- 1Faculty of Kinesiology, Sport, and Recreation, College of Health Sciences, University of Alberta, Edmonton, AB, Canada
- 2Department of Physical Therapy, Faculty of Rehabilitation Medicine, University of Alberta, Edmonton, AB, Canada
- 3Supportive Care Services and Patient Experience, Cancer Care Alberta, Edmonton, AB, Canada
- 4Department of Oncology, Queen’s University, Kingston, ON, Canada
- 5Cancer Care and Epidemiology, Cancer Research Institute, Queen’s University, Kingston, ON, Canada
- 6Department of Cancer Epidemiology and Prevention Research, Alberta Health Services, Calgary, AB, Canada
- 7Departments of Oncology and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Exercise plays many important roles across the entire cancer continuum that have been described in previous frameworks. These frameworks, however, have generally provided a simplified description of the roles of exercise postdiagnosis. The modern cancer treatment landscape has become complex and often consists of multiple lines of multimodal treatments combined concurrently and/or sequentially and delivered over many months or years. This complexity requires a more multifaceted and targeted approach to the study of exercise after a cancer diagnosis. Here, we propose a new integrated framework—Exercise Across the Postdiagnosis Cancer Continuum (EPiCC)—that highlights the distinct roles of exercise for disease treatment and supportive care from diagnosis until death. We also propose new terminology to clarify the distinct roles of exercise that emerge in the context of the modern cancer treatment landscape. The EPiCC Framework is structured around multiple sequential cancer treatments that highlight six distinct cancer treatment-related time periods for exercise—before treatments, during treatments, between treatments, immediately after successful treatments, during longer term survivorship after successful treatments, and during end of life after unsuccessful treatments. The EPiCC Framework proposes that the specific roles of exercise as a disease treatment and supportive care intervention will vary depending on its positioning within different cancer treatment combinations. As a cancer treatment, exercise may serve as a “priming therapy”, primary therapy, neoadjuvant therapy, induction therapy, “bridging therapy”, adjuvant therapy, consolidation therapy, maintenance therapy, and/or salvage therapy. As a supportive care intervention, exercise may serve as prehabilitation, intrahabilitation, interhabilitation, rehabilitation, “perihabilitation”, health promotion/disease prevention, and/or palliation. To date, exercise has been studied during all of the cancer treatment-related time periods but only in relation to some cancer treatments and combinations. Moreover, fewer studies have examined exercise across multiple cancer treatment-related time periods within any cancer treatment combination. Future research is needed to study exercise as a disease treatment and supportive care intervention within and across the distinct cancer treatment-related time periods contained within different cancer treatment combinations. The aim of the EPiCC Framework is to stimulate a more targeted, integrated, and clinically-informed approach to the study of exercise after a cancer diagnosis.
Introduction
Exercise plays many important roles across the entire cancer continuum. In 2001, the Physical Exercise Across the Cancer Experience (PEACE) Framework was proposed to organize these roles, bring structure to the field, and highlight new opportunities for exercise oncology research (1). On the postdiagnosis side, the PEACE Framework focused on the supportive care role of exercise across a simple clinical scenario of a single nonsurgical treatment that resulted in either cure or persistent disease. Based on this simple clinical scenario, the PEACE Framework proposed that postdiagnosis exercise may play a supportive care role before treatment (buffering), during treatment (coping), immediately after successful treatment (rehabilitation), immediately after failed treatment (palliation), and during long-term survivorship for patients who were cured (health promotion and survival).
The PEACE Framework was updated in 2007 as the Physical Activity and Cancer Control (PACC) Framework (2) to incorporate new ideas regarding the cancer continuum and to highlight the potential role of exercise in affecting clinical cancer outcomes proposed in the Organizational Framework of Physical Activity and Clinical Endpoints in Cancer Survivors (3). On the postdiagnosis side, PACC was still based on the simple clinical scenario of a single nonsurgical treatment with exercise proposed to play a potential role before treatment (treatment preparation/coping), during treatment (treatment effectiveness/coping), immediately after treatment (recovery/rehabilitation), during long-term survivorship (disease prevention/health promotion), and at the end of life (palliation and survival). Although the PEACE and PACC Frameworks acknowledged the complexity of cancer treatments, this complexity was not explicitly incorporated into the frameworks. Consequently, both frameworks provided a relatively simple description of the potential roles of exercise after a cancer diagnosis.
More recently, the Multiphasic Prehabilitation Framework was proposed to describe the potential roles of multimodal prehabilitation (including exercise) for both patients and caregivers across the postdiagnosis cancer continuum (4). This framework highlighted the potential roles of multimodal prehabilitation across multiple consecutive cancer treatments from diagnosis until treatment completion. Although this framework described the potential role of exercise across a more complicated treatment landscape, it did not explicitly address the posttreatment phase of the cancer continuum or the advanced/metastatic disease setting. Moreover, the framework primarily described opportunities for prehabilitation during the treatment phase, and did not address the potential role of exercise as a disease treatment.
In 2022, the Exercise as Cancer Treatment (EXACT) Framework was proposed to provide a more detailed assessment of the potential role of exercise as a cancer treatment from a clinical oncology perspective (5, 6). The EXACT framework proposed that exercise may have effects on the primary tumor, micrometastases, and metastatic disease before, during, and/or after other cancer treatments (5, 6). Although the EXACT Framework provided a more clinically focused assessment of exercise as a cancer treatment and highlighted the issue of treatment sequencing, it did not fully incorporate the complexity of multiple cancer treatments and it did not address the supportive care role of exercise.
Here, we propose a new integrated framework—Exercise Across the Postdiagnosis Cancer Continuum (EPiCC)—that highlights the dual roles of postdiagnosis exercise for supportive care and disease treatment across the modern cancer treatment landscape from diagnosis until death. The primary goal of proposing this new framework is to better characterize existing exercise oncology research and to identify new opportunities for future research that emerge in the context of the modern cancer treatment landscape. A secondary goal is to propose terminology for describing the complicated supportive care and disease treatment roles of exercise across the postdiagnosis cancer continuum to foster consistency and specificity across studies.
The modern cancer treatment landscape
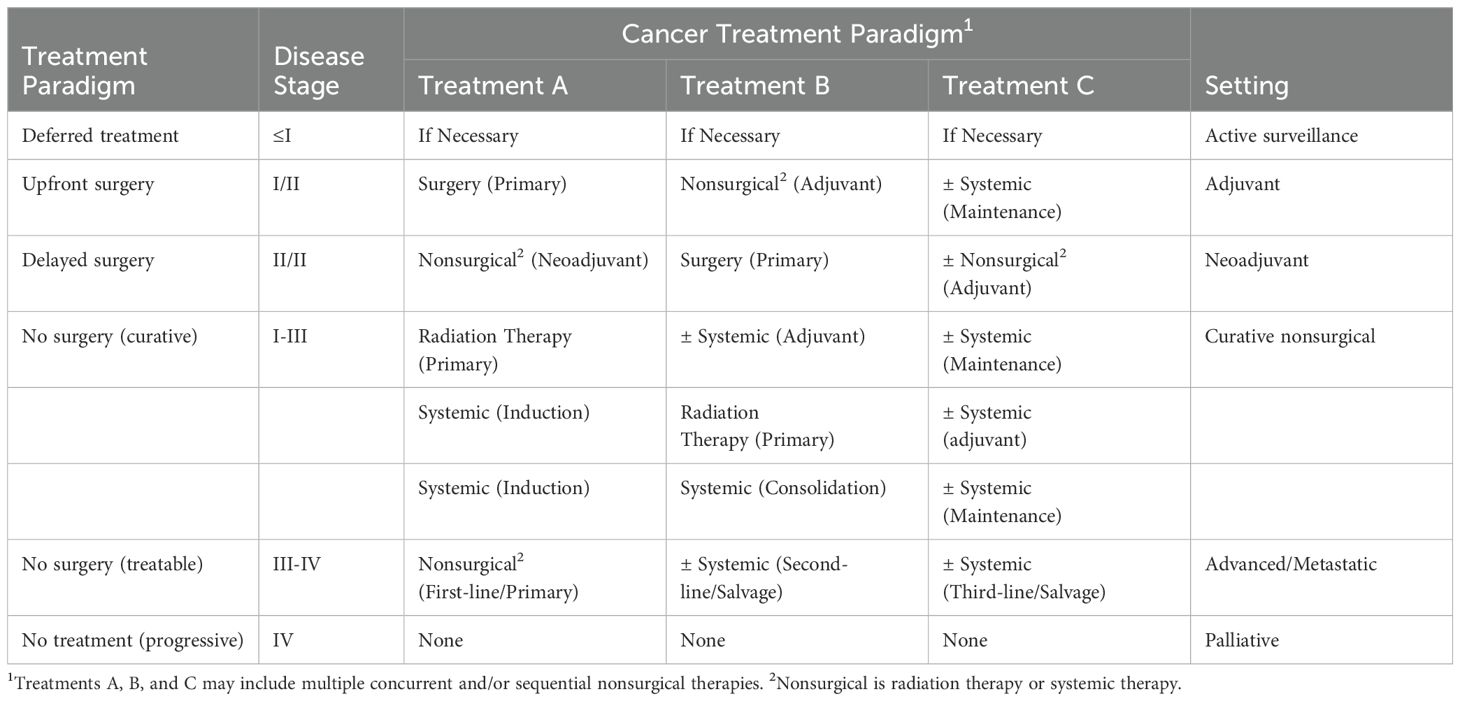
The modern cancer treatment landscape has become complex with the addition of newer treatments such as immunotherapies and targeted therapies to previously established treatments such as surgery, radiation therapy, chemotherapy, and endocrine (hormone) therapy. With the rapid expansion of treatment options, the treatment landscape for many cancers has become saturated and complicated. As a result, the optimal combination and sequencing of cancer treatments has become a critical issue in clinical oncology with implications for treatment efficacy as well as safety and tolerability (7–9). Modern cancer treatment approaches often consist of multiple lines of multimodal treatments combined concurrently and/or sequentially to treat a disease that can progress or recur multiple times. Cancer patients may receive many different treatments over many months or years. The terminology used to describe this increasingly complex cancer treatment landscape is not fully standardized and is sometimes inconsistent (10). Table 1 provides definitions for some of the key terms used to describe cancer treatment combinations and sequencing that are important to understand when evaluating the role of exercise as a cancer treatment in the modern treatment landscape.
Integrating exercise into this convoluted cancer treatment landscape has itself become more complicated. As a result, it is no longer adequate to describe the role of postdiagnosis exercise in reference to a single nonsurgical cancer treatment (i.e., before, during, or after) or in reference to an isolated cancer treatment-related time period (e.g., exercise during chemotherapy, postsurgical exercise). Exercise must be situated within the entirety of the broader cancer treatment landscape. Moreover, it is insufficiently precise to discuss exercise as a “cancer treatment” or to refer to its supportive care roles as “rehabilitation” and “prehabilitation”. The complexity of the modern cancer treatment landscape requires a more multifaceted and sophisticated approach to the study of exercise after a cancer diagnosis. To articulate the role of exercise in the modern cancer treatment landscape, it is important to first characterize the properties of exercise as a cancer treatment.
Exercise as a cancer treatment
As noted in the EXACT Framework (5), the potential roles of exercise as a cancer treatment are determined by its unique characteristics. Exercise is essentially a single drug (energy expenditure) whose biological effects can be manipulated based on the type, dose, frequency, intensity, and duration (i.e., the exercise prescription). Like other drugs, exercise has systemic effects which means it can potentially act as a treatment for local, regional, and distant disease. Although a single administration of exercise (i.e., an exercise session) can have acute biological effects (11), it will not instantly eliminate an existing tumor like a surgical resection or ablative therapy. In this sense, exercise is like other nonsurgical therapies such as radiation therapy or chemotherapy that require multiple administrations over an extended period of time to achieve a clinical benefit. In terms of cancer treatment terminology (Table 1), exercise may be considered a systemic therapy that is its own treatment modality (i.e., exercise therapy).
Integrating exercise into the modern cancer treatment landscape
From an exercise perspective, there are two important features of current cancer treatments that influence how exercise may be integrated into the modern cancer treatment landscape. With respect to cancer treatment modalities, the important distinction is between treatment modalities that involve a single administration of the therapy with immediate therapeutic effects (usually surgery or ablative therapies) versus treatment modalities that involve multiple administrations of a therapy with gradual or continuous therapeutic effects over time (usually radiation therapy and systemic therapies). Exercise generally cannot be performed during the actual administration of any cancer therapy (except perhaps during a continuous infusion), however, it can be performed within (between) multiple administrations of the same cancer therapy while the treatment is having therapeutic effects (i.e., during treatment). Conceptually, exercise during treatment refers to exercising while the treatment is working or having therapeutic effects. Pragmatically, however, exercise during treatment may refer to exercise between the first and last administrations of a therapy that is administered on multiple occasions over time. The critical point is that exercise can be performed before, during, and after therapies that require multiple administrations over time (usually nonsurgical therapies) whereas it can only be performed before and after therapies that consist of a single administration (usually surgery or ablative therapies).
With respect to cancer treatment combinations, the important distinction is between concurrent (at the same time) and sequential (one after the other) combinations. Exercise can be performed between sequential combinations that have a sufficient break between them (e.g., 4-6 weeks) but not between concurrent combinations or sequential treatments with minimal breaks between them. Consequently, exercise may be performed before, during, between, and after sequential combinations whereas it can only be performed before, during, and after concurrent combinations. These two important distinctions influence the potential roles of exercise across the modern cancer treatment landscape. Table 2 provides suggested terminology and definitions regarding the timing of exercise in relation to the modern cancer treatment landscape.
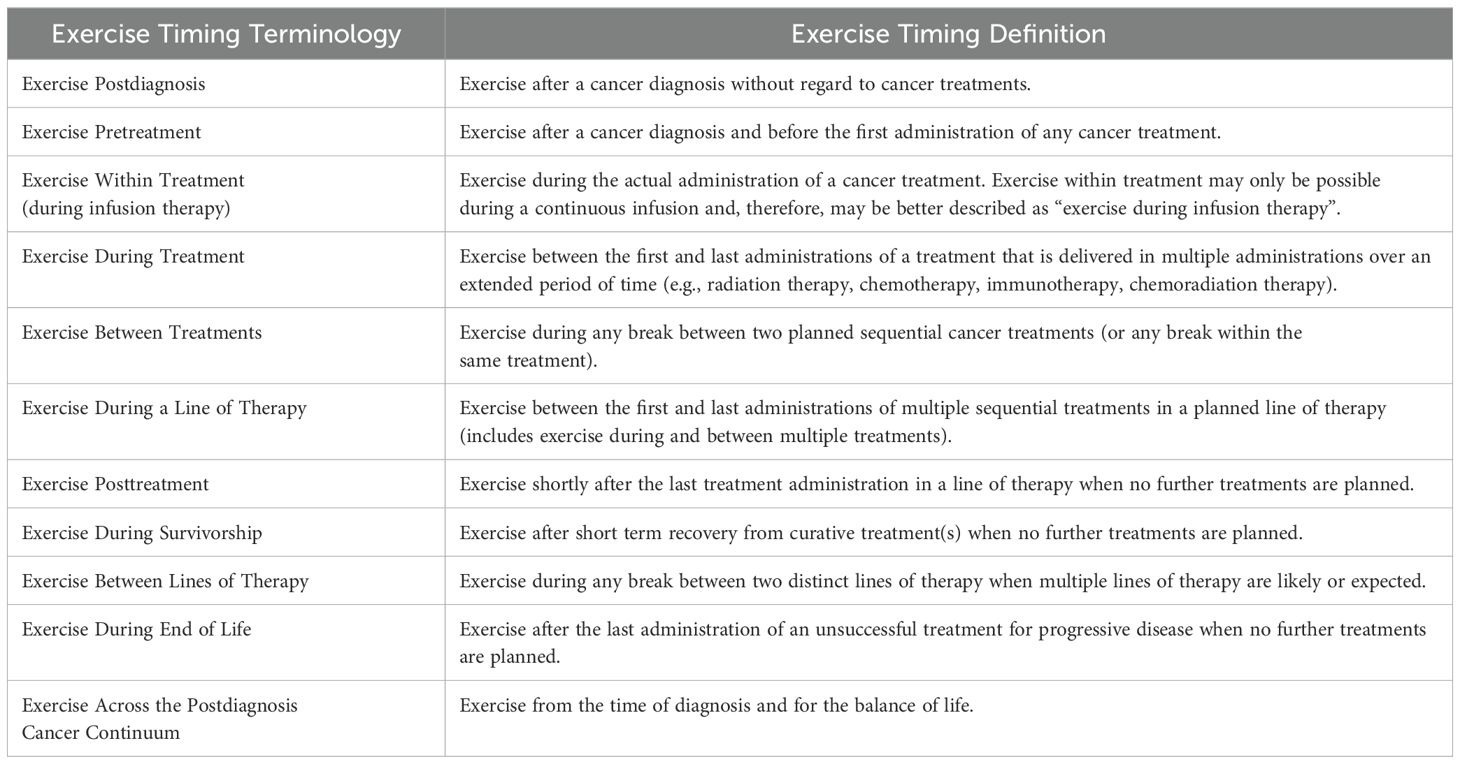
Table 2. Proposed terminology and definitions for describing the timing of exercise across the postdiagnosis cancer continuum.
Common modern cancer treatment paradigms
There are endless treatment combinations for different cancers and disease stages that make it virtually impossible to describe the roles of exercise succinctly across all clinical scenarios. Nevertheless, there are some general treatment paradigms that can help exercise oncology researchers think through the role of exercise after a cancer diagnosis. Table 3 presents six common cancer treatment paradigms (and their terminology) that are typical for different disease stages in solid tumors and hematologic cancers. The major distinction among the modern cancer treatment paradigms from an exercise perspective is the occurrence and timing of surgery.
For many early stage solid tumors (stages I and II), surgery occurs first (upfront) followed by possible nonsurgical therapies (i.e., the adjuvant setting). For some locally advanced solid tumors (stages II and III), surgery may occur after nonsurgical therapies (i.e., the neoadjuvant setting) and be followed by additional nonsurgical therapies. For hematological cancers and some early stage solid tumors, curative treatment may not include surgery at all (i.e., curative settings without surgery). Similarly, surgery is uncommon in the metastatic setting (stage IV) where the treatment sequence typically involves multiple lines of systemic therapies, although in some circumstances local therapies may be used to treat limited metastases (i.e., “metastasis-directed therapy”) (12). Finally, there are two scenarios in which treatment may not occur at all. For some very small low grade cancers (stage ≤I), treatment may only occur if necessary (i.e., the active surveillance setting) whereas for some widely disseminated cancers there may be no effective treatments available (i.e., the palliative setting).
Exercise across the postdiagnosis cancer continuum framework
The EPiCC Framework integrates and expands upon previous frameworks that have described the roles of exercise across the postdiagnosis cancer continuum (1–6). More specifically, the EPiCC Framework proposes two broad roles for exercise—supportive care and disease treatment—across the postdiagnosis cancer care continuum from diagnosis until death (Figure 1). The supportive care role of exercise generally focuses on quality of life issues and includes outcomes such as health-related fitness, physical functioning, symptom/side effect management, treatment tolerance, and psychosocial functioning. The disease treatment role of exercise generally focuses on quantity of life issues and includes outcomes related to tumor control, disease control, and survival. Although the modern cancer treatment landscape is diverse and complex, the EPiCC Framework portrays three sequential treatments as sufficient to capture the diverse roles exercise might play across multiple sequential cancer treatments. The EPiCC Framework highlights six distinct cancer treatment-related time periods for exercise—before treatments, during treatments, between treatments, immediately after successful treatments, during survivorship after successful treatments, and during end of life after unsuccessful treatments. The primary proposition of the EPiCC Framework is that the effects of exercise on disease and supportive care outcomes will vary depending on its positioning within different cancer treatment combinations. For example, even across a clinical oncology scenario of three sequential nonsurgical cancer treatments as depicted in the EPiCC Framework, there are 255 possible exercise combinations (including all 1-way to 8-way combinations from pretreatment to survivorship).
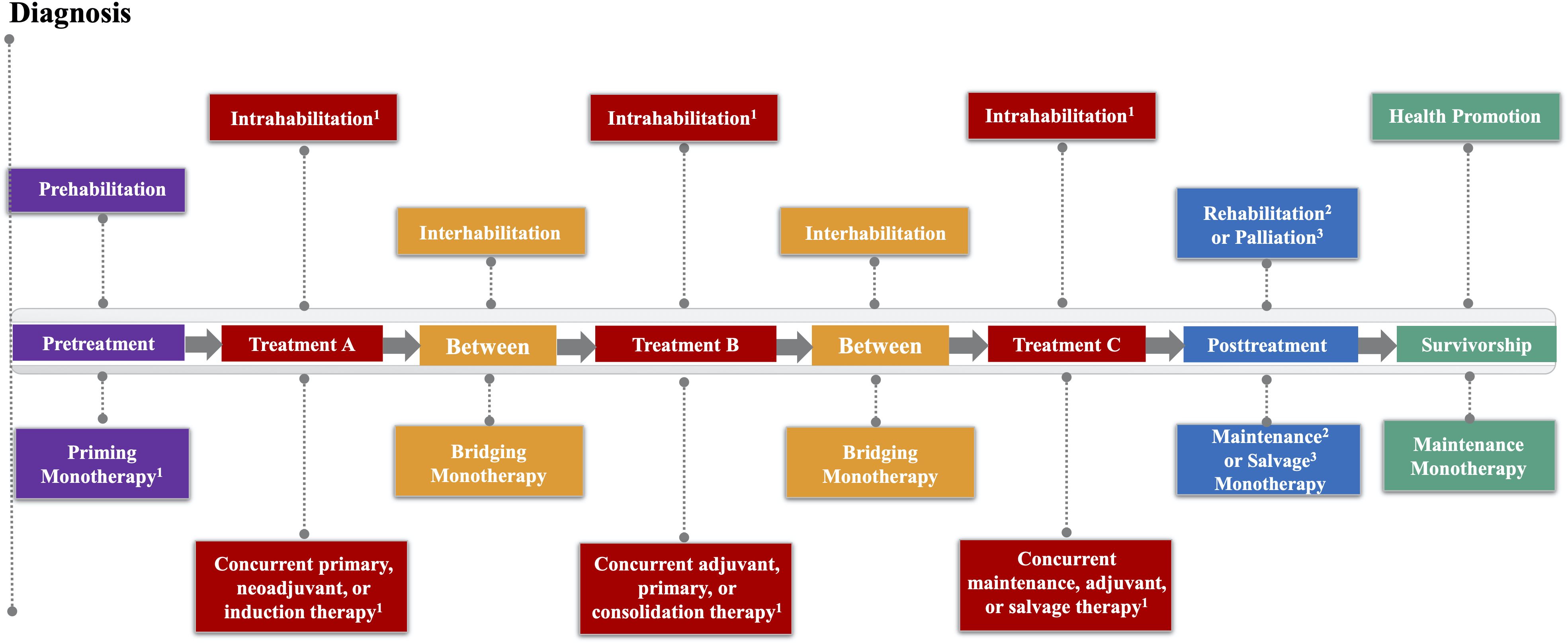
Figure 1. Exercise Across the Postdiagnosis Cancer Continuum (EPiCC) Framework. 1If treatment is not surgery; 2If cure/remission; 3If progressive disease.
As a cancer treatment, exercise may serve as a “priming therapy”, primary therapy, neoadjuvant therapy, induction therapy, “bridging therapy”, adjuvant therapy, consolidation therapy, maintenance therapy, and/or salvage therapy depending on its positioning within different cancer treatment combinations (Table 1). As a supportive care intervention, exercise may serve as prehabilitation, intrahabilitation, interhabilitation, rehabilitation, “perihabilitation”, health promotion/disease prevention, and/or palliation depending on its timing in relation to cancer treatments (Table 4). Figure 2 provides adaptations of the general EPiCC framework to the four main cancer treatment paradigms that involve multiple sequential cancer treatments: (a) the adjuvant setting (upfront surgery), (b) the neoadjuvant setting (delayed surgery), (c) the nonsurgical setting (curative), and (d) the metastatic setting (treatable). The following sections provide a general description and conceptual overview of exercise as a disease treatment and supportive care intervention in the EPiCC Framework.

Table 4. Proposed terminology and definitions for describing the supportive care roles of exercise across the postdiagnosis cancer continuum.
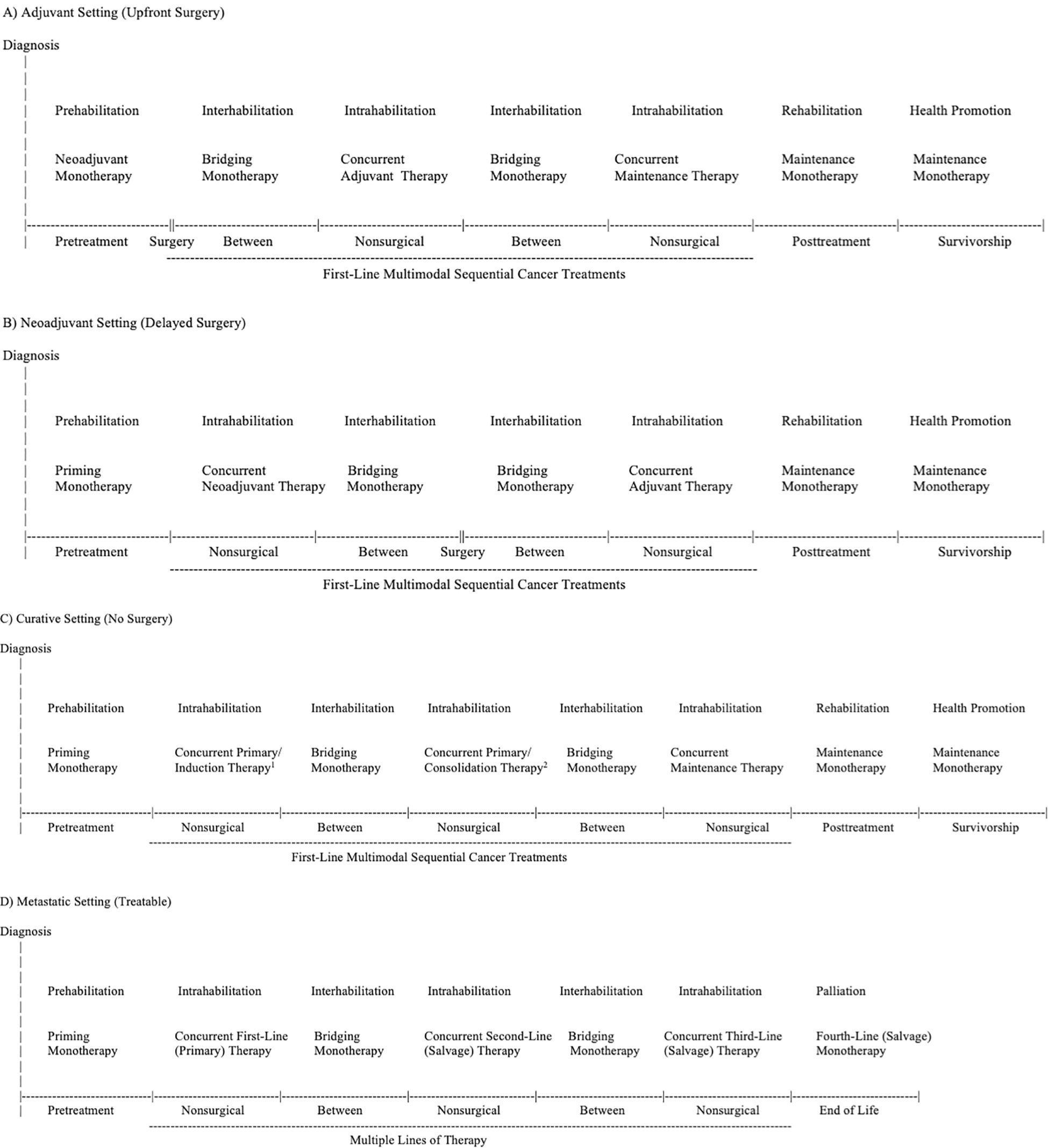
Figure 2. Exercise Across the Postdiagnosis Cancer Continuum (EPiCC) Framework Tailored to the Adjuvant (Upfront Surgery) Setting (A), Neoadjuvant (Delayed Surgery) Setting (B), Curative (No Surgery) Setting (C), and Metastatic (Treatable) Setting (D). 1Primary therapy if radiation therapy; induction therapy if systemic therapy. 2Primary therapy if radiation therapy; consolidation therapy if systemic therapy.
Exercise as treatment within the modern cancer treatment landscape
The expanding cancer treatment landscape offers new opportunities for exercise to serve as a cancer treatment by demonstrating independent, additive, and/or synergistic effects with existing cancer treatments (13). The role of exercise as a cancer treatment may vary across the postdiagnosis cancer continuum depending on its combination and sequencing with other cancer treatments. Exercise mechanisms may inform the optimal positioning of exercise in relation to different cancer treatments (i.e., exercise sequencing). In general, exercise may cause biological and/or hemodynamic effects in the tumor microenvironment and systemically that independently, or in combination with other cancer treatments, affect the growth and spread of cancer cells (6). It is unlikely, however, that exercise alone is curative for any cancer. Preclinical studies have shown that exercise alone, at best, slows the progression of cancer (14–17). No preclinical studies have demonstrated that exercise alone produces complete responses, disease regression (partial responses), or even stable disease. Consequently, for clinical benefit, exercise will need to be combined with other cancer treatments either concurrently or sequentially.
In the following sections, we briefly describe the potential roles of exercise as a cancer treatment based on its positioning within different treatment combinations. We use the term “concurrent exercise therapy” to refer to exercise that is administered at the same time as another cancer treatment (i.e., during that treatment) whereas we use the term “exercise monotherapy” to refer to exercise that is administered asynchronously (i.e., by itself) even though it is part of a sequential treatment combination (i.e., before, between, or after other treatments).
Exercise as primary therapy
Primary therapy generally refers to the most definitive treatment(s) for a cancer with the goal of cure or remission and is usually reserved for surgery or radiation therapy in the early stage setting (Table 1). In the advanced/metastatic setting, primary therapy may be used to describe a systemic therapy. Exercise monotherapy is unlikely to be considered a primary therapy for any cancer because it does not eliminate or even dramatically reduce cancerous tumors in preclinical models (14–17). Moreover, most cancers have at least one effective treatment that makes it unlikely exercise would even be examined as a primary monotherapy. The only clinical scenario that provides an opportunity to examine exercise as a primary monotherapy is the active surveillance setting in which no immediate treatments are provided. Similar to preclinical models, however, exercise as a primary monotherapy may slow the progression of cancer but it is unlikely to eliminate or reverse the disease (18, 19).
It is possible that exercise could serve as a concurrent primary therapy with radiation therapy (or a systemic therapy) based on preclinical studies suggesting that exercise may enhance radiosensitivity and drug sensitivity/delivery through improvements in tumor vasculature, perfusion, and hypoxia (20). Conceptually, this treatment role for exercise is similar to neoadjuvant and induction therapy because exercise is combined concurrently with the first upfront nonsurgical treatment. It is different, however, because a primary therapy is expected to be the definitive treatment for the disease whereas neoadjuvant and induction therapies are considered preliminary therapies to be followed by definitive surgery or nonsurgical therapies, respectively.
Exercise monotherapy prior to a nonsurgical primary therapy (or neoadjuvant or induction therapy) may be effective because alterations in the tumor microenvironment may enhance the effectiveness of subsequent nonsurgical therapies such as radiation therapy, chemotherapy, and immunotherapy. This possible treatment role for exercise may be better described as a “priming monotherapy” (21) rather than a primary (or neoadjuvant or induction monotherapy) because exercise may alter the cancer or tumor microenvironment to be more responsive to the upfront nonsurgical therapy but it will not induce a remission or cure itself. This treatment role for exercise has not been studied in preclinical models because exercise is usually not administered between the time of an established primary tumor and an upfront nonsurgical cancer treatment. It has also not been studied in clinical research because exercise interventions have generally not occurred before an upfront nonsurgical therapy. It is unclear, therefore, if there is sufficient time between diagnosis and upfront nonsurgical therapies (whether primary, neoadjuvant, or induction) for exercise interventions to be feasible and effective as a priming monotherapy.
Exercise as neoadjuvant therapy
Neoadjuvant therapy generally refers to upfront nonsurgical therapies given for nonmetastatic disease with the goal of reducing the size/extent of the primary tumor and micrometastases prior to definitive surgery (Table 1). Exercise is unlikely to serve as a neoadjuvant monotherapy because it fails to produce any tumor shrinkage on its own in preclinical models (14–17). Moreover, exercise effects on the tumor microenvironment may not be clinically helpful in the neoadjuvant setting because the tumor is usually completely resected after the neoadjuvant therapy. It is possible that improved tumor vasculature may reduce the number of tumor cells being shed into the surrounding tissue and blood vessels before surgery, however, the window for such an effect may be short. Nevertheless, studies of exercise monotherapy prior to surgery may provide important mechanistic evidence on the biological effects of exercise on human in vivo tumors (22, 23). As noted earlier, exercise as a “priming monotherapy” prior to a neoadjuvant therapy may enhance the efficacy of the neoadjuvant therapy through tumor vasculature mechanisms, however, the feasibility and efficacy of such a treatment role for exercise has not been tested in preclinical or clinical models.
Exercise as a concurrent neoadjuvant therapy may be clinically efficacious because alterations in the tumor microenvironment may enhance the effectiveness of the concurrent neoadjuvant therapies such as radiation therapy, chemotherapy, and immunotherapy. These effects have been demonstrated in preclinical and clinical studies (24). In preclinical studies, exercise has improved the delivery and efficacy of some systemic therapies by improving tumor vasculature and perfusion (25). In small exploratory clinical studies, exercise has improved the response to neoadjuvant chemoradiation and chemotherapy (24).
For example, Morielli et al. (26) examined the effects of exercise in 36 rectal cancer patients receiving neoadjuvant chemoradiotherapy prior to definitive surgery and reported that the number of patients achieving a pathologic complete or near complete response was significantly (p=0.020) higher in the exercise group (10/18 = 56%) compared to the usual care group (3/17 = 18%). Similarly, in a non-randomized trial involving 39 esophageal cancer patients during neoadjuvant chemotherapy (27), the exercise group compared to nonexercise group achieved higher rates of tumor regression (75% vs. 37%; p=0.025) and combined tumor and node downstaging (43% vs. 16%; p=0.089). Most recently, Sanft et al. (28) examined the effects of a combined diet and exercise intervention on chemotherapy tolerance and pathologic complete response in 173 breast cancer patients initiating chemotherapy. In a subgroup analysis of the 72 women who received neoadjuvant chemotherapy, the intervention group was significantly (p=0.037) more likely to achieve a pathologic complete response (53%) compared to the usual care group (28%). Based on these preliminary data, a definitive phase III trial is examining the effects of exercise as a concurrent neoadjuvant therapy on pathologic complete response in 790 breast cancer patients receiving neoadjuvant chemotherapy (29).
Exercise as induction therapy
Induction therapy generally refers to upfront systemic treatment(s) in a sequence of systemic treatments for a hematological (blood) cancer or advanced solid cancer with the goal of remission or cure (Table 1). Exercise is unlikely to serve as an induction monotherapy because it will not produce a remission or cure on its own (14–17). As noted earlier, exercise monotherapy prior to an induction therapy may be effective because alterations in multiple tumor microenvironments (typical of hematologic cancers or advanced solid tumors) may enhance the effectiveness of subsequent induction therapies such as chemotherapy and immunotherapy. Again, this possible treatment role for exercise may be better described as a “priming monotherapy” rather than an induction monotherapy because it may alter the tumor microenvironment to be more responsive to an induction therapy but it will not induce a remission or cure itself. As noted earlier, this treatment role for exercise has not been studied in preclinical or clinical models. Exercise may be more feasible and helpful as a concurrent induction therapy because alterations in multiple tumor microenvironments (20) may also enhance concurrent therapies, similar to its potential role as a concurrent primary or neoadjuvant therapy (although primary and neoadjuvant therapy usually target a single primary tumor).
Exercise as adjuvant therapy
Adjuvant therapy generally refers to nonsurgical therapies after primary surgery (or after primary radiation therapy) for nonmetastatic disease with the goal of killing any remaining cancer cells (Table 1). Exercise may serve as an adjuvant monotherapy because of the limited number of disseminated cancer cells that typically remain after definitive surgery (30). Exercise as a monotherapy may act as a treatment for treatment naïve micrometastases through various systemic mechanisms including increased fluid shear stress, enhanced immune surveillance, reduced inflammation, and improved insulin sensitivity (31). Exercise has been studied as an adjuvant monotherapy in preclinical animal models that have injected a small number of cancer cells that disseminate in the absence of a primary tumor (32). These studies have generally shown that exercise alone can reduce the number and size of metastatic tumors (i.e., slow disease progression) but not eliminate the remaining cancer cells (32).
There are some clinical scenarios where exercise could be tested as an adjuvant monotherapy although many early stage disease settings with complete surgical resection are followed by some adjuvant therapy. Nevertheless, some observational studies have demonstrated associations between exercise and cancer recurrence or death in clinical scenarios that involved only surgery or limited adjuvant therapy. For example, Friedenreich et al. (33) examined the associations between postdiagnosis physical activity and survival in 425 endometrial cancer patients who had received surgery but only about one-third received any adjuvant therapy. Higher postdiagnosis recreational physical activity was strongly associated with improved disease-free survival (HR, 0.33; 95% CI, 0.17 to 0.64) suggesting that exercise may be active as an adjuvant monotherapy in this clinical setting. Similarly, Lee et al. (34) examined the associations between physical activity and cancer outcomes in 43,596 colorectal cancer survivors from the Korean National Health Insurance Service database. In a subgroup analysis of survivors who had received surgery only, higher physical activity was associated with a significantly lower risk of mortality (HR=0.75; 95% CI, 0.65 to 0.87) suggesting that exercise may serve as an adjuvant monotherapy in this clinical setting.
Exercise that occurs between the first therapy (e.g., primary or neoadjuvant or induction) and second therapy (e.g., adjuvant or primary or consolidation), or between any two therapies, may be considered a “bridging monotherapy” (Table 1). The goal of a bridging therapy is to contain or control the cancer while waiting for the next major therapy to be administered. This treatment role for exercise has not been studied in preclinical models because exercise is usually not administered between two sequential cancer treatments. There is a modest amount of clinical research that has examined exercise between neoadjuvant therapy and primary (surgical) therapy which has yielded promising results (26, 35). The relative utility of exercise as a bridging monotherapy, however, may depend on the length of time between two sequential therapies.
Exercise may also be helpful as a concurrent adjuvant therapy. As noted earlier, exercise may directly treat micrometastases through various mechanisms (31), however, it may also potentiate the effects of existing cancer treatments through similar mechanisms (e.g., increased fluid shear stress may make cancer cells more vulnerable to chemotherapy (36), or increased immune surveillance may enhance the effectiveness of immunotherapies (37). Exercise might also improve tumor vasculature and blood flow to small micrometastatic formations depending on their location and, therefore, enhance delivery of systemic therapies to these undetected lesions. Some preclinical studies have examined this scenario by using metastasis models treated with chemotherapy and shown reduced tumor growth and spread with exercise (24). In one clinical study (38), 242 early stage breast cancer patients receiving chemotherapy were randomized to usual care (n=82), aerobic exercise (n=78) or resistance exercise (n=82). In an exploratory follow-up (39), disease-free survival 8 years later was 82.7% for the two exercise groups combined compared with 75.6% for the usual care group (Hazard ratio =0.68, 95% CI=0.37-1.24; log-rank p=0.21) suggesting that exercise may be beneficial as a concurrent adjuvant therapy in this clinical setting.
Exercise as consolidation therapy
Consolidation therapy generally refers to systemic treatment(s) after upfront (induction) systemic treatment(s) for a hematological (blood) cancer or metastatic disease with the goal of killing any remaining cancer cells after successful induction treatment. It is possible that exercise may serve as a consolidation monotherapy if there are few remaining cancer cells throughout the body after the induction therapy, similar to its role as an adjuvant therapy. Nevertheless, the induction therapy may alter the genetics, biology, tumor microenvironment, and location of any remaining cancer cells making them more or less sensitive to exercise (40). Exercise may also be effective as a concurrent consolidation therapy based on mechanisms previously discussed for concurrent adjuvant therapy. To date, exercise has not been studied as a consolidation therapy (concurrent or monotherapy) in any preclinical or human studies.
Exercise as maintenance therapy
Maintenance therapy generally refers to systemic treatment(s) after successful primary/adjuvant or induction/consolidation therapies for any stage cancer given at a lower dose for an extended period of time with the goal of maintaining cure, remission, or stable disease (Table 1). Exercise may be an effective maintenance monotherapy in the curative setting because of the very limited disease that typically remains after primary and adjuvant treatments. There are possible mechanisms for exercise as a maintenance monotherapy similar to adjuvant monotherapy (31), however, as a maintenance therapy exercise needs to be able to eliminate previously treated micrometastases which may have acquired new mutations or reside in difficult to reach locations (41). Exercise has not been shown to serve as a maintenance monotherapy in preclinical models because studies have not examined exercise after successful previous treatments.
Most current observational studies have tested exercise as a maintenance monotherapy because postdiagnosis assessments of physical activity have generally occurred well after treatments have been completed (e.g., between 2 to 5 years postdiagnosis) (6). Friedenreich et al. (42) summarized 136 studies on PA and cancer outcomes across various cancer types. Results showed that higher postdiagnosis physical activity was significantly associated with a lower risk of cancer-specific mortality (HR=0.66; 95% CI=0.59-0.73), suggesting that exercise may serve as a maintenance monotherapy across a wide range of early stage clinical settings and different treatment combinations.
The Colon Health and Life-Long Exercise Change (CHALLENGE) trial is examining exercise as a maintenance monotherapy in colon cancer survivors (43). CHALLENGE will examine the effects of a 3-year structured exercise program on disease-free survival in high risk stage II or stage III colon cancer patients who have completed surgical resection and adjuvant chemotherapy within the past 2-6 months. CHALLENGE will answer the question of whether exercise as a maintenance monotherapy can lower the risk of recurrence and death in colon cancer patients after surgical resection and adjuvant chemotherapy.
Exercise may also serve as a maintenance monotherapy in the active surveillance setting where newly diagnosed cancer patients do not immediately receive any treatments. Unlike other settings, the maintenance monotherapy would be for treatment naïve disease. Moreover, any cancer treatment in this setting would need to be highly tolerable because treatment toxicities would not be acceptable given the very low risk of cancer death in these patients. Nevertheless, many of these patients will experience cancer progression and will ultimately require cancer treatments (44). For these patients, exercise may also serve as a “priming monotherapy” because it may alter the tumor microenvironment to make it more susceptible to future nonsurgical treatments (radiation therapy, hormone therapy). Given that it is unknown ahead of time which patients might progress to definitive treatment, exercise for cancer treatment during active surveillance may serve as both a maintenance monotherapy and priming monotherapy.
Exercise may be helpful as a concurrent maintenance therapy based on biological mechanisms and mechanical sheer stress (31) which may make micrometastases more vulnerable to other maintenance therapies such as such as chemotherapy, immunotherapy, or hormone therapy, similar to the concurrent adjuvant therapy setting. Some observational studies in breast cancer may have addressed this treatment role because exercise was likely assessed during long-term endocrine therapy, however, most studies have not explicitly analyzed exercise as a maintenance monotherapy versus concurrent therapy (6).
Exercise as salvage therapy
Salvage therapy generally refers to second-line (or later-line) nonsurgical treatments for any stage cancer that has recurred, not responded, or stopped responding to the previous treatment (Table 1). Exercise is unlikely to serve as a salvage monotherapy given that patients have stopped responding to treatments and have extensive disease that has likely acquired many resistance mechanisms. Exercise as a concurrent salvage therapy may be more likely to be effective given its possible role in remodeling tumor microenvironments and facilitating drug delivery to existing tumors (20). No previous preclinical studies and very few observational or intervention studies have tested exercise as a salvage therapy.
Some human studies have examined the effects of exercise during treatment for metastatic disease but few have reported whether the treatments were first-line (primary) or later-line (salvage) therapies. In a systematic review and meta-analysis of 11 studies of exercise and survival outcomes in patients with advanced cancer, exercise was associated with improved survival in 7 observational studies but not in 4 randomized controlled trials (45). The Healthy Exercise for Lymphoma Patients (HELP) trial (46) randomized 122 lymphoma patients to usual care or 12 weeks of supervised aerobic exercise. At the time of the exercise intervention, 54 lymphoma patients were receiving chemotherapy, about half as a first-line therapy (primary therapy) and half as a later-line therapy (salvage therapy). An exploratory analysis revealed that the exercise group had a non-statistically significant (p=0.24) higher clinical complete response to chemotherapy (13/28 = 46.4%) compared to the usual care group (8/26 = 30.8%) suggesting the possibility that exercise may serve as a concurrent salvage therapy (or primary therapy) with chemotherapy in patients with lymphoma cancer.
Exercise as supportive care within the modern cancer treatment landscape
The expanded cancer treatment landscape also provides many additional opportunities to study exercise as a supportive care intervention. Multiple sequential cancer treatments may make the supportive care benefits of exercise even more important. Exercise may produce even larger and more clinically relevant benefits for health-related fitness, physical functioning, psychological functioning, quality of life, symptom/side effects, treatment tolerance, and possibly even treatment delays (within and between multiple sequential treatments or lines of therapy). In the EPiCC Framework, exercise may play distinct supportive care roles before treatments, during treatments, between treatments, immediately after successful treatments, during long term survivorship after successful treatments, and during end of life after unsuccessful treatments.
Currently, the supportive care terminology for exercise oncology has generally been limited to “prehabilitation” and “rehabilitation”, which were originally used to describe exercise interventions before and after surgery, respectively (4). While these terms can easily be extended to describe exercise interventions before and after nonsurgical cancer treatments (4) (e.g., exercise prehabilitation for chemotherapy or exercise rehabilitation after radiation therapy), they do not adequately describe exercise interventions during or between cancer treatments, during survivorship, or during end of life. Some researchers have proposed that terms such as rehabilitation (47–49), prehabilitation (4, 49), palliative care (50), or health promotion (51) can be expanded to include most or all of the postdiagnosis cancer continuum, however, these expanded definitions may mask the distinct supportive care roles of exercise across the postdiagnosis cancer continuum. Although there may be some overlap of supportive care goals across the postdiagnosis cancer continuum, the EPiCC Framework proposes that each cancer treatment-related time period has a distinct primary supportive care goal directly related to the treatment period.
Conceptually, the goals of prehabilitation exercise are to improve function before an invasive cancer treatment to help buffer or reduce impending treatment complications and side effects. Conversely, the goals of rehabilitation exercise are to recover or restore function after an invasive cancer treatment to help ameliorate any resulting treatment complications and side effects. Seemingly, the goals of exercise during an invasive cancer treatment would be to prevent decline or maintain function to help manage or mitigate emerging treatment complications and side effects. Preventing decline during a cancer treatment is distinct from prehabilitation and rehabilitation.
The concept of “habilitation” typically refers to helping persons with disabilities keep, learn, or improve skills and functioning for daily living (52). Another broader definition of habilitation, however, is the act or process of becoming fit or of making fit for a particular purpose (Dictionary.com). This latter definition fits the general goals of exercise for supportive care in cancer. Moreover, habilitation is the root word of prehabilitation and rehabilitation and can easily be modified to accommodate exercise during or within treatment (intrahabilitation), between treatments (interhabilitation), and even around treatments (perihabilitation) (53).
In the EPiCC Framework, supportive care terminology for exercise is based on the cancer treatment-related time periods. That is, exercise may be performed before treatments (prehabilitation), during treatments (intrahabilitation), between treatments (interhabilitation), immediately after successful treatments (rehabilitation), around treatments (perihabilitation), during longer term survivorship after successful treatments (health promotion/disease prevention), and during end of life after unsuccessful treatments (palliation). Table 4 provides suggested terminology and definitions for the various supportive care roles of exercise across the postdiagnosis cancer continuum. We now discuss these concepts and the distinct supportive care roles of exercise in more detail.
Prehabilitation and rehabilitation
As noted previously, the most common terms used to describe exercise after a cancer diagnosis have been prehabilitation and rehabilitation (4). There is no consensus on the definition and scope of prehabilitation (54–56), however, the classic definition restricts it to the period after diagnosis and before the first cancer treatment (57). Others restrict it to the period after diagnosis and before primary surgery which allows exercise during and after neoadjuvant therapy to be considered prehabilitation (58). Still, others include multiple periods from cancer diagnosis until adjuvant therapy which includes before, during, and between multiple sequential cancer treatments (4). Alternatively, given that the term prehabilitation implies preparation for treatments, it could be argued that there must be at least one remaining treatment modality for exercise to be considered prehabilitation in the modern cancer treatment landscape. These different conceptualizations of prehabilitation are depicted in Table 5.
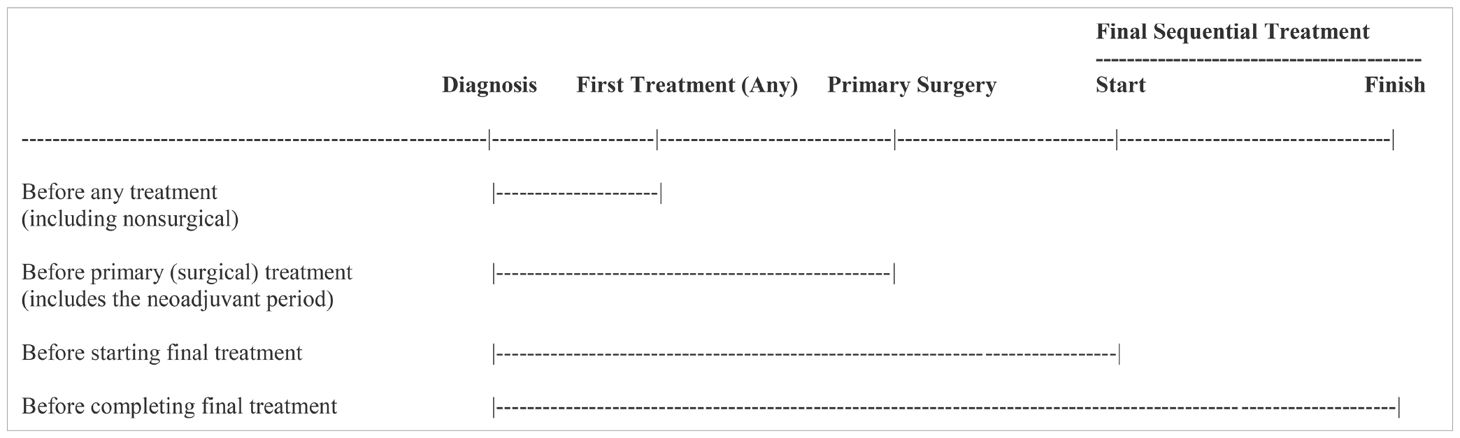
Table 5. Different conceptualizations of exercise prehabilitation in the context of the modern cancer treatment landscape.
In theory, exercising at any time before a planned cancer treatment may include prehabilitation goals even if it is the second or third cancer treatment in a sequence (Table 6). The time between a cancer diagnosis and first cancer treatment is often fairly short (days to weeks) whereas the time between a cancer diagnosis and the second or third cancer treatment in a sequential combination may be substantial (weeks to months). This extended time period makes prehabilitation an even more powerful concept that could play a critical role in cancer care because of the extended sequential cancer treatment protocols (4). The concept of prehabilitation could even be extended to clinical scenarios with multiple lines of therapy where further lines are expected (e.g., bladder, ovarian) or to the active surveillance setting where definitive treatment may be anticipated at some point (59).
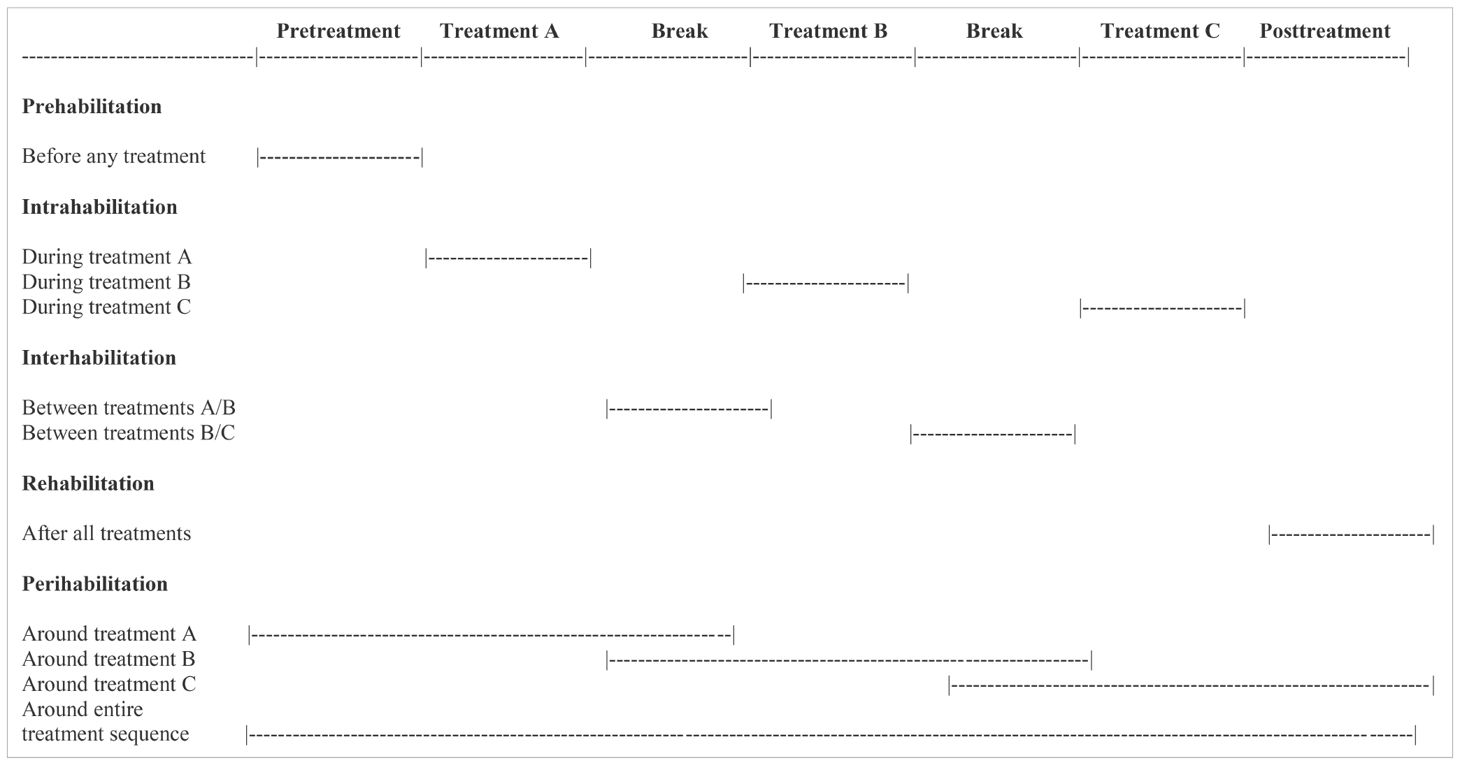
Table 6. Exercise habilitation concepts according to the Exercise Across the Postdiagnosis Cancer Continuum (EPiCC) framework.
The classic definition of rehabilitation is to restore or recover function after an adverse event (48). Theoretically, rehabilitation may be needed after the very first cancer treatment. Patients can be rehabilitated from a single treatment or from multiple sequential treatments as part of a line of therapy (Table 6). Rehabilitation may extend for several months after the final treatment in a multiple sequential treatment paradigm until the patient is fully recovered from treatment effects. Therefore, rehabilitation may start after the first treatment in a series of sequential cancer treatments and extend several months after the final treatment.
In juxtaposing the two concepts, prehabilitation may begin immediately after the cancer diagnosis and extend until just before the last administration of the final sequential therapy (e.g., last chemotherapy infusion, last immunotherapy injection, last radiation therapy fraction). Conversely, rehabilitation may begin immediately after the first administration of the first sequential therapy (e.g., surgery, first chemotherapy infusion, first radiation therapy fraction) and extend beyond the last administration of the final sequential therapy (e.g., last chemotherapy infusion, last immunotherapy injection, last radiation therapy fraction) until full recovery. Consequently, prehabilitation and rehabilitation goals may overlap substantially in the context of multiple sequential cancer treatments (4).
In classical conceptualizations of prehabilitation and rehabilitation based on acute insult/injury, rehabilitation begins when prehabilitation ends. In the EPiCC Framework, the relative emphasis on prehabilitation and rehabilitation may gradually change over the course of multiple sequential cancer treatments and may depend on the particular combination and sequencing of cancer treatments. The shift from prehabilitation to rehabilitation may happen gradually across multiple sequential treatments rather than abruptly as typically happens after surgical monotherapy. In general, prehabilitation may be more important early in the treatment sequence and diminish in importance later in the treatment sequence, whereas the opposite may be true for rehabilitation (Figure 3). Given the diverse roles of exercise for supportive care across multiple sequential cancer treatments, the term prehabilitation in the EPiCC Framework is restricted to the time between diagnosis and the first treatment. This phase of the postdiagnosis cancer continuum is primarily focused on prehabilitation. Similarly, the term rehabilitation in the EPiCC Framework is restricted to the time after the completion of the final treatment in a sequence. This phase of the postdiagnosis cancer continuum is primarily focused on rehabilitation.
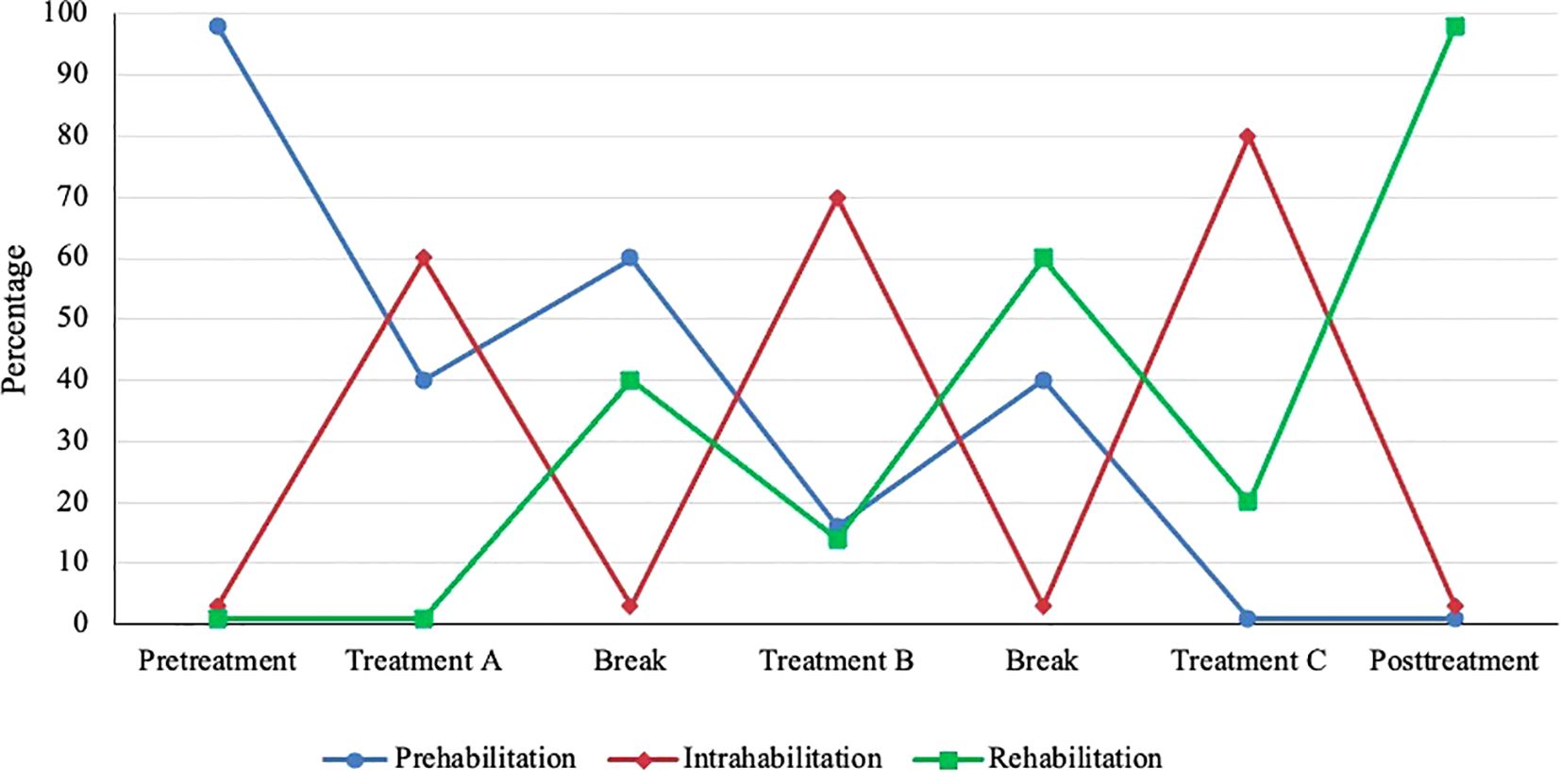
Figure 3. Hypothetical emphasis on prehabilitation, intrahabilitation, and rehabilitation across multiple sequential cancer treatments. Relative emphasis may vary depending on the combination and sequencing of treatments.
Intrahabilitation exercise
In the EPiCC Framework, exercise during nonsurgical cancer treatment(s) modalities is referred to as intrahabilitation (Table 6). Intrahabilitation may occur during a single nonsurgical treatment modality (e.g., chemotherapy) or during a concurrent combination of nonsurgical treatment modalities (e.g., chemoradiation therapy, chemoimmunotherapy). The primary goal of exercise during any (toxic) nonsurgical cancer treatment is to prevent decline and manage (tolerate) the current treatment(s). In the context of multimodal sequential cancer treatments, exercise during a cancer treatment may also include rehabilitation from any previous treatment(s) and/or prehabilitation for any subsequent treatment(s). The relative importance of prehabilitation, intrahabilitation, and rehabilitation may vary depending on the particular combination and sequencing of cancer treatment modalities (Figure 3). For example, during adjuvant chemotherapy after a conservative surgery and before radiation therapy, intrahabilitation during chemotherapy may be more important than rehabilitation from surgery or prehabilitation for radiation therapy. Conversely, during adjuvant radiation therapy after radical surgery and before adjuvant chemotherapy, rehabilitation from surgery and prehabilitation for chemotherapy may be more important than intrahabilitation for radiation therapy. Moreover, the relative importance of intrahabilitation may increase over the course of multiple sequential cancer treatments because of accumulating or compounding toxicities (Figure 3).
Interhabilitation exercise
In the EPiCC Framework, exercising between cancer treatments is referred to as interhabilitation (Table 6). Although the time period between successive cancer treatments may be short, gaps of 4-6 weeks may be sufficient for a meaningful exercise intervention (60). Planned treatment breaks may occur between two different treatment modalities or even within a single treatment modality such as endocrine therapy or immunotherapy (i.e., structured treatment interruptions or “drug holidays”). By definition, exercise between treatments would serve the primary goals of both rehabilitation and prehabilitation. The relative importance of prehabilitation and rehabilitation may depend on the specific combination and sequence of treatments. For example, during the window between surgery and adjuvant chemotherapy, rehabilitation from surgery may be more important than prehabilitation for chemotherapy after radical surgery whereas the opposite may be true after conservative surgery. Moreover, the relative importance of prehabilitation and rehabilitation may also depend on whether the interhabilitation occurs early or late in the treatment sequence (Figure 3).
Interhabilitation exercise specifically targets the time period between two predefined treatments. For example, an exercise intervention may target the time period between neoadjuvant chemoradiation therapy and radical surgery to help patients recover from the neoadjuvant therapy and prepare for surgery (35). Conversely, an exercise intervention that targets patients after a radical surgery without regard for subsequent treatments is more likely focused on rehabilitation than interhabilitation (61). If there is no meaningful break between two sequential cancer treatments, then the supportive care role for exercise is successive intrahabilitation (e.g., intrahabilitation for chemotherapy followed by intrahabilitation for radiation therapy).
Perihabilitation exercise
Although perihabilitation exercise is not its own discrete cancer treatment-related time period, it may be used to describe exercise interventions that occur across or around multiple contiguous cancer treatment-related time periods (e.g., before, during, between, and/or after treatments) (53) (Tables 6, 7). Exercise oncology researchers may study perihabilitation exercise for a specific treatment (e.g., surgery, stem cell transplant, chemotherapy) or even a combination of treatments (e.g., surgery followed by chemotherapy). Perihabilitation goals may include combinations of prehabilitation, intrahabilitation, and/or rehabilitation. The emergence of the modern cancer treatment landscape makes perihabilitation research in exercise oncology increasingly necessary and important.
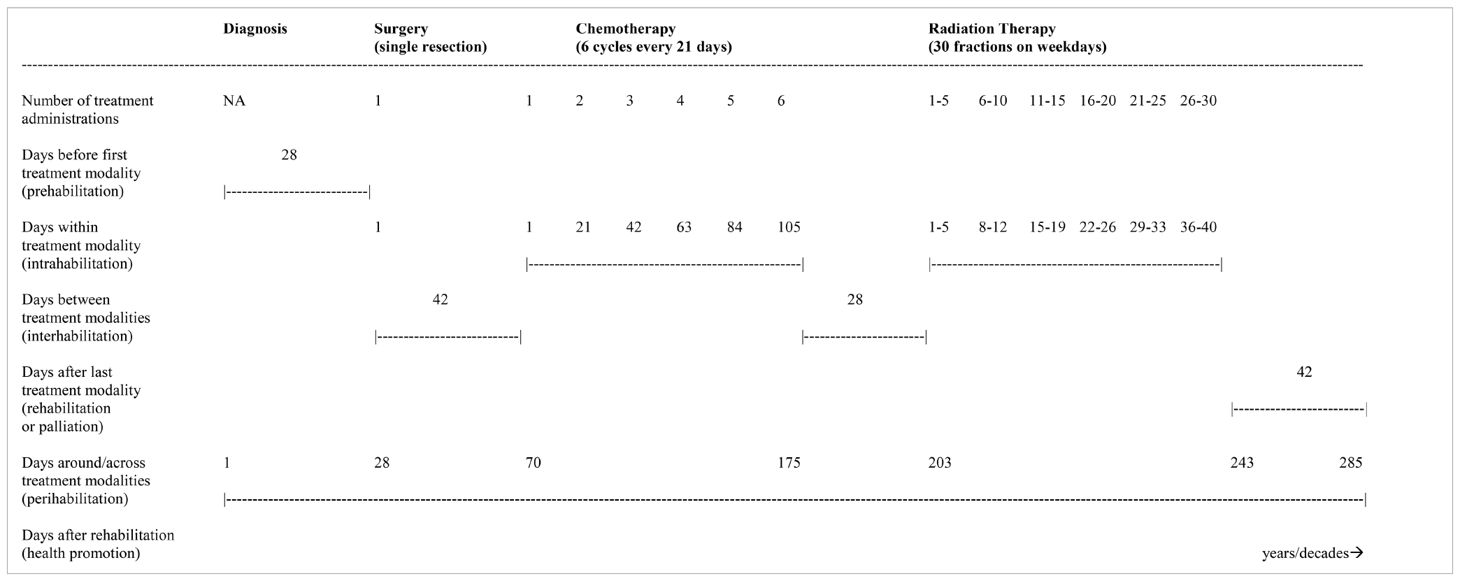
Table 7. Hypothetical treatment sequence of surgical resection followed by chemotherapy and radiation therapy.
For example, Morielli et al. (26) examined the effects of exercise in rectal cancer patients during and after neoadjuvant chemoradiation therapy prior to surgery compared to usual care (i.e., perihabilitation for neoadjuvant chemoradiation therapy). This study examined intrahabilitation exercise during neoadjuvant chemoradiation therapy and interhabilitation exercise between neoadjuvant chemoradiation therapy and surgery to help patients manage the neoadjuvant chemoradiation therapy, recover from the neoadjuvant chemoradiation therapy, and prepare for surgery. Similarly, Scott et al. (62) examined the effects of exercise in breast cancer patients during chemotherapy, after chemotherapy, or both during and after chemotherapy compared to usual care. This study, therefore, compared whether perihabilitation exercise for chemotherapy (during and after) was superior to intrahabilitation exercise alone or rehabilitation exercise alone in helping breast cancer patients manage (tolerate) and recover from chemotherapy.
Health promotion/disease prevention exercise
In the EPiCC Framework, exercise for health promotion/disease prevention begins after rehabilitation from successful cancer treatments and continues during survivorship until or unless a recurrence or second cancer occurs. Exercise during this phase focuses on improving overall health and preventing secondary diseases/late effects. The primary goals of exercise during cancer survivorship, therefore, are to improve general health outcomes such as quality of life, psychological well-being, and energy levels, as well as prevent secondary diseases/late effects. This distinct supportive care role for exercise is important because many cancer patients are cured of their cancer, experience minimal long-term complications, and return to normal life.
The goal of preventing secondary diseases/late effects is important during survivorship because some cancer patients are at higher risk of secondary diseases such as cardiovascular disease, diabetes, and osteoporosis because of their cancer and treatments. Moreover, many early stage cancer patients are more likely to die from other secondary diseases than cancer. Consequently, disease prevention may be a critical focus during the survivorship phase depending on the combination and sequencing of previous treatments. Although the prevention of secondary diseases/late effects is the primary concern of the survivorship phase, it may also be a focus earlier in the treatment trajectory for treatment(s) that are known to increase the risk of secondary diseases (e.g., some hormone therapies and chemotherapies).
For cancer survivors with lingering or chronic health effects after successful treatments, exercise during survivorship may consist of a combination of rehabilitation and health promotion/disease prevention goals. The relative emphasis on rehabilitation versus health promotion/disease prevention after successful cancer treatments may depend on the cancer treatment combination. In general, rehabilitation exercise may be more important early after treatment and diminish further out from treatment whereas the opposite may be true for health promotion/disease prevention exercise. For cancer survivors with persistent or permanent side effects, rehabilitation goals may remain important throughout survivorship and the transition from rehabilitation to health promotion/disease prevention may never occur completely.
Exercise for health promotion/disease prevention may also be the primary focus of the unique setting of active surveillance where newly diagnosed cancer patients do not immediately receive any treatments. These patients essentially enter the “survivorship” phase at the time of diagnosis and, therefore, the supportive care role of exercise in this setting may focus immediately on health promotion and disease prevention. Nevertheless, many of these patients will experience cancer progression and will ultimately require cancer treatments (44). For these patients, the supportive care role of exercise may also include prehabilitation. Given that it is unknown ahead of time which patients might progress, exercise for supportive care during active surveillance may include both prehabilitation and health promotion/disease prevention goals. Once the decision is made to undergo cancer treatment, exercise goals may shift entirely to prehabilitation.
Palliative exercise
In the EPiCC Framework, exercise for palliation begins after stopping all life-prolonging cancer treatments for progressive disease and continues until death. Palliation is generally defined as relieving symptoms, suffering, and improving quality of life without curing the underlying terminal or life-limiting disease (63). The primary goal of exercise for palliation at the end of life is to relieve symptoms and suffering, and to maintain physical functioning and quality of life for as long as possible. This distinct supportive care role for exercise is important because almost half of all cancer patients ultimately die from the disease and are likely in need of palliation. Exercise for palliation may be anticipated as patients with advanced cancer undergo their final life-prolonging treatments. Therefore, exercise during the final treatments may consist of a combination of intrahabilitation and palliation goals. The relative importance of intrahabilitation and palliation in patients with advanced cancer may shift over successive lines of therapy and depend on the particular treatment combinations. Once the decision is made to stop cancer treatments altogether, exercise goals may shift entirely to palliation.
Discussion
The EPiCC Framework provides structure to the field of exercise oncology and highlights the potential roles of exercise after a cancer diagnosis. In the following sections, we provide a brief discussion of future research directions although there are many more that we do not discuss. Moreover, we note some of the limitations of the EPiCC framework and how it might be applied in future systematic reviews and meta-analyses. Finally, we provide a conclusion and succinct summary of the key propositions of the EPiCC Framework.
Emerging research questions and future directions
The EPiCC Framework highlights many new research questions and future directions for the field of exercise oncology that emerge in the context of multimodal sequential cancer treatments. Table 8 highlights some of these key questions. Answers to these questions will help exercise oncology researchers determine the optimal positioning of exercise within different cancer treatment combinations and sequences. From a treatment perspective, (a) preclinical in vitro studies have studied exercise as an adjuvant monotherapy (64), (b) preclinical animal studies have studied exercise as a monotherapy (14) or concurrent therapy (24) for primary tumors (i.e., primary, neoadjuvant, or induction therapy) and/or disseminated tumor cells (i.e., adjuvant therapy), (c) observational studies have primarily examined exercise as a maintenance monotherapy (6), and (d) intervention studies have primarily studied exercise as a concurrent neoadjuvant therapy although isolated studies have been conducted as concurrent adjuvant therapy, primary monotherapy, and maintenance monotherapy (24).

Table 8. Exercise oncology research questions in the context of the modern cancer treatment landscape.
Studying exercise as a cancer treatment across the modern cancer treatment landscape may be challenging for preclinical studies. It may be feasible to examine exercise as a combination therapy in animal models in relation to a single cancer treatment (or a concurrent combination of cancer treatments), however, it may be more difficult to examine exercise in relation to multiple sequential treatments. That is, exercise may be tested before, during, and/or after a single (or concurrently combined) nonsurgical therapy as has been demonstrated in preclinical drug studies (65–67) but perhaps not across multiple sequential cancer treatments. Another possible approach to studying exercise after cancer treatments in preclinical models may be to use patient derived xenografts that have been previously treated. Identifying the mechanisms of action for any exercise effects will be a critical component of research integrating exercise into existing cancer treatment combinations.
Observational studies of exercise as a cancer treatment need to be initiated at diagnosis and tie assessments to the treatment-related time periods (6). Observational studies are well-positioned to study the specific role of exercise as a cancer treatment in single discrete treatment-related time periods or across multiple contiguous or disconnected treatment-related time periods to determine the best combination and sequencing of exercise within different cancer treatment combinations (6). Such studies may also become a model for how to integrate exercise assessments into routine clinical practice at critical phases of the cancer treatment experience. Intervention studies should be able to study the effects of exercise in single discrete treatment-related time periods or across multiple consecutive treatment-related time periods that are close together.
The supportive care role of exercise in cancer has been well-established across many different patient groups and treatment modalities. Nevertheless, most supportive care research has examined exercise within a single discrete treatment-related period such as before surgery, during nonsurgical therapy (especially chemotherapy and radiation therapy), long-term after curative treatments, or in patients with metastatic cancer. That is, exercise has been primarily studied as prehabilitation for surgery (68), intrahabilitation for chemotherapy (69) and radiation therapy (70), health promotion for long-term cancer survivors (71), and palliation for patients with advanced cancer (72). Some studies have examined exercise interhabilitation between neoadjuvant therapy and surgery (35).
Studies are needed to target other single discrete treatment-related periods (e.g., before, during, between, or after a specific treatment) and multiple contiguous or disconnected treatment-related periods (e.g., before, during, and between two or more sequential treatments). For example, few studies have examined exercise during other single discrete treatment-related periods such as prehabilitation for nonsurgical therapies, intrahabilitation for newer nonsurgical therapies (e.g., immunotherapy, targeted therapy), intrahabilitation for combined concurrent therapies (e.g., chemoradiation therapy, chemoimmunotherapy), or interhabilitation between sequential treatments. Moreover, when examining exercise for intrahabilitation, few studies have distinguished between the particular sequence of a therapy (e.g., chemotherapy as primary, neoadjuvant, induction, adjuvant, consolidation, maintenance, or salvage therapy) or the particular sequencing combination (e.g., adjuvant chemotherapy after radical surgery versus after primary radiation therapy). Finally, few studies have examined exercise across multiple contiguous or disconnected treatment-related periods such as before, during, between, and/or after multiple sequential treatments (i.e., perihabilitation) (4).
Perihabilitation studies in exercise oncology may be particularly informative because they evaluate the relative effects of performing exercise during single versus multiple contiguous cancer treatment-related time periods (i.e., simple versus “compound” research questions). For example, a study comparing exercise during and after chemotherapy (perihabilitation) to exercise after chemotherapy (rehabilitation) can shed light on which benefits of exercise during chemotherapy (intrahabilitation) can be recouped quickly, slowly, or not at all with rehabilitation exercise (73). The relative importance of exercise during chemotherapy depends not just on its demonstrated benefits but also on how quickly (and completely) those benefits can be recouped with exercise after chemotherapy. Similarly, a study comparing exercise before and after surgery (perihabilitation for surgery) to exercise after surgery (rehabilitation) can shed light on which benefits of exercise before surgery (prehabilitation) can be recouped quickly, slowly, or not at all with rehabilitation exercise.
In general, the case for exercise early in the cancer treatment sequence is strengthened if there are benefits that cannot be recouped completely or at all by exercise later in the cancer treatment sequence. Such information may allow exercise oncology practitioners to reduce or “de-escalate” the exercise prescription at certain times during a particular cancer treatment combination rather than promote the “maximally tolerated dose” of exercise across the entire postdiagnosis cancer continuum. Finally, from a treatment perspective, exercise effects earlier in the treatment sequence may alter exercise effects later in the treatment sequence (i.e., “compound” research questions). It is unclear if continuous exercise from the time of diagnosis and for the balance of life is optimal from a treatment perspective or whether there are critical time points during a treatment sequence when exercise may exert optimal treatment effects (i.e., exercise breaks with rechallenge).
Future studies are also needed that examine the effects of different exercise prescriptions on supportive care and disease outcomes including the type, frequency, intensity, duration, progression, and periodization. Moreover, it will be important to examine the specific timing of individual exercise administrations in relation to different cancer treatment schedules. That is, it will be important to specify how to integrate the treatment schedule of exercise with the treatment schedule of the cancer therapy.
Finally, the modern cancer treatment landscape also has significant implications for the feasibility and sustainability of exercise across multiple sequential cancer treatments. Exercise motivation and behavior change is difficult during a single cancer treatment and may become even more challenging in the context of the modern cancer treatment landscape. Conceivably, the determinants of exercise may vary depending on the combination and sequencing of cancer treatments. For example, the determinants of exercise during neoadjuvant chemotherapy may be different than during adjuvant chemotherapy. Similarly, the determinants of exercise during radiation therapy after surgery may be different than during radiation therapy after chemotherapy. Exercise behavior change interventions may need to take into account the particular combination and sequencing of cancer treatments.
Limitations
There are important limitations of the EPiCC Framework and our overview of the field of exercise oncology. First, our proposed framework does not reflect all of the clinical scenarios in oncology, although we believe it covers the major current treatment paradigms. Moreover, we believe our framework may be extrapolated to other less common treatment combinations or to new treatment combinations as they emerge in the future. Second, the roles of exercise as a disease treatment and supportive care intervention will vary by the specific types of cancer treatments within the cancer treatment combinations (e.g., type of surgery, type of chemotherapy, type of immunotherapy). Third, the roles of exercise as a disease treatment and supportive care intervention will also vary by the type and subtype of cancer (e.g., pancreatic, liver, brain, diffuse large B-cell lymphoma) and not just by the cancer treatment combinations. This precision medicine approach to exercise oncology is critical as the response to exercise may be quite different for different cancers, subtypes, cell lines, molecular profiles, and mutational statuses. Therefore, the EPiCC Framework will need to be applied and tested for specific cancers and subtypes. Fourth, it is unclear how feasible it will be to conduct preclinical in vitro, preclinical animal, observational, and clinical studies within and across multiple sequential cancer treatments. Innovative ideas will be needed to overcome logistical and methodological challenges in future research. Finally, we did not conduct systematic reviews on the effects of exercise within each of the different cancer treatment-related time periods and cancer treatment combinations. Such an undertaking was deemed impractical and beyond the scope of this paper. Future systematic reviews may consider using the EPiCC Framework to target their reviews to a specific treatment-related time period or across multiple contiguous treatment-related time periods within a specific cancer treatment combination. Alternatively, future systematic reviews may consider using the EPiCC Framework to organize and structure their reviews for a specific type of cancer (e.g., bladder, pancreatic, ovarian).
Conclusions
In conclusion, the modern cancer treatment landscape has become saturated and complicated for many cancers. Exercise may play many important distinct roles across the postdiagnosis cancer continuum, however, studies need to account for the positioning of exercise in relation to the combination and sequencing of other cancer treatments. The EPiCC Framework provides a conceptual model to organize the potential roles of exercise across the postdiagnosis cancer continuum (Figure 4). Before cancer treatments, exercise may serve as prehabilitation for any cancer therapy and as a possible priming monotherapy for any nonsurgical therapy. During cancer treatments, exercise may serve as intrahabilitation and as a concurrent therapy for any nonsurgical therapy at any point in the treatment sequence (e.g., primary, neoadjuvant, induction, adjuvant, consolidation, maintenance, or salvage). Between cancer treatments, exercise may serve as interhabilitation and as a bridging monotherapy between any two treatments in the treatment sequence. Immediately after successful treatments, exercise may serve as rehabilitation and as a maintenance monotherapy. During survivorship, exercise may be implemented for health promotion/disease prevention as well as a maintenance monotherapy. During end of life, exercise may serve as palliation but it is unlikely to serve as a salvage monotherapy. We propose the EPiCC Framework to stimulate a more targeted, integrated, and clinically-informed approach to the study of exercise after a cancer diagnosis.
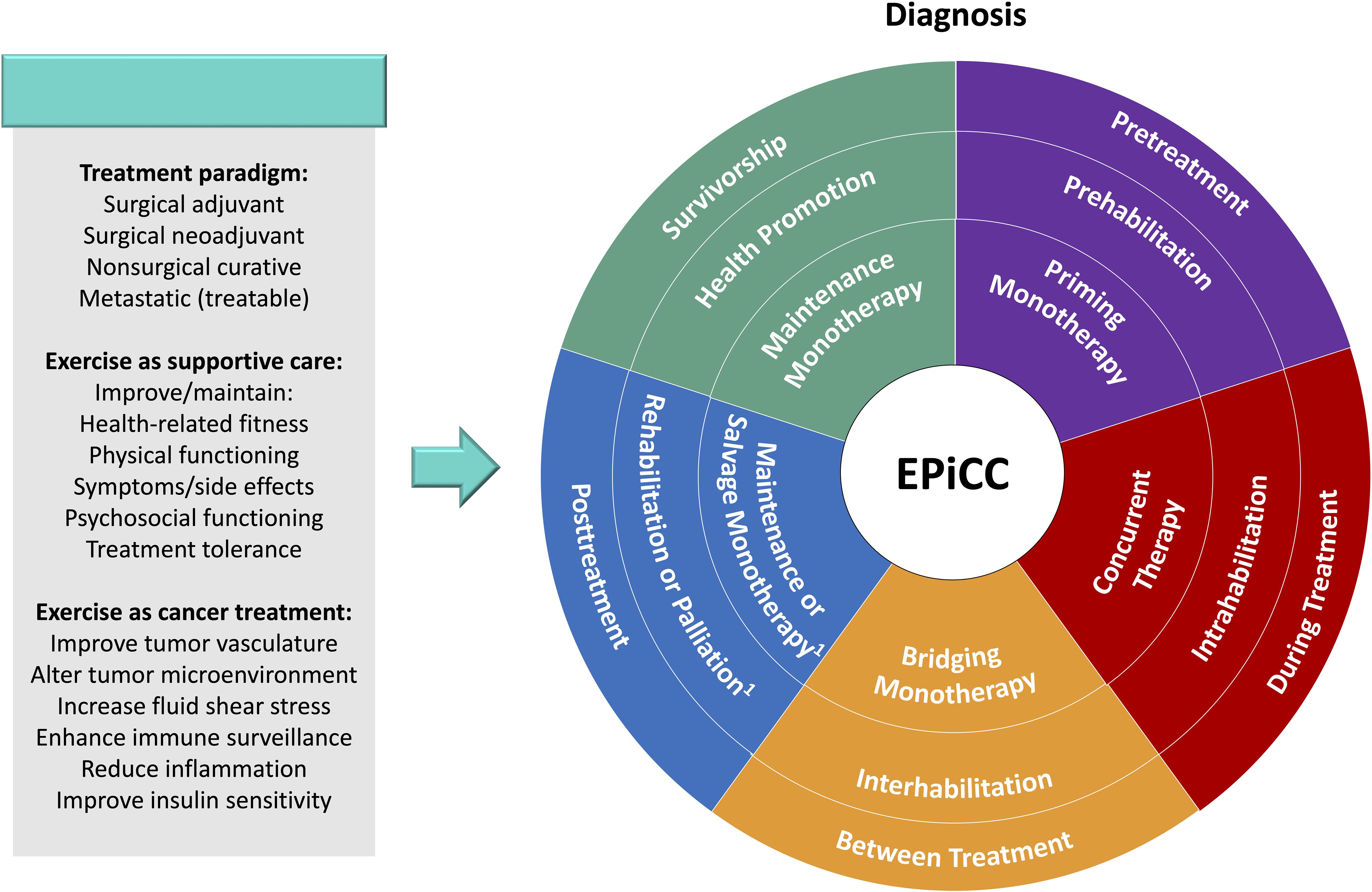
Figure 4. Exercise Across the Postdiagnosis Cancer Continuum (EPiCC) Wheel. Outer ring contains treatment-related time periods. Middle ring contains supportive care roles for exercise. Inner ring contains cancer treatment roles for exercise. 1Rehabilitation and maintenance monotherapy if successful treatment; palliation and salvage monotherapy if unsuccessful treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
KC: Conceptualization, Writing – original draft, Writing – review & editing. MM: Writing – review & editing. CB: Writing – review & editing. CF: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. KC is supported by the Canada Research Chairs Program and a Foundation Grant from the Canadian Institutes of Health Research (159927).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Courneya KS, Friedenreich CM. Framework PEACE: an organizational model for examining physical exercise across the cancer experience. Ann Behav Med. (2001) 23:263–72. doi: 10.1207/S15324796ABM2304_5
2. Courneya KS, Friedenreich CM. Physical activity and cancer control. Semin Oncol Nurs. (2007) 23:242–52. doi: 10.1016/j.soncn.2007.08.002
3. Courneya K, Jones LW, Fairey AS, Campbell KL, Ladha AB, Friedenreich CM, et al. Physical activity in cancer survivors: implications for recurrence and mortality. Cancer Ther. (2004) 2:1–12.
4. Santa Mina D, van Rooijen SJ, Minnella EM, Alibhai SMH, Brahmbhatt P, Dalton SO, et al. Multiphasic prehabilitation across the cancer continuum: A narrative review and conceptual framework. Front Oncol. (2020) 10:598425. doi: 10.3389/fonc.2020.598425
5. Courneya KS, Booth CM. Exercise as a cancer treatment: A clinical oncology framework for exercise oncology research. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.957135
6. Courneya KS, Friedenreich CM. Designing, analyzing, and interpreting observational studies of physical activity and cancer outcomes from a clinical oncology perspective. Front Oncol. (2023) 13:1098278. doi: 10.3389/fonc.2023.1098278
7. Catenacci DV, Chao J, Muro K, Al-Batran SE, Klempner SJ, Wainberg ZA, et al. Toward a treatment sequencing strategy: A systematic review of treatment regimens in advanced gastric cancer/gastroesophageal junction adenocarcinoma. Oncologist. (2021) 26:e1704–e29. doi: 10.1002/onco.13907
8. Caffo O. Treatment sequencing in oncology: balancing clinical trial and real-world evidence. Future Oncol. (2019) 15:2887–9. doi: 10.2217/fon-2019-0415
9. Provencio M, Calvo V, Romero A, Spicer JD, Cruz-Bermudez A. Treatment sequencing in resectable lung cancer: the good and the bad of adjuvant versus neoadjuvant therapy. Am Soc Clin Oncol Educ Book. (2022) 42:1–18. doi: 10.1200/EDBK_358995
10. Devisetty K, Wong SJ. Neoadjuvant versus induction chemotherapy: more than semantics. J Clin Oncol. (2013) 31:2971–2. doi: 10.1200/JCO.2013.50.2674
11. Fiuza-Luces C, Valenzuela PL, Galvez BG, Ramirez M, Lopez-Soto A, Simpson RJ, et al. The effect of physical exercise on anticancer immunity. Nat Rev Immunol. (2024) 24:282–93. doi: 10.1038/s41577-023-00943-0
12. Lynch C, Arshad M, Katipally RR, Pitroda SP, Weichselbaum RR. Sharing the burden: the case for definitive local therapy in place of immune checkpoint blockade for patients with a low-volume burden of metastatic disease. J Clin Oncol. (2024), JCO2400549. doi: 10.1200/JCO.24.00549
13. Plana D, Palmer AC, Sorger PK. Independent drug action in combination therapy: implications for precision oncology. Cancer Discovery. (2022) 12:606–24. doi: 10.1158/2159-8290.CD-21-0212
14. Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: A critical systematic review of in vivo preclinical data. Cancer Res. (2016) 76:4032–50. doi: 10.1158/0008-5472.CAN-16-0887
15. Eschke RK, Lampit A, Schenk A, Javelle F, Steindorf K, Diel P, et al. Impact of physical exercise on growth and progression of cancer in rodents-A systematic review and meta-analysis. Front Oncol. (2019) 9:35. doi: 10.3389/fonc.2019.00035
16. Figueira ACC, Cortinhas A, Soares JP, Leitao JC, Ferreira RP, Duarte JA. Efficacy of exercise on breast cancer outcomes: A systematic review and meta-analysis of preclinical data. Int J Sports Med. (2018) 39:327–42. doi: 10.1055/s-0044-101149
17. Li Y, Xiao X, Zhang Y, Tang W, Zhong D, Liu T, et al. Effect of exercise on breast cancer: A systematic review and meta-analysis of animal experiments. Front Mol Biosci. (2022) 9:843810. doi: 10.3389/fmolb.2022.843810
18. Kang DW, Fairey AS, Boule NG, Field CJ, Wharton SA, Courneya KS. Effects of exercise on cardiorespiratory fitness and biochemical progression in men with localized prostate cancer under active surveillance: the ERASE randomized clinical trial. JAMA Oncol. (2021) 7:1487–95. doi: 10.1001/jamaoncol.2021.3067
19. Brassetti A, Cacciatore L, Bove AM, Anceschi U, Proietti F, Misuraca L, et al. The impact of physical activity on the outcomes of active surveillance in prostate cancer patients: A scoping review. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16030630
20. Esteves M, Monteiro MP, Duarte JA. The effects of physical exercise on tumor vasculature: systematic review and meta-analysis. Int J Sports Med. (2021) 42:1237–49. doi: 10.1055/a-1533-1876
21. Scandura JM, Roboz GJ, Moh M, Morawa E, Brenet F, Bose JR, et al. Phase 1 study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood. (2011) 118:1472–80. doi: 10.1182/blood-2010-11-320093
22. Ligibel JA, Dillon D, Giobbie-Hurder A, McTiernan A, Frank E, Cornwell M, et al. Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: results from the pre-operative health and body (PreHAB) study. Clin Cancer Res. (2019) 25:5398–406. doi: 10.1158/1078-0432.CCR-18-3143
23. Houben LHP, Tuytten T, Holwerda AM, Wisanto E, Senden J, Wodzig W, et al. A low or high physical activity level does not modulate prostate tumor tissue protein synthesis rates. Med Sci Sports Exerc. (2024) 56:635–43. doi: 10.1249/MSS.0000000000003349
24. Yang L, Morielli AR, Heer E, Kirkham AA, Cheung WY, Usmani N, et al. Effects of exercise on cancer treatment efficacy: A systematic review of preclinical and clinical studies. Cancer Res. (2021) 81:4889–95. doi: 10.1158/0008-5472.CAN-21-1258
25. Florez Bedoya CA, Cardoso ACF, Parker N, Ngo-Huang A, Petzel MQ, Kim MP, et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci Rep. (2019) 9:13966. doi: 10.1038/s41598-019-49582-3
26. Morielli AR, Usmani N, Boule NG, Severin D, Tankel K, Joseph K, et al. Feasibility, safety, and preliminary efficacy of exercise during and after neoadjuvant rectal cancer treatment: A phase II randomized controlled trial. Clin Colorectal Cancer. (2021) 20:216–26. doi: 10.1016/j.clcc.2021.05.004
27. Zylstra J, Whyte GP, Beckmann K, Pate J, Santaolalla A, Gervais-Andre L, et al. Exercise prehabilitation during neoadjuvant chemotherapy may enhance tumour regression in oesophageal cancer: results from a prospective non-randomised trial. Br J Sports Med. (2022) 56:402–9. doi: 10.1136/bjsports-2021-104243
28. Sanft T, Harrigan M, McGowan C, Cartmel B, Zupa M, Li FY, et al. Randomized trial of exercise and nutrition on chemotherapy completion and pathologic complete response in women with breast cancer: the lifestyle, exercise, and nutrition early after diagnosis study. J Clin Oncol. (2023) 41:5285–95. doi: 10.1200/JCO.23.00871
29. de Boniface J, Altena R, Haddad Ringborg C, Bolam KA, Wengstrom Y. Physical exercise during neoadjuvant chemotherapy for breast cancer as a mean to increase pathological complete response rates: Trial protocol of the randomized Neo-ACT trial. PloS One. (2022) 17:e0274804. doi: 10.1371/journal.pone.0274804
30. Dall'Olio FG, Marabelle A, Caramella C, Garcia C, Aldea M, Chaput N, et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2022) 19:75–90. doi: 10.1038/s41571-021-00564-3
31. Brown JC, Gilmore LA. Physical activity reduces the risk of recurrence and mortality in cancer patients. Exerc Sport Sci Rev. (2020) 48:67–73. doi: 10.1249/JES.0000000000000214
32. Rincon-Castanedo C, Morales JS, Martin-Ruiz A, Valenzuela PL, Ramirez M, Santos-Lozano A, et al. Physical exercise effects on metastasis: a systematic review and meta-analysis in animal cancer models. Cancer Metastasis Rev. (2020) 39:91–114. doi: 10.1007/s10555-020-09851-4
33. Friedenreich CM, Cook LS, Wang Q, Kokts-Porietis RL, McNeil J, Ryder-Burbidge C, et al. Prospective cohort study of pre- and postdiagnosis physical activity and endometrial cancer survival. J Clin Oncol. (2020) 38:4107–17. doi: 10.1200/JCO.20.01336
34. Lee M, Lee Y, Jang D, Shin A. Physical activity after colorectal cancer diagnosis and mortality in a nationwide retrospective cohort study. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13194804
35. West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. (2015) 114:244–51. doi: 10.1093/bja/aeu318
36. Regmi S, Fung TS, Lim S, Luo KQ. Fluidic shear stress increases the anti-cancer effects of ROS-generating drugs in circulating tumor cells. Breast Cancer Res Treat. (2018) 172:297–312. doi: 10.1007/s10549-018-4922-8
37. Shaver AL, Sharma S, Nikita N, Lefler DS, Basu-Mallick A, Johnson JM, et al. The effects of physical activity on cancer patients undergoing treatment with immune checkpoint inhibitors: A scoping review. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13246364
38. Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. (2007) 25:4396–404. doi: 10.1200/JCO.2006.08.2024
39. Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. (2014) 46:1744–51. doi: 10.1249/MSS.0000000000000297
40. Nabrinsky E, Macklis J, Bitran J. A review of the abscopal effect in the era of immunotherapy. Cureus. (2022) 14:e29620. doi: 10.7759/cureus.29620
41. Masuda H, Harano K, Miura S, Wang Y, Hirota Y, Harada O, et al. Changes in triple-negative breast cancer molecular subtypes in patients without pathologic complete response after neoadjuvant systemic chemotherapy. JCO Precis Oncol. (2022) 6:e2000368. doi: 10.1200/PO.20.00368
42. Friedenreich CM, Stone CR, Cheung WY, Hayes SC. Physical activity and mortality in cancer survivors: A systematic review and meta-analysis. JNCI Cancer Spectr. (2020) 4:pkz080. doi: 10.1093/jncics/pkz080
43. Courneya KS, Booth CM, Gill S, O'Brien P, Vardy J, Friedenreich CM, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. (2008) 15:279–85. doi: 10.3747/co.v15i6.378
44. Newcomb LF, Schenk JM, Zheng Y, Liu M, Zhu K, Brooks JD, et al. Long-term outcomes in patients using protocol-directed active surveillance for prostate cancer. JAMA. (2024) 331:2084–93. doi: 10.1001/jama.2024.6695
45. Takemura N, Chan SL, Smith R, Cheung DST, Lin CC. The effects of physical activity on overall survival among advanced cancer patients: a systematic review and meta-analysis. BMC Cancer. (2021) 21:242. doi: 10.1186/s12885-021-07988-1
46. Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. (2009) 27:4605–12. doi: 10.1200/JCO.2008.20.0634
47. Alfano CM, Cheville AL, Mustian K. Developing high-quality cancer rehabilitation programs: A timely need. Am Soc Clin Oncol Educ Book. (2016) 35:241–9. doi: 10.1200/EDBK_156164
48. Chowdhury RA, Brennan FP, Gardiner MD. Cancer rehabilitation and palliative care-exploring the synergies. J Pain Symptom Manage. (2020) 60:1239–52. doi: 10.1016/j.jpainsymman.2020.07.030
49. Arora A, Chang P, Asher A. The role of rehabilitation for the dying cancer patient. Curr Phys Med Rehab. (2024) 12:119–25. doi: 10.1007/s40141-024-00433-x
50. Partridge AH, Seah DS, King T, Leighl NB, Hauke R, Wollins DS, et al. Developing a service model that integrates palliative care throughout cancer care: the time is now. J Clin Oncol. (2014) 32:3330–6. doi: 10.1200/JCO.2013.54.8149
51. Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. (2017) 9:e1029. doi: 10.7759/cureus.1029
52. McNeely MD N. Principles of physical and occupational therapy in cancer. Cancer Rehabilitation: Principles Practice. (2018).
53. Yang X, Li M, Zhang A, Huang X, Jiang Y, Zou F, et al. Perihabilitation: A holistic perspective on rehabilitation and prehabilitation. World J Phys Rehabil Med. (2021) 5:1–5.
54. Crevenna R, Palma S, Licht T. Cancer prehabilitation - a short review. Magazine Eur Med Oncol. (2021) 14:39–43. doi: 10.1007/s12254-021-00686-5
55. Flores LE, Westmark D, Katz NB, Hunter TL, Silver EM, Bryan KM, et al. Prehabilitation in radiation therapy: a scoping review. Support Care Cancer. (2024) 32:83. doi: 10.1007/s00520-023-08262-9
56. Seth I, Bulloch G, Qin KR, Xie Y, Sebastian B, Liew H, et al. Pre-rehabilitation interventions for patients with head and neck cancers: A systematic review and meta-analysis. Head Neck. (2024) 46:86–117. doi: 10.1002/hed.27561
57. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. (2013) 92:715–27. doi: 10.1097/PHM.0b013e31829b4afe
58. Moug SJ, Mutrie N, Barry SJE, Mackay G, Steele RJC, Boachie C, et al. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: results from the REx trial. Colorectal Dis. (2019) 21:548–62. doi: 10.1111/codi.2019.21.issue-5
59. Kang DW, Fairey AS, Boule NG, Field CJ, Courneya KS. Exercise duRing Active Surveillance for prostatE cancer-the ERASE trial: a study protocol of a phase II randomised controlled trial. BMJ Open. (2019) 9:e026438. doi: 10.1136/bmjopen-2018-026438
60. Inglis EC, Iannetta D, Rasica L, Mackie MZ, Keir DA, MacInnis MJ, et al. Heavy-, Severe-, and Extreme-, but not Moderate-Intensity Exercise Increase V̇o2max and Thresholds after 6 Weeks of Training. Med Sci Sports Exerc. (2024) 56:1307–16. doi: 10.1249/MSS.0000000000003406
61. Min J, Kim J, Ryu J, Park S, Courneya K, Ligibel J, et al. Early implementation of exercise to facilitate recovery after breast cancer surgery: a randomized controlled trial. JAMA Surg. (2024) 159:872–80. (in press). doi: 10.1001/jamasurg.2024.1633
62. Scott JM, Lee J, Herndon JE, Michalski MG, Lee CP, O'Brien KA, et al. Timing of exercise therapy when initiating adjuvant chemotherapy for breast cancer: a randomized trial. Eur Heart J. (2023) 44:4878–89. doi: 10.1093/eurheartj/ehad085
63. Applequist H, Daly BJ. Palliation: A concept analysis. Res Theory Nurs Pract. (2015) 29:297–305. doi: 10.1891/1541-6577.29.4.297
64. Bettariga F, Taaffe DR, Galvao DA, Bishop C, Kim JS, Newton RU. Suppressive effects of exercise-conditioned serum on cancer cells: A narrative review of the influence of exercise mode, volume, and intensity. J Sport Health Sci. (2023) 13:484–98. doi: 10.1016/j.jshs.2023.12.001
65. Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discovery. (2016) 6:1382–99. doi: 10.1158/2159-8290.CD-16-0577
66. Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res. (2017) 23:6165–77. doi: 10.1158/1078-0432.CCR-16-2677
67. Ottewell PD, Monkkonen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. (2008) 100:1167–78. doi: 10.1093/jnci/djn240
68. Meneses-Echavez JF, Loaiza-Betancur AF, Diaz-Lopez V, Echavarria-Rodriguez AM, Triana-Reina HR. Prehabilitation programs for individuals with cancer: a systematic review of randomized-controlled trials. Syst Rev. (2023) 12:219. doi: 10.1186/s13643-023-02373-4
69. Kearney N, Connolly D, Begic S, Mockler D, Guinan E. Feasibility metrics of exercise interventions during chemotherapy: A systematic review. Crit Rev Oncol Hematol. (2024) 195:104272. doi: 10.1016/j.critrevonc.2024.104272
70. Trommer M, Marnitz S, Skoetz N, Rupp R, Niels T, Morgenthaler J, et al. Exercise interventions for adults with cancer receiving radiation therapy alone. Cochrane Database Syst Rev. (2023) 3:CD013448. doi: 10.1002/14651858.CD013448.pub2
71. Gao R, Yu T, Liu L, Bi J, Zhao H, Tao Y, et al. Exercise intervention for post-treatment colorectal cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. (2020) 14:878–93. doi: 10.1007/s11764-020-00900-z
72. Toohey K, Chapman M, Rushby AM, Urban K, Ingham G, Singh B. The effects of physical exercise in the palliative care phase for people with advanced cancer: a systematic review with meta-analysis. J Cancer Surviv. (2022) 17:399–415. doi: 10.1007/s11764-021-01153-0
Keywords: cancer treatments, combination therapy, exercise, physical activity, multimodality therapy, prehabilitation, rehabilitation
Citation: Courneya KS, McNeely ML, Booth CM and Friedenreich CM (2024) An integrated framework for the study of exercise across the postdiagnosis cancer continuum. Front. Oncol. 14:1432899. doi: 10.3389/fonc.2024.1432899
Received: 14 May 2024; Accepted: 05 September 2024;
Published: 23 September 2024.
Edited by:
Sarah J. Hardcastle, Sheffield Hallam University, United KingdomReviewed by:
Anastassios Philippou, National and Kapodistrian University of Athens, GreeceMichele Milella, University of Verona, Italy
Copyright © 2024 Courneya, McNeely, Booth and Friedenreich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerry S. Courneya, a2VycnkuY291cm5leWFAdWFsYmVydGEuY2E=
 Kerry S. Courneya
Kerry S. Courneya Margaret L. McNeely2,3
Margaret L. McNeely2,3