- 1Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, United States
- 2Dorothy M. Davis Heart and Lung Research Institute, Department of Surgery, Division of General and Gastrointestinal Surgery, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 3Department of Pathology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, United States
Triple-negative breast cancer (TNBC) is one of the most aggressive subtypes of breast cancer with higher rates of recurrence and distant metastasis, as well as decreased 5-year survival rates. Racial disparities are evident in the incidence and mortality rates of triple negative breast cancer particularly increased in young African American women. Concurrently, young African American women have multiple risk factors for TNBC including higher rates of premenopausal abdominal obesity (higher waist-hip ratio) and lower rates of breastfeeding with higher parity, implicating these factors as potentially contributors to poor outcomes. By understanding the mechanisms of how premenopausal obesity and lack of breastfeeding may be associated with increased risk of triple negative breast cancer, we can determine the best strategies for intervention and awareness to improve outcomes in TNBC.
1 Introduction
Breast cancer is the most commonly occurring form of cancer internationally, with more than 1 in 8 women diagnosed in their lifetime (1, 2). The chances that a woman will die of breast cancer is approximately 1 in 39, making it globally the second deadliest form of cancer in women (3). In the US, incidence rates of breast cancer diagnosis grew dramatically from the 1940s to the 1990s but have stabilized in the last two decades at approximately 130 new cases per 100,000 people (2, 4). In 2019, there were 268,000 new cases of breast cancer and 41,000 women died as a direct result of this disease (5). Predicted numbers for 2023 are similar, with 297,790 estimated new cases and 34,020 deaths (5, 6). Recent estimates suggest that nearly 4 million women with a history of breast cancer are likely currently living in the US (7).
Triple negative breast cancer (TNBC) cases generally have worse prognosis as they are characterized by aggressive growth and invasiveness compared to any other subtype of breast cancer (8, 9). This breast cancer subtype is negative for the presence of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) (10) and do not have any targeted therapy. There are numerous risk factors for TNBC including age, age at menarche, multiparity, premenopausal obesity, lack of breastfeeding following full-term pregnancy, and duration of breastfeeding (11). TNBC is more common among women with BRCA1 mutations (8). The African American Breast Cancer Epidemiology and Risk (AMBER) consortium utilized data collected through the Women’s Circle of Health Study, the Black Women’s Health Study, the Carolina Breast Cancer Study, and Multiethnic Cohort Study and found that the lack of breastfeeding following full-term pregnancy was associated with an increased risk of ER negative breast cancer but not ER+ breast cancer (12, 13). Additionally, this data revealed an additional risk of ER- breast cancer following each consecutive parity coupled with the absence of breastfeeding (12).
While obesity is a multifaceted, complex state that has been identified as an independent risk factor for various diseases and cancers, its relationship with breast cancer is more controversial. Several epidemiological studies have found no relationship or even a beneficial effect of higher body mass index (BMI) on breast cancer diagnosis and related outcomes (14–16). Particularly, obesity rates (BMI > 30kg/m2) in premenopausal breast cancer have been negatively correlated with breast cancer risk (14–16). However, several studies have shown that BMI may not be the best measurement of obesity in some populations. For instance, African American women (AAW) have higher levels of abdominal adiposity that is not accounted for in BMI measurement (17). In the East Carolina Breast Cancer Study a higher waist to hip ratio (WHR), a measurement focused on abdominal adiposity, was associated with a higher risk of TNBC in premenopausal women (18). These findings were similarly shown in the Women’s Circle of Health Study, that AAW premenopausal women with a higher WHR had a greater risk of breast cancer (19, 20). Therefore, revaluation of WHR and its association with breast cancer risk could be more appropriate in the future.
Although there have been new insights in understanding how the lack of breastfeeding impacts breast cancer risk (21), there has been no research on how obesity in combination with a lack of breastfeeding can further increase the risk of breast cancer. The lack of breastfeeding and obesity contribute to aberration in several common pathways for developing TNBC. It is important to understand how these two risk factors may interact and augment the risk. In this review, we summarize the population and biological literature on the overlapping pathways affected by lack of breastfeeding and premenopausal obesity that are associated with an increased risk of TNBC.
2 Breast Involution
Literature on the relationship between the lack of breastfeeding and cancer risk point towards the process of involution as being the critical window for changes increasing the risk (22). Involution of the mammary gland or breast tissue is a post-lactation process that remodels the tissue to its near pre-pregnancy state for subsequent pregnancies and lactation (23). While majority of the involution process has been elucidated using rodent models, several studies have confirmed the process of involution in humans (23–26). The process of involution is initiated by the absence of suckling and occurs in two distinct phases (27). The first phase of involution that lasts over a period of 2-3 days is reversible with re-initiation of suckling (27, 28). During this initial phase, epithelial cells undergo programmed cell death (28) and alveolar cell detachment, and dead cells accumulate in the lumen (27). Adipocytes begin to re-differentiate and re-populate the mammary gland (28). The second irreversible phase occurs over a 4-7-day period that is initiated by the breakdown of the extracellular matrix and leads to a second round of programmed cell death (27, 28). The second phase also includes collapsing of the alveoli, tissue remodeling, and adipocyte hypertrophy to a state very similar to pre-pregnancy state. The involution process is illustrated in Figure 1.
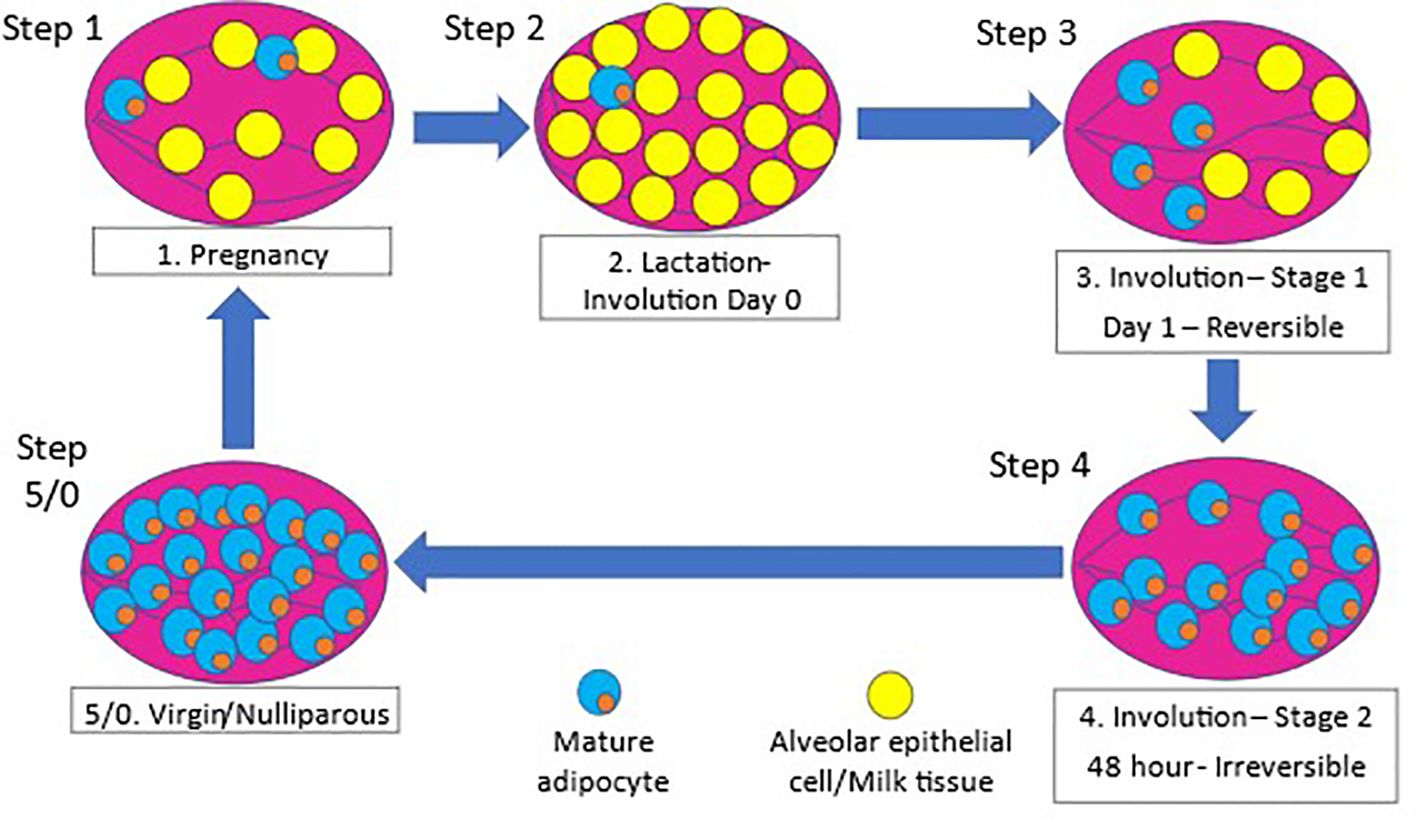
Figure 1. Remodeling of mammary gland during pregnancy and involution. 1) The mammary gland undergoes epithelial to alveolar differentiation during pregnancy preparing for lactation at birth, followed by massive cell death (22, 27, 29, 30). The mammary gland undergoes massive cell death and tissue remodeling as it transitions from a lactating state to a prepregnant state upon cessation of lactation after birth (22). 2) During pregnancy, the mammary gland expands dramatically through extensive epithelial cell proliferation and differentiation to alveolar cells in preparation for milk production and secretion, and this process continues throughout the period of lactation (27, 29). 3) However, upon weaning of the offspring, the gland undergoes a reversible phase of involution, where apoptotic alveolar cells shed in the lumen (27, 30). 4) This is followed by the second phase of involution within 48-72 hours of weaning, characterized by massive cell death, collapse of the alveolar structure, and adipocyte repopulation when the tissue is structurally remodeled back to its prepregnant state (27, 30).
Through the development and use of mouse models to study this phenomenon, two distinct types of involution have been conceptualized. The term “abrupt involution” has been used to describe instances when breastfeeding is not initiated after birth or there is a short period of lactation less than 3 months (28, 31). Abrupt involution forces the mammary gland to undergo the involution and remodeling process at the peak of milk production (28, 31). On the contrary, during gradual involution when breastfeeding is prolonged greater than 6 months, alveolar cell death and remodeling of the mammary gland is more orchestrated and gradual (31). The process of gradual involution leads to remodeling of the mammary gland over a longer period of time compared to glands forced through abrupt involution (28, 31).
3 Overlapping link between obesity and abrupt involution on increased risk of TNBC
There is overwhelming scientific evidence on role of obesity role in development of TNBC (32–37). But unlike obesity, there is limited research on abrupt involution and how lack of breastfeeding impacts the risk of developing breast cancer. Numerous epidemiological studies point to a link between lack of breastfeeding following full-term pregnancy and breast cancer risk (10–12, 21); however, majority of mechanistic studies to understand this correlation have been conducted in rodent and bovine models. Comparison of underlying mechanisms connecting the two independent TNBC risk factors, lack of breastfeeding/abrupt involution and premenopausal obesity revealed significant overlap in processes that link each factor to higher breast cancer risk (Figure 2). A recent study has shown that obesity induced inflammation resulted in premature involution and attributed it to zinc-mediated stress on endoplasmic reticulum (32). However, combined impacts of these two risk factors and long-term changes within the breast tissue and breast cancer risk, specifically TNBC, has yet to be studied.
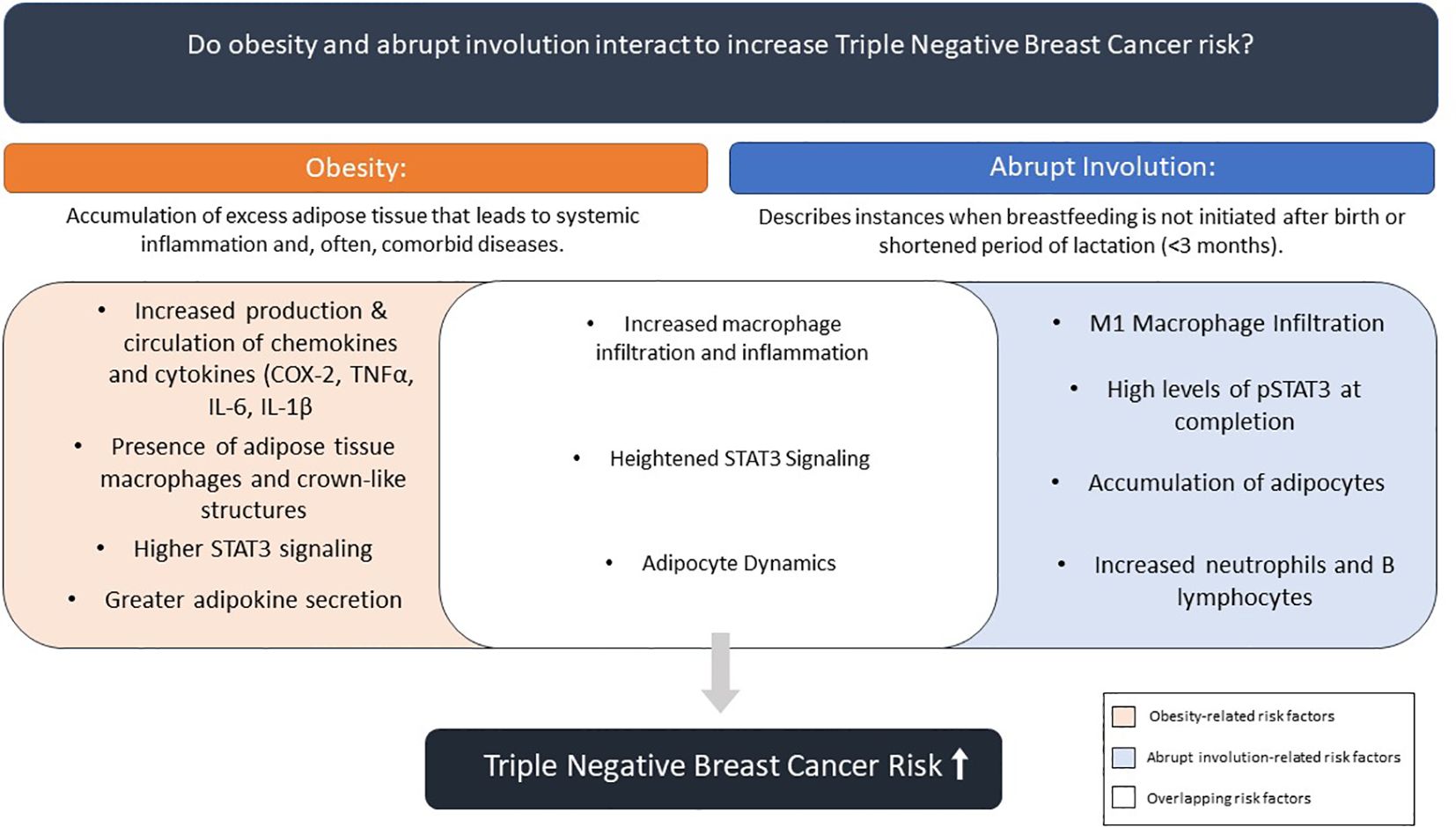
Figure 2. Summary of effects of obesity and abrupt involution on combined contribution to breast cancer risk.
3.1 Mammary gland Inflammation
Both obesity and abrupt involution can lead to states of increased inflammation in breast tissue (32). Obesity, on its own, is characterized as a chronic state of systemic inflammation that leads to an increase in local inflammation enhancing the risk for cancer development (34–36). Abrupt involution leads to acute inflammation in the mammary gland that sustains over time (31). However, unpublished data from our laboratory suggests abrupt involution may lead to systemic inflammation as well.
3.1.1 Upregulation of cytokines and macrophage population in obesity
Multiple studies using mouse models and human tissues have shown increased inflammation, in particular macrophage infiltration, in the mammary gland of obese mice and individuals (28, 33, 35, 38). What is the link between adiposity and inflammation? In both humans and mice, as adipocytes expand in number and size, blood vessel development lags leading to a hypoxic environment (39). The lack of oxygen and over-crowding of the adipocytes causes the adipocytes to produce cytokines that increase the inflammatory state within the tissue and circulation (39). Lack of oxygen and nutrients during aberrant expansion of adipose tissue leads to adipocyte death (38, 40, 41). Infiltration of macrophages within adipose tissue, and the mammary gland, have the ability to gather around dying adipocytes (38). Increased macrophage populations may lead to a self-sustaining immune response, as macrophage infiltration leads to increased production and secretion of pro-inflammatory cytokines, such as tumor necrosis factor α (TNFα), interleukin 6 (IL-6), and interleukin 1β (IL-1β) (38). When encircling necrotic adipocytes, the macrophages form what is called a crown-like structure (CLS) (38, 40, 41). Increased inflammation, infiltration of macrophages, and formation of CLS can lead to dysregulation of adipokines, disruption of adipocyte differentiation, and changes in estrogen signaling, which are associated with increased TNBC risk (38).
Murine models of obesity have refined our understanding of cytokines in the mammary gland. In a diet-induced obesity study, C57BL/6J ovariectomized mice consuming a high-fat diet for 10 and 24 weeks showed increased levels of NFκB, IL-1β, TNFα, and COX-2 in the mammary gland compared to low-fat non-ovariectomized controls (33). This effect was further explored using a diet-induced obesity mouse model where mice were injected with E0771 cells which caused accelerated rates of TNBC development correlated with high IL-6 levels within the adipose tissue and tumor (36). Additionally, the TNBC tumors displayed increased infiltration of cancer associated adipocytes (36).
Studies have found increased CLS in the mammary gland of mice on high-fat diets (33), demonstrating an association between obesity and macrophage infiltration. In addition to an increase in the macrophage population, macrophage phenotype changes have been reported in the mammary glands of obese mice. These adipose tissue macrophages (ATM) shift to metabolically active macrophages (MMe), which are proinflammatory. MMe have been found to overexpress GPR130 ligands and produce cytokines, like IL-6, to promote stemness in cells that promote the development of TNBC. Increased concentration of MMe has been reported in breast tissues collected from obese women and was positively correlated to BMI. When cultured in vitro, MMe were able to activate signal transducer and activator of transcription 3 (STAT3) signaling (42), which is a pathway of interest in the development of breast cancer.
In women with TNBC, visceral adipose tissue size positively correlated with tumor size and inversely correlated with blood vessel density within the tumor (36). Additionally, pro-inflammatory cytokine IL-6 produced by the adipose tissue was higher correlated to levels within circulation and the tumor (36). In women diagnosed with breast cancer, there was a positive correlation for the presence of CLS in the breast tissue with obesity, insulin resistance, and poor prognosis (38, 40). A comparative study of breast cancer patients found that AAW had more CLS in the breast tissue than non-Hispanic black and Caucasian women (41). The macrophages surrounding adipocytes in the AAW population were shown to be highly proliferative (41).
3.1.2 Upregulation of cytokines and macrophage population in abrupt involution
The inflammatory effects associated with obesity are similar to what was observed in the mammary gland following abrupt involution (31). Mouse models of involution provide evidence of an acute inflammatory response during and shortly after involution that sustains to long-term time points. For example, to understand the inflammatory process during abrupt involution of mammary gland, Stein et al. used female Balb/C mice that were forced to undergo involution on day 7 of lactation (43). During the involution process, mice displayed activation of B-cell lymphocytes and STAT3 pathways followed by increased infiltration of neutrophils and F4/80 positive macrophages in mammary gland (43). Similarly, a model using C57bl/6 and Mafia transgenic mice that forced abrupt involution at day 10 postpartum, found an increase in macrophage infiltration in wild-type mammary glands and depletion of macrophages in the transgenic mice inhibited the involution process (44). In our lab, a similar mouse model of abrupt involution was developed using FVB/n mice where forced involution was induced by removal of pups on day 7 of lactation (31). The mammary glands of these mice showed sustained activation of STAT3 pathway at day 28 postpartum and increased F4/80 macrophage infiltration even at day 56 postpartum that continued long-term to 120 days postpartum (31). In a mouse obesity model, C57bl/6 female mice on high-fat diets were found to have a higher number of macrophages in the mammary glands and this accumulation was partially responsible for induction of early involution (32).
High levels of macrophages within the mammary gland during involution has been thought to be related to upregulation of glycoprotein semaphorin A (SEMA7A) that is found on T lymphocytes (45). In C57bl/6 undergoing abrupt involution, podoplanin (PDPN), a lymphatic system marker, and marker of macrophages CD68+, were associated with SEMA7A (45). The co-expression of these gene markers is associated with increased risk and poor prognosis of breast cancer (45). This gene signature has been confirmed in breast biopsies of women undergoing involution (45). The infiltration of immune cells during abrupt involution mimics the wound healing process and promote tumor progression in D2A1 injected mice (46). Characterization of the immune environment and wound healing-like process in breast tissue was confirmed in women undergoing involution (26). Alterations of the immune environment were consistent with pro-tumorigenic conditions (26).
3.2 STAT3 pathway activation
STAT3 signaling pathway is an important pathway during the involution process necessary to facilitate programmed cell death. Stein et al. and Basree et al. found upregulation of STAT3 pathway activation during and after abrupt mammary gland involution (31, 43). In Balb/c mice, STAT3 mRNA levels doubled one day following forced involution and continued to be upregulated until day 3 of involution before returning towards pre-pregnancy levels (43). This early upregulation of STAT3 during involution was associated with increases in acute phase response genes such as lipopolysaccharide as well as its receptor CD14, a monocyte marker, in the luminal epithelial population (43). While STAT3 signaling was not explored during the involution process in FVB/n mice, STAT3 and phosphorylated STAT3 (pSTAT3) were found to be highly expressed in abruptly involuted mammary glands 28 days postpartum that continued to be elevated through day 56 postpartum (31). Combined, these studies suggest an early increase in STAT3 activation that helps mammary glands to return to pre-pregnancy state is re-activated when investigated after up to 120 days postpartum, demonstrating sustained inflammation.
STAT3 signaling pathway has been shown to be elevated in breast cancer, particularly TNBC (47). Cytokine IL-6 is a known transporter of STAT3 into the nucleus, showcasing a role of inflammation on the STAT3 pathway (48). In a mouse xenograft model of TNBC, blocking of STAT3 signaling was found to reduce STAT3 translocation into the nucleus, reduction of the epithelial mesenchymal transition, and promoted apoptosis of TNBC cells (48). As STAT3 has been indicated in both obesity and involution, further exploration of the combined effect of these two independent variables is warranted.
3.3 Adipokine alterations
In obesity, or under positive energy balance, adipocytes continuously take up free fatty acids from circulation (49). This leads to an increase in adipocyte size and crowding of adipocytes within tissues (49). As adipocytes expand, there is a decrease in oxygen availability for normal metabolic processes, which leads to hypoxia (49). Depletion or lack of oxygen available to adipose tissue has been shown to increase the development of insulin resistance and extra cellular matrix remodeling through increased secretion of leptin and decreased adiponectin gene expression (50). All these changes contribute to a pro-tumorigenic environment.
Mammary glands are composed predominantly of adipose tissue (28). In individuals who are considered overweight or obese, there is increased adiposity in the mammary glands (51). Adipose tissue secretes hormones and cytokines called adipokines (51). These hormones are a relatively new discovery with the first adipokine, leptin, being identified in the 1990s (52). While several adipokines and cytokines are released from the adipose tissue in the mammary gland, breast cancer research has focused predominantly on the effects of leptin and adiponectin, with new research emerging on the role of resistin (37, 53, 54). The role these adipokines play during and after involution has not been studied.
Leptin: It has been shown that pre-adipocytes and mature adipocytes secrete leptin (53). Secretion of leptin triggered by excess energy binds to the leptin receptor on the cell membrane (53) and signals the brain to reduce energy intake (55). However, overexpression of the leptin receptor and overstimulation of leptin secretion has been linked to the development of breast cancer, which could be related to higher calorie intake leading to increased adiposity (54). In addition, in postmenopausal breast cancer patients (n=42) there was increased leptin secretion compared to healthy controls (53). This increased leptin secretion was positively correlated to faster cancer progression, metastasis, and poor survival rates (53). Leptin-deficient mouse models have demonstrated reduced tumor growth rates and decrease in tumor size (56). Binding of leptin to its receptor activates multiple signaling pathways such as PI3K/AKT, MAPK/ERK1/2 and JAK/STAT, the key signaling pathways in TNBC leading to cell proliferation, migration, differentiation, anti-apoptosis, and stemness (54), connecting obesity, specifically leptin to increased tumorigenesis.
Adiponectin: Adiponectin is produced and secreted by adipose tissue, and an inverse correlation between adiponectin and breast cancer risk has been shown (57). It is known for its insulin-sensitizing properties, as well as regulating immune responses (56). Adiponectin was shown to prevent cell proliferation and promote apoptosis through inhibiting the AKT pathway as well as promoting increased reliance on fatty acids for energy in TNBC cell lines (57). While adiponectin can promote TNBC cell death, the role of adiponectin in breast cancer is still controversial and warrants further investigation. One study of breast cancer patients in Germany a positive correlation of adiponectin levels and increased breast cancer related mortality (58).
Resistin: Resistin is a relatively newer adipokine that has been investigated in connection to breast cancer risk (53). Resistin is secreted by peripheral blood mononuclear cells and macrophages in humans (53). It plays a role in the inflammatory process by targeting immune cells to increase proinflammatory cytokine production (59, 60). Studies have shown increased levels of resistin in obese humans and rodents (59). Based on the role of resistin in inflammation, a link has been proposed between resistin and insulin resistance, although overall data is inconclusive (61, 62). Resistin has been shown to increase stemness in TNBC cell populations through activation of STAT3 (59, 60). In adipocyte stem cells, resistin enhanced properties of invasion, proliferation, and mesenchymal transition when co-cultured with a TNBC cell line (60).
4 Discussion
Short-term breastfeeding (abrupt mammary gland involution) and obesity are highly metabolic processes that have been independently associated with increased breast cancer risk (10, 12, 21, 35, 36). In this review, we sought to provide a comparison of the mechanisms underlying these two independent risk factors, revealing numerous overlapping impacts within the breast tissue. Obesity and lack of breastfeeding following full-term pregnancy both appear lead to greater acute and chronic localized inflammation within the mammary gland related to macrophage infiltration, changes in epithelial cell population, and STAT3 activation. This supports the need to consider these two separate processes in concert and, further, whether the inflammatory effects are overlapping, additive, or synergistic.
In the US, racial disparities in mortality rates are significant after breast cancer diagnosis (8). Even with similar rates of incidence between non-Hispanic White women (NHW) and AAW, the latter face 40% higher death rates (6). This can be attributed to multiple factors, including higher poverty rates and pervasive systemic racism that undermines access to screenings and superior treatment options (6). Further incidences for high mortality rates for AAW include lack of medical coverage, barriers in accessibility to early detection and screening, more advanced stage when diagnosed, and unequal access to improvements in treatment (63).
A biological reason that contributes to the higher mortality seen in AAW is the higher incidence of the aggressive TNBC (64). While 48% of patients diagnosed with TNBC were found to have BRCA1 mutation, the frequency of BRCA1 mutations in AAW with TNBC is 27.9% compared to 46.2% in NHW women (65–68). AAW have multiple overlapping modifiable behavioral risk factors that contribute to increased risk of TNBC and worse outcomes, including: lack of breastfeeding, shorter duration of breastfeeding, as well as higher rates of premenopausal obesity (BMI ≥ 30 kg/m2) (11). Breastfeeding rates amongst AAW are far below the recommendation from pediatric health experts (69). There are many factors that lead to this disparity, such as a lack of breastfeeding education and social and familial breastfeeding support (69). The AMBER consortium was a large collaborative initiative funded by the National Cancer Institute (NCI) to understand lifestyle and genetic risk factors of breast cancer in the AAW population (13). Studies have shown that among AAW increases number of parities led to reduction of breastfeeding initiation and shorter duration of breastfeeding (70). Interestingly, the heightened risk of TNBC due to multiparity in AAW is reversed if individuals chose to breastfeed (18, 71).
While more research needs to be conducted on the lasting effects of abrupt involution, research on the combination of these two independent risk factors is imperative. By understanding the individual and combination effects of obesity and abrupt involution, intervention strategies against the detrimental effects can be developed for women who cannot or chose not to breastfeed. Research at the intersection of these two independent risk factors can impact all women who cannot or choose not to breastfeed and particularly impact AAW who have higher rates of pre-menopausal obesity, lower rates of breastfeeding, and higher incidence and mortality associated with TNBC (12). Ultimately, understanding of the combinatorial effect and development of an intervention may help to reduce racial disparities in breast cancer.
Author contributions
KO: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AK: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. GS: Visualization, Writing – review & editing. SA: Writing – review & editing. ES: Conceptualization, Writing – review & editing, Supervision. SM: Conceptualization, Writing – review & editing. KS: Supervision, Writing – review & editing. RG: Funding acquisition, Writing – review & editing. BR: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding provided through NIH RO1CA231857yg from joint MPI of BR and RG.
Acknowledgments
The authors would like to thank Angela Dahlberg, editor in the Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, for editing the manuscript. Authors KO and BR co-directed this work.
In memoriam
We would like to dedicate this work to Dr. Bhuvaneswari Ramaswamy, who passed away after a heroic battle with breast cancer. She was incredibly passionate about her patients and research to help those with breast cancer.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kibugu-Decuir P. Breast Cancer now most common form of cancer: WHO taking action. Switzerland: The New Times (2021). Available at: https://www.proquest.com/docview/2494896663.
2. Cancer of the Breast (Female) - Cancer Stat Facts. SEER. Available at: https://seer.cancer.gov/statfacts/html/breast.html.
3. Breast cancer statistics. In: How Common is Breast Cancer? (2024). Atlanta, GA. Available at: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html.
4. Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer (1). N Engl J Med. (1992) 327:319–28. doi: 10.1056/NEJM199207303270505
5. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
7. American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc (2019).
8. Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. (2015) 15:248–54. doi: 10.1038/nrc3896
9. Hon JDC, Singh B, Sahin A, Du G, Wang J, Wang VY, et al. Breast cancer molecular subtypes: from TNBC to QNBC. Am J Cancer Res. (2016) 6:1864–72.
10. Shinde SS, Forman MR, Kuerer HM, Yan K, Peintinger F, Hunt KK, et al. Higher parity and shorter breastfeeding duration: Association with triple-negative phenotype of breast cancer. Cancer. (2010) 116:4933–43. doi: 10.1002/cncr.v116:21
11. Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE. Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer. (2014) 14:62. doi: 10.1186/1471-2407-14-62
12. Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. (2014) 106:dju237. doi: 10.1093/jnci/dju237
13. Allott EH, Geradts J, Cohen SM, Khoury T, Zirpoli GR, Bshara W, et al. Frequency of breast cancer subtypes among African American women in the AMBER consortium. Breast Cancer Res. (2018) 20:1–9. doi: 10.1186/s13058-018-0939-5
14. van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. (2000) 152:514–27. doi: 10.1093/aje/152.6.514
15. Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. (2001) 91:421–30. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1053>3.0.CO;2-T
16. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. (2008) 371:569–78. doi: 10.1016/S0140-6736(08)60269-X
17. Rahman M, Temple JR, Breitkopf CR, Berenson AB. Racial differences in body fat distribution among reproductive-aged women. Metabolism. (2009) 58:1329–37. doi: 10.1016/j.metabol.2009.04.017
18. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. (2006) 295:2492–502. doi: 10.1001/jama.295.21.2492
19. McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev. (2013) 22:1227–38. doi: 10.1158/1055-9965.EPI-12-1432
20. Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. (2015) 150:655–66. doi: 10.1007/s10549-015-3353-z
21. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet Lond Engl. (2002) 360:187–95. doi: 10.1016/S0140-6736(02)09454-0
22. Radisky DC, Hartmann LC. Mammary involution and breast cancer risk: transgenic models and clinical studies. J Mammary Gland Biol Neoplasia. (2009) 14:181–91. doi: 10.1007/s10911-009-9123-y
23. Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res. (2014) 16:1–14. doi: 10.1186/bcr3633
24. Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest. (2014) 124. doi: 10.1172/JCI73777
25. O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. (2010) 176:1241–55. doi: 10.2353/ajpath.2010.090735
26. Jindal S, Narasimhan J, Borges VF, Schedin P. Characterization of weaning-induced breast involution in women: implications for young women’s breast cancer. NPJ Breast Cancer. (2020) 6:55. doi: 10.1038/s41523-020-00196-3
27. Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. (2012) 1:533–57. doi: 10.1002/wdev.v1.4
28. Zwick RK, Rudolph MC, Shook BA, Holtrup B, Roth E, Lei V, et al. Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nat Commun. (2018) 9:3592. doi: 10.1038/s41467-018-05911-0
29. Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. (2015) 142:1028–42. doi: 10.1242/dev.087643
30. Guo Q, Betts C, Pennock N, Mitchell E, Schedin P. Mammary gland involution provides a unique model to study the TGF-β Cancer paradox. J Clin Med. (2017) 6:10. doi: 10.3390/jcm6010010
31. Basree MM, Shinde N, Koivisto C, Cuitino M, Kladney R, Zhang J, et al. Abrupt involution induces inflammation, estrogenic signaling, and hyperplasia linking lack of breastfeeding with increased risk of breast cancer. Breast Cancer Res BCR. (2019) 21:80. doi: 10.1186/s13058-019-1163-7
32. Hennigar SR, Velasquez V, Kelleher SL. Obesity-induced inflammation is associated with alterations in subcellular zinc pools and premature mammary gland involution in lactating mice. J Nutr. (2015) 145:1999–2005. doi: 10.3945/jn.115.214122
33. Chamberlin T, Thompson V, Hillers-Ziemer LE, Walton BN, Arendt LM. Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage-mediated extracellular matrix remodeling. FASEB J. (2020) 34:8611–24. doi: 10.1096/fj.202000228RR
34. Berger ER, Iyengar NM. Obesity and energy balance considerations in triple-negative breast cancer. Cancer J. (2021) 27:17–24. doi: 10.1097/PPO.0000000000000502
35. Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. (2015) 7:301ra130. doi: 10.1126/scitranslmed.3010467
36. Incio J, Ligibel JA, McManus DT, Suboj P, Jung K, Kawaguchi K, et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci Trans Med. (2018) 10:eaag0945. doi: 10.1126/scitranslmed.aag0945
37. Sabol RA, Bowles AC, Côté A, Wise R, O'Donnell B, Matossian MD, et al. Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Res. (2019) 21:67. doi: 10.1186/s13058-019-1153-9
38. Chang MC, Eslami Z, Ennis M, Goodwin PJ. Crown-like structures in breast adipose tissue of breast cancer patients: associations with CD68 expression, obesity, metabolic factors and prognosis. NPJ Breast Cancer. (2021) 7:97. doi: 10.1038/s41523-021-00304-x
39. Li Q, Spalding KL. The regulation of adipocyte growth in white adipose tissue. Front Cell Dev Biol. (2022) 10:1003219. doi: 10.3389/fcell.2022.1003219
40. Maliniak ML, Cheriyan AM, Sherman ME, Liu Y, Gogineni K, Liu J, et al. Detection of crown-like structures in breast adipose tissue and clinical outcomes among African-American and White women with breast cancer. Breast Cancer Res. (2020) 22:65. doi: 10.1186/s13058-020-01308-4
41. Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat. (2016) 158:113–26. doi: 10.1007/s10549-016-3847-3
42. Tiwari P, Blank A, Cui C, Schoenfelt KQ, Zhou G, Xu Y, et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med. (2019) 216:1345–58. doi: 10.1084/jem.20181616
43. Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. (2004) 6:1–17. doi: 10.1186/bcr753
44. O'Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. (2012) 139:269–75. doi: 10.1242/dev.071696
45. Elder AM, Tamburini BA, Crump LS, Black SA, Wessells VM, Schedin PJ, et al. Semaphorin 7A promotes macrophage-mediated lymphatic remodeling during postpartum mammary gland involution and in breast cancer. Cancer Res. (2018) 78:6473–85. doi: 10.1158/0008-5472.CAN-18-1642
46. Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer. (2015) 136:1803–13. doi: 10.1002/ijc.v136.8
47. Ibrahim SA, Gadalla R, El-Ghonaimy EA, Samir O, Mohamed HT, Hassan H, et al. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer. (2017) 16:57. doi: 10.1186/s12943-017-0621-z
48. Qi Y, Wu H, Zhu T, Liu Z, Liu C, Yan C, et al. Acetyl-cinobufagin suppresses triple-negative breast cancer progression by inhibiting the STAT3 pathway. Aging (Albany NY). (2023) 15:8258–74. doi: 10.18632/aging.204967
49. Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. (2014) 157:1339–52. doi: 10.1016/j.cell.2014.05.012
50. Ali MM, Hassan C, Masrur M, Bianco FM, Naquiallah D, Mirza I, et al. Adipose tissue hypoxia correlates with adipokine hypomethylation and vascular dysfunction. Biomedicines. (2021) 9:1034. doi: 10.3390/biomedicines9081034
51. Hillers-Ziemer LE, Arendt LM. Weighing the risk: effects of obesity on the mammary gland and breast cancer risk. J Mammary Gland Biol Neoplasia. (2020) 25:115–31. doi: 10.1007/s10911-020-09452-5
52. Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. (2015) 36:461–70. doi: 10.1016/j.tips.2015.04.014
53. Li J, Han X. Adipocytokines and breast cancer. Curr Probl Cancer. (2018) 42:208–14. doi: 10.1016/j.currproblcancer.2018.01.004
54. Thiagarajan PS, Zheng Q, Bhagrath M, Mulkearns-Hubert EE, Myers MG, Lathia JD, et al. STAT3 activation by leptin receptor is essential for TNBC stem cell maintenance. Endocrine-related Cancer. (2017) 24:415–26. doi: 10.1530/ERC-16-0349
55. Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. (2015) 64:24–34. doi: 10.1016/j.metabol.2014.08.004
56. Tumminia A, Vinciguerra F, Parisi M, Graziano M, Sciacca L, Baratta R, et al. Adipose tissue, obesity and adiponectin: role in endocrine cancer risk. Int J Mol Sci. (2019) 20:2863. doi: 10.3390/ijms20122863
57. Pham DV, Park PH. Adiponectin triggers breast cancer cell death via fatty acid metabolic reprogramming. J Exp Clin Cancer Res. (2022) 41:9. doi: 10.1186/s13046-021-02223-y
58. Obi N, Jung AY, Maurer T, Huebner M, Johnson T, Behrens S, et al. Association of circulating leptin, adiponectin, and resistin concentrations with long-term breast cancer prognosis in a German patient cohort. Sci Rep. (2021) 11:23526. doi: 10.1038/s41598-021-02958-w
59. Deshmukh SK, Srivastava SK, Zubair H, Bhardwaj A, Tyagi N, Al-Ghadhban A, et al. Resistin potentiates chemoresistance and stemness of breast cancer cells: Implications for racially disparate therapeutic outcomes. Cancer Lett. (2017) 396:21–9. doi: 10.1016/j.canlet.2017.03.010
60. Wang YY, Hung AC, Wu YC, Lo S, Chen HD, Chen YK, et al. ADSCs stimulated by resistin promote breast cancer cell Malignancy via CXCL5 in a breast cancer coculture model. Sci Rep. (2022) 12:15437. doi: 10.1038/s41598-022-19290-6
61. Su KZ, Li YR, Zhang D, Yuan JH, Zhang CS, Liu Y, et al. Relation of circulating resistin to insulin resistance in type 2 diabetes and obesity: a systematic review and meta-analysis. Front Physiol. (2019) 10:1399. doi: 10.3389/fphys.2019.01399
62. Siddiqui K, Joy SS, George TP. Circulating resistin levels in relation with insulin resistance, inflammatory and endothelial dysfunction markers in patients with type 2 diabetes and impaired fasting glucose. Endocrine Metab Sci. (2020) 1:100059. doi: 10.1016/j.endmts.2020.100059
63. Yedjou C, Tchounwou P, Payton M, Miele L, Fonseca D, Lowe L, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health. (2017) 14:486. doi: 10.3390/ijerph14050486
64. Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. (2009) 113:357–70. doi: 10.1007/s10549-008-9926-3
65. Lee E, McKean-Cowdin R, Ma H, Spicer DV, Van Den Berg D, Bernstein L, et al. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young women. J Clin Oncol. (2011) 29:4373. doi: 10.1200/JCO.2010.33.6446
66. Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. (2009) 115:2222–33. doi: 10.1002/cncr.24200
67. Nanda R, Schumm LP, Cummings S, Fackenthal JD, Sveen L, Ademuyiwa F, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. Jama. (2005) 294:1925–33. doi: 10.1001/jama.294.15.1925
68. Greenup R, Buchanan A, Lorizio W, Rhoads K, Chan S, Leedom T, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol. (2013) 20:3254–8. doi: 10.1245/s10434-013-3205-1
69. Spinelli MG, Endicott J, Goetz RR. Increased breastfeeding rates in black women after a treatment intervention. Breastfeed Med Off J Acad Breastfeed Med. (2013) 8:479–84. doi: 10.1089/bfm.2013.0051
70. Kim JH, Fiese BH, Donovan SM. Breastfeeding is natural but not the cultural norm: A mixed-methods study of first-time breastfeeding, African American mothers participating in WIC. J Nutr Educ Behav. (2017) 49:S151–S161.e1. doi: 10.1016/j.jneb.2017.04.003
Keywords: racial disparities, triple negative breast cancer, obesity, involution, breastfeeding
Citation: Ormiston K, Kulkarni A, Sarathy G, Alsammerai S, Shankar E, Majumder S, Stanford KI, Ganju RK and Ramaswamy B (2024) Obesity and lack of breastfeeding: a perfect storm to augment risk of breast cancer? Front. Oncol. 14:1432208. doi: 10.3389/fonc.2024.1432208
Received: 13 May 2024; Accepted: 30 September 2024;
Published: 25 October 2024.
Edited by:
Liza Makowski, University of Tennessee Health Science Center (UTHSC), United StatesCopyright © 2024 Ormiston, Kulkarni, Sarathy, Alsammerai, Shankar, Majumder, Stanford, Ganju and Ramaswamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anagh Kulkarni, YW52a2t1bGthcm5pQGdtYWlsLmNvbQ==; Sara Alsammerai, c2FyYWxzYW1teTE2QGdtYWlsLmNvbQ==
†Deceased
 Kate Ormiston
Kate Ormiston Anagh Kulkarni1*
Anagh Kulkarni1* Kristin I. Stanford
Kristin I. Stanford Ramesh K. Ganju
Ramesh K. Ganju Bhuvaneswari Ramaswamy
Bhuvaneswari Ramaswamy