335–41. doi: 10.1007/s11255-023-03883-9
25. Sun KN, Wu JH, Chen ZH, He YJ, Chen YL, Hu JZ, et al. Predictive value of flank pain and gross hematuria on long-term survival in patients with upper tract urothelial carcinoma treated by radical nephroureterectomy. Clin Med Insights Oncol. (2023) 17:11795549221147993. doi: 10.1177/11795549221147993
26. Lin T-W, Lee H-Y, Yang S-F, Li C-C, Ke H-L, Li W-M, et al. Perineural invasion is a powerful prognostic factor for upper tract urothelial carcinoma following radical nephroureterectomy. Ann Surg Oncol. (2022) 29:3306–17. doi: 10.1245/s10434-021-11265-7
27. Li X, Ma X, Tang L, Wang B, Chen L, Zhang F, et al. Prognostic value of neutrophil-to-lymphocyte ratio in urothelial carcinoma of the upper urinary tract and bladder: a systematic review and meta-analysis. Oncotarget. (2017) 8:62681–92. doi: 10.18632/oncotarget.17467
28. Shao Y, Li W, Wang D, Wu B. Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta-analysis. World J Surg Oncol. (2020) 18:273. doi: 10.1186/s12957-020-02048-7
30. Michelson N, Rincon-Torroella J, Quiñones-Hinojosa A, Greenfield Jp. Exploring the role of inflammation in the Malignant transformation of low-grade gliomas. J neuroimmunology. (2016) 297. doi: 10.1016/j.jneuroim.2016.05.019
31. Rink M, Sharifi N, Fritsche Hm, Aziz A, Miller F, Kluth La, et al. Impact of preoperative anemia on oncologic outcomes of upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol. (2014) 191. doi: 10.1016/j.juro.2013.09.010
32. Morizane S, Iwamoto H, Masago T, Yao A, Isoyama T, Sejima T, et al. Preoperative prognostic factors after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Int Urol Nephrol. (2013) 45. doi: 10.1007/s11255-012-0347-1
33. Weng P-H, Hung K-Y, Huang H-L, Chen J-H, Sung P-K, Huang K-C. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. (2011) 6:1121–8. doi: 10.2215/cjn.09011010
34. Chen J-S, Lu C-L, Huang L-C, Shen C-H, Chen SC-C. Chronic kidney disease is associated with upper tract urothelial carcinoma: A nationwide population-based cohort study in Taiwan. Medicine. (2016) 95:e3255. doi: 10.1097/md.0000000000003255
35. Hung Ph, Shen Ch, Chiu Yl, Jong Ic, Chiang Pc, Lin Ct, et al. The aggressiveness of urinary tract urothelial carcinoma increases with the severity of chronic kidney disease. BJU Int. (2009) 104. doi: 10.1111/j.1464-410X.2009.08636.x
Keywords: upper urinary tract urothelial carcinoma, radical nephroureterectomy, extra-urinary recurrence, prediction model, online network calculator
Citation: Wu H, Jia D, Dai X, Cao H, Wang F, Yang T, Wang L, Xu T and Gao B (2024) A new nomogram for predicting extraurothelial recurrence in patients with upper urinary tract urothelial carcinoma following radical nephroureterectomy. Front. Oncol. 14:1442168. doi: 10.3389/fonc.2024.1442168
Received: 01 June 2024; Accepted: 11 October 2024;
Published: 06 November 2024.
Edited by:
Sabine Doris Brookman-May, Ludwig Maximilian University of Munich, GermanyReviewed by:
Panagiotis Mourmouris, National and Kapodistrian University of Athens, GreeceCopyright © 2024 Wu, Jia, Dai, Cao, Wang, Yang, Wang, Xu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoshan Gao, Z2FvYnNAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.