- 1Department of Radiology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Ultrasound, Ya’an People’s Hospital, Ya’an, China
- 3Department of Pathology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
Extraskeletal Ewing’s sarcoma (ES) has been reported to originate from various sites. Primary endobronchial ES is an extremely rare bronchial tumor, especially multifocal lesions. This report describes a rare presentation of primary bronchial ES in a 31-year-old female who was referred to the emergency department of our hospital due to suspicion of a foreign body in the bronchus. Computed tomography and bronchoscopy revealed multiple polypoid nodules in the middle bronchus of her right lung, thus excluding the initial diagnosis. Infection-related laboratory tests and serum tumor markers were normal. The bronchial sleeve resection was performed to remove the tumor completely and the patient’s clinical symptoms obviously improved. Subsequent imaging, histopathological, immunohistochemical and genetic analyses made a conclusive diagnosis of primary endobronchial ES. To our knowledge, this is the eighth case of primary bronchial ES reported in medical literature.
Introduction
The Ewing sarcoma family of tumors (ESFT) is a group of small round cell neoplasms that have similar neuroectodermal origin and chromosomal translocation (1).While the vast majority of ESFT cases occur in children and in bones, approximately 15%-20% originate outside the skeleton as extraskeletal Ewing’s sarcoma (EES) (2). EES was first described by Angervall and Enzinger (3) in 1975. EES typically originates from the deep soft tissues of the trunk, extremities, retroperitoneum, and chest wall. EES has also been reported in the kidneys, adrenal glands, pancreas, uterus, gastrointestinal tract, and other visceral organs (4). EES that arises in the bronchus are extremely rare, especially multifocal lesions. To our knowledge, primary endobronchial multifocal ES in the middle bronchus of the right lung has not previously been reported. This case report describes a rare primary endobronchial ES in the middle bronchus of the right lung in a 31-year-old female. The patient underwent thoracic surgical exploration and sleeve bronchial resection, and preoperative imaging studies, postoperative pathological results and genetic analysis confirmed the diagnosis of primary bronchial ES.
Case report
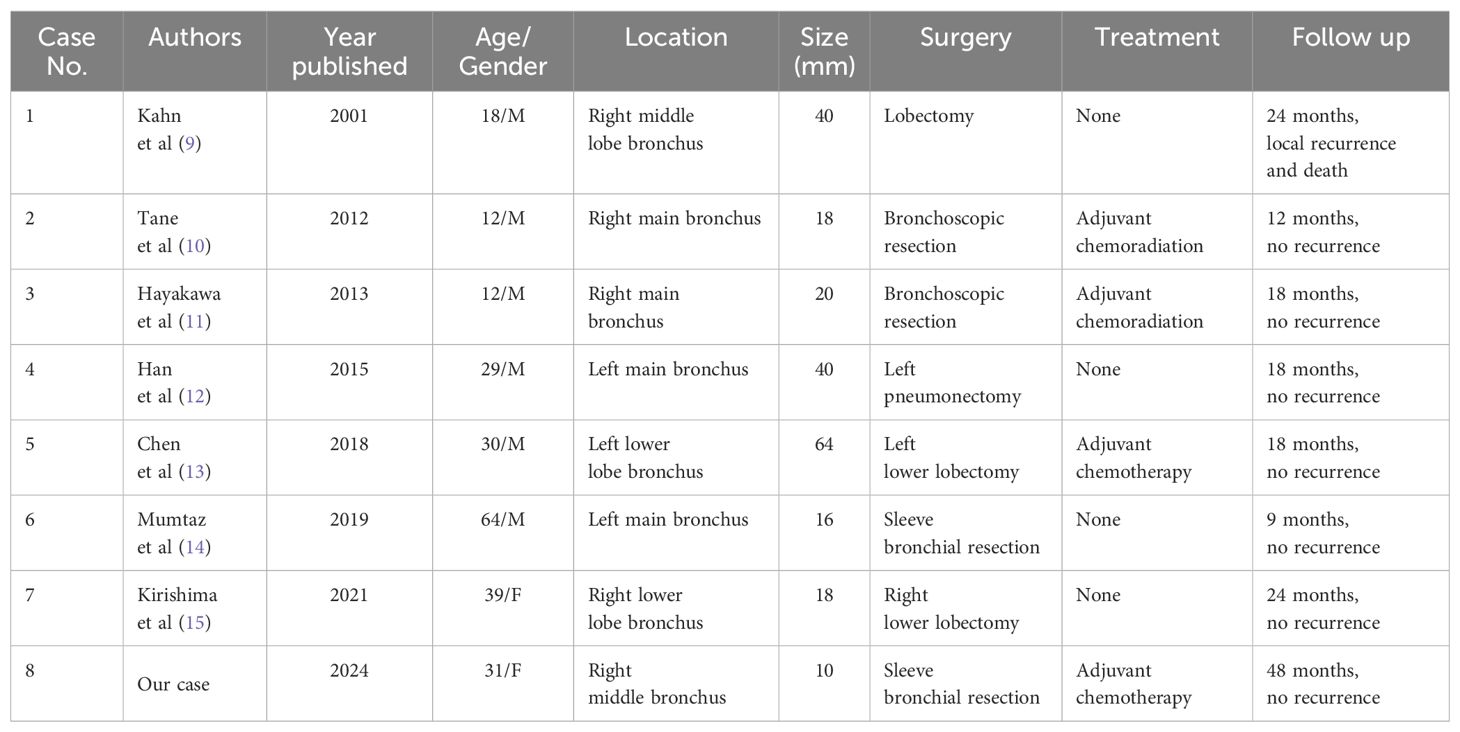
A 31-year-old female presented with a one-week history of slight pharyngeal discomfort and choking cough while lying supine. She had an occasional audible whistle when she spoke loudly. Her symptoms worsened after eating fish with black beans 3 days previously. Computed tomography (CT) performed at another hospital detected multiple polypoid lesions in the middle bronchus of the right lung, the largest measuring approximately 0.7 cm×0.7 cm×0.6 cm (Figure 1). The tumors exhibited well-defined borders and homogeneous density. The walls of bronchi were smooth. She was admitted to the emergency department of our hospital due to suspicion of a foreign body in the bronchi. The initial diagnosis was ruled out after scanning the CT images. Tuberculosis was suspected based on the patient’s age. Infection-related laboratory tests and serum tumor markers were normal. She denied any prior history of fever, recent chest trauma, contact with tuberculosis, or exposure to toxins. Her family denied a history of malignancy. The vital signs were stable, the thoracic contour was normal; there were no dry or moist rales and pleural friction sounds. More prevalent low-grade malignant tumors, such as squamous-cell carcinoma, mucoepidermoid carcinoma, and carcinoid tumor, which prompted bronchoscopic biopsy, were also doubted. Bronchoscopy revealed grey-white polypoid masses based on the wall of right middle bronchus. The lesions partially obstructed the middle bronchus with no evidence of invasion (Figure 2A). Transbronchial needle aspiration was performed and histological results revealed typical small blue tumor cells without necrosis and then the patient was referred to West China Hospital for further treatment. Right bronchial sleeve resection was performed, and polypoid endobronchial nodules were observed, the largest measuring approximately 1.0 cm×1.0 cm×1.0 cm. Grossly, the tumors appeared to be in situ, however, microscopically, they infiltrated the submucosa, muscles, and cartilages. No perineural, vascular, or lymphatic invasion was observed. Hematoxylin and eosin (H&E) staining revealed monomorphic population of small blue cells with variably conspicuous nucleoli and scant cytoplasm (Figure 2B). Immunohistochemistry analysis demonstrated strong positivity for CD99, NKX2.2, CD56, P63, INI-1, and negativity for PCK, Syn, CgA, EMA, DSE, Myogenin, S100, SMA, WT-1, LCA, CK8/18 (Figures 2C–E). The Ki-67 labeling index was approximately 50% (Figure 2F). Fluorescence in situ hybridization (FISH) for EWSR1 gene translocation was positive, and next-generation sequencing confirmed EWSR1-FLI1 gene fusion. ECT, MRI of the head and neck and multisite ultrasonography results were normal. Therefore, this tumor was eventually identified as a primary endobronchial ES. The patient underwent 17 courses of chemotherapy after resection including 2 alternating blocks: VDC (vincristine 2 mg/m2, doxorubicin 60 mg/m2, cyclophosphamide 200 mg/m2, respectively, on day 1, every 4 weeks) and IE (ifosfamide 2600 mg/m2, etoposide 150 mg/m2, respectively, on days 1 to 5, every 4 weeks). The radiotherapy was not performed. Chest CT, abdominal MRI, and bronchoscopy were performed annually. At the 48-month follow-up after the operation, the patient remained disease-free.
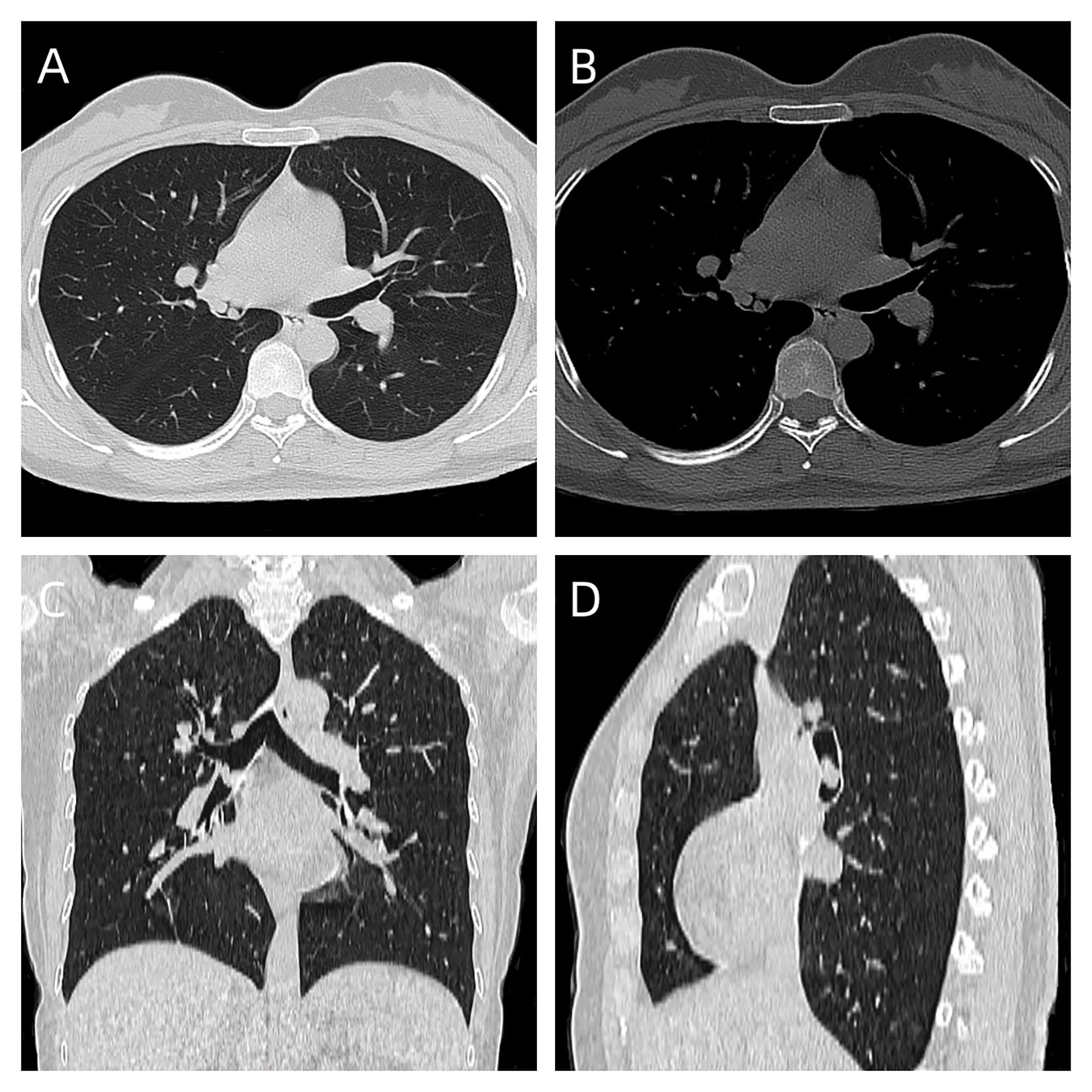
Figure 1. (A) axial lung window, CT showed multiple polypoid lesions in the middle bronchus of the right lung; (B) axial bone window; (C) coronal scan; (D) saggital scan.
Figure 2. (A) Bronchoscopic images demonstrated polypoid endobronchial tumors that originated in the middle bronchus of the right lung. (B) The tumor cells show small, round-to-oval nuclei without rosette-like arrangement (HE, original magnification 200). (C–E) Immunohistochemical findings showed strongly positivity for CD99, NKX2.2 and CD56 (F) The Ki-67 labeling index was approximately 50%.
Discussion
ES is a highly aggressive malignancy with poor prognosis (5) that usually affects the bones, particularly the femur, pelvis or axial skeleton. It is most commonly diagnosed in the second decade of life, with a slight male predominance (6). The majority of patients with EES are young, with a median age of 20 years, they are 5–10 years older than those with ES of the bone (ESB); the age range is also much wider. Compared with ESB, EES demonstrates a less strong predilection for male patients, with a more even distribution among men and women (7). For bronchial ES, including our case (a 31-year-old female), reports in the literature describe the details of 6 males and 2 females aged 12-65 years (mean age 29.5 years). Our findings are consistent with those of previous reports.
EES has been reported in the thorax, extremities, head and neck, pelvis, abdomen, spine, intracranial space, orbit and so on (8). Bronchial ES is extremely rare, with only 7 cases reported in the literature, and our patient is the eighth such documented case of primary bronchial ES (Table 1) (9–15). Moreover, to the best of our knowledge, an ESS originating in the right lung’s middle bronchus has never been reported. Due to the low incidence and atypical clinical manifestations of EES, accurate diagnosis, differential diagnosis, and subsequent treatment options are challenging.
The clinical symptoms vary depending on the region of involvement. Symptoms include irritable cough (87.5%), dyspnea (62.5%), fever (25%), hemoptysis (12.5%), chest pain (12.5%), and pharyngeal discomfort(12.5%). These clinical manifestations of bronchial ES are atypical and may be easily mistaken for other bronchial tumors or diseases. As in our case, the patient initially complained of some throat irritation and a choking cough. After consuming fish with black beans, the patient’s symptoms worsened, and she was mistaken for a body foreign in her bronchus. Subsequent comprehensive analyses led to the definitive diagnosis of primary endobronchial ES. It should be distinguished from squamous cell carcinoma, cartinoid tumor, mucoepidermoid carcinoma, adenoid cystic carcinoma, small cell carcinoma, adenocarcinoma, lymphoma rhabdomyosarcoma and leiomyosarcoma (16).
Chest radiography may provide less information for primary bronchial ES due to shading of the mediastinal structures. However, direct radiographic evidence, such as a pulmonary hilar mass and indirect manifestations, such as obstructive atelectasis and obstructive pneumonia may still be found, if the tumor has grown to a considerable size or into the lumen, even in a small volume. In our study, 6 of 8 patients underwent this examination; pulmonary hilar mass was observed in 3 (37.5%); obstructive atelectasis in 4 (50%) and pneumonia in 2 (25%).
Chest CT, particularly three-dimensional imaging, offers superior clarity in visualizing intrabronchial tumors and can assist in the early suggestive diagnosis of primary bronchial ES (17). In our study and the literature, 5 of 8 (62.5%) were located in the right bronchus, and the remaining 3 (37.5%) were found in the left bronchus. Of these cases, 5 (62.5%) invaded the bronchial wall with or without extension to the pulmonary parenchyma; these tumors presented with irregular shape, ill-defined borders, and homogeneous or heterogeneous density (average diameter, 32mm).The remaining 3 (37.5%) were confined to the bronchial lumen, showing nodular shape, smooth, and well-defined margins with homogeneous density (average diameter, 22.7mm). Three of 8 patients underwent CT-enhanced scanning, in which 1 of 3 tumors (33.3%) exhibited homogeneous enhancement, while 2 (66.7%) exhibited multiple focal heterogeneous enhancements. Furthermore, we discovered multifocal tumors in our case, a finding that has not been previously reported in the literature. The etiology of this phenomenon remains unknown.
Currently, ES diagnosis depends mainly on the comprehensive histopathological, immunohistochemical, and genetic analyses.
Histopathologically, ES is characterized by an abundance of small round blue cells arranged in compact sheets with a low amount of eosinophilic cytoplasm and a high nuclear-to-cytoplasmic volume ratio (18). ES must be differentiated from malignant lymphoma, neuroblastoma, and embryonal rhabdomyosarcoma (19). The differentiation of ES from the other entities may occasionally be difficult, especially in the soft tissue variants. The individual cells in ES are round, of moderate size, have clear and frequently quite scant cytoplasm, and round to oval nucleus. Classical ES cells are frequently positive for glycogen. The final diagnosis depends on the immunohistochemical and genetics findings.
ES is driven by a non-random chromosomal translocation events involving the EWSR1 gene located on chromosome 22, and members of the ETS family of transcription factors, most commonly FLI1 on chromosome 11 (85%), Other fusion partners include ERG (10%), ETV1 (<1%), and ETV4 (<1%) and so on (20). In our study, genetic testing was performed in 4 of the 8 cases of bronchial ES, and the EWSR1-FLI-1 fusion formed by t (11, 21)(q24;12) chromosomal translocation was found in 3 patients, including our case, while the results in other patients were negative.
CD99 (the product of the MIC2 antigen) represents the most useful molecular marker for ES diagnosis, although its diagnostic specificity and sensitivity for ES are not 100% (21). Strong CD99 membrane immunopositivity is seen in practically all examples of ES. In our study, CD99 was found to be strongly positive in all 8 reported cases. Due to its remarkable sensitivity, CD99 immunonegativity would strongly argue against a diagnosis of ES. FLI1 and ERG immunopositivity can be seen in those ES harboring EWSR1-FLI1 (22) and EWSR1-ERG gene fusions (23), respectively. NKX2.2, a homeodomain transcription factor involved in neuroendocrine/glial differentiation and a downstream target of EWSR1-FLI1, is recognized as a specific molecular marker in the diagnosis of ES, and its combination with CD99 makes this test highly specific (24). In our study, we observed 1 case each with positive FLI-1 and NKX2.2 expression. In addition, we detected vimentin(3/8), synaptophysin (3/8), cytokeratin (2/8), P63 (2/8) and CD56 (2/8). However, the diagnostic utility of these markers requires further investigation.
ES is generally difficult to treat. Patients frequently relapse and require complex treatment regimens including surgery, radiotherapy, and chemotherapy (25). Owing to the rarity of bronchial ES tumor, discrepancies in their clinical presentation and differences in patient characteristics, the optimal management of bronchial ES tumors remains unclear (26).
For bronchial ES tumors, surgical resection with negative margins, when possible, appears to offer the best chance for disease-free survival compared with additional use of neoadjuvant or adjuvant therapy. The treatment and prognosis of 8 bronchial ES tumors reported in the literature is summarized in Table 1. Eight patients all underwent different surgical procedures with or without adjuvant therapy, only 1 of whom experienced recurrence and death. This result suggests that although the bronchial ES is an aggressive tumor, the prognosis was better than those originating in other organs.
The surgical strategy for bronchial ES is contingent on the specific bronchial segment affected. Tumors in the distal bronchial airway and pulmonary parenchyma may requires lobectomy to maintain negative margins. For tumors located proximally in the bronchus, bronchoscopic snare resection may be an option. However, this method increases the risk of local recurrence because bronchial ES tumors usually present as pedunculated, exophytic tumors which can spread into the submucosal layer, muscle, and cartilage (12). In the present case, preoperative CT and bronchoscopy identified multiple polypoid lesions characterized by smooth adjacent bronchial walls. Macroscopically, these tumors appeared to be confined in the bronchial wall; however, microscopic evaluation revealed infiltration into the submucosa, muscle layers, and cartilages. Bronchial sleeve resection is a surgical option to remove the tumor completely and preserves as much healthy lung tissue as possible.
Prognosis depends on stage, tumor location and size, patient age, and response to chemotherapy. The multimodal approach to the treatment of ES has allowed the 5-year survival rate for localized tumors to be 70% to 80%. EES tends to have a better prognosis than osseous ES (27). Our patient, a 31-year-old female, is the second reported case of primary bronchial ES treated safely by sleeve resection with chemotherapy, and the patient was alive with no evidence of recurrence on imaging scans and bronchoscopy at 48 month’s follow up.
Although rare, we should consider the possibility of bronchial EES when we are evaluating the bronchial lesions. Further in-depth research and accumulation of data from similar cases are essential to refine our understanding and management strategies for bronchial ES.
Conclusion
Our case highlights the complex diagnosis and management of primary bronchial ES, an uncommon and highly aggressive malignancy with an atypical presentation. We aimed to expand the existing knowledge on this unusual entity and promote further research to enhance therapeutic strategies for primary bronchial ES. Furthermore, it underscores the indispensable significance of an interdisciplinary approach in handling rare and intricate cases of thoracic surgery and oncology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Sichuan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DL: Conceptualization, Project administration, Writing – original draft, Writing – review & editing, Data curation. XGL: Data curation, Investigation, Writing – original draft. XL: Data curation, Investigation, Writing – review & editing. YL: Supervision, Writing – review & editing. JY: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We greatly appreciate the assistance of the staff of the Department of Thoracic Surgery, West China Hospital and thank them for their efforts. The authors would like to thank the patient who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maire G, Brown CW, Bayani J, Pereira C, Gravel DH, Bell JC, et al. Complex rearrangement of chromosomes 19, 21, and 22 in Ewing sarcoma involving a novel reciprocal inversion-insertion mechanism of EWS-ERG fusion gene formation: a case analysis and literature review. Cancer Genet Cytogenet. (2008) 181:81–92. doi: 10.1016/j.cancergencyto.2007.11.002
2. Jawad MU, Cheung MC, Min ES, Schneiderbauer MM, Koniaris LG, Scully SP. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database,1973-2005. Cancer. (2009) 115:3526–36. doi: 10.1002/cncr.24388
3. Angervall L, Enzinger FM. Extraskeletal neoplasm resembling Ewing’s sarcoma. Cancer. (1975) 36:240–51. doi: 10.1002/(ISSN)1097-0142
4. Alexandra W, Madhura D, Candice WB, Mohamed B, Jeffrey G, Brinda RK, et al. Extraskeletal ewing sarcoma from head to toe: multimodality imaging review. Radiographics. (2022) 42:1145–60. doi: 10.1148/rg.210226
5. Sparreboom BD, Trautman J, Yaxley J. Ewing sarcoma: A pictorial review of typical and atypical locations with reference to the updated 2020 WHO classification system. J Med Imaging Radiat Oncol. (2022) 66:812–18. doi: 10.1111/1754-9485.13456
6. Haveman LM, van Ewijk R, van Dalen EC, Breunis WB, Kremer LC, van den Berg H, et al. High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents, and young adults with primary metastatic Ewing sarcoma. Cochrane Database Syst Rev. (2021) 9:CD011405. doi: 10.1002/14651858.CD011405.pub2
7. Murphey MD, Senchak LT, Mambalam PK, Logie CI, Klassen-Fischer MK, Kransdorf MJ. From the radiologic pathology archives: ewing sarcoma family of tumors: radiologicpathologic correlation. RadioGraphics. (2013) 33:803–31. doi: 10.1148/rg.333135005
8. Ghandour M, Lehner B, Klotz M, Geisbüsch A, Bollmann J, Renkawitz T, et al. Extraosseous ewing sarcoma in children: A systematic review and meta-analysis of clinicodemographic characteristics. Children. (2022) 9:1859. doi: 10.3390/children9121859
9. Kahn AG, Avagnina A, Nazar J, Elsner B. Primitive neuroectodermal tumor of the lung. Arch Pathol Lab Med. (2001) 125:397–9. doi: 10.5858/2001-125-0397-PNTOTL
10. Tane S, Nishio W, Hashimoto S, Hokka D, Maniwa Y, Funada Y, et al. Ewing’s sarcoma family of tumors originating in the main bronchus. Thorac Cancer. (2012) 3:353–6. doi: 10.1111/j.1759-7714.2011.00105.x
11. Hayakawa A, Hirase S, Matsunoshita N, Yamamoto N, Kubokawa I, Mori T, et al. Primary pediatric endobronchial Ewing sarcoma family of tumors. Am J Case Rep. (2013) 14:67–9. doi: 10.12659/AJCR.883821
12. Han W, Huh D, Kim B, Kwak E, Lee S. Endobronchial primitive neuroectodermal tumor with pneumothorax ex vacuo. Ann Thorac Surg. (2015) 100:1455–8. doi: 10.1016/j.athoracsur.2014.11.073
13. Chen J, Yuan T, Liu X, Hua B, Dong C, Liu Y, et al. Ewing’s sarcoma and/or peripheral primitive neuroectodermal tumors in bronchus. Am J Med Sci. (2019) 357:75–80. doi: 10.1016/j.amjms.2018.08.009
14. Mumtaz H, Khalil F, Tandon A, Toloza E, Fontaine JP. Primary bronchial Ewing sarcoma. Int J Surg Case Rep. (2019) 61:230–3. doi: 10.1016/j.ijscr.2019.07.062
15. Kirishima M, Kato I, Hisaoka M, Nakatani Y, Takeda AH, Mizuno K, et al. Solid endobronchial tumor with EWSR1-FLI1 fusion gene-A diagnostically challenging case of the Ewing sarcoma. Pathol Int. (2021) 71:488–90. doi: 10.1111/pin.13109
16. Roby BB, Drehner D, Sidman JD. Pediatric tracheal and endobronchial tumors: an institutional experience. Arch Otolaryngol Head Neck Surg. (2011) 137:925–9. doi: 10.1001/archoto.2011.153
17. Bakir B, Tüzün U, Terzibaşioğlu E, Dursun M, Güven K, Salmaslioğlu A, et al. The diagnostic efficiency of multislice CT virtual bronchoscopy in detecting endobronchial tumors. Tuberk Toraks. (2008) 56:43–9.
18. Marcilla D, MaChado I, Grunewald TGP, Llombart-Bosch A, de Alava E. (Immuno) histological analysis of ewing sarcoma. Methods Mol Biol. (2021) 2226:49–64. doi: 10.1007/978-1-0716-1020-6_5
19. Dehner LP. Primitive neuroectodermal tumor and Ewing’s sarcoma. Am J Surg Pathol. (1993) 17:1–13. doi: 10.1097/00000478-199301000-00001
20. Kumar S, Pack S, Kumar D, Walker R, Quezado M, Zhuang Z, et al. Detection of EWS-FLI-1 fusion in Ewing sarcoma/peripheral primitive neuroectodermal tumor by fluorescence in situ hybridization using formalin-fixed paraffin-embedded tissue. Hum Pathol. (1999) 30:324–30. doi: 10.1016/s0046-8177(99)90012-6
21. Folpe AL, Hill CE, Parham DM, O’Shea PA, Weiss SW. Immunohistochemical detection of FLI-1 protein expression: A study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing’s sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol. (2000) 24:165762. doi: 10.1097/00000478-200012000-00010
22. Choi YL, Chi JG, Suh YL. CD99 immunoreactivity in ependymoma. Appl Immunohistochem Mol Morphol. (2001) 9:125–9. doi: 10.1097/00129039-200106000-00004
23. Wang WL, Patel NR, Caragea M, Hogendoorn PC, López-Terrada D, Hornick JL, et al. Expression of ERG, an Ets family transcription factor, identifies ERG-rearranged Ewing sarcoma. Mod Pathol. (2012) 25:1378–83. doi: 10.1038/modpathol.2012.97
24. Yoshida A, Sekine S, Tsuta K, Fukayama M, Furuta K, Tsuda H. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. (2012) 36:993–9. doi: 10.1097/PAS.0b013e31824ee43c
25. Van Mater D, Wagner L. Management of recurrent Ewing sarcoma: challenges and approaches. Onco Targets Ther. (2019) 12:2279–88. doi: 10.2147/OTT.S1705
26. Salah S, Abuhijla F, Ismail T, Yaser S, Sultan I, Halalsheh H, et al. Outcomes of extraskeletal vs. skeletal Ewing sarcoma patients treated with standard chemotherapy protocol. Clin Transl Oncol. (2020) 22:878–83. doi: 10.1007/s12094-019-02202-y
Keywords: Ewing’s sarcoma, endobronchus, thoracic tumor, EWSR1-FLI1 fusion gene, case report
Citation: Liu D, Liu X, Li X, Liu Y and Yu J (2024) Primary endobronchial multifocal Ewing’s sarcoma: a rare case report. Front. Oncol. 14:1431950. doi: 10.3389/fonc.2024.1431950
Received: 13 May 2024; Accepted: 25 June 2024;
Published: 30 August 2024.
Edited by:
David Jon Gordon, The University of Iowa, United StatesReviewed by:
Jenna Gedminas, The University of Iowa, United StatesLenka Součková, Masaryk University, Czechia
Copyright © 2024 Liu, Liu, Li, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yisha Liu, bHlzMTM0MDg0NjI5NDNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Dan Liu
Dan Liu Xiaoge Liu2†
Xiaoge Liu2†