- 1Department of Parathyroid Pathology and Mineral Disorders, Endocrinology Research Center, Moscow, Russia
- 2Laboratory of Pathomorphology, Endocrinology Research Center, Moscow, Russia
- 3Department of Сomputed Tomography and Magnetic Resonance Imaging, Endocrinology Research Center, Moscow, Russia
- 4Laboratory of General, Molecular and Population Genetics, Endocrinology Research Center, Moscow, Russia
- 5Administration, Endocrinology Research Center, Moscow, Russia
Parathyroid carcinoma (PC) is one of the rarest malignant neoplasms of the human endocrine system, with a prevalence of approximately 0.005% of all oncological diseases. Despite its indolent course, PC generally relapses in about 40%–60% of cases. The severity of the disease is usually determined by uncontrolled life-threatening hypercalcemia. Currently, there are no reliable criteria for preoperative diagnosis of PC; moreover, topical diagnosis and morphologic examination remain challenges. Surgery remains the gold standard for the treatment of both primary tumors and distant metastases. Other treatment options, such as chemotherapy or immunotherapy, are limited. Targeted therapy is considered a promising direction for disseminated tumors. We present a clinical case of a 70-year-old female patient with recurrent intrathyroidal PC and distant lung metastases, with novel variants in the MET and CDKN1C genes.
Introduction
Parathyroid cancer (PC) is a rare endocrine malignancy, diagnosed in approximately 1%–5% of patients with primary hyperparathyroidism (PHPT) (1). The etiology of the disease is still unknown. Germline inactivating mutations of the tumor suppressor gene CDC73, with somatic loss of heterozygosity at the 1q31.2 locus, account for about 50%–75% of familial cases, and more than 75% of sporadic PC cases have somatic biallelic inactivation/loss of this gene. Recurrent mutations of the PRUNE2 and the ADCK1 genes, CCND1 gene amplification, changes in the PI3K/AKT/mTOR signaling pathway, as well as modifications of the microRNA expression profile and hypermethylation of CpG islands in the promoter regions, have also been described in patients with PC (2). PC is characterized by predominantly local tumor growth. Recurrence of the disease occurs on average 2–4 years after the initial surgical treatment, and distant metastases are detected in 30%–60% of cases (3, 4). Surgery remains the “gold standard” approach for both primary and secondary foci (4), but in some cases, it may not be appropriate. The more severe course of PHPT due to PC, compared to benign parathyroid tumors, is usually caused by life-threatening hypercalcemia. We present a case of a patient with a relapse of intrathyroidal PC and distant lung metastases, with novel variants in the MET and the CDKN1C genes.
Case report
A 75-year-old woman had a long history of recurrent urolithiasis (including transurethral resection of the ureter due to obstruction by a large stone measuring 39 mm and several laparoscopic lithotripsies). Since 2002, the patient has regularly undergone ultrasound (US) for a thyroid goiter. According to 2018 results, the intrathyroidal nodule in the right lobe measured 26 mm × 24 mm. In the same year, scintigraphy (radiopharmaceutical is unknown) suggested hyperfunctional parathyroid tissue on the right. Laboratory tests showed a high parathyroid hormone (PTH) level of 423 pg/ml, 25(OH)vitamin D deficiency at 14.26 ng/ml, and calcium levels were not determined.
In February 2019, she was admitted to the endocrinological department with severe joint and bone pain and marked weakness, where PHPT was confirmed: PTH, 496 pg/ml (15–65); total calcium, 3.43 mmol/L (2.15–2.55); ionized calcium, 1.88 mmol/L (1.03–1.29); 24-h urine calcium, 11.0 mmol/day (2.5–8); and 25(OH)vitamin D, 17.44 ng/ml. Thyroid function tests were within reference values (TSH, 1.4 mMe/L; calcitonin, 5.2 pg/ml). The neck US did not reveal altered parathyroid glands. Fine-needle aspiration (FNA) cytology of a right thyroid formation was classified as Bethesda V; PTH washout from needle aspiration was not performed.
In October 2019, the patient was admitted to the Endocrinology Research Center. Laboratory findings demonstrated PTH at 521.8 pg/ml (15–65), corrected total calcium (Ca corr.) at 3.74 mmol/L (2.15–2.55), phosphorus at 0.68 mmol/L (0.74-1.52), and creatinine at 70.1 μmol/L, with the estimated glomerular filtration rate (eGFR) (CKD-EPI) at 78 ml/min/1.73 m2. Cinacalcet 60 mg/day and saline infusion were prescribed to control hypercalcemia, but without any effect.
Computed tomography (CT) and renal US showed multiple stones in the left kidney and hydronephrosis (staghorn stone, 28 mm × 18 mm × 19 mm in the pelvis, and about 12 stones, 2–12 mm, in the calyces). BMD assessed by Dual-energy X-ray absorptiometry (DXA) scan demonstrated a T-score of − 4.0 SD at the lumbar spine, − 2.2 SD at the femur neck, and − 5.5 SD at the radius.
Contrast-enhanced US and MRI detected a neoplasm with clear contours located posterior to the right thyroid lobe, measuring 3.1 cm × 2.9 cm × 2.7 cm in diameter, without diffusion restriction on MRI (diffusion-weighted imaging).
Intraoperative neck revision revealed a tumor of 4.5 cm × 3.5 cm × 2.5 cm near the lower right thyroid pole, infiltrating the surrounding tissues and muscles, with no boundaries with the thyroid tissue. Thus, en bloc resection with ipsilateral lymphadenectomy was performed.
Histopathological and immunohistochemical (IHC) examination showed a 27-g intrathyroidal PC measuring 6.5 cm × 4.5 cm × 4.0 cm (Figure 1), with diffuse expression of PTH, Ki-67 was 1.5% (Figures 2A, B), and there was loss of parafibromin immunoreactivity with internal positive tissue control.
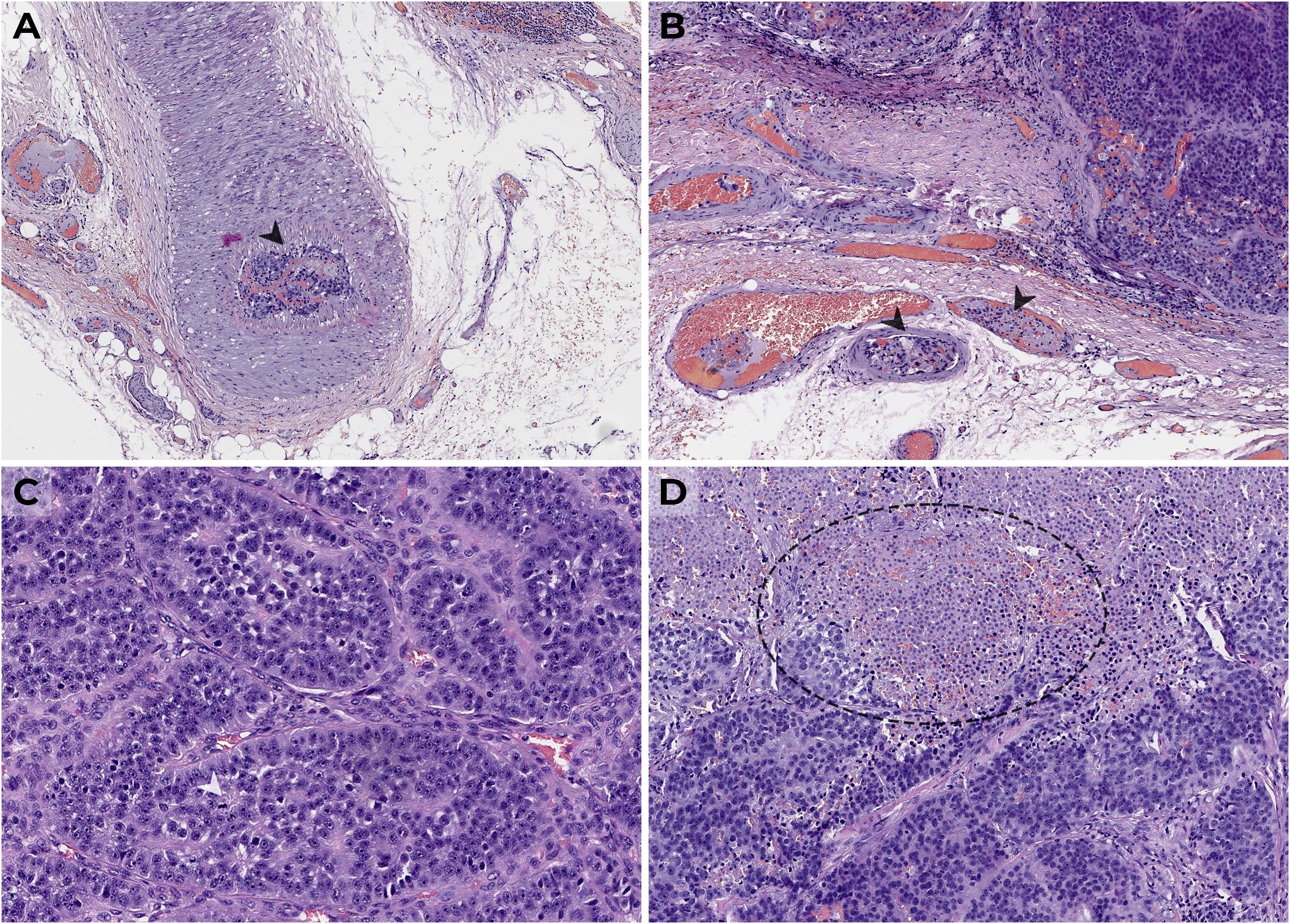
Figure 1. Parathyroid carcinoma (H&E, × 400) from large cells with moderate nuclei polymorphism forming a solid-alveolar structure with thickened stroma, vascular (A, B) and fatty tissue invasion, atypical mitoses (C), and foci of necrosis (D); lymph nodes without signs of metastasis are shown.
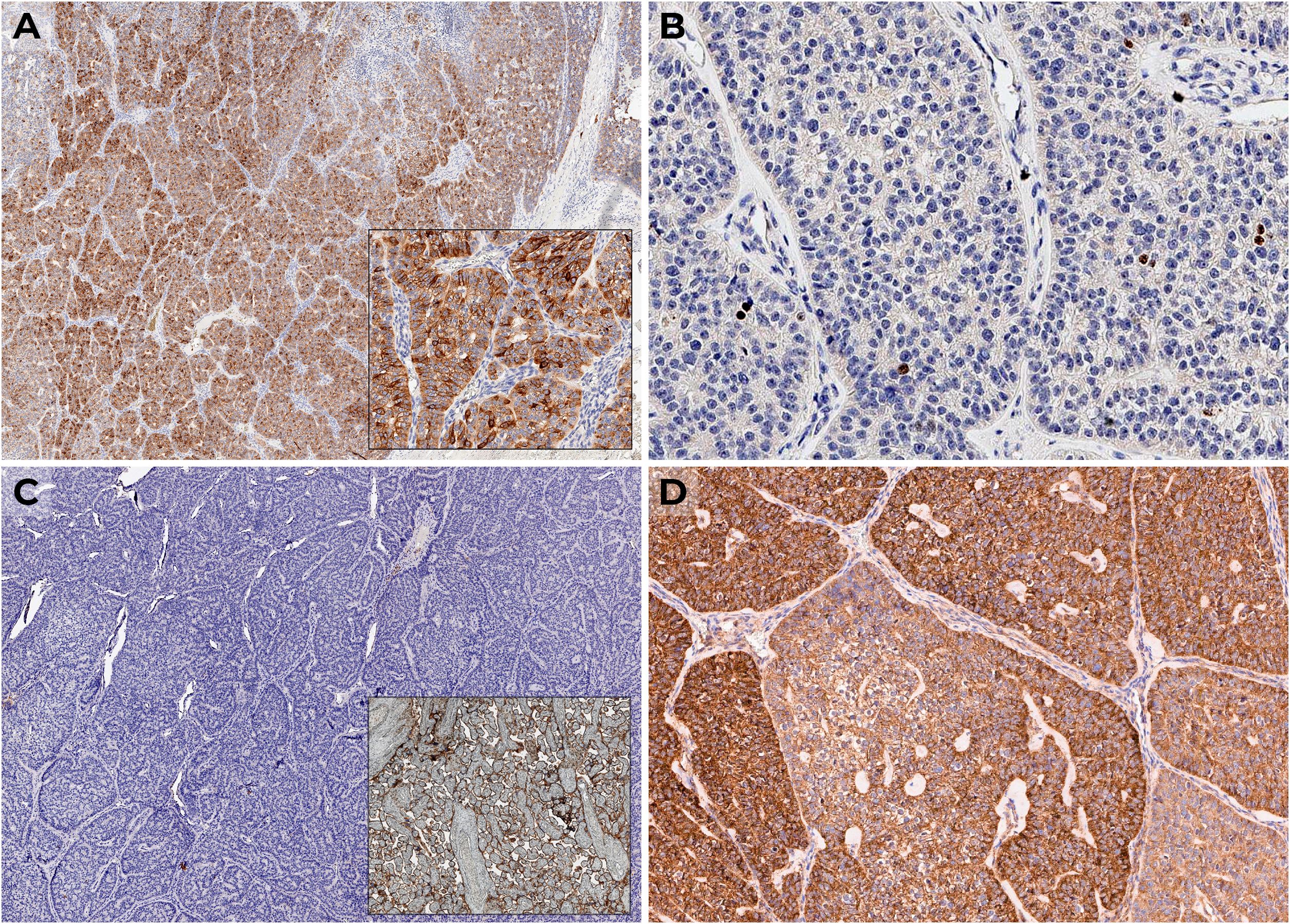
Figure 2. IHC examination: (A) diffuse expression of PTH; (B) Ki-67 1.5%; (C) negative expression of PD-L1 with positive control (placenta); (D) positive VEGF expression.
Histopathological diagnosis of parathyroid tumors was established according to the WHO classification criteria. Sections, 3–3.5 μm thick, were made from formalin-fixed paraffin-embedded (FFPE) blocks of tumor tissue samples. Dewaxing and unmasking of antigens were carried out using high- and low-pH buffers (Leica, Wetzlar, Germany). Sections were stained with anti-PTH (MRQ-31, 1:100; USA, Cell Marque), Ki-67 (MIB-1, 1:100; Denmark, DAKO), and parafibromin (2H1, 1:50; USA, Santa Cruz Biotechnology).
Postoperatively, PTH decreased to 2.21 pg/ml (15–65), total calcium decreased to 2.42 mmol/l (2.15–2.55), and ionized calcium decreased to 1.25 mmol/L (1.03–1.29). She was prescribed alfacalcidol 2 µg/day and calcium carbonate 1,000 mg/day, which she received for several months. No regular follow-up has been carried out.
In October 2022, blood tests showed remarkably elevated total calcium of 3.87 mmol/L (2.15–2.55) and PTH of 500 pg/ml (16–65). Subsequent scintigraphy with 99mTc-MIBI revealed two round foci up to 10 mm in diameter in the thyroid gland region. PHPT recurrence was diagnosed.
Since February 2023, the patient has noticed a general deterioration, hoarseness, and dysphagia. She also experienced increased joint, lower back, and muscle pain. On admission to our center in July 2023, we observed a deterioration in calcium–phosphorus metabolism: PTH, 1,148 pg/ml (15–65); severe hypercalcemia, 4.04 mmol/L (2.15–2.55); and eGFR (CKD-EPI), 32 ml/min/1.73 m2. To prevent a hypercalcaemic crisis, the patient received a 60-mg single dose of denosumab. She was also treated with isotonic saline and cinacalcet up to 90 mg/day, with a positive effect (Graph 1).
We also noted the progression of PHPT-associated complications. Contrast-enhanced CT showed bilateral nephrolithiasis, pelvic ectasia on the right, and ureteral ectasia on the left. Despite no significant dynamics in the DXA scans, X-ray revealed multiple vertebral compressions (Th7–9 up to 28%, Th10–11, L2 up to 17%). Bone turnover markers were elevated: alkaline phosphatase, 463 U/L (40–150); C-terminal telopeptide of type I collagen, 5.02 ng/ml (0.3–1.1); and osteocalcin, 300 ng/ml (15–46).
US showed a hypoechogenic zone measuring 0.3 cm × 0.3 cm × 0.3 cm (EU-TIRADS 5) in the left thyroid lobe and a hypoechogenic nodule measuring 0.6 cm× 0.5 cm × 0.5 cm behind the left thyroid lobe, which correlated with the results of scintigraphy and CT (Figures 3A, B). The сontrast-enhanced CT also showed multiple metastases up to 16 mm in diameter in both lungs (Figures 3C, E), which were confirmed by PET-CT with 11C-choline in September 2023 (in S9 of the right lung, 16 mm × 17 mm, SUVmax = 2.96; in S6 of the left lung, up to 11 mm × 10 mm, SUVmax = 1.93) (Figures 3D, F).
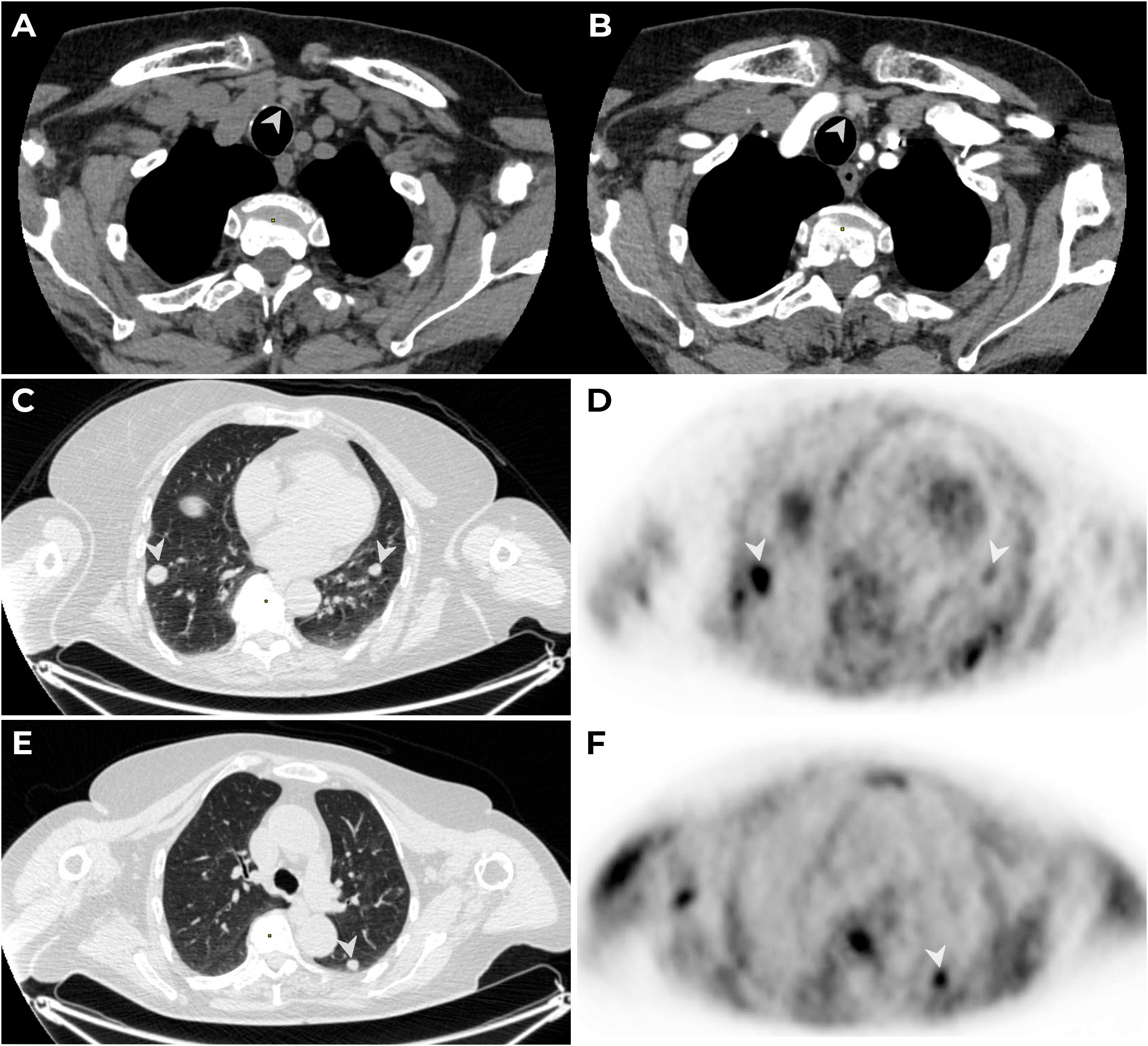
Figure 3. MSCT: native (A) and arterial (B) phases of contrast enhancement. A lesion with an oval shape, smooth clear contours, 6 mm × 5 mm × 5 mm in diameter (native density: 83 HU, 104 HU in the arterial phase and 93 HU in the venous phase) is detected paratracheal to the left (→) along the lower contour of the left lobe of the thyroid gland. PET/CT with 11C-choline. The CT thorax (C, D) visualizes secondary lesions (mts) (→) in S9 of the right and left lungs (C, D) and in S6 of the left lung (E, F), with hyperfixation (→) of the radiopharmaceutical on PET.
We performed genetic testing to exclude the hereditary disease. Genomic DNA was isolated from whole blood according to standard protocols. Molecular genetic analysis was performed using a specifically developed NGS gene panel for the diagnosis of endocrinopathies, developed in our center and capable of simultaneously detecting variants in several genes within a single study (see Supplementary Materials for a list of genes included in the panel). DNA libraries were prepared by hybridization enrichment of targets using probe panels, following the manufacturer’s instructions. Sequencing of libraries was performed on the Illumina Nextseq 550 platform (USA) in paired-end mode (150 bp reads using v.2.5 Nextseq 500/550 Mid Output Reagent Kit). The read sequences were aligned to the human genome reference sequence (GRCh38) and processed using an automated bioinformatics algorithm. Detected variants were classified as pathogenic, likely pathogenic, or of uncertain clinical significance according to the recommendations of the American College of Medical Genetics and Genomics (ACMG) and the Russian Institute of Genetics and Genomics (RIMG).
Genetic analysis showed heterozygous MET gene (NM_000245.4) mutation in exon 2 (HG38, chr7:116699952T>C, c.868 T>C), p.(Ser290Pro), and a previously unreported heterozygous CDKN1C gene (NM_001122630.2) mutation in exon 2 (HG38, chr11:2884735G>A, c.722 C>T), p.(Ala241Val)—both variants of uncertain clinical significance.
During the last follow-up consultation in October 2023, the patient complained of severe muscle weakness and, as a consequence, loss of self-care ability. Laboratory tests showed increased PTH levels of up to 1,718 pg/ml (15–65) and significant hypercalcemia of 4.13 mmol/L (2.15–2.55) (Graph 1), with eGFR (CKD-EPI) at 27 ml/min/1.73 m2.
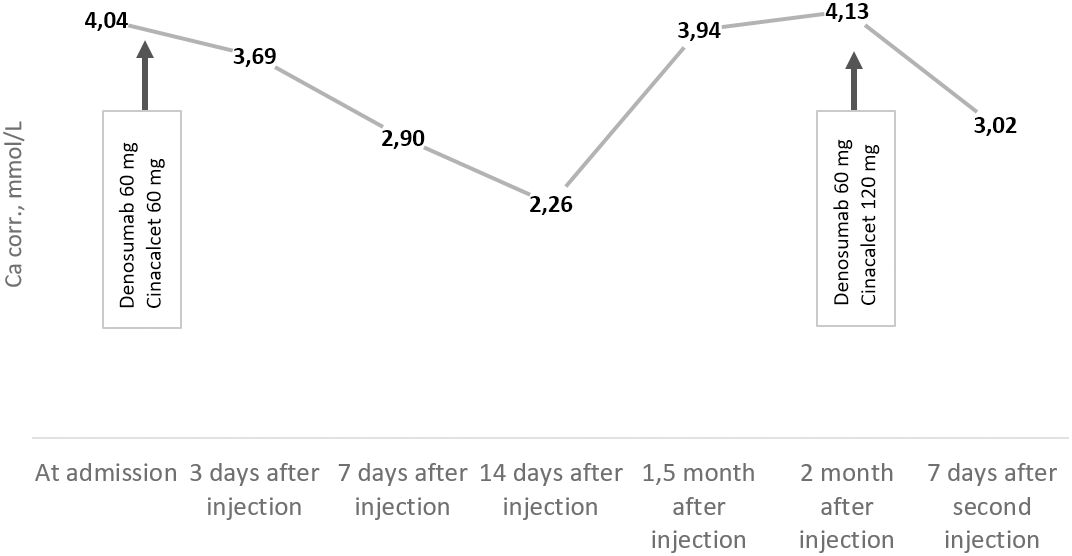
Graph 1. Trends of albumin-corrected calcium levels (reference range: 2.15–2.55 mmol/L) from July to October 2023.
Considering the lack of data on lung metastasis progression, the minimal size of the neck lesions without 11C-choline accumulation, and the expected resistance of metastases to radiotherapy and chemotherapy, we recommended continuing symptomatic treatment of hypercalcemia with denosumab 120 mg every 1–2 months and cinacalcet 120 mg/day, with regular blood calcium level monitoring. We performed an additional IHC study of the primary tumor to identify potential targets for future therapy. For this purpose, we used antibodies for PD-L1 (SP142, 1:100; UK, Abcam Inc.) and VEGF (VG1, 1:100; USA, Diagnostic BioSystems). There was no PD-L1 expression (Figure 2C), but diffuse positive expression of VEGF was observed (Figure 2D).
Discussion
Ectopic parathyroid glands occur in 22% of cases, with the most common location being intrathyroidal (5, 6). About 30 cases of intrathyroidal PC have been described in the literature (7, 5, 8). Preoperative diagnosis of PC is challenging and, therefore, is usually made postoperatively through histology. It may be difficult to determine intrathyroidal PC. Our patient had a long history of nodular thyroid goiter and recurrent urolithiasis, but calcium and PTH levels were not assessed. FNA does not allow differentiation between thyroid and parathyroid neoplasms due to considerable morphologic overlap (presence of papillary structures, microfollicular cells, macrophages, compacted colloid-like material, oxyphilic cytoplasm, and exposed nuclei), and PC may be mistaken for a thyroid malignant lesion (9, 10). In our case, FNA showed a lesion suspicious of malignancy (Bethesda V). Considering the scintigraphy results and laboratory findings, an intrathyroidal parathyroid lesion was suspected and confirmed by PTH washout. PTH washout from needle aspiration is useful, though there is still a risk of tumor seeding (9).
Surgery remains the treatment of choice for both local and metastatic PC. Remission is only possible with complete removal of the tumor (R0). If PC is suspected, most experts recommend parathyroidectomy with the ipsilateral thyroid lobe, adjacent adipose tissue, and resection of altered lymph nodes (en bloc resection). Taking into account the tumor size and its adhesion to the surrounding tissues (assessed intraoperatively as parathyroid carcinoma), the surgeon decided to perform ipsilateral lymphadenectomy to ensure the radicality of the operation. Despite the extended volume of surgery and a postoperative decrease in PTH, we confirmed PHPT recurrence with distant metastases 3 years later. There is still debate about the optimal management strategy. Studies differ on whether local resection alone, en bloc resection (including ipsilateral thyroidectomy), or radical surgery (including central and lateral neck dissection) is the correct approach, although there is a tendency toward the first approach. According to the recent systematic review by McInerney et al., there is no survival difference between local resection and extensive radical surgery (p < 0.005). However, across all studies, parathyroidectomy alone (local resection) was the most frequently utilized approach (11).
The tumor usually spreads locally, but distant metastases in the lungs, bones, and, less commonly, the brain and liver, can be found in 30% of cases. With timely surgery, the survival rates at 5 and 10 years are 77%–100% and 49%–91%, respectively. Recurrence usually occurs after 2–4 years, although the longest disease-free survival time recorded was 23 years (12–14). Methods such as US, CECT, and MRI are useful in detecting metastases, but scintigraphy with 99mTc-MIBI and PET-CT with 18F-FDG or 11C-choline are more accurate (15). In our case, PET-CT with 11C-choline allowed us to detect functionally active nodules in the lungs, while small foci in the neck, detected by US and CT, did not accumulate radioisotopes.
In PC patients, it is usually not the tumor load but hypercalcemia that determines the severity of disease. If surgery is not appropriate, saline infusion with concomitant loop diuretics, calcimimetics, and antiresorptive drugs (bisphosphonates or denosumab) are required to control life-threatening hypercalcemia. Cinacalcet not only controls blood calcium levels but also lowers PTH levels by modulating calcium-sensitive receptors (16, 17). The effectiveness of calcimimetics does not diminish over time and remains effective as long as treatment continues (18), although patients may experience dose-dependent side effects (19, 20). Due to its pronounced hypocalcemic effect, denosumab is the therapy of choice for bisphosphonate/calcimimetics-refractory hypercalcemia and low eGFR (a common complication of severe PHPT), administered at a maximum dose of 120 mg every 28 days (21, 22). In the case described by Roukain, therapy with denosumab resulted in long-term control of hypercalcemia in a patient with relapsed PC (23). Karuppiah et al. reported successful use of denosumab in a patient with biochemical PC relapse, intolerance to cinacalcet, and resistance to the hypocalcaemic effect of bisphosphonates, as well as negative findings on 18F-FDG PET-CT and 99mTc-MIBI scintigraphy (24). In our case, the combination of cinacalcet and denosumab was justified by several factors: severe hypercalcemia, markedly decreased GFR, and severe osteoporosis. Due to failure to achieve significant calcemia reduction, we prescribed denosumab 120 mg/28 days along with cinacalcet titration to 120 mg/day, under regular laboratory follow-up.
In our case, genetic testing did not reveal any germline mutation in CDC73; however, we observed a loss of parafibromin IHC expression. Although this typically implies biallelic CDC73 inactivation, it is important to note that parafibromin IHC can be technically challenging and difficult to interpret (25). We identified heterozygous variants in the MET and the CDKN1C genes, which have not been previously described in the literature, and their clinical significance remains uncertain. The MET gene is a proto-oncogene that encodes c-Met, a member of the receptor tyrosine kinase family. The c-Met protein is involved in several canonical signaling pathways (Ras-CDC42-PAK-Rho kinase, Ras-MAPK, PI3K-AKT-mTOR, and β-catenin). Driver mutations of the MET gene, such as amplification and loss of exon 14, activate cell transformation, promote oncogenic activity, and worsen patient prognosis. Genetic alterations in MET can promote resistance to targeted therapies using first-, second-, and third-generation tyrosine kinase inhibitors; therefore, combination therapy is currently prescribed (26). The CDKN1C gene, which encodes the CDKN1C protein, is imprinted, with preferential expression of the maternal allele. The CDKN1C binds to the cyclin/cyclin-dependent kinase complex and inhibits DNA replication, thereby suppressing cell proliferation (27). Heterozygous mutations in the MET gene have been described in lung adenocarcinoma, arthrogryposis, hepatocellular carcinoma, and papillary renal cell carcinoma (OMIM:164860). However, the phenotypic features of these conditions were not observed in our case. CDKN1C mutations cause intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital abnormalities (IMAGE) syndrome (OMIM:300290) and Beckwith–Wiedemann syndrome (OMIM:600856). Variants in the MET and CDKN1C genes are described here for the first time in PC. Due to their uncertain clinical significance, we are unable to determine their role in PC. Further genetic studies in patients with PC are promising, as they may potentially influence diagnostic and treatment strategies.
Conventional adjuvant therapy, radiotherapy, and chemotherapy have proven ineffective in metastatic PC (28). Molecular profiling of metastatic PC offers important insights into disease pathogenesis and aids in identifying potential therapeutic targets.
The use of targeted therapy, such as kinase inhibitors (sorafenib, lenvatinib) and immune checkpoint inhibitors (pembrolizumab), in advanced PC has been described in the literature (29, 30). Makino et al. presented a case of sorafenib use in a 61-year-old man with metastatic PC and a somatic mutation in the CDC73 gene (c.126_131 + 9delinsCT). Multiple pulmonary metastases and refractory hypercalcemia appeared shortly after en bloc` parathyroidectomy. Combination therapy with sorafenib (400 mg twice daily), evocalcet, and denosumab successfully controlled blood calcium levels, which was vital for the patient. It is worth noting that immunohistochemical expression of VEGFR-2, but not PDGFR-α, was detected in the tumor cells in this case (31). Before prescribing kinase inhibitor therapy, it is necessary to assess disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria and consider the clinical picture, including the response to symptomatic treatment for the management of hypercalcemia.
Patient perspective
In this case, repeated surgical treatment was not pursued due to the small size of the neck lesions without 11C-choline accumulation and the presence of metastases in both lungs. At the time of the last admission, the medical council did not prescribe the targeted therapy, primarily because no targeted lesions were identified according to the RECIST classification. Continuing treatment with denosumab and cinacalcet for the management of hypercalcemia was recommended, along with dynamic assessment of tumor foci in accordance with RECIST guidelines.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report
Author contributions
EK: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. AL: Conceptualization, Writing – original draft, Writing – review & editing. OS: Conceptualization, Writing – original draft, Writing – review & editing. AE: Supervision, Writing – review & editing, Data curation. RS: Visualization, Writing – review & editing, Conceptualization. LU: Writing – review & editing, Investigation, Visualization. NT: Writing – review & editing, Investigation, Visualization. SP: Writing – review & editing, Formal analysis, Investigation. VZ: Writing – review & editing, Formal analysis, Investigation. NM: Supervision, Writing – review & editing, Data curation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was funded by the Government of the Russian Federation (Agreement No. 075-15-2022-310, dated 20 April 2022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1441083/full#supplementary-material
References
1. Kong SH, Kim JH, Park MY, Kim SW, Shin CS. Epidemiology and prognosis of parathyroid carcinoma: real-world data using nationwide cohort. J Cancer Res Clin Oncol. (2021) 147:3091–7. doi: 10.1007/s00432-021-03576-9
2. Marini F, Giusti F, Palmini G, Perigli G, Santoro R, Brandi ML. Genetics and epigenetics of parathyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:834362. doi: 10.3389/fendo.2022.834362
3. Hu Y, Cui M, Chang X, Wang O, Chen T, Xiao J, et al. Patterns and predictors of cervical lymph node metastasis in parathyroid carcinoma. Cancers (Basel). (2022) 14:4004. doi: 10.3390/cancers14164004
4. Salcuni AS, Cetani F, Guarnieri V, Nicastro V, Romagnoli E, de Martino D, et al. Parathyroid carcinoma. Best Pract Res Clin Endocrinol Metab. (2018) 32:877–89. doi: 10.1016/j.beem.2018.11.002
5. Kim E, Bondarenko E, Eremkina A, Nikiforovich P, Mokrysheva N. Silent intrathyroid parathyroid carcinoma. Endocrinol Diabetes Metab Case Rep. (2023) 2023: 23, 0027. doi: 10.1530/EDM-23-0027
6. Mokrysheva NG, Krupinova JA, Voronkova IA. Parathyroid glands: the normal development, anatomy and histological structure. Endocr Surg. (2019) 12:178–87. doi: 10.14341/serg10039
7. Balakrishnan M, George SA, Rajab SH, Francis IM, Kapila K. Cytological challenges in the diagnosis of intrathyroidal parathyroid carcinoma: A case report and review of literature. Diagn Cytopathol. (2018) 46:47–52. doi: 10.1002/dc.23847
8. Salimkhanov R, Bondarenko E, Eremkina A, Bibik E, Kim E, Begova K, et al. Case report: Sagliker syndrome in the patient with recurrent tertiary hyperparathyroidism due to intrathyroidal parathyroid carcinoma. Front Endocrinol (Lausanne). (2023) 14:1292993. doi: 10.3389/fendo.2023.1292993
9. Ha HJ, Kim EJ, Kim J-S, Shin M-S, Noh I, Park S, et al. Major clues and pitfalls in the differential diagnosis of parathyroid and thyroid lesions using fine needle aspiration cytology. Medicina (B Aires). (2020) 56:558. doi: 10.3390/medicina56110558
10. Mir F, Rohra P, Aakash N, Furlan K, Ghai R, Reddy V, et al. Fine needle aspiration of an intrathyroidal parathyroid carcinoma mimicking a primary thyroid anaplastic carcinoma: A case report with review of the literature. Diagn Cytopathol. (2021) 49. doi: 10.1002/dc.24560
11. McInerney NJ, Moran T, O’Duffy F. Parathyroid carcinoma: Current management and outcomes – A systematic review. Am J Otolaryngol - Head Neck Med Surg. (2023) 44:103843. doi: 10.1016/j.amjoto.2023.103843
12. Cetani F, Pardi E, Marcocci C. "Parathyroid carcinoma". In: Brandi ML, editor. Parathyroid Disorders: Focusing on Unmet Needs. S. Karger AG. (2019), 63–76. doi: 10.1159/000491039
13. Kebebew E. Parathyroid carcinoma. Curr Treat Options Oncol. (2001) 2:347–54. doi: 10.1007/s11864-001-0028-2
14. Shane E. Parathyroid carcinoma. J Clin Endocrinol Metab. (2001) 86:485–93. doi: 10.1210/jcem.86.2.7207
15. Hatzl M, Röper-Kelmayr JC, Fellner FA, Gabriel M. 18F-fluorocholine, 18F-FDG, and 18F-fluoroethyl tyrosine PET/CT in parathyroid cancer. Clin Nucl Med. (2017) 42:448–50. doi: 10.1097/RLU.0000000000001652
16. Marcocci C, Bollerslev J, Khan AA, Shoback DM. Medical management of primary hyperparathyroidism: proceedings of the fourth international workshop on the management of asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. (2014) 99:3607–18. doi: 10.1210/jc.2014-1417
17. Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM, Smallridge RC, et al. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab. (2007) 92:3803–8. doi: 10.1210/jc.2007-0585
18. National Institute for Health and Care Excellence [NICE]. Hyperparathyroidism (primary): diagnosis, assessment and initial management. NG132. (2019). Available at: https://www.nice.org.uk/guidance/ng132(Accessed: 15 May 2024).
19. Betea D, Potorac I, Beckers A. Parathyroid carcinoma: Challenges in diagnosis and treatment. Ann Endocrinol (Paris). (2015) 76:169–77. doi: 10.1016/j.ando.2015.03.003
20. Rodrigo JP, Hernandez-Prera JC, Randolph GW, Zafereo ME, Hartl DM, Silver CE, et al. Parathyroid cancer: An update. Cancer Treat Rev. (2020) 86:102012. doi: 10.1016/j.ctrv.2020.102012
21. Hu MI, Glezerman IG, Leboulleux S, Insogna K, Gucalp R, Misiorowski W, et al. Denosumab for treatment of hypercalcemia of Malignancy. J Clin Endocrinol Metab. (2014) 99:3144–52. doi: 10.1210/jc.2014-1001
22. Thosani S, Hu MI. Denosumab: A new agent in the management of hypercalcemia of Malignancy. Futur Oncol. (2015) 11:2865–71. doi: 10.2217/fon.15.232
23. Roukain A, Alwan H, Bongiovanni M, Sykiotis GP, Kopp PA. Denosumab for the treatment of hypercalcemia in a patient with parathyroid carcinoma: A case report. Front Endocrinol (Lausanne). (2022) 12:794988. doi: 10.3389/fendo.2021.794988
24. Karuppiah D, Thanabalasingham G, Shine B, Wang LM, Sadler GP, Karavitaki N, et al. Refractory hypercalcaemia secondary to parathyroid carcinoma: response to high-dose denosumab. Eur J Endocrinol. (2014) 171:K1–5. doi: 10.1530/EJE-14-0166
25. Gill AJ, Lim G, Cheung VKY, Andrici J, Perry-keene JL, Paik J, et al. Parafibromin-deficient (HPT-JT type, CDC73 mutated) parathyroid tumors demonstrate distinctive morphologic features. Am J Surg Pathol. (2019) 43:35–46. doi: 10.1097/PAS.0000000000001017
26. Rivas S, Marín A, Samtani S, González-Feliú E, Armisén R. MET signaling pathways, resistance mechanisms, and opportunities for target therapies. Int J Mol Sci. (2022) 23:13898. doi: 10.3390/ijms232213898
27. Li J, Chen L-N, He H-L. CDKN1C gene mutation causing familial Silver–Russell syndrome: A case report and review of literature. World J Clin cases. (2023) 11:4655–63. doi: 10.12998/wjcc.v11.i19.4655
28. Christakis I, Silva AM, Kwatampora LJ, Warneke CL, Clarke CN, Williams MD, et al. Oncologic progress for the treatment of parathyroid carcinoma is needed. J Surg Oncol. (2016) 114:708–13. doi: 10.1002/jso.24407
29. Park D, Airi R, Sherman M. Microsatellite instability driven metastatic parathyroid carcinoma managed with the anti-PD1 immunotherapy, pembrolizumab. BMJ Case Rep. (2020) 13:e235293. doi: 10.1136/bcr-2020-235293
30. Kim EI, Krupinova JA, Belousov PV, Trutneva KA, Mokrysheva NG. Treatment of parathyroid cancer: current status and near-term prospects. Vestn Ross Akad Meditsinskikh Nauk. (2022) 77:362–70. doi: 10.15690/vramn2132
Keywords: primary hyperparathyroidism, parathyroid cancer, metastases, hypercalcemia, case report
Citation: Kim E, Lavreniuk A, Spasskaya O, Eremkina A, Salimkhanov R, Urusova L, Tarbaeva N, Popov S, Zakharova V and Mokrysheva N (2025) Case report: Relapse of intrathyroidal parathyroid carcinoma in a patient with novel variants in MET and CDKN1C genes. Front. Oncol. 14:1441083. doi: 10.3389/fonc.2024.1441083
Received: 30 May 2024; Accepted: 23 December 2024;
Published: 16 January 2025.
Edited by:
Richard Yuxiong Su, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Jessica Costa-Guda, University of Connecticut, United StatesSmita Jha, National Institutes of Health (NIH), United States
Copyright © 2025 Kim, Lavreniuk, Spasskaya, Eremkina, Salimkhanov, Urusova, Tarbaeva, Popov, Zakharova and Mokrysheva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anastasiia Lavreniuk, bGF2YW5hc3RhLmJveEBnbWFpbC5jb20=
 Ekaterina Kim
Ekaterina Kim Anastasiia Lavreniuk
Anastasiia Lavreniuk Olga Spasskaya
Olga Spasskaya Anna Eremkina
Anna Eremkina Rustam Salimkhanov
Rustam Salimkhanov Liliya Urusova2
Liliya Urusova2 Victoria Zakharova
Victoria Zakharova Natalia Mokrysheva
Natalia Mokrysheva