- 1The First School of Clinical Medicine, Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Nuclear Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 3Department of Oncology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
Mammary-like adenocarcinoma of the vulva is a malignancy with a low incidence rate compared with the squamous cell carcinoma occurring at the same site. We present a rare case of mammary-like adenocarcinoma of the vulva with multiple-organ involvement using 18F-FDG PET/CT. This study indicates that 18F-FDG PET/CT can not only detect the primary lesion but also distinguish the stage of the mammary-like adenocarcinoma of the vulva.
Introduction
The mammary-like adenocarcinoma (MLA) of the vulva is a rare primary vulvar tumor occurring in ectopic vulvar breast tissue (1), and it shows a low incidence rate, and has immunohistochemical profiles and aggressive behaviors similar to those of breast cancers, which make it challenging for clinical diagnosis and differential diagnosis. PET/CT plays an important role in the management of vulvar cancer (2–4). However, at present, there is little literature on the detection of MLA of the vulva with 18F-FDG PET/CT imaging.
In this case, the 18F-FDG PET/CT imaging indicated a malignant tumor according to morphological manifestations and metabolic characteristics, which was definitely diagnosed as primary MLA of the vulva by the pathological examination.
Case description
A 68-year-old woman presented with low back pain for 1 month, and a non-contrast-enhanced abdominal CT revealed several lesions on her spine bones, suggesting multiple metastatic lesions. Furthermore, laboratory examinations showed increased tumor markers: CA125: 50.7 U/ml (normal value < 35 U/ml), Ferritin: 539 ng/ml (normal value 13-150 ng/ml). This patient had no previous history of malignancy. For evaluating the general condition, this patient underwent 18F-FDG PET/CT imaging, which demonstrated multiple fluorodeoxyglucose (FDG) uptake in the liver, bones and lymph nodes in the PET/CT MIP image (Figure 1A). Multiple foci of abnormal activity (broken arrows) were seen in the liver (Figures 1B, D), although there was no clear evidence of anatomical abnormality in the corresponding CT image (Figure 1C). Multiple hypermetabolic foci (solid arrows) were also seen in the bones (Figures 1E–P), with mixed bone destruction on CT (Figure 1L). Enlarged retroperitoneal and bilateral inguinal lymph nodes (dashed arrows) with increased activities were seen on PET/CT (Figures 1E–J). An additional focal activity (Figures 1N, P, curved arrows) with SUVmax of 6.2 was also observed in the vulva, although CT indicated no clear evidence of anatomical abnormality in the corresponding area (Figure 1O).
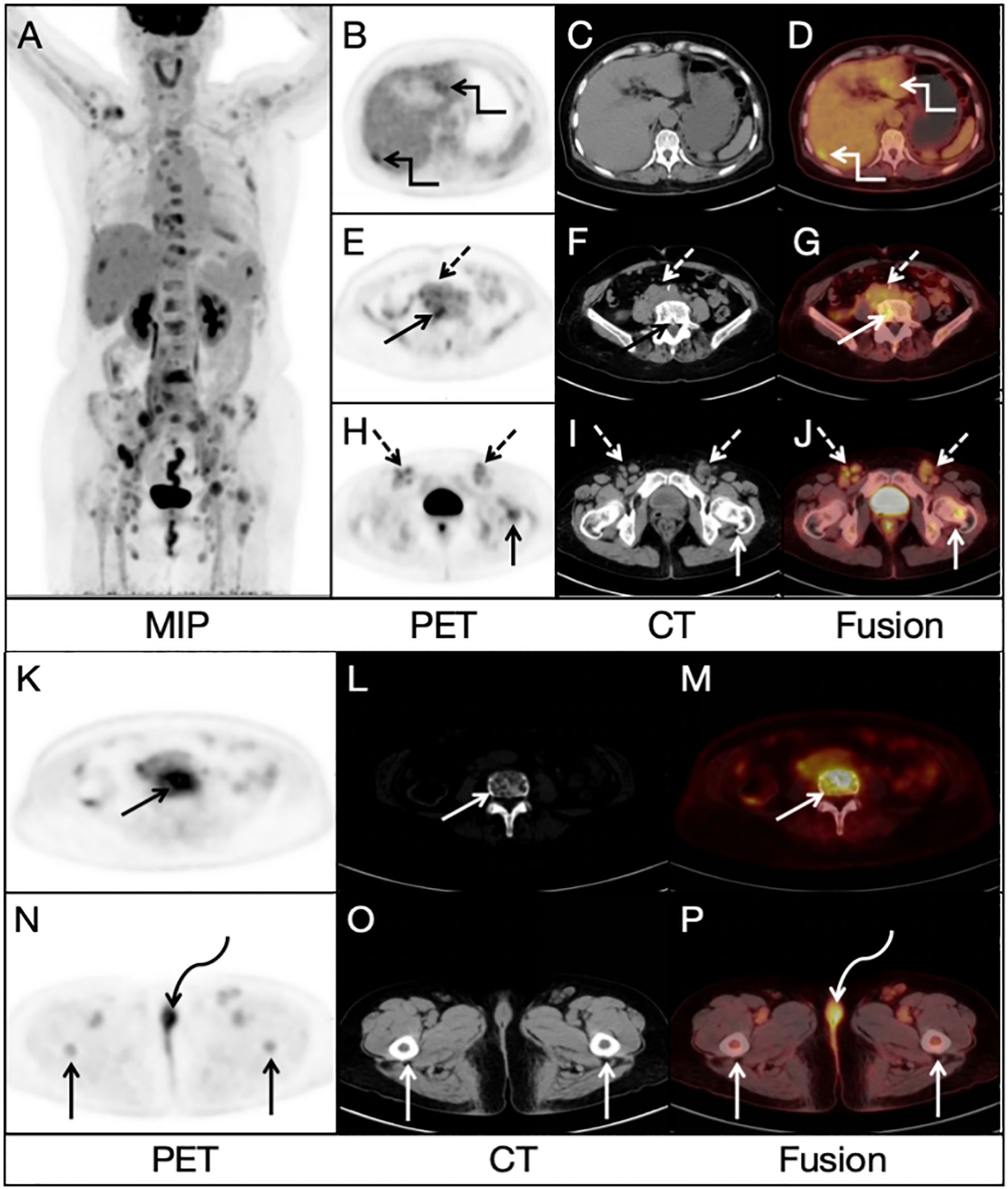
Figure 1. Radiological findings on 18FDG PET/CT image: (A) Multiple lesions in the bones, liver and lymph nodes. (B, D) Multiple foci of abnormal activity (broken arrows) in the liver. (C) No clear evidence of anatomical abnormality in the corresponding area on CT image. (E–J) Enlarged retroperitoneal and bilateral inguinal lymph nodes (dashed arrows). (K–M) Multiple hypermetabolic foci (solid arrows) in the bones (N, P) An additional focal activity (curved arrows) with a SUVmax of 6.2 in the vulva. (O) No clear evidence of anatomical abnormality in the corresponding area on CT image.
FDG uptake in many lesions in the liver, bones and lymph nodes might suggest multiple metastases, but the location of primary lesion was unclear. Due to the existence of bilateral inguinal lymph node metastases, the vulvar lesion was suspected to be the primary lesion. To further clarify the diagnosis, this patient underwent a delayed pelvic FDG PET/CT imaging at 2 h after FDG administration, and the results demonstrated multiple possible malignant lesions in the vulva (Figures 2A, C, curved arrows), with a furtherly increased SUVmax of 10.2 while no evidence of anatomical abnormality in the corresponding area on CT image (Figure 2B). A more detailed medical history was then taken. The woman had been complaining of vulvar inflammation and pain for years. The physical examination had revealed the presence of stiffness and swelling in the mons pubis, accompanied by two erythematous lesions of 1.0 cm in diameter. According to the medical history and findings of dual-time-point 18F-FDG PET/CT, vulvar carcinoma with liver, bone and lymph node metastasis was suggested.
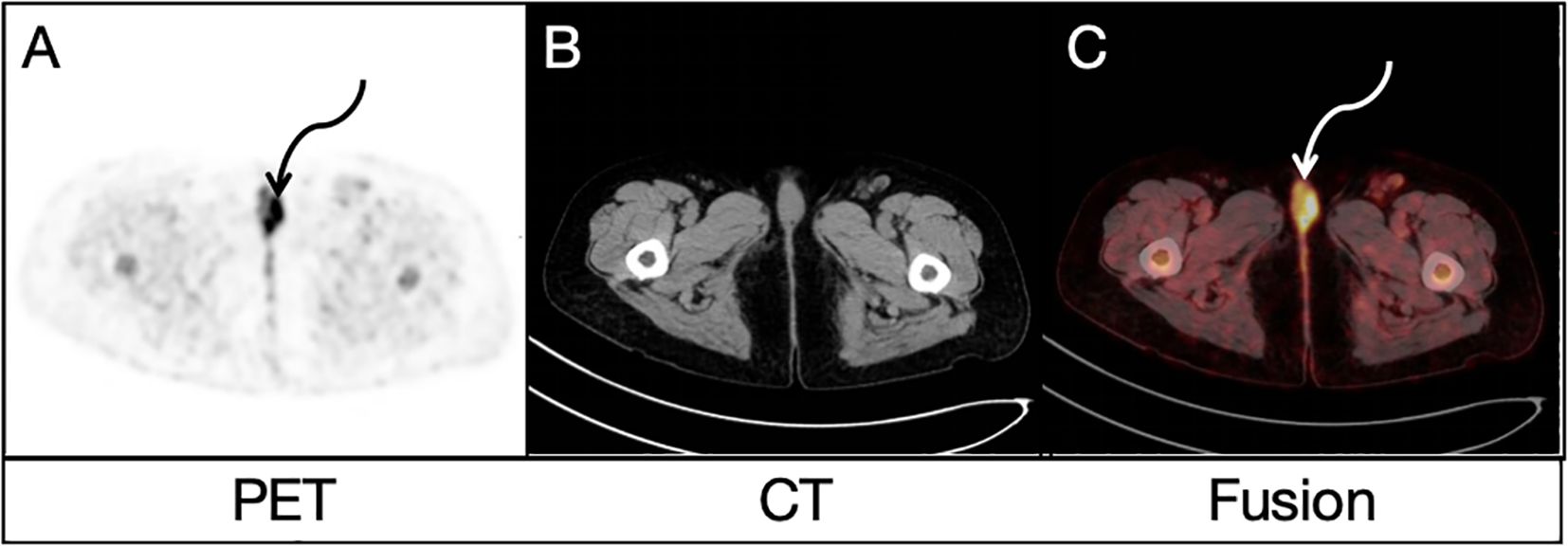
Figure 2. Delayed pelvic FDG PET/CT findings: Both PET image (A) and PET/CT fusion image (C) demonstrated similar possible malignant lesions (curved arrows) in the vulva, with a SUVmax of 10.2 while no evidence of anatomical abnormality in the corresponding area on CT image (B).
Subsequently, biopsy and pathological examination of affected vulva (Figure 3D) and right inguinal lymph nodes (Figures 3A–C) were performed, indicating that the vulvar lesion was an infiltrative, moderately/poorly differentiated adenocarcinoma arising from mammary gland-like epithelium of the vulva. In addition, the immunohistochemical results showed that the estrogen receptor/progesterone receptor and positive common breast cancer markers such as epithelial membrane antigen (EMA), carcinoembryonic antigen (CEA), cytokeratin 7(CK7), GATA-binding protein 3(GATA3) and Ki-67 were increasingly expressed by more than 40%. The morphology and immunoprofile of the tumor cells suggested the incidence of MLA.
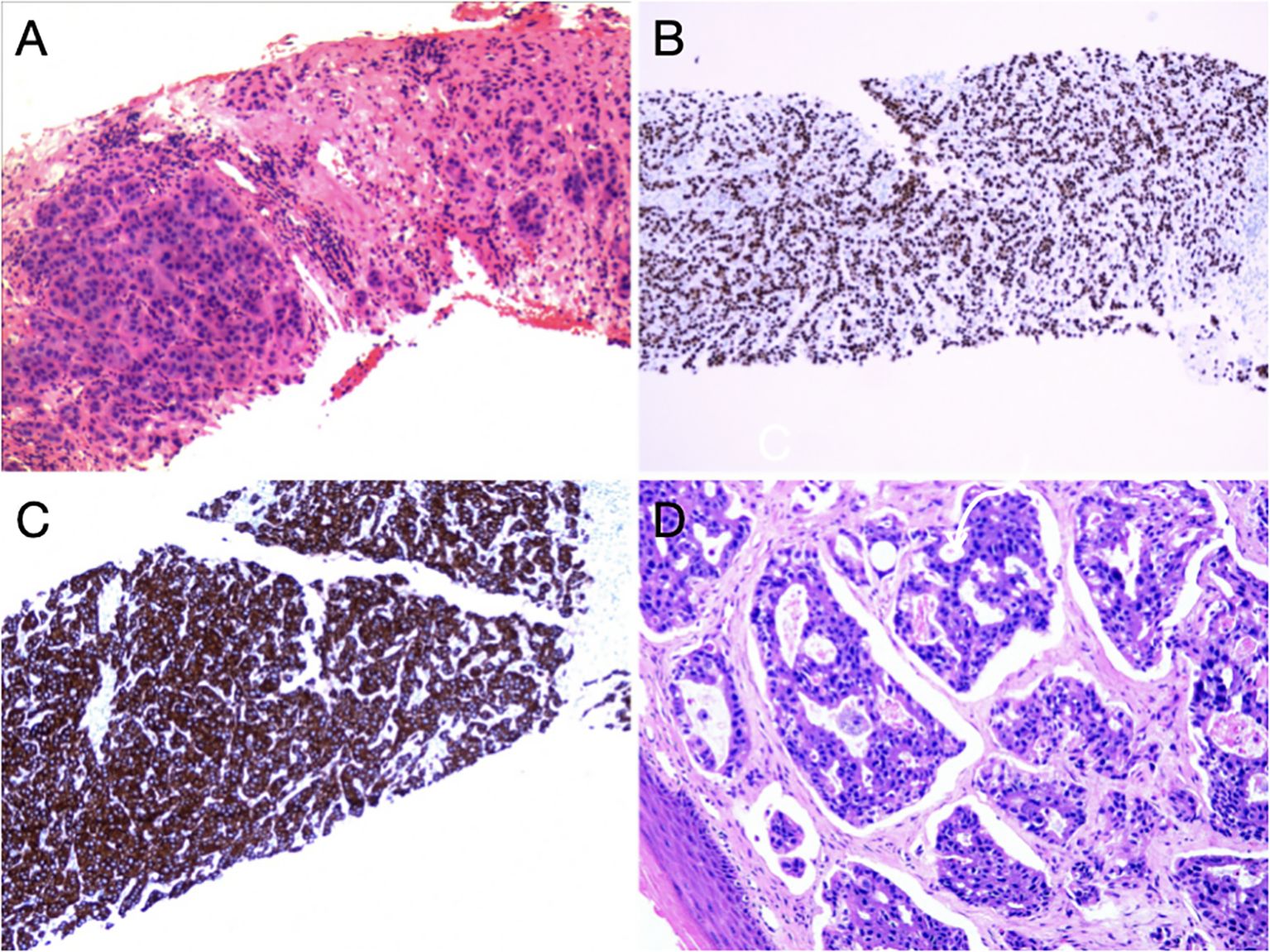
Figure 3. Pathohistological findings of inguinal lymph nodes and vulva: (A) HE staining of right inguinal lymph nodes. In fibrous connective tissue and lymphoid tissue, there were nests and cords of heterogeneous cells with enlarged nuclei and an increased nucleo-plasmatic ratio, and these features were consistent with those of malignant epithelial tumors. (B) Positive expression of GATA3. (C) Strongly positive expression of CK7. (D) HE staining of the vulva. There were cribriform hyperplasia and nest-shaped clusters of cells, local necrosis and vascular cancer thrombus in the tissues.
This patient had no history of breast malignancy, and no abnormal 18F-FDG uptake was observed in bilateral breasts. Finally, this patient was diagnosed with a primary MLA of the vulva, with multiple metastases in liver, bones and lymph nodes. At present, the patient is receiving palliative radiotherapy in local lesion of vulvar MLA (with a prescription dose of 50 Gy in 25 fractions) after 6 cycles of chemotherapy with paclitaxel and carboplatin. The outcome after chemotherapy was assessed as stable.
Discussion
It has been reported that the squamous cell carcinoma is the most common malignant tumor in the vulva, and accounts for 76% of vulvar cancers, while the adenocarcinoma only accounts for less than 2% of vulvar cancers (5). Van der Linden et al. (6) have observed that the majority of glandular malignancies in the vulva are primary tumors, which account for 55%. Primary adenocarcinomas in the vulva predominantly include extramammary Paget’s disease and sweat gland carcinomas. In addition, a rare form of adenocarcinoma is known as MLA of the vulva, which originates from putative mammary-like glands of the vulva, and exhibits a spectrum of pathological reactions that are similar to those in their mammary counterparts (7–9). Notably, the extramammary Paget’s disease can also present as a manifestation of underlying breast carcinoma, thus contributing to the differential diagnosis (10). The diagnosis of vulvar malignancies is obviously dependent on pathological evaluation (11). Since vulvar MLA and breast cancer have morphological similarities and similar aggressive behaviors, the criteria for diagnosing vulvar malignancy were defined by referring to breast carcinoma (12): in the absence of concurrent breast carcinoma, a primary adenocarcinoma of mammary-like glands of the vulva can be diagnosed when its morphological pattern is consistent with that of the breast carcinoma, with the presence of estrogen/progesterone receptors and positive markers such as CK 7 and GATA 3, which are common in breast cancers. In this case, the immunohistochemical results were measured against the above-mentioned criteria.
It is worth noting that HER-2, as one of the molecular typing markers of breast cancer, theoretically has an important reference value in the diagnosis and treatment of vulvar MLA (13). Unfortunately, the HER-2 expression was not detected in this case. Among previous reports (14), HER-2 expression was detected in a few cases, indicating a potential value of HER-2 in the diagnosis and treatment of vulvar MLA.
Ki67 is widely recognized as the best indicator of cell proliferation activity. Rolfe et al. (15) demonstrated that the increased expression of Ki67 reflects the changes in the proliferation during vulvar carcinogenesis, suggesting that Ki67 can serve as an indicator of tumor invasiveness. This patient is still under treatment, and attention will be paid to the correlation between tumor progression and ki67 expression. However, a study (16) has shown that Ki67 has no prognostic value in patients with vulvar Paget’s disease (PDV) or mammary Paget’s disease (PDB). Therefore, further study is needed to explore the correlation between Ki67 expression and this disease in the future.
The MLA originating from the vulva is exceedingly uncommon, and fewer than 40 cases have been reported since 1935 (14, 17). Only two patients in these reports underwent FDG PET/CT to assess the stage of the adenocarcinoma, and the results revealed that one patient had no metastatic disease, while the other patient exhibited distant metastases in lymph nodes and bones (18, 19). PET/CT plays an important role in the management of vulvar cancer. In the case reported by Patel D et al. (20), PET/CT exerted an important effect in staging of an identified vulvar cancer. Moreover, Triumbari EKA et al. (21) demonstrated that a negative preoperative PET/CT imaging may indicate no groin metastases in early-stage vulvar cancer patients, thus it’s not necessary for them to undergo sentinel node biopsy. And Treglia G et al. (22) reported a case of vulvar extramammary Paget’s disease (EMPD) restaged by PET/CT. In this case, PET/CT scan was helpful in locating the primary tumor. The manifestations of some inflammatory lesions and tumors on PET/CT are similar. Zhuang H et al. (23) have proved that the SUVs of delayed images of malignant lesions increase over time compared with those of earlier images, while the SUVs of the inflammatory lesions and benign lesions remain stable or decrease slightly over time. Another clinical trial (24) also showed that dual-time-point FDG-PET imaging has a potential to improve the accuracy of distinguishing between inflammation and tumor lesions. In this case, the manifestations of vulvar lesions on dual-time-point FDG PET/CT clearly showed this feature, and the SUVmax increased from 6.2 on the initial image to 10.2 on the delayed image. Furthermore, some studies (25, 26) have shown that the radiomics features of PET/CT images have an important value in lymph node assessment and prognosis prediction in patients with vulvar cancers.
Because of the rarity of this disease and the lack of definitive treatment guidelines, this type of cancers is currently staged and treated according to the treatment method for primary breast cancers (27, 28). Surgical excision with adjuvant therapies such as radiation, anthracycline-based chemotherapy and hormonal therapy is a common option (29). Benito V et al. (30) presented a case of an elderly patient with metastatic vulvar adenocarcinoma arising from mammary-like glands successfully treated with a combination of surgery and hormonal therapy. And Tanaka H et al. (31) reported a case of MLA successfully treated by paclitaxel weekly without excision. In addition, Butler B et al. (32) explored the application of sentinel lymph node mapping in patients with MLA, this technique has been almost exclusively applied in patients with breast carcinoma. It is very suitable to use sentinel lymph node mapping in the patients with vulvar cancers, which can predict the lymphatic drainage of the vulva, with a lower false negative rate and a substantially reduced adverse reaction rate (e.g. lymphedema) compared with the total groin lymph node dissection (33, 34). Tessier-Cloutier B et al. (35) observed that the breast carcinoma and MLA of the vulva had similar intrinsic luminal molecular subtypes, and thus proposed a new treatment strategy, that is, molecular subtyping of breast cancer can be performed to optimize individual treatment.
To sum up, we report a rare case of MLA of the vulva with multiple-organ involvement detected by 18F-FDG PET/CT. In this case, according to the distribution characteristics of lesions on PET/CT, the primary tumor was found, indicating the important role of PET/CT. Furthermore, dual-time-point PET/CT imaging demonstrates a great diagnostic value and potential significance in differential diagnosis. The effects of PET/CT imaging in prognostic prediction of MLA remain to be confirmed in future studies. This case is described based on the real world, and there are some deficiencies in the diagnosis of MLA of the vulva, such as insufficient collection of detailed medical histories and a lack of physical examination before PET/CT imaging, which have increased the difficulty of diagnosis. The study of this case reveals that optimized diagnostic strategy and appropriate imaging technique are especially important in the clinical diagnosis of rare diseases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
QS: Writing – original draft. BZ: Writing – review & editing. YG: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Nanjing University of Chinese Medicine (GrantNo.XZR2020006) and Special Project for Director Promotion of Jiangsu Province Hospital of Chinese Medicine (GrantNo.Y2021ZR26).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McMaster J, Dua A, Dowdy SC. Primary breast adenocarcinoma in ectopic breast tissue in the vulva. Case Rep Obstet Gynecol. (2013) 2013:721696. doi: 10.1155/2013/721696
2. Peiró V, Chiva L, González A, Bratos R, Alonso S, Márquez R, et al. Utility of the PET/CT in vulvar cancer management. Rev espanola medicina Nucl e imagen Mol. (2014) 33:87–92. doi: 10.1016/j.remn.2013.07.009
3. Dolanbay M, Ozcelik B, Abdulrezzak U, Serin IS, Kutuk MS, Uludag S. F-18 fluoro-D-glucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in planning of surgery and sentinel lymph node screening in vulvar cancers. Arch gynecology obstetrics. (2016) 293:1319–24. doi: 10.1007/s00404-015-3927-3
4. Cohn DE, Dehdashti F, Gibb RK, Mutch DG, Rader JS, Siegel BA, et al. Prospective evaluation of positron emission tomography for the detection of groin node metastases from vulvar cancer. Gynecologic Oncol. (2002) 85:179–84. doi: 10.1006/gyno.2002.6605
5. Wohlmuth C, Wohlmuth-Wieser I. Vulvar Malignancies: an interdisciplinary perspective. J Dtsch Dermatol Ges. (2019) 17:1257–76. doi: 10.1111/ddg.13995
6. van der Linden M, Schuurman M, Bulten J, van der Aa M, Massuger L, de Hullu J. Incidence and survival of glandular vulvar Malignancies in the Netherlands. Gynecol Oncol. (2017) 144:553–7. doi: 10.1016/j.ygyno.2017.01.020
7. van der Putte SC, van Gorp LH. Adenocarcinoma of the mammary-like glands of the vulva: a concept unifying sweat gland carcinoma of the vulva, carcinoma of supernumerary mammary glands and extramammary Paget’s disease. J Cutan Pathol. (1994) 21:157–63. doi: 10.1111/j.1600-0560.1994.tb00251.x
8. Van der Putte SC. Mammary-like glands of the vulva and their disorders. Int J Gynecol Pathol. (1994) 13:150–60. doi: 10.1097/00004347-199404000-00009
9. Desouki MM, Fadare O. Primary adenocarcinomas of the vulva and related structures: an enigmatic and diverse group of tumors. Semin Diagn Pathol. (2021) 38:71–84. doi: 10.1053/j.semdp.2020.09.011
10. Ohira S, Itoh K, Osada K, Oka K, Suzuki A, Osada R, et al. Vulvar Paget’s disease with underlying adenocarcinoma simulating breast carcinoma: case report and review of the literature. Int J Gynecol Cancer. (2004) 14:1012–7. doi: 10.1111/j.1048-891X.2004.14544.x
11. Michalski BM, Pfeifer JD, Mutch D, Council ML. Cancer of the vulva: a review. Dermatol Surg. (2021) 47:174–83. doi: 10.1097/DSS.0000000000002584
12. Irvin WP, Cathro HP, Grosh WW, Rice LW, Andersen WA. Primary breast carcinoma of the vulva: a case report and literature review. Gynecol Oncol. (1999) 73:155–9. doi: 10.1006/gyno.1998.5269
13. Lob S, Linsmeier E, Herbert SL, Schlaiss T, Kiesel M, Wischhusen J, et al. Prognostic effect of HER2 evolution from primary breast cancer to breast cancer metastases. J Cancer Res Clin Oncol. (2023) 149:5417–28. doi: 10.1007/s00432-022-04486-0
14. Niakan S, Love H, Cao Q, Kawar N. Primary invasive lobular carcinoma arising in mammary-like glands of the vulva managed with neoadjuvant trastuzumab-based chemotherapy, excision, and sentinel lymph node biopsy. Clin Case Rep. (2021) 9:118–22. doi: 10.1002/ccr3.3475
15. Rolfe KJ, Eva LJ, MacLean AB, Crow JC, Perrett CW, Reid WM. Cell cycle proteins as molecular markers of Malignant change in vulvar lichen sclerosus. Int J Gynecol Cancer. (2001) 11:113–8. doi: 10.1046/j.1525-1438.2001.011002113.x
16. Ellis PE, Fong LF, Rolfe KJ, Crow JC, Reid WM, Davidson T, et al. The role of P53 and Ki67 in Paget’s disease of the vulva and the breast. Gynecol Oncol. (2002) 86:150–6. doi: 10.1006/gyno.2002.6629
17. Abbott JJ, Ahmed I. Adenocarcinoma of mammary-like glands of the vulva: report of a case and review of the literature. Am J Dermatopathol. (2006) 28:127–33. doi: 10.1097/01.dad.0000171601.25315.2b
18. Deshmukh AA, Greenwalt J, Whitworth JM, Fox MD, Crozier JA. Mammary-like carcinoma of the vulva: A diagnostic challenge. Breast J. (2020) 26:1242–4. doi: 10.1111/tbj.13831
19. Ananthula A, Lockwood B, Savage J, Malak S, Chen C, Makhoul I, et al. Primary breast carcinoma of the vulva metastatic to lymph nodes and bones: a case report and literature review. Perm J. (2020) 24:19.084. doi: 10.7812/TPP/19.084
20. Patel D, Anderson TM, Lu Y. FDG-PET/CT of vulvar adenocarcinoma with diffuse metastases. Clin Nucl Med. (2016) 41:710–1. doi: 10.1097/RLU.0000000000001257
21. Triumbari EKA, de Koster EJ, Rufini V, Fragomeni SM, Garganese G, Collarino A. 18F-FDG PET and 18F-FDG PET/CT in vulvar cancer: a systematic review and meta-analysis. Clin Nucl Med. (2021) 46:125–32. doi: 10.1097/RLU.0000000000003411
22. Treglia G, Giovannini E, Bertagna F, Giovanella L, Malaggese M. An unusual case of metastatic extramammary Paget’s disease of the vulva identified by 18F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol. (2013) 32:402–3. doi: 10.1016/j.remn.2013.05.001
23. Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating Malignant from inflammatory processes. J Nucl Med. (2001) 42:1412–7.
24. Xiu Y, Bhutani C, Dhurairaj T, Yu JQ, Dadparvar S, Reddy S, et al. Dual-time point FDG PET imaging in the evaluation of pulmonary nodules with minimally increased metabolic activity. Clin Nucl Med. (2007) 32:101–5. doi: 10.1097/01.rlu.0000252457.54929.b7
25. Collarino A, Garganese G, Fragomeni SM, Pereira Arias-Bouda LM, Ieria FP, Boellaard R, et al. Radiomics in vulvar cancer: first clinical experience using (18)F-FDG PET/CT Images. J Nucl Med. (2018) 60:199–206. doi: 10.2967/jnumed.118.215889
26. Collarino A, Garganese G, Valdes Olmos RA, Stefanelli A, Perotti G, Mirk P, et al. Evaluation of dual-timepoint (18)F-FDG PET/CT imaging for lymph node staging in vulvar cancer. J Nucl Med. (2017) 58:1913–8. doi: 10.2967/jnumed.117.194332
27. Meddeb S, Rhim MS, Mestiri S, Kouira M, Bibi M, Khairi H, et al. Mammary-like adenocarcinoma of the vulva associated to Paget’s disease: a case report. Pan Afr Med J. (2014) 19:188. doi: 10.11604/pamj.2014.19.188.4521
28. Morais M, Vaz Silva J, Tavares MV. Diagnosis and management of primary vulvar adenocarcinoma of mammary gland type: report of two distinct cases. BMJ Case Rep. (2022) 15:6. doi: 10.1136/bcr-2021-245580
29. Lopes G, DeCesare T, Ghurani G, Vincek V, Jorda M, Glück S, et al. Primary ectopic breast cancer presenting as a vulvar mass. Clin Breast Cancer. (2006) 7:278–9. doi: 10.3816/CBC.2006.n.041
30. Benito V, Arribas S, Martínez D, Medina N, Lubrano A, Arencibia O. Metastatic adenocarcinoma of mammary-like glands of the vulva successfully treated with surgery and hormonal therapy. J Obstet Gynaecol Res. (2013) 39:450–4. doi: 10.1111/j.1447-0756.2012.01937.x
31. Tanaka H, Umekawa T, Nagao K, Ishihara A, Toyoda N. Adenocarcinoma of mammary-like glands in the vulva successfully treated by weekly paclitaxel. Int J Gynecol Cancer. (2005) 15:568–71. doi: 10.1111/j.1525-1438.2005.15328.x
32. Butler B, Leath CA 3rd, Barnett JC. Primary invasive breast carcinoma arising in mammary-like glands of the vulva managed with excision and sentinel lymph node biopsy. Gynecol Oncol Case Rep. (2013) 7:7–9. doi: 10.1016/j.gynor.2013.09.001
33. Collarino A, Fuoco V, Garganese G, Pereira Arias-Bouda LM, Perotti G, Manca G, et al. Lymphoscintigraphy and sentinel lymph node biopsy in vulvar carcinoma: update from a European expert panel. Eur J Nucl Med Mol Imaging. (2020) 47:1261–74. doi: 10.1007/s00259-019-04650-8
34. Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J Clin Oncol. (2012) 30:3786–91. doi: 10.1200/JCO.2011.41.2528
Keywords: mammary-like adenocarcinoma, vulvar adenocarcinoma, FDG, PET/CT, vulvar cancer
Citation: Song Q, Zhang B and Gu Y (2024) 18F-FDG PET/CT detection of primary mammary-like adenocarcinoma of the vulva with multiple metastases: a case report. Front. Oncol. 14:1441064. doi: 10.3389/fonc.2024.1441064
Received: 30 May 2024; Accepted: 25 October 2024;
Published: 14 November 2024.
Edited by:
Eric Edward Sigmund, New York University, United StatesReviewed by:
Chunyin Zhang, Southwest Medical University, ChinaGiorgia Garganese, Mater Olbia Hospital, Italy
Copyright © 2024 Song, Zhang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biyun Zhang, YmlhbmthMDgzMEAxMjYuY29t
 Qing Song1
Qing Song1 Biyun Zhang
Biyun Zhang