
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 01 October 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1437574
Background: The role of high-dose chemotherapy followed by autologous hematopoietic cell transplantation in the management of patients with relapsed/refractory germ-cell tumors has not been established in prospective studies. Our aim was to estimate the benefits and harm of this treatment in men with relapsed/refractory germ-cell tumors.
Methods: Electronic databases, conference proceedings, and trial registers until April 30, 2023, were searched. Randomized and non-randomized prospective controlled trials were included. Risk of bias assessments were performed using either RoB2 or ROBINS-I tools. The certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach. Time-to-event data were analyzed using the hazard ratio. The primary outcome was overall survival, and a meta-analysis was not conducted to assess it because non-randomized trials were judged to have a critical risk of bias. Categorical data were analyzed using a risk ratio. All results are presented with the corresponding 95% confidence interval.
Results: Four out of 3,824 records met the inclusion criteria, and three out of four were used to assess primary and secondary outcomes. Based on the IT94 study (N = 263 participants), single high-dose chemotherapy followed by autologous hematopoietic cell transplantation may have little to no effect on overall survival [hazard ratio (HR) 0.98, 95%CI 0.68 to 1.42; p = 0.916]. Non-randomized trials (N = 43 participants) showed contrasting results, which may be explained by the number of cycles of high-dose chemotherapy administered in each study. Regarding secondary outcomes, information was only provided for event-free survival, response rate, and acute toxicities.
Conclusions: Based on prospective data, there is insufficient evidence to support or refute the proposal that high-dose chemotherapy with autologous hematopoietic cell transplantation improves survival in men with relapsed/refractory germ-cell tumors. If this treatment is considered essential, the choice should be made by experienced clinicians at high-volume cancer centers.
The role of high-dose chemotherapy (HDCT) followed by autologous hematopoietic cell transplantation (AHCT) in the management of patients with relapsed or refractory germ-cell tumors (GCTs) has not been established in prospective studies. This intervention is expensive and may be associated with severe hematological and non-hematological toxicities, as well as death, while the impact of HDCT on quality of life (QoL) is largely unknown.
Testicular GCT is a highly curable malignancy. In fact, roughly 90% of all testicular GCTs may be cured with medical treatment (1, 2). The majority of stage I testicular GCT patients are cured after radical orchiectomy. Stage II and III (metastatic) GCT patients undergo further treatments after surgery, including platinum-based chemotherapy, radiotherapy, or retroperitoneal lymph node dissection.
Experience matters in GCT management. Analyses from the Swedish Norwegian Testicular Cancer Project and Scotland showed that patients with metastatic GCTs had better survival rates when they were treated at high-volume centers compared with those treated at low-volume hospitals (3).
According to the The International Germ Cell Cancer Collaborative Group (IGCCCG) Update Consortium, progression-free survival (PFS) and overall survival (OS) for metastatic seminoma and non-seminoma significantly improved between 1990 and 2013 (1, 2). Despite these improvements, the 3-year overall survival of metastatic patients with relapsed or refractory disease after first-line platinum-based chemotherapy ranges from 6.1% to 77% (4). Regrettably, it is a matter of debate which is the best course of action across prognostic categories. The most relevant clinical practice guidelines in the management of germ-cell malignancies do not show a clear recommendation regarding the treatment of relapsed/refractory GCTs after first-line chemotherapy (5, 6). Guidelines and experts do provide a clear statement in two rare clinical scenarios: “growing teratoma” syndrome during or after platinum-based chemotherapy and resectable late relapsed GCTs, where salvage surgery would be the best treatment option (7, 8). In situations where chemotherapy is the cornerstone salvage therapy, it is still controversial if HDCT has a superior effect to conventional-dose chemotherapy (CDCT). In fact, several studies with contradictory results have been published on this topic, including phase I, II, and III clinical trials and retrospective studies as well as systematic reviews.
In theory, increasing the dose of chemotherapy may amplify the treatment efficacy. High doses of chemotherapy may overcome the resistance of germ-cell tumor cells to conventional-dose regimens and improve survival. However, HDCT produces dose-limiting toxicities, making the bone marrow the most affected tissue that can be salvaged using stem cell transplant (9).
The ability to safely deliver HDCT followed by AHCT is strongly associated with the medical team’s experience. Actually, it is well known that death rates due to toxicity are largely related to expertise (10, 11). Patients receiving HDCT require several supportive treatments and teamwork. Supportive care needed during treatment includes AHCT, granulocyte-colony stimulating factor, transfusion of blood products, and intensive care unit support.
Many unknowns exist regarding HDCT regimens, including the number of cycles, drugs, and the need for induction chemotherapy. This reflects what happens in daily clinical practice worldwide (12).
Two phase III randomized controlled trials (RCTs) have tested the efficacy of HDCT in previously treated GCT patients (13, 14). In addition, a current international phase III RCT is assessing the efficacy of Paclitaxel, Ifosfamide and Cisplatin (TIP) given at first relapse compared with Paclitaxel and Ifosfamide followed by Carboplatin and Etoposide (TI-CE) followed by AHCT (15). The IT94 trial used four cycles of Etoposide, Ifosfamide and Cisplatin (VIP)/Vinblastin, Ifosfamide and Cisplatin (VeIP) compared with three cycles of VIP/VeIP followed by one cycle of HDCT with AHCT. The second phase III trial compared one cycle of VIP followed by three cycles of high-dose chemotherapy with carboplatin and etoposide (arm A) versus three cycles of VIP and one cycle of HDCT while adding cyclophosphamide to carboplatin and etoposide (arm B). This study was terminated prematurely due to excess deaths in arm B. None of these studies produced meaningful results in terms of OS.
The most meaningful data regarding HDCT come from retrospective studies. A study conducted at Indiana University treated 364 patients with either one or two courses of carboplatin and etoposide followed by AHCT between 2004 and 2014. The 2-year PFS was 60%, and the 2-year OS was 66% at a median follow-up of 3.3 years (16). Furthermore, Feldman et al. (17) reported a full dataset of 107 patients treated with the TI-CE regimen. This study showed a 5-year disease-free survival (DFS) and OS of 47% and 52% (median follow-up, 61 months), respectively.
Finally, systematic reviews (SRs) already published have based their conclusions mainly on survival endpoints (e.g., OS), forgetting other patient-important outcomes, including adverse events and QoL. Furthermore, those reviews have some methodological limitations, namely, no available protocol, no adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, no attempt to perform risk of bias assessment in included studies, and did not assess the certainty of the evidence (18, 19).
This SR and meta-analysis aimed to assess the efficacy of HDCT followed by AHCT versus CDCT in improving OS, event-free survival (EFS), response rate, and PFS, determine the treatments’ impact on QoL and describe the treatment-related toxicities in men with relapsed or refractory GCTs.
This SR was developed with the guidance of the PRISMA 2020 statement (20, 21). The full protocol is available in the Supplementary Material.
Does high-dose chemotherapy followed by autologous hematopoietic cell transplantation as initial salvage chemotherapy in patients with relapsed or refractory germ-cell tumors improve outcomes compared with conventional-dose chemotherapy?
Primary outcome: overall survival.
Secondary outcomes: quality of life, event-free survival, response rate, progression-free survival, and acute and chronic toxicities.
Randomized and non-randomized prospective controlled trials comparing the effectiveness of HDCT followed by AHCT with CDCT for people with relapsed/refractory GCTs were included.
Participants were men (≥15 years old at the date of diagnosis) with a diagnosis of GCTs, confirmed by either pathology or elevated tumor markers plus suggestive imaging, and patients with unequivocal evidence of relapse or progression after first-line platinum-based chemotherapy for metastatic GCTs. Patients with extragonadal GCTs [retroperitoneum, mediastinum, or central nervous system (CNS)] were included if they met the aforementioned criteria.
We used the intervention HDCT followed by AHCT (i.e., experimental arm) and CDCT (i.e., control arm). There are various HDCT and CDCT regimens; however, most of them share at least two chemotherapy drugs. Most HDCT regimens incorporate etoposide and carboplatin as cornerstone drugs. In contrast, the majority of CDCT regimens also include two keystone chemotherapies: ifosfamide and cisplatin. Hence, we considered all of them as one experimental arm (HDCT) and one control arm (CDCT).
We also decided to include in the experimental arm the HDCT regimens that incorporated induction chemotherapy as well as HDCT regimens given in either a single or sequential way.
MEDLINE PubMed, Embase, and CENTRAL electronic databases were searched from inception to April 30, 2023 (see searching strategy in Appendix 1–3 in the Supplementary Material). Language restrictions were not imposed.
Reference lists of selected review articles were searched (9, 19, 22). Conference proceedings of the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), the International Society for Paediatric Oncology, the American Society of Pediatric Hematology/Oncology, the American Society for Blood and Marrow Transplantation, and the European Society for Blood and Marrow Transplantation were also searched from inception to April 30, 2023. ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform were scanned for ongoing trials (from inception to April 30, 2023).
We uploaded all titles and abstracts retrieved by electronic searching into Covidence and removed any duplicates. One reviewer (JB) examined the remaining references and excluded any studies that did not meet the inclusion criteria. Then, we collected the full text of the studies that met the inclusion criteria based on the title, abstract, or both for detailed inspection. Two reviewers (JB and PD) independently assessed the eligibility of the retrieved papers and resolved any discrepancies through discussion. A PRISMA flow diagram was produced (Figure 1).
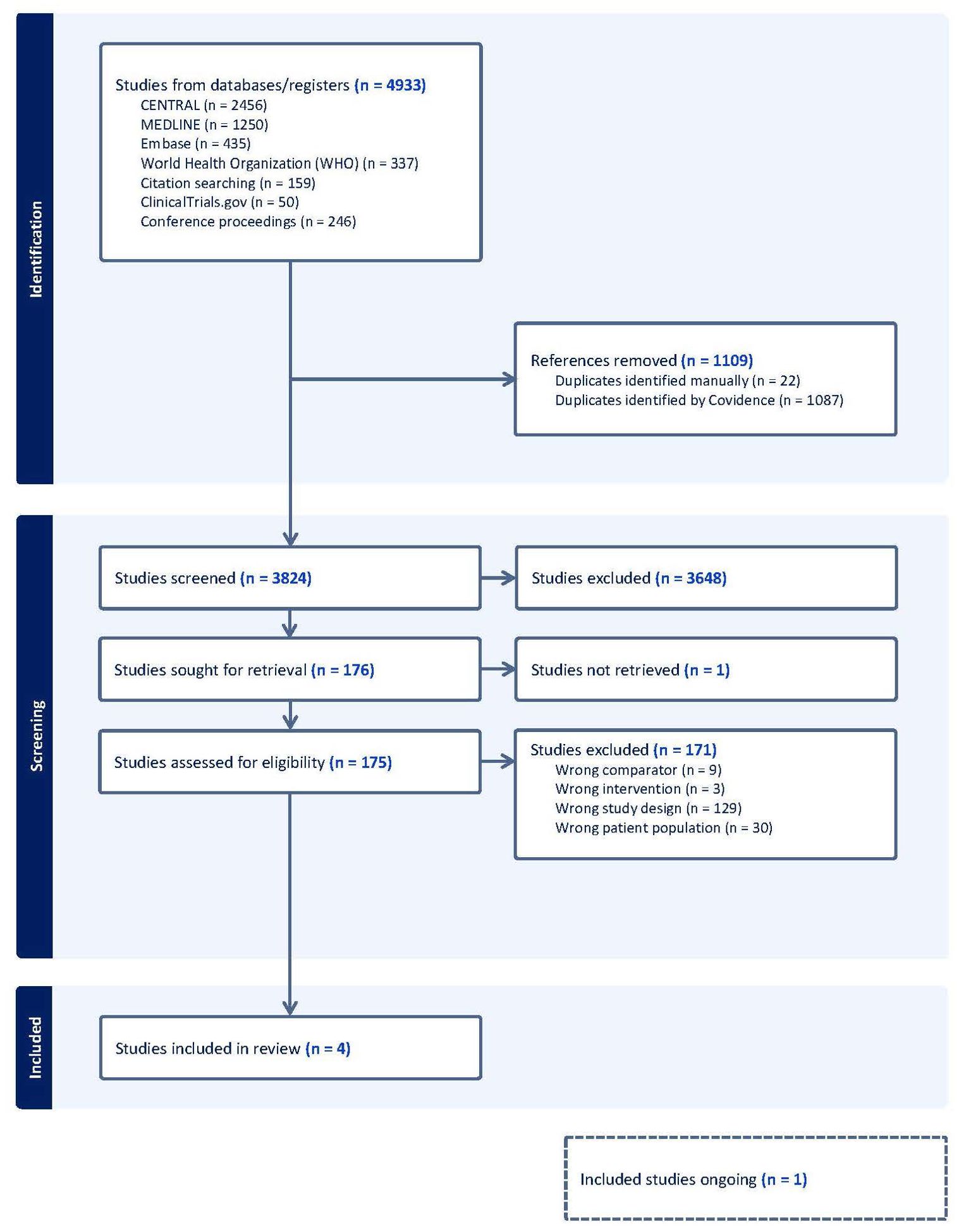
Figure 1. PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Two authors (JB and PD) independently extracted data using a customized template. Discrepancies were resolved between reviewers by discussion. Data on the characteristics of the study, participants, interventions, methods, outcomes, and details of funding sources for the included studies were extracted. Hazard ratio (HR) calculations spreadsheet was used to facilitate the estimation of HRs from included studies (23).
We extracted data to enable intention-to-treat (ITT) and per-protocol analyses.
Two authors (JB and PD) independently assessed the risk of bias in the included studies using either RoB2 (24) or ROBINS-I (25) tools according to the study design. Discrepancies were resolved between reviewers by discussion. The risk of bias was separately assessed for each outcome.
Time-to-event data were analyzed using HR, and categorical data were analyzed using Response rate (RR). All results are presented with the corresponding 95% confidence interval. A p-value ≤ 0.05 was considered statistically significant.
The outcomes of the included studies did not have homogeneous definitions. Therefore, we decided to accept the definitions provided by the authors of the original studies.
ITT analysis was applied to all outcomes when reporting results. A per-protocol analysis was utilized to assess adverse events (AEs). We would attempt to contact the study authors of the included studies.
Meta-analysis for OS was not conducted because only one study had reliable data for analysis. The generic inverse–variance method was used to calculate time-to-event outcomes, considering that we could obtain log HR and standard errors from included studies.
For categorical data, we decided to conduct meta-analyses using the Mantel–Haenszel method since it is more robust for sparse data (26).
We employed STATA 17.0 to perform statistical analysis (see STATA commands in the Supplementary Material).
Variability would have been investigated and handled following the Ann Arbor Heterogeneity Consensus Group recommendations (27, 28) if we had been capable of conducting a meta-analysis of OS (see the protocol in the Supplementary Material for further details).
-Prespecified clinical covariates.
We planned to investigate five variables with strong rationale: histological subtype, baseline risk as per International Prognostic Factors Study Group (IPFSG) prognostic score, type of CDCT regimen, type of HDCT regimen, and number of cycles of HDCT.
Regrettably, we were not able to conduct subgroup analysis and meta-regression as we stated in the protocol.
-Role of statistical heterogeneity. A non-significant Cochran’s Q test (p-value ≥ 0.1) or a small I2 (<25%) would not have avoided the need to investigate clinical and methodological heterogeneity if we had been able to perform a meta-analysis of OS.
We did conduct meta-analyses to explore some secondary outcomes further. However, due to the exploratory nature of secondary outcomes, clinical and methodological heterogeneities were not investigated.
-Plotting and visual aids. We used a graphical presentation of the data included from studies (e.g., forest plots). A funnel plot was not used due to few available studies.
We followed the next strategies for dealing with each source of multiplicity as we explained in detail in the protocol:
-Multiple outcomes. We classified the outcomes into primary and secondary outcomes. Secondary outcomes were not part of the main conclusion of the review.
-Multiple groups. There are several HDCT and CDCT regimens; however, most of them share at least two chemotherapy drugs. Thus, we considered all of them as one intervention group and one control group.
-Multiple time points. We combined information at different time points by assuming that the HR (as a summary estimate effect) is constant over time (29).
-Multiple effect measures. OS and EFS can be measured using different metrics (e.g., median, 5-year rate, or HR). Since sufficient data were available, we calculated the HR for each time-to-event outcome.
-Subgroup analyses. Subgroup analyses were not conducted.
-Multiple sources. We maximized the information yield by collating all available data (duplicate publications, companion documents, or multiple reports of a primary trial). We used the most complete dataset aggregated across all known publications.
We prepared a summary of the findings table using GRADEpro (30). For each outcome, a reviewer (JB) assessed the certainty of the evidence using the five GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) considerations (31).
Four trials fulfilled the inclusion criteria (Figure 1) (13, 15, 32, 33). One of them is not published yet (15). Therefore, three out of four were used to assess primary and secondary outcomes. The characteristics of the included studies are summarized in Tables 1A–D. See the characteristics of the excluded studies in the Supplementary Material (Supplementary Table S1).
The risk of bias assessment and support judgments of the included RCTs (13) can be found in Supplementary Tables S2A–E and Figures 2, 3.
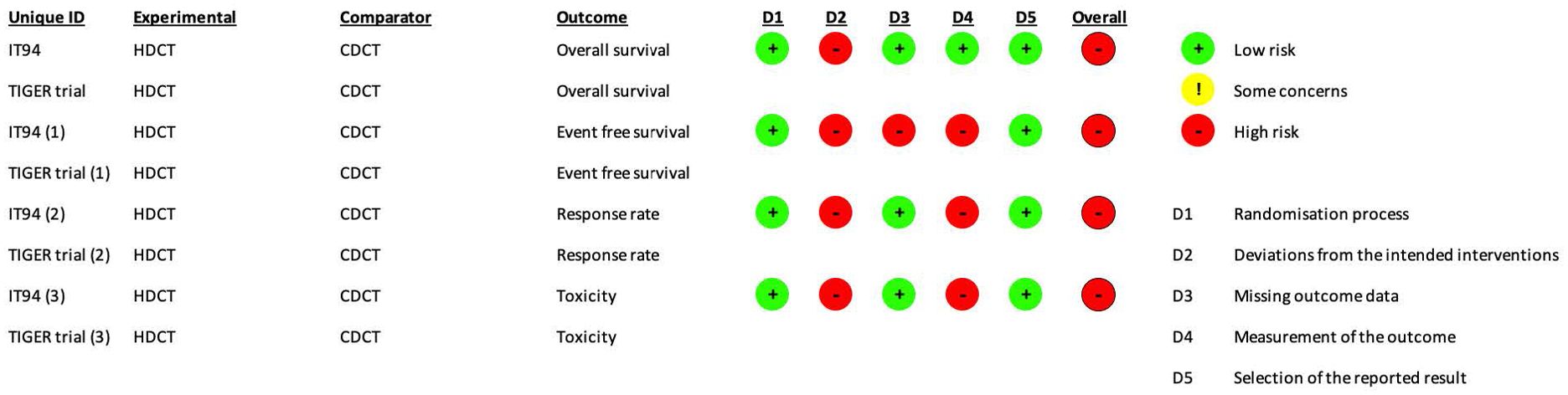
Figure 2. RoB2 risk of bias assessment of randomized controlled trials (intention-to-treat analysis). Risk of bias was separately assessed for each outcome.
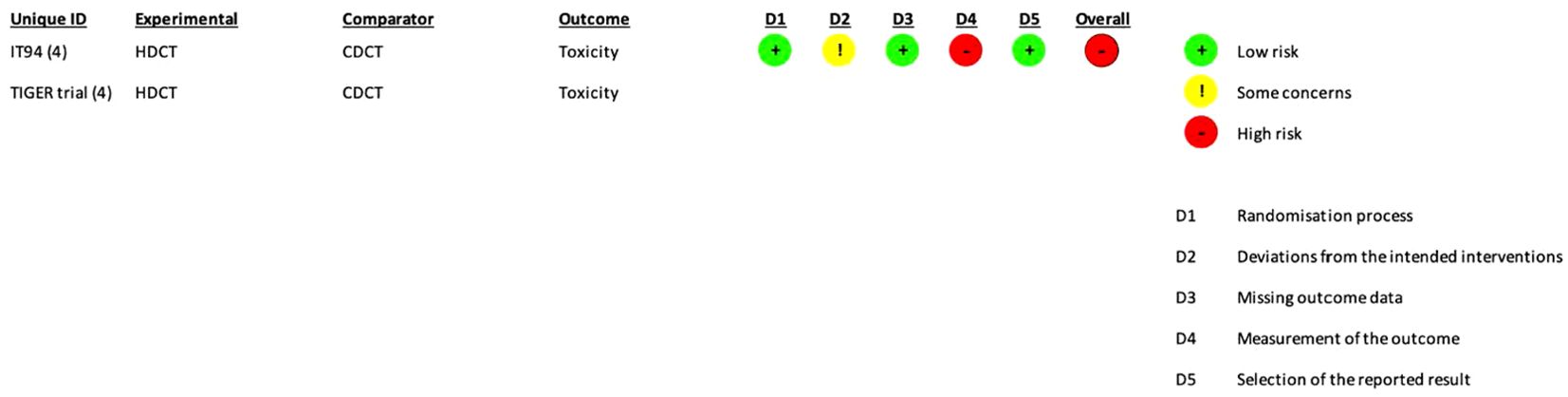
Figure 3. RoB2 risk of bias assessment of randomized controlled trials (per-protocol analysis). Risk of bias was only assessed for toxicity.
The risk of bias was separately assessed for each outcome. For all outcomes, the aim was to assess the effect of assignment to intervention except toxicity (the effect of adhering to intervention was evaluated).
The risk of bias assessment and support judgments of the included non-randomized prospective controlled trials (32, 33) can be found in Supplementary Tables S3A–E and Figure 4 (34).
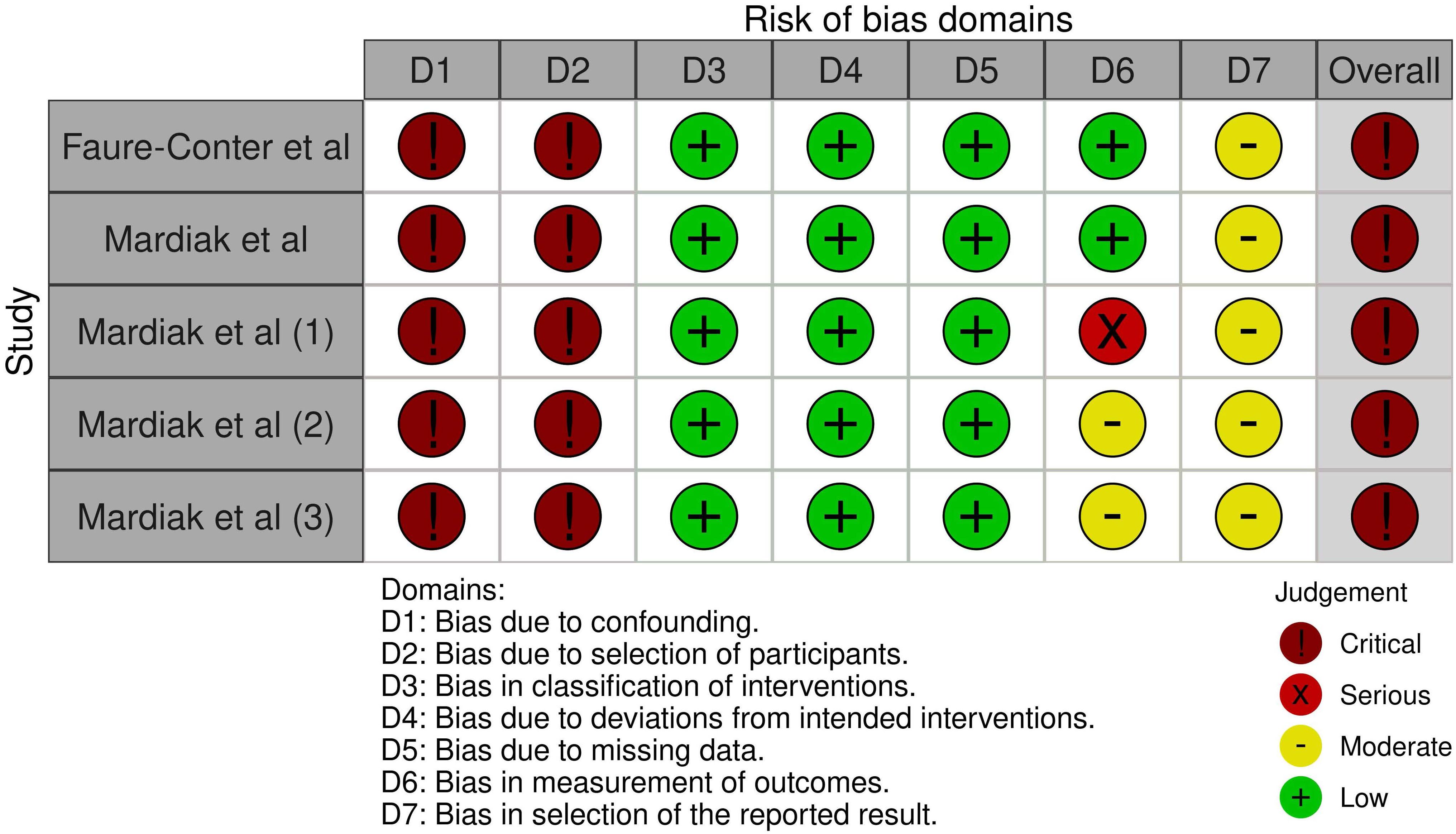
Figure 4. ROBINS-I risk of bias assessment of non-randomized controlled trials. Risk of bias was separately assessed for each outcome. Faure-Conter et al. (ITT analysis): overall survival. Mardiak et al. (ITT analysis): overall survival. Mardiak et al. (1) (ITT analysis): response rate. Mardiak et al. (2) (ITT analysis): toxicity. Mardiak et al. (3) (per-protocol analysis): toxicity. ITT, intention-to-treat.
See Table 2.
Of 280 patients, 263 were evaluated for OS, considering data from the IT94 trial. After a median follow-up of 45 months, single HDCT followed by AHCT may have little to no effect on OS of men with relapsed GCTs (HR 0.98, 95%CI 0.68 to 1.42; p = 0.916; very low-certainty evidence).
Mardiak’s (HR 0.25, 95%CI 0.07 to 0.86) and Faure-Conter’s (HR 0.61, 95%CI 0.20 to 1.82) studies showed contrasting and imprecise results, which may be explained by the number of cycles of high-dose chemotherapy administered in each study, patient’s characteristics, and the small sample size (Figure 5 and Table 3).
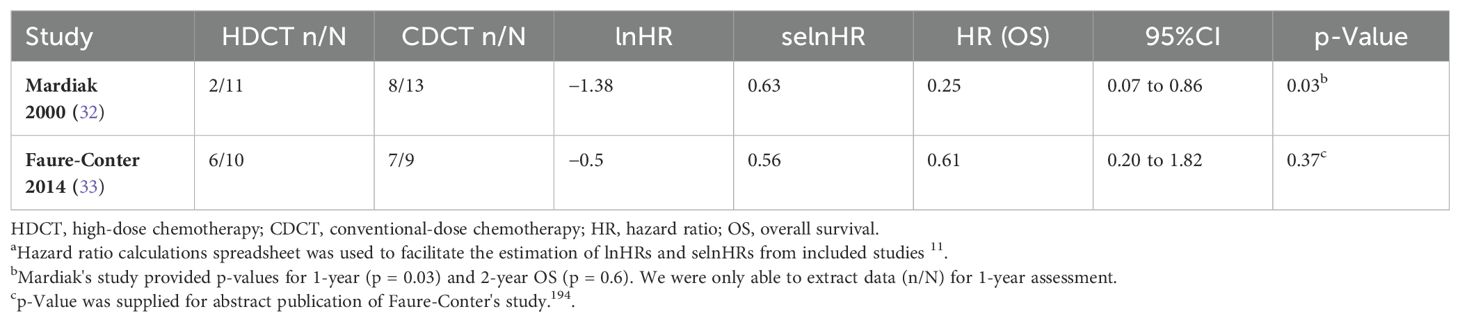
Table 3. Overall survival based on data provided by non-randomized controlled trialsa.
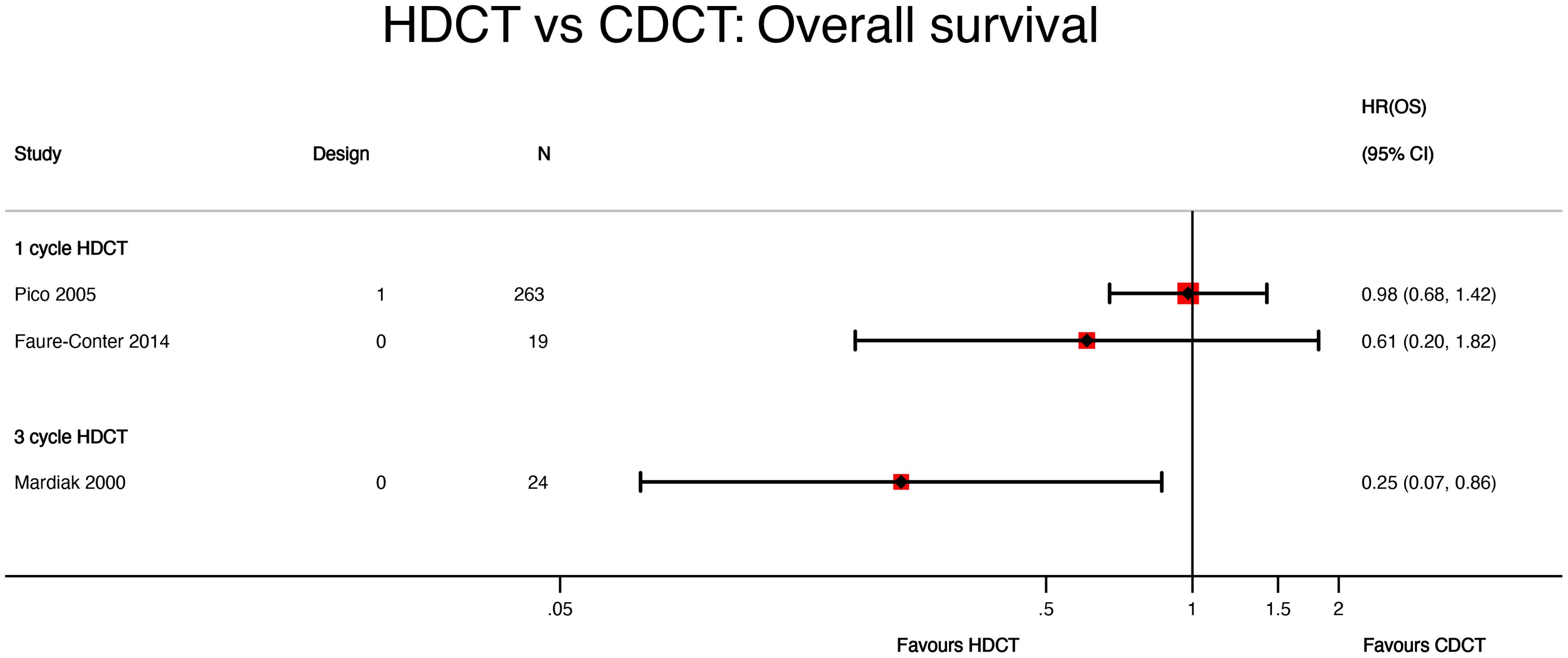
Figure 5. HDCT vs. CDCT: overall survival. HDCT, high-dose chemotherapy; CDCT, conventional-dose chemotherapy.
The lack of granularity of time-to-event data reported by Mardiak’s and Faure-Conter’s studies and their critical risk of bias impeded us from conducting a meta-analysis of OS.
The included studies did not provide data on QoL, PFS, and chronic toxicities.
Considering only data supplied by the IT94 study, 263 out of 280 patients were evaluable for EFS. After a median follow-up of 45 months, single HDCT followed by AHCT may improve the EFS of men with relapsed GCTs (HR 0.80, 95%CI 0.59 to 1.10; p = 0.169; low-certainty evidence).
Mardiak’s study did not report data on EFS. On the contrary, Faure-Conter’s trial did display EFS information. However, the lack of detail on time-to-event data hindered us from obtaining EFS for experimental and control arms.
Of 280 patients from the IT94 trial, 247 were evaluated for response rate (Table 4). Single HDCT followed by AHCT may result in little to no difference in the overall response rate (ORR) of men with relapsed GCTs (RR 1.03, 95%CI 0.84 to 1.26; p = 0.775; low-certainty evidence). Additionally, single HDCT with AHCT may not reduce failure of men with relapsed GCTs (RR 0.96, 95%CI 0.71 to 1.30; p = 0.775; low-certainty evidence). For further details about other response rate outcomes, see Table 2.

Table 4. Response rate outcomes only considering data from Pico 2005 (13).
Mardiak’s and Faure-Conter’s studies supplied data to explore response rate outcomes further. Considering the limitations of data reported from those trials, we found no evidence that HDCT followed by AHCT improves response rate outcomes, including complete response (CR), ORR, and failure (Table 5).
See meta-analyses of complete response, overall response rate, and failure considering all included studies (Figures 6–8).
The included studies did not comprehensibly report acute AEs. The IT94 trial only reported some severe and life-threatening (G3≥) acute hematological and gastrointestinal toxicities. Mardiak’s study described acute hematological AEs and mentioned that non-hematological toxicities were rare during the study (e.g., two patients developed ischemic heart disease, and one patient had thrombophlebitis). Regarding Faure-Conter’s trial, the authors did not characterize acute toxicities whatsoever.
Acute toxicities were evaluated as per ITT and protocol analyses (see Supplementary Tables S4 and S5 in the Supplementary Material).
Of 280 patients from the IT94 study, 274 were evaluable for acute hematological toxicities. Single HDCT followed by AHCT probably results in little to no difference in neutropenia ≥ G3 of men with relapsed GCTs (RR 1.06, 95%CI 0.98 to 1.14; p = 0.134; moderate-certainty evidence). However, single HDCT with AHCT likely increases febrile neutropenia ≥ G3 (RR 1.57, 95%CI 1.30 to 1.91; p < 0.001; moderate-certainty evidence) as well as thrombocytopenia ≥ G3 (RR 1.54, 95%CI 1.30 to 1.82; p < 0.001; moderate-certainty evidence) of men with relapsed GCTs.
Mardiak’s study found no evidence that HDCT, followed by AHCT, increases acute hematological toxicities of patients with GCTs (see Supplementary Table S6 in the Supplementary Material). This is probably explained by the fact that the control arm did not receive the standard dose of VIP but 1.6 VIP (i.e., etoposide 600 mg/m2, ifosfamide 8,000 mg/m2, and cisplatin 100 mg/m2). See Meta-Analysis of Febrile Neutropenia and Thrombocytopenia considering all included studies (Figures 9, 10).
Of 280 patients from the IT94 trial, 274 were evaluable for acute gastrointestinal toxicities. Single HDCT followed by AHCT may result in a large increase in nausea/vomiting ≥ G3 (RR 3.30, 95%CI 2.03 to 5.38; p < 0.001; low-certainty evidence), diarrhea ≥ G3 (RR 9.36, 95%CI 2.22 to 39.42; p = 0.002; low-certainty evidence), and mucositis ≥ G3 (RR 16.43, 95%CI 5.25 to 51.39; p < 0.001; low-certainty evidence) of men with relapsed GCTs.
The IT94 trial reported 9/138 deaths due to toxicities in the experimental arm and 4/136 deaths in the control arm. The causes of death due to toxicity can be found in Supplementary Table S7 in the Supplementary Material.
Based on data from Pico’s trial, we concluded that single HDCT followed by AHCT may increase death due to toxicity of men with relapsed GCTs (RR 2.22, 95%CI 0.70 to 7.03; p = 0.176; low-certainty evidence).
Mardiak’s and Faure-Conter’s studies reported one death each. In the first study, the patient died due to toxicity (i.e., septic shock) during the first cycle of 1.6 VIP. On the contrary, one patient died of renal and myocardial acute failure after receiving CarboPEC in Faure-Conter’s trial.
The main objective of this SR and meta-analysis was to evaluate the efficacy of HDCT followed by AHCT versus CDCT in improving OS in men with relapsed or refractory GCTs. Three out of four studies were used to assess prespecified outcomes: one randomized (13) and two non-randomized prospective controlled trials (32, 33).
The IT94 trial (13) enrolled 280 participants, 263 of whom had data available for OS analysis. This study compared VIP/VeIP for four cycles with VIP/VeIP for 3 cycles, followed by one cycle of CarboPEC with AHCT. The IT94 study excluded patients with refractory disease. Based on the data provided by this study, we concluded that single HDCT followed by AHCT may have little to no effect on OS in relapsed GCT patients.
Non-randomized trials revealed contradictory results. Mardiak’s trial used three cycles of HDCT followed by AHCT. It showed that HDCT improves survival compared with CDCT. However, the OS benefit was lost at the 2-year assessment. On the contrary, Faure-Conter’s trial did not show statistical evidence that single HDCT improves OS in patients with GCTs.
Data from the IT94 trial (13) were utilized to produce the main conclusions about EFS, response rate, and acute toxicities. However, we conducted additional exploratory analysis using data from the included non-randomized studies (32, 33).
Based on the IT94 trial, we determined that single HDCT followed by AHCT may improve the EFS of men with relapsed GCTs. No significant differences were found in ORR and failure comparing single HDCT followed by AHCT with CDCT. Exploring the impact of data from included no-randomized trials on ORR and failure, we found no evidence that HDCT followed by AHCT improves CR or decreases failure.
As for acute toxicity ≥ G3, 274 out of 280 participants had data accessible for analysis. We concluded that single HDCT followed by AHCT likely increases the risk of developing febrile neutropenia and thrombocytopenia and may result in a large increase in nausea/vomiting, diarrhea, and mucositis. Exploring the influence of data from included no-randomized trials on febrile neutropenia and thrombocytopenia, we found that our conclusions did not change much. Finally, we also found that single HDCT followed by AHCT may increase death due to toxicity.
The conclusion about the primary outcome was supported by very-low-certainty evidence; thus, it should be interpreted cautiously. All secondary outcomes were exploratory and, therefore, are only hypothesis-generating.
The certainty of evidence was very low for OS due to study limitations and imprecision. Despite this, the IT94 trial provides the best available prospective evidence comparing the efficacy of single HDCT followed by AHCT with CDCT in men with relapsed GCTs.
The certainty of evidence-supported secondary outcomes such as EFS, response rate, acute gastrointestinal toxicity, and death due to toxicity was rated as low due to trial limitations and imprecision. On the contrary, due to study limitations, we rated the certainty of evidence for neutropenia, febrile neutropenia, and thrombocytopenia as moderate.
Despite that we included two non-randomized prospective controlled trials in our systematic review, data from these studies were not utilized to build the main conclusions. According to the ROBINS-I tool, the risk of bias in Mardiak’s and Faure-Conter’s studies was considered critical. Additionally, Mardiak’s trial had a small sample size (n = 25), included people with treatment-naïve GCTs (n = 9), and did not provide sufficient data to calculate time-to-event outcomes for relapsed/refractory GCT population. Faure-Conter’s trial also had a small sample size (n = 19) and included male and female infants (n = 9) as well as participants with ovarian and sacrococcygeal primary sites.
Finally, it is worth noting that included studies used HDCT and CDCT regimens, which are no longer utilized at high-volume cancer centers with experience in the management of GCTs (10, 17).
In view of the very-low-certainty evidence supporting OS, the current body of prospective data does not allow a definitive conclusion.
The results of this SR provide a clear overview of the currently available prospective data regarding the value of HDCT with AHCT compared to CDCT in men with relapsed GCTs. We used a broad search strategy to identify eligible studies, searching three citation databases, two trial registers, and conference proceedings and checking the reference lists of previous reviews. We also considered non-English studies and used Google Translate when required. However, although it is unlikely that we missed eligible randomized controlled trials, we cannot be sure regarding non-randomized prospective controlled studies due to the search strategy utilized, issues related to indexing this type of study, and the quality of reporting. Regardless of these problems, it is improbable that we had missed a non-randomized prospective controlled study with a low risk of bias. In addition, we were not able to find updated data from included studies. Other limitations were that only one reviewer performed the screening of the studies and assessed the certainty of the evidence.
In addition, another source of potential bias was the decision to group different regimens of HDCT and CDCT in one experimental and one control arm, respectively. We made this pragmatic choice with the aim of conducting a subgroup analysis to further explain this source of variability. Nonetheless, we did not perform a meta-analysis of OS for the reason mentioned above.
As we stated above, the aim of our systematic review was to assess the efficacy of high-dose chemotherapy followed by autologous hematopoietic cell transplantation versus conventional-dose chemotherapy in improving survival and other patient-important outcomes. Compared with previous reviews, ours was focused on summarizing the best available prospective evidence and addressing outcomes such as quality of life and toxicities.
The only available phase III randomized controlled trial (i.e., IT94 study), which compared CDCT with HDCT followed AHCT, failed to show a significant survival benefit from HDCT. Single-center studies conducted at high-volume academic cancer centers did show promising results utilizing HDCT with AHCT in this population (16, 17). Along the same lines, Lorch and colleagues found that HDCT, given as the first salvage therapy, improved PFS and OS in patients with relapsed/refractory germ cell tumors compared with CDCT regardless of IPFSG prognostic category except among low-risk patients (35). In contrast, a recent retrospective study found that patients with favorable and unfavorable-risk disease as per Memorial Sloan Kettering Cancer Center (MSKCC) criteria can also achieve durable responses with initial salvage TIP (36). These results show that experience matters, perhaps reflecting a better selection of patients, surgical expertise in managing residual mass, and the ability to safely deliver HDCT followed by AHCT. It is well known that death rates due to HDCT-related toxicity are largely related to expertise, as shown by Lorch et al. (35).
Systematic reviews have been published on this topic so far. They have shown results in keeping with our findings. For instance, Husnain et al. (18) failed to show any significant results for overall survival in patients with relapsed/refractory germ cell tumors treated with HDCT with AHCT. Petrelli and colleagues (22) found comparable efficacy when CDCT and HDCT were used as salvage therapies in relapsed/refractory germ cell tumors. Interestingly, Bin Riaz et al. (19) established that a single cycle of HDCT with AHCT does not improve survival compared with CDCT. However, the authors did find that two or three cycles of HDCT play a positive role in this population.
There is insufficient prospective evidence to support or refute the proposal that HDCT with AHCT improves outcomes in men with relapsed or refractory GCTs.
If HDCT with AHCT is considered essential to treat people with relapsed/refractory GCTs, the choice should be made by experienced clinicians, taking into consideration the very-low-certainty evidence on OS and potential severe toxicities associated with this treatment. In addition, they must discuss with their patients the unknown impact of HDCT on QoL and long-term toxicities.
If salvage chemotherapy is considered essential to treat people with relapsed/refractory GCTs, clinicians should make a referral to a high-volume cancer center.
This review revealed a lack of high-quality prospective research in the relapsed/refractory GCT population. However, the IT94 and TIGER trials show that it is possible to conduct phase III randomized controlled trials in a rare patient population.
We believe there is still room for an SR and meta-analysis of this topic. When the TIGER trial is published, a network meta-analysis using individual participant data should be performed comparing CDCT, single, and sequential HDCT to better understand the role of adding further cycles of HDCT in the efficacy of this therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JB: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. PD: Investigation, Methodology, Validation, Writing – review & editing. BN: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We acknowledge that COVIDENCE and GRADEpro software were used during the systematic review process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1437574/full#supplementary-material
1. Beyer J, Collette L, Sauvé N, Daugaard G, Feldman DR, Tandstad T, et al. Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-update consortium. J Clin Oncol. (2021) 39:1553–62. doi: 10.1200/JCO.20.03292
2. Gillessen S, Sauvé N, Collette L, Daugaard G, De Wit R, Albany C, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG update consortium. J Clin Oncol. (2021) 39:1563–74. doi: 10.1200/JCO.20.03296
3. Tandstad T, Kollmannsberger CK, Roth BJ, Jeldres C, Gillessen S, Fizazi K, et al. Practice makes perfect: the rest of the story in testicular cancer as A model curable neoplasm. J Clin Oncol. (2017) 35:3525–8. doi: 10.1200/JCO.2017.73.4723
4. International Prognostic Factors Study Group, Lorch A, Beyer J, Bascoul-Mollevi C, Kramar A, Einhorn LH, et al. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol. (2010) 28:4906–11. doi: 10.1200/JCO.2009.26.8128
5. Honecker F, Aparicio J, Berney D, Beyer J, Bokemeyer C, Cathomas R, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:1658–86. doi: 10.1093/annonc/mdy217
6. Gilligan T, Lin DW, Aggarwal R, Chism D, Cost N, Derweesh IH, et al. Testicular cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2019) 17:1529–54. doi: 10.6004/jnccn.2019.0058
7. Oldenburg J, Martin JM, Fosså SD. Late relapses of germ cell Malignancies: incidence, management, and prognosis. J Clin Oncol. (2006) 24:5503–11. doi: 10.1200/JCO.2006.08.1836
8. Lorch A, Beyer J. High-dose chemotherapy as salvage treatment in germ-cell cancer: when, in whom and how. World J Urol. (2017) 35:1177–84. doi: 10.1007/s00345-016-1941-0
9. Chovanec M, Adra N, Abu Zaid M, Abonour R, Einhorn L. High-dose chemotherapy for relapsed testicular germ cell tumours. Nat Rev Urol. (2023) 20:217–25. doi: 10.1038/s41585-022-00683-1
10. Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. New Engl J Med. (2007) 357:340–8. doi: 10.1056/NEJMoa067749
11. Zschäbitz S, Distler FA, Krieger B, Wuchter P, Schäfer-Eckart K, Jenzer M, et al. Survival outcomes of patients with germ cell tumors treated with high-dose chemotherapy for refractory or relapsing disease. Oncotarget. (2018) 9:22537–45. doi: 10.18632/oncotarget.25162
12. Al-Ezzi EM, Zahralliyali A, Hansen AR, Hamilton RJ, Crump M, Kuruvilla J, et al. The use of salvage chemotherapy for patients with relapsed testicular germ cell tumor (GCT) in Canada: A national survey. Curr Oncol. (2023) 30:6166–76. doi: 10.3390/curroncol30070458
13. Pico JL, Rosti G, Kramar A, Wandt H, Koza V, Salvioni R, et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol. (2005) 16:1152–9. doi: 10.1093/annonc/mdi228
14. Lorch A, Kollmannsberger C, Hartmann JT, Metzner B, Schmidt-Wolf IG, Berdel WE, et al. Single versus sequential high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: a prospective randomized multicenter trial of the German Testicular Cancer Study Group. J Clin Oncol. (2007) 25:2778–84. doi: 10.1200/JCO.2006.09.2148
15. ACTRN12618001236280. Standard-dose combination chemotherapy or high-dose combination chemotherapy and stem cell transplant in treating patients with relapsed or refractory germ cell tumors. Standard-Dose Combination Chemotherapy or High-Dose Combination Chemotherapy and Stem Cell Transplant in Treating Patients With Relapsed or Refractory Germ Cell Tumors. (2018).
16. Adra N, Abonour R, Althouse SK, Albany C, Hanna NH, Einhorn LH. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: the Indiana university experience. J Clin Oncol. (2017) 35:1096–102. doi: 10.1200/JCO.2016.69.5395
17. Feldman DR, Sheinfeld J, Bajorin DF, Fischer P, Turkula S, Ishill N, et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol. (2010) 28:1706–13. doi: 10.1200/JCO.2009.25.1561
18. Husnain M, Riaz IB, Kamal MU, Gondal FR, Ali Z, Sipra Q-U-AR, et al. High dose chemotherapy with autologous stem cell transplant in treatment of germ cell tumors: A systematic review and meta-analysis. Blood. (2016) 128:5829–9. doi: 10.1182/blood.V128.22.5829.5829
19. Bin Riaz I, Umar M, Zahid U, Husnain M, Iftikhar A, Mcbride A, et al. Role of one, two and three doses of high-dose chemotherapy with autologous transplantation in the treatment of high-risk or relapsed testicular cancer: a systematic review. Bone Marrow Transplant. (2018) 53:1242–54. doi: 10.1038/s41409-018-0188-3
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. (2021) 18(3):e1003583.
21. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4(1):1.
22. Petrelli F, Coinu A, Rosti G, Pedrazzoli P, Barni S. Salvage treatment for testicular cancer with standard- or high-dose chemotherapy: a systematic review of 59 studies. Med Oncol. (2017) 34:133. doi: 10.1007/s12032-017-0990-6
23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
24. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898.
25. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
26. Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane (2023). Available from www.training.cochrane.org/handbook.
27. Gagnier JJ, Moher D, Boon H, Beyene J, Bombardier C. Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Methodol. (2012) 12:111. doi: 10.1186/1471-2288-12-111
28. Gagnier JJ, Morgenstern H, Altman DG, Berlin J, Chang S, Mcculloch P, et al. Consensus-based recommendations for investigating clinical heterogeneity in systematic reviews. BMC Med Res Methodol. (2013) 13:106. doi: 10.1186/1471-2288-13-106
29. Proschan MA, Waclawiw MA. Practical guidelines for multiplicity adjustment in clinical trials. Control Clin Trials. (2000) 21:527–39. doi: 10.1016/S0197-2456(00)00106-9
30. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. (2024). Available from gradepro.org.
31. Schünemann H, Brożek J, Guyatt G, Oxman A. editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. (2013). Available from�guidelinedevelopment.org/handbook.
32. Mardiak J, Fuchsberger P, Lakota J, Sálek T, Sycová-Milá Z, Drahokoupilová M, et al. Sequential intermediate high-dose therapy with etoposide, ifosfamide and cisplatin for patients with germ cell tumors. Neoplasma. (2000) 47:239–43.
33. Faure-Conter C, Orbach D, Cropet C, Baranzelli MC, Martelli H, Thebaud E, et al. Salvage therapy for refractory or recurrent pediatric germ cell tumors: the French SFCE experience. Pediatr Blood Cancer. (2014) 61:253–9. doi: 10.1002/pbc.24730
34. Mcguinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synthesis Methods. (2021) 12(1):55–61. doi: 10.1002/jrsm.1411
35. Lorch A, Bascoul-Mollevi C, Kramar A, Einhorn L, Necchi A, Massard C, et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol. (2011) 29:2178–84. doi: 10.1200/JCO.2010.32.6678
36. Gleeson JP, Knezevic A, Bromberg M, Patil S, Sheinfeld J, Carver BS, et al. Paclitaxel, ifosfamide, and cisplatin as initial salvage chemotherapy for germ cell tumors: long-term follow-up and outcomes for favorable- and unfavorable-risk disease. J Clin Oncol. (2024) 0:JCO.23.02542. doi: 10.1200/JCO.23.02542
Keywords: refractory germ cell tumors, relapsed germ cell tumor, high dose chemotherapy with autologous stem cell transplantation, chemotherapy, survival, adverse (side) effects
Citation: Briones J, Diaz P and Nicholson BD (2024) High-dose chemotherapy as initial salvage chemotherapy in patients with relapsed or refractory testicular cancer: a systematic review and meta-analysis. Front. Oncol. 14:1437574. doi: 10.3389/fonc.2024.1437574
Received: 24 May 2024; Accepted: 26 August 2024;
Published: 01 October 2024.
Edited by:
Alcides Chaux, Universidad del Norte, ParaguayReviewed by:
Patrizia Giannatempo, Fondazione Istituto Nazionale dei Tumori, ItalyCopyright © 2024 Briones, Diaz and Nicholson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Briones, anJicmlvbmVzQHVjLmNs
†ORCID: Juan Briones, orcid.org/0000-0003-4373-0014
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.