- The First Affiliated Hospital of Guangxi University of Science and Technology, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
Introduction: This meta-analysis aims to evaluate the complications associated with prepectoral breast reconstruction (PBR) compared to subpectoral breast reconstruction (SBR) in patients diagnosed with breast cancer.
Materials and methods: A comprehensive search was performed in four databases, including Medline, Embase, Web of Science and CENTRAL, to collect literature published up until December 31, 2024. In addition, we conducted a thorough manual examination of the bibliographies of the identified papers, as well as pertinent reviews and meta-analyses. We conducted a search on three clinical trial registries, namely ClinicalTrials.gov, Controlled-trials.com, and Umin.ac.jp/ctr/index.htm. Meta-analyses were conducted on total complications, hematoma, infection, wound healing issues, necrosis, capsular contracture, rippling, animation deformity, and reoperation.
Results: A total of 40 studies were included in the meta-analysis. Compared with SBR, PBR significantly reduced the incidence of animated malformations (OR=0.37, 95% CI: 0.19 to 0.70, P=0.003, I ²=12%), but increased the incidence of ripples (OR=2.39, 95% CI: 1.53 to 3.72, P=0.0001, I ²=10%) and seroma (OR=1.55, 95% CI: 1.02 to 2.35, P=0.04, increasing I ²=70%).
Conclusions: Our findings indicate that PBR and SBR have comparable safety profiles, with similar total complication rates. Specifically, PBR is more likely to cause rippling and seroma, whereas SBR is more prone to causing animation deformity.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024565837, identifier CRD42024565837.
1 Introduction
Breast cancer is a prevalent malignancy among women, ranking highest in newly diagnosed cases of female cancers. The incidence of breast cancer in women increases with age, particularly post-menopause, posing a significant threat to women’s health and well-being. According to a 2021 World Health Organization survey on breast cancer incidence, approximately 2.3 million women were diagnosed with breast cancer globally in 2020, with a mortality rate of about 30%. Between 2016 and 2020, around eight million women were diagnosed with breast cancer (1). In developed regions such as North America, Europe, and Australia, breast cancer remains a common cancer among women, with high annual incidence rates. Conversely, certain Asian and African countries have lower breast cancer incidence rates.
Historically, the primary treatment for breast cancer involved the highly invasive radical mastectomy, which often resulted in significant psychological distress, including feelings of humiliation and diminished self-worth due to societal stigma. This psychological burden frequently led to low self-esteem, social withdrawal, and delays in seeking necessary follow-up care (2). Recent advancements in surgical techniques, including breast-conserving surgery, breast reconstruction post-mastectomy, and breast cancer endoscopy, have improved survival rates and reduced the psychological burden on patients (3–5).
Prosthetic breast reconstruction is a major method for breast reconstruction following breast cancer surgery (6). At present, the positions for implant placement can be divided into anterior pectoralis major and inferior pectoralis major. Prepectoral Breast Reconstruction (PBR): Involves placing the implant above the pectoralis major muscle, directly under the skin and subcutaneous tissue. PBR has gained popularity with the advent of advanced surgical techniques and improved implant technology. This method avoids disruption of the pectoralis major muscle, potentially reducing postoperative pain. PBR may lead to a quicker recovery and less postoperative discomfort, but it requires adequate soft tissue coverage and careful patient selection to minimize the risk of complications such as implant visibility and rippling. Subpectoral Breast Reconstruction (SBR): Involves placing the implant beneath the pectoralis major muscle. Traditionally, it has been the standard approach due to the muscle providing additional coverage and support for the implant. This method may reduce the risk of implant visibility and palpable edges, potentially leading to more natural aesthetic outcomes. However, SBR can be associated with postoperative pain and longer recovery times due to the manipulation of the pectoralis major muscle. This approach can be classified into immediate reconstruction, delayed reconstruction, and phased immediate reconstruction based on the timing, and into autologous tissue reconstruction, prosthesis reconstruction, and a combination of both based on the materials used (7).
Subpectoral breast reconstruction has traditionally been favored due to its provision of better vascularized soft tissue coverage. However, plastic surgeons have increasingly preferred prepectoral breast reconstruction to reduce animation deformity, perioperative narcotic use, and chest wall morbidity (2, 8). PBR was widely used in the early stages of alloplastic surgery but posed risks such as implant exposure, skin breakdown, wrinkling, rippling, palpability, visibility, and misalignment (6, 9). While the subpectoral plane offers advantages over the prepectoral plane, it also presents challenges such as pain, mobility issues, and insufficient breast projection (7, 10). The choice between SBR and PBR remains a topic of ongoing debate among surgeons. While SBR has been the traditional approach with a well-documented safety profile, PBR offers potential advantages in terms of reduced postoperative pain and quicker recovery. However, PBR’s long-term outcomes and complication rates compared to SBR are not yet fully understood, necessitating further research. By synthesizing available data, a meta-analysis can inform evidence-based clinical practices, guiding surgeons in making informed decisions about the most appropriate reconstruction technique for their patients.
2 Materials and methods
2.1 Search strategy
This meta-analysis adhered to the 2020 guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The study was registered with PROSPERO (registration number CRD42024565837). We conducted a comprehensive search of PubMed, Embase, Web of Science, and the Cochrane Library for literature published up to January 31, 2024. The search strategy followed the PICOS principle, utilizing a combination of MeSH terms and free text. Keywords included “Breast Cancer”, “Mastectomy”, “Breast Implants”, “Prepectoral”, and “Subpectoral”. Supplementary Table 1 provides a detailed search record. We also manually reviewed the bibliographies of identified studies, relevant reviews, and meta-analyses to uncover additional eligible research. Additionally, we searched three clinical trial registries: ClinicalTrials.gov, Controlled-trials.com, and Umin.ac.jp/ctr/index.htm, to include unpublished clinical studies.
2.2 Inclusion and exclusion criteria
The inclusion criteria are as follows: (1) Patients diagnosed with breast cancer who underwent mastectomy. (2) Intervention group patients received prepectoral prosthesis implantation. (3) Control group patients received subpectoral prosthesis implantation. (4) At least one of the following outcomes was reported: total complication, hematoma, infection, wound healing issues, necrosis, capsular contracture, rippling, animation deformity, and reoperation. (5) Study design: randomized controlled trials, prospective studies, and retrospective studies. The exclusion criteria are as follows: (1) Articles such as case reports, protocols, letters, editorials, comments, reviews, and meta-analyses. (2)Non-breast cancer studies. (3)Studies not comparing prepectoral versus subpectoral prosthesis implantation. (4) No relevant outcomes reported. (5) Duplicate patient cohorts. (6) Data could not be extracted.
2.3 Selection of studies
The literature selection process was executed using EndNote (Version 20; Clarivate Analytics) to eliminate duplicate entries. Two independent reviewers conducted the initial search. Redundant items were removed, and the titles and abstracts were screened for relevance. Subsequently, each study was classified as either included or excluded. Discrepancies were resolved through consensus, and if consensus was not achieved, a third reviewer acted as a mediator.
2.4 Data extraction
Data were extracted independently by two reviewers. The extracted data included: (1) Basic characteristics of the studies: author, nationality, year of publication. (2) Baseline characteristics of study subjects: age, sample size, tumor stage. (3) Outcome indicators: total complication, hematoma, infection, wound healing issues, necrosis, capsular contracture, rippling, animation deformity, and reoperation.
2.5 Quality assessment
The quality of the included studies was assessed independently by two reviewers using the Newcastle-Ottawa Scale (NOS) for retrospective studies. Discrepancies were resolved through discussion and consensus.
2.6 Statistical analysis
The study findings were analyzed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). Continuous variables were compared using the weighted mean difference (WMD) with a 95% confidence interval (CI). Binary variables were compared using the relative ratio (RR) with a 95% CI. Medians and interquartile ranges of continuous data were converted to means and standard deviations. Statistical heterogeneity among studies was evaluated using Cochrane’s Q test and the I² index. Given the diversity of the included studies, the random effects model was primarily used. A p-value below 0.05 was considered statistically significant.
3 Results
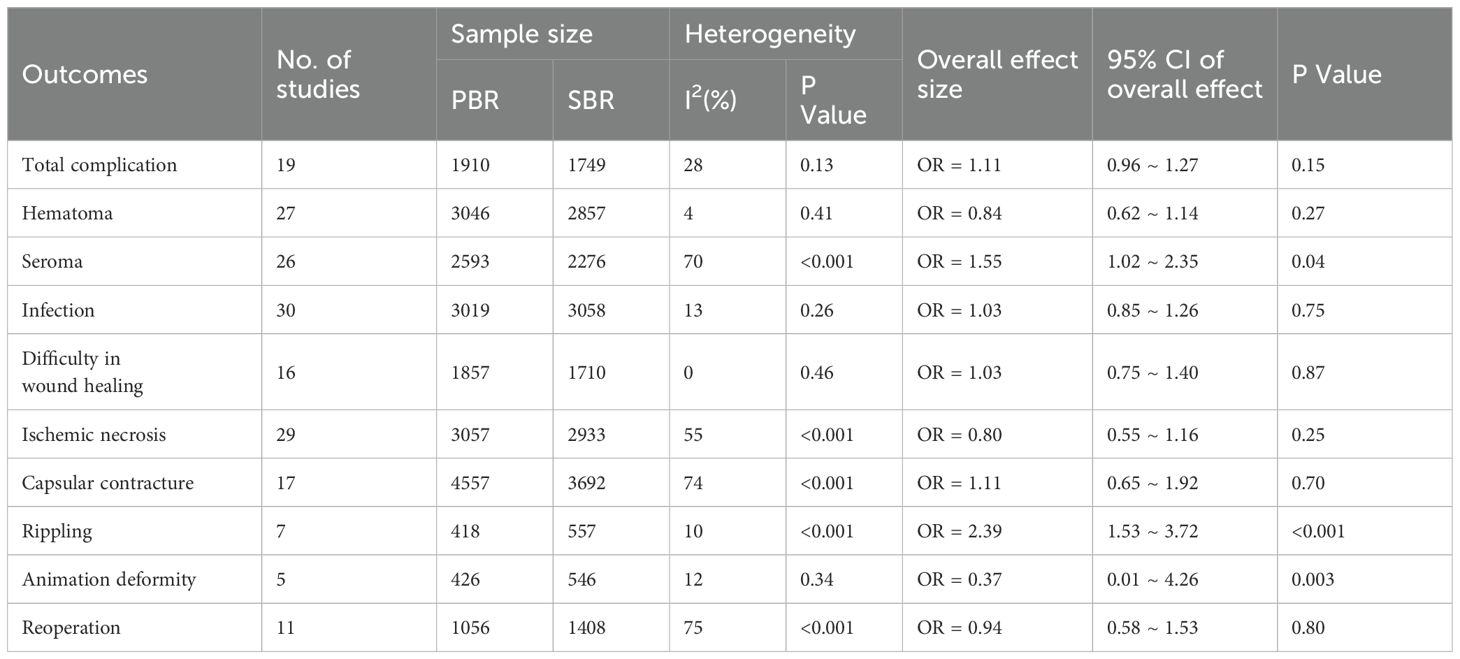
All results of the meta-analysis were summarized in Table 1.
3.1 Search results
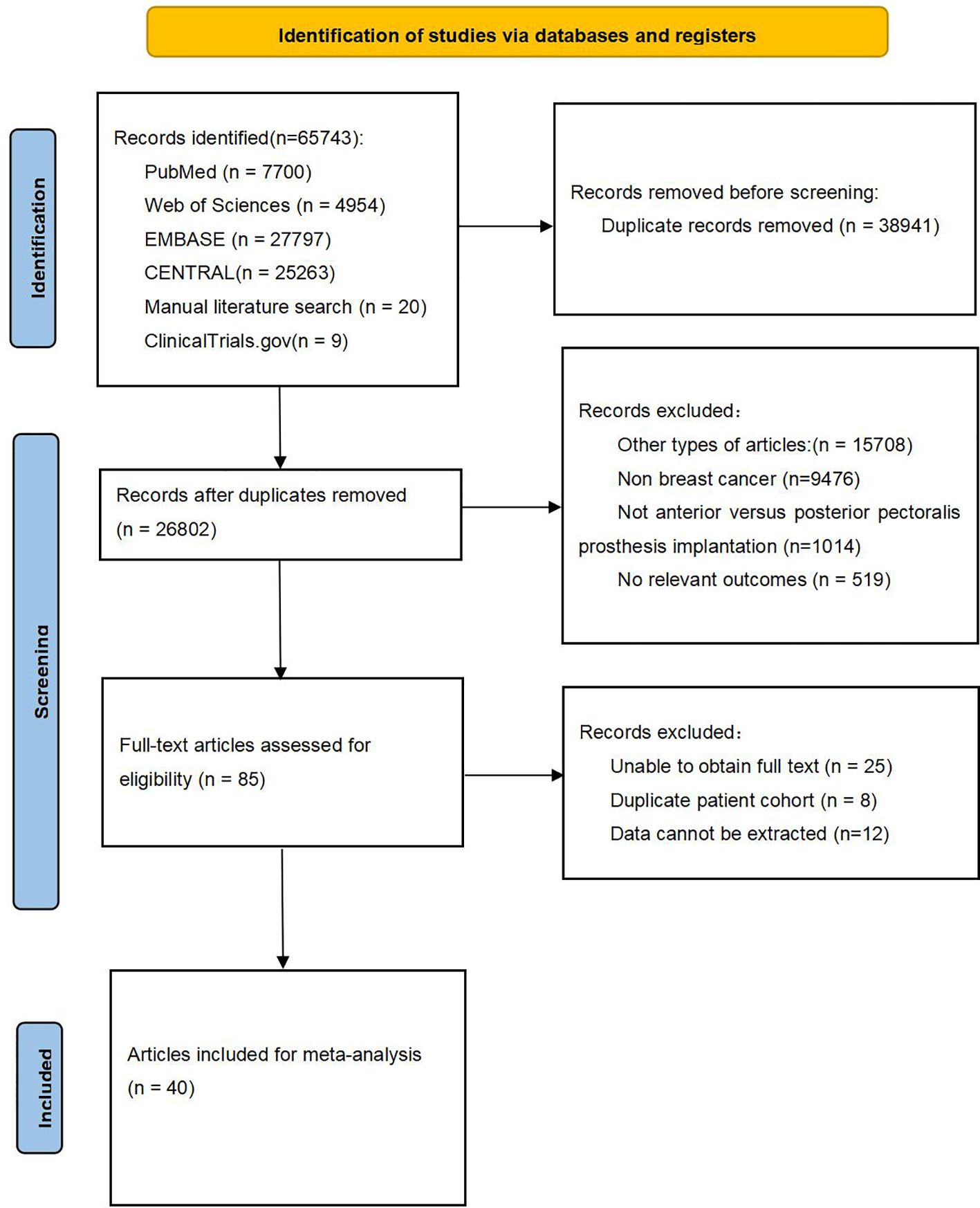
A total of 65,743 publications were retrieved from four databases and three clinical trial registries. After applying inclusion and exclusion criteria, 40 articles (6, 11–49) were included in the final meta-analysis. The selection and inclusion process are illustrated in Figure 1.
3.2 Study characteristics
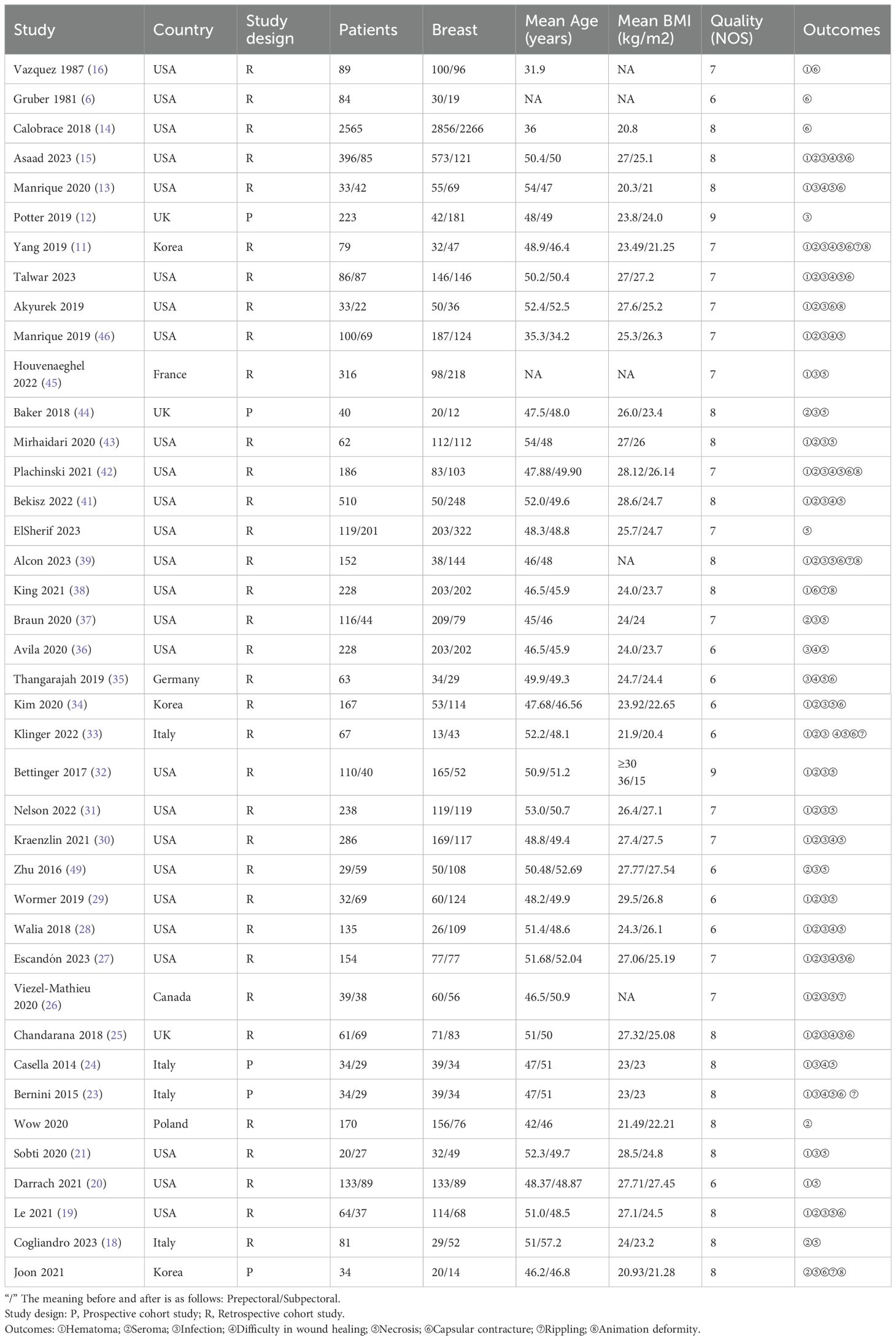
The meta-analysis included 40 studies: 5 prospective and 35 retrospective studies, with a total of 8,632 participants. A total of 12,943 breasts underwent prosthesis implantation, with 6,749 in the PBR group and 6,194 in the SBR group. The studies were conducted in the USA, UK, France, Germany, Italy, Canada, Poland, and Korea. Detailed patient characteristics are provided in Table 1, with additional details in Supplementary Table 2.
3.3 Quality assessment
The quality assessment, using the NOS, rated two studies at 9 points, sixteen studies at 8 points, thirteen studies at 7 points, and nine studies at 6 points, indicating high quality for all included studies. Detailed quality assessments are presented in Table 2.
3.4 Complications
3.4.1 Total complication
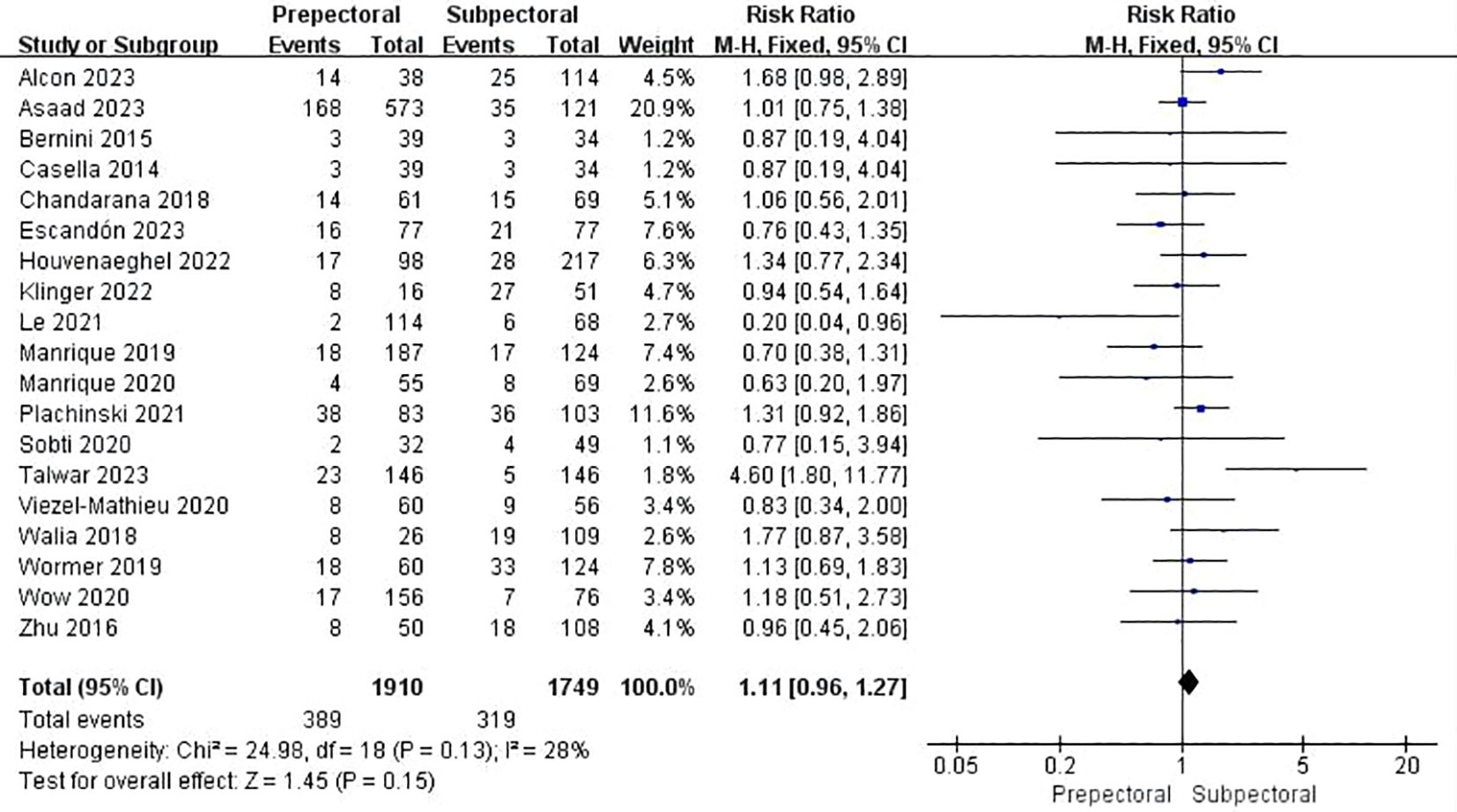
Nineteen studies documented total complications (13, 15, 19, 21–29, 33, 39, 42, 45, 46, 48, 49). The pooled analysis showed no significant difference between PBR and SBR (OR = 1.11, 95% CI: 0.96 to 1.27, P=0.15, I²=28%) (Figure 2).
3.4.2 Hematoma
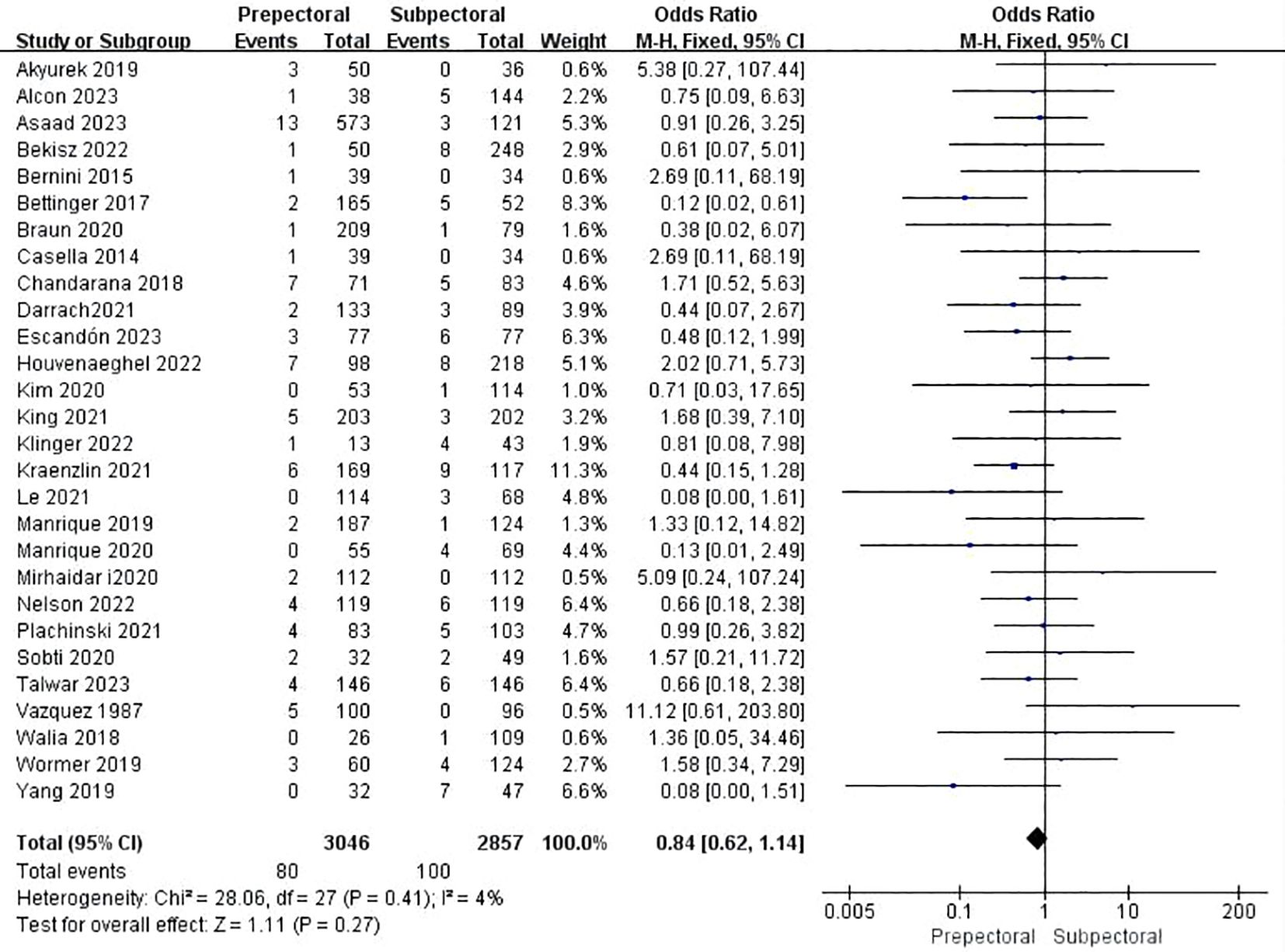
Twenty-eight studies reported hematoma (11, 13, 15, 16, 19–21, 23–34, 38, 39, 41–43, 45–48). The pooled analysis indicated no significant difference between PBR and SBR (OR = 0.84, 95% CI: 0.62 to 1.14, P=0.27, I²=4%) (Figure 3).
3.4.3 Seroma
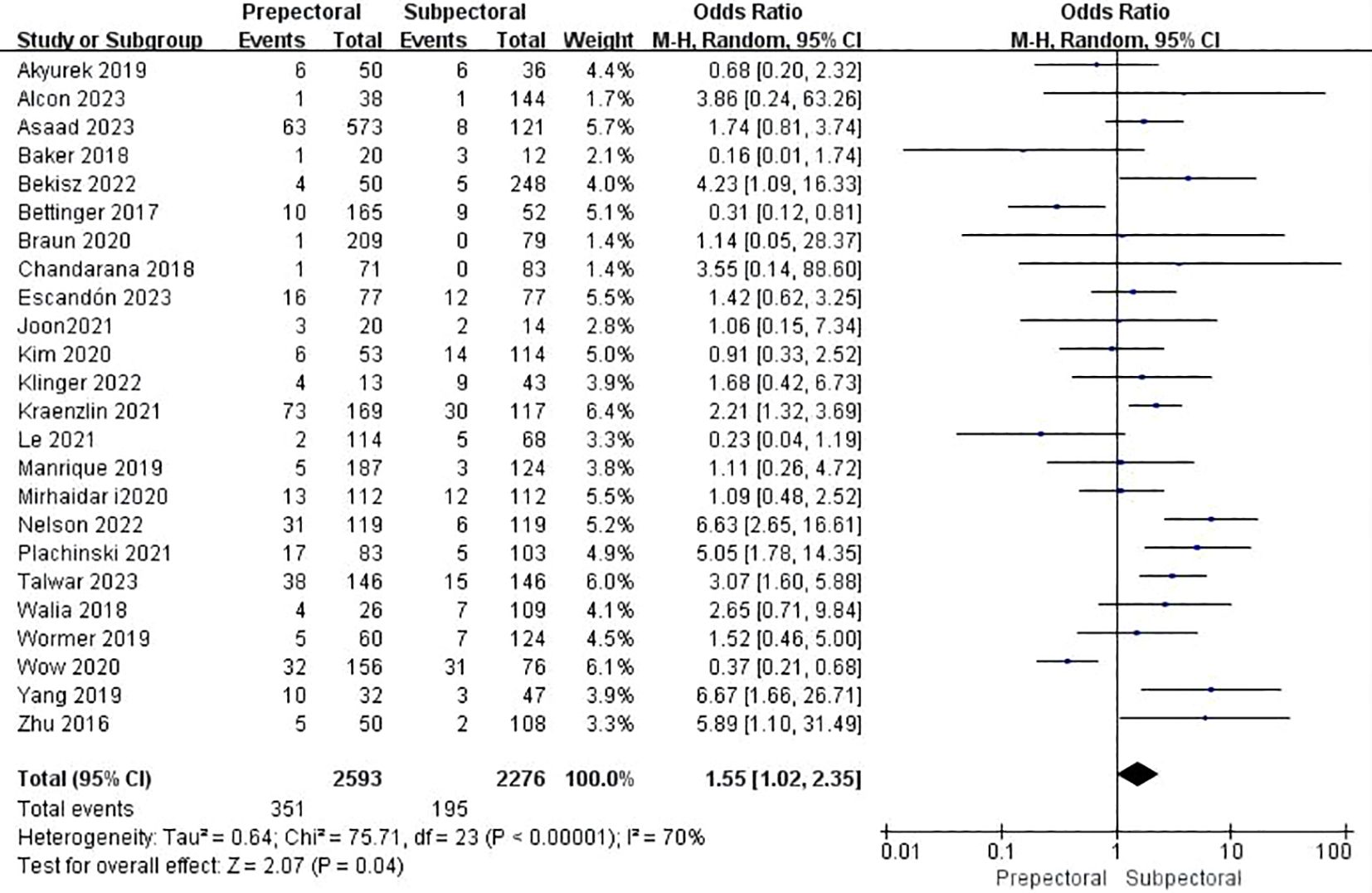
Twenty-six studies reported seroma (11, 15, 17–19, 22, 25–34, 37, 39, 41–44, 46–49). The pooled analysis showed a significantly higher occurrence of seroma in the PBR group (OR = 1.55, 95% CI: 1.02 to 2.35, P=0.04, I²=70%) (Figure 4).
3.4.4 Infection
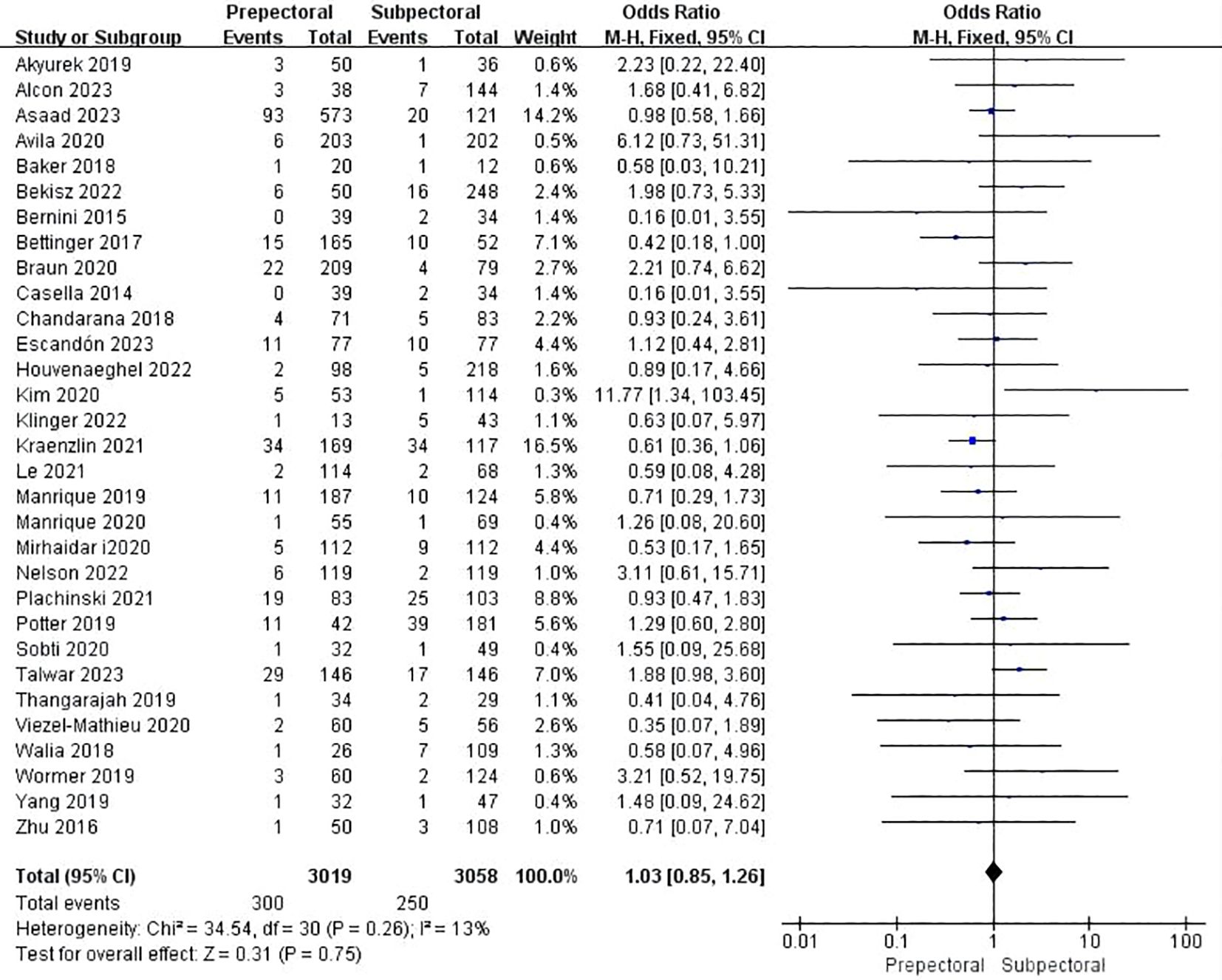
Thirty studies reported infection (11–13, 15, 19, 21, 23–37, 39, 41–49). The pooled analysis indicated no significant difference between PBR and SBR (OR = 1.03, 95% CI: 0.85 to 1.26, P=0.73, I²=13%) (Figure 5).
3.4.5 Wound healing issues
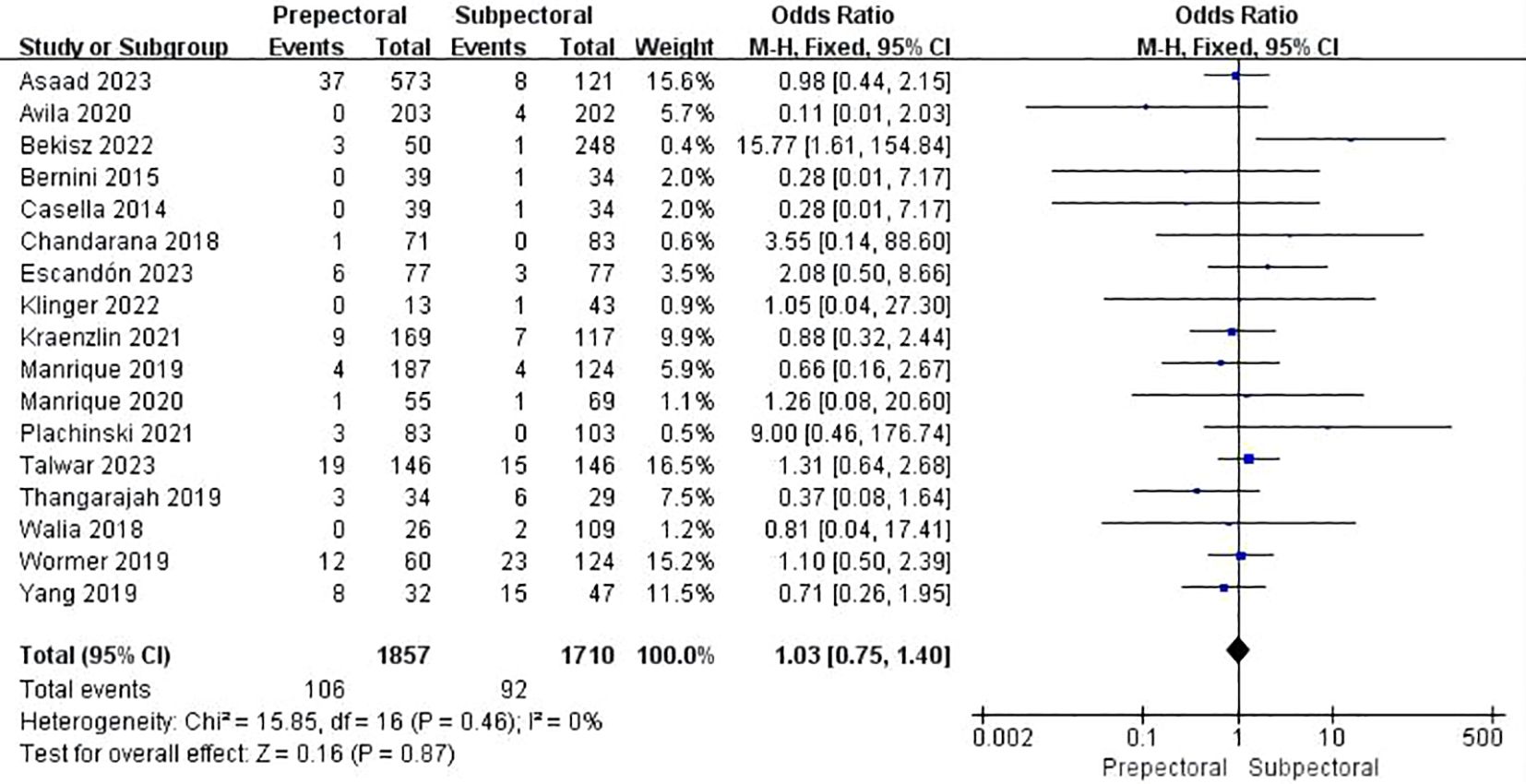
Sixteen studies reported wound healing issues (11, 13, 15, 23–25, 27, 28, 30, 33, 35, 36, 41, 42, 46, 48). The pooled analysis showed no significant difference between PBR and SBR (OR = 1.03, 95% CI: 0.75 to 1.40, P=0.87, I²=0%) (Figure 6).
3.4.6 Necrosis
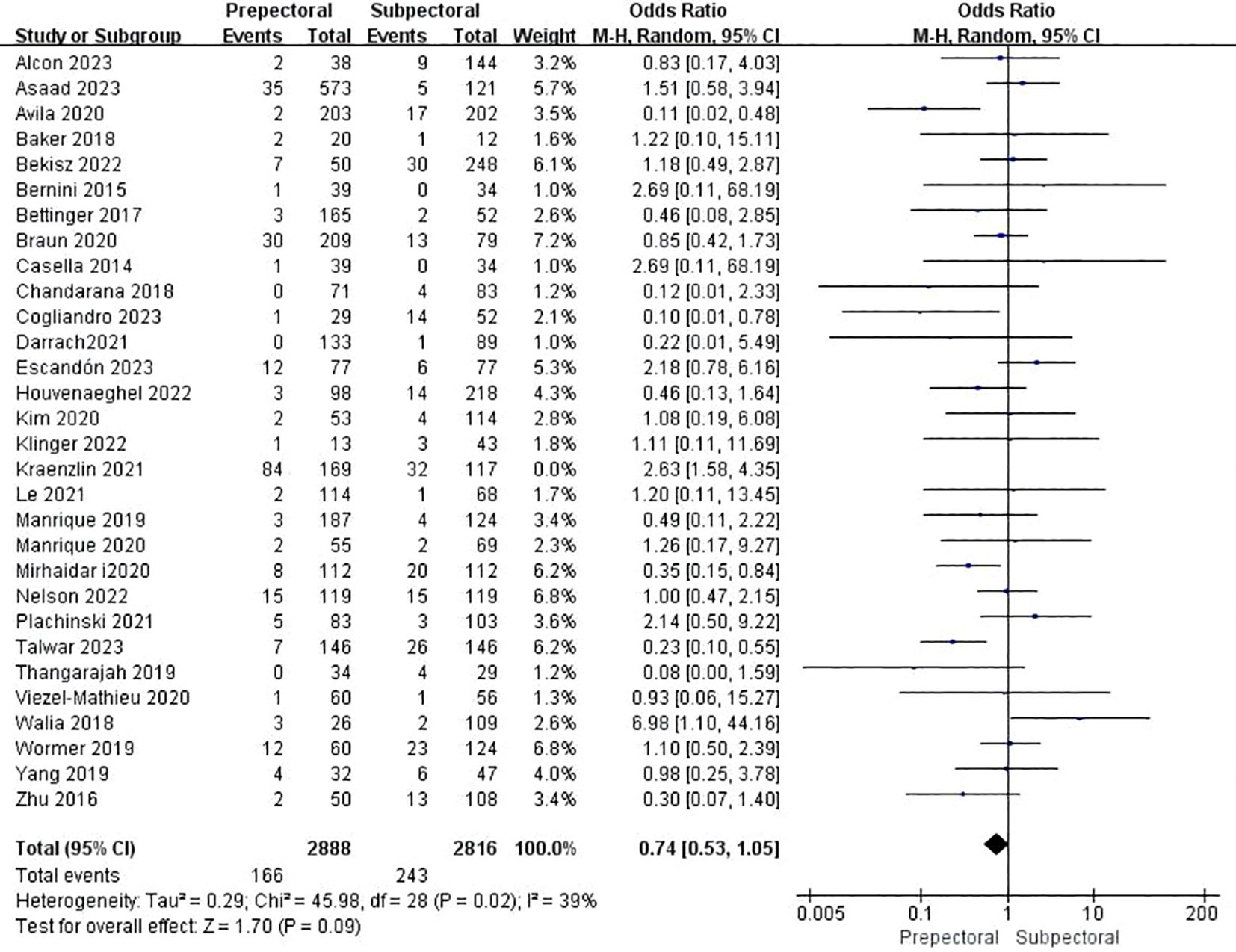
Thirty studies reported necrosis (11, 13, 15, 18–20, 23–37, 39, 41–46, 48, 49). The pooled analysis showed no significant difference between PBR and SBR (OR = 0.74, 95%CI: 0.53 to 1.05, P=0.09, I2 = 39%) (Figure 7).
3.4.7 Capsular contracture
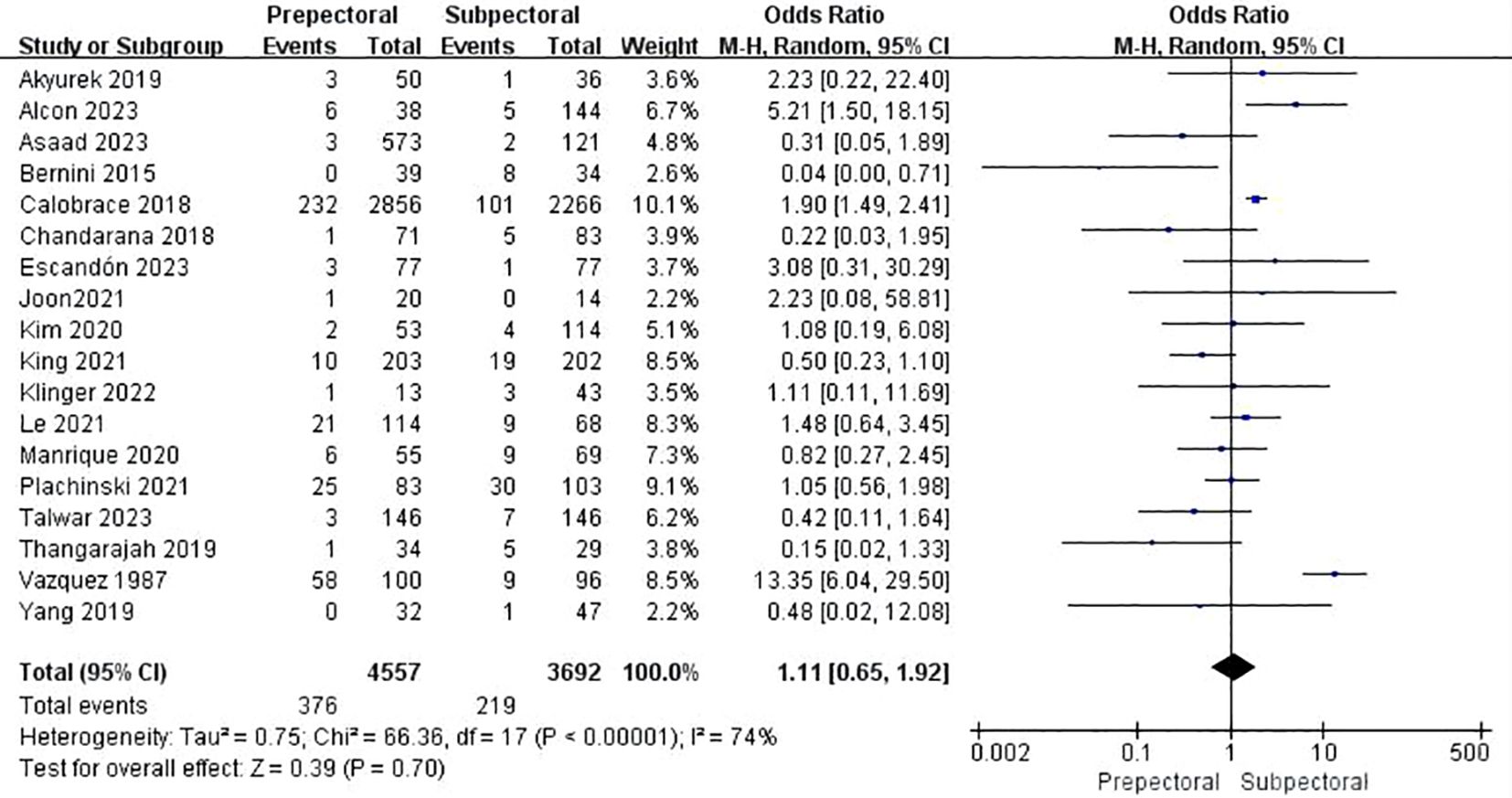
Eighteen studies reported capsular contracture (11, 13–17, 19, 23, 25, 27, 33–35, 38, 39, 42, 47, 48). The pooled analysis showed no significant difference between PBR and SBR (OR = 1.11, 95% CI: 0.65 to 1.92, P=0.70, I²=74%) (Figure 8).
3.4.8 Rippling
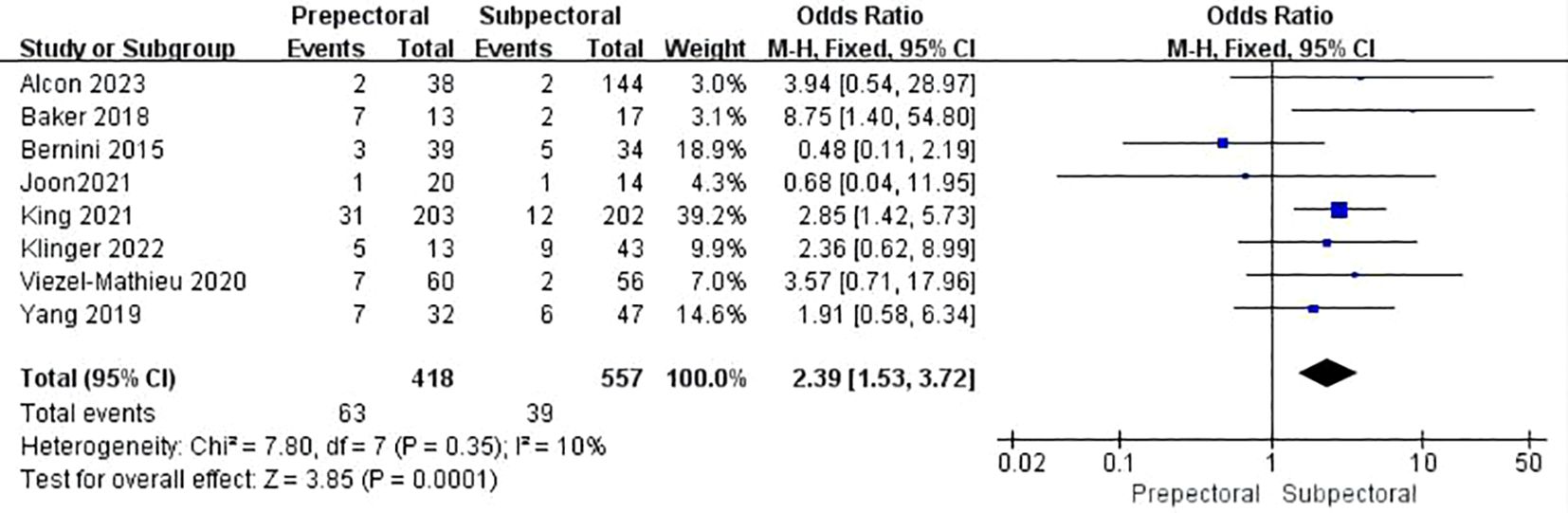
Seven studies reported rippling (11, 17, 23, 26, 33, 38, 39). The pooled analysis showed a significantly higher incidence of rippling in the PBR group compared to the SBR group (OR = 2.39, 95% CI: 1.53 to 3.72, P=0.0001, I²=10%) (Figure 9).
3.4.9 Animation deformity
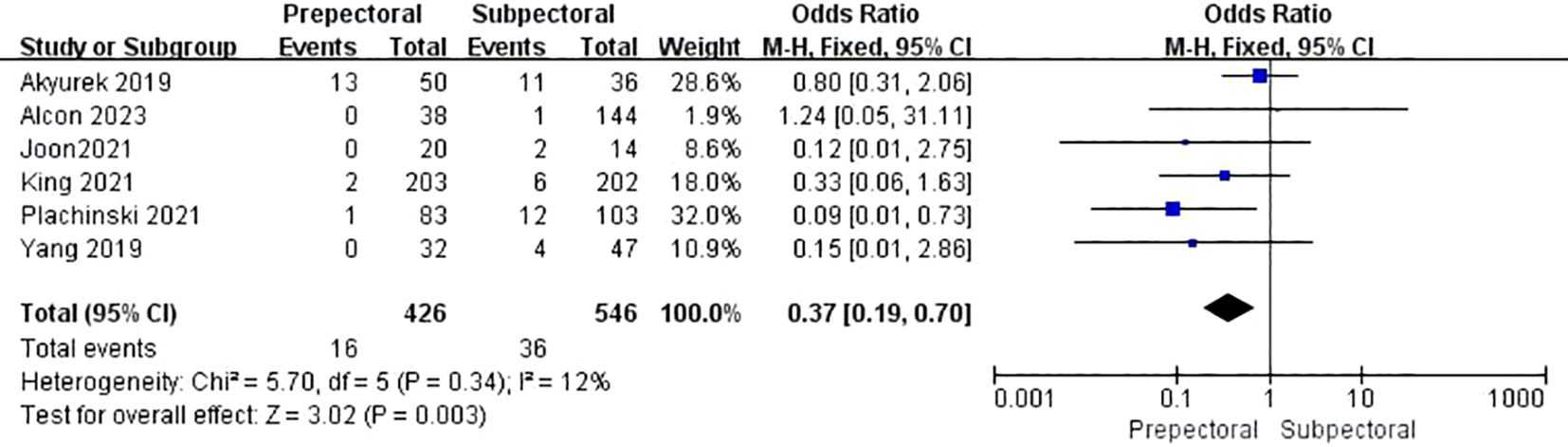
Five studies reported animation deformity (11, 17, 38, 39, 42). The pooled analysis showed a significantly lower occurrence of animation deformity in the PBR group compared to the SBR group (OR = 0.37, 95% CI: 0.19 to 0.70, P=0.003, I²=12%) (Figure 10).
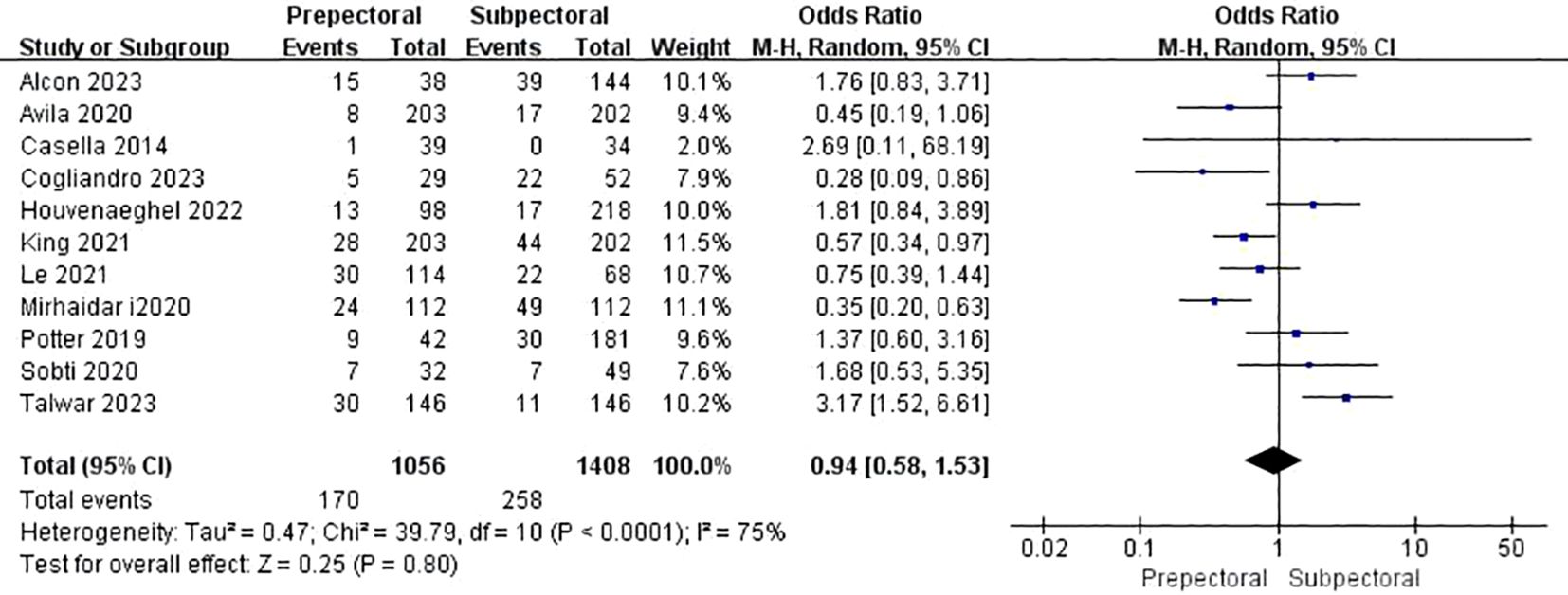
3.4.10 Reoperation
Eleven studies reported reoperation (12, 18, 19, 21, 24, 36, 38, 39, 43, 45, 48). The pooled analysis showed no significant difference between PBR and SBR (OR = 1.11, 95% CI: 0.96 to 1.27, P=0.15, I²=28%) (Figure 11).
3.5 Publication bias
Funnel plots (Figure 12) were used to evaluate publication bias. The symmetrical funnel plots indicated no apparent publication bias for hematoma (Figure 12A), seroma (Figure 12B), infection (Figure 12C), wound healing issues (Figure 12D), rippling (Figure 12G), reoperation (Figure 12H), animation deformity (Figure 12I), and total complication (Figure 12J). However, the funnel plots for necrosis (Figure 12E) and capsular contracture (Figure 12F) showed significant asymmetry, indicating potential publication bias.
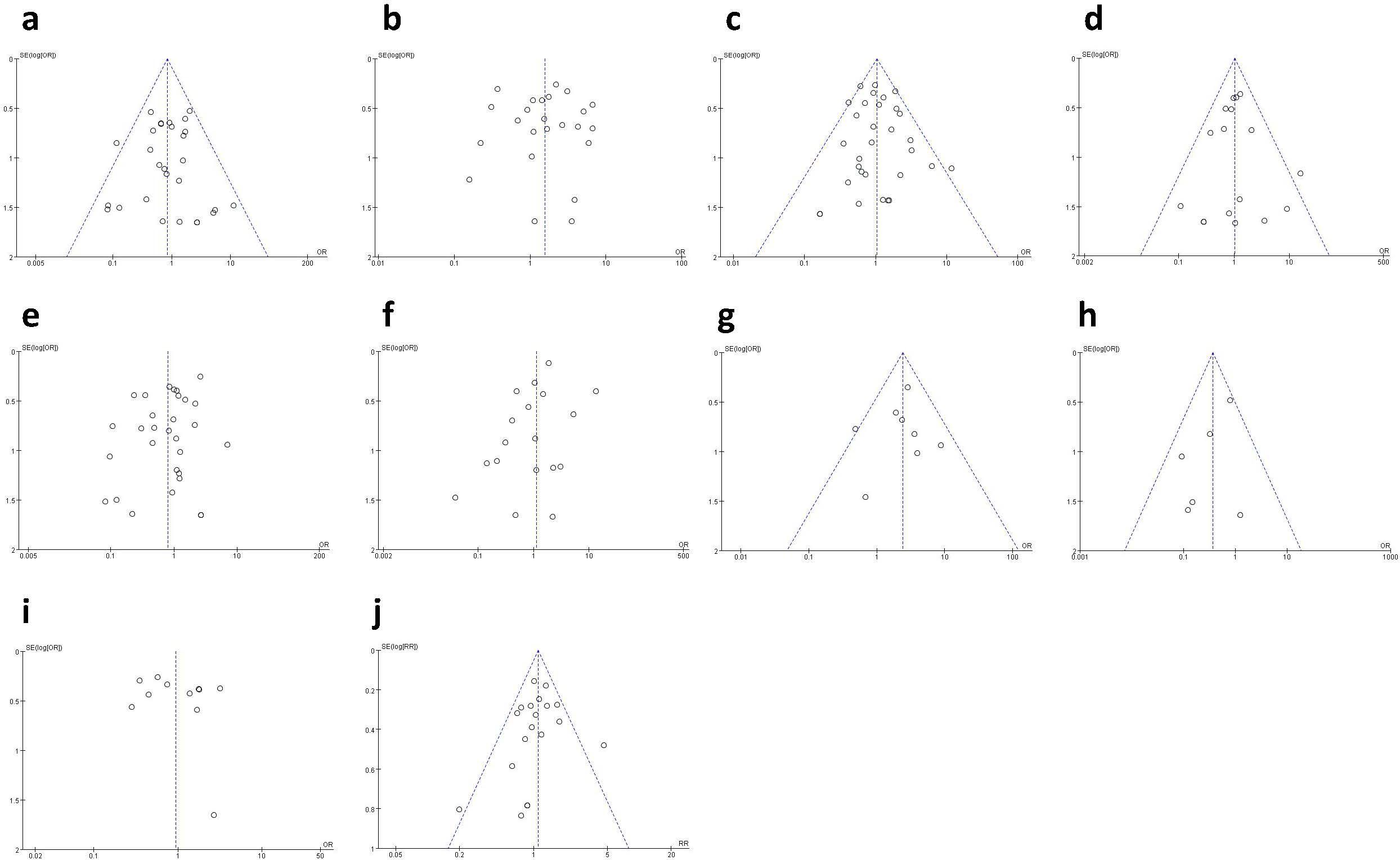
Figure 12. Funnel plot: (A) funnel plot for hematoma; (B) funnel plot for seroma; (C) funnel plot for infection; (D) funnel plot for wound healing issues; (E) funnel plot for necrosis; (F) funnel plot for capsular contracture; (G) funnel plot for rippling; (H) funnel plot for reoperation; (I) funnel plot for animation deformity; (J) funnel plot for total complication.
4 Discussion
This meta-analysis aimed to evaluate the complications associated with prepectoral breast reconstruction (PBR) compared to subpectoral breast reconstruction (SBR) in breast cancer patients. Our results indicated no statistically significant difference between the two groups concerning overall complications, hematoma, infection, wound healing issues, reoperation, animation abnormalities, necrosis, and capsular contracture. However, the aggregated data revealed a significantly higher incidence of seroma in the PBR group compared to the SBR group. Additionally, there was a markedly higher occurrence of rippling in the PBR group compared to the SBR group.
Implant-based breast reconstruction was initially performed from the 1960s to 1970s, predominantly utilizing the pectoralis major muscle. The first documentation of this procedure appeared in 1971. However, it was discontinued due to the emergence of numerous complications (2, 50). To prevent ripple deformity and the development of capsular contracture, a subpectoral major implant graft was devised (6, 51) Direct subpectoral muscle reconstruction is becoming increasingly common in many medical institutions. This procedure involves placing a permanent implant or expander along the breast using a biomaterial or synthetic mesh, typically following breast cancer treatment (52). Subpectoral major muscle implantation can effectively address several complications. However, it may also lead to animation deformity or breast deformity due to the contraction of the chest muscle after subpectoral muscle reconstruction surgery. This, in turn, can result in new issues, primarily recurrent pain (10, 53–55).
Due to advancements in technology for pectoralis major anterior implant grafts, many surgeons have reevaluated the positioning of implants in the chest plane (56). Additionally, the integration of contemporary tissue vascularization techniques and the utilization of novel surgical materials have been combined to enhance the outcomes of prepectoral restoration (57). Hence, the selection of the implantation plane should be approached with careful consideration, as each plane offers distinct advantages and noticeable drawbacks. Wrapping acellular dermal matrix (ADM) improves the resolution of issues. The use of ADM has been found to significantly decrease the incidence of capsular contracture, potentially due to a reduction in the production of granulation tissue (21, 58, 59). ADM can be derived from human, bovine, or porcine sources and must undergo biotechnological processes to eliminate cell antigens and prevent antibody reactions. However, it retains a structural matrix that supports and enhances tissue regeneration (25). Despite these advancements, patients undergoing breast reconstruction still face substantial issues such as animation deformity, corrugated malformations, and capsular contracture. The advancement of the times has facilitated progress in tissue expanders, ADM, and breast cancer surgery, thus propelling the advancement of implant-based reconstructive surgery (13, 60, 61).
Previous meta-analyses have compared prepectoral breast reconstruction (PBR) with subpectoral breast reconstruction (SBR). A meta-analysis by Li et al. found no significant differences in overall complications between PBR and SBR. Additionally, there were no significant differences in the incidence of tissue necrosis, hematoma, seroma, infection, and wound dehiscence. However, the incidence of capsular contracture was lower in the PBR group compared to the SBR group (62). The meta-analysis also found that capsular contracture (OR 0.26) and hematoma (OR 0.35) were significantly lower in SBR compared with PBR, but there was a higher incidence of implant displacement (OR) and animation deformity (OR 14.47). No significant differences were found in seroma (OR 1.06), ripple deformity (OR 1.39), and infection (OR 1.21) between the two groups. Implant-based breast reconstruction carries a risk of tissue ischemia and necrosis, which is increased by conditions such as patient smoking, advanced age, hypertension, diabetes, and obesity (63, 64). Additionally, several studies have documented that the surgical approach plays a crucial role in tissue necrosis (65, 66). The surgical approach is a crucial determinant of tissue necrosis. For instance, the selection of the incision type and the decrease in the thickness of the mastectomy flap are factors that influence the eventual loss of blood supply to the tissue, resulting in local ischemia and tissue necrosis (65, 66). Another meta-analysis (67) revealed that the incidence of capsular contracture (OR 0.26) and hematoma (OR 0.35) was significantly reduced in SBR compared to PBR. The results of capsule contracture are vastly different from previous clinical and evidence-based medicine conclusions. However, there was a higher likelihood of implant displacement (OR) and animation deformity (OR 14.47) in SBR. No significant differences were found in the incidence of seroma (OR 1.06), ripple deformity (OR 1.39), and infection (OR 1.21) between the two groups. Chatterjee et al. (68) conducted a meta-analysis of 14 trials with a total of 654 breasts. The study revealed that tissue necrosis was the most prevalent problem prior to chest reconstruction, occurring in 7.8% of cases. Seroma and capsular contracture followed closely behind, with incidences of 6.7% and 5.8% respectively. No notable disparities were observed in the rates of infection (OR 0.46) and dehiscence (OR 1.84). It is important to mention that our study incorporated a significantly larger number of articles compared to previous meta-analyses. This enables us to obtain more dependable and trustworthy conclusions.
The SBR approach is widely recognized as a significant risk factor for animation deformity. This is due to its impact on the stability of the pectoralis major muscle in its natural state, leading to the repair and fibrosis of the muscle (69). Consequently, this results in an unnatural change in breast shape when the muscle contracts. Animation deformity negatively impacts aesthetics and significantly impairs quality of life and comfort. Becker et al. found that 80% of surveyed patients experienced negative effects due to animation deformity, with 45% experiencing substantial impacts (70). Furthermore, nearly half of the patients reported that animation deformity negatively impacted their academic and professional pursuits, as well as their daily activities. Additionally, approximately one-third of patients expressed a desire to undergo reconstructive treatment when surveyed.
This meta-analysis includes the highest number of studies comparing the complications of PBR and SBR in the treatment of breast cancer, to the best of our knowledge. This has the potential to result in more dependable conclusions. Our findings provide valuable insights into the clinical outcomes of surgical procedures that contribute to both clinical practice and research in the field of breast cancer. However, we acknowledge the possible limitations of our research. Firstly, the studies included in the analysis were not randomized controlled trials (RCTs), but rather prospective or retrospective investigations. This introduces the possibility of selective bias in patient selection, which reduces the reliability and trustworthiness of the findings. Furthermore, the omission of confounding factors, such as differences in nations, case inclusion criteria, medical equipment, adjuvant therapy, mastectomy, implant, mean implant size, and surgical procedures, can result in research heterogeneity and bias.
In conclusion, our research indicates that both PBR and SBR have similar safety profiles, with comparable incidence rates of total complications. More precisely, PBR is more susceptible to rippling and seroma, while SBR is more susceptible to causing animation deformity.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
YW: Data curation, Conceptualization, Formal analysis, Funding acquisition, Writing – review & editing. LY: Conceptualization, Data curation, Formal analysis, Writing – original draft. MH: Conceptualization, Data curation, Formal analysis, Writing – original draft. YH: Investigation, Methodology, Project administration, Writing – original draft. CL: Investigation, Methodology, Project administration, Writing – original draft. YL: Software, Supervision, Validation, Writing – original draft. WL: Funding acquisition, Resources, Visualization, Writing – review & editing. TQ: Funding acquisition, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors disclose the receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Key Laboratory Construction Project of Guangxi Health Commission (ZPZH2020007), the Scientific Research Foundation of Guangxi University of Science and Technology(20Z13),the Scientific Research Foundation of Guangxi Health Commission (Z-B20220927) and the Scientific Research Foundation of Guangxi Health Commission (Z-B20180136).
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1439293/full#supplementary-material
References
1. Who. (2024). Breast cancer . Available online at: https://www.Who.Int/Zh/News-Room/Fact-Sheets/Detail/Breast-Cancer. (Accessed March 20, 2024).
2. Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg. (1971) 47:565–7. doi: 10.1097/00006534-197106000-00008
3. Hird RB, Rousseau J, Nguyen C, Lettieri J, Orr RK. Skin sparing mastectomy with delayed implant reconstruction. Am Surg. (2010) 76:785–7. doi: 10.1177/000313481007600746
4. Nava MB, Catanuto G, Pennati A, Garganese G, Spano A. Conservative mastectomies. Aesthetic Plast Surg. (2009) 33:681–6. doi: 10.1007/s00266-009-9382-4
5. Nava MB, Rocco N, Catanuto G. Conservative mastectomies: an overview. Gland Surg. (2015) 4:463–6. doi: 10.3978/j.issn.2227-684X.2015.04.06
6. Gruber RP, Kahn RA, Lash H, Maser MR, Apfelberg DB, Laub DR. Breast reconstruction following mastectomy: A comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. (1981) 67:312–7. doi: 10.1097/00006534-198103000-00007
7. Gabriel A, Sigalove S, Sigalove NM, Storm-Dickerson TL, Rice J, Pope N, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: A review of 102 reconstructions. Aesthet Surg J. (2018) 38:519–26. doi: 10.1093/asj/sjx261
8. Sigalove S, Maxwell GP, Sigalove NM, Storm-Dickerson TL, Pope N, Rice J, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg. (2017) 139:287–94. doi: 10.1097/prs.0000000000002950
9. Artz JS, Dinner MI, Foglietti MA, Sampliner J. Breast reconstruction utilizing subcutaneous tissue expansion followed by polyurethane-covered silicone implants: A 6-year experience. Plast Reconstr Surg. (1991) 88:635–9; discussion 40-1.
10. Spear SL, Schwartz J, Dayan JH, Clemens MW. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. (2009) 33:44–8. doi: 10.1007/s00266-008-9275-y
11. Yang JY, Kim CW, Lee JW, Kim SK, Lee SA, Hwang E. Considerations for patient selection: prepectoral versus subpectoral implant-based breast reconstruction. Arch Plast Surg. (2019) 46:550–7. doi: 10.5999/aps.2019.00353
12. Potter S, Conroy EJ, Cutress RI, Williamson PR, Whisker L, Thrush S, et al. Short-term safety outcomes of mastectomy and immediate implant-based breast reconstruction with and without mesh (Ibra): A multicentre, prospective cohort study. Lancet Oncol. (2019) 20:254–66. doi: 10.1016/s1470-2045(18)30781-2
13. Manrique OJ, Kapoor T, Banuelos J, Jacobson SR, Martinez-Jorge J, Nguyen MT, et al. Single-stage direct-to-implant breast reconstruction: A comparison between subpectoral versus prepectoral implant placement. Ann Plast Surg. (2020) 84:361–5. doi: 10.1097/sap.0000000000002028
14. Calobrace MB, Stevens WG, Capizzi PJ, Cohen R, Godinez T, Beckstrand M. Risk factor analysis for capsular contracture: A 10-year sientra study using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. (2018) 141:20s–8s. doi: 10.1097/prs.0000000000004351
15. Asaad M, Yu JZ, Tran JP, Liu J, O'Grady B, Clemens MW, et al. Surgical and patient-reported outcomes of 694 two-stage prepectoral versus subpectoral breast reconstructions. Plast Reconstr Surg. (2023) 152:43s–54s. doi: 10.1097/prs.0000000000010380
16. Vazquez B, Given KS, Houston GC. Breast augmentation: A review of subglandular and submuscular implantation. Aesthetic Plast Surg. (1987) 11:101–5. doi: 10.1007/bf01575494
17. Lee JS, Park E, Lee JH, Lee J, Park HY, Yang JD, et al. A prospective comparison study of early functional outcomes after implant-based breast reconstruction: subpectoral versus prepectoral technique. Ann Palliat Med. (2021) 10:2520–9. doi: 10.21037/apm-20-1550
18. Cogliandro A, Salzillo R, De Bernardis R, Loria FS, Petrucci V, Barone M, et al. Prepectoral versus subpectoral direct-to-implant breast reconstruction: evaluation of patient's quality of life and satisfaction with breast-Q. Aesthetic Plast Surg. (2023) 47:1291–9. doi: 10.1007/s00266-023-03316-z
19. Le NK, Persing S, Dinis J, Gabrick KS, Wu RT, Sinnott CJ, et al. A comparison of breast-Q scores between prepectoral and subpectoral direct-to-implant breast reconstruction. Plast Reconstr Surg. (2021) 148:708e–14e. doi: 10.1097/prs.0000000000008410
20. Darrach H, Kraenzlin FS, Khavanin N, He W, Lee E, Sacks JM. Pectoral placement of tissue expanders affects inpatient opioid use. Breast J. (2021) 27:126–33. doi: 10.1111/tbj.14149
21. Sobti N, Weitzman RE, Nealon KP, Jimenez RB, Gfrerer L, Mattos D, et al. Evaluation of capsular contracture following immediate prepectoral versus subpectoral direct-to-implant breast reconstruction. Sci Rep. (2020) 10:1137. doi: 10.1038/s41598-020-58094-4
22. Wow T, Kolacinska-Wow A, Wichtowski M, Boguszewska-Byczkiewicz K, Nowicka Z, Ploszka K, et al. A retrospective study assessing the outcomes of immediate prepectoral and subpectoral implant and mesh-based breast reconstruction. Cancers (Basel). (2022) 14:1–12. doi: 10.3390/cancers14133188
23. Bernini M, Calabrese C, Cecconi L, Santi C, Gjondedaj U, Roselli J, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open. (2015) 3:e574. doi: 10.1097/gox.0000000000000533
24. Casella D, Bernini M, Bencini L, Roselli J, Lacaria MT, Martellucci J, et al. Tiloop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg. (2014) 37:599–604. doi: 10.1007/s00238-014-1001-1
25. Chandarana MN, Jafferbhoy S, Marla S, Soumian S, Narayanan S. Acellular dermal matrix in implant-based immediate breast reconstructions: A comparison of prepectoral and subpectoral approach. Gland Surg. (2018) 7:S64–s9. doi: 10.21037/gs.2018.03.05
26. Viezel-Mathieu A, Alnaif N, Aljerian A, Safran T, Brabant G, Boileau JF, et al. Acellular dermal matrix-sparing direct-to-implant prepectoral breast reconstruction: A comparative study including cost analysis. Ann Plast Surg. (2020) 84:139–43. doi: 10.1097/sap.0000000000001997
27. Escandón JM, Sweitzer K, Christiano JG, Gooch JC, Olzinski AT, Prieto PA, et al. Subpectoral versus prepectoral two-stage breast reconstruction: A propensity score-matched analysis of 30-day morbidity and long-term outcomes. J Plast Reconstr Aesthet Surg. (2023) 76:76–87. doi: 10.1016/j.bjps.2022.10.028
28. Walia GS, Aston J, Bello R, Mackert GA, Pedreira RA, Cho BH, et al. Prepectoral versus subpectoral tissue expander placement: A clinical and quality of life outcomes study. Plast Reconstr Surg Glob Open. (2018) 6:e1731. doi: 10.1097/gox.0000000000001731
29. Wormer BA, Valmadrid AC, Ganesh Kumar N, Al Kassis S, Rankin TM, Kaoutzanis C, et al. Reducing expansion visits in immediate implant-based breast reconstruction: A comparative study of prepectoral and subpectoral expander placement. Plast Reconstr Surg. (2019) 144:276–86. doi: 10.1097/prs.0000000000005791
30. Kraenzlin F, Darrach H, Khavanin N, Kokosis G, Aliu O, Broderick K, et al. Tissue expander-based breast reconstruction in the prepectoral versus subpectoral plane: an analysis of short-term outcomes. Ann Plast Surg. (2021) 86:19–23. doi: 10.1097/sap.0000000000002415
31. Nelson JA, Shamsunder MG, Vorstenbosch J, Polanco TO, Matros E, Coriddi MR, et al. Prepectoral and subpectoral tissue expander-based breast reconstruction: A propensity-matched analysis of 90-day clinical and health-related quality-of-life outcomes. Plast Reconstr Surg. (2022) 149:607e–16e. doi: 10.1097/prs.0000000000008892
32. Bettinger LN, Waters LM, Reese SW, Kutner SE, Jacobs DI. Comparative study of prepectoral and subpectoral expander-based breast reconstruction and Clavien iiib score outcomes. Plast Reconstr Surg Glob Open. (2017) 5:e1433. doi: 10.1097/gox.0000000000001433
33. Klinger F, Lisa A, Testori A, Vaccari S, Bandi V, Lorenzano V, et al. Immediate direct-to-implant breast reconstruction: A single center comparison between different procedures. Front Surg. (2022) 9:935410. doi: 10.3389/fsurg.2022.935410
34. Kim JH, Hong SE. A comparative analysis between subpectoral versus prepectoral single stage direct-to-implant breast reconstruction. Medicina (Kaunas). (2020) 56:1–10. doi: 10.3390/medicina56100537
35. Thangarajah F, Treeter T, Krug B, Hellmich M, Eichler C, Hanstein B, et al. Comparison of subpectoral versus prepectoral immediate implant reconstruction after skin- and nipple-sparing mastectomy in breast cancer patients: A retrospective hospital-based cohort study. Breast Care (Basel). (2019) 14:382–7. doi: 10.1159/000496696
36. Avila A, Bartholomew AJ, Sosin M, Deldar R, Griffith KF, Willey SC, et al. Acute postoperative complications in prepectoral versus subpectoral reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. (2020) 146:715e–20e. doi: 10.1097/prs.0000000000007326
37. Braun SE, Dreicer M, Butterworth JA, Larson KE. Do nipple necrosis rates differ in prepectoral versus submuscular implant-based reconstruction after nipple-sparing mastectomy? Ann Surg Oncol. (2020) 27:4760–6. doi: 10.1245/s10434-020-08887-8
38. King CA, Bartholomew AJ, Sosin M, Avila A, Famiglietti AL, Dekker PK, et al. A critical appraisal of late complications of prepectoral versus subpectoral breast reconstruction following nipple-sparing mastectomy. Ann Surg Oncol. (2021) 28:9150–8. doi: 10.1245/s10434-021-10085-z
39. Alcon A, Rosser M, Gedallovich J, Foster RD, Sbitany H, Piper ML. Long-term outcomes in prepectoral versus subpectoral two-stage implant-based breast reconstruction after nipple-sparing mastectomy. Plast Reconstr Surg. (2023) 152:273–80. doi: 10.1097/prs.0000000000010251
40. ElSherif A, Bernard S, Djohan R, Atallah A, Tu C, Valente SA. Nipple necrosis rate with submuscular versus prepectoral implant-based reconstruction in nipple sparing mastectomy: does it differ? Am J Surg. (2024) 230:57–62. doi: 10.1016/j.amjsurg.2023.11.039
41. Bekisz JM, Salibian AA, Frey JD, Choi M, Karp NS. Picking the right plane: A comparison of total submuscular, dual-plane, and prepectoral implant-based breast reconstruction. Plast Reconstr Surg. (2022) 150:737e–46e. doi: 10.1097/prs.0000000000009537
42. Plachinski SJ, Boehm LM, Adamson KA, LoGiudice JA, Doren EL. Comparative analysis of prepectoral versus subpectoral implant-based breast reconstruction. Plast Reconstr Surg Glob Open. (2021) 9:e3709. doi: 10.1097/gox.0000000000003709
43. Mirhaidari SJ, Azouz V, Wagner DS. Prepectoral versus subpectoral direct to implant immediate breast reconstruction. Ann Plast Surg. (2020) 84:263–70. doi: 10.1097/sap.0000000000002059
44. Baker BG, Irri R, MacCallum V, Chattopadhyay R, Murphy J, Harvey JR. A prospective comparison of short-term outcomes of subpectoral and prepectoral strattice-based immediate breast reconstruction. Plast Reconstr Surg. (2018) 141:1077–84. doi: 10.1097/prs.0000000000004270
45. Houvenaeghel G, Cohen M, Sabiani L, Van Troy A, Quilichini O, Charavil A, et al. Mastectomy and immediate breast reconstruction with pre-pectoral or sub-pectoral implant: assessing clinical practice, post-surgical outcomes, patient's satisfaction and cost. J Surg Res (Houst). (2022) 5:500–10. doi: 10.26502/jsr.10020250
46. Manrique OJ, Banuelos J, Abu-Ghname A, Nguyen MD, Tran NV, Martinez-Jorge J, et al. Surgical outcomes of prepectoral versus subpectoral implant-based breast reconstruction in young women. Plast Reconstr Surg Glob Open. (2019) 7:e2119. doi: 10.1097/gox.0000000000002119
47. Akyurek M, Dowlatshahi S, Quinlan RM. Two-stage prosthetic breast reconstruction with latissimus flap: prepectoral versus subpectoral approach. J Plast Reconstr Aesthet Surg. (2020) 73:501–6. doi: 10.1016/j.bjps.2019.10.021
48. Talwar AA, Lanni MA, Ryan IA, Kodali P, Bernstein E, McAuliffe PB, et al. Prepectoral versus submuscular implant-based breast reconstruction: A matched-pair comparison of outcomes. Plast Reconstr Surg. (2024) 153:281e–90e. doi: 10.1097/prs.0000000000010618
49. Zhu L, Mohan AT, Abdelsattar JM, Wang Z, Vijayasekaran A, Hwang SM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. (2016) 69:e77–86. doi: 10.1016/j.bjps.2016.01.006
50. Nava MB, Pennati AE, Lozza L, Spano A, Zambetti M, Catanuto G. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. (2011) 128:353–9. doi: 10.1097/PRS.0b013e31821e6c10
51. Kelly AP Jr., Jacobson HS, Fox JI, Jenny H. Complications of subcutaneous mastectomy and replacement by the Cronin silastic mammary prosthesis. Plast Reconstr Surg. (1966) 37:438–45. doi: 10.1097/00006534-196605000-00011
52. Sobti N, Ji E, Brown RL, Cetrulo CL Jr., Colwell AS, Winograd JM, et al. Evaluation of acellular dermal matrix efficacy in prosthesis-based breast reconstruction. Plast Reconstr Surg. (2018) 141:541–9. doi: 10.1097/prs.0000000000004109
53. Glasberg SB, Light D. Alloderm and strattice in breast reconstruction: A comparison and techniques for optimizing outcomes. Plast Reconstr Surg. (2012) 129:1223–33. doi: 10.1097/PRS.0b013e31824ec429
54. Tasoulis MK, Iqbal FM, Cawthorn S, MacNeill F, Vidya R. Subcutaneous implant breast reconstruction: time to reconsider? Eur J Surg Oncol. (2017) 43:1636–46. doi: 10.1016/j.ejso.2017.04.008
55. Nealon KP, Weitzman RE, Sobti N, Gadd M, Specht M, Jimenez RB, et al. Prepectoral direct-to-implant breast reconstruction: safety outcome endpoints and delineation of risk factors. Plast Reconstr Surg. (2020) 145:898e–908e. doi: 10.1097/prs.0000000000006721
56. Ter Louw RP, Nahabedian MY. Prepectoral breast reconstruction. Plast Reconstr Surg. (2017) 140:51s–9s. doi: 10.1097/prs.0000000000003942
57. Nahabedian MY, Cocilovo C. Two-stage prosthetic breast reconstruction: A comparison between prepectoral and partial subpectoral techniques. Plast Reconstr Surg. (2017) 140:22s–30s. doi: 10.1097/prs.0000000000004047
58. Breuing KH, Colwell AS. Inferolateral alloderm hammock for implant coverage in breast reconstruction. Ann Plast Surg. (2007) 59:250–5. doi: 10.1097/SAP.0b013e31802f8426
59. Spear SL, Parikh PM, Reisin E, Menon NG. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. (2008) 32:418–25. doi: 10.1007/s00266-008-9128-8
60. Schaeffer CV, Dassoulas KR, Thuman J, Campbell CA. Early functional outcomes after prepectoral breast reconstruction: A case-matched cohort study. Ann Plast Surg. (2019) 82:S399–s403. doi: 10.1097/sap.0000000000001669
61. Momeni A, Remington AC, Wan DC, Nguyen D, Gurtner GC. A matched-pair analysis of prepectoral with subpectoral breast reconstruction: is there a difference in postoperative complication rate? Plast Reconstr Surg. (2019) 144:801–7. doi: 10.1097/prs.0000000000006008
62. Li L, Su Y, Xiu B, Huang X, Chi W, Hou J, et al. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: A systematic review and meta analysis. Eur J Surg Oncol. (2019) 45:1542–50. doi: 10.1016/j.ejso.2019.05.015
63. Ito H, Ueno T, Suga H, Shiraishi T, Isaka H, Imi K, et al. Risk factors for skin flap necrosis in breast cancer patients treated with mastectomy followed by immediate breast reconstruction. World J Surg. (2019) 43:846–52. doi: 10.1007/s00268-018-4852-y
64. Sue GR, Long C, Lee GK. Management of mastectomy skin necrosis in implant based breast reconstruction. Ann Plast Surg. (2017) 78:S208–s11. doi: 10.1097/sap.0000000000001045
65. Endara M, Chen D, Verma K, Nahabedian MY, Spear SL. Breast reconstruction following nipple-sparing mastectomy: A systematic review of the literature with pooled analysis. Plast Reconstr Surg. (2013) 132:1043–54. doi: 10.1097/PRS.0b013e3182a48b8a
66. Daar DA, Abdou SA, Rosario L, Rifkin WJ, Santos PJ, Wirth GA, et al. Is there a preferred incision location for nipple-sparing mastectomy? A systematic review and meta-analysis. Plast Reconstr Surg. (2019) 143:906e–19e. doi: 10.1097/prs.0000000000005502
67. Li S, Mu D, Liu C, Xin M, Fu S, Xu B, et al. Complications following subpectoral versus prepectoral breast augmentation: A meta-analysis. Aesthetic Plast Surg. (2019) 43:890–8. doi: 10.1007/s00266-019-01404-7
68. Chatterjee A, Nahabedian MY, Gabriel A, Macarios D, Parekh M, Wang F, et al. Early assessment of post-surgical outcomes with pre-pectoral breast reconstruction: A literature review and meta-analysis. J Surg Oncol. (2018) 117:1119–30. doi: 10.1002/jso.24938
69. Fracol M, Feld LN, Chiu WK, Kim JYS. An overview of animation deformity in prosthetic breast reconstruction. Gland Surg. (2019) 8:95–101. doi: 10.21037/gs.2018.09.09
Keywords: breast cancer, reconstruction, prepectoral, subpectoral, complication, meta-analysis
Citation: Wu Y, Yu L, Huang M, Huang Y, Li C, Liang Y, Liang W and Qin T (2024) Comparative complications of prepectoral versus subpectoral breast reconstruction in patients with breast cancer: a meta-analysis. Front. Oncol. 14:1439293. doi: 10.3389/fonc.2024.1439293
Received: 27 May 2024; Accepted: 06 August 2024;
Published: 26 August 2024.
Edited by:
Hao-yu Lin, First Affiliated Hospital of Shantou University Medical College, ChinaReviewed by:
Emanuele Garreffa, Derby Hospitals NHS Foundation Trust, United KingdomKe Xie, University of Electronic Science and Technology of China, China
Copyright © 2024 Wu, Yu, Huang, Huang, Li, Liang, Liang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Liang, TGlhbmd3bTIyQGljbG91ZC5jb20=; Tian Qin, cWludGlhbjIwMjRrZEAxNjMuY29t
†These authors share first authorship
 Yongxiao Wu†
Yongxiao Wu† Lizhi Yu
Lizhi Yu Miaoyan Huang
Miaoyan Huang Weiming Liang
Weiming Liang Tian Qin
Tian Qin