- 1Department of Internal Medicine, King Hussein Cancer Center, Amman, Jordan
- 2School of Medicine, The University of Jordan, Amman, Jordan
- 3Department of Cell Therapy and Applied Genomics, King Hussein Cancer Center, Amman, Jordan
- 4Department of Radiology, King Hussein Cancer Center, Amman, Jordan
Breast cancer is the most common cancer among women worldwide, and its incidence rate is still increasing, especially among younger women. Nationally, it constitutes one-fifth of all cancer cases and almost 40% of all female cancers. With a median age of 51 years, breast cancer is diagnosed at least a decade earlier, and at more advanced stages compared to Western societies. Hereditary cancers account for 10% or more of all cancer burden worldwide. With expanded indications, increased number of genes tested, and significant decline in cost of testing, such proportion will probably increase. Individuals with pathogenic variants of BRCA1 and BRCA2 are at higher risk of breast, ovarian, pancreatic and many other cancers. Over the past two decades, several highly penetrant cancer-susceptibility genes were identified across almost all tumor sites, thus increasing the need for comprehensive cancer genetic programs that address the testing process, counselling patients and at-risk family members, and then deal with all testing results and its consequences. In addition to its important role in preventing more cancers in index patients themselves and among their close relatives, identification of pathogenic or likely pathogenic variants, mostly in BRCA1 or BRCA2, may inform therapeutic decisions in common cancers including breast, ovarian, prostate and pancreatic cancers. In this manuscript, we describe the experience of a comprehensive cancer center, in a resource-limited country in establishing a comprehensive clinical cancer genetics program that can serve as an example for others who share similar demographic and financial restrains.
1 Introduction
Jordan is a small country (89,000 square km) located in the west part of the Middle East. The population has grown recently to over 11 million, affected mostly by the influx of refugees from neighboring countries (1). Much of the population is heavily concentrated around Amman, the capital, and in the northwest cities. Population of Jordan is relatively young, with a median age of 22.5 years and only 3.5% are 65 years and older (2). Jordan’s economy is among the smallest in the region with insufficient supplies of water and limited natural resources. High rate of unemployment, especially among younger individuals, adds to the economic challenges (3). The country is classified by the World Bank as a “lower middle-income country (LMIC)” with a Gross Domestic Product (GDP) of $48.65 billion and $4311.0 per capita (4).
King Hussein Cancer Center (KHCC) is a 352-bed stand-alone tertiary cancer center established in 2001 to serve Jordanians and patients from the region. The center covers cancer care dimensions across the continuum including screening and early detection, cancer prevention, active treatment, palliative/hospice care and survivorship. Additionally, the center is actively engaged in clinical research and serves as the regional hub for cancer-related medical training and education (5, 6).
Breast cancer is the most common cancer among women worldwide (7, 8). Recent data have suggested that the incidence rate of breast cancer increased during 2015-2019 by almost 1% annually (6, 7). Nationally, over 1750 new breast cancer cases were diagnosed and reported by the Jordan Cancer Registry in 2022. As such, it constitutes one-fifth of all cancer cases and almost 40% of all female cancers. Additionally, one fourth of all cancer-related mortality in women are due to breast cancer (9). With a median age of 50-52 years, breast cancer in Jordan, and most of the Arab world, is diagnosed at least a decade earlier compared to the West. To complicate the issue further, more than a third of patients present late with metastatic or locally advanced disease (10, 11).
2 Hereditary cancers
Hereditary cancers account for 10% or more of cancer burden worldwide (12). With our improving understanding of molecular biology, wider access to diagnostic technology and expanded indications for testing, such proportion will significantly increase. Over the past two decades, several highly penetrant cancer-susceptibility genes were identified, thus establishing the nidus for a new dimension in counselling and cancer prevention (13, 14). Additionally, identification of pathogenic or likely pathogenic (P/LP) variants may inform therapeutic decisions in common cancers including breast, ovarian, prostate and pancreatic cancers (15, 16).
Individuals with pathogenic variants of BRCA1 and BRCA2 are at higher risk of breast and ovarian cancers (17, 18). In one study that prospectively included a cohort of 978 BRCA1 and 909 BRCA2 pathogenic carriers from the United Kingdom, the average cumulative risks by age 70 years for BRCA1 carriers were estimated to be 60% (95% confidence interval [CI], 44%-75%) for breast cancer and 59% (95% CI, 43%-76%) for ovarian cancer. Women with BRCA2 pathogenic variants had a corresponding risk of 55% (95% CI, 41%-70%) and 16.5% (95% CI, 7.5%-34%) for breast and ovarian cancers, respectively (19). The estimated risk for contralateral breast cancer is 83% (95% CI, 69%-94%) for BRCA1 carrier and 62% (95% CI, 44%-79.5%) for BRCA2. A meta-analysis of ten eligible studies that looked at the penetrance rates of BRCA1 and BRCA2 reached similar conclusions (20).
Despite the undebatable importance of germline genetic testing, access and uptake of such services is limited and mostly dependent on financial and psychosocial structure of particular society or country.
3 Genetic testing
3.1 The start: from research to clinical practice
The process of genetic counselling and testing at KHCC started as a research project in 2016. Through a competitive grant from KHCC and MD Anderson Cancer Center Sister Institution Network Fund (SINF), we enrolled 100 young Jordanian breast cancer women (median age, 40 years) who were considered at higher risk of harboring a germline variant and testing was carried at a commercial lab (Myriad Genetics, Salt Lake City, Utah). In total, 27 (27.0%) patients had pathogenic or likely pathogenic BRCA1 or BRCA2 variants; the only two variants were tested at that time. Higher mutation rates were observed among patients with triple negative disease and those with positive family history of breast or ovarian cancers (21). Giving the research setup under which the genetic testing was performed, the process was highly regulated and monitored by our Institutional Review Board (IRB) which enhanced our capabilities to convert the testing process to a clinical service. Several issues including patients’ consenting, pre- and post-genetic testing counselling, communication with close relatives and cascade testing were all improved.
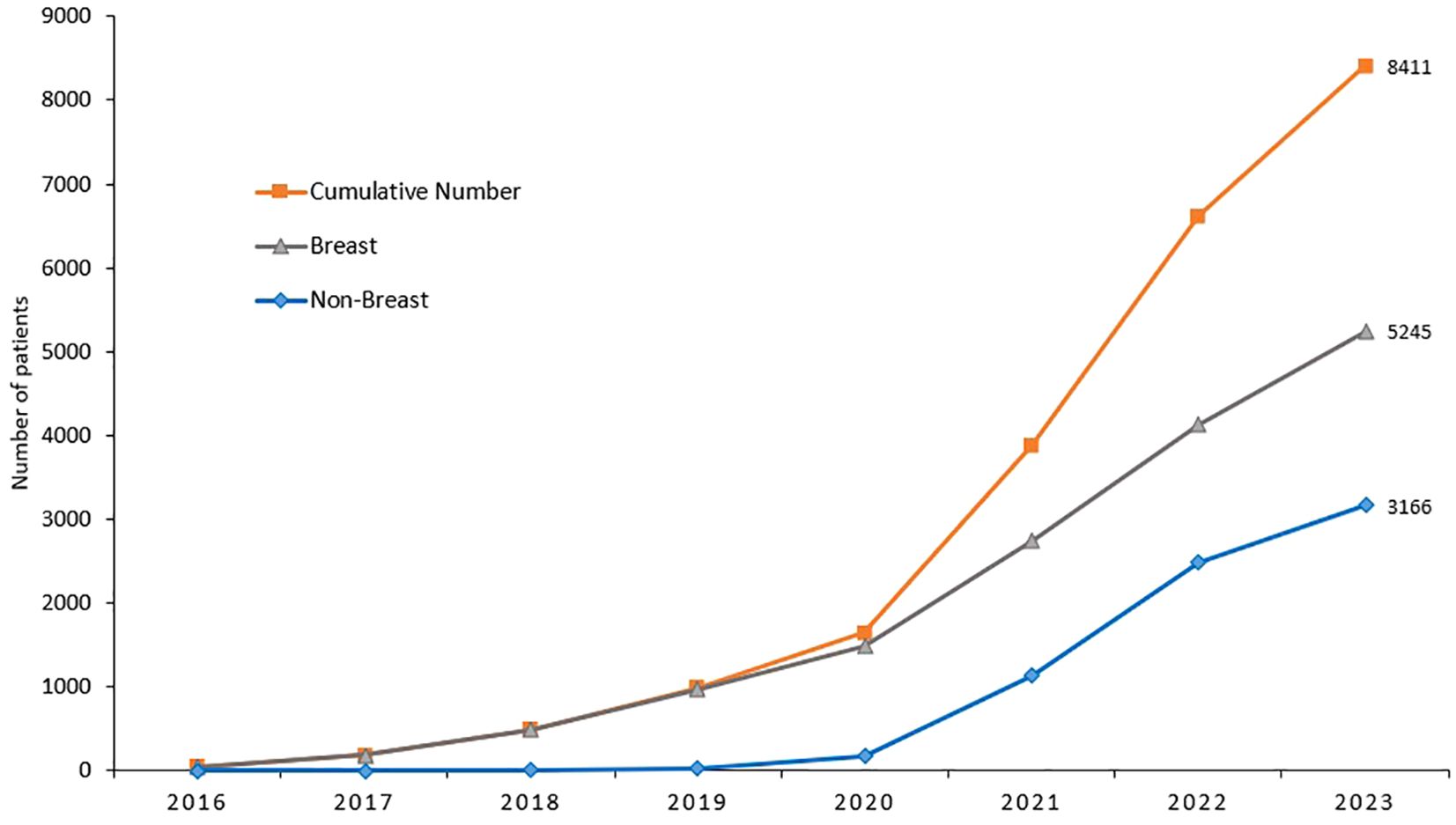
Though the cost of genetic testing has declined significantly over the past few years, access can still be a financial burden. Recent advances in molecular diagnostics and its clinical implications on cancer management have put lots of pressure on resource-limited countries. Soon after the completion of our study, genetic testing and counselling are routinely practiced in our center across all primary tumor sites, not only breast cancer, and according to international guidelines. Cost of germline genetic testing is mostly covered by cancer-specific insurance. Since we started, over 9,000 cancer patients were tested; over half of them are breast cancers, Figures 1, 2. It’s important to highlight here that very few patients refuse germline genetic testing, both for research purposes or when clinically indicated. However, the uptake of cascade testing for at-risk “healthy” relative is lower than anticipated. Cost of testing and potential risk-reducing interventions continue to be important barriers.
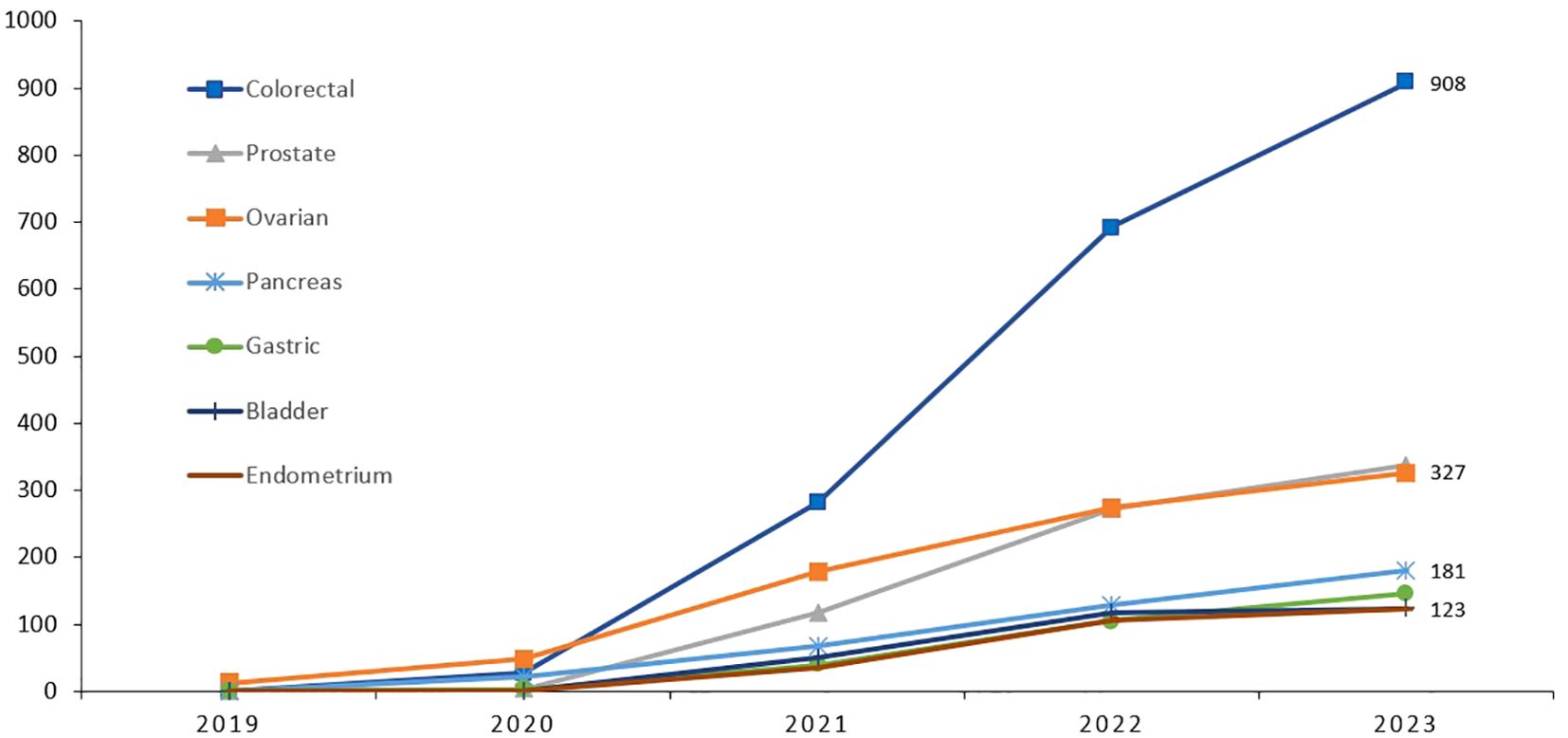
Figure 2. Cumulative number of patients with cancers other than breast, who had germline genetic testing illustrating the significant increase in uptake.
Such “comprehensive” service is not readily available elsewhere in the country for almost half of cancer patients treated outside our institution and when performed, it’s only the testing part and mostly without proper pre- or post-testing counselling and mostly without cascade testing for at-risk family members. Patients’ referral to centers where such service is provided is not easy; logistics related to insurance coverage and communication with patients themselves and close relatives are among the main issues encountered.
3.2 Eligible patients
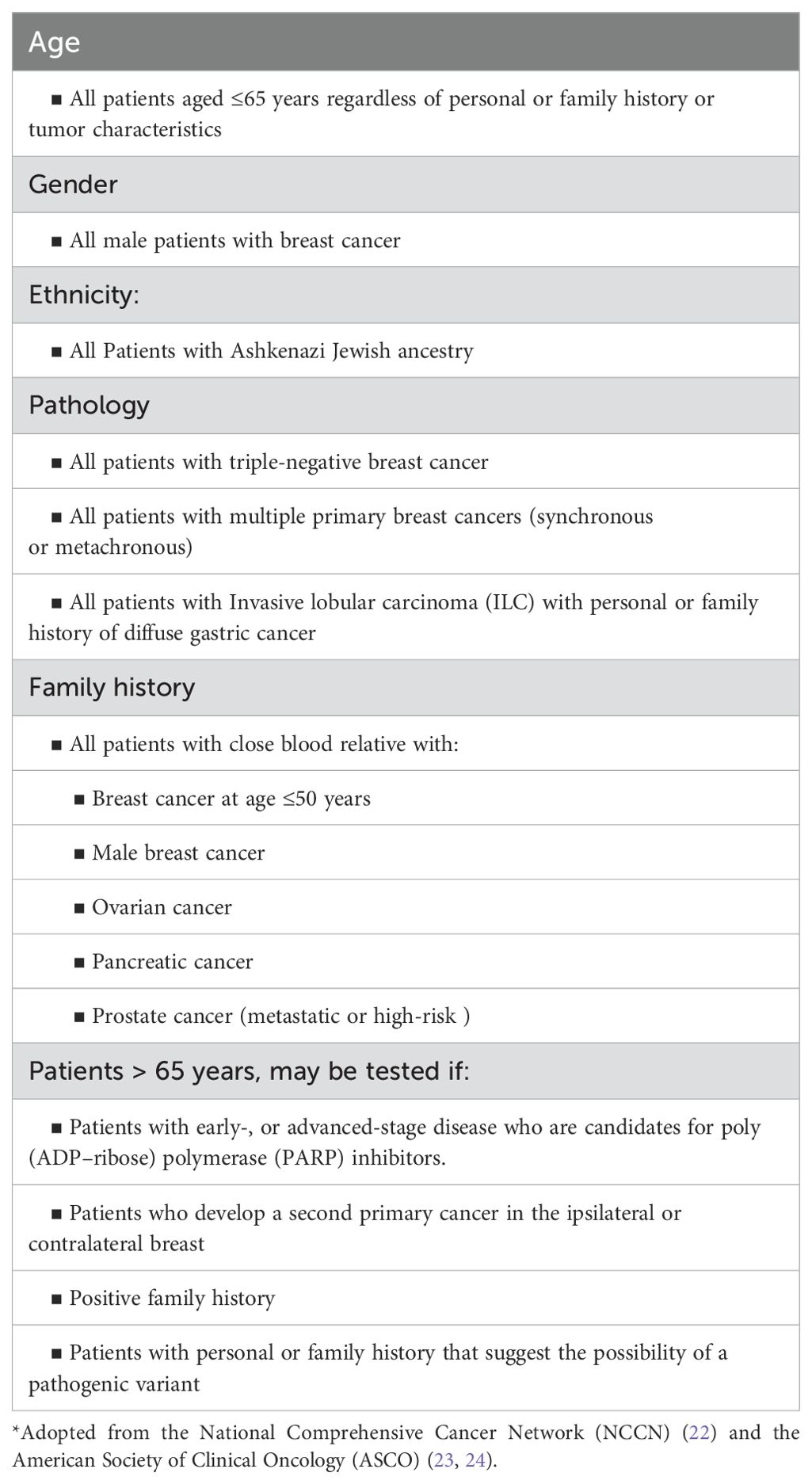
We follow the National Comprehensive Cancer Network (NCCN) and other international guidelines, including the American Society of Clinical Oncology (ASCO), for germline genetic testing (22–24). These guidelines are frequently updated and not all physicians, including medical and surgical oncologists, are familiar with such very frequent updates; a factor that may contribute to the lower referral of eligible patients for testing and counselling. To enhance referral rate, germline genetic testing was included as a KPI (Key Performance Indicator) and was added to the discussion points during our weekly multidisciplinary team meetings of each new breast cancer patient. Over the past few years, the age at which the NCCN recommends commence testing, regardless of personal or family history of cancer, was raised from 40 years, to 45, then 50, and more recently was raised to 65 by the ASCO and the Society of Surgical Oncology (ASO). Current indications for genetic testing are summarized in Table 1.
Though we advocate using guidelines-based testing, some clinicians and researchers advocate universal testing of all women with breast cancer. Such a new direction is supported by several recent publications that showed higher rates of missed opportunities, should we restrict testing to those suggested by the guidelines (25–27). The American Society of Breast Surgeons endorsed this universal testing approach (28).
The new expanded testing guidelines will probably include over 80% of patients with breast cancer. Given the younger age at breast cancer diagnosis, and the very low percentage of Jordanians above the age of 65 years (less than 5%), we expect that current guidelines would cover over 90 or 95% of all newly diagnosed breast cancer patients.
3.3 Variants of uncertain significance
At the initial phases of setting up our program, genetic testing was restricted to BRCA1 and BRCA2 which was expanded initially to include PALB2 and CHEK2. However, currently we test for 19 genes (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, TP53). With expanded gene testing, we faced the problem with high percentage of Variants of Uncertain Significance (VUS) which reached rates beyond 50%. Such high rates can be explained by the under representation of “Arabs” in international genetic variants libraries (29). As more information becomes available, some of the VUS can be modified and upgraded to “pathogenic”. Though few, so far, such changes put lots of pressure on health care systems to be able to communicate such upgrades with patients tested months or even years later. To help avoid any mishaps in communicating these results, and its associated clinical decisions, we involve the patients themselves and recommend they also check with their health care providers with subsequent follow up visits; a statement that is included in the consent form, too.
3.4 Genetic counselling
There isn’t any formal educational or training programs in the country that graduate genetic counselors. As such, medical and surgical oncologists were the ones who started the process of pre- and post-testing counselling. However, as we can imagine, time needed for this process is difficult to allocate in a busy schedule of a surgeon or a medical oncologist. To help solve this problem, we managed to train health care workers with basic education in molecular biology, pharmacy or nursing, to become “clinical counsellors”. Currently, 3 genetic counselors are running daily “genetic counseling clinics” independently. However, and given the increasing number of patients tested, we also established cancer genetics clinics that are run daily by medical oncologists; some were trained at one of our international collaborative institutions. Our hybrid approach (counselors and medical oncologists) is unique and can serve as an example of a multidisciplinary approach, especially in resource-limited countries.
4 Local data
In one of our studies, a total of 1310 patients with breast cancer were tested as per the NCCN guidelines. Age ≤ 45 years was the most common indication for testing (n= 800, 61.1%), while positive family history of breast, ovarian, pancreatic or prostate cancers, and triple-negative disease were among other common indications. Among the whole group, 184 (14.0%) patients had P/LP variants with only 90 (48.9%) were in BRCA1 or BRCA2, while the others had pathogenic variants in other genes including APC, TP53, CHEK2 and PALB2. Variants of uncertain significance (VUS) were reported in 559 (42.7%) patients; majority (90.7%) were in genes other than BRCA1 or BRCA2 (30).
In another study that involved 616 younger patients diagnosed with breast cancer at age 40 or younger; 75 (12.2%) patients had P/LP variants; two of the BRCA2 mutations were novel. In multivariate analysis, triple−negative disease (Odd Ratio [OR]: 5.37; 95% CI, 2.88–10.02, P< 0.0001), family history of breast cancer in ≥ 2 family members (OR: 4.44; 95% CI, 2.52–7.84, P< 0.0001), and a personal history ≥ 2 primary breast cancers (OR: 3.43; 95% CI, 1.62–7.24, P= 0.001) were associated with higher mutation rates (31).
More recently, our group enrolled a total of 3,319 unselected patients with solid tumors, regardless of their personal or family history of cancer on a universal cancer germline genetic testing study, 1672 (50.4%) had breast cancer. Patients were classified into two groups; those who met the NCCN 2020 testing criteria and those who did not. Pathogenic germline variants were detected in 14.5% of the 1125 breast cancer who met the testing criteria compared to 8.0% of 547 patients who did not. Common variants identified are summarized in Figure 3 (32).
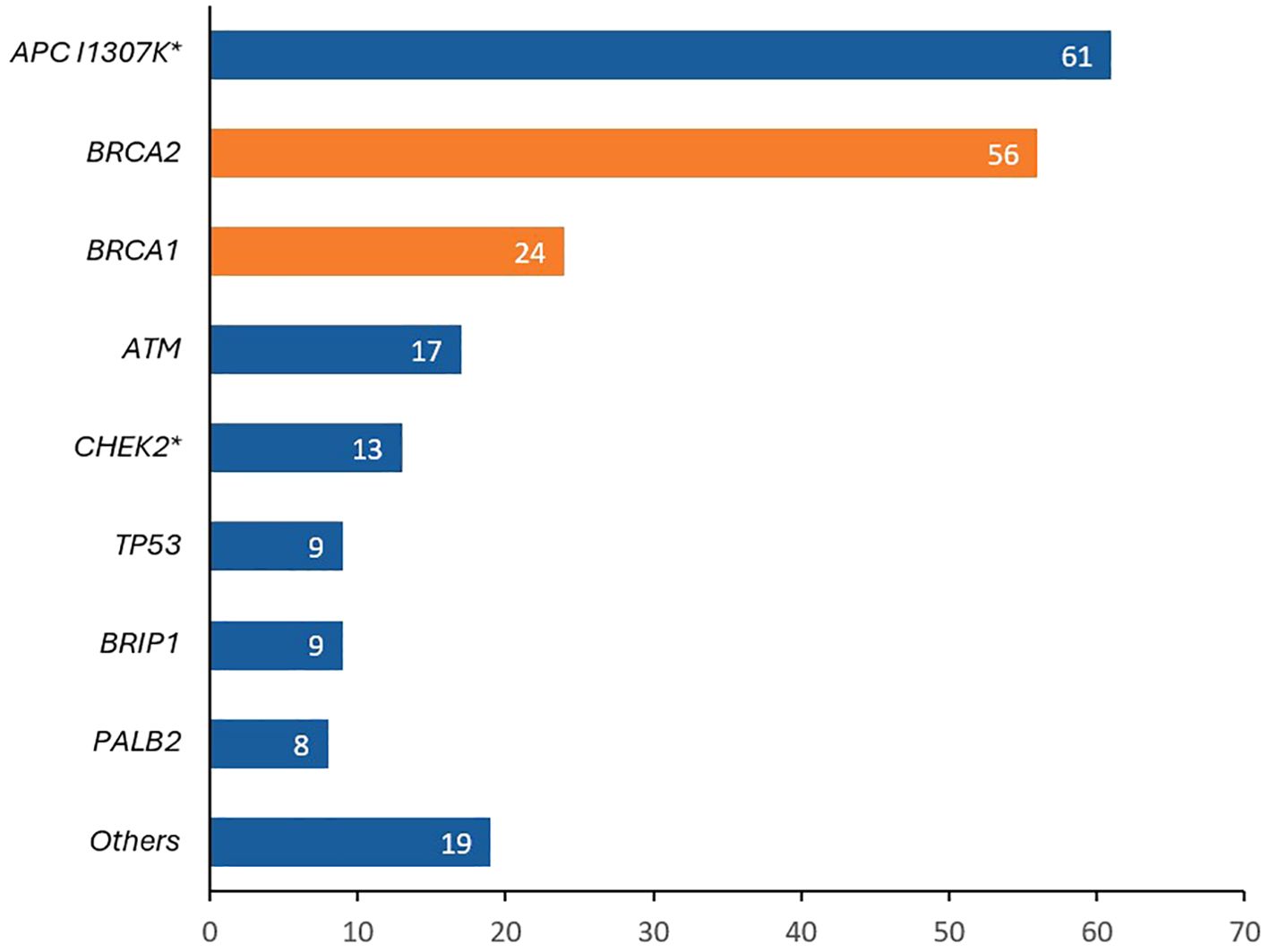
Figure 3. Pathogenic/likely pathogenic (P/LP) variants detected in patients with breast cancer patients tested as part of a universal germline genetic testing program. APC I1307K was the most identified variant (more common than BRCA1 and BRCA2). *8 patients had biallelic APC I1307K, 1 patient had a biallelic CHEK2 and 1 patient had a biallelic PMS2.
5 Cascade testing
Following the establishment of testing guidelines across all primary cancer sites, the identification of the index case is never an issue. Patients’ pre-testing assessment includes a three-generation family tree and at-risk family members are identified by the genetic counselor. Patients with positive germline mutation are asked to communicate such results with at-risk relatives. Appointments with the genetic counselling clinic, and occasionally with the primary oncologist, are arranged. Those who wish to be tested, after appropriate consenting, are offered the test almost at no-cost, if performed within 90 days of the index case. The uptake rate for cascade testing was low but improving.
Additionally, we face significant obstacles in dealing with positive pathogenic mutation among unaffected carriers, too. In one observational cross-sectional study performed by our group to assess level of communication between patients and their at-risk relatives, 169 breast cancer patients with positive breast cancer-susceptibility germline variants, were invited to participate. The cohort included 42 (24.9%) with P/LP BRCA1 variants, 84 (49.7%) with BRCA2 and 43 (25.4%) with non-BRCA variants. Results of P/LP variants were communicated with family members by majority (n= 160, 94.7%), including 642 first degree female relatives, however, 286 (44.5%) of them have taken no actions. Fear of positive test results, cost of testing, unwillingness to undergo preventive measures, and social stigma were cited as barriers to genetic testing in 54%, 50%, 34% and 15%, respectively (33).
Extensive work is needed to increase awareness of patients and relatives about the associated risk and high impact of risk-reducing interventions. However, such awareness campaigns won’t be effective until health care systems provide access to genetic testing and risk-reducing interventions at reasonable cost.
6 Impact of genetic testing on medical management
Both BRCA1 and BRCA2 encode proteins that are critical for homologous recombination DNA repair (34). Thus, homologous recombination repair is defective in breast cancers with germline P/LP BRCA1 or BRCA2 variants (35). Poly(adenosine diphosphate-ribose) polymerase (PARP) is an intracellular enzyme that helps repair damaged DNA. Thus, PARP inhibitors can selectively damage tumor cells that is deficient in homologous recombination repair (36, 37).
Several randomized controlled clinical trials investigated the role of PARP inhibitors in patients with P/LP BRCA1 or BRCA2 variants. Based on positive outcomes in two phase 3 trials; OlympiAD (38, 39) and EMBRACA (40, 41), two PARP inhibitors, olaparib and talazoparib, have been approved by the European Medicines Agency (EMA) the US Food and Drug Administration (FDA) for use in patients with P/LP BRCA-mutated, HER2-negative metastatic breast cancer. In the OlympiaAD trial, olaparib improved progression-free survival (PFS) compared with chemotherapy [7.0 months vs 4.2 months; hazard ratio (HR) for disease progression or death, 0.58; 95% CI, 0.43-0.80; P< 0.001)]. Talazoparib, in the EMBRACA trial, prolonged PFS (HR= 0.542, 95% CI, 0.413-0.711; P< 0.0001). A meta-analysis had shown that the efficacy, safety and tolerability of talazoparib and olaparib were found to be comparable (42). Another PARP inhibitor, veliparib, was tried in the BROCADE3 trial conducted in a similar patient population of P/LP BRCA1/2 with advanced HER2-negative breast cancer. The addition of veliparib to platinum-based doublet chemotherapy, led to significant and durable PFS improvement (43, 44).
PARP inhibitors were also shown to be effective when used in the adjuvant setting in patients with early stage high-risk HER2-negative breast cancer with P/LP BRCA1/2 variants; patients in the OlympiA trial receiving adjuvant olaparib had significantly longer overall survival (OS); 4-year OS was 89.8% in the olaparib group and 86.4% in the placebo group (95% CI, 0.1% - 6.8%) (45, 46). Based on the above data, PARP inhibitors are currently recommended by the NCCN guidelines for all patients with P/LP BRCA1/2 variants with recurrent or stage IV disease and to a select of patients with early-stage disease, Table 2 (47).
7 International collaboration
Many researchers and scientists believe that germline genetic data can be viewed as a national commodity and can be considered a commercial enterprise. De-identified data should be made public and shared across the globe. However, international rules and regulations should be established at a global level to regulate the research conduct and ensure no deviations from the set goals. It’s anticipated that such data may help advance cancer research, accelerate discoveries, precision medicine and innovation at diagnostic, prognostic and therapeutic levels.
Such data sharing is more important in societies like ours, the population of which is underrepresented in the international genetic library and associated clinical trials (48). As an institution, through our journey to establish our program, we teamed up with internationally recognized centers including, MD. Anderson Cancer Center, Leeds cancer center and Princess Margert Hospital in Toronto.
8 Challenges
8.1 Awareness
Genetic counseling and testing, early detection, prevention, treatment and risk-reduction strategies in patients with pathogenic BRCA1/2 variants are not sufficiently known by most patients and family members. More importantly, knowledge of primary health care providers and “general” surgeons who deal with high number of breast cancer patients in their first encounter is often suboptimal (49–51). Following the full integration of our clinical cancer genetics program, detailed family history is fully integrated in patients’ assessment (52). To enhance referral to genetic testing and counselling, appropriate national guidelines, linked to action plans, are highly needed.
8.2 The stigma
Patients may experience constant anxiety about the genetic test results, associated cancer diagnosis and genetic transmission to their children. Fears of being stigmatized by friends, relatives, and partners may push high-risk individuals to refuse to be tested, and when tested might not share positive results with their at-risk family members. Through proper counselling, such fears might be lessened by experienced medical staff.
8.3 Confidentiality
The confidentiality of doctor-patient relationship was never an issue; however, confidentiality of medical records, being electronic, was highly considered a potential hazard. Access to medical records is granted to all health care workers and leak of information, though strictly prohibited by the law, is a possibility. During our initial phases of germline genetic testing, and to encourage patients to undergo testing, results were not entered into electronic records and were kept literally in a “safe closet”. However, as we gained experience with the counselling process and gained patients’ confidence and to improve communicating testing results that may affect patients’ medical and surgical management across different services, results are now made available in medical records similar to all other laboratory testing.
8.4 Financial coverage
There is no clear guidance within the national healthcare legislation and governmental insurance in Jordan regarding the genetic testing. Testing is not available in any of the MOH or military (Royal Medical Services) hospitals. Germline genetic testing, however, was done at one of the university hospitals as part of a research project (53). Patients treated in the private sector may elect to do the test, at their own cost, as almost all private insurance providers don’t cover for testing and its associated preventive strategies.
9 Risk-reducing surgeries
9.1 Contralateral mastectomy
Breast cancer patients with BRCA1/2 pathogenic variants are at higher risk for contralateral breast cancer. Risk-reducing mastectomy among such patients showed a significant reduction in contralateral disease and some studies showed a significant improvement in the risk of breast cancer-related death (54–56).
9.2 Bilateral mastectomy
Many studies, including two meta-analyses, showed that prophylactic bilateral mastectomy reduces the risk for breast cancer in unaffected women with BRCA1 [relative risk (RR)= 0.134, 95% CI, 0.019–0.937] and BRCA2 (RR= 0.183, 95% CI, 0.072–0.468) (54, 57). However, the effect of such prophylactic surgery on survival is debatable. Only one of the above cited meta-analyses showed improved survival. Extent of surgery can be as aggressive as total mastectomy, though less aggressive surgeries including skin-sparing mastectomy (SSM) and nipple-sparing mastectomy (NSM), both associated with better cosmoses, are considered safe (58).
No clear recommendation on the age of risk-reducing surgery (RRM), however, given the increasing risk of breast cancer with age, it’s recommended to consider women age, preference, life expectancy and family history including age of onset. Access to these procedures is limited, and such procedures for “unaffected” carriers are not covered by public or private insurance.
9.3 Salpingo-oophorectomy
In addition to reducing the risk of ovarian cancer, risk-reducing salpingo-oophorectomy (RRSO) may have beneficial effect on breast cancer risk reduction; more so when done at younger age (59).
One prospective, multicenter study included 2482 women with BRCA1 or BRCA2 P/LP variants from 22 centers in Europe and North America to assess the relationship of risk-reducing mastectomy or salpingo-oophorectomy with cancer outcomes. In addition to lowering the risk of ovarian cancer, RRSO resulted in significant reduction in new breast cancer cases; compared with women who did not undergo RRSO. Women who underwent RRSO had a lower risk of first diagnosis of breast cancer in BRCA1 mutation carriers; 20% vs 14% (HR= 0.63, 95% CI, 0.41-0.96) and BRCA2 mutation carriers; 23% vs 7% (HR= 0.36, 95% CI, 0.16-0.82). Additionally, women who underwent RRSO had lower all-cause mortality, compared to those who didn’t; 10% vs 3% (HR= 0.40, 95% CI, 0.26-0.61), breast cancer-specific mortality; 6% vs 2% (HR= 0.44, 95% CI, 0.26-0.76), and ovarian cancer-specific mortality; 3% vs 0.4% (HR= 0.21, 95% CI, 0.06-0.80) (60).
In a meta-analysis that included 13,965 BRCA1 and 7,057 BRCA2 mutation carriers enrolled on 14 observational studies, the breast cancer risk among BRCA1 mutation carriers was lowered by RRSO (HR = 0.63, 95% CI, 0.49-0.81, P < 0.01) and BRCA2 mutation carriers (HR= 0.51, 95% CI, 0.34-0.75, P < 0.01). This risk reduction was more apparent among younger patients and was apparent in the immediate 5 years after surgery in both BRCA1 and BRCA2 mutation carriers (61).
These findings should encourage physicians to recommend RRSO for BRCA1 and BRCA2 carriers while young, however not before they reach the age of 40-44 years, to allow them to finalize their family planning, if they wish to.
10 Conclusions
Breast cancer germline genetic testing, as part of a more comprehensive program, is a national priority. In addition to its valuable impact in preventing additional cancer diagnoses in patients themselves and at-risk relatives, identification of P/LP variants may inform clinical management and choice of therapy among affected patients. Access to such testing is improving, however, it needs to be done in an organized way that ensures appropriate counselling of patients and family members. Risk-reducing strategies, including prophylactic surgeries, when needed, should be addressed by the national health care system, and a highly needed cancer control program.
Author contributions
HA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. BS: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. FT: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. HH: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. OA: Writing – review & editing. HK: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. MA: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. SE: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. AM: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors acknowledge the support of Mrs. Alice Haddadin and Ms. Doaa Alsadi in preparing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. About Jordan (2016). UNFPA Jordan. Available online at: https://Jordan.unfpa.org/en/about-Jordan (Accessed April 30, 2024).
2. Jordan | Economic indicators. Moody’s analytics. Available online at: https://www.economy.com/Jordan/indicators (Accessed April 30, 2024).
3. Unemployment rate for Jordanians (Q4, 2023). Department of Statistics. Available online at: https://dosweb.dos.gov.jo/ (Accessed April 13, 2024).
4. World Bank Open Data. World bank open data. Available online at: https://data.worldbank.org/country/JO (Accessed January 16, 2024).
5. King Hussein Cancer Center. Available online at: https://www.khcc.jo/en (Accessed April 13, 2024).
6. Abdel-Razeq H, Barbar M, Shamieh O, Mansour A. Oncology medical training and practice: Managing Jordan’s brain drain through brain train-The King Hussein Cancer Center experience. JCO Glob Oncol. (2020) 6:1041–5. doi: 10.1200/GO.20.00141
7. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
8. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
9. Jordan Cancer Registry. Cancer incidence in Jordan (2015). Non-Communicable Diseases Directorate Ministry of Health, Jordan. Available online at: https://chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.moh.gov.jo/ebv4.0/root_storage/ar/eb_list_page/%D8%A7%D9%84%D8%AA%D9%82%D8%B1%D9%8A%D8%B1_%D8%A7%D9%84%D8%B3%D9%86%D9%88%D9%8A_%D9%84%D8%A7%D8%B5%D8%A7%D8%A8%D8%A7%D8%AA_%D8%A7%D9%84%D8%B3%D8%B1%D8%B7%D8%A7%D9%86_%D8%A7%D9%84%D9%85%D8%B3%D8%AC%D9%84%D8%A9_%D9%81%D9%8A_%D8%A7%D9%84%D8%A7%D8%B1%D8%AF%D9%86_%D9%84%D8%B9%D8%A7%D9%85_2022.pdf (Accessed 21 Sep, 2024).
10. Chouchane L, Boussen H, Sastry KSR. Breast cancer in Arab populations: molecular characteristics and disease management implications. Lancet Oncol. (2013) 14:e417–24. doi: 10.1016/S1470-2045(13)70165-7
11. Al-Shamsi HO, Abdelwahed N, Abyad A, Abu-Gheida I, Afrit M, Abu ElFuol T, et al. Breast cancer in the Arabian gulf countries. Cancers. (2023) 15:5398. doi: 10.3390/cancers15225398
12. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer — Analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. (2000) 343:78–85. doi: 10.1056/NEJM200007133430201
13. Couch FJ, Nathanson KL, Offit K. Two decades after BRCA: Setting paradigms in personalized cancer care and prevention. Science. (2014) 343:1466–70. doi: 10.1126/science.1251827
14. Kurian AW, Ward KC, Howlader N, Deapen D, Hamilton AS, Mariotto A, et al. Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J Clin Oncol. (2019) 37:1305–15. doi: 10.1200/JCO.18.01854
15. Maxwell KN, Domchek SM. Cancer treatment according to BRCA1 and BRCA2 mutations. Nat Rev Clin Oncol. (2012) 9:520–8. doi: 10.1038/nrclinonc.2012.123
16. Stoppa-Lyonnet D. The biological effects and clinical implications of BRCA mutations: where do we go from here? Eur J Hum Genet. (2016) 24:S3–9. doi: 10.1038/ejhg.2016.93
17. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. (2017) 317:2402. doi: 10.1001/jama.2017.7112
18. Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, Eisen A, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. (2006) 24:863–71. doi: 10.1200/JCO.2005.03.6772
19. Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers, results from prospective analysis of EMBRACE. J Natl Cancer Inst. (2013) 105:812–22. doi: 10.1093/jnci/djt095
20. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. (2007) 25:1329–33. doi: 10.1200/JCO.2006.09.1066
21. Abdel-Razeq H, Al-Omari A, Zahran F, Arun B. Germline BRCA1/BRCA2 mutations among high risk breast cancer patients in Jordan. BMC Cancer. (2018) 18:152. doi: 10.1186/s12885-018-4079-1
22. National Comprehensive Cancer Network (NCCN). Breast, ovarian and pancreatic cancer. Genetic/familial high risk assessment. Available online at: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (Accessed April 18, 2024).
23. Bedrosian I, Somerfield MR, Achatz MI, Boughey JC, Curigliano G, Friedman S, et al. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology Guideline. J Clin Oncol. (2024) 42:584–604. doi: 10.1200/JCO.23.02225
24. Kurian AW, Bedrosian I, Kohlmann WK, Somerfield MR, Robson ME. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology Guideline Q and A. JCO Oncol Pract. (2024) 20:466–71. doi: 10.1200/OP.23.00771
25. Sun L, Brentnall A, Patel S, Buist DSM, Bowles EJA, Evans DGR, et al. A cost-effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol. (2019) 5:1718. doi: 10.1001/jamaoncol.2019.3323
26. De Silva DL, Stafford L, Skandarajah AR, Sinclair M, Devereux L, Hogg K, et al. Universal genetic testing for women with newly diagnosed breast cancer in the context of multidisciplinary team care. Med J Aust. (2023) 218:368–73. doi: 10.5694/mja2.51906
27. Culver JO, Freiberg Y, Ricker C, Comeaux JG, Chang EY, Banerjee V, et al. Integration of universal germline genetic testing for all new breast cancer patients. Ann Surg Oncol. (2023) 30:1017–25. doi: 10.1245/s10434-022-12595-w
28. Manahan ER, Kuerer HM, Sebastian M, Hughes KS, Boughey JC, Euhus DM, et al. Consensus guidelines on genetic` testing for hereditary breast cancer from the American society of breast surgeons. Ann Surg Oncol. (2019) 26:3025–31. doi: 10.1245/s10434-019-07549-8
29. Abdel-Razeq H, Tamimi F, Abujamous L, Abdel-Razeq R, Abunasser M, Edaily S, et al. rates of variants of uncertain significance among patients with breast cancer undergoing genetic testing: regional perspectives. Front Oncol. (2022) 12:673094. doi: 10.3389/fonc.2022.673094
30. Abdel-Razeq H, Abujamous L, Al-Azzam K, Abu-Fares H, Bani Hani H, Alkyam M, et al. Guideline-based, Multi-gene panel germline genetic testing for at-risk patients with breast cancer. Breast Cancer (Dove Med Press). (2023) 15:1–10. doi: 10.2147/BCTT.S394092
31. Abdel-Razeq H, Abujamous L, Abunasser M, Edaily S, Bater R. Prevalence and predictors of germline BRCA1 and BRCA2 mutations among young patients with breast cancer in Jordan. Sci Rep. (2021) 11:14906. doi: 10.1038/s41598-021-94403-1
32. Abdel-Razeq H, Nielsen SM, Tbakhi A, Bani Hani H, Sharaf B, Abu Hijlih R, et al. Implementation of universal pan-cancer germline genetic testing in an Arab population: The Jordanian exploratory cancer genetics (Jo-ECAG) study. JCO. (2023) 41:10591–1. doi: 10.1200/JCO.2023.41.16_suppl.10591
33. Abdel-Razeq H, Mustafa R, Abdel-Razeq S, Abu-Fares H, Al Masri S, Damsees R, et al. Pathogenic germline variants in patients with breast cancer: conversations across generations, practices and patients’ attitude. Front Genet. (2023) 14:1194075. doi: 10.3389/fgene.2023.1194075
34. Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. (2002) 8:571–6. doi: 10.1016/S1471-4914(02)02434-6
35. Davies H, Glodzik D, Morganella S, Yates LR, Staaf J, Zou X, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med. (2017) 23:517–25. doi: 10.1038/nm.4292
36. Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. (2005) 434:917–21. doi: 10.1038/nature03445
37. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. (2005) 434:913–7. doi: 10.1038/nature03443
38. Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. (2010) 376:235–44. doi: 10.1016/S0140-6736(10)60892-6
39. Robson ME, Tung N, Conte P, Im S-A, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. (2019) 30:558–66. doi: 10.1093/annonc/mdz012
40. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. (2018) 379:753–63. doi: 10.1056/NEJMoa1802905
41. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee K-H, Gonçalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. (2020) 31:1526–35. doi: 10.1016/j.annonc.2020.08.2098
42. Wang J, Zhang Y, Yuan L, Ren L, Zhang Y, Qi X. Comparative efficacy, safety, and acceptability of single-agent poly (ADP-ribose) polymerase (PARP) inhibitors in BRCA-mutated HER2-negative metastatic or advanced breast cancer: a network meta-analysis. Aging. (2020) 13:450–9. doi: 10.18632/aging.202152
43. Han HS, Arun BK, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, et al. Veliparib monotherapy following carboplatin/paclitaxel plus veliparib combination therapy in patients with germline BRCA-associated advanced breast cancer: results of exploratory analyses from the phase III BROCADE3 trial. Ann Oncol. (2022) 33:299–309. doi: 10.1016/j.annonc.2021.11.018
44. Diéras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2020) 21:1269–82. doi: 10.1016/S1470-2045(20)30447-2
45. Geyer CE, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. (2022) 33:1250–68. doi: 10.1016/j.annonc.2022.09.159
46. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. (2021) 384:2394–405. doi: 10.1056/NEJMoa2105215
47. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast cancer. Version 2.2024. Available online at: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed April 16, 2024).
48. Bertagnolli MM, Sartor O, Chabner BA, Rothenberg ML, Khozin S, Hugh-Jones C, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. (2017) 376:1178–81. doi: 10.1056/NEJMsb1702054
49. Linfield DT, Rothberg MB, Pfoh ER, Noss R, Cassard L, Powers JC, et al. Primary care physician referral practices regarding BRCA1/2 genetic co unseling in a major health system. Breast Cancer Res Treat. (2022) 195:153–60. doi: 10.1007/s10549-022-06523-5
50. Offit K, Groeger E, Turner S, Wadsworth EA, Weiser MA. The ‘duty to warn’ a patient’s family members about hereditary disease risks. JAMA. (2004) 292:1469–73. doi: 10.1001/jama.292.12.1469
51. Vande Perre P, Toledano D, Corsini C, Escriba E, Laporte M, Bertet H, et al. Role of the general practitioner in the care of BRCA1 and BRCA2 mutation carriers: General practitioner and patient perspectives. Molec Gen Gen Med. (2018) 6:957–65. doi: 10.1002/mgg3.464
52. Seven M, Bağcivan G, Akyuz A, Bölükbaş F. Women with family history of breast cancer: How much are they aware of their risk? J Canc Educ. (2018) 33:915–21. doi: 10.1007/s13187-017-1226-3
53. Abu-Helalah M, Azab B, Mubaidin R, Ali D, Jafar H, Alshraideh H, et al. BRCA1 and BRCA2 genes mutations among high risk breast cancer patients in Jordan. Sci Rep. (2020) 10:17573. doi: 10.1038/s41598-020-74250-2
54. Li X, You R, Wang X, Liu C, Xu Z, Zhou J, et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: a meta-analysis and systematic review. Clin Cancer Res. (2016) 22:3971–81. doi: 10.1158/1078-0432.CCR-15-1465
55. Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. (2014) 348:g226–6. doi: 10.1136/bmj.g226
56. Abdel-Razeq H. Surgical options for patients with early-stage breast cancer and pathogenic germline variants: an oncologist perspectives. Front Oncol. (2023) 13:1265197. doi: 10.3389/fonc.2023.1265197
57. Honold F, Camus M. Prophylactic mastectomy versus surveillance for the prevention of breast cancer in women’s BRCA carriers. Medwave. (2018) 18:e7161. doi: 10.5867/medwave.2018.04.7160
58. Jakub JW, Peled AW, Gray RJ, Greenup RA, Kiluk JV, Sacchini V, et al. Oncologic safety of prophylactic nipple-sparing mastectomy in a population with BRCA mutations: a multi-institutional study. JAMA Surg. (2018) 153:123. doi: 10.1001/jamasurg.2017.3422
59. Heemskerk-Gerritsen BAM, Seynaeve C, Van Asperen CJ, Ausems MGEM, Collée JM, Van Doorn HC, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. (2015) 107. doi: 10.1093/jnci/djv033
60. Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. (2010) 304:967–75. doi: 10.1001/jama.2010.1237
Keywords: clinical cancer genetics program, BRCA, inherited breast cancer, low-income countries, germline mutation
Citation: Abdel-Razeq H, Sharaf B, Tamimi F, Hani HB, Alsmadi O, Khalil H, Abunasser M, Edaily S and Mansour A (2024) Establishment of a clinical cancer genetics program for breast cancer in a resource-limited country; challenges and opportunities. Front. Oncol. 14:1431985. doi: 10.3389/fonc.2024.1431985
Received: 13 May 2024; Accepted: 07 October 2024;
Published: 23 October 2024.
Edited by:
Enrique Soto-Perez-de-Celis, National Institute of Medical Sciences and Nutrition Salvador Zubirán, MexicoReviewed by:
Muhammad Usman Rashid, Shaukat Khanum Memorial Cancer Hospital and Research Center, PakistanYanin Chavarri Guerra, National Institute of Medical Sciences and Nutrition Salvador Zubirán, Mexico
Copyright © 2024 Abdel-Razeq, Sharaf, Tamimi, Hani, Alsmadi, Khalil, Abunasser, Edaily and Mansour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hikmat Abdel-Razeq, aGFiZGVscmF6ZXFAa2hjYy5qbw==
 Hikmat Abdel-Razeq
Hikmat Abdel-Razeq Baha Sharaf
Baha Sharaf Faris Tamimi
Faris Tamimi Hira Bani Hani
Hira Bani Hani Osama Alsmadi
Osama Alsmadi Hanan Khalil1
Hanan Khalil1 Mahmoud Abunasser
Mahmoud Abunasser Sarah Edaily
Sarah Edaily Asem Mansour
Asem Mansour