- 1Department of Urology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
- 2Department of Urology, Medical School of Southeast University Nanjing Drum Tower Hospital, Nanjing, China
Objective: To analyze changes in renal function and associated risk factors in patients with bladder cancer undergoing robot-assisted radical cystectomy (RARC) with intracorporeal or extracorporeal urinary diversion (ICUD or ECUD).
Methods: Clinical-pathological data was extracted from electronic medical records of 266 patients with bladder cancer who underwent RARC at our institution between August 2015 and August 2022. Postoperative renal function was assessed using the estimated glomerular filtration rate (eGFR).
Result: Patients were classified into ECUD and ICUD groups based on the surgical approach. Significant differences in eGFR were observed between the two groups at 1, 2, and 3 years postoperatively. Moreover, 112 patients (42.1%) experienced long-term renal function injury. Independent risk factors for long-term renal function injury included the type of surgical approach, ureteroenteric anastomotic strictures, and pathological stage T3 or above. In terms of short-term renal function, 30 cases of acute kidney injury (AKI) were observed, with an incidence rate of 11.3%. No difference in AKI incidence was found between the groups.
Conclusions: Postoperative AKI and chronic kidney injury are prevalent complications following RC. This study highlights that pathological stage, ureteroenteric anastomotic strictures, and ECUD significantly impact long-term renal function, but the type of urinary diversion (ileal conduit or orthotopic neobladder) had no effect on renal function, and ICUD was superior in terms of long-term renal injury rate. Therefore, precise preoperative assessment and the selection of appropriate surgical approach are crucial for preserving renal function in patients with bladder cancer.
1 Introduction
Bladder cancer (BCa) is a prevalent neoplasm of the urinary system. In China, it is the fourth most common malignancies in males and tenth in females. BCa is classified into non-muscle-invasive bladder cancer and muscle-invasive bladder cancer. For locally muscle invasive bladder cancer without distant metastasis, the standard treatment involves cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy (RC), which also applies to certain high-risk and very high-risk non-muscle-invasive cases (1, 2). With the advent of robotic-assisted laparoscopic surgical systems in China, robot-assisted radical cystectomy (RARC) has seen increased adoption and has become the preferred method in numerous medical centers (3). Initially, most surgeons performed extracorporeal urinary diversion (ECUD). However, the use of intracorporeal urinary diversion (ICUD) is steadily increasing. Comparative studies, both domestic and international, have reported no significant differences between RARC with ICUD and RARC with ECUD in terms of surgical duration, positive margin rate, number of lymph nodes retrieved, and survival rates. Additionally, RARC with ICUD is associated with less blood loss and faster bowel recovery (4, 5). Postoperative renal injury incidence varies from 20% to 70% across different surgical approaches (6–8). This variation is likely due to differences in definitions, measurements, and follow-up periods across studies. However, regardless of the surgical approaches, most patients experience renal injury, which is linked to increased hospitalization rates, all-cause mortality, and cancer-specific mortality (9). While several studies have evaluated long-term renal function changes following open radical cystectomy (ORC), a single institution in the United States has compared long-term renal function outcomes and progression to CKD stages 3B and higher after intracorporeal RARC, extracorporeal RARC, and ORC (10). However, the risk factors for renal injury following RARC remain contentious, and there is a paucity of research comparing ICUD with ECUD in China.
To address these gaps, this study conducts a retrospective analysis of patient data undergoing RARC at the Affiliated Drum Tower Hospital of Nanjing University Medical School. We aim to compare perioperative outcomes, renal function changes, and associated risk factors between different urinary diversion methods and surgical approaches.
2 Methods
We reviewed the data of 266 patients who underwent RARC at the Department of Urology, Affiliated Drum Tower Hospital of Nanjing University Medical School, from August 2015 to August 2022. We collected demographic data and perioperative outcomes including surgical duration, estimated blood loss, transfusion rate, pathological stage, and clinical parameters potentially affecting postoperative renal function. This study evaluates the surgical approaches, renal function changes, and factors influencing renal function changes.
Inclusion criteria: (1) Patients with a preoperative diagnosis of locally resectable muscle-invasive bladder cancer (T2-4a, N0-x, M0) or high-risk non-muscle-invasive bladder cancer. (2) Patients who underwent RARC.
Exclusion criteria: (1) Patients undergoing bladder cystectomy for reasons other than bladder cancer, with or without nephroureterectomy. (2) Patients with a preoperative estimated glomerular filtration rate (eGFR) ≤45 mL/min/1.73m2 or severe hydronephrosis. (3) Patients with incomplete demographic data or those who did not participate in postoperative follow-up.
eGFR was calculated using the CKD-Epidemiology Collaboration formula (11). Baseline eGFR was determined from a single preoperative measurement, while postoperative eGFR was recorded by surgeons during follow-up, with a minimum follow-up period of at least one year. Ureteroenteric anastomotic strictures were identified as asymptomatic or symptomatic hydronephrosis in patients with radiological evidence of obstruction at the ureterointestinal anastomosis, primarily based on CT urography or confirmed during surgery. Long-term renal injury was defined as a reduction in eGFR of ≥10 mL/min/1.73m2 from baseline (10, 12). Acute kidney injury (AKI) was defined according to KDIGO guidelines as an increase in serum creatinine to more than 1.5 times the baseline value, with this increase occurring or presumed to have occurred within the preceding seven days (13). All patients receiving neoadjuvant and adjuvant chemotherapy were treated with a gemcitabine and cisplatin regimen, with neoadjuvant chemotherapy administered every three weeks for 3-4 cycles and adjuvant chemotherapy every three weeks for 4-6 cycles. Postoperative follow-up was conducted every three months in the first year and at least annually thereafter.
Continuous variables were presented as mean ± standard deviation and compared between groups using an independent sample t-test. Categorical variables were presented as frequencies (percentages) and compared using the chi-square test. Logistic regression analysis was used to identify factors influencing renal function. All statistical analyses were performed using SPSS version 25.0, with two-tailed p-values < 0.05 considered statistically significant.
3 Result
3.1 Baseline characteristics
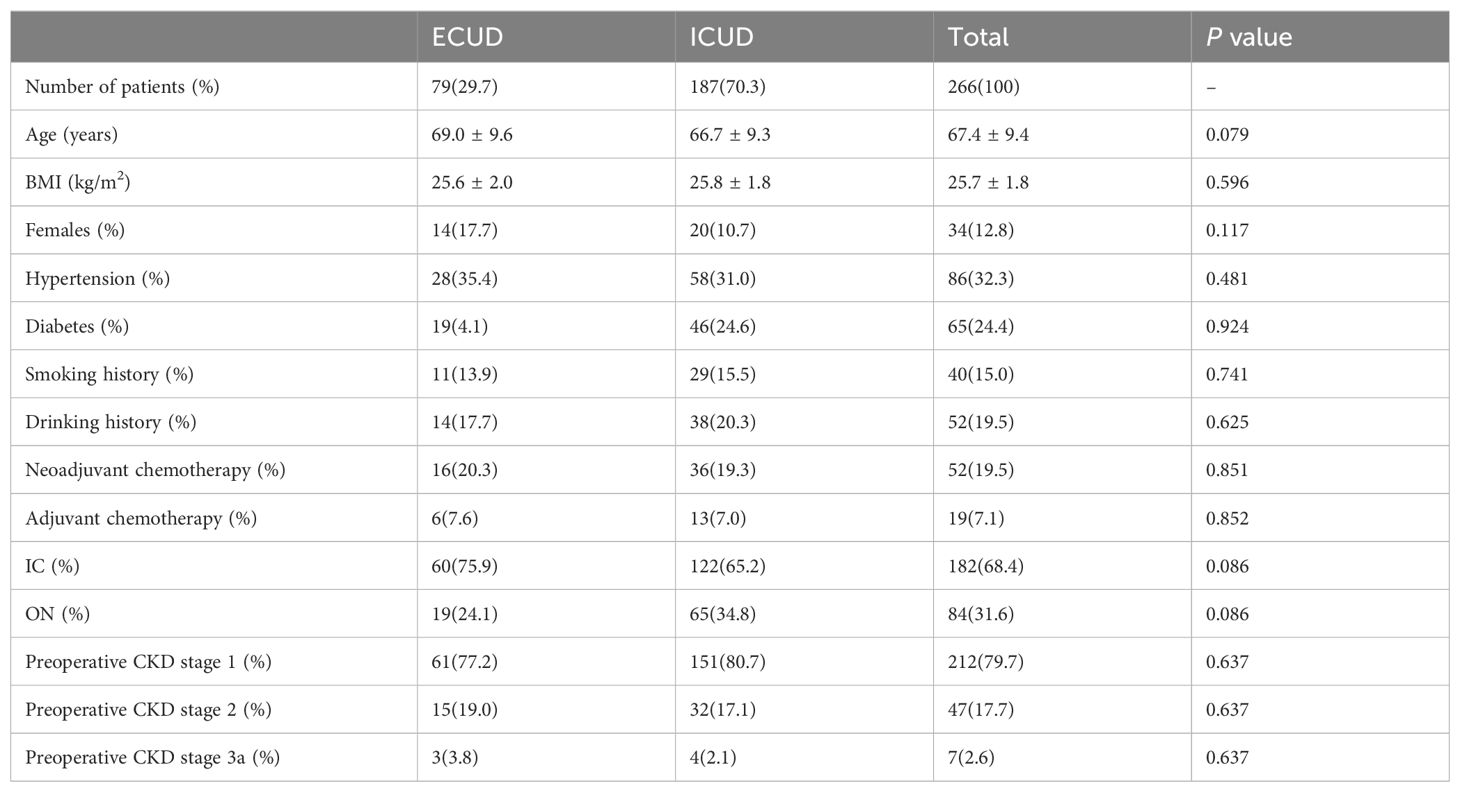
Patients were categorized into two groups based on their surgical approach for urinary diversion: ECUD and ICUD groups. The study included 266 patients with a follow-up duration of 36 months. Among these, 84 underwent orthotopic neobladder (ON) reconstruction and 182 underwent ileal conduit (IC) reconstruction. The cohort comprised 232 males and 34 females. Demographic and clinical characteristics are detailed in Table 1. In the ECUD and ICUD groups, the majority of patients underwent IC (68.4% vs. 31.6%, P= 0.086). The baseline estimated glomerular filtration rate (eGFR) for all patients was 107.3 ± 23.3 mL/min/1.73m2 (ECUD vs. ICUD: 104.7 ± 24.1 vs. 107.5 ± 23.0, P=0.377), with preoperative creatinine levels of 70.1 ± 16.5umol/L and 69.5 ± 15.9umol/L respectively (ECUD vs. ICUD, P=0.770). Neoadjuvant chemotherapy was administered to 19.5% of patients, with no statistically significant difference in the distribution of CKD stages 1-3a between the two groups preoperatively (CKD stage 1: 79.7%, CKD stage 2: 17.7%, CKD stage 3a: 2.6%, P=0.637). Among the cases, there were 84 ON and 182 IC reconstructions, with 65 (34.8%) and 122 (65.2%) ICUD and 19 (24.1%) and 60 (75.9%) ECUD, respectively. Notably, significant differences in bladder reconstruction methods between the two groups was not observed (P=0.086).
3.2 Perioperative outcomes and complications
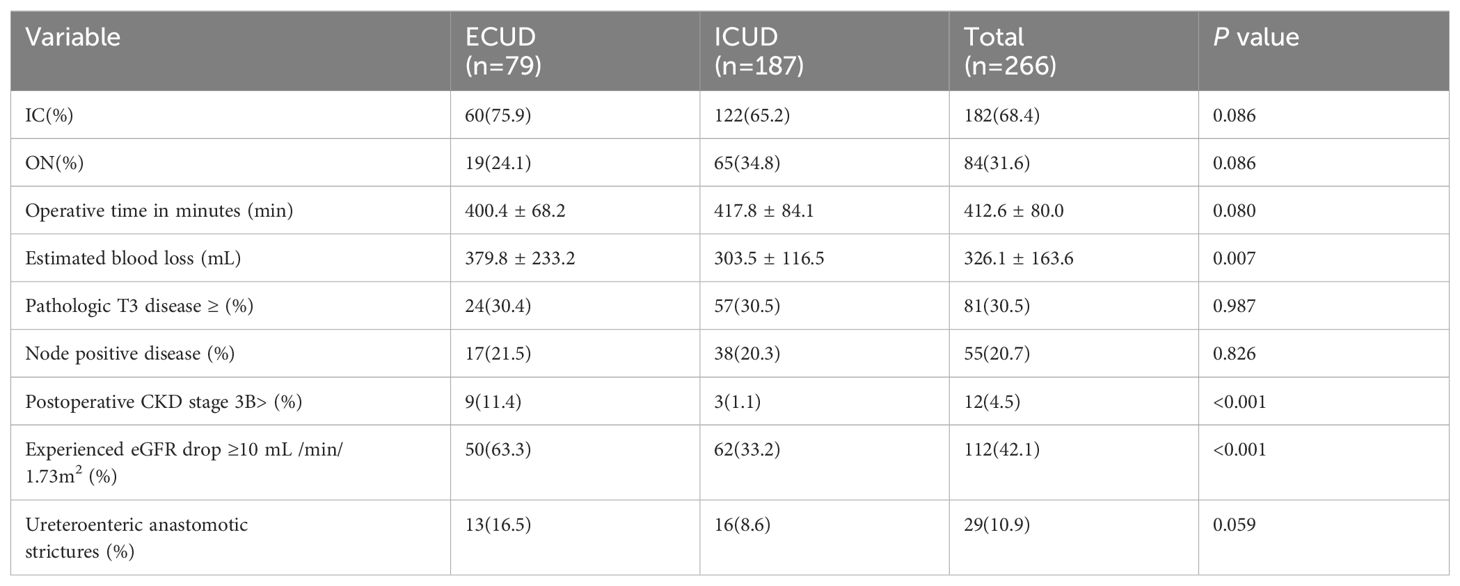
ICUD was associated with a longer operative time compared to ECUD (P=0.080), although this difference was not statistically significant. Estimated blood loss was significantly lower in the ICUD group compared to the EUCD group (303.5 ± 116.5 vs. 379.8 ± 233.3 mL, P=0.007). However, transfusion rates did not differ significantly between the groups (ECUD vs. ICUD 24.1% vs. 20.3%, P=0.498). There were no significant differences in the distribution of pathological T3 stage or higher (P=0.987) and lymph node positivity rate (P=0.826) between the two groups. The incidence of ureteroenteric anastomotic strictures was lower in the ICUD group compared to the ECUD group, though this difference was not statistically significant (ICUD: 8.6%; ECUD: 16.5%, P=0.059), as shown in Table 2.
Overall, 37.6% of patients experienced complications within the first 30 days postoperatively, with 35.3% classified as minor complications (Grade I-II) and 2.3% as major complications (Grade III-V). The most common early complication was urinary tract infection (UTI), affecting 34 patients (12.8%), with 38.2% of these receiving intravenous antibiotic treatment. The incidence of late complications (beyond 90 days) was 38.7%, with hydronephrosis (21.4%) and UTI (17.7%) being the most frequent. None of the patients with late complications experienced major complications.
3.3 Renal function outcomes
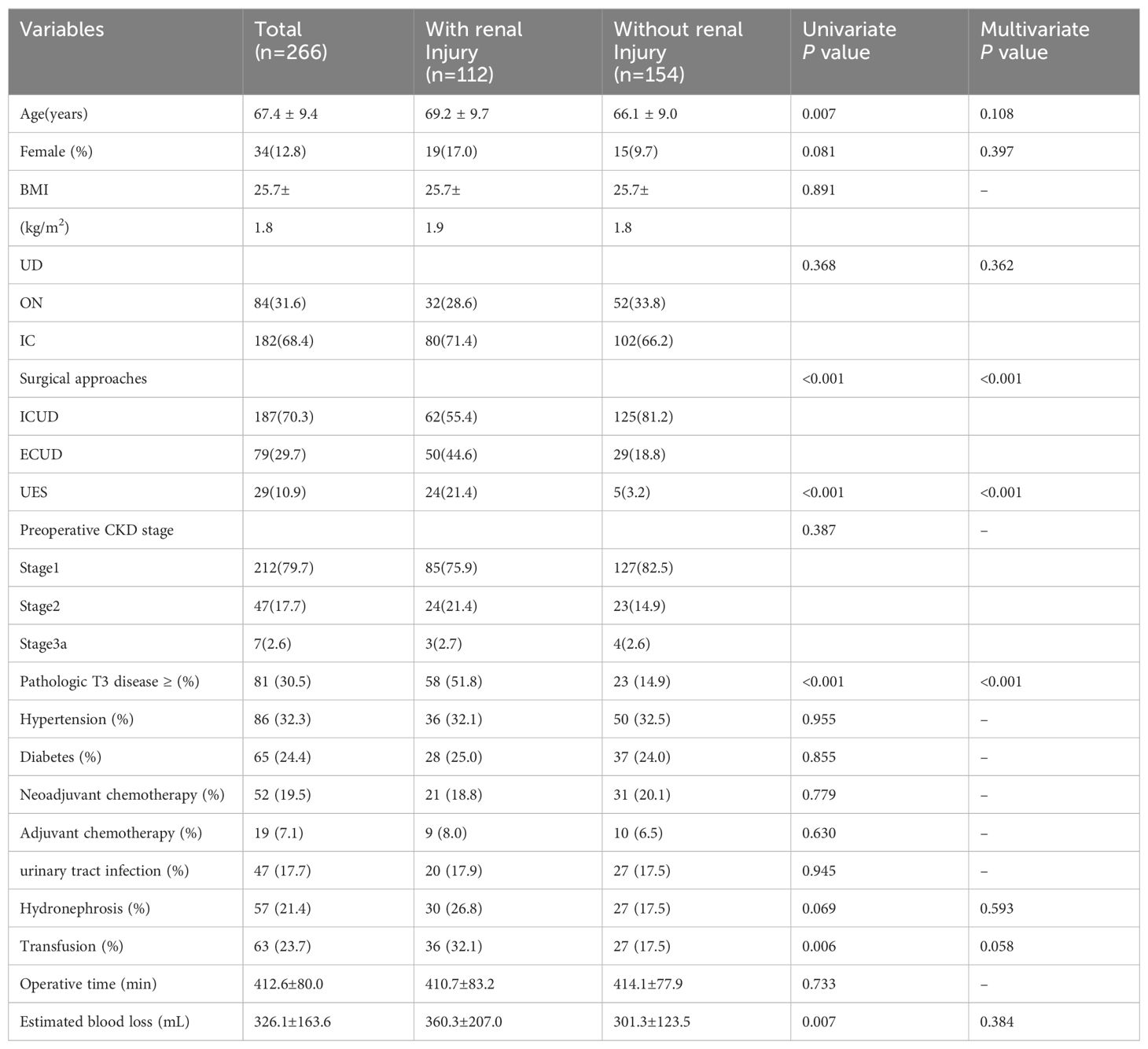
Renal function was monitored at specified intervals through eGFR measurements (Figure 1). There was no significant difference in the average preoperative eGFR between the two groups. Postoperative eGFR values for the ECUD group were 100.3 ± 25.0, 97.1 ± 25.3, 92.3 ± 26.5, 90.4 ± 26.5, and 88.1 ± 28.3 mL/min/1.73m2 at 3, 6, 12, 24, and 36 months, respectively. For the ICUD group, the values were 103.9 ± 23.1, 101.3 ± 23.0, 98.9 ± 23.3, 97.3 ± 23.3, and 95.7 ± 23.5 mL/min/1.73m2 at the same points. Significant differences in eGFR between the two groups were observed at 1 (P=0.042), 2 (P=0.034), and 3 years (P=0.037) postoperatively. During the follow-up period, 112 patients (42.1%) experienced long-term renal injury, with 12 cases (4.5%) progressing to CKD3B or higher. Of these, nine cases (11.4%) were in the ECUD group and three cases (1.1%) were in the ICUD group, with a statistically significant difference (P<0.001). Patients were classified into renal injury and non-renal injury groups based on the presence of long-term renal injury. Univariate analysis revealed significant differences between the two groups in terms of age (P=0.007), surgical approach (P<0.001), ureteroenteric anastomotic strictures (P<0.001), pathological stage T3 or higher (P<0.001), postoperative hydronephrosis (P=0.006), and blood loss (P=0.007). Multivariate logistic regression analysis revealed that different surgical approaches (OR 0.24, 95% CI: 0.12-0.46, P< 0.001), ureteroenteric anastomotic strictures (OR 4.37, 95% CI: 1.32-14.45, P= 0.016), and pathological stage T3 or higher (OR 6.21, 95% CI: 3.20-12.07, P<0.001) were independent risk factors for long-term renal injury postoperatively (Tables 3, 4).
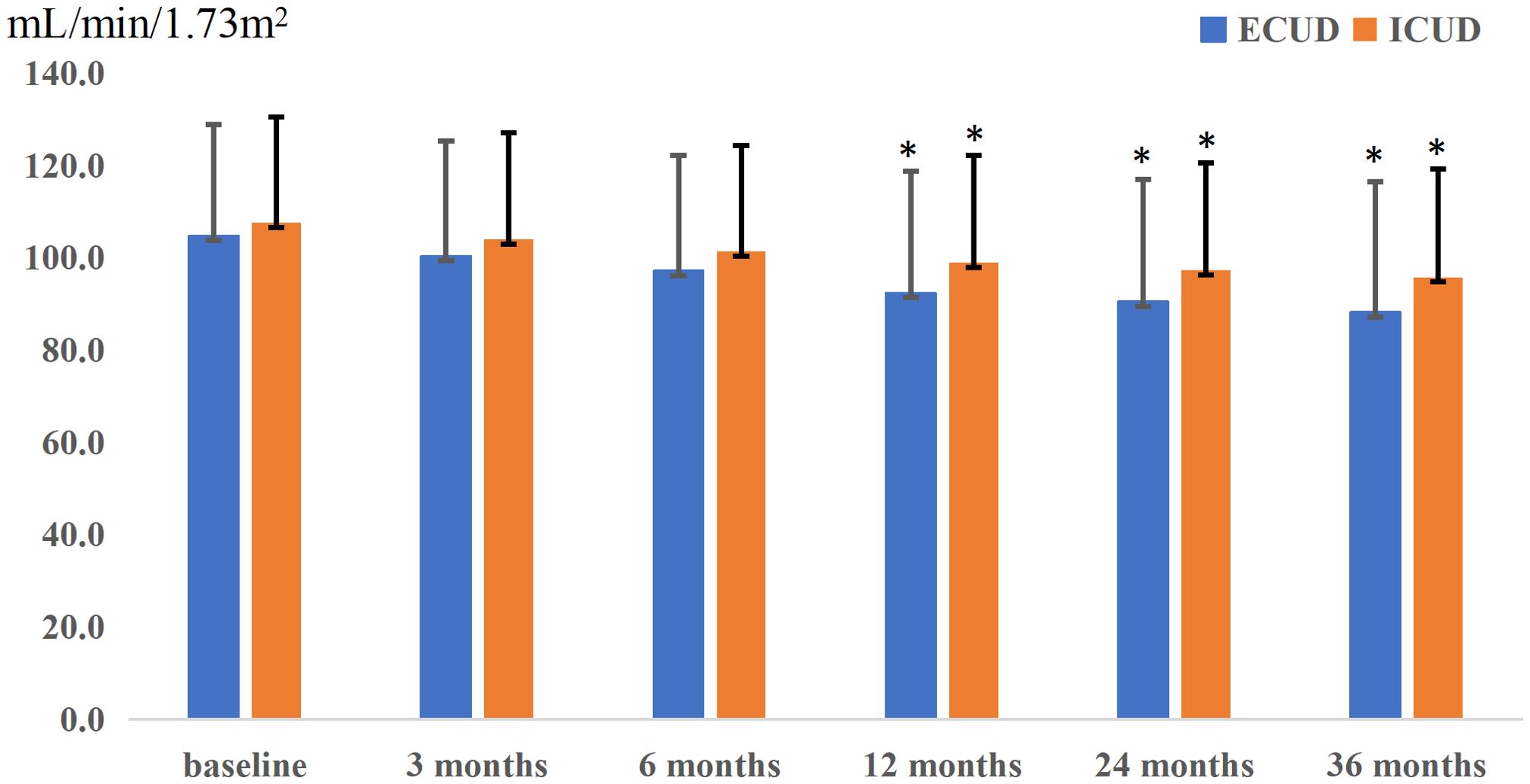
Figure 1. Postoperative renal function changes in two groups, presenting the trend of renal function changes over time for patients undergoing different surgical approaches. The horizontal axis represents follow-up time, while the vertical axis represents the estimated glomerular filtration rate (eGFR). The extracorporeal urinary diversion (ECUD) group is depicted in blue, and the intracorporeal urinary diversion (ICUD) group is depicted in orange. Additionally, error bars are represented by black lines. The * indicates that the P-value is less than 0.05.
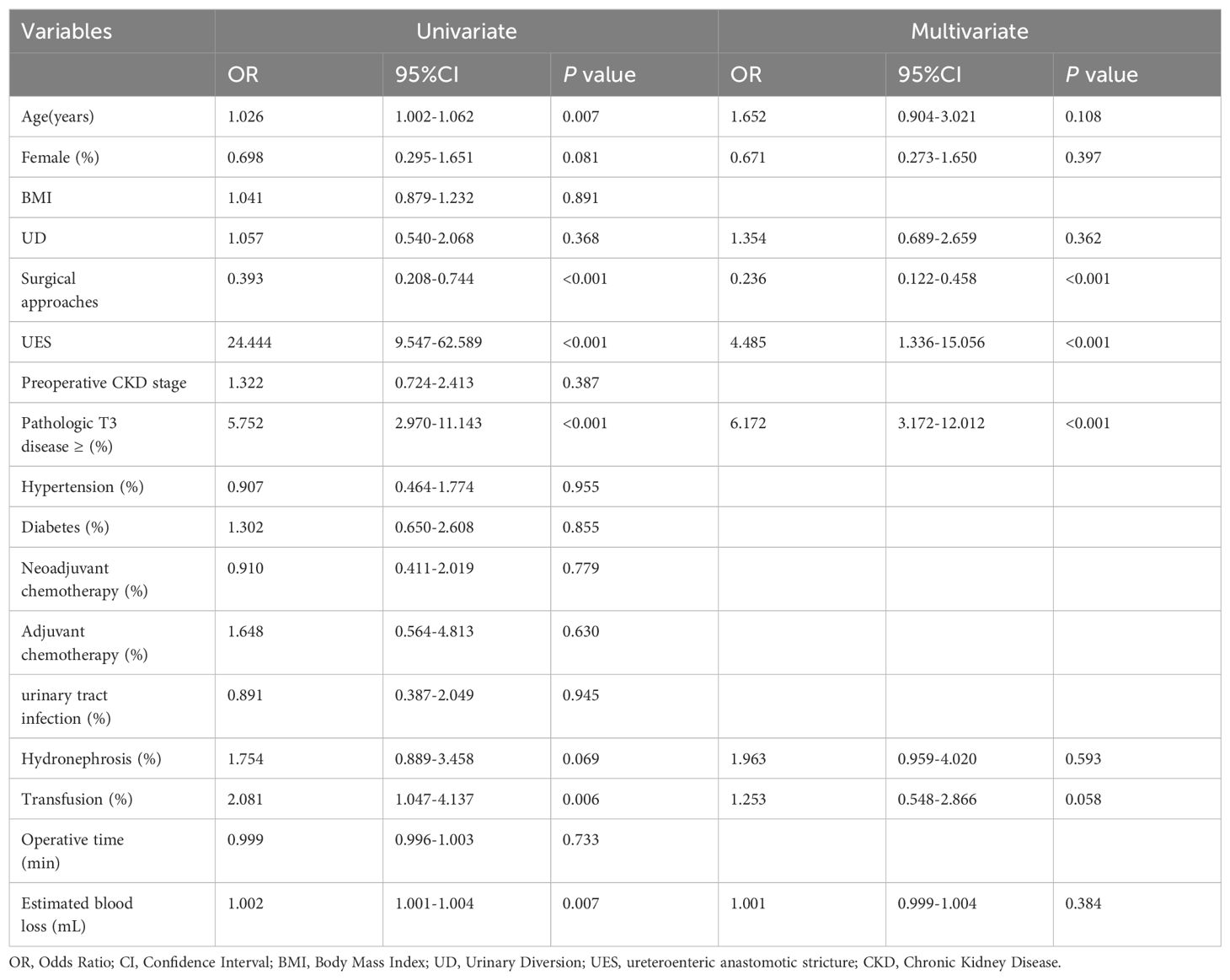
Table 4. univariate and multivarite logistic regressions analysis to predict long-term renal impairment after RARC.
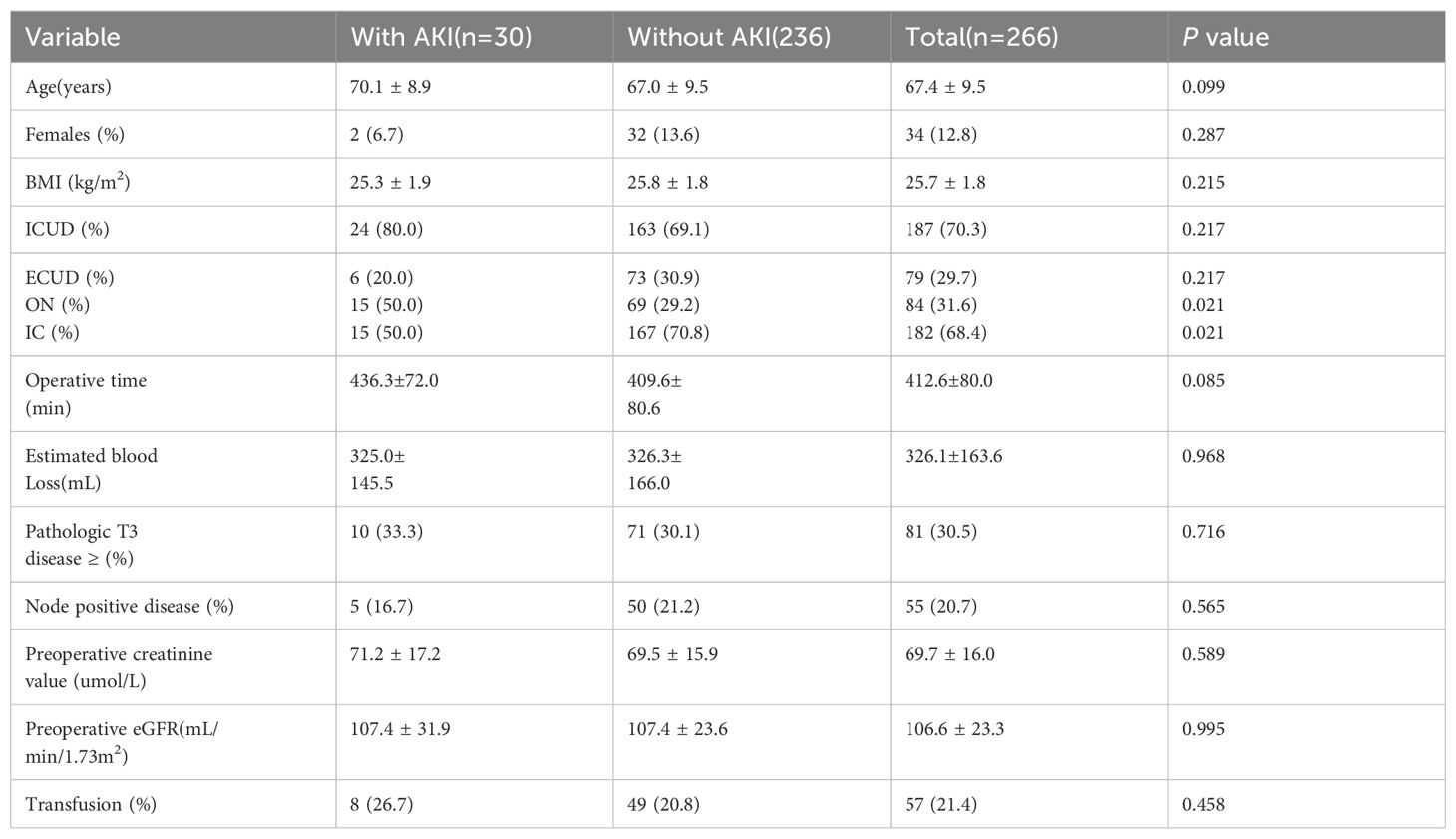
Regarding short-term postoperative renal function, 30 patients (11.3%) experienced AKI during the follow-up period, with 20% in the ECUD group and 80% in the ICUD group (P= 0.217). No statistically significant differences in parameter were observed among patients with AKI, as shown in Table 5.
4 Discussion
In this study, 4.5% of patients progressed to CKD3B or higher stages of CKD following RARC, a proportion lower than some reported data (7). This difference may be attributed to the higher baseline renal function of patients in our study compared to those in international studies. Additionally, 42.1% of patients experienced postoperative renal function injury, which aligns with or slightly lower than findings from other retrospective studies. Variations in these outcomes may be attributed to differences in inclusion/exclusion criteria, such as excluding patients with severe preoperative hydronephrosis, variations in follow-up duration, or differences in renal function assessment methods. Nonetheless, these findings underscore that a considerable proportion of patients experience renal function injury following RARC.
Our study confirms that ECUD is an independent risk factor for long-term renal function injury following RARC. Although there were no significant differences in preoperative eGFR or at 3 and 6 months postoperatively between the two surgical approaches, significant differences emerged at 1, 2, and 3 years postoperatively, with the ECUD group showing a more rapid decline in eGFR compared to the ICUD group. Razdan et al. previously reported that ICUD has a protective effect against eGFR decline at 24 months postoperatively (14). Our study extends these findings by demonstrating that ECUD remains a significant risk factor for long-term renal function injury over a longer follow-up period. Given that RARC with ICUD is associated with less blood loss and no significant differences in operative time, positive margin rate, lymph node yield, and survival rates compared to RARC with ECUD, it is recommended that medical facilities with the capability should consider prioritizing RARC with ICUD as the preferred surgical approach.
Eisenberg et al. identified postoperative ureteroenteric anastomotic strictures as an independent risk factor for renal function impairment following ORC in a cohort of 1631 patients treated at a single institution from 1980 to 2006 (12). Similarly, our study corroborates this finding. The lack of corresponding force feedback in robotic surgical systems may lead to excessive ureteral mobilization during anastomoses in robot-assisted surgery. In ECUD, excessive stretching of the ureters compromises their blood supply. Desai et al. conducted a prospective study involving 132 patients undergoing RARC with intracorporeal IC urinary diversion, reporting a stricture rate of 3.8% at 90 days postoperatively (15). This stricture rate is lower than the 9.4% reported by Anderson et al, suggesting that ICUD, which requires a shorter length of the ureter, may reduce the likelihood of excessive dissection and subsequent ureteral adventitia. Excessive dissection can lead to compromised ureteral blood supply, leading to ischemia at the anastomotic site, perianastomotic fibrosis, and scar formation, resulting in ureteroenteric anastomotic strictures. These structures can further cause ureteral and renal pelvis dilation and hydroureteronephrosis, leading to long-term renal function injury (16). Currently, there is limited evidence on how factors such as urinary diversion, choice of intestinal segment, surgical approaches, ureteroenteric anastomosis, and suturing techniques influence stricture outcomes.
In addition to identifying ureteroenteric anastomotic strictures as an independent risk factor for long-term renal function injury postoperatively, this study also found that pathological stages at T3 or higher are an independent risk factor for long-term renal injury. This association may be due, in part, to the postoperative adjuvant therapy received by patients at this stage, as 19.5% of those with pathological stages at T3 or higher received such treatment. Adjuvant therapy, typically including gemcitabine plus cisplatin (GC) chemotherapy, may impact renal function. Further analysis revealed that the incidence of postoperative hydronephrosis was 35.8% in the T3 or higher group compared to 18.4% in the T2 or lower group, with a statistically significant difference observed. Therefore, the influence of pathological stage at T3 or higher on long-term renal function injury may be multifactorial. Additionally, Nishikawa et al. identified infection-related complications, such as pyelonephritis, as risk factors for renal function impairment in a retrospective analysis of 169 patients (6). In our study, 17.7% of patients experienced postoperative UTI; however, specific data on pyelonephritis were not recorded, largely due to follow-up at other medical institutions. Identifying and managing complications following RC is crucial. Our findings indicate that 37.6% of patients experienced complications within 30 days postoperatively, of which 2.3% were high-grade. The incidence of late complications was 38.7%, with no major complications reported among patients with late complications. These results are consistent with or slightly lower than those reported in previous studies (17, 18).
Regarding short-term postoperative renal function, 11.3% of patients experienced AKI, with no significant difference in AKI incidence between ICUD and ECUD. Hyllested et al. demonstrated that RARC is associated with a higher risk of AKI compared to ORC in a study of 755 patients (19). The learning curve associated with RARC and its representation of only 22% of surgeries during their study period may contribute to the higher risk of AKI. Furrer et al. also reported an increased risk of AKI in patients with surgical time exceeding 400 minutes (20). However, this study did not identify specific factors influencing AKI.
This study has several limitations. It is a retrospective analysis and did not collect data prospectively, which may affect the accuracy of the results. Additionally, non-nadir eGFR values obtained during postoperative follow-up may influence the findings. Furthermore, patients undergoing benign or palliative cystectomy, as well as those with preoperative CKD stages 3B, 4, and 5 who may experience renal function injury postoperatively, were excluded from this study, limiting the generalizability of the findings to these groups. RARC at our institution was performed primarily by three experienced urologists, each having completed a minimum of 15 RARC procedures, typically supported by a consistently reliable team. Nevertheless, variations in surgical techniques and learning curves among practitioners could still impact outcomes. Finally, due to the limited number of patients and the short postoperative follow-up period, further prospective studies with multicenter participation, larger sample sizes, and longer follow-up durations are needed.
5 Conclusion
Postoperative AKI and long-term renal function impairment are common complications following RC for bladder cancer, significantly affecting patients’ quality of life. Long-term follow-up is essential for the early detection and effective management of these complications. This study demonstrates that tumor characteristics (pathological stage), surgery-related complications (ureteroenteric anastomotic strictures), and the choice of surgical approaches (ECUD and ICUD) can impact long-term renal function. But the type of urinary diversion (IC or ON) had no effect on renal function, and ICUD was superior in terms of long-term renal injury rate. Therefore, precise preoperative assessment and careful selection of appropriate surgical approaches are crucial for preserving renal function in patients with bladder cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by The Committee on Medical Ethics of Nanjing Drum Tower Hospital for the studies involving humans. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HW: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. WW: Data curation, Methodology, Writing – original draft. XW: Data curation, Investigation, Writing – original draft. CF: Data curation, Formal Analysis, Writing – original draft. KZ: Investigation, Methodology, Writing – original draft. TC: Investigation, Methodology, Writing – original draft. CZ: Formal Analysis, Project administration, Writing – original draft. SZ: Resources, Supervision, Writing – review & editing. HG: Resources, Supervision, Writing – review & editing. GZ: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burger M, Catto JWF, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. (2013) 63:234–41. doi: 10.1016/j.eururo.2012.07.033
2. Cochetti G, Rossi de Vermandois JA, Maulà V, Cari L, Cagnani R, Suvieri C, et al. Diagnostic performance of the Bladder EpiCheck methylation test and photodynamic diagnosis-guided cystoscopy in the surveillance of high-risk non-muscle invasive bladder cancer: A single centre, prospective, blinded clinical trial. Urol Oncol. (2022) 40:105.e11–105.e18. doi: 10.1016/j.urolonc.2021.11.001
3. Hussein AA, May PR, Jing Z, Ahmed YE, Wijburg CJ, Canda AE, et al. Outcomes of intracorporeal urinary diversion after robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. J Urol. (2018) 199:1302–11. doi: 10.1016/j.juro.2017.12.045
4. Yuh B, Wilson T, Bochner B, Chan K, Palou J, Stenzl A, et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. Eur Urol. (2015) 67:402–22. doi: 10.1016/j.eururo.2014.12.008
5. Lenfant L, Verhoest G, Campi R, Parra J, Graffeille V, Masson-Lecomte A, et al. Perioperative outcomes and complications of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy for bladder cancer: a real-life, multi-institutional french study. World J Urol. (2018) 36:1711–8. doi: 10.1007/s00345-018-2313-8
6. Nishikawa M, Miyake H, Yamashita M, Inoue TA, Fujisawa M. Long-term changes in renal function outcomes following radical cystectomy and urinary diversion. Int J Clin Oncol. (2014) 19:1105–011. doi: 10.1007/s10147-014-0661-y
7. Vejlgaard M, Maibom SL, Stroomberg HV, Poulsen AM, Thind PO, Røder MA, et al. Long-term renal function following radical cystectomy for bladder cancer. Urology. (2022) 160:147–53. doi: 10.1016/j.urology.2021.11.015
8. Zahran MH, Harraz AM, Baset MA, El-Baz R, Shaaban AA, Ali-El-Dein B, et al. Voiding and renal function 10 years after radical cystectomy and orthotopic neobladder in women. BJU Int. (2023) 132:291–7. doi: 10.1111/bju.16011
9. Fujita N, Hatakeyama S, Okita K, Momota M, Narita T, Tobisawa Y, et al. Impact of chronic kidney disease on oncological outcomes in patients with high-risk non-muscle-invasive bladder cancer who underwent adjuvant bacillus Calmette-Guérin therapy. Urol Oncol. (2021) 39:191.e9–191.e16. doi: 10.1016/j.urolonc.2020.06.032
10. Lone Z, Murthy PB, Zhang JH, Ericson KJ, Thomas L, Khanna A, et al. Comparison of renal function after open radical cystectomy, extracorporeal robot assisted radical cystectomy, and intracorporeal robot assisted radical cystectomy. Urol Oncol. (2021) 39:301.e1–9. doi: 10.1016/j.urolonc.2020.09.018
11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
12. Eisenberg MS, Thompson RH, Frank I, Kim SP, Cotter KJ, Tollefson MK, et al. Long-term renal function outcomes after radical cystectomy. J Urol. (2014) 191:619–25. doi: 10.1016/j.juro.2013.09.011
13. Levey AS. Defining AKD: the spectrum of AKI, AKD, and CKD. Nephron. (2022) 146:302–5. doi: 10.1159/000516647
14. Razdan S, Eilender B, Pfail JP, Garcia M, Ranti D, Rosenzweig S, et al. Higher preoperative eGFR is a predictor of worse renal function decline after robotic assisted radical cystectomy: Implications for postoperative management. Urol Oncol. (2022) 40:275.e11–275.e18. doi: 10.1016/j.urolonc.2022.02.011
15. Desai MM, Gill IS, De Castro Abreu AL, Hosseini A, Nyberg T, Adding C, et al. Robotic intracorporeal orthotopic neobladder during radical cystectomy in 132 patients. J Urol. (2014) 192:1734–40. doi: 10.1016/j.juro.2014.06.087
16. Ahmed K, Khan SA, Hayn MH, Agarwal PK, Badani KK, Balbay MD, et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. (2014) 65:340–7. doi: 10.1016/j.eururo.2013.09.042
17. Cochetti G, Paladini A, Del Zingaro M, Ciarletti S, Pastore F, Massa G, et al. Robot-assisted radical cystectomy with intracorporeal reconstruction of urinary diversion by mechanical stapler: prospective evaluation of early and late complications. Front Surg. (2023) 10:1157684. doi: 10.3389/fsurg.2023.1157684
18. Kauffman EC, Ng CK, Lee MM, Otto BJ, Portnoff A, Wang GJ, et al. Critical analysis of complications after robotic-assisted radical cystectomy with identification of preoperative and operative risk factors. BJU Int. (2010) 105:520–7. doi: 10.1111/j.1464-410X.2009.08843.x
19. Hyllested E, Vejlgaard M, Stroomberg HV, Maibom SL, Joensen UN, Røder A. Acute kidney injury within 90Days of radical cystectomy for bladder cancer: incidence and risk factors. Urology. (2023) 182:181–9. doi: 10.1016/j.urology.2023.07.047
Keywords: bladder cancer, robot-assisted radical cystectomy, urinary diversion, acute kidney injury, chronic kidney injury
Citation: Wang H, Wang W, Wang X, Fang C, Zhao K, Chen T, Zhang C, Zhang S, Guo H and Zhang G (2024) Intracorporeal urinary diversion offers the advantage of delaying postoperative renal function injury in patients undergoing robot-assisted radical cystectomy. Front. Oncol. 14:1435050. doi: 10.3389/fonc.2024.1435050
Received: 19 May 2024; Accepted: 13 August 2024;
Published: 04 September 2024.
Edited by:
Salvatore Siracusano, University of L’Aquila, ItalyReviewed by:
Murat Akand, University Hospitals Leuven, BelgiumAndrey O. Morozov, I.M. Sechenov First Moscow State Medical University, Russia
Alessio Paladini, University of Perugia, Italy
Copyright © 2024 Wang, Wang, Wang, Fang, Zhao, Chen, Zhang, Zhang, Guo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gutian Zhang, emhhbmcuZ3V0aWFuQG5qdS5lZHUuY24=
 Hao Wang
Hao Wang Wendi Wang2
Wendi Wang2 Chengwei Zhang
Chengwei Zhang Hongqian Guo
Hongqian Guo