- 1The First School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
- 2The Third Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 3Department of Medical Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, China
Purpose: The Systemic Immuno-Inflammation Index (SII) is a crucial clinical measure of inflammation, and there is currently no solid evidence linking SII to an increased risk of prostate cancer (PCa). Through the analysis of serum total prostate-specific antigen (tPSA), free prostate-specific antigen (fPSA), and the tPSA/fPSA (fPSA%) ratio, this study sought to investigate the relationship between SII and PCa risk among the U.S. elderly.
Methods: Elderly male participants were gathered from the NHANES database between 2001 and 2010.SII was calculated by platelet count * neutrophil count/lymphocyte count. High risk individuals for prostate cancer were defined as those with tPSA > 4 ng/ml and fPSA% < 16%. Multivariate logistic regression models, restricted cubic spline curves, and subgroup analyses were used to assess the relationship between SII and PCa risk.
Results: This research comprised 2664 people in total, 137 (5.14%) of whom were deemed to be at high risk of developing PCa. Multivariate logistic regression analysis, after controlling for variables, revealed a significant positive correlation between high PCa risk and an increase in SII (p = 0.009). The RCS suggested a turning point at 9.01. Restricted cubic spline curves revealed a non-linear U-shaped association between SII and high PCa risk (p for nonlinear = 0.028). Education level, marital status, PIR, alcohol status, smoking status, rheumatoid arthritis status, and heart problem were not significantly correlated with this positive connection, according to subgroup analyses and interaction tests.
Conclusion: The results of this study suggest that inflammation represented by SII is associated with high PCa risk.
1 Introduction
Prostate cancer is the second most common cancer in men worldwide and the fifth leading cause of cancer-related deaths in men, with its incidence and mortality rates rising annually in recent years (1, 2). The measurement of serum prostate-specific antigen (PSA) concentration plays an irreplaceable role in the early screening of prostate cancer. However, various factors, such as smoking habits, can affect PSA levels (3). As a result, widespread PSA screening in men may lead to false positives and the overdiagnosis of non-aggressive conditions (4), and a PSA value between 4 and 10 is a gray area for distinguishing between prostate cancer and benign prostatic diseases. Therefore, current studies often use an fPSA% of less than 0.16 as a standard for high-risk prostate cancer (5, 6). Recent studies have found that novel combinations of serum markers have higher clinical potential for distinguishing between high and low risk of prostate cancer (7).
The Systemic Immune-Inflammation Index (SII) is a unique composite index based on neutrophil, platelet, and lymphocyte counts, which accurately reflects the systemic inflammatory state (8). The interaction between systemic inflammation and local immune response is often considered the seventh hallmark of cancer and has been shown to be involved in the development and progression of various cancers (9). SII objectively reflects the inflammation-immune balance in cancer patients and can thus be considered an effective marker for measuring inflammation levels in cancer patients.
Many studies have shown that inflammation is closely related to the occurrence, development, invasion, and metastasis of prostate cancer (PCa) (10). Research indicates that internal inflammation of the prostate is a risk factor for PCa. The “inflammatory storm” within the prostate has been shown to accelerate the development of PCa by causing DNA damage and overexpression of anti-cancer genes (11, 12). However, there is currently no direct method for assisting prostate cancer screening through inflammation markers.
Recent studies have found a potential positive causal relationship between SII and PCa (13). There is a single-center small-sample retrospective study have further found that SII may be an independent risk factor for diagnosing PCa (14). In addition, research have found a significant association between higher SII levels and poorer prognosis in bladder cancer patients undergoing radical cystectomy (15). Another study based on the UK Biobank found a strong association between SII and the risk of colorectal cancer and lung cancer (16). However, previous studies predicting the relationship between SII and prostate cancer risk based solely on tPSA concentration lack rigor and there is no clear evidence yet on whether SII can serve as a predictive marker for assessing the risk of prostate cancer. Therefore, we collected relevant indicators from the publicly available National Health and Nutrition Examination Survey (NHANES) data from 2001 to 2010. This approach seeks to integrate new inflammatory markers like SII to enhance the precision of prostate cancer screening and risk stratification, offering varied evidence from an inflammatory perspective. It also lays the groundwork for broader studies on the association of SII with the risk of different cancers.
2 Materials and methods
2.1 Study population
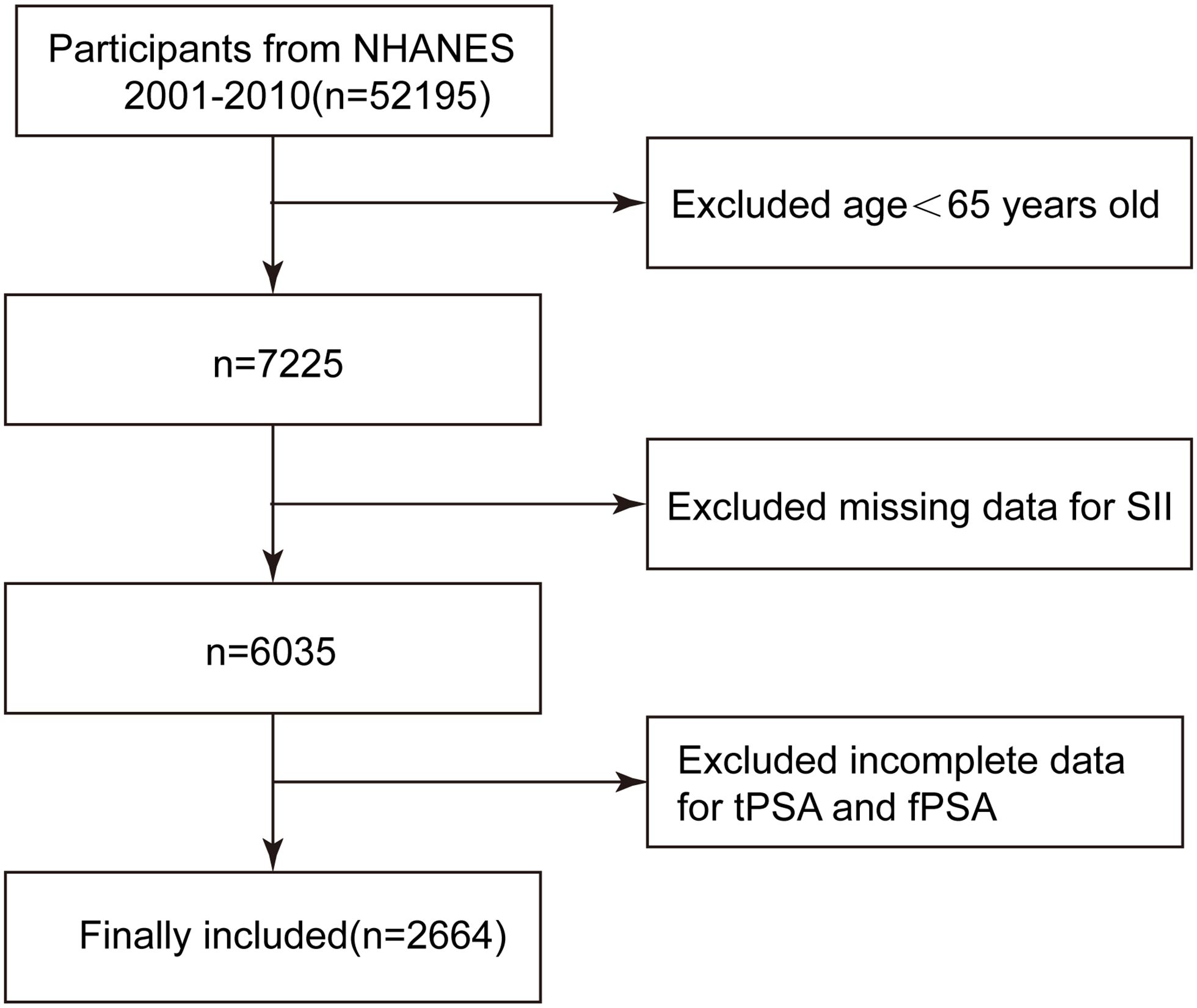
This study is a cross-sectional study based on the population included in the NHANES database from 2001 to 2010. The NHANES database is a project of the National Center for Health Statistics in the United States, dedicated to collecting extensive health information from the population to support various research studies. The NHANES study adheres to the ethical guidelines set forth in the 1975 Helsinki Declaration and received informed consent from all participants. This research was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies. A total of 52,195 participants were obtained from the NHANES database from 2001 to 2010, among which 7,225 participants were aged ≥65 years. Among them, 1,190 individuals did not have complete information on platelet (PLT), lymphocyte, and neutrophil counts. After screening, 3,371 participants still lacked complete information on tPSA and fPSA. Therefore, our final analysis included 2,664 participants with complete information. See Figure 1 for details.
2.2 Definitions of SII and the high risk population for prostate cancer
The participants undergoing PSA testing were all 65 years old and above, and individuals with current prostate infection or inflammation, rectal examination within the past week, prostate biopsy within the past month, cystoscopy within the past month, or a history of prostate cancer were excluded. tPSA was obtained using the Hybritech PSA method on Beckman Access, and fPSA was detected using the Access Hybritech free PSA assay kit. Multiple clinical studies have shown that when tPSA is >4 and t/fPSA is less than 16 (17, 18), the probability of patients having prostate cancer greatly increases, and further diagnostic procedures such as enhanced MR or biopsy should be conducted to confirm the diagnosis. This screening method has become the mainstream approach in clinical practice for defining individuals at high risk of prostate cancer. Therefore, we defined such patients as the extremely high risk group for clinical prostate cancer, while others were considered to have a lower risk of prostate cancer.
SII is commonly used to assess patients’ immune function and inflammatory status, with a high SII often indicating immune dysfunction and inflammation progression. According to previous research findings (19, 20), we calculated SII using the formula platelet count * neutrophil count/lymphocyte count. To simplify the calculation of the data, we performed a logarithmic transformation on SII. After consulting earlier research (20)and taking into account the various definitions of SII’s crucial values, we finally split the log-transformed SII into four equal sections using quartile averages: low SII (Q1), low-medium SII (Q2), medium-high SII (Q3), and high SII (Q4).
2.3 Covariates
We extensively collected covariates that could potentially influence the relationship between SII and PSA. Individual information included race, education level, marital status, poverty status, BMI, smoking and drinking status. Among them, we distinguished the impoverished population based on whether the PIR was greater than 1. Besides demographic characteristics, laboratory tests known from previous studies to influence prostate cancer—such as cholesterol, total bilirubin, glucose, lactate dehydrogenase, and creatinine—were included to adequately control for confounding variables (21, 22). The specific detection methods for the above laboratory tests are all documented in NHANES. Additionally, extensive research has established that conditions like hypertension, diabetes, stroke, hyperlipidemia, arthritis, and heart disease independently affect the survival of prostate cancer patients (23, 24). Even though some variables did not achieve statistical significance in univariate analysis, they were still included as covariates due to their potential influence, as indicated by earlier studies.
2.4 Statistical analysis
Study’s statistical analysis took sample weights into account and used “survey” package to perform weighted computations in R. Continuous variables are described as mean (Standard Deviation), while categorical variables are represented using numbers (Percentages). Differences in all influencing factors between high risk and low risk prostate cancer populations were compared using Kruskal-Wallis rank-sum test for continuous variables and chi-square test for categorical variables. The log-transformed SII was divided into quartiles, with low SII (Q1) as the reference. Multiple linear regression was used to calculate the β values or OR values and corresponding 95% confidence intervals for both unadjusted and adjusted models, thereby examining the significant correlation and trend between different levels of SII and high risk prostate cancer. Restricted cubic spline (RCS) regression models were applied to analyze the 10th, 50th, and 90th percentiles of prostate cancer risk groups, tPSA, fPSA, and fPSA%, to explore linear/non-linear relationships. To further explore potential factors influencing LogSII and high/low risk prostate cancer populations, some covariates that might have an impact were included in subgroup analyses. Regression analyses were performed on adjusted models with categorical variables added as effect modifiers to observe significant interactions between different categories.
In this study, we fitted two adjusted statistical models using regression analysis. In Adjusted I Model, adjustments were made for race, education level, poverty level, marital status, BMI, smoking and drinking status. Adjusted II Model, adjustments were made for the variables included in Adjusted I Model, as well as for cholesterol, total bilirubin, glucose, lactate dehydrogenase, creatinine, hyperlipidemia status, diabetes status, stroke status, rheumatoid arthritis status, and heart disease status. Adjusted I Model focuses on adjusting for demographic and lifestyle factors to minimize their confounding effects on the outcomes. Building on this, Adjusted II Model includes additional adjustments for laboratory tests and comorbidities, representing the fully adjusted model in this study. This comprehensive adjustment allows for a more accurate estimation of odds ratio (OR), enhancing the robustness of the findings. All statistical analyses were performed using R version 4.2.3. A p value less than 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of the study population
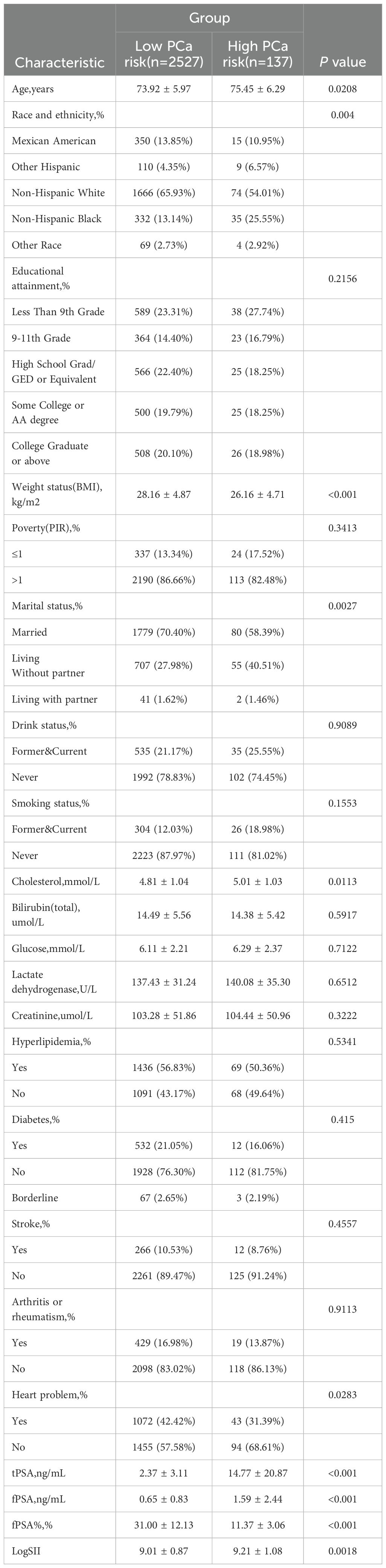
From 2001 to 2010, a total of 2,664 elderly participants were included in our study, with an average age of 73.99 years. Among them, the population with a high incidence rate of prostate cancer accounted for 5.14% of the total population. The average levels of tPSA and fPSA in the included population were 3.00 and 0.70, respectively, while the fPSA% was 29.99%. The average value of LogSII in the included population was 9.02. In the baseline characteristic table stratified by high and low risk of prostate cancer, we found no significant statistical differences in Educational attainment, Poverty status, Drink status, Smoking status, total Bilirubin, Glucose, Lactate dehydrogenase level, Creatinine, Hyperlipidemia status, Diabetes status, Arthritis or rheumatism status, and Stroke between groups, while significant differences were observed in the remaining characteristics. See Table 1 for details.
3.2 SII is associated with increased likelihood of the risk for PCa
Through multivariable logistic regression analysis, we found that higher levels of SII were associated with the high risk group of prostate cancer (Table 2). This association was significant in the Non-adjusted model, Adjust I model, and Adjust II model. In the Adjust II model, the likelihood of high risk prostate cancer was significantly increased by 96% for participants in the highest LogSII quartile compared to those in the lowest LogSII quartile. Moreover, as LogSII increased, the likelihood of being at high risk for prostate cancer also showed an upward trend (p for trend = 0.009).
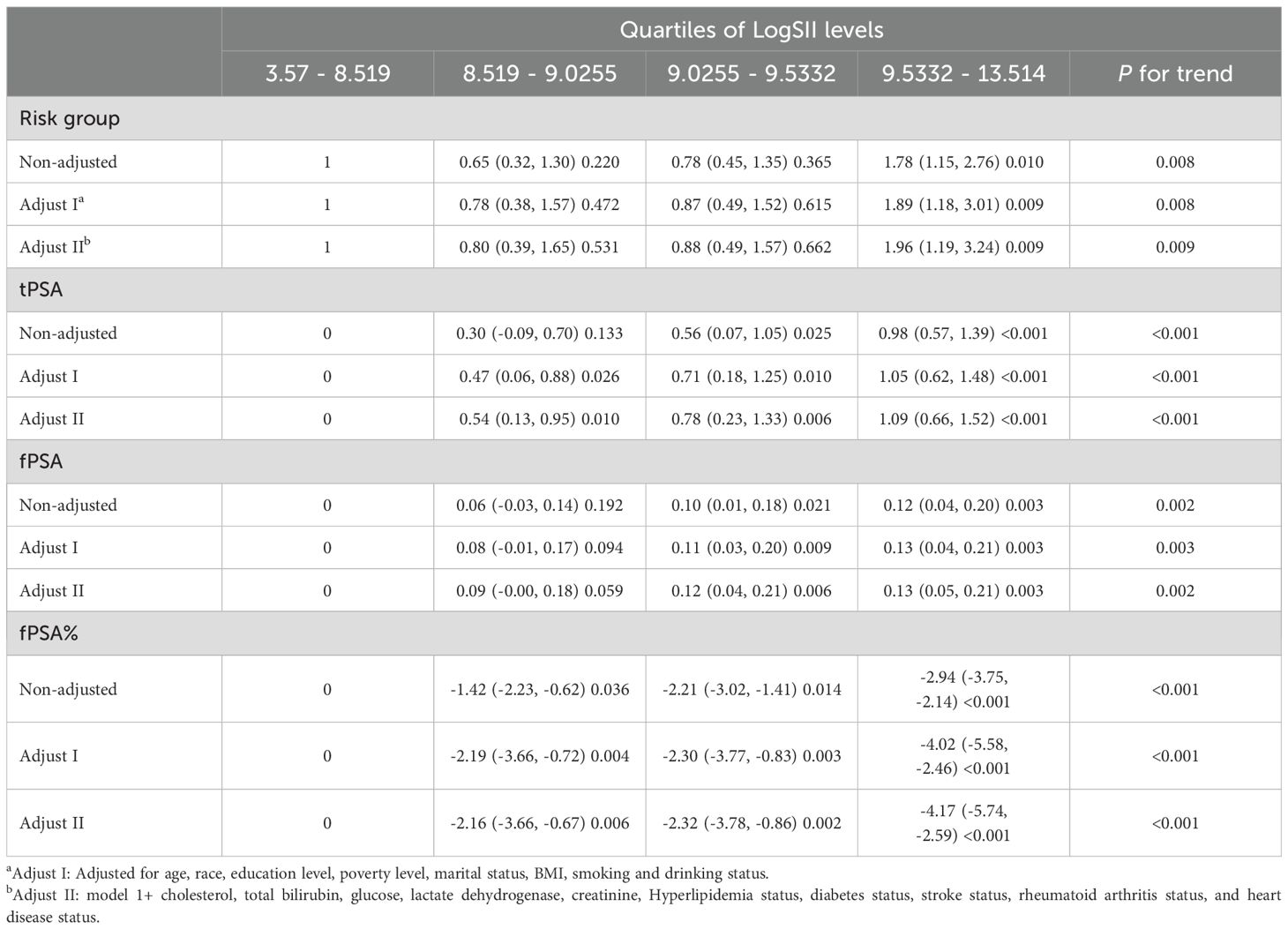
Table 2. Associations of LogSII with PCa risk and serum PSA levels by linear regression in NHANES 2001-2010 (weighted).
Additionally, we conducted multivariable linear regression analyses on LogSII quartiles and tPSA, fPSA, and fPSA%. Higher LogSII levels were associated with higher levels of tPSA and fPSA, and this association was significant in Adjust II model. In the Adjust II model, tPSA increased by 1.09 and fPSA increased by 0.13 for participants in the highest LogSII quartile compared to those in the lowest LogSII quartile. Moreover, there was an upward trend observed in the tPSA levels as LogSII increased (p for trend < 0.001). There was also an increased trend in the fPSA (p for trend = 0.003).
Higher LogSII quartiles were associated with lower levels of fPSA%, and this association was significant in all three models for participants in the second, third, and highest quartiles compared to those in the lowest LogSII quartile. In the Adjust II model, fPSA% decreased by 4.17% for participants in the highest LogSII quartile compared to those in the lowest LogSII quartile. Additionally, as LogSII increased, the levels of fPSA% showed a downward trend (p for trend < 0.001).
3.3 The nonlinear relationship between SII and the risk for PCa
For the Adjust II model, we used regression cubic splines to demonstrate the relationship between LogSII and PCa risk groups (Figure 2A), tPSA (Figure 2B), fPSA (Figure 2C), and fPSA% (Figure 2D). LogSII showed a linear positive correlation with tPSA and fPSA, and a linear negative correlation with fPSA%. There was a non-linear positive correlation between LogSII and high/low PCa risk groups (p for nonlinear = 0.030).
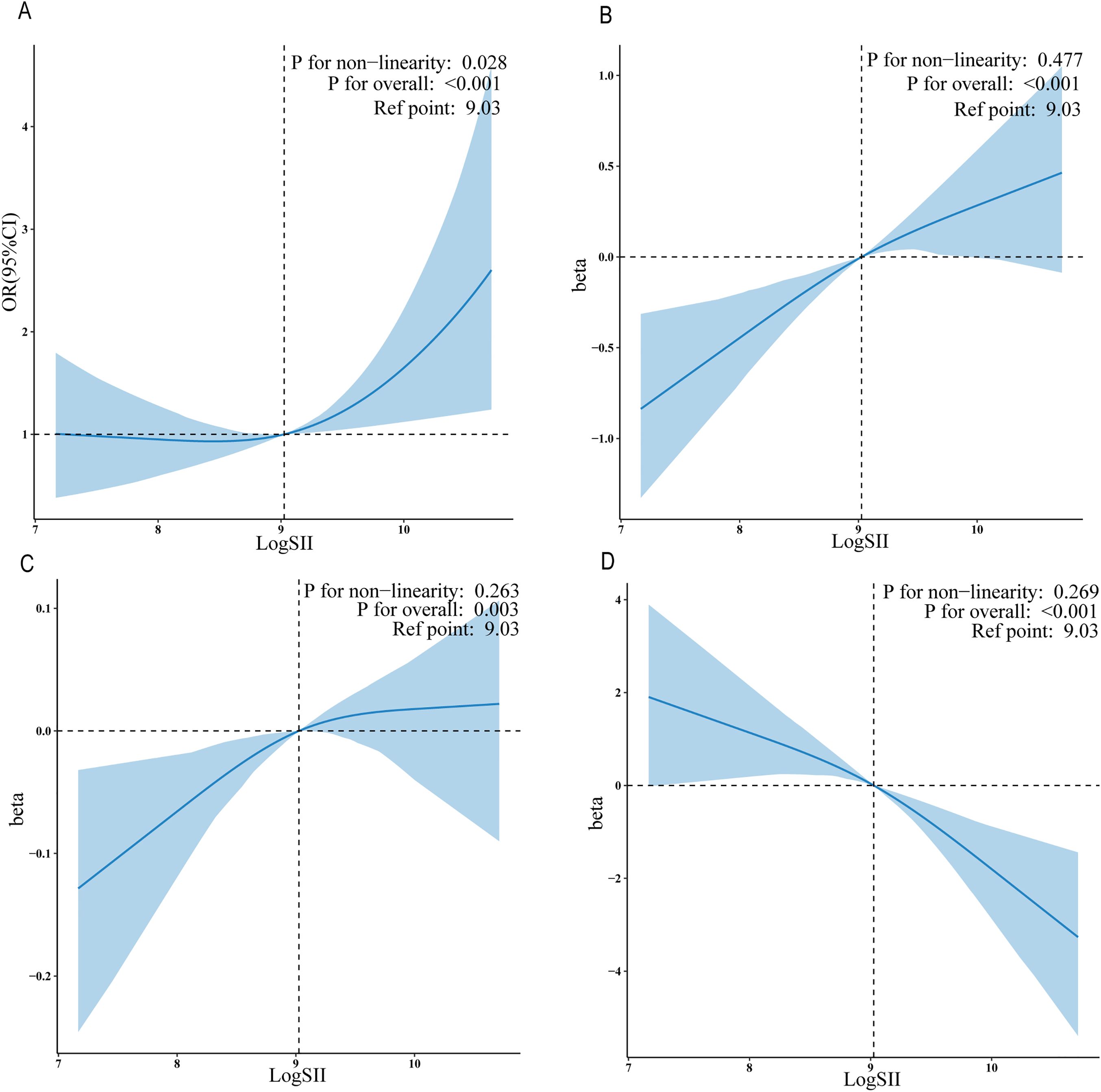
Figure 2. The exposure-response associations of the SII with serum PSA levels and PCa by restricted cubic spline model (weighted). Association of LogSII with the PCa risk group (A), tPSA (B), fPSA (C) and fPSA% (D).
Furthermore, we identified that a LogSII value of 9.03 may serve as a clinically significant threshold. Results from the aforementioned RCS analysis consistently indicated that 9.03 is the inflection point of the threshold. When LogSII is less than 9.03, there is no significant association with a high risk of prostate cancer. However, when LogSII exceeds 9.03, a significant positive correlation with a high risk of prostate cancer is observed. This overall relationship exhibits a non-linear U-shaped pattern.
Association of LogSII with the PCa risk group (A), tPSA (B),fPSA (C)and fPSA%(D).
3.4 Subgroup analysis
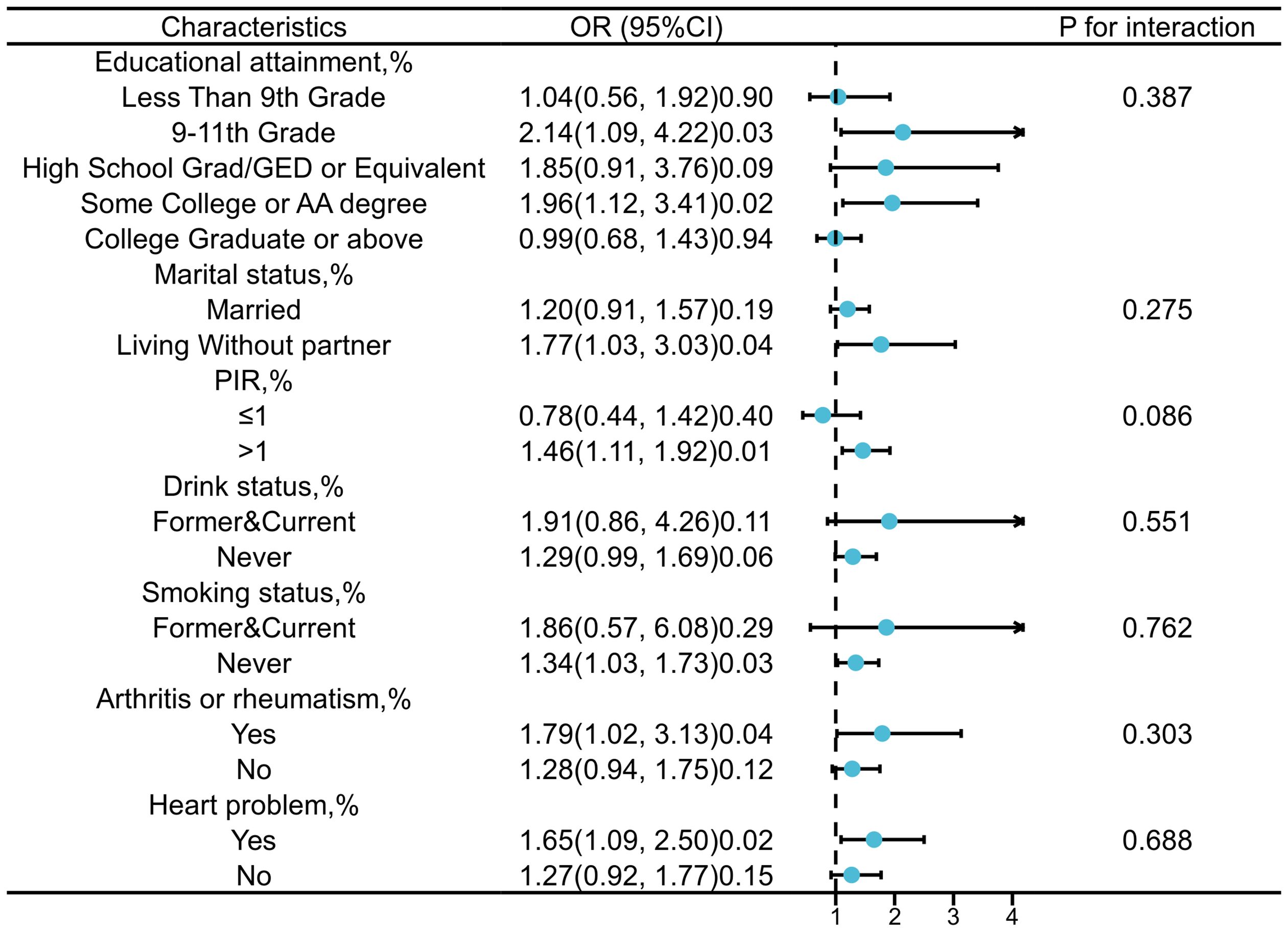
The stratified analysis based on education level, marital status, PIR, alcohol status, smoking status, rheumatoid arthritis status, and heart problem was conducted to further investigate the relationship between SII and PCa (Figure 3). Interaction tests indicated that there were no statistically significant differences in the association between SII and PCa risk across the different strata, suggesting that these stratifying factors do not have a statistically significant impact on the observed positive correlation. Nonetheless, we observed higher OR values in subgroups with PIR greater than 1, a history of smoking, alcohol consumption, rheumatoid arthritis, and heart problem.
4 Discussion
This study collected 2664 participants aged 65 and above from the NHANES database to explore the association between SII and PCa risk in high and low risk groups. The findings indicated a positive linear correlation between SII and both tPSA and fPSA. Additionally, a notable negative linear correlation was observed between SII and the percentage of fPSA%. However, it exhibited a non-linear U-shaped association with high and low risk PCa subgroups. Subgroup analysis revealed that variables like race, marital status, and stroke status, among others, did not significantly differ between subgroups.
Previous studies utilizing NHANES data have investigated the relationship between the Systemic Immune-Inflammation Index (SII) and prostate cancer, using the incidence of prostate cancer as the outcome measure. Although the study found that SII was associated with a 7% increased risk of prostate cancer, this association did not reach statistical significance (25). Some reports have identified SII as a significant diagnostic marker for patients with PSA levels below 10 ng/ml in prostate fusion biopsy (26), with the combined diagnostic efficiency of SII and PSA for prostate cancer surpassing that of PSA alone (27). Conversely, Murray et al. argued that SII cannot distinguish clinically significant prostate cancer from indolent cancer or benign diseases during the initial biopsy (28). As a result, the clinical findings on the relationship between SII and prostate cancer or PSA remain controversial. This study observed that a significant increase in SII levels is associated with a higher risk of prostate cancer. SII could serve as a crucial marker for distinguishing between high-risk and low-risk prostate cancer, offering potential value in supporting prostate cancer diagnosis.
Inflammation has long been recognized as a crucial factor influencing the development and progression of prostate cancer (PCa). Numerous clinical studies have demonstrated the association between inflammation and the development and progression of PCa (29). A retrospective study from Korea, which analyzed 746,176 patients, found a significant association between prostatic inflammation and an increased incidence of PCa, with acute prostatitis posing a higher risk than chronic inflammation (30). Furthermore, a study based on U.S. Medicare data from 1999 to 2010, involving 2,701,782 osteoarthritis patients, 13,044 ankylosing spondylitis patients, and 10,859,304 controls, found that both osteoarthritis and ankylosing spondylitis increase the risk of developing prostate cancer (31).
The mechanisms by which inflammation induces the occurrence of prostate cancer (PCa) remain a hot research topic. One mainstream view of the inflammation-cancer transformation mechanism is that long-term chronic inflammation promotes mutations in human cells and the genome (32). Reports indicate that the overexpression of inflammation-related genes, along with prolonged activation of various inflammatory signaling pathways, growth proteins, and cellular messengers, promotes cellular mutations and structural variations, leading to prostate cancer as well as castration resistance, metabolic reprogramming, and immunosuppression (33). Furthermore, research indicates that chronic inflammation can influence the tumor immune microenvironment and the urinary microbiome (34, 35). Inflammatory reactive oxygen species (ROS) can mediate oxidative stress, leading to prostatic inflammatory atrophy, which eventually contributes to the development of prostatic adenocarcinoma (36). SII is derived from the counts of neutrophils, platelets, and lymphocytes. Studies have identified neutrophil polarization as a critical factor in cancer development (37), with neutrophils not only producing ROS that cause DNA mutations but also generating inflammatory cytokines that create an immunosuppressive environment (38). Platelets and their byproducts can impact the coagulation cascade, activate oncogenic mutations, and maintain proliferative signals, while also inducing angiogenesis via factors like vascular endothelial growth factor, which promotes tumor metastasis (39). Lymphocytes are intricately linked to cancer; their reduction can lead to immune evasion and tumor progression, whereas their abnormal proliferation may exacerbate immune-related adverse events (40).
Compared with previous studies, this study revealed new associations between SII and high and low risk prostate cancer populations, as well as the association patterns with different prostate cancer biomarkers, providing a deeper understanding of the relationship between immunity, inflammation, and prostate cancer risk, and bringing a new perspective to research on prostate cancer screening. This study quantitatively analyzed the potential relationship between SII and prostate cancer risk by calculating the significant inflection point at which SII is associated with prostate cancer risk. This finding provides a new perspective on the contributing factors to prostate cancer risk and may pave the way for more refined screening and diagnostic strategies in the future. Additionally, the large sample size of this study enhances the reliability of its conclusions. Subgroup analyses also highlighted the influence of age and marital status. However, before SII can be adopted as a routine clinical marker, further longitudinal studies and validations across different populations are essential. Longitudinal cohort studies can monitor how SII levels change over time and their impact on prostate cancer risk, while randomized controlled trials can assess the effectiveness of interventions targeting SII in reducing this risk. These approaches will not only strengthen the evidence for a causal relationship but also explore whether SII could be a viable target for preventive strategies. This will be crucial in confirming the reliability of SII in predicting prostate cancer risk and ensuring its practical application.
This study has several limitations. The cross-sectional design of the study limits the ability to draw causal conclusions. While analysis reveals associations between the SII and PCa risk, it cannot infer causality due to the simultaneous measurement of exposure and outcome variables. The reliance on data from the 2001-2010 NHANES survey may not fully reflect recent trends in PSA screening practices or the latest advancements in prostate cancer management. Although these historical data provide valuable insights into the relationship between SII and PCa risk, the temporal gap could affect the generalizability of our findings to the current population. Additionally, due to the lack of data on prostate cancer subtypes and stage, we were unable to analyze the specific impact of pathological types and tumor stages. Furthermore, as this is a cross-sectional study, it precludes further discussion incorporating patients’ subsequent diagnostic outcomes. The exclusion criteria were established to ensure the reliability and accuracy of our analyses by focusing on participants with complete and valid data. However, excluding individuals with incomplete data could introduce selection bias, as it may result in a study sample that is not fully representative of the general population. In cross-sectional studies, data are gathered at one specific time point, a method frequently used in epidemiological research with the NHANES database. However, since factors like platelet, lymphocyte, and neutrophil counts, along with tPSA and fPSA levels, can vary over time, this might introduce some variability in the results. Finally, although we controlled for some confounding variables, we could not account for other potential unknown confounders in this study.
5 Conclusion
The results of this study indicate that among elderly, SII is significantly positively correlated with serum levels of tPSA and fPSA, and significantly negatively correlated with fPSA%. It also shows a nonlinear U-shaped correlation with high risk PCa. SII may serve as an effective indicator for identifying high risk populations for PCa from an inflammatory perspective. Further prospective studies are needed to confirm our conclusions.
Data availability statement
In this study, publicly accessible datasets were evaluated. Visit https://www.cdc.gov/nchs/nhanes/index.htm to get this data.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics Research Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YY: Funding acquisition, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. QZ: Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. CX: Funding acquisition, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. National Famous and Old Chinese Medicine Experts Inheritance Studio Construction Project(G.TCM.R.J.H.(2022)75); Natural Science Foundation of Zhejiang Province(NO.LY21H270015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chakroborty D, Singh AP. Prostate cancer: insights into disease progression and therapeutic challenges. Int J Mol Sci. (2024) 25:2451. doi: 10.3390/ijms25052451(2024
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Tarantino G, Crocetto F, Vito CD, Martino R, Pandolfo SD, Creta M, et al. Clinical factors affecting prostate-specific antigen levels in prostate cancer patients undergoing radical prostatectomy: a retrospective study. Future Sci OA. (2021) 7:FSO643. doi: 10.2144/fsoa-2020-0154.(2021
4. Kalavacherla S, Riviere P, Javier-DesLoges J, Banegas MP, McKay RR, Murphy JD, et al. Low-value prostate-specific antigen screening in older males. JAMA network Open. (2023) 6:e237504. doi: 10.1001/jamanetworkopen.2023.7504.(2023
5. Filella X, Foj L, Wijngaard R, Luque P. Value of PHI and PHID in the detection of intermediate- and high-risk prostate cancer. Clinica chimica acta; Int J Clin Chem. (2022) 531:277–82. doi: 10.1016/j.cca.2022.04.992
6. Ferraro S, Biganzoli D, Rossi RS, Palmisano F, Bussetti M, Verzotti E, et al. Individual risk prediction of high grade prostate cancer based on the combination between total prostate-specific antigen (PSA) and free to total PSA ratio. Clin Chem Lab Med. (2023) 61:1327–34. doi: 10.1515/cclm-2023-0008
7. McNally CJ, Watt J, Kurth MJ, Lamont JV, Moore T, Fitzgerald P, et al. A novel combination of serum markers in a multivariate model to help triage patients into "Low-" and "High-risk" Categories for prostate cancer. Front Oncol. (2022) 12:837127. doi: 10.3389/fonc.2022.837127
8. Qin Z, Li H, Wang L, Geng J, Yang Q, Su B, et al. Systemic immune-inflammation index is associated with increased urinary albumin excretion: A population-based study. Front Immunol. (2022) 13:863640. doi: 10.3389/fimmu.2022.863640
9. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23:6261–72. doi: 10.3748/wjg.v23.i34.6261
10. Rani A, Dasgupta P, Murphy JJ. Prostate cancer: the role of inflammation and chemokines. Am J Pathol. (2019) 189:2119–37. doi: 10.1016/j.ajpath.2019.07.007
11. de Bono JS, Guo C, Gurel B, De Marzo AM, Sfanos KS, Mani RS, et al. Prostate carcinogenesis: inflammatory storms. Nat Rev Cancer. (2020) 20:455–69. doi: 10.1038/s41568-020-0267-9
12. Crocetto F, Boccellino M, Barone B, Di Zazzo E, Sciarra A, Galasso G, et al. The crosstalk between prostate cancer and microbiota inflammation: nutraceutical products are useful to balance this interplay? Nutrients. (2020) 12:2648. doi: 10.3390/nu12092648
13. Qi W, Zhou Y, Liu Z, Wang J, Lv G, Zhong M, et al. Revealing the prognostic and clinicopathological significance of systemic immune-inflammation index in patients with different stage prostate cancer: A systematic review and meta-analysis. Front Med (Lausanne). (2022) 9:1052943. doi: 10.3389/fmed.2022.1052943
14. Wang S, Ji Y, Chen Y, Du P, Cao Y, Yang X, et al. The values of systemic immune-inflammation index and neutrophil-lymphocyte ratio in the localized prostate cancer and benign prostate hyperplasia: A retrospective clinical study. Front Oncol. (2022) 11:812319. doi: 10.3389/fonc.2021.812319
15. Russo P, Marino F, Rossi F, Bizzarri FP, Ragonese M, Dibitetto F, et al. Is systemic immune-inflammation index a real non-invasive biomarker to predict oncological outcomes in patients eligible for radical cystectomy? Medicina (Kaunas). (2023) 59:2063. doi: 10.3390/medicina59122063.(2023
16. Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. (2021) 36:841–8. doi: 10.1007/s10654-021-00752-6
17. Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. (1998) 279:1542–7. doi: 10.1001/jama.279.19.1542.(1998
18. Kou J, Huang J, Li J, Wu Z, Ni L. Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: a systematic review and meta-analysis. Clin Exp Med. (2023) 23:3895–905. doi: 10.1007/s10238-023-01035-y.(2023
19. Hirahara N, Tajima Y, Matsubara T, Fujii Y, Kaji S, Kawabata Y, et al. Systemic immune-inflammation index predicts overall survival in patients with gastric cancer: a propensity score-matched analysis. J Gastrointest Surg. (2021) 25:1124–33. doi: 10.1007/s11605-020-04710-7.(2021
20. Cao Y, Li P, Zhang Y, Qiu M, Li J, Ma S, et al. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: Results from NHANES. Front Immunol. (2023) 14:1087345. doi: 10.3389/fimmu.2023.1087345
21. Zapata D, Howard LE, Allott EH, Hamilton RJ, Goldberg K, Freedland SJ. Is PSA related to serum cholesterol and does the relationship differ between black and white men? Prostate. (2015) 75:1877–85. doi: 10.1002/pros.23069.(2015
22. Wang A, Lazo M, Carter HB, Groopman JD, Nelson WG, Platz EA. Association between liver fibrosis and serum PSA among U.S. Men: national health and nutrition examination survey (NHANES), 2001-2010. Cancer Epidemiol Biomarkers Prev. (2019) 28:1331–8. doi: 10.1158/1055-9965.EPI-19-0145.(2019
23. Littlejohns TJ, Travis RC, Key TJ, Allen NE. Lifestyle factors and prostate-specific antigen (PSA) testing in UK Biobank: Implications for epidemiological research. Cancer Epidemiol. (2016) 45:40–6. doi: 10.1016/j.canep.2016.09.010.(2016
24. Gacci M, Russo GI, De Nunzio C, Sebastianelli A, Salvi M, Vignozzi L, et al. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. (2017) 20:146–55. doi: 10.1038/pcan.2017.1.(2017
25. Luo Z, Wang W, Xiang L. amp]]amp; Jin, T. Association between the systemic immune-inflammation index and prostate cancer. Nutr Cancer. (2023) 75:1918–25. doi: 10.1080/01635581.2023.2272800
26. Sonmez G, Demirtas T, Tombul ST, Akgun H. amp]]amp; Demirtas, A. Diagnostic efficiency of systemic immune-inflammation index in fusion prostate biopsy. Actas Urol Esp (Engl Ed). (2021) 45:359–65. doi: 10.1016/j.acuro.2020.08.015
27. Zhu M, Zhou Y, Liu Z, Jiang Z, Qi W, Chen S, et al. Diagnostic efficiency of pan-immune-inflammation value to predict prostate cancer in patients with prostate-specific antigen between 4 and 20 ng/mL. J Clin Med. (2023) 12:820. doi: 10.3390/jcm12030820.(2023
28. Murray NP, Fuentealba C, Salazar A. amp]]amp; Reyes, E. Platelet-to-lymphocyte ratio and systemic immune-inflammation index versus circulating prostate cells to predict significant prostate cancer at first biopsy. Turk J Urol. (2020) 46:115–22. doi: 10.5152/tud.2020.19203
29. Fujita K, Hayashi T, Matsushita M, Uemura M, Nonomura N. Obesity, inflammation, and prostate cancer. J Clin Med. (2019) 8:201. doi: 10.3390/jcm8020201
30. Jung G, Kim JK, Kim H, Lee J. amp]]amp; Hong, S. K. The association between prostatitis and risk of prostate cancer: a National Health Insurance Database study. World J Urol. (2022) 40:2781–7. doi: 10.1007/s00345-022-04165-2
31. Ward MM, Alehashemi S. Risks of solid cancers in elderly persons with osteoarthritis or ankylosing spondylitis. Rheumatol (Oxf Engl). (2020) 59:3817–25. doi: 10.1093/rheumatology/keaa166
32. Klapp V, Álvarez-Abril B, Leuzzi G, Kroemer G, Ciccia A, Galluzzi L. The DNA damage response and inflammation in cancer. Cancer Discovery. (2023) 13:1521–45. doi: 10.1158/2159-8290.CD-22-1220.(2023
33. Oseni SO, Naar C, Pavlović M, Asghar W, Hartmann JX, Fields GB, et al. The molecular basis and clinical consequences of chronic inflammation in prostatic diseases: prostatitis, benign prostatic hyperplasia, and prostate cancer. Cancers. (2023) 15:3110. doi: 10.3390/cancers15123110
34. Chen C, Wang Z, Ding Y, Qin Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. (2023) 14:1133308.(2023. doi: 10.3389/fimmu.2023.1133308.(2023
35. Katongole P, Sande OJ, Joloba M, Reynolds SJ. amp]]amp; Niyonzima, N. The human microbiome and its link in prostate cancer risk and pathogenesis. Infect Agent Cancer. (2020) 15:53. doi: 10.1186/s13027-020-00319-2
36. D'Este F, Della Pietra E, Badillo Pazmay GV, Xodo LE, Rapozzi V. Role of nitric oxide in the response to photooxidative stress in prostate cancer cells. Biochem Pharmacol. (2020) 182:114205. doi: 10.1016/j.bcp.2020.114205.(2020
37. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017.(2009
38. Sounbuli K, Mironova N, Alekseeva L. Diverse neutrophil functions in cancer and promising neutrophil-based cancer therapies. Int J Mol Sci. (2022) 23:15827. doi: 10.3390/ijms232415827.(2022
39. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. (2015) 126:582–8. doi: 10.1182/blood-2014-08-531582.(2015
Keywords: systemic immune-inflammation index, total prostate-specific antigen, free prostatespecific antigen, fPSA%, prostate cancer, cross-sectional study
Citation: He R, Ye Y, Zhu Q and Xie C (2024) Systemic immune-inflammation index is associated with high risk for prostate cancer among the U.S. elderly: Evidence from NHANES 2001-2010. Front. Oncol. 14:1441271. doi: 10.3389/fonc.2024.1441271
Received: 31 May 2024; Accepted: 05 September 2024;
Published: 23 September 2024.
Edited by:
Jorge Adrián Ramírez De Arellano Sánchez, University of Guadalajara, MexicoReviewed by:
Savio Domenico Pandolfo, Federico II University Hospital, ItalyXiuzhi Duan, Zhejiang University, China
Copyright © 2024 He, Ye, Zhu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changsheng Xie, MTk5MTMwMzBAemNtdS5lZHUuY24=
†These authors contributed equally to this work and share first authorship
 Ran He
Ran He Youjun Ye2†
Youjun Ye2†