- 1Department of Radiotherapy, General Hospital of Ningxia Medical University, Yinchuan, China
- 2Department of Orthopedic, General Hospital of Ningxia Medical University, Yinchuan, China
- 3First Clinical Medical College, General Hospital of Ningxia Medical University, Yinchuan, China
Background: Bone metastases of lung cancer (BMLC) severely diminish patients’ quality of life due to bone-related events, and the lack of clear guidelines globally regarding medical and surgical treatment significantly reduces patient survival. While knowledge about BMLC has grown exponentially over the past two decades, a comprehensive and objective bibliometric analysis remains absent.
Methods: A comprehensive bibliometric analysis was conducted on relevant literature on BMLC extracted from the Web of Science database from 2004 to 2023 by Biblioshiny, VOSviewer, Scimago Graphica, CiteSpace, and Microsoft Office Excel Professional Plus 2016 software. 936 papers related to BMLC were extracted from the Web of Science Core Collection (WoSCC). The number of publications, countries, institutions, global collaborations, authors, journals, keywords, thematic trends, and cited references were then visualized. Finally, the research status and development direction in the last 20 years were analyzed.
Results: This study included a total of 936 papers on BMLC from 2004 to 2023. There has been a steady increase in global publications each year, peaking in 2021. China had the highest number of publications, followed by Japan and the United States. Additionally, China had the most citations with an H-index of 35, while the US followed with an H-index of 34, highlighting their significant contributions to the field. “Frontiers in Oncology” had the highest number of publications. CiteSpace analysis identified “lung cancer,” “bone metastasis,” and “survival” as the top high-frequency keywords, encapsulating the core research focus. Keyword clustering analysis revealed six main clusters representing the primary research directions. Burst analysis of keywords showed that “skeletal complications” had the highest burst intensity from 2005 to 2013, while recent research trends include “immunotherapy” and “denosumab,” with bursts from 2021 to 2023. Trend topic analysis indicated that “non-small cell lung cancer,” “immunotherapy,” and “immune checkpoint inhibitors” represent the cutting-edge research directions in this field.
Conclusion: This article reveals the current status and trend of research on BMLC, which is increasing worldwide. China and the United States have contributed the most, but international cooperative research on BMLC should be strengthened. The pathogenesis, early prevention, and individualized treatment of BMLC need to be strengthened for further study, and immunotherapy is the next hotspot of lung cancer bone metastasis research.
1 Introduction
Lung cancer is the malignant tumor with the highest rate of morbidity and mortality worldwide today and represents a severe threat to the life and health of human beings. According to the American Cancer Society’s Global Cancer Statistics Report 2022, lung cancer is both the most common type of cancer worldwide and the leading cause of death among people living with cancer (1). Lung cancer has an insidious onset and is challenging to diagnose at an early stage. Approximately 50% of lung cancers are advanced (stage IV) at diagnosis, and bone metastasis is one of the significant sites of hematogenous metastasis (2). With the progress and development of precision medicine technology, there has been a gradual improvement in the 5-year survival rate for patients with advanced lung cancer (3, 4). The prolonged survival of patients is accompanied by an increased risk of bone metastasis and skeletal-related events (SREs) (5, 6).
BMLC often causes SREs, such as bone pain, pathologic fracture, spinal cord compression or paraplegia, hypercalcemia, and pain caused by related treatments. These SREs seriously affect patients’ quality of life and shorten patients’ survival. The favorable sites of lung cancer bone metastasis are in the proximal part of the spine and trunk bone, and those occurring in the spine account for 50%, the femur account for 25%, and the ribs and sternum account for 12% (7–9). Studies have shown that the survival time of patients with BMLC can be reduced by half once SREs occur (10). Patient survival is further shortened when combined with severe SREs such as hypercalcemia, pathologic fracture, spinal cord compression, and other complications (11, 12). Therefore, active prevention and treatment of bone metastases, as well as effective control of the primary disease, are of paramount importance. However, the current research literature and data on the pathogenesis, diagnosis, and treatment of BMLC are scattered, which could be more conducive to relevant researchers efficiently grasping the main results of previous studies and refining the hotspots for future prospective studies.
Although Meta-analysis and traditional reviews have conducted relevant studies on BMLC, they only focus on specific perspectives or indicators, with a narrower scope of research design and more limited analysis and discussion. Bibliometrics focuses on the quantification of a complete body of knowledge. By analyzing the existing quantitative literature, it is possible to use intuitive maps to predict the future direction of a research field and systematically analyze the research progress of various countries, institutions, authors, and disciplines. Only a few scientists have provided a comprehensive and quantitative estimate of the scientific output of bone metastasis’s performance in lung cancer through bibliometrics. Therefore, this paper aims to comprehensively and objectively analyze the indicators of the documents related to lung cancer bone metastasis through bibliometrics to elucidate the trends, hotspots, and directions of the research related to lung cancer bone metastasis to the researchers and also to provide new clues and ideas for the future study, diagnosis, and treatment of lung cancer bone metastasis.
2 Materials and methods
2.1 Data collection
The literature extracted from the Science Citation Index Expanded in the Web of Science Core Collection (WosCC) database compiles comprehensive citation and publication information across various scientific fields.
2.2 Search strategies
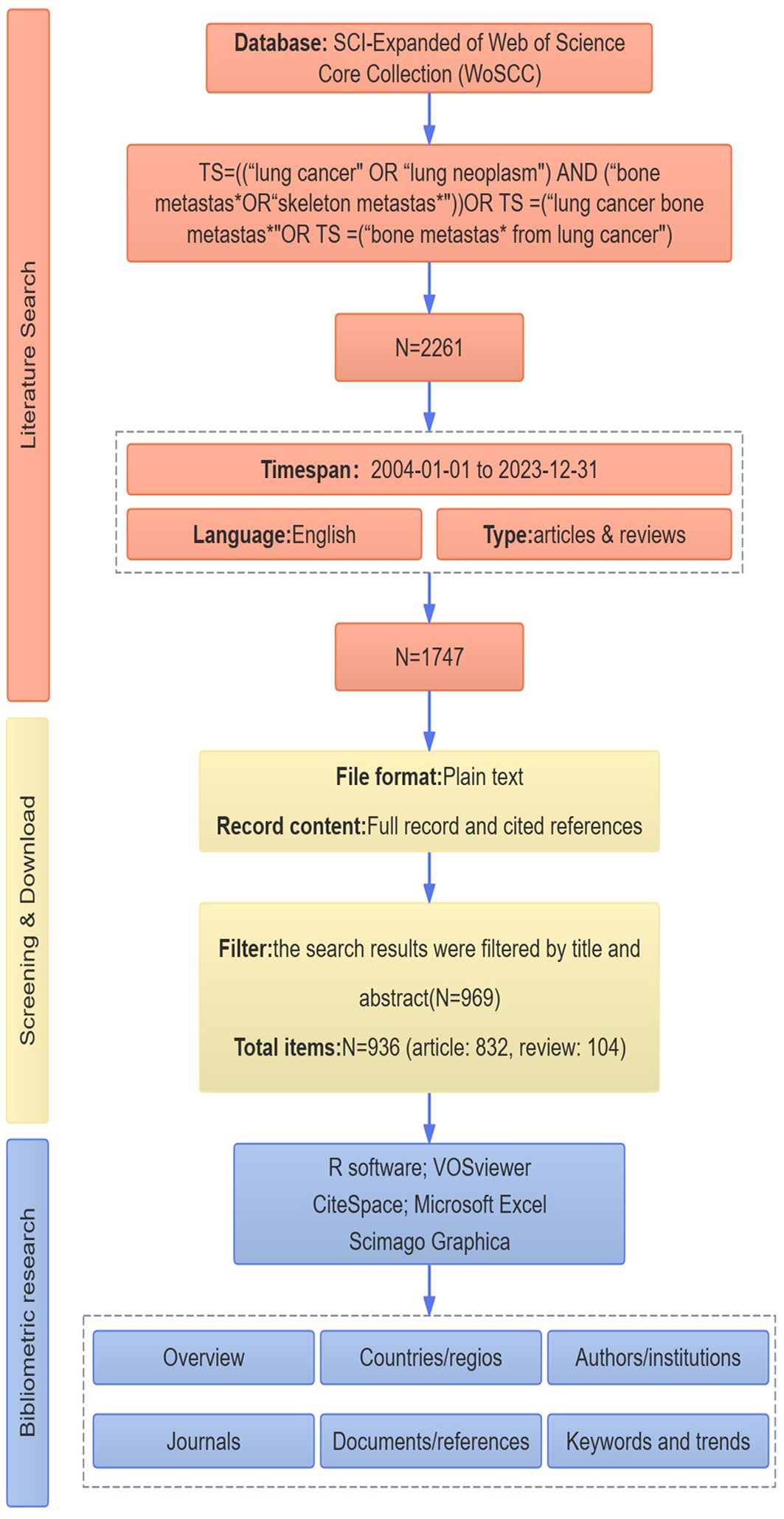
All published papers were collected from WoSCC, and two investigators identified relevant published between January 01, 2004, and December 31, 2023, using the following search strategy: TS = ((“lung cancer” OR “lung neoplasm”) AND (“bone metastas*” OR “skeleton metastas*”)) OR TS = (“lung cancer bone metastas*”) OR TS = (“bone metastas* from lung cancer”) AND publishing year = (2004–2023) AND document types = (articles & reviews) AND language = (English). After the preliminary data retrieval, two researchers (ZG Gu and ZQ Yang) screened all manuscripts separately to ensure they were relevant to the theme of this study, with any discrepancies being resolved by the experienced corresponding author (JD Shi, NK Niu, YY Wang). The flowchart of the study is shown in Figure 1.
All the records of publications, including year of publication, title, author names, affiliations, countries/regions, abstract, keywords, and name of publishing journals, The included records were exported and saved as plain text files with “Full Record and Cited References” for further bibliometric analysis.
2.3 Bibliometric analysis
Our bibliometric analysis followed a general to detailed path, including an overview of countries/regions, institutions/authors, journal distribution, documents/references, keywords, and trends. The collected data were later imported into Biblioshiny (R version 4.3.3), VOSviewer (version 1.6.20), Scimago Graphica (Version 1.0.41), CiteSpace (version 6.3.R1), and Microsoft Office Excel Professional Plus 2019 (Redmond WA, USA) for further analysis.
3 Results
3.1 Overall publication of global literature
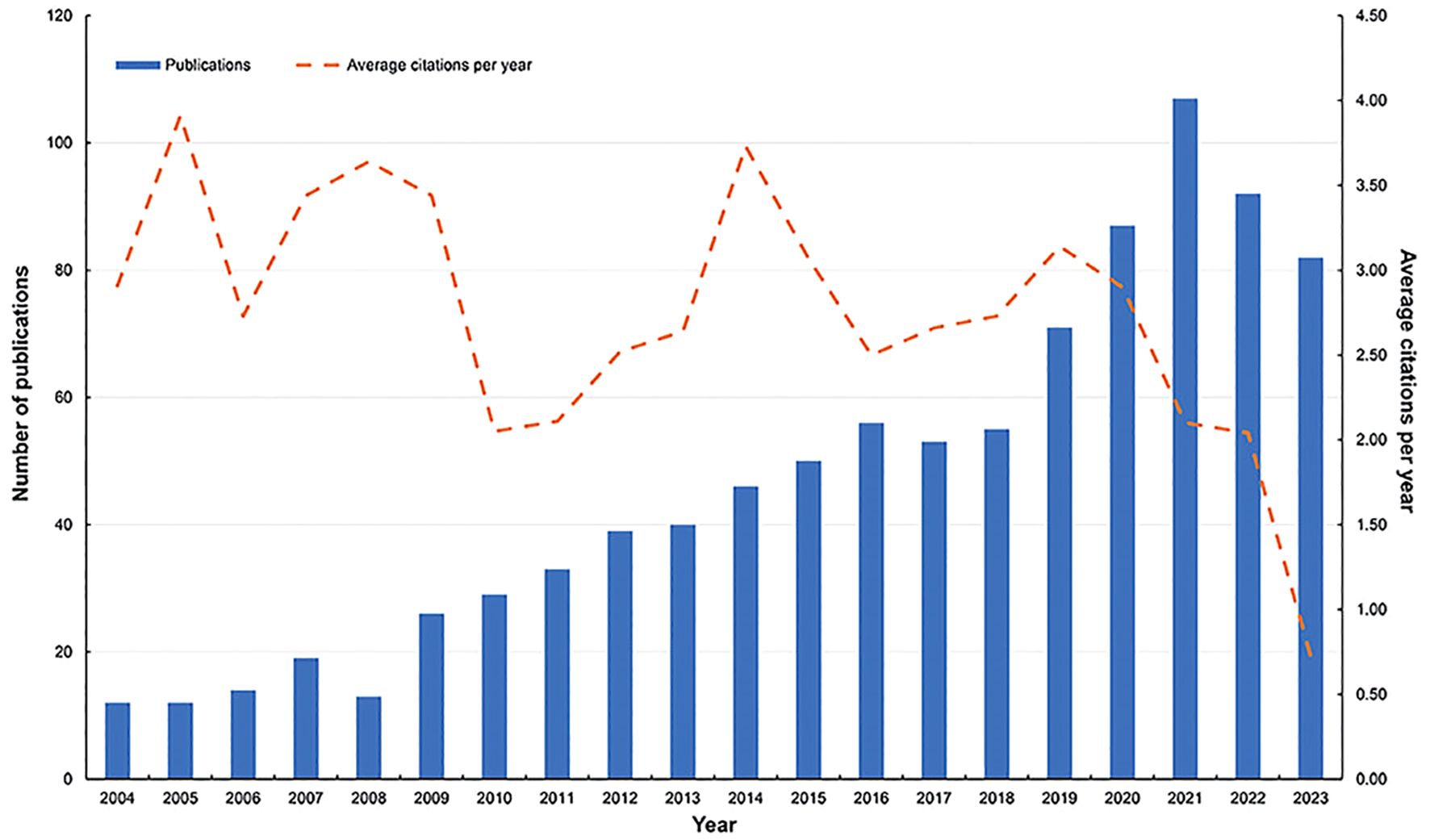
The flowchart of the study is shown in Figure 1. This study included 936 BMLC articles, 832 articles, and 104 reviews. The annual number of publications (NP) related to BMLC is shown in Figure 2. Annual publications increased from 12 in 2004 to 82 in 2023. However, there is an upward trend, peaking in 2021 (107 articles).
3.2 Analysis of countries/regions and institutions
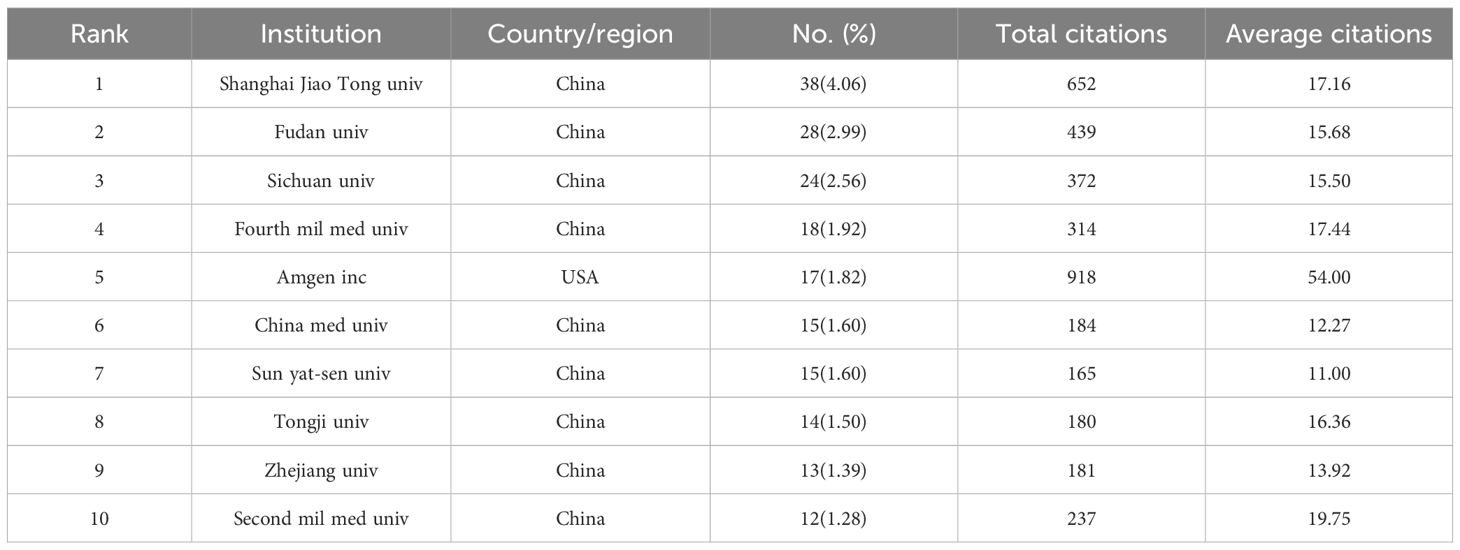
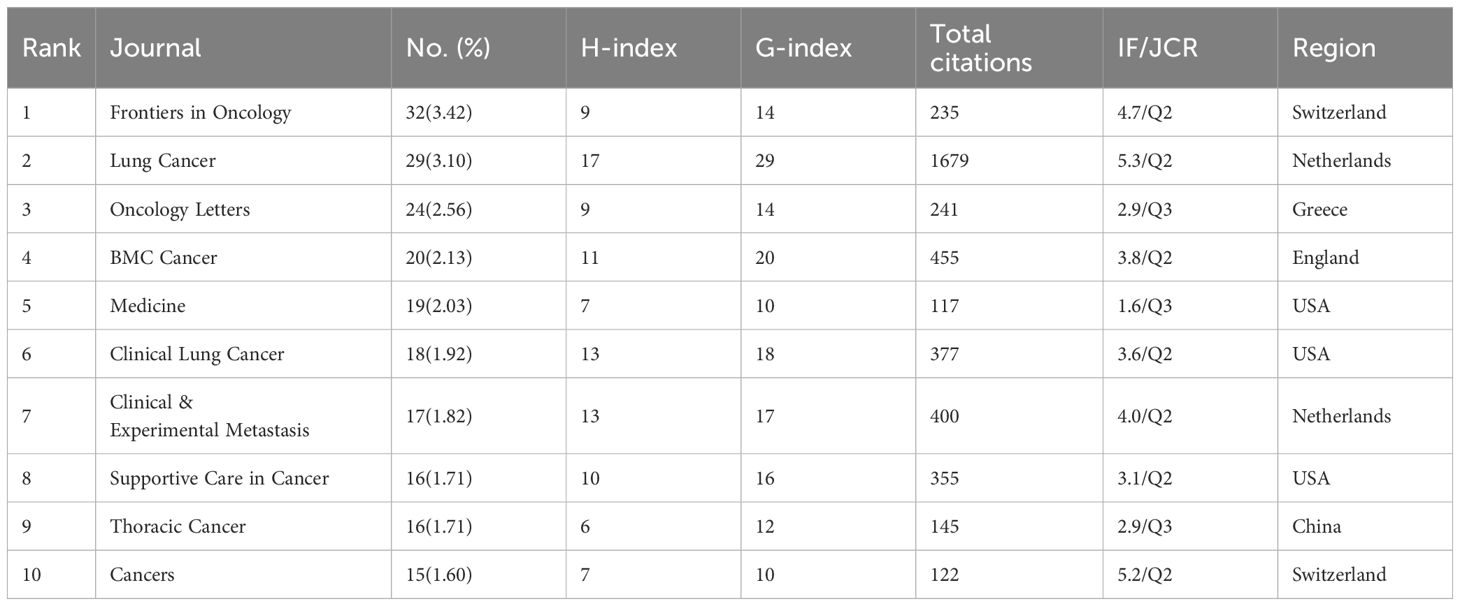
The number of publications in each country was analyzed to identify the countries/regions that have been the major contributors to the field. Researchers from 1543 institutions in 58 countries participated in the study of BMLC. The ten most important countries/regions for production were analyzed according to the country of residence of the corresponding author (Table 1). China published the highest number of articles (411, 43.91%). This was followed by Japan (111, 11.86%) and the United States (89, 9.51%) (Figure 3A). In addition, China ranked first among all participating countries/regions with 5,387 citations and an H-index of 35. The United States ranks second with 4,439 citations and an H-index of 34. This shows that China and the United States are the most significant contributors to the field. The total number of publications from these two countries exceeds half of the total. The 58 countries are divided into 16 clusters. The country of authorship in the articles and the circle size in the figure represent the number of publications (Figure 3B). The geographic map of the number of publications and the intensity of cooperation of each country is shown in (Figure 3C), and the United States is the country that initiates and participates in the most international collaborations. Figure 3D shows a network of collaborative relationships between leading institutions (with at least three publications). Table 2 shows the top 10 universities with the highest number of published papers, of which nine are from China, and one is from the United States. Shanghai Jiao Tong University published the most articles (38, 4.06%), followed by Fudan University (28, 3.00%) and Sichuan University (24, 2.56%). Among the top ten organizations regarding the number of articles published, Amgen Inc. ranked first with Average citations of 54.00, followed by Second Mil Med univ (19.75) and Shanghai Jiao Tong University (17.16).
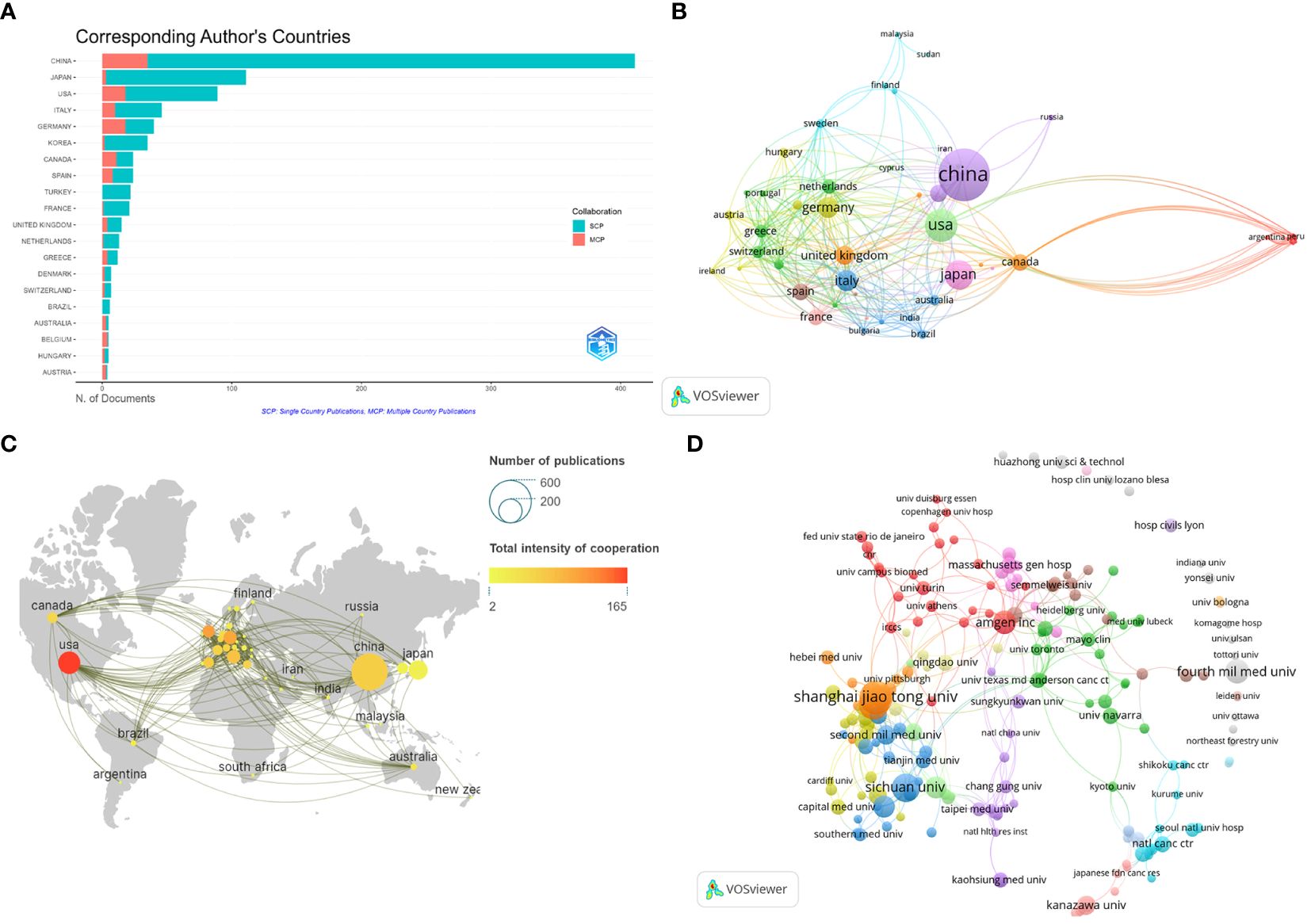
Figure 3 Visualize and analyze active countries and institutions. (A) Top 20 corresponding author’s countries;(B) The co-authorship map of countries/regions involved in BMLC; (C) Country cooperation map; (D) The co-authorship map of institutions engaged in BMLC research.
3.3 Authors and co-authors
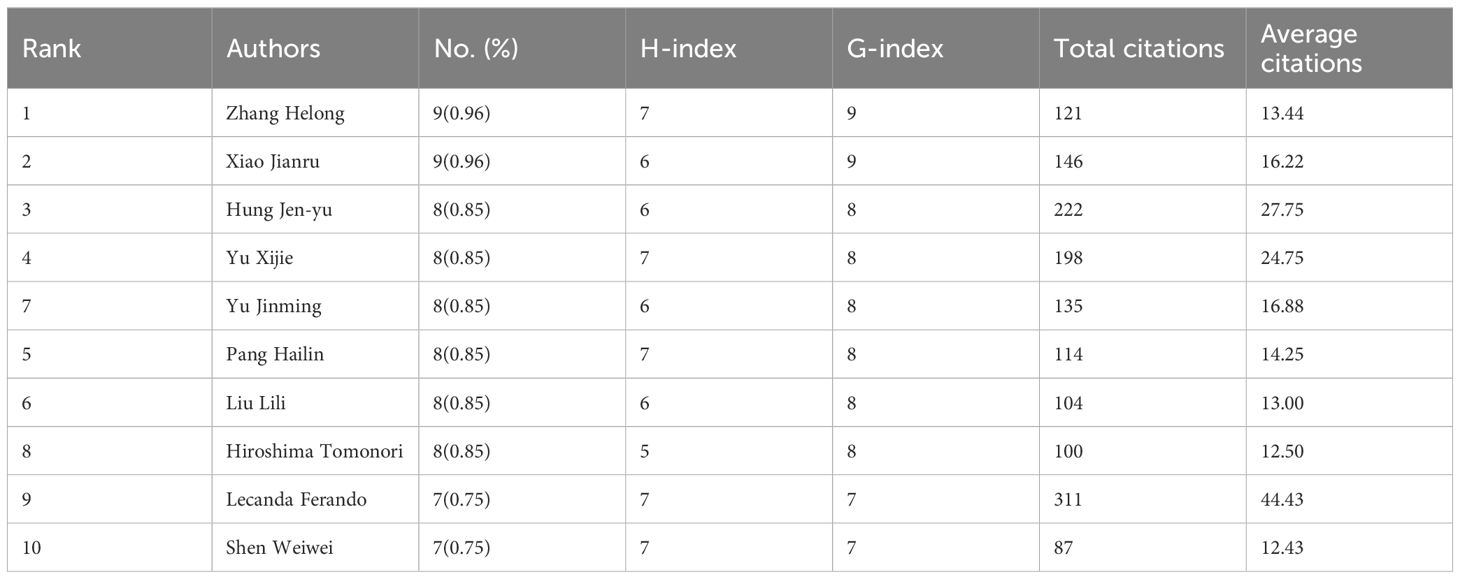
A total of 6,012 authors published research papers on bone metastasis in lung cancer, of which 257 scholars published three or more. 6,012 authors published research papers on BMLC, with 257 authors publishing three or more articles. Table 3 shows the 10 most published authors in this field. Zhang Helong and Xiao Jianru published the most articles (n = 9), and Lecanda Fernando ranked first in total citations (311) and average citations (44.43). Among the top ten authors, Hong Jenyu and Yu Jinming started their research earlier and continued their contribution to the field in 2022–2023 (Figure 4A). A total of 257 authors with at least three publications were grouped into 36 clusters (clusters), and we plotted the authors’ collaborative network graph (co-authors network graph) (Figure 4B). A total of 257 authors with at least three publications were divided into 36 clusters. We plotted the collaborative network graph of the authors (co-authors network graph) (Figure 4B). Each node represents an author, and larger nodes represent more published papers. Thicker lines indicate closer cooperation between authors. Different colors represent different clusters, and a relatively strong collaboration exists between authors in the same cluster.
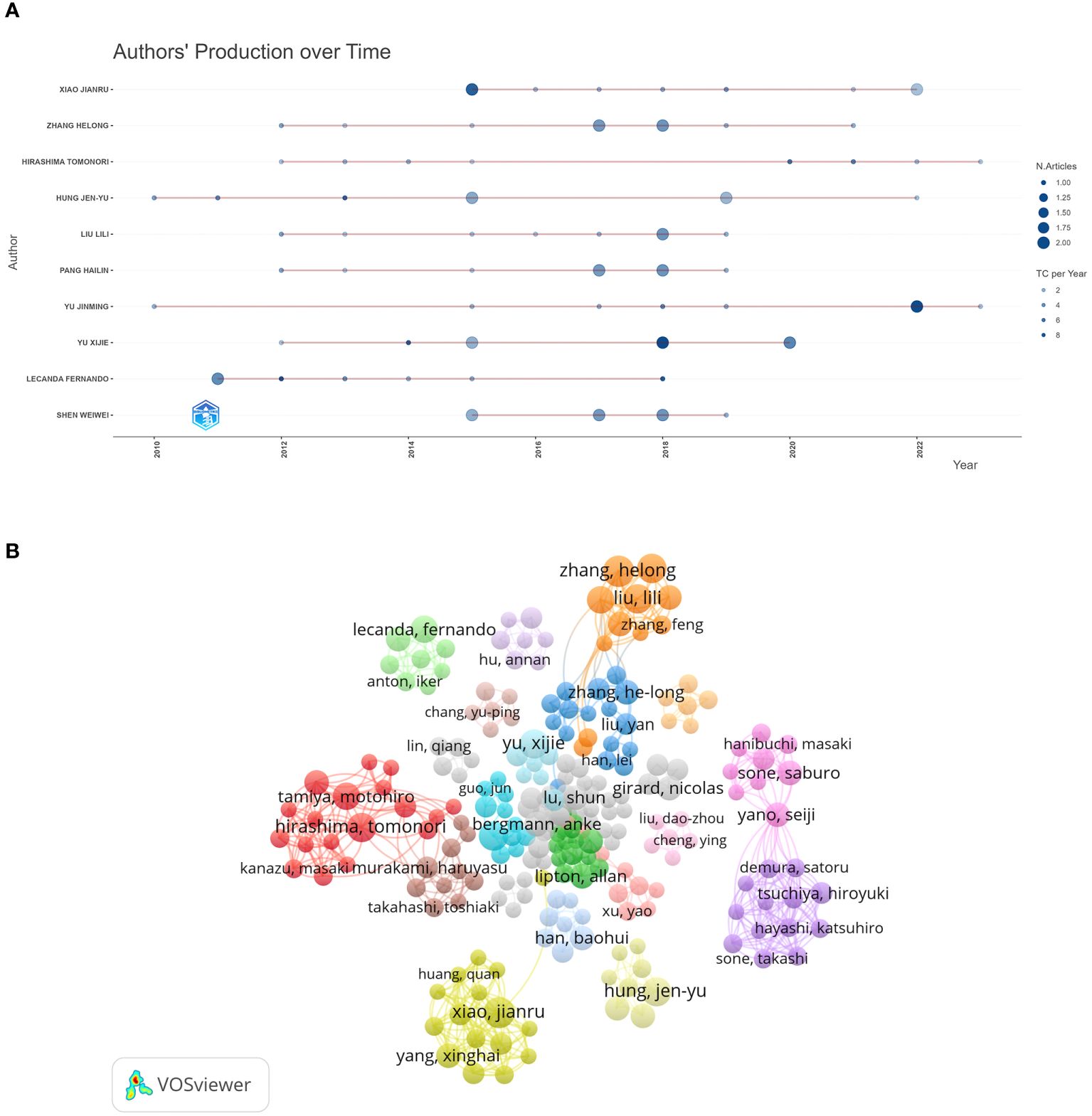
Figure 4 Visualization of active authors analysis. (A) Authors’ production over time. (B) The visualization map of authors’ co-authorship.
3.4 Journal distribution
A total of 267 journals were involved in publishing articles on bone metastasis research in lung cancer. Table 4 shows the top 10 journals and citations for BMLC. In addition, the top-ranked journals have high H-indexes and citation counts. This indicates that they play a crucial role in research in the field. Frontiers in Oncology has published the most, followed by Lung Cancer and Oncology Letters (Table 4).Figure 5A illustrates the authors’ publishing patterns in related topics and journals. Critical research keywords for the top 20 authors included bone metastasis/lung cancer/bone metastases/non-small cell lung cancer/metastasis/prognosis/chemotherapy/small cell lung cancer/denosumab/skeletal-related events/lung adenocarcinoma/zoledronic acid/survival/cancer. Their research is usually published in Medicine, Oncology Reports, and Tumor Biology journals. Among them, Medicine has become the journal that publishes the most articles on bone metastasis of lung cancer. Figure 5B shows the dual-map overlay of journals. On the left are the journals that have published articles on BMLC, and on the right is a map of cited journals.
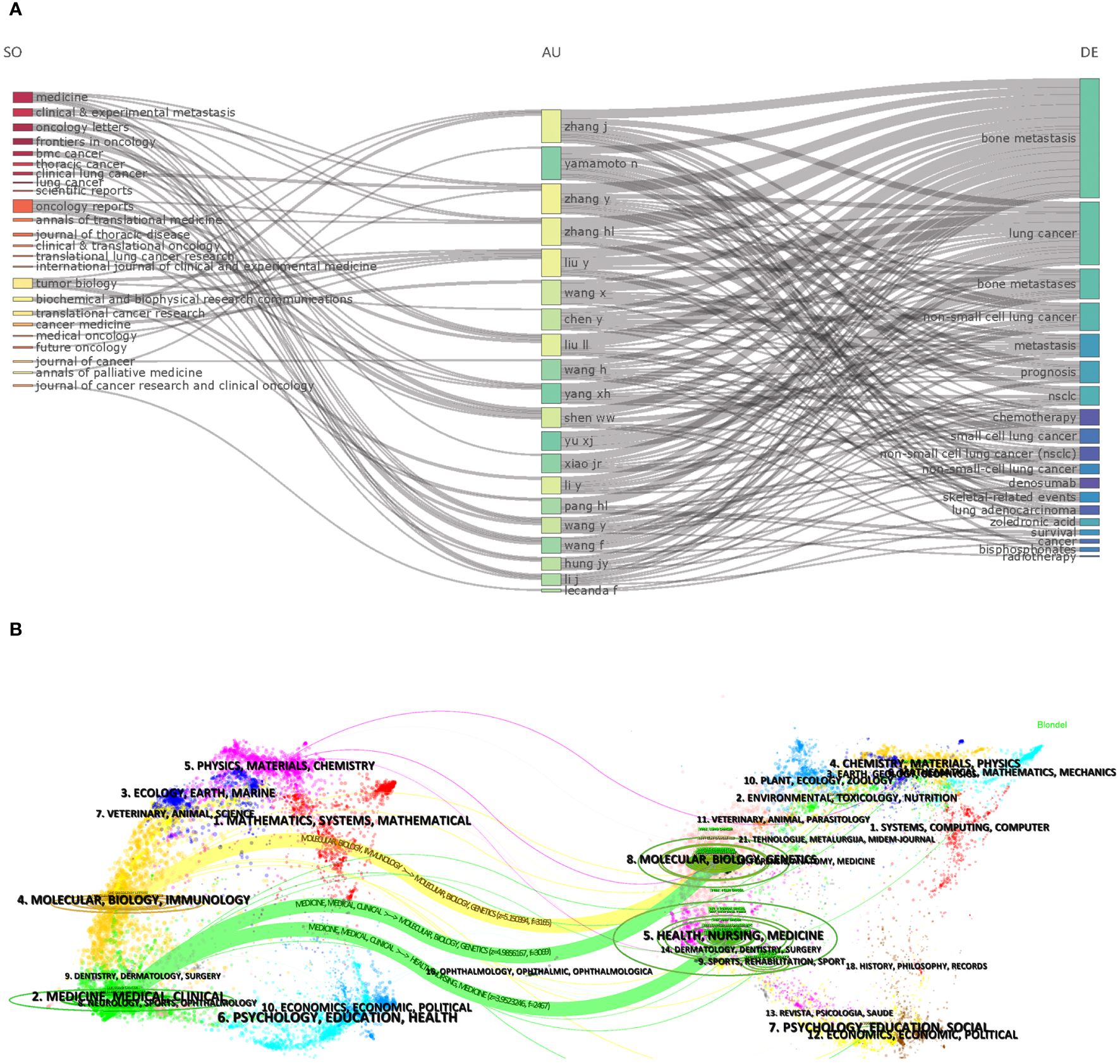
Figure 5 (A) Three-field plot (middle field: Authors; left field: Sources; right field: Keywords). (B) The dual-map overlay of journals related to LCBM.
3.5 Document citation and reference co-citation
We ranked the included papers according to their citation data in WoSCC. The top 10 papers were listed according to their number of citations, with the three most cited papers having 528 citations (Pathologic Fractures Correlate With Reduced Survival in Patients With Malignant Bone Disease (13), 460 (Metastatic Sites and Survival in lung cancer (14), and 441 (Prospective Fracture Correlation in Patients With Malignant Bone Disease (15). Metastatic sites and survival in lung cancer pointed out that bone metastasis in lung cancer had the most nervous system (47%) followed by bone (39%). We also assessed the impact of each paper in the field by counting the number of local citations in the current dataset. The ranking of locally cited literature differs slightly from the total cited literature. The highest-ranked paper in the locally cited literature was a study on bone metastases in non-small cell lung cancer by Asuka Tsuya, and the second and third-highest-ranked studies in terms of local citations were both studies on the clinical features, pathophysiology, and therapeutic strategies for metastatic bone disease by author R. E. Coleman. This represents a recognition of the significance and direction of research in this area. Figures 6A, B show the ten most cited publications globally and the ten most cited locally.
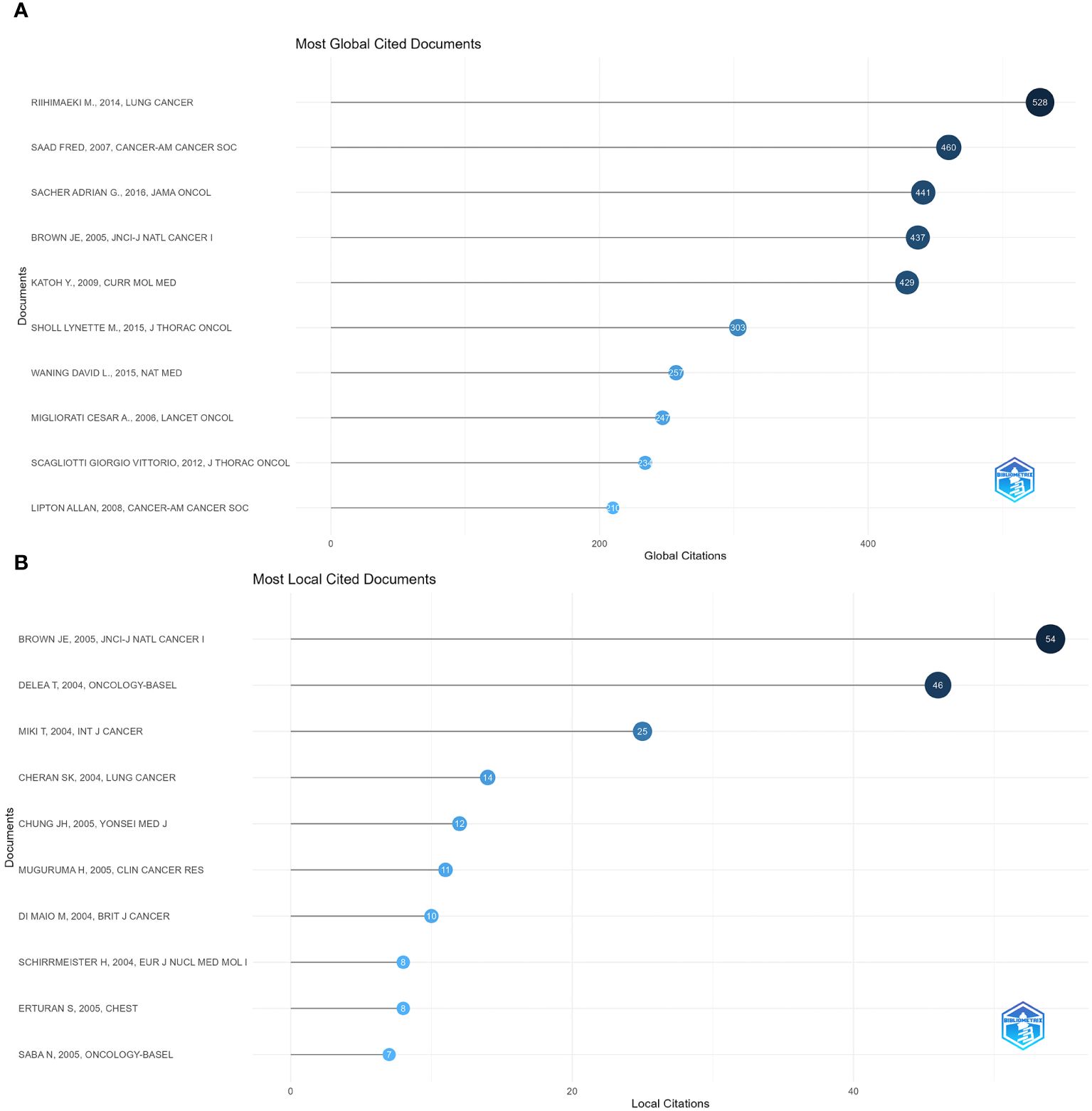
Figure 6 Visualization of document analysis. (A) Most global cited documents. (B) Most local cited documents.
In Figure 7A, co-cited references are presented as nodes. The most co-cited reference was “ Cancer statistics, 2019 (16)”, followed by “ Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity (17)”, “ Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma (18)” was the third most co-cited article. CiteSpace identified the top 25 references and depicted them in Figure 7B, demonstrating the strength and duration of the citation bursts for these references. The top 10 papers are landmarks in studying bone metastasis in lung cancer in order of citation (Supplementary Table 1).
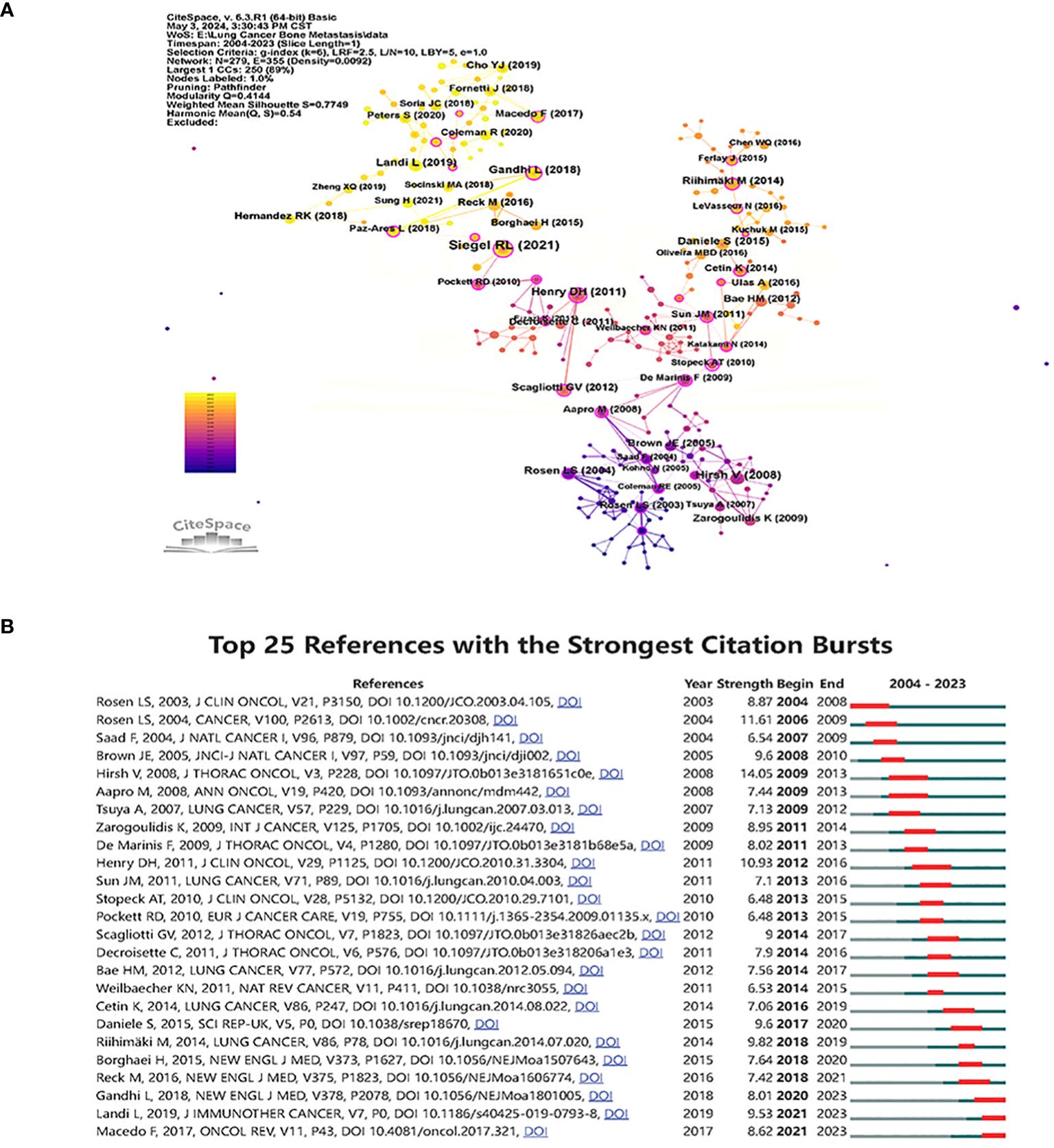
Figure 7 Visualize and analyze references analysis (A) Cluster analysis of co-cited references. (B) Top 25 references with the strongest citation bursts.
3.6 Keywords and trends
Figure 8A shows the high-frequency keywords analyzed by CiteSpace, with “lung cancer,” “bone metastasis,” and “survival” being the top three (larger circles in Figure 8A). These keywords summarize the topic. They also represent the research focus of the field. The details of the top 10 keywords are shown in Supplementary Table 1. Keyword clustering shows the frequency and distribution of two keywords that occur together. It forms a cluster representing the main research directions in the field (Figure 8B). These keywords formed six clusters (Clusters), including “non-small cell lung cancer,” “zoledronic acid,” “small cell cancer,” “lung cancer,” and “bone metastases.” Figure 8C shows the top 25 keywords with the strongest keyword bursts, which are intended to reflect research hotspots and trends. Among them, “skeletal complications (2005–2013, intensity 10.91)” was the keyword with the strongest burst intensity. The citation explosion of keywords “immunotherapy” and “denosumab” lasts from 2021 to 2023. From the timeline of keywords regarding treating BMLC, early studies (2005–2012) were focused on phosphonates’ efficacy in solid tumors with bone metastases (Figure 8D). Over time, there has been a gradual increase in studies of radiotherapy and chemotherapy (2010–2013). Pembrolizumab, Denosumab, Docetaxel, and nivolumab gradually came into the picture as drugs were developed. The keywords were analyzed by trend topic in Biblioshiny (Figure 9A). The top three by frequency of occurrence were “bone metastasis” (247), “lung cancer” (222), and “bone metastases” (147). Biblioshiny analysis of keywords shows that “non-small cell lung cancer” (2020–2022), “immunotherapy” (2021–2023), and “immune checkpoint inhibitors, ICIs” (2020–2022) may represent the frontiers of this research area. The abstracts were analyzed using the trend topic in Biblioshiny (Figure 9B). The top three by frequency of occurrence were “cell lung cancer” (395), “non-small cell lung” (318), and “lung cancer nsclc” (304). Biblioshiny analysis of abstracts shows that “serum tumor markers” (2021–2023), “vertebral body height” (2023–2023), and “nsclc patients receiving” (2023–2023) may represent the frontiers of this research area (Supplementary Table 2).
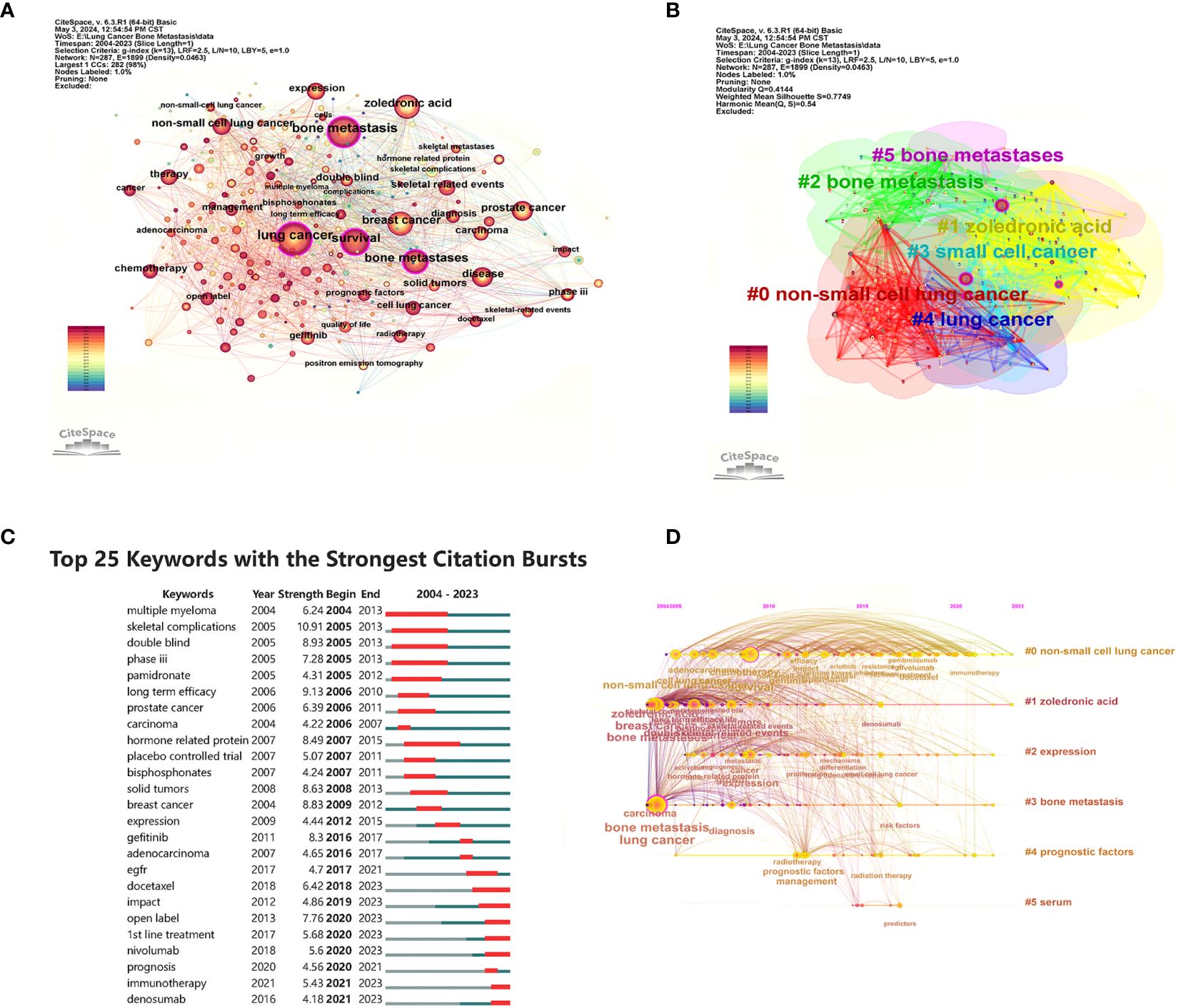
Figure 8 Visualization of keywords analysis. (A) The network map of keywords. (B) Clusters of keywords; (C) Top 25 keywords with the strongest citation bursts. (D) Keyword timeline view in LCBM research.
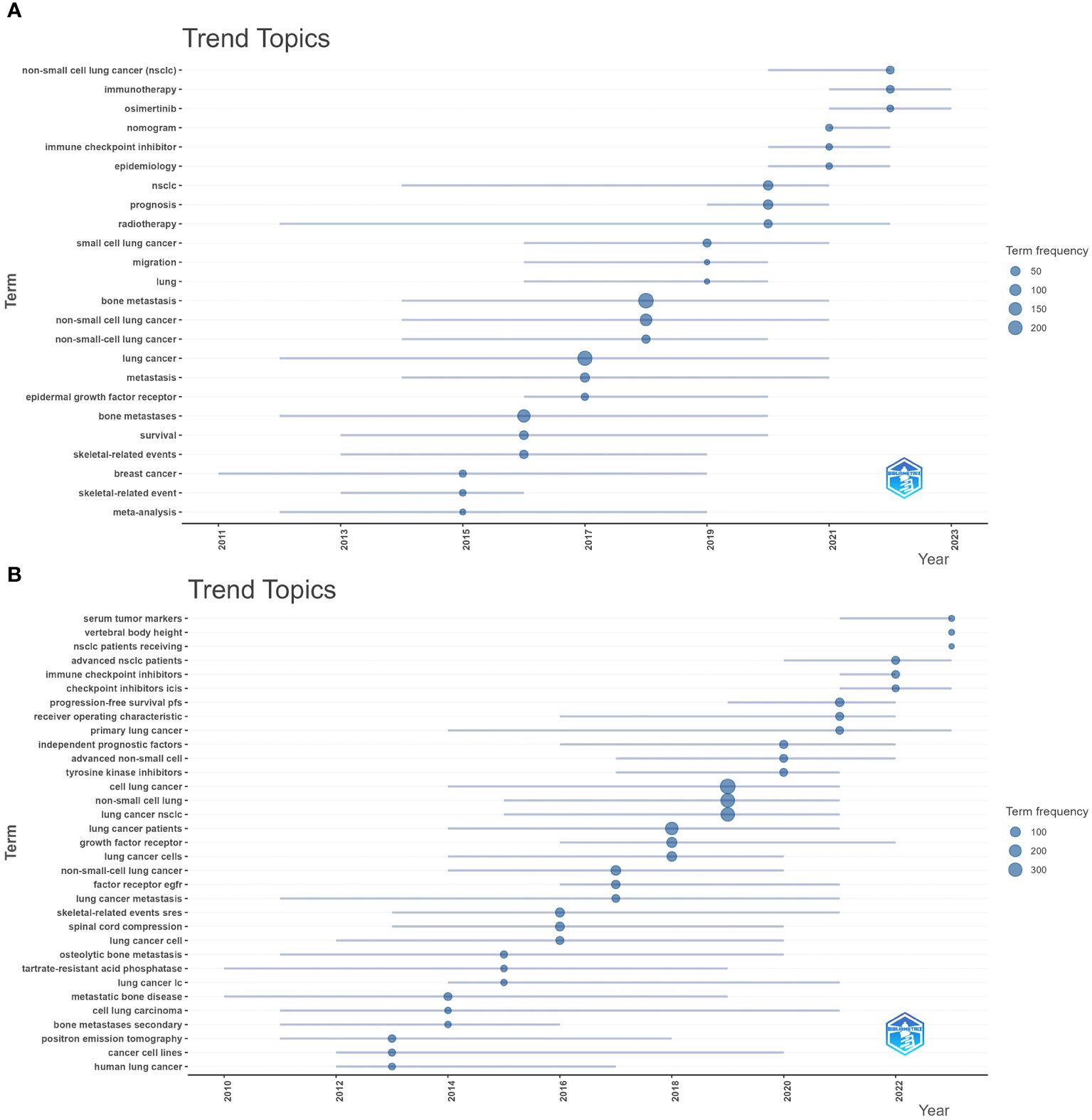
Figure 9 Visualize and analyze Trend Topics (A) Author’s keywords (Circle size represents frequency) (B). Visualization of Trend Topics. Abstracts (trigram).
4 Discussion
Patients with BMLC have an extremely high mortality rate and face serious treatment challenges. Relevant research on BMLC has developed rapidly in recent years. Bibliometrics, as an emerging method, can assess the current status and trend of research in a particular field. Bibliometrics, as a new method, can evaluate the research)) status and trends in a certain field. This paper can provide a reference for researchers to better grasp the development trend and emerging research hotspots through the bibliometric analysis of BMLC-related studies from 2004–2023 in the WoSCC database and the visualization of the international research status in this field.
In this study, we used VOSviewer, CiteSpace, and R to study the research dynamics and hotspots of BMLC in SCI-Expanded. A total of 936 papers were obtained from WoSCC. Between 2004 and 2023, the number of papers published yearly is expected to increase. This is consistent with the increasing trend of new cancers worldwide. The number of new cancer cases worldwide will increase from 18.7 million in 2010 to 23.6 million in 2019 (19). In general, there are fewer publications on BMLC than on solid tumor bone metastases such as breast cancer and lung metastases in prostate cancer (20, 21). This may be because when bone metastases occur in lung cancer, it means that patients have a shorter survival (14), with reported data ranging from 3 to 12 months, depending on the patient’s treatment (22).
China has the most significant publications and has contributed significantly to BMLC research. In addition, nine of the top 10 high-yielding institutions are from China, including Shanghai Jiao Tong University, Fudan University, and Sichuan University. This indicates that LCBM has received increasing attention from Chinese research institutions in recent years. Meanwhile, China ranked first in the H-index. However, the quality of Chinese BMLC research still needs to be improved, as the average number of citations is still at the bottom of the list. The reason for the high number of publications in China may be linked to the high incidence of lung cancer, which continues to have a high incidence (815,000 new lung cancer cases in 2020) and mortality rate. The burden of lung cancer in China is directly related to the high prevalence of smoking, especially among men. The prevalence of smoking among Chinese men, although declining, is still high (50% in 2019) (23). In addition, this has been associated with China’s large population, low rates of early lung cancer screening, and economic development (24). Despite the relatively small number of studies, the United States ranked first in the average number of citations and second in the H-index. This indicates a high level of research quality in the United States. At the same time, the United States plays a central role in the cooperation between countries/regions. Europe and other developed countries cooperate closely, while developing countries cooperate sparsely, as shown in Figure 3C.
Therefore, we should strengthen international cooperation to help developing countries. As far as authors are concerned, those experienced in the BMLC have become highly recognized, with Lecanda Fernando being the most cited international author. Among the top two authors with publications in BMLC, Zhang Helong et al. conducted an in-depth study on gene regulation and biomarkers in lung cancer bone metastasis (25–27). Xiao Jianru et al. found that surgery can improve patients’ quality of life with non-small cell lung cancer bone metastasis (28, 29).
Articles of BMLC are most often published in Frontiers in Oncology, followed by Lung Cancer and Oncology Letters. As a high-quality, influential journal in the field, Lung Cancer has the highest impact factor of 5.3 and is the most cited journal. The journals on the list will likely be the significant publication outlets in the field in the future. The most cited paper states that fractures are associated with an increased risk of death in patients with malignant bone disease. Therefore, prevention of fractures is an essential goal of treatment. Zoledronic acid significantly benefits patients with bone metastases by preventing skeletal complications and reducing bone pain. Thus, it allows patients to maintain mobility and functional independence and reduces the risk of death due to fracture complications (13). Nitrogen-containing bisphosphonates inhibit osteoclast-mediated bone destruction and block the release of bone-destroying cytokines and growth factors, thereby retarding the growth of bone tumors. It also inhibits tumor cell functions such as tumor cell adhesion, migration, invasion and proliferation, as well as bone matrix adhesion by inhibiting farnesyl diphosphate synthase (FPPS), thereby inducing apoptosis (30).
In Figure 8A, high-frequency keywords reveal the research hotspots of BMLC-related research fields to some extent. The top three high-frequency keywords were “lung cancer,” “bone metastasis,” and “survival.” They represent the most commonly used terms and research hotspots in BMLC research, and “survival” has become a research hotspot in this field. Treatment is the basis for improving survival, and the treatment of lung cancer bone metastasis can be divided into radiotherapy, chemotherapy, targeted therapy, immunotherapy, and multidisciplinary treatment. Radiotherapy can effectively reduce the damage caused by the disease and prolong the life of patients to a certain extent.
In recent years, with the advent of new radiotherapy techniques (such as stereotactic radiotherapy (31) and multidisciplinary oncology management (32), the combination of radiotherapy with new systemic, integrated treatment options (such as targeted therapy (33) and immunotherapy (34) can improve patient’s quality of life to a greater extent, thereby contributing to remission and significantly extending survival—reduction of treatment-related toxicity (35). Adjuvant chemotherapy, surgery, and phosphonate-targeted therapy also improved therapeutic effects (36–38).
The epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) gefitinib and erlotinib, used in adjuvant chemotherapy, showed significant antitumor activity in lung cancer with EGFR gene mutation (39). It has also been suggested that CT-guided 125 I brachytherapy may be a reasonable alternative for relieving painful bone metastases secondary to lung cancer after one cycle of chemotherapy progression. It can accelerate pain relief, improve quality of life, reduce pain recurrence, and reduce cost-effectiveness and similar complications (40), but it needs to be validated in a larger cohort. In recent years, basic research has helped better understand the relevant mechanisms, and targeted therapies are becoming increasingly influential. Receptor activators of the nuclear factor-κB ligand (RANKL)/RANK signaling pathway are critical mediators of bone remodeling. Targeting RANKL with the antibody denosumab is part of the standard treatment for bone loss diseases, including bone metastases (41). However, as with phosphonates, adverse consequences such as hypocalcemia, atypical femur fractures, and jaw osteonecrosis should be noted in clinical practice (42, 43).
Analyze the direction of abstract and keyword trends by Trend Topics. “non-small cell lung cancer,” “immunotherapy,” and “ immune checkpoint inhibitors, ICIs” may herald the frontiers of this research area. Bone metastases occur in more than 50% of patients with non-small cell lung cancer (NSCLC) (44). Adjuvant preoperative chemotherapy is poorly tolerated by patients due to its more pronounced toxicities, with a 5% improvement in 5-year survival (45). The emergence of immune checkpoint inhibitors (ICIs) has dramatically changed the therapeutic outlook for NSCLC patients, and preoperative neoadjuvant immunotherapy regimens have gradually gained attention as an effective and safe treatment option (46). By blocking programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated antigen-4, PD-L1 and cytotoxic T lymphocyte-associated antigen-4, PD-L1, and cytotoxic T lymphocyte-associated antigen-4, PD-L1 and PD-L1, NSCLC patients have been treated with a new preoperative adjuvant immunotherapy. -associated antigen-4 (CTLA-4) pathways interacting with inhibitory antibodies to enable activated T cells to release cytokines, perforins, granzymes, etc., to kill tumor cells in a rapidly evolving immunotherapeutic regimen (47). Preoperative neoadjuvant immunotherapy and postoperative adjuvant immunotherapy regimens using ICIs have also been investigated as alternatives to preoperative neoadjuvant chemotherapy and postoperative adjuvant chemotherapy regimens. Currently, ICI therapy, either as monotherapy or in combination with platinum-based chemotherapy, is approved as a standard of care for advanced NSCLC (48). Regarding the therapeutic role of ICIs for bone metastases, Asano Y et al. (49) used the PD-1 inhibitors natalizumab and pembrolizumab as well as the PD-L1 inhibitors atezolizumab and durvalumab for the treatment of In patients with advanced bone metastases of NSCLC, the results of the study showed that treatment with ICIs produced favorable therapeutic effects and improved prognosis in patients with advanced bone metastases of NSCLC. After the excellent progress of immunotherapy, many studies have focused on immunotherapy combined with chemotherapy or other drugs in combination regimens. Ma K et al. (50) showed that immunotherapy combined with antiangiogenic therapy is an option for patients with advanced NSCLC with reasonable safety and tolerability and pointed out that bone metastasis is a potential independent negative predictor of median overall survival. However, most of the current studies in this area are phase I and II studies, and in terms of data from phase III studies, the study by Forde P M et al. made significant progress on the regimen of preoperative natalizumab and platinum-containing drug chemotherapy in patients with NSCLC (51). However, experimental data still need to be published because phase III studies of other types of ICIs are still ongoing. More studies are required in order to explore the various kinds of ICIs, their cycles of use, and the choice of LCBM. It is worth proposing that with the development of precision medicine and artificial intelligence, the development of individualized column-line diagrams for BM risk stratification (52–54) and multiple machine learning algorithms provide new research ideas in identifying and predicting the occurrence of bone metastasis and the final clinical outcome of lung cancer (55–59).
5 Limitations
This research carries some limitations. Firstly, our study only included the WoSCC database, which may have led to the exclusion of important studies that were not included in this database. Secondly, the exclusion of non-English literature may have an impact on the overall results. Thirdly, our analysis was limited to bibliometric data and did not include a detailed qualitative analysis of the content of the studies, which may provide deeper insights into the topic.
6 Conclusion
This study used bibliometrics to reveal lung cancer bone metastasis’s research status and trend. International cooperation in LCBM research should be further strengthened. Our study provides valuable information for understanding and dealing with lung cancer bone metastasis and provides a basis for conducting more in-depth research and searching for more effective treatments. Further research should focus on the pathogenesis, early prevention, and individualized treatment of lung cancer bone metastases to improve patient survival and quality of life. There are some limitations to the study. First, the analyzed data was retrieved only from WoSCC, which may have excluded essential studies not included in that database. Second, only English articles were included in this study, which may have led to the exclusion of some critical studies in non-English language publications. Third, citations can be influenced by outdated research and publication dates. Finally, despite our standardized data processing, the analysis software’s mechanical nature may lead to inaccurate keyword extraction and incomplete article content analysis. However, the impact of the above deficiencies on the overall trend of the article is still within the acceptable range.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JT: Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing, Software. ZG: Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ZY: Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. LM: Writing – review & editing, Methodology. QL: Methodology, Writing – review & editing. JS: Methodology, Supervision, Visualization, Writing – review & editing. NN: Methodology, Supervision, Visualization, Writing – review & editing. YW: Methodology, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by the Ningxia Natural Science Foundation (2023AAC03591).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1439209/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Li S, Peng Y, Weinhandl ED, Blaes AH, Cetin K, Chia VM, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. (2012) 4:87–93. doi: 10.2147/CLEP.S28339
3. Stopeck A, Brufsky A, Kennedy L, Bhatta S, Bhowmik D, Buchanan J, et al. Cost-effectiveness of denosumab for the prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. (2020) 23:37–47. doi: 10.1080/13696998.2019.1651122
4. Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology-basel. (2004) 67:390–6. doi: 10.1159/000082923
5. Shiraishi Y, Hakozaki T, Nomura S, Kataoka T, Tanaka K, Miura S, et al. A multicenter, randomized phase III study comparing platinum combination chemotherapy plus pembrolizumab with platinum combination chemotherapy plus nivolumab and ipilimumab for treatment-naive advanced non-small cell lung cancer without driver gene alterations: JCOG2007 (NIPPON study). Clin Lung Cancer. (2022) 23:e285–8. doi: 10.1016/j.cllc.2021.10.012
6. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol. (2023) 41:2458–66. doi: 10.1200/JCO.22.02544
7. Quint LE, Tummala S, Brisson LJ, Francis IR, Krupnick AS, Kazerooni EA, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg. (1996) 62:246–50. doi: 10.1016/0003-4975(96)00220-2
8. Zhang S, Zhang X, Zhang D, Wei L, Xiong B, Meng Q, et al. Synergistic effect of docetaxel and gambogic acid on bone metastasis of lung cancer. B Cancer. (2023) 110:478–86. doi: 10.1016/j.bulcan.2023.02.007
9. Jin PC, Gou B, Qian W. Urinary markers in treatment monitoring of lung cancer patients with bone metastasis. Int J Biol Markers. (2019) 34:243–50. doi: 10.1177/1724600819848762
10. Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. (2018) 18:44. doi: 10.1186/s12885-017-3922-0
11. Knapp BJ, Devarakonda S, Govindan R. Bone metastases in non-small cell lung cancer: a narrative review. J Thorac Dis. (2022) 14:1696–712. doi: 10.21037/jtd-21-1502
12. Kim J, Jeong C, Lee J, Ha J, Baek K-H, Kim S, et al. Bone-modifying agents for non-small-cell lung cancer patients with bone metastases during the era of immune checkpoint inhibitors: a narrative review. Semin Oncol. (2023) 50:105–12. doi: 10.1053/j.seminoncol.2023.09.002
13. Saad F, Lipton A, Cook R, Chen Y-M, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with Malignant bone disease. Cancer-am Cancer Soc. (2007) 110:1860–7. doi: 10.1002/cncr.22991
14. Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer (Amst Neth). (2014) 86:78–84. doi: 10.1016/j.lungcan.2014.07.020
15. Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. (2016) 2:1014–22. doi: 10.1001/jamaoncol.2016.0173
16. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
17. Hirsh V, Major PP, Lipton A, Cook RJ, Langer CJ, Smith MR, et al. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. (2008) 3:228–36. doi: 10.1097/JTO.0b013e3181651c0e
18. Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol: Off J Am Soc Clin Oncol. (2011) 29:1125–32. doi: 10.1200/JCO.2010.31.3304
19. Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. (2022) 8:420. doi: 10.1001/jamaoncol.2021.6987
20. Chen Y, Guo Z-N, He R-Q, Huang Z-G, Luo J-Y, Tang W, et al. How has the field of metastatic breast cancer in bones evolved over the past 22 years? J Bone Oncol. (2023) 40:100480. doi: 10.1016/j.jbo.2023.100480
21. Chen Y, Tang C, Shen Z, Peng S, Wu W, Lei Z, et al. Bibliometric analysis of the global research development of bone metastases in prostate cancer: a 22-year study. Front Oncol. (2022) 12:947445. doi: 10.3389/fonc.2022.947445
22. Socinski MA, Morris DE, Masters GA, Lilenbaum R, American College of Chest Physicians. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. (2003) 123:226S–43S. doi: 10.1378/chest.123.1_suppl.226S
23. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
24. Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. (2023) 20:624–39. doi: 10.1038/s41571-023-00798-3
25. Duan L, Pang H-L, Chen W-J, Shen W-W, Cao P-P, Wang S-M, et al. The role of GDF15 in bone metastasis of lung adenocarcinoma cells. Oncol Rep. (2019) 41:2379–88. doi: 10.3892/or.2019.7024
26. Ma N-Q, Liu L-L, Min J, Wang J-W, Jiang W-F, Liu Y, et al. The effect of down regulation of calcineurin aα by lentiviral vector-mediated RNAi on the biological behavior of small-cell lung cancer and its bone metastasis. Clin Exp Metastasis. (2011) 28:765–78. doi: 10.1007/s10585-011-9408-6
27. Chen P, Min J, Wu H, Zhang H, Wang C, Tan G, et al. Annexin A1 is a potential biomarker of bone metastasis in small cell lung cancer. Oncol Lett. (2021) 21:141. doi: 10.3892/ol.2020.12402
28. Tang Y, Qu J, Wu J, Li S, Zhou Y, Xiao J. Metastatic spinal cord compression from non-small-cell lung cancer treated with surgery and adjuvant therapies: a retrospective analysis of outcomes and prognostic factors in 116 patients. J Bone Joint Surg Am. (2015) 97:1418–25. doi: 10.2106/JBJS.N.01124
29. Tang Y, Qu J, Wu J, Liu H, Chu T, Xiao J, et al. Effect of surgery on quality of life of patients with spinal metastasis from non-small-cell lung cancer. J Bone Joint Surg Am. (2016) 98:396–402. doi: 10.2106/JBJS.O.00629
30. Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM, et al. Metastasis and bone loss: Advancing treatment and prevention. Cancer Treat Rev. (2010) 36:615–20. doi: 10.1016/j.ctrv.2010.04.003
31. Thureau S, Marchesi V, Vieillard M-H, Perrier L, Lisbona A, Leheurteur M, et al. Efficacy of extracranial stereotactic body radiation therapy (SBRT) added to standard treatment in patients with solid tumors (breast, prostate and non-small cell lung cancer) with up to 3 bone-only metastases: study protocol for a randomised phase III trial (STEREO-OS). BMC Cancer. (2021) 21:117. doi: 10.1186/s12885-021-07828-2
32. D’Oronzo S, Coleman R, Brown J, Silvestris F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol. (2019) 15:4–4. doi: 10.1016/j.jbo.2018.10.004
33. Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. (2014) 40:558–66. doi: 10.1016/j.ctrv.2013.10.001
34. Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest. (2020) 130:3603–20. doi: 10.1172/JCI133334
35. Hendriks LEL, Hermans BCM, van den Beuken-van Everdingen MHJ, Hochstenbag MMH, Dingemans A-MC. Effect of bisphosphonates, denosumab, and radioisotopes on bone pain and quality of life in patients with non-small cell lung cancer and bone metastases: A systematic review. J Thorac Oncol (2016) 11:155–73. doi: 10.1016/j.jtho.2015.10.001
36. Yu M, Su Y, Cui D, Sun Q, Luan B, Zhao D. Chemotherapy effectively suppresses interleukin-20, receptor activator of nuclear factor kappa-B ligand, and osteoprotegerin levels in patients with lung adenocarcinoma and bone metastasis. J Cancer Res Ther. (2016) 12:963–8. doi: 10.4103/0973-1482.179085
37. Takahashi Y, Adachi H, Mizukami Y, Yokouchi H, Oizumi S, Watanabe A. Patient outcomes post-pulmonary resection for synchronous bone-metastatic non-small cell lung cancer. J Thorac Dis. (2019) 11:3836–45. doi: 10.21037/jtd.2019.09.17
38. Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer-am Cancer Soc. (2008) 113:193–201. doi: 10.1002/cncr.23529
39. Inomata M, Shukuya T, Takahashi T, Ono A, Nakamura Y, Tsuya A, et al. Continuous administration of EGFR-TKIs following radiotherapy after disease progression in bone lesions for non-small cell lung cancer. Anticancer Res. (2011) 31:4519–23.
40. Xiang Z, Wang L, Yan H, Zhong Z, Liu W, Mo Z, et al. 125I seed brachytherapy versus external beam radiation therapy for the palliation of painful bone metastases of lung cancer after one cycle of chemotherapy progression. OncoTargets Ther. (2018) 11:5183–93. doi: 10.2147/OTT.S154973
41. Casimiro S, Vilhais G, Gomes I, Costa L. The roadmap of RANKL/RANK pathway in cancer. Cells. (2021) 10:1978. doi: 10.3390/cells10081978
42. Cheng X, Wei J, Ge Q, Xing D, Zhou X, Qian Y, et al. The optimized drug delivery systems of treating cancer bone metastatic osteolysis with nanomaterials. Drug Delivery. (2021) 28:37–53. doi: 10.1080/10717544.2020.1856225
43. Cheung E, Borno HT. The limitations of today’s clinical guidance: atypical femoral fracture and long-term bone-modifying agents in the oncology setting. J Oncol Pharm Pract: Off Publ Int Soc Oncol Pharm Pract. (2020) 26:1180–9. doi: 10.1177/1078155220907965
44. Del Conte A, De Carlo E, Bertoli E, Stanzione B, Revelant A, Bertola M, et al. Bone metastasis and immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): microenvironment and possible clinical implications. Int J Mol Sci. (2022) 23:6832. doi: 10.3390/ijms23126832
45. NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. (2014) 383:1561–71. doi: 10.1016/S0140-6736(13)62159-5
46. Zhou J, Lu X, Zhu H, Ding N, Zhang Y, Xu X, et al. Resistance to immune checkpoint inhibitors in advanced lung cancer: clinical characteristics, potential prognostic factors and next strategy. Front Immunol. (2023) 14:1089026. doi: 10.3389/fimmu.2023.1089026
47. Ji J, Zhang C, Peng L, Jiao W. [research progress, benefit groups, treatment cycle and efficacy prediction of neoadjuvant immunotherapy for non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi = Chin J Lung Cancer. (2022) 25:92–101. doi: 10.3779/j.issn.1009-3419.2022.101.01
48. Liu C, Wang M, Xu C, Li B, Chen J, Chen J, et al. Immune checkpoint inhibitor therapy for bone metastases: specific microenvironment and current situation. J Immunol Res. (2021) 2021:8970173. doi: 10.1155/2021/8970173
49. Asano Y, Yamamoto N, Demura S, Hayashi K, Takeuchi A, Kato S, et al. Novel predictors of immune checkpoint inhibitor response and prognosis in advanced non-small-cell lung cancer with bone metastasis. Cancer Med. (2023) 12:12425–37. doi: 10.1002/cam4.5952
50. Ma K, Guo Q, Li X. Efficacy and safety of combined immunotherapy and antiangiogenic therapy for advanced non-small cell lung cancer: a real-world observation study. BMC Pulm Med. (2023) 23:175. doi: 10.1186/s12890-023-02470-z
51. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
52. Hu X, Huang W, Sun Z, Ye H, Man K, Wang Q, et al. Predictive factors, preventive implications, and personalized surgical strategies for bone metastasis from lung cancer: population-based approach with a comprehensive cancer center-based study. EPMA J. (2022) 13:57–75. doi: 10.1007/s13167-022-00270-9
53. Zeng H, Huang W-W, Liu Y-J, Huang Q, Zhao S-M, Li Y-L, et al. Development and validation of a nomogram for predicting prognosis to immune checkpoint inhibitors plus chemotherapy in patients with non-small cell lung cancer. Front Oncol. (2021) 11:685047. doi: 10.3389/fonc.2021.685047
54. Dong Q, Deng J, Mok TN, Chen J, Zha Z. Construction and validation of two novel nomograms for predicting the overall survival and cancer-specific survival of NSCLC patients with bone metastasis. Int J Gen Med. (2021) 14:9261–72. doi: 10.2147/IJGM.S342596
55. Yu C-C, Ting C-Y, Yang M-H, Chan H-P. Comparison of irregular flux viewer system with BONENAVI version for identification of tc-99m MDP whole body bone scan metastasis images. J X-Ray Sci Technol. (2021) 29:617–33. doi: 10.3233/XST-200834
56. Gao H, He Z-Y, Du X-L, Wang Z-G, Xiang L. Machine learning for the prediction of synchronous organ-specific metastasis in patients with lung cancer. Front Oncol. (2022) 12:817372. doi: 10.3389/fonc.2022.817372
57. Li M-P, Liu W-C, Sun B-L, Zhong N-S, Liu Z-L, Huang S-H, et al. Prediction of bone metastasis in non-small cell lung cancer based on machine learning. Front Oncol. (2022) 12:1054300. doi: 10.3389/fonc.2022.1054300
58. Zhou C-M, Wang Y, Xue Q, Zhu Y. Differentiation of bone metastasis in elderly patients with lung adenocarcinoma using multiple machine learning algorithms. Cancer Control: J Moffitt Cancer Cent. (2023) 30:10732748231167958. doi: 10.1177/10732748231167958
59. Cui Y, Shi X, Wang S, Qin Y, Wang B, Che X, et al. Machine learning approaches for prediction of early death among lung cancer patients with bone metastases using routine clinical characteristics: an analysis of 19,887 patients. Front Public Health. (2022) 10:1019168. doi: 10.3389/fpubh.2022.1019168
Keywords: lung cancer, bone metastasis, bibliometrics, current state, research
Citation: Tang J, Gu Z, Yang Z, Ma L, Liu Q, Shi J, Niu N and Wang Y (2024) Bibliometric analysis of bone metastases from lung cancer research from 2004 to 2023. Front. Oncol. 14:1439209. doi: 10.3389/fonc.2024.1439209
Received: 27 May 2024; Accepted: 22 July 2024;
Published: 06 August 2024.
Edited by:
Xi Yang, Fudan University, ChinaReviewed by:
Keqiang Zhang, City of Hope National Medical Center, United StatesJiahua Lyu, Sichuan Cancer Hospital, China
Copyright © 2024 Tang, Gu, Yang, Ma, Liu, Shi, Niu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiandang Shi, c2hpX2ppYW5kYW5nQG54bXUuZWR1LmNu; Ningkui Niu, bmluZ2t1aW5pdUAxNjMuY29t; Yanyang Wang, ZmR3eXkxOTgxQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Jing Tang
Jing Tang Zhangui Gu
Zhangui Gu Zongqiang Yang
Zongqiang Yang Long Ma3
Long Ma3 Qiang Liu
Qiang Liu Jiandang Shi
Jiandang Shi Ningkui Niu
Ningkui Niu Yanyang Wang
Yanyang Wang