- 1Department of Radiotherapy, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Cancer Hospital of Dalian University of Technology, Shenyang, Liaoning, , China
- 2School of Graduate, China Medical University, Shenyang, China
- 3Faculty of Medicine, Dalian University of Technology, Dalian, China
Purpose: Lung cancer is a devastating disease, with brain metastasis being one of the most common distant metastases of lung adenocarcinoma. This study aimed to investigate the prognostic characteristics of individuals with brain metastases originating from invasive lung adenocarcinoma of distinct pathological subtypes, providing a reference for the management of these patients.
Methods: Clinical data from 156 patients with lung adenocarcinoma-derived brain metastases were collected, including age, sex, smoking status, Karnofsky Performance Status scores, pathological subtype, lymph node metastasis, tumor site, treatment mode, T stage, and N stage. Patients were classified into two groups (highly differentiated and poorly differentiated) based on their pathological subtypes. Propensity score matching was used to control for confounding factors. The prognostic value of pathological subtypes was assessed using Kaplan-Meier analysis and Cox proportional hazards regression modeling.
Results: Kaplan-Meier analysis indicated that patients in the moderately to highly differentiated group had better prognoses. Multivariate analysis revealed that being in the poorly differentiated group was a risk factor for poorer prognosis. Thoracic tumor radiation therapy, chemotherapy, and surgery positively influenced the time interval between lung cancer diagnosis and brain metastasis.
Conclusions: The pathological subtypes of lung adenocarcinoma-derived brain metastases are associated with patient prognosis. Patients in the poorly differentiated group have worse prognoses compared to those in the moderately to highly differentiated group. Therefore, patients in the poorly differentiated group may require more frequent follow-ups and aggressive treatment.
1 Introduction
Lung cancer is a worldwide population health concern with a mortality rate higher than that of breast, prostate, and colorectal cancers combined (1). In China, lung cancer has the highest incidence and mortality rate among all cancer types (2, 3). Approximately 85% of lung cancer diagnoses are non-small-cell lung carcinomas (NSCLC) (4). Between 10% and 20% of patients with NSCLC develop brain metastases at presentation, and up to 50% of patients will develop brain metastases during the course of the disease (5, 6). Patients with brain metastases have a poor prognosis and a shortened median survival (7). With the continued development of molecular targeted therapies and immunotherapies, patients with lung cancer are living longer and, therefore, are at greater risk for brain metastases. Although the deleterious effects of brain metastases from lung cancer are widely understood, patients with brain metastases are less sensitive to drug therapy, and surgical interventions are limited. The median survival of patients with brain metastases is typically 4–9 months (8). Brain metastasis significantly impacts patient quality of life and has become a serious global social health problem (9–11).
Lung adenocarcinoma has a greater risk of brain metastasis among patients with NSCLC, according to a long-term follow-up in the US SEER database (12). However, the number of studies on prognostic factors for brain metastases in lung adenocarcinoma is limited. Cell type, primary tumor size, and lymph node stage have been associated with the probability of lung adenocarcinoma brain metastasis (13). Pathological subtypes of lung adenocarcinoma play a considerable role in cancer progression. Invasive non-mucinous adenocarcinomas are divided into five types: lepidic, acinar, papillary, micropapillary, and solid (14). Each pathological subtype has unique histological features that impact patient survival and treatment. Numerous studies have found that micropapillary and solid types are associated with a poorer prognosis, whereas the lepidic growth type is associated with a better prognosis (15–17). Therefore, lung adenocarcinomas with micropapillary and solid components are considered high-risk and require more thorough treatments. In contrast, the other subtypes are categorized as low risk.
Considering that NSCLC comprises numerous types that may be affected by various confounding factors, we selected lung adenocarcinoma, which is prone to brain metastases, as the study subject to reduce interference and improve the accuracy of the study. Moreover, to date, the impact of different pathologic subtypes on the prognosis of patients with brain metastases from aggressive lung adenocarcinoma has not been reported. Therefore, we retrospectively analyzed the relationship between pathologic subtypes of lung adenocarcinoma and survival after brain metastasis. In addition, we evaluated the relationship between pathologic subtypes and the time interval between lung cancer diagnosis and brain metastasis (brain metastasis interval). We evaluated 156 patients with brain metastases from invasive lung adenocarcinoma admitted to our hospital to provide a theoretical basis for future treatment approaches.
2 Methods
2.1 Study subjects
The clinical data of 156 patients with brain metastases from invasive lung adenocarcinoma were assessed. The patients were treated at the Liaoning Provincial Tumor Hospital (2008–2017). The inclusion criteria were as follows: (1) a clear diagnosis of lung adenocarcinoma and the existence of subtype classification according to clinicopathology or cytology; (2) brain metastasis confirmed by magnetic resonance imaging; and (3) age ≥18 years. The exclusion criteria were as follows: (1) no clear pathological diagnosis or secondary lung cancer; (2) primary tumor at other sites; and (3) incomplete clinical data.
2.2 Data collection
The relevant patient information was collected, including pathological type, sex, age, smoking status, Karnofsky Performance Status (KPS) score, lymph node metastasis, tumor site, treatment modality, T stage (size and extent of the primary tumor), and N stage (number of affected lymph nodes).
2.3 Grouping method
Lung cancer is divided into three grades: grade 1 indicates high differentiation, with predominantly lepidic growth type and a high-grade pattern (solid, micropapillary, or complex glandular) not exceeding 20%; grade 2 indicates moderate differentiation, with acinar or papillary predominance and a high-grade pattern not exceeding 20%; and grade 3 indicates poor differentiation, where the high-grade pattern is ≥20%. Patients were divided into two groups: a moderate- to high-differentiation group and a poor-differentiation group, according to their pathological subtypes.
2.4 Follow-up
The date of the patient’s death or last follow-up was used as the cutoff date.
2.5 Statistical methods
After propensity score matching (PSM), the patients from the poor-differentiation group (n = 59) and moderate- to high-differentiation group (n = 59) were matched using a 1:1 ratio. The parameters of patient clinicopathological characteristics and the distinct pathological subtypes were compared using the chi-squared test. Multivariate Cox regression analysis was used to determine the prognostic risk factors. The Kaplan–Meier method was applied to the survival curves to calculate the survival rates (0: moderate- to high-differentiation group; 1: poor-differentiation group), and the difference in survival was compared using the log-rank test. Differences were considered statistically significant when P < 0.05. All statistical analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS) version 25.0.
3 Results
3.1 Patient characteristics
Hundred fifty-six patients who met the inclusion criteria were recruited for this analysis. Of those patients, 89 (57.1%) had moderately to highly differentiated invasive lung adenocarcinoma, and the remaining 67 (42.9%) had poorly differentiated invasive lung adenocarcinoma. More than half (66.7%) of the individuals were younger than 60 years, 79 (50.6%) were female, and the majority (76.2%) underwent chest chemotherapy. In the poor-differentiation group, patients were more likely to be male (P < 0.05). Lymph node involvement (N2–3; P < 0.05) was more severe in the moderate- to high-differentiation group than in the poor-differentiation group (Table 1).
3.2 Survival analysis of overall survival and the brain metastasis interval before PSM
Case subtype was linked to brain metastasis survival in univariate analyses (hazard ratio [HR]: 1.421; 95% confidence interval [CI]: 1.020–1.981; P = 0.038) but not to the brain metastasis interval (HR: 1.190; 95% CI: 0.853–1.660; P = 0.305). The KPS, primary tumor site, T stage, thoracic tumor stage, thoracic tumor chemotherapy, thoracic tumor radiation therapy, and thoracic tumor surgical therapy were related to the brain metastasis interval (P < 0.05). Patients in the poor-differentiation group had a lower survival rate after brain metastasis (HR: 1.421; 95% CI: 1.020-1.981; P = 0.038) than those in the moderate- to high-differentiation group. As shown by the multivariate analysis, a peripheral primary tumor site (HR: 0.516; 95% CI: 0.331–0.802; P = 0.003), chemotherapy (HR: 0.415; 95% CI: 0.276–0.624; P < 0.001), and surgical treatment for thoracic tumors (HR: 0.266; 95% CI: 0.151–0.469; P < 0.001) were positive prognostic factors for the brain metastasis interval (Tables 2, 3).
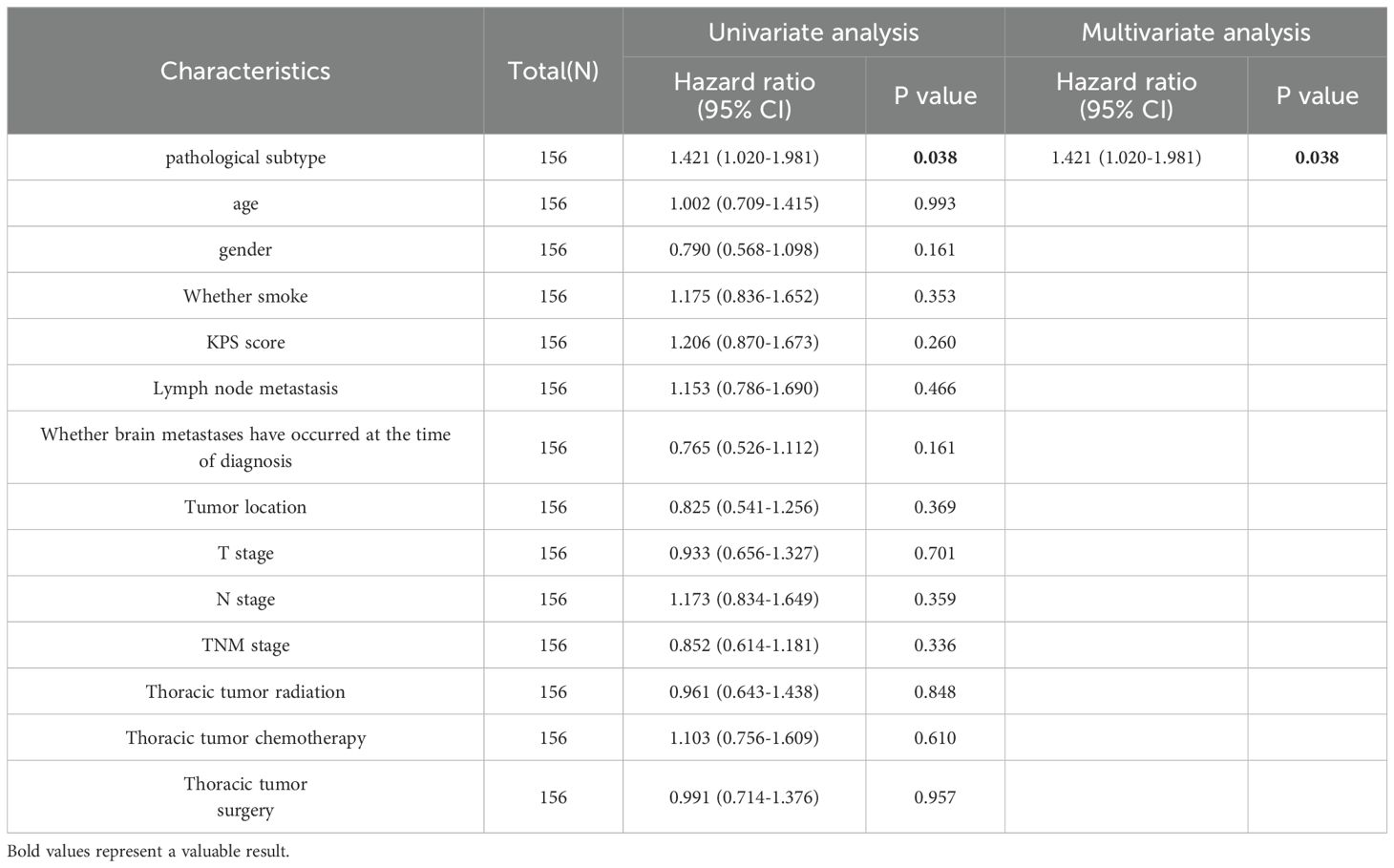
Table 2. Univariate and multivariate Cox regression analyses before propensity score matching to examine the overall survival.

Table 3. Univariate and multivariate Cox regression analyses before propensity score matching to examine the brain metastasis interval.
3.3 Survival analysis of overall survival and the brain metastasis interval after PSM
The basic principle of PSM is to replace multiple covariates with a single score that equalizes the covariate distribution between the treatment and control groups. Before PSM, the moderate- to high-differentiation group included 89 patients, and the poor-differentiation group included 67 patients. After PSM, 59 clinically homogeneous patients were included in each group. In the moderate- to high-differentiation and poor-differentiation groups, the risk factors (age, sex, smoking status, the KPS score, lymph node metastasis, whether brain metastasis occurred at the time of diagnosis, location of the tumor in the thorax, T stage, N stage, tumor stage, chemotherapy, radiotherapy, and surgical treatment) influencing patient survival and the brain metastasis interval were balanced. Kaplan–Meier curves showed that patients with brain metastases in the moderate- to high-differentiation group had a longer overall survival (OS; P < 0.05) with a median OS (mOS) of 25.00 months (95% CI: 19.55–30.45 months) compared with an mOS of 14.67 months in the poor-differentiation group (95% CI: 11.80–17.53 months) (Figure 1).
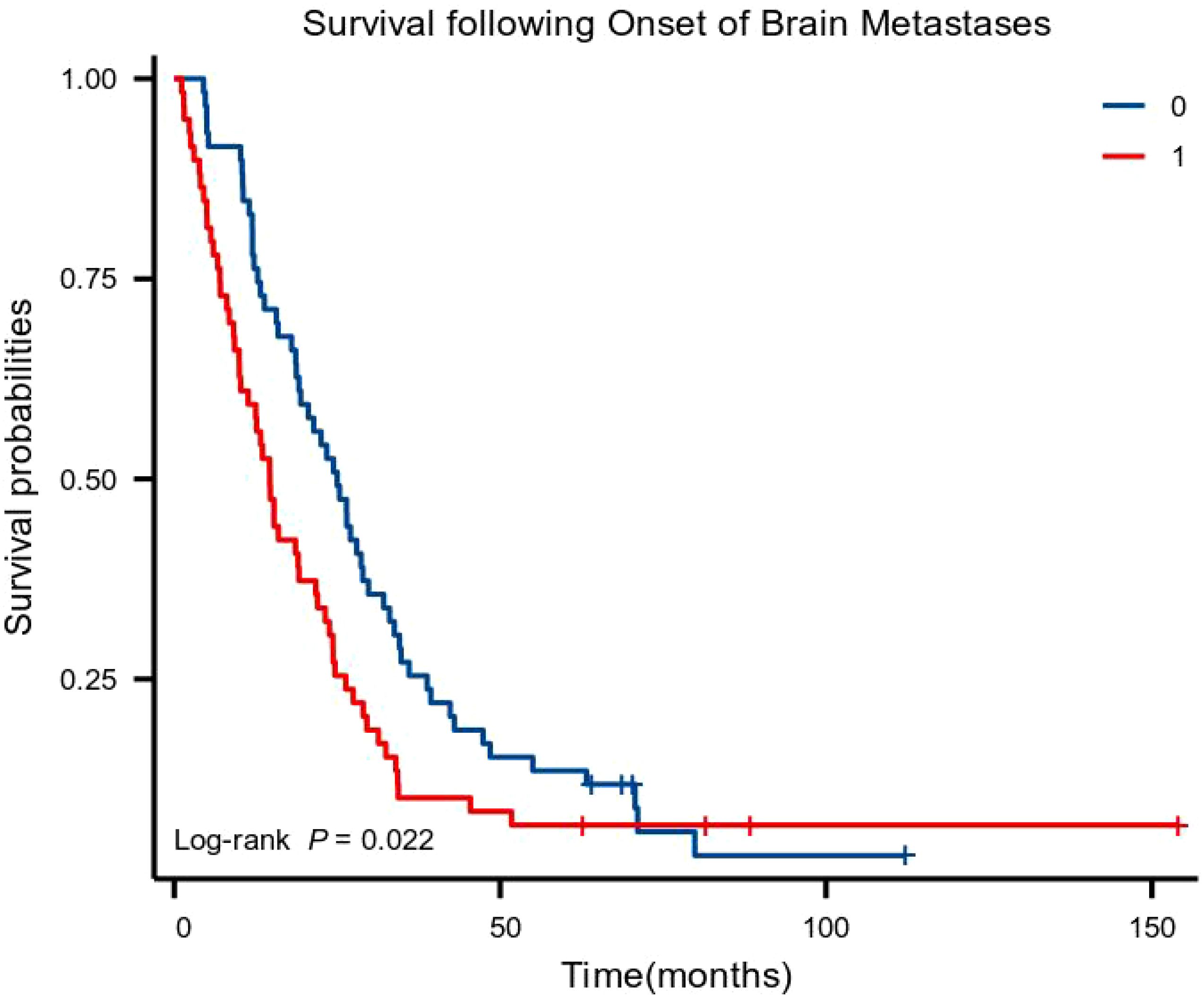
Figure 1. Kaplan–Meier curves for overall survival (0: moderate- to high-differentiation group; 1: poor-differentiation group).
In the univariate analysis, the pathological subtype was related to brain metastasis survival (HR: 1.546, 95% CI: 1.061–2.254; P = 0.023) but not to the brain metastasis interval (HR: 1.112; 95% CI: 0.762–1.621; P = 0.583). KPS, thoracic tumor staging, thoracic tumor chemotherapy, thoracic tumor radiation therapy, and thoracic tumor surgical treatment were related to the brain metastasis interval (P < 0.05). The survival of patients with brain metastases was lower in the poor-differentiation group (HR: 1.546; 95% CI: 1.061–2.254; P = 0.023) than in the moderate- to high-differentiation group in the multivariate analysis. Thoracic tumor radiation therapy (HR: 0.492; 95% CI: 0.282–0.858; P = 0.012), chemotherapy for thoracic tumors (HR: 0.399; 95% CI: 0.252–0.632; P < 0.001), and surgical treatment for thoracic tumors (HR: 0.198; 95% CI: 0.102–0.387; P < 0.001) were positive prognostic factors for the brain metastasis interval (Tables 4, 5).
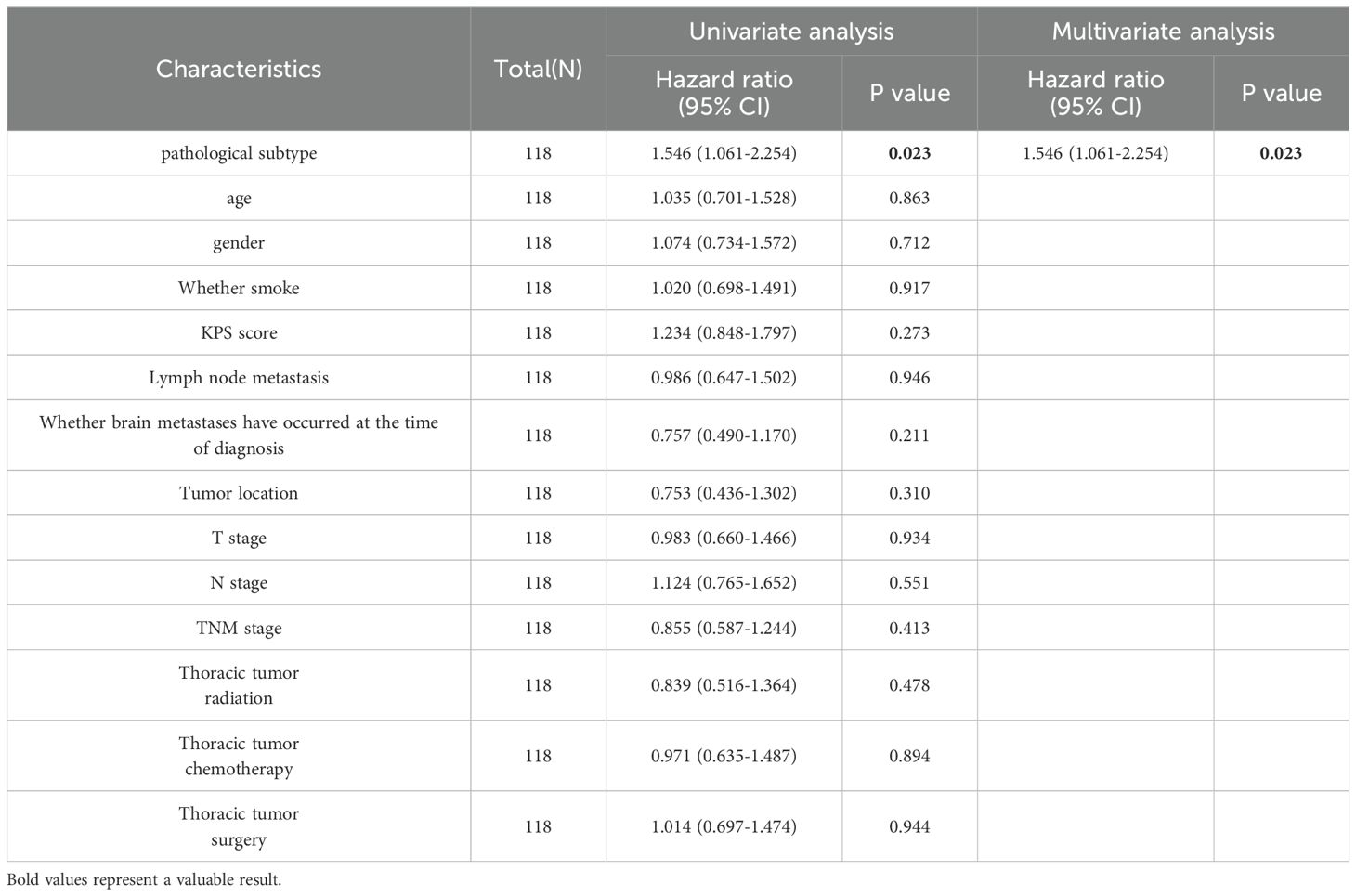
Table 4. Univariate and multivariate Cox regression analyses after propensity score matching to examine overall survival.

Table 5. Univariate and multivariate Cox regression analyses after propensity score matching to examine the brain metastasis interval.
4 Discussion
We evaluated the relationship between pathological subtype, patient survival after brain metastasis, and the interval between lung cancer diagnosis and brain metastases. This study revealed a longer mOS and OS in the moderate- to high-differentiation group. Furthermore, the pathological subtype of lung adenocarcinoma (P = 0.023) was revealed as an independent factor impacting survival time. Independent factors affecting the brain metastasis interval included radiation therapy for thoracic tumors (P = 0.012), chemotherapy for thoracic tumors (P < 0.001), and surgical treatment for thoracic tumors (P < 0.001).
In studies focusing on the interval between diagnosis and brain metastasis, the lung adenocarcinoma pathological subtype did not influence the brain metastasis interval; however, treatments targeting thoracic tumors (surgical treatment, chemotherapy, and radiation) tended to delay the development of brain metastases. Similarly, some studies have found that patients with lung adenocarcinoma who did not receive complementary treatments were more prone to develop brain metastasis after a definitive diagnosis of lung cancer (18, 19). However, most studies evaluating the time interval between diagnosis and brain metastasis did not group patients by pathological subtypes (no comparative data have been found at this time). Yang et al. (20) observed that shorter brain metastasis time intervals adversely affect the survival of patients undergoing surgery. Hence, our understanding of the relationship between brain metastasis time intervals and pathological subtypes needs to be improved to ensure timely treatment.
Various histological subtypes of lung adenocarcinoma show diverse clinical features, and the risk of recurrence and prognosis also differ. Yaldız et al. (21) found that solid and micropapillary histological subtypes were poor prognostic factors in invasive lung adenocarcinomas undergoing surgical treatment. Additionally, Russell et al. (22) found that micropapillary adenocarcinomas had a lower survival rate than papillary- and vesicular-dominant adenocarcinomas. Several studies exploring the relationship between pathological subtypes and brain metastasis found that patients with micropapillary and solid types are prone to brain metastases with lower survival (23–25). Consistent with previous studies, this study found a significant correlation between pathological subtypes and the progression and prognosis of brain metastases in invasive lung adenocarcinoma. Therefore, patients with a pathology suggestive of solid and micropapillary types should be closely followed up with postoperative examinations to observe tumor metastasis, and these should be treated aggressively.
The pathological subtypes also offer insights into the clinical treatment options. The response of distinct lung adenocarcinoma subtypes to targeted therapy and immunotherapy requires further investigation. For patients with invasive adenocarcinoma, there are a number of conventional targets for mutation detection related to the specific histological growth pattern of adenocarcinomas. The epidermal growth factor receptor (EGFR) gene is the most well-studied molecular target in lung cancer and is a biomarker for predicting the effectiveness of targeted therapies (26). Various studies have reported that EGFR mutations have the highest incidence rate in micropapillary tumors, followed by alveolar and solid tumor types (27–29). Studying the relationship between pathological subtype and gene mutation status provides important information to assist patients in their therapeutic choices. The expression of programmed death-ligand 1 (PD-L1) in invasive lung adenocarcinomas also varies greatly according to the histological type. PD-L1-positive tumors are more common in alveolar and solid adenocarcinomas than in other adenocarcinoma subtypes (30). This pathological classification can have a meaningful impact when screening patients for lung adenocarcinomas who are more suitable for immunotherapy, both for postoperative adjuvant chemotherapy and for treatment after recurrence. The patient’s pathological subtype may assist in selecting the most appropriate treatment regimen.
Each histological subtype of invasive lung adenocarcinoma is associated with a distinct prognosis, and the underlying mechanisms have been somewhat elucidated. One study found that, at the single-cell level, the tumor microenvironment in solid-type invasive lung adenocarcinoma was more hypoxic and acidic than that in other histological subtypes. This leads to fewer T cells, an increase in immunosuppressive myeloid cells, and a higher incidence of tumor metastasis (31). In addition, when typing lung adenocarcinomas according to different DNA methylation levels, the hypermethylated subtypes tend to be micropapillary-predominant cases. This demonstrates that patients with brain metastases from invasive pulmonary adenocarcinomas of the solid and micropapillary types have a worse prognosis, adding to the credibility of our study (32). Patients with early-stage cancer have a higher risk of metastasis if their tumors contain a highly aggressive component, requiring more stringent adjuvant therapy and close follow-up.
This study has some limitations. It was a single-center retrospective study; therefore, selection bias and confounding factors are unavoidable. Furthermore, follow-ups were not continued to obtain patient survival data. Moreover, in our cohort, the proportion of patients with gene mutations was not analyzed.
In conclusion, the findings indicate that pathological subtype is an independent risk factor affecting prognosis. Additionally, patients with poorly differentiated pathological subtypes had a lower survival rate. This provides important information for clinicians to judge patient prognosis without the need for other auxiliary techniques, as well as a reliable experimental basis for guiding the time window of treatment for this type of patient. Future prospective randomized cohort studies with large sample sizes need to be conducted for more detailed analyses. In addition, further research should consider the proportion of patients with gene mutations to draw relevant conclusions. In the near future, we expect to be able to analyze the progression of lung adenocarcinoma in terms of tumor recurrence, lymph node metastasis, and hematopoietic metastasis according to pathological subtypes, thereby leading to more accurate diagnosis and treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Liaoning Cancer Hospital and the number is 2020X0102. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CZ: Writing – original draft. XZ: Writing – original draft. XY: Writing – original draft. HX: Writing – review & editing. HT: Writing – review & editing. YS: Writing – review & editing. ML: Writing – review & editing. YJ: Writing – review & editing. TW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Fundamental Research Funds for the Central Universities [LD2023032, LD2023011, LD202221], Ministry of Science and Technology of the People’s Republic of China [G2023127016L].
Acknowledgments
We are very grateful to the participants who participated in the study because they spend precious time participating in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (London England). (2022) 42:937–70. doi: 10.1002/cac2.12359
3. Lu S, Yu Y, Yang Y. Retrospect and prospect for lung cancer in China: clinical advances of immune checkpoint inhibitors. oncologist. (2019) 24:S21–s30. doi: 10.1634/theoncologist.2019-IO-S1-s02
4. de Castro J, Tagliaferri P, de Lima VCC, Ng S, Thomas M, Arunachalam A, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care. (2017) 26:e12734. doi: 10.1111/ecc.12734
5. Page S, Milner-Watts C, Perna M, Janzic U, Vidal N, Kaudeer N, et al. Systemic treatment of brain metastases in non-small cell lung cancer. Eur J Cancer (Oxford England: 1990). (2020) 132:187–98. doi: 10.1016/j.ejca.2020.03.006
6. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clinic Proc. (2019) 94:1623–40. doi: 10.1016/j.mayocp.2019.01.013
7. Farris JC, Hughes RT, Razavian NB, Pearce JB, Snavely AC, Chan MD, et al. Brain metastasis incidence and patterns of presentation after definitive treatment of locally advanced non-small cell lung cancer: A potential argument for brain magnetic resonance imaging surveillance. Adv Radiat Oncol. (2023) 8:101058. doi: 10.1016/j.adro.2022.101058
8. Peters S, Bexelius C, Munk V, Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev. (2016) 45:139–62. doi: 10.1016/j.ctrv.2016.03.009
9. Gupta S, Singh S, Chophy A, Nair S, Ahuja R, Kusum K, et al. Analysis of prognostic factors in patients with brain metastases affecting survival. J Egyptian Natl Cancer Institute. (2022) 34:45. doi: 10.1186/s43046-022-00146-z
10. Zhu H, Zhou L, Guo Y, Yang G, Dong Q, Zhang Z, et al. Factors for incidence risk and prognosis in non-small-cell lung cancer patients with synchronous brain metastasis: a population-based study. Future Oncol (London England). (2021) 17:2461–73. doi: 10.2217/fon-2021-0103
11. Han X, Li H. Research progress in the treatment of brain metastases from non-small cell lung cancer. Zhongguo Fei Ai Za Zhi Chin J Lung Cancer. (2020) 23:1087–94. doi: 10.3779/j.issn.1009-3419.2020.102.39
12. Jena A, Taneja S, Talwar V, Sharma JB. Magnetic resonance (MR) patterns of brain metastasis in lung cancer patients: correlation of imaging findings with symptom. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. (2008) 3:140–4. doi: 10.1097/JTO.0b013e318161d775
13. Won YW, Joo J, Yun T, Lee GK, Han JY, Kim HT, et al. A nomogram to predict brain metastasis as the first relapse in curatively resected non-small cell lung cancer patients. Lung Cancer (Amsterdam Netherlands). (2015) 88:201–7. doi: 10.1016/j.lungcan.2015.02.006
14. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
15. Chang C, Sun X, Zhao W, Wang R, Qian X, Lei B, et al. Minor components of micropapillary and solid subtypes in lung invasive adenocarcinoma (≤ 3 cm): PET/CT findings and correlations with lymph node metastasis. La Radiol Med. (2020) 125:257–64. doi: 10.1007/s11547-019-01112-x
16. Xu L, Zhou H, Wang G, Huang Z, Xiong R, Sun X, et al. The prognostic influence of histological subtypes of micropapillary tumors on patients with lung adenocarcinoma ≤ 2 cm. Front Oncol. (2022) 12:954317. doi: 10.3389/fonc.2022.954317
17. Takeno N, Tarumi S, Abe M, Suzuki Y, Kinoshita I, Kato T. Lung adenocarcinoma with micropapillary and solid patterns: Recurrence rate and trends. Thorac Cancer. (2023) 14:2987–92. doi: 10.1111/1759-7714.15087
18. Ilhan-Mutlu A, Osswald M, Liao Y, Gömmel M, Reck M, Miles D, et al. Bevacizumab prevents brain metastases formation in lung adenocarcinoma. Mol Cancer Ther. (2016) 15:702–10. doi: 10.1158/1535-7163.Mct-15-0582
19. Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQ. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro-oncology. (2017) 19:i1–i24. doi: 10.1093/neuonc/now197
20. Yang Z, Chen H, Jin T, Sun L, Li L, Zhang S, et al. The impact of time interval on prognosis in patients with non-small cell lung cancer brain metastases after metastases surgery. World Neurosurg. (2023) 180:e171–82. doi: 10.1016/j.wneu.2023.09.021
21. Yaldız D, Örs Kaya Ş, Ceylan KC, Acar A, Aydoğdu Z, Gürsoy S, et al. Prognostic effects of predominant histologic subtypes in resected pulmonary adenocarcinomas. Balkan Med J. (2019) 36:347–53. doi: 10.4274/balkanmedj.galenos.2019.2019.1.130
22. Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. (2011) 6:1496–504. doi: 10.1097/JTO.0b013e318221f701
23. Lengel HB, Mastrogiacomo B, Connolly JG, Tan KS, Liu Y, Fick CN, et al. Genomic mapping of metastatic organotropism in lung adenocarcinoma. Cancer Cell. (2023) 41:970–985.e973. doi: 10.1016/j.ccell.2023.03.018
24. Arrieta O, Salas AA, Cardona AF, Díaz-García D, Lara-Mejía L, Escamilla I, et al. Risk of development of brain metastases according to the IASLC/ATS/ERS lung adenocarcinoma classification in locally advanced and metastatic disease. Lung Cancer (Amsterdam Netherlands). (2021) 155:183–90. doi: 10.1016/j.lungcan.2021.01.023
25. Casteillo F, Guy JB, Dal-Col P, Karpathiou G, Pommier B, Bayle-Bleuez S, et al. Pathologic subtypes of lung adenocarcinoma brain metastasis is a strong predictor of survival after resection. Am J Surg Pathol. (2018) 42:1701–7. doi: 10.1097/pas.0000000000001161
26. Araghi M, Mannani R, Heidarnejad maleki A, Hamidi A, Rostami S, Safa SH, et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. (2023) 23:162. doi: 10.1186/s12935-023-02990-y
27. Chen Z, Liu X, Zhao J, Yang H, Teng X. Correlation of EGFR mutation and histological subtype according to the IASLC/ATS/ERS classification of lung adenocarcinoma. Int J Clin Exp Pathol. (2014) 7(11):8039–45.
28. Kim HJ, Choi EY, Jin HJ, Shin KC. Relationship between EGFR mutations and clinicopathological features of lung adenocarcinomas diagnosed via small biopsies. Anticancer Res. (2014) 34:3189–95.
29. Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol (Northwood London England). (2013) 30:645. doi: 10.1007/s12032-013-0645-1
30. Miyazawa T, Marushima H, Saji H, Kojima K, Hoshikawa M, Takagi M, et al. PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surgery: Off J Assoc Thorac Cardiovasc Surgeons Asia. (2019) 25:1–9. doi: 10.5761/atcs.oa.18-00163
31. Li D, Yu H, Hu J, Li S, Yan Y, Li S, et al. Comparative profiling of single-cell transcriptome reveals heterogeneity of tumor microenvironment between solid and acinar lung adenocarcinoma. J Trans Med. (2022) 20:423. doi: 10.1186/s12967-022-03620-3
Keywords: invasive lung adenocarcinoma, brain metastasis, pathological subtype, prognosis, survival
Citation: Zhou C, Zhang X, Yan X, Xie H, Tan H, Song Y, Li M, Jin Y and Wang T (2024) Impact of lung adenocarcinoma subtypes on survival and timing of brain metastases. Front. Oncol. 14:1433505. doi: 10.3389/fonc.2024.1433505
Received: 16 May 2024; Accepted: 19 August 2024;
Published: 03 September 2024.
Edited by:
Jianxin Xue, Sichuan University, ChinaReviewed by:
Weimin Gao, Barrow Neurological Institute (BNI), United StatesShih-Ying Wu, Wake Forest Baptist Medical Center, United States
Copyright © 2024 Zhou, Zhang, Yan, Xie, Tan, Song, Li, Jin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianlu Wang, d2FuZ3RpYW5sdUBjYW5jZXJob3NwLWxuLWNtdS5jb20=
†These authors have contributed equally to this work
 Chuyan Zhou1,2†
Chuyan Zhou1,2† Xiaofang Zhang
Xiaofang Zhang Yingqiu Song
Yingqiu Song Tianlu Wang
Tianlu Wang