
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 03 October 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1439984
This article is part of the Research Topic Advances of Brain Metastasis in Breast Cancer View all 5 articles
 Zeyu Liu1
Zeyu Liu1 Ming Li1
Ming Li1 Ziyi Zhao2
Ziyi Zhao2 Aina Liu1*
Aina Liu1* Ping Sun1*
Ping Sun1*Background: The anti-angiogenic agent anlotinib offers a new treatment option for triple-negative breast cancer (TNBC) patients with brain metastases. This study aimed to evaluate the efficacy and safety of anlotinib in the treatment of TNBC patients with brain metastases.
Methods: Between October 2019 and April 2024, 29 TNBC patients with brain metastases who had failed prior therapy and were treated with anlotinib were retrospectively analyzed. The primary endpoint was central nervous system (CNS) progression-free survival (PFS), and secondary endpoints included overall survival (OS), intracranial disease control rate (iDCR), intracranial objective response rate (iORR), and safety.
Results: The median CNS PFS of 29 patients was 7.2 months (95% confidence interval [CI], 3.5-10.9 months), and the median OS was 10.2 months (95% CI, 5.6-14.8 months). The iORR and iDCR were 31.0% and 86.2%, respectively. Five patients (17.2%) experienced grade 3-4 adverse events (AEs), with bone marrow suppression (2/29, 6.9%) being the most common. Most AEs were clinically manageable, and no treatment-related death was observed.
Conclusion: Anlotinib demonstrated encouraging efficacy and manageable toxicity in the treatment of TNBC patients with brain metastases who had failed standard treatment.
Breast cancer remains the most prevalent form of cancer in women and is the second most common cause of the development of brain metastases after lung cancer (1, 2). Triple-negative breast cancer (TNBC) accounts for 15-20% of all breast cancer cases. Compared with other subtypes, metastases from TNBC are more common with a worse prognosis. Since patients with TNBC are unable to benefit from endocrine therapy or human epidermal growth factor receptor 2 (HER2)-targeted therapy, the standard of care for nonsurgical TNBC remains nonspecific chemotherapy. In addition to chemotherapeutic agents such as paclitaxel and cisplatin, the main treatments for TNBC include immunotherapeutic agents targeting PD-L1 such as atezolizumab and pembrolizumab (3) Thus, more effective therapies for TNBC remain to be developed. With the prolonged overall survival (OS) of patients with advanced breast cancer, the incidence of brain metastases has increased remarkably. Brain metastases occur in about 25% of patients with advanced breast cancer and even up to 40% of patients with TNBC, greatly influencing the quality of life of patients (4, 5).
The blood-brain barrier hinders the entry of numerous drugs into the brain, limiting the efficacy of drug therapy in patients with brain metastases (6). As a consequence of these constraints, radiotherapy or surgical interventions are typically utilized for treating patients with brain metastases. Therapies utilizing drugs for controlling brain metastases are still being investigated. Although traditional chemotherapy has not yielded optimal results in addressing brain metastases, tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor (VEGF) have proved to be efficacious in controlling brain metastases.
Neovascularization is a key step in the proliferation of malignant tumors, making anti-angiogenesis an important strategy in anti-tumor therapy. Tumor growth requires an adequate blood supply to provide nutrients. It has been shown that angiogenesis not only affects tumor growth but also is an important factor in promoting tumor metastases. Thus, it is possible to inhibit tumor growth by suppressing angiogenesis (7, 8). The growth and metastasis of breast cancer are dependent on angiogenesis, and the expression of VEGF in breast cancer tissues far exceeds that in normal tissues (9). Moreover, TNBC had significantly higher VEGF expression levels than patients with non-TNBC. VEGF secreted by breast cancer tissues acts on vascular endothelial growth factor receptor (VEGFR), which promotes the division and proliferation of vascular endothelial cells, and induces tumor angiogenesis, thus providing sufficient blood supply for breast cancer progression. Bevacizumab is an anti-angiogenic agent that is commonly used in metastatic breast cancer. Previous studies have shown that bevacizumab improves central nervous system (CNS) objective response rate (ORR) and median progression-free survival (PFS) in patients with brain metastases from breast cancer, but along with a high risk of grade 3-4 toxicity (10, 11).
Anlotinib is a novel small-molecule TKI with anti-angiogenic properties and the ability to cross the blood-brain barrier. It targets more sites than bevacizumab and exhibits better systemic efficacy than bevacizumab. The primary targets of anlotinib include VEGFR, fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and stem cell growth factor receptor (c-Kit) (12–14). Anlotinib can reduce the levels of proangiogenic factors and enhance the expression of immune cell adhesion molecules, chemokines, and their receptors. Additionally, it can effectively impede both tumor angiogenesis and growth (12). As a result, it exhibits encouraging efficacy in anti-tumor therapy with minimal toxicity. Besides, anlotinib is administered orally and is therefore more acceptable to patients than bevacizumab, without the risk of injections. A phase II study has established the positive impact of anlotinib in addressing HER2-negative advanced or metastatic breast cancer (15). Furthermore, anlotinib also shows promising efficacy in brain metastases from lung cancer (16). Recently, it has been observed to be effective in treating TNBC patients with brain metastases. In this context, a retrospective study was conducted to illustrate brain metastases from TNBC. The results showed that anlotinib yielded outstanding anti-tumor activity without inducing severe treatment-related adverse events (AEs).
This was a single-center retrospective study conducted in The Affiliated Yantai Yuhuangding Hospital of Qingdao University. From October 2019 to April 2024, TNBC patients with brain metastases who had received second-line or subsequent treatment with anlotinib at our hospital were included and analyzed for efficacy and safety. Eligible patients were 30-70 years old and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Patients with leptomeningeal disease were not included in this study. Steroids were used in some patients due to severe cases of cerebral edema. Patients who have been previously submitted to CNS radiotheraphy for more than six months and have experienced progression of intracranial lesions will be included in the study. The patterns of technique used in terms of radiotheraphy depends on the patient’s intracranial lesions; patients with 1-2 intracranial lesions were treated with Stereotactic Radio-Surgery (SRS), patients with more than 5 intracranial lesions were treated with Whole Brain Radiotheraphy (WBRT), and patients with 3-5 intracranial lesions were given the option of using either SRS or WBRT, depending on the specifics of the lesions.
All patients received anlotinib monotherapy or in combination with chemotherapy. The primary chemotherapeutic agents included capecitabine (1000 mg/m2 twice daily for 14 days followed by 7 days of rest), gemcitabine (1000 mg/m2 on days 1 and 8), nab-paclitaxel (125 mg/m2 on days 1 and 8), vinorelbine (25 mg/m2 on days 1 and 8), and eribulin (1.4 mg/m2 on days 1 and 8). Anlotinib was administered at a dosage of 12 mg, 10 mg, or 8 mg orally on days 1-14 of a 21-day cycle. The recommended dosage for anlotinib was 12 mg. When the patient experienced AEs of grade 2 or higher, anlotinib was suspended, resumed when the AEs dropped below grade 2, and the dose of anlotinib was adjusted downward to 10 mg, and when this event occurred a second time, the dose of anlotinib was adjusted downward to 8 mg.
Efficacy was assessed every two cycles according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). Response to treatment was categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The primary endpoint was CNS PFS, which was defined as the time from the start of treatment to intracranial disease progression or death. Secondary endpoints included OS (the time from the start of treatment to death from any cause), intracranial ORR (iORR; the proportion of patients who had an intracranial PR [iPR] or iCR at the best response), intracranial disease control rate (iDCR; the proportion of patients who had an iPR, iCR, or iSD at the best response), and safety.
All data were statistically analyzed using SPSS version 27.0 (IBM, New York, USA). PFS and OS were estimated by the Kaplan-Meier method. The effect of clinical factors on PFS and OS was examined by a stratified Cox proportional hazards regression model. The significance level was set at p<0.05.
The median age of the 29 patients was 57 years (95% CI, 52.3-59.5 years). The majority of patients were younger than 60 years of age (19/29, 65.5%), had an ECOG performance status of 0-1 (24/29, 82.8%), had multiple brain metastases (16/29, 55.2%), had ≥3 metastatic sites (21/29, 72.4%), and received anlotinib as third-line or subsequent treatment (20/29, 69.0%). The clinical characteristics of the 29 patients are summarized in Table 1.
The treatment response to anlotinib is summarized in Table 2. The median CNS PFS of 29 patients was 7.2 months (95% confidence interval [CI], 3.5-10.9 months; Figure 1A), and the median OS was 10.2 months (95% CI, 5.6-14.8 months; Figure 1B). Systemic ORR and DCR were 34.5% and 82.8%, respectively. Among the 29 patients, 1 achieved an iCR, 8 achieved an iPR, 16 had iSD, and 4 had iPD, with an iORR of 31.0% and an iDCR of 86.2%. In addition, Among 16 patients who had iSD, 9 patients had multiple BMs, 7 patients had single BM, 2 patients had received prior antinagiogenic therapies. Among 8 patients who had iPR, 3 patients had multiple BMs, 5 patients had single BM, 1 patient had received prior antinagiogenic therapies. The patient with iCR had multiple BMs and had not received prior antinagiogenic therapies. Figure 2 shows the typical and clear magnetic resonance imaging (MRI) images of one patient before and after the administration of anlotinib. This patient has never been previously submitted to CNS radiotheraphy treatment.
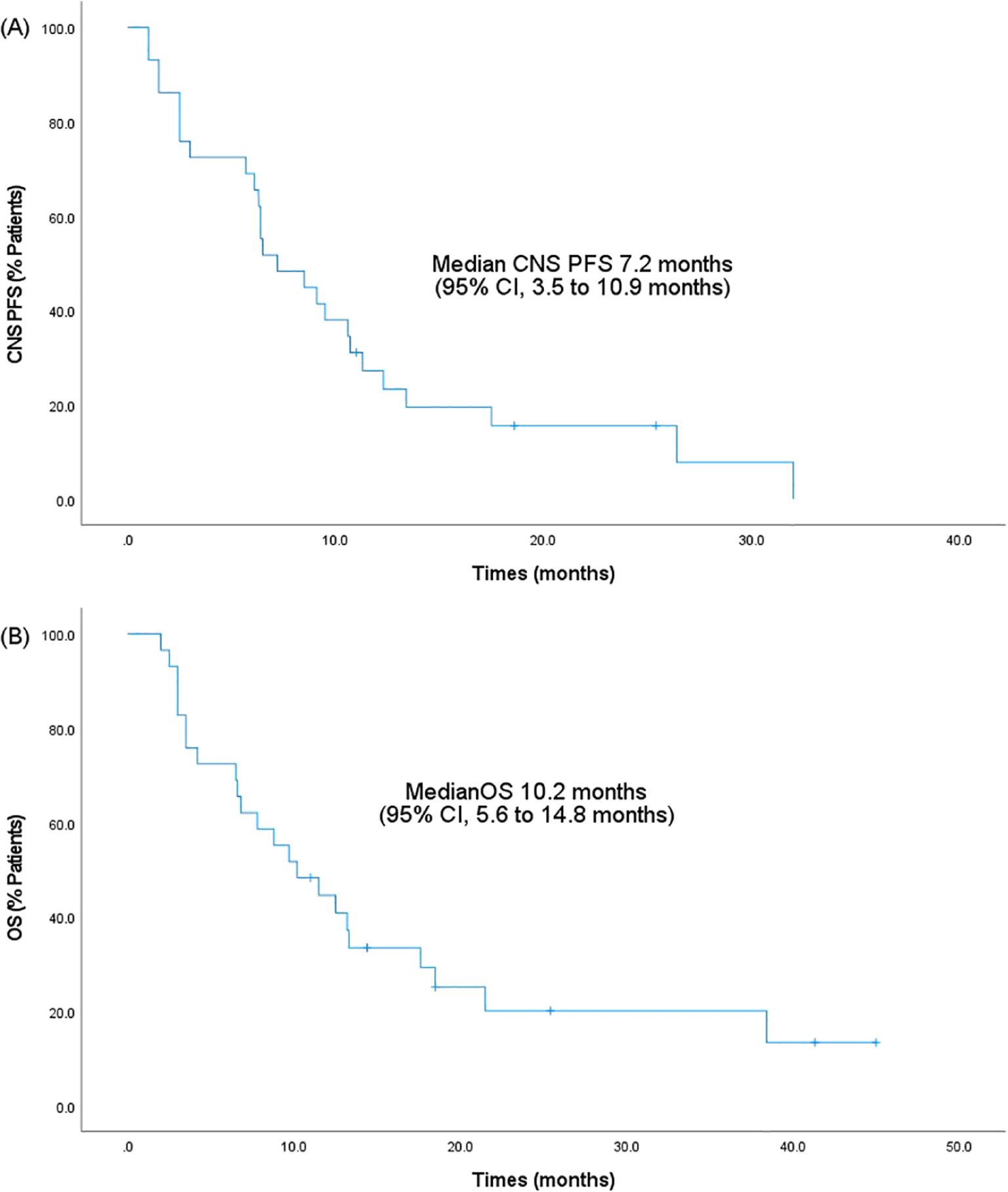
Figure 1. Kaplan-Meier survival analysis. (A) CNS PFS. (B) OS. PFS, progression-free survival; 95% CI, 95% confidence intervals; OS, overall survival; CNS, central nervous system.
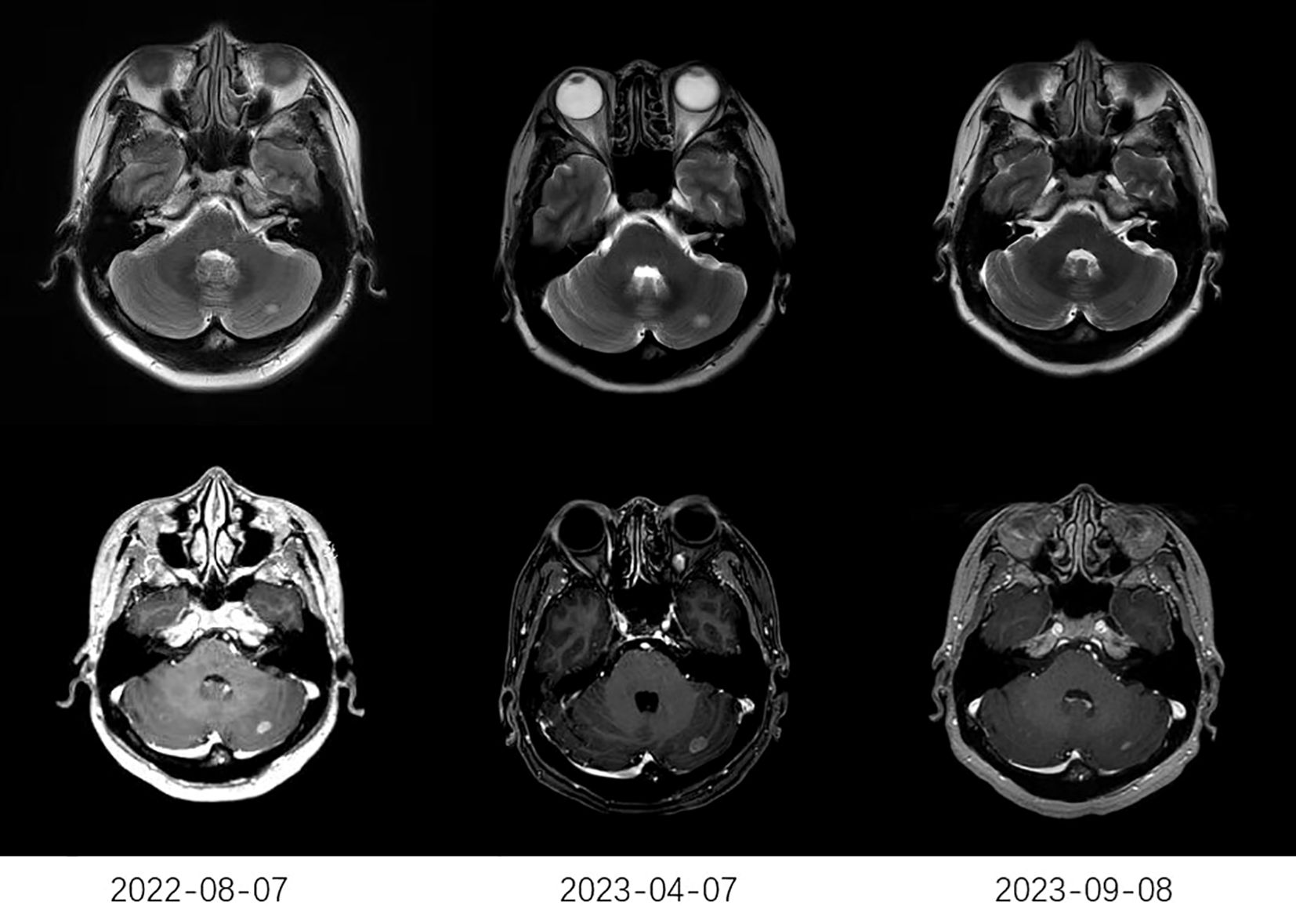
Figure 2. The typical MRI images of one patient. The MRI on April 7, 2023, showed a larger lesion than that on August 7, 2022. After receiving anlotinib (2023-09-08), MRI showed a significant reduction in the lesion compared to that before receiving anlotinib (2023-04-07). MRI, typical magnetic resonance imaging.
A stratified Cox proportional hazards regression model was performed to assess the effect of different clinical factors on CNS PFS and OS. All variables that were significant at p<0.05 in univariate analysis were included in the multivariate analysis (Tables 3, 4). ECOG performance status, treatment regimens, and prior anti-angiogenesis therapy after metastases were statistically significant in the univariate analysis for the CNS PFS (all p<0.05). After including the above clinical factors in the multivariate analysis, ECOG performance status of 2 (hazard ratio [HR]=4.8; 95% CI, 1.3-17.5; p=0.02) and prior anti-angiogenesis therapy after metastases (HR=4.4; 95% CI, 1.4-13.5; p=0.01) were proved to be independent and meaningful unfavorable prognostic factors for the CNS PFS. In the univariate analysis for the OS, treatment regimens, prior anti-angiogenesis therapy after metastases, and symptomatic brain metastases were estimated to be statistically significant (all p<0.05). After including the above clinical factors in multivariate analysis, prior anti-angiogenesis therapy after metastases (HR=4.3; 95% CI, 1.3-14.1; p=0.02) and symptomatic brain metastases (HR=4.2; 95% CI, 1.5-12.1; p=0.01) were independent and meaningful prognostic factors for the OS.
Kaplan-Meier survival analysis was performed based on significant predictors in the multivariate analysis. As shown in Figure 3, shorter CNS PFS was more likely to occur in patients with an ECOG performance status of 2 (6.1 vs. 9.1 months, p=0.01) or in patients who had received prior anti-angiogenesis therapy after brain metastases development (1.5 vs. 9.1 months, p=0.01). Besides, the OS was significantly shorted in cases with symptomatic brain metastases (6.5 vs. 13.3 months, p=0.01) or in cases who had received prior anti-angiogenesis therapy after metastases (4.2 vs. 12.5 months, p=0.04).
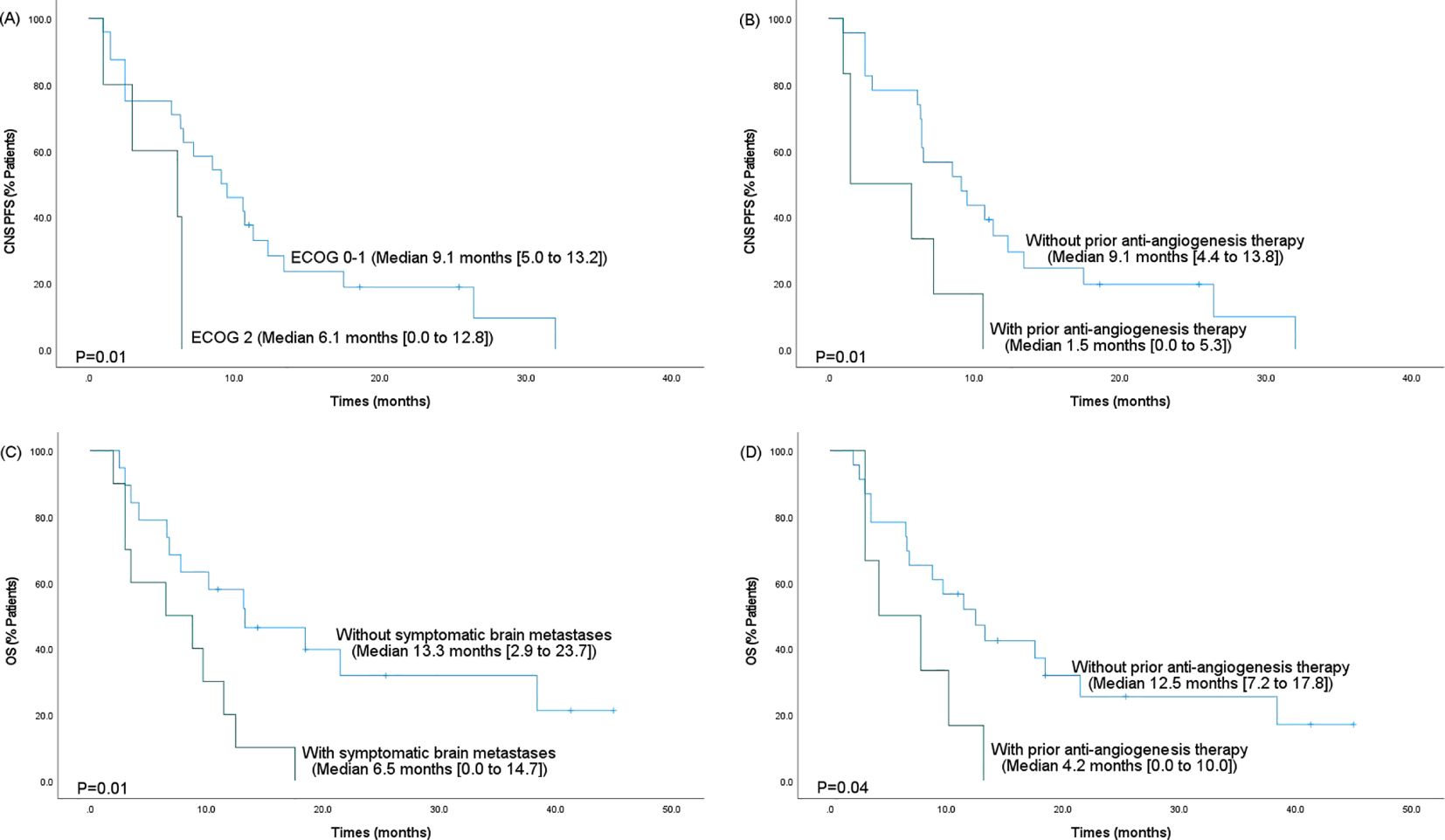
Figure 3. Kaplan-Meier survival analysis for the CNS PFS and OS based on significant predictors in the multivariate analysis. (A) The CNS PFS based on ECOG performance status. (B) The CNS PFS based on prior anti-angiogenesis therapy after metastases. (C) The OS based on the presence of symptomatic brain metastases. (D) The OS based on prior anti-angiogenesis therapy after metastases. PFS, progression-free survival; 95% CI, 95% confidence intervals; OS, overall survival; CNS, central nervous system.
AEs that occurred during treatment with anlotinib in the 29 patients were summarized in Table 5. The most common AEs of all grades were bone marrow suppression (8/29, 27.6%), fatigue (7/29, 24.1%), hypertension (6/29, 20.7%), gastrointestinal response (6/29, 20.7%), and hand-foot syndrome (6/29, 20.7%). A total of 5 patients (17.2%) experienced grade 3-4 AEs. The most common grade 3-4 AE was bone marrow suppression (2/29, 6.9%). No treatment-related death was observed.
The presence of brain metastases from breast cancer is associated with a poor prognosis, and the incidence of this complication has been increasing in recent years (4). Given the challenge of penetrating the blood-brain barrier with traditional chemotherapeutic drugs, prevailing treatment options for brain metastases mainly include surgical interventions and radiotherapy. Exploration of drug-based therapies for optimal and sustained control of brain metastases remains a subject of ongoing research.
Anti-angiogenic agents represented by bevacizumab have shown promising efficacy in the treatment of brain metastases from breast cancer, suggesting that anti-angiogenesis therapy can inhibit the progression of brain metastases (10). Meanwhile, previous studies have demonstrated the favorable efficacy of anti-angiogenic agents such as bevacizumab and apatinib in TNBC (17, 18). However, the incidence of AEs related to bevacizumab is high and bevacizumab has difficulty crossing the blood-brain barrier (11, 19), as well as fewer targets for apatinib than anlotinib. TKIs have exhibited promising efficacy in the management of brain metastases. The necessity for agents to cross the blood-brain barrier to reach the CNS has limited the employment of several chemotherapeutics and targeted agents for CNS diseases. Whole-brain radiotherapy has been reported to be associated with a risk of neurotoxicity as well as stereotactic radiosurgery may be correlated with an increased risk of radionecrosis (20). Thus, there is an urgent need to develop novel and more effective drugs against brain metastases. Increasing evidence suggests the potential role of TKIs in controlling tumors within the CNS (21). Furthermore, results from the post hoc analysis of a phase III trial have suggested that anlotinib demonstrates activity in the brain and plays a potential role in the treatment of intracranial lesions (16). In this case, anlotinib has exhibited notable efficacy against brain metastases from breast cancer.
As of the data cut-off on March 2024, to the best of our knowledge, this is the first study to explore the efficacy and safety of anlotinib in the treatment of brain metastases from TNBC. In our study, the median CNS PFS was 7.2 months (95% CI, 3.5-10.9 months), and the median OS was 10.2 months (95% CI, 5.6-14.8 months). Moreover, the iORR and iDCR were 31.0% and 86.2%, respectively. These results indicated that anlotinib exhibited activity in the brain and had a positive impact on brain metastases from TNBC. Nevertheless, due to the limited availability of pertinent research, further investigations are required to gain a deepen knowledge of the specific mechanism of action. Furthermore, as a retrospective study, our study has limitations and still lacks the diagnosis of CNS injury at different moments of oncological treatment.
Few prior studies have addressed the treatment of brain metastases from breast cancer with anlotinib. In the study by Qian et al. on the efficacy and safety of anlotinib in metastatic breast cancer, 15 patients with brain metastases were included. The results showed that 7 patients achieved an SD, 5 patients had a PR, and 3 patients experienced a PD, with an ORR of 33%, a DCR of 80%, and a CNS PFS of 9.4 months, which were consistent with our findings (22). However, there was no distinction for TNBC in the study by Qian et al. Furthermore, the role of anlotinib in controlling various other intracranial tumors has been explored in several studies. The post hoc analysis of a phase III trial showed that anlotinib prolonged PFS and OS in non-small cell lung cancer (NSCLC) patients with brain metastases compared with placebo, while patients without brain metastases showed comparable improvements. The iORR was 14.3% and the iDCR was 85.7% in patients with brain metastases who were treated with anlotinib, indicating effective control of brain metastases. Additionally, anlotinib was associated with a higher incidence of neural toxicities (18.4% vs. 8.4%) and neurological symptoms (49.3% vs. 35.7%) than placebo, with no association with infarction or cerebral hemorrhage (16). Some studies and case reports have shown that the combination of anlotinib and radiotherapy may provide additional survival benefits in NSCLC patients with brain metastases. This combination therapy is effective in controlling both brain metastases and associated edema (23–27). Anlotinib can substantially reduce the proliferation of tumor microvessels, alleviate hypoxia in the tumor microenvironment, and enhance the efficacy of radiotherapy. The relief of hypoxia by anlotinib may result from vascular normalization, direct inhibition, or a combination of both (28–31). Early case reports have indicated that combination therapy with anlotinib and other drugs, including immune checkpoint inhibitors such as toripalimab and durvalumab, has proven to be effective in controlling brain metastases from small-cell lung cancer (32, 33). Moreover, anlotinib shows the ability of reducing or avoiding steroids and effectively mitigating cerebral edema caused by brain metastases (34). In the studies mentioned above, anlotinib has displayed an acceptable safety profile. Overall, these studies have shown that anlotinib is effective against brain metastases from lung cancer, suggesting its potential role in brain metastases from breast cancer. Some authors have found that the combination of anlotinib with other treatments can achieve better therapeutic effects on glioblastoma (35–37), suggesting the ability of anlotinib to penetrate the blood-brain barrier to act on intracranial tumors. Hence, we argued that in this retrospective cohort, anlotinib cross the blood-brain barrier and inhibit the growth of brain metastases, leading to a positive outcome from patients with brain metastases from breast cancer. Moreover, AEs that occurred during the treatment with anlotinib were acceptable in our study. However, a more detailed and precise understanding of the treatment mechanism requires further investigation.
The number of cases outlined in our description was relatively limited and the duration of anlotinib treatment is not entirely uniform, making the potential for some deviation in the data. However, based on the current study and other previous studies, it is evident that anlotinib holds significant promise in the treatment of brain metastases from TNBC. Moreover, anlotinib was generally safe and well tolerated, with a low incidence of treatment-related AEs.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The study was reviewed and approved by the Ethics Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University (No. 2024-249). Due to the retrospective nature of the study, informed consent was waived. The MRI images used in the manuscript were obtained with the patient's written informed consent.
ZL: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. ML: Data curation, Writing – original draft. ZZ: Writing – original draft. AL: Conceptualization, Writing – review & editing. PS: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Special Fund for Clinical Research on Dynamic Monitoring of Lymphocyte Subsets by Flow Cytometry of Shandong Medical Association (No. YXH2022ZX03218).
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal arlix A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. (2004) 22:2865–72. doi: 10.1200/JCO.2004.12.149
3. Li Y, Zhang H, Merkher Y, Chen L, Liu N, Leonov S, et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. (2022) 15:121. doi: 10.1186/s13045-022-01341-0
4. Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. (2019) 121:991–1000. doi: 10.1038/s41416-019-0619-y
5. Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. (2015) 143:471–8. doi: 10.1309/AJCPYO5FSV3UPEXS
6. Mo F, Pellerino A, Soffietti R, Rudà R. Blood-brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci. (2021) 22:12654. doi: 10.3390/ijms222312654
7. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. (1971) 285:1182–6. doi: 10.1056/NEJM197111182852108
8. Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. (2012) 72:1909–14. doi: 10.1158/0008-5472.CAN-11-3406
9. Yoshiji H, Gomez DE, Shibuya M, Thorgeirsson UP. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res. (1996) 56:2013–6. doi: 10.1007/s002620050279
10. Leone JP, Emblem KE, Weitz M, Gelman RS, Schneider BP, Freedman RA, et al. Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res. (2020) 22:131. doi: 10.1186/s13058-020-01372-w
11. Dickler MN, Barry WT, Cirrincione CT, Ellis MJ, Moynahan ME, Innocenti F, et al. Phase III trial evaluating letrozole as first-line endocrine therapy with or without bevacizumab for the treatment of postmenopausal women with hormone receptor-positive advanced-stage breast cancer: CALGB 40503 (Alliance). J Clin Oncol. (2016) 34:2602–9. doi: 10.1200/JCO.2015.66.1595
12. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. (2018) 11:120. doi: 10.1186/s13045-018-0664-7
13. Lin B, Song X, Yang D, Bai D, Yao Y, Lu N, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. (2018) 654:77–86. doi: 10.1016/j.gene.2018.02.026
14. Sun Y, Niu W, Du F, Du C, Li S, Wang J, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. (2016) 9:105. doi: 10.1186/s13045-016-0332-8
15. Hu N, Si Y, Yue J, Sun T, Wang X, Jia Z, et al. Anlotinib has good efficacy and low toxicity: a phase II study of anlotinib in pre-treated HER-2 negative metastatic breast cancer. Cancer Biol Med. (2021) 18:849–59. doi: 10.20892/j.issn.2095-3941.2020.0463
16. Jiang S, Liang H, Liu Z, Zhao S, Liu J, Xie Z, et al. The impact of anlotinib on brain metastases of non-small cell lung cancer: post hoc analysis of a phase III randomized control trial (ALTER0303). Oncologist. (2020) 25:e870–4. doi: 10.1634/theoncologist.2019-0838
17. Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. (2011) 29:1252–60. doi: 10.1200/JCO.2010.28.0982
18. Li DD, Tao Z, Wang B, Wang L, Cao J, Hu X, et al. Apatinib plus vinorelbine versus vinorelbine for metastatic triple-negative breast cancer who failed first/second-line treatment: the NAN trial. NPJ Breast Cancer. (2022) 8:110. doi: 10.1038/s41523-022-00462-6
19. Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. (2014) 15:1269–78. doi: 10.1016/S1470-2045(14)70439-5
20. Hoffmann C, Distel L, Knippen S, Gryc T, Schmidt MA, Fietkau R, et al. Brain volume reduction after whole-brain radiotherapy: quantification and prognostic relevance. Neuro Oncol. (2018) 20:268–78. doi: 10.1093/neuonc/nox150
21. Franchino F, Rudà R, Soffietti R. Mechanisms and therapy for cancer metastasis to the brain. Front Oncol. (2018) 8:161. doi: 10.3389/fonc.2018.00161
22. Qian Y, Lou K, Zhou H, Zhang L, Yuan Y. Efficacy and safety of anlotinib-based treatment in metastatic breast cancer patients. Front Oncol. (2022) 12:1042451. doi: 10.3389/fonc.2022.1042451
23. Zhong Q, Liu Z. Efficacy and safety of anlotinib in patients with advanced non-small cell lung cancer: A real-world study. Cancer Manag Res. (2021) 13:4115–28. doi: 10.2147/CMAR.S304838
24. Kong C, Yu S, Qian P, Song X, Wen J, Jiang M, et al. Anlotinib combined with whole-brain radiotherapy in non-small cell lung cancer with multiple brain metastases that progressed or developed after at least one lines of prior treatment. Front Oncol. (2023) 13:1169333. doi: 10.3389/fonc.2023.1169333
25. He Z, Liu J, Ma Y, Jiang H, Cui Z, Wang G, et al. Anlotinib combined with cranial radiotherapy for non-small cell lung cancer patients with brain metastasis: A retrospectively, control study. Cancer Manag Res. (2021) 13:6101–11. doi: 10.2147/CMAR.S319650
26. Liu J, Xu J, Ye W, Zhong W, Zhang X, Mao J, et al. Whole-brain radiotherapy combined with anlotinib for multiple brain metastases from non-small cell lung cancer without targetable driver mutation: A single-arm, phase II study. Clin Med Insights Oncol. (2022) 16:11795549221079185. doi: 10.1177/11795549221079185
27. Wang Y, Wang X, Guan Y, Song Y, Zhuang H, Wang E, et al. Stereotactic radiosurgery combined with anlotinib for limited brain metastases with perilesional edema in non-small cell lung cancer: Rvision-001 study protocol. Thorac Cancer. (2020) 11:1361–4. doi: 10.1111/1759-7714.13386
28. Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. (2018) 109:1207–19. doi: 10.1111/cas.13536
29. Lu J, Zhong H, Chu T, Zhang X, Li R, Sun J, et al. Role of anlotinib-induced CCL2 decrease in anti-angiogenesis and response prediction for nonsmall cell lung cancer therapy. Eur Respir J. (2019) 53:1801562. doi: 10.1183/13993003.01562-2018
30. Shi J, Zhang Y, Wang J, Li J, Li Z. Anlotinib combined with chemoradiotherapy exhibits significant therapeutic efficacy in esophageal squamous cell carcinoma. Front Oncol. (2020) 10:995. doi: 10.3389/fonc.2020.00995
31. Li PJ, Lai S, Jin T, Ying H, Chen Y, Zhang P, et al. Radiotherapy opens the blood-brain barrier and synergizes with anlotinib in treating glioblastoma. Radiother Oncol. (2023) 183:109633. doi: 10.1016/j.radonc.2023.109633
32. Wu Y, Zhang T, Liu Y, Wang J, Bi N. Anlotinib combined with durvalumab in a patient with recurrent multifocal brain metastases of small cell lung cancer after definitive concurrent chemoradiotherapy and palliative radiotherapy of the lung and brain: a case report. Ann Palliat Med. (2021) 10:2379–86. doi: 10.21037/apm
33. Huang F, Tang J, Lou J, Wang Q, Ma K, Qiao R, et al. Intracranial complete response to toripalimab and anlotinib in a patient with recurrent brain metastases of small cell lung cancer after failure of second-line maintenance therapy: a case report. Transl Cancer Res. (2022) 11:3337–42. doi: 10.21037/tcr
34. Zhuang H, Wang Y, Cheng C, Shi S. The efficacy of anlotinib instead of glucocorticoids for edema induced by brain metastases in NSCLC patients with anti-PD1/PDL-1 immunotherapy. Neuro Oncol. (2021) 23:169–71. doi: 10.1093/neuonc/noaa236
35. Lv Y, Zhang J, Liu F, Song M, Hou Y, Liang N, et al. Targeted therapy with anlotinib for patient with recurrent glioblastoma: A case report and literature review. Med (Baltimore). (2019) 98:e15749. doi: 10.1097/MD.0000000000015749
36. Shen F, Li J, Liu F, Sun N, Qiu X, Ding W, et al. The efficacy and adverse effects of anlotinib in the treatment of high-grade glioma: A retrospective analysis. Front Oncol. (2023) 13:1095362. doi: 10.3389/fonc.2023.1095362
Keywords: brain metastases, triple-negative breast cancer, anlotinib, tyrosine kinase inhibitor, antiangiogenesis, side effect
Citation: Liu Z, Li M, Zhao Z, Liu A and Sun P (2024) Efficacy and safety of anlotinib for triple-negative breast cancer with brain metastases. Front. Oncol. 14:1439984. doi: 10.3389/fonc.2024.1439984
Received: 28 May 2024; Accepted: 11 September 2024;
Published: 03 October 2024.
Edited by:
Wenjie Lv, Shanghai Jiao Tong University, ChinaReviewed by:
Alessia Pellerino, University Hospital of the City of Health and Science of Turin, ItalyCopyright © 2024 Liu, Li, Zhao, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aina Liu, bmFuYTQzMTJAc2luYS5jb20=; Ping Sun, c3VucGluZzIwMDM5QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.