
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 October 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1431920
This article is part of the Research Topic Comprehensive Treatment Strategy for Improving Surgical Resection Rate of Retroperitoneal Sarcomas View all 10 articles
 Xiao Zhou1,2†
Xiao Zhou1,2† Aobo Zhuang1,2†
Aobo Zhuang1,2† Xi Li3†
Xi Li3† Zhe Xi1,2
Zhe Xi1,2 Yingxue Cheng1,2
Yingxue Cheng1,2 Guangting Yan1,2
Guangting Yan1,2 Yue Wang1,2
Yue Wang1,2 Gen Zhang1,2
Gen Zhang1,2 Yangyang Huang2
Yangyang Huang2 Chenhe Zhang1,2
Chenhe Zhang1,2 Fuan Xie1,2
Fuan Xie1,2 Xin Ma2*
Xin Ma2* Ting Wu1*
Ting Wu1* Wengang Li1,2*
Wengang Li1,2*Background: Difficulty in achieving complete resection leads to a poor prognosis for retroperitoneal soft tissue sarcoma, hence emphasizing the significance of adjuvant treatment. The benefit of preoperative radiotherapy for retroperitoneal liposarcoma was initially demonstrated by the STRASS trial. However, the impact of intraoperative radiotherapy (IORT) on retroperitoneal liposarcoma remains unexplored.
Method: Patients with retroperitoneal liposarcoma were identified in the Surveillance, Epidemiology, and End Results (SEER) database, treated between 2000 and 2019. Subsequently, a 1:1 propensity score-matched (PSM) analysis was conducted based on variables identified from a multivariate analysis. T-tests were used to assess differences in normally distributed continuous variables, while the rank-sum test was applied to variables that did not follow a normal distribution. The chi-squared test was utilized to evaluate differences in categorical variables. Ultimately, survival analysis was performed using SPSS to evaluate patient prognosis.
Result: A total of 2129 patients with retroperitoneal liposarcoma were included in our study. Age, sex, histology, grading, chemotherapy, and tumor size as independent prognostic risk factors for these patients through multivariate Cox regression analysis. Subsequently, 66 patients were included in the survival analysis through PSM, with 33 patients receiving IORT. Finally, the survival analysis revealed that there was no difference in overall survival among patients with retroperitoneal liposarcoma, regardless of whether they received IORT or not (p= 0.711).
Conclusion: As an exploratory study, our findings suggest that patients may not derive benefit from intraoperative radiotherapy. These observations are intended to lay the groundwork for future prospective clinical studies.
Soft tissue sarcomas (STS) are heterogeneous tumors that arise from mesenchymal cells, including muscle, fat, cartilage, nerve, and vascular tissue. Consequently, STS occur in all body parts, with a higher prevalence in the lower and upper limbs, and a comparatively lower prevalence in the retroperitoneum, chest wall, and head and neck (1). STS accounts for approximately 1% of all newly diagnosed malignant solid tumors, equating to approximately 12,000 cases annually in the United States (2). Despite the low incidence rate of STS, retroperitoneal sarcoma (RPS) still contributes to approximately 15% of all STS cases, with an average annual incidence of 2.7 per million people (3). Among adults, the most common histological type is liposarcoma (approximately 50-70%), which is further subdivided into well-differentiated liposarcoma (WDLPS) (synonymous with atypical lipoma tumors [ALT] when diagnosed in the extremities) and dedifferentiated liposarcoma (DDLPS) (4).
Local area recurrence (LAR) is the dominant form of recurrence in patients with RPS and often leads to death (5). Therefore, reducing LAR is an important goal for patients with RPS (5). The primary and only treatment for localized RPS is surgical excision, with the major oncological goal being to achieve complete resection (R0+R1) (6).
Although many people have undergone multiple sequential excisions of multiple organs, the outcomes of RPS are generally less satisfactory than those of other soft tissue sarcomas (7). Hence, adjuvant treatment with surgery holds significance; nonetheless, there is insufficient evidence to support the effectiveness of chemotherapy in retroperitoneal liposarcoma (RPL) (6). Surgeons have, therefore, begun to experiment with radiotherapy. Multiple randomized trials have confirmed that preoperative or postoperative radiotherapy during limb-sparing surgery significantly reduces the risk of local recurrence (LR) in patients with STS in the extremities (5). In recent years, there has been a growing trend among academics and experts to utilize preoperative radiotherapy as a prominent approach in the treatment of RPS. The STRASS trial was also the first to demonstrate the benefits of preoperative radiotherapy for RPL (8).
An advantage of intraoperative radiotherapy (IORT) is the ability of surgeon to remove critical organs and attempt to irradiate only the tumor bed. This advantage allows the dose to be selectively increased in the risk area, thereby increasing the treatment ratio between target and normal tissues (5). However, research into IORT in patients with retroperitoneal soft tissue sarcoma needs to be improved, and the number of patients included in the prospective only studies must be expanded (5).
Although the STRASS trial demonstrated that patients with RPL may benefit from neoradiotherapy, there are currently no studies on the prognostic impact of IORT in patients with RPL. Therefore, we conducted the exploratory study by reviewing bulk data through searching the Seer database to address the gaps in IORT of RPL patients and provide direction for further prospective clinical research.
According to the Figure 1, patient data were collected from the Incidence - SEER database, 17 registries from the National Cancer Institute SEER Stat software with additional treatment fields added. According to the third edition of the International Classification of Diseases for Oncology (ICD-O-3), patients diagnosed with RPL and underwent surgery in 2000 and 2019 were incorporated into the study cohort. Inclusion criteria were as follows: (1) primary focus in the retroperitoneum; (2) patients undergoing surgery; (3) pathological diagnosis with well-differentiated liposarcoma, dedifferentiated liposarcoma, and unknown; (4) The age of the patient is between 18 and 80. Exclusion criteria were: (1) patients who did not undergo surgery; (2) patients with histologically non-RPL; (3) incomplete treatment and follow-up information. The variables chosen for analysis include the year of diagnosis, age, sex, race (Caucasians, African-Americans, or other), site code ICD-0-3, tissue grade, histology record ICD-0-3, radiotherapy (sequence of radiotherapy with surgery), status of lymph node dissection, chemotherapy (whether or not), month of survival, COD to site rec KM, and vital status record.
The characteristics that are extracted include year of diagnosis, age, sex, race, histology, grade, radiotherapy, lymph node dissection status, chemotherapy, and tumor size. Univariate Cox regression analysis is conducted on the entire cohort, and multivariate Cox regression analysis is performed on statistically significant variables to evaluate the impact of variables on the prognosis of RPL patients.
As this study is a retrospective analysis using the SEER database, the implementation of IORT may be subject to selection bias and potential confounding factors. To address this issue, we balanced the baseline characteristics between different treatment groups through 1:1 propensity score matching (PSM), making the cohorts more comparable and the results more reliable. Using the Cox regression model, we identified key prognostic factors affecting the prognosis of patients with retroperitoneal liposarcoma, such as tumor size, grade, histological type, and patient baseline characteristics (e.g., age, gender, race). Based on these variables, we calculated the propensity score for each patient and employed a 1:1 nearest neighbor matching algorithm, setting the maximum allowable propensity score difference (caliper width) at 0.01, and divided the patients into two groups based on the treatment methods. Subsequently, we validated the matching quality by performing comparative analysis on the matched characteristics to ensure that the baseline features of the two groups were similar.
Overall survival (OS) of RPL is defined as the duration between the initial diagnosis and the occurrence of death from any cause or the most recent follow-up. Using survival status as the dependent variable and treatment modality as the independent variable, 1:1 PSM was performed with a clamp value of 0.01 to calibrate for the effect of baseline clinicopathological differences. The normality test was used to test whether the continuous variables fit the normal distribution (e.g., age, tumor size), the t-test was used to assess whether there were differences between groups for the continuous variables that fit the normal distribution while the rank sum test is used for variables that do not conform to a normal distribution, and the chi-squared test was used to assess whether there were differences between groups for categorical variables (e.g., gender, race, sex, histology, grade, chemotherapy, treatment). Survival analysis was performed by plotting the Kaplan-Meier curve to compare whether there were differences in OS between groups. To eliminate the confounding effects of adjuvant radiotherapy, patients were stratified into two groups based on their exposure to adjuvant radiotherapy (which included preoperative, postoperative, and combined IORT with pre- and postoperative treatments). Subsequently, within these two groups, patients were further matched on a 1:1 PSM based on whether they received IORT as the independent variable. Following this matching, survival analyses were conducted once more to evaluate the outcomes. SPSS (Version 27.0. Armonk, NY: IBM Corp) statistical software was used for statistical analysis and presentation. Double-tailed p < 0.05 was considered statistically significant.
As shown in Table 1, 2129 patients who met the inclusion criteria were included, the age distribution exhibited a median value of 63 (IQR: 54, 71) years and the tumor size distribution had a median value of 208 (IQR: 130, 300) mm. The patient population exhibited minimal disparity in terms of sex, with 1199 male patients and 930 female patients. The most common histological type was dedifferentiation (960 cases, 45.09%), followed by well-differentiation (787 cases, 36.97%), and the most common tumor grade was FNCLCC I (976 cases, 45.84%). Most patients did not receive chemotherapy (1914 cases, 89.9%) and most were Caucasians (1786 cases, 83.89%). There were 18 cases (0.85%) of IORT, 15 cases (0.71%) of Intraoperative radiation with other radiation before/after surgery, 9 cases (0.42%) of preoperative and postoperative radiotherapy, 138 cases (6.48%) of preoperative radiotherapy, 281 cases (13.20%) of postoperative radiotherapy and 1666 cases (78.25%) of no radiotherapy.
As shown in Table 2, univariate Cox regression analysis was conducted using variables including year of diagnosis, age, sex, race, histology, grade, radiotherapy, lymph node dissection status, chemotherapy, and tumor size. The study revealed that patients’ prognosis were affected by age (p<0.001), sex (p<0.001), histology (p<0.001), grade (p<0.001), chemotherapy (p<0.001), and tumor size (p= 0.003). Upon inclusion of all aforementioned variables in the multivariate Cox regression analysis, the findings revealed that age (HR = 1.041, 95% CI 1.035-1.047, p<0.001), sex (HR = 1.230, 95% CI 1.080-1.402, p<0. 001), histology (p = 0. 029), grade (p<0.001), chemotherapy (HR = 1.947, 95% CI 1.608-2.357, p<0.001), and tumor size (HR = 1.001, 95% CI 1.000-1.001, p<0.001) were identified as independent risk factors for the prognosis of patients.
Due to the significant disparity in the number of patients undergoing IORT relative to the entire cohort, we implemented a 1:1 PSM strategy to eliminate potential confounders by using independent risk factors derived from the previous step of the analysis. After matching, there were no statistically significant differences between the two groups.
Baseline characteristics of the population after PSM are shown in Table 3. The median age of patients who underwent IORT was 61 years old, while the median tumor size was 200 mm. The patient population consisted predominantly of individuals exhibiting both well-differentiated and dedifferentiated characteristics, with comparable frequencies observed for each group. The majority of patients exhibited a tumor grade of FNCLCC I. A limited number of patients received chemotherapy. The majority of patients were Caucasians.
The survival analysis was conducted using SPSS software and a survival curve was plotted. According to the Kaplan-Meier analysis, there was no statistically significant disparity in OS between patients who received IORT and those who did not (Figure 2: P= 0.711). To eliminate the confounding effects of adjuvant radiotherapy, patients were stratified into two groups based on their exposure to adjuvant radiotherapy (which included preoperative, postoperative, and combined IORT with pre- and postoperative treatments). Subsequently, patients were further matched on a 1:1 PSM based on whether they only received IORT as the independent variable. Following this matching, survival analyses were conducted once more to evaluate the outcomes. And as shown in Figures 3, 4, IORT did not affect patient prognosis, regardless of whether the patient receives adjuvant radiotherapy (P= 0.45, P= 0.899).
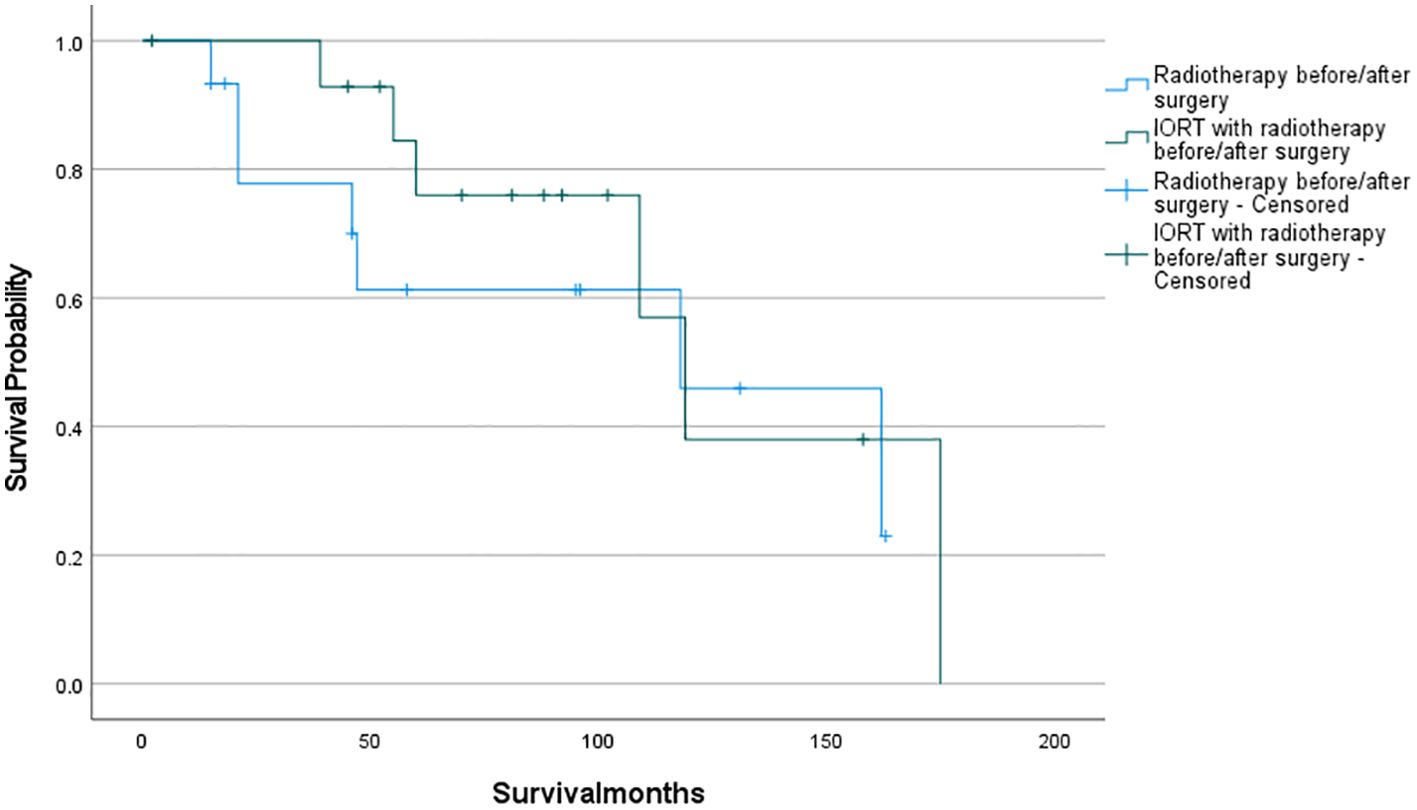
Figure 3. Patients who received IORT with radiotherapy before/after surgery compared to those who received radiotherapy before/after surgery (P= 0.45).
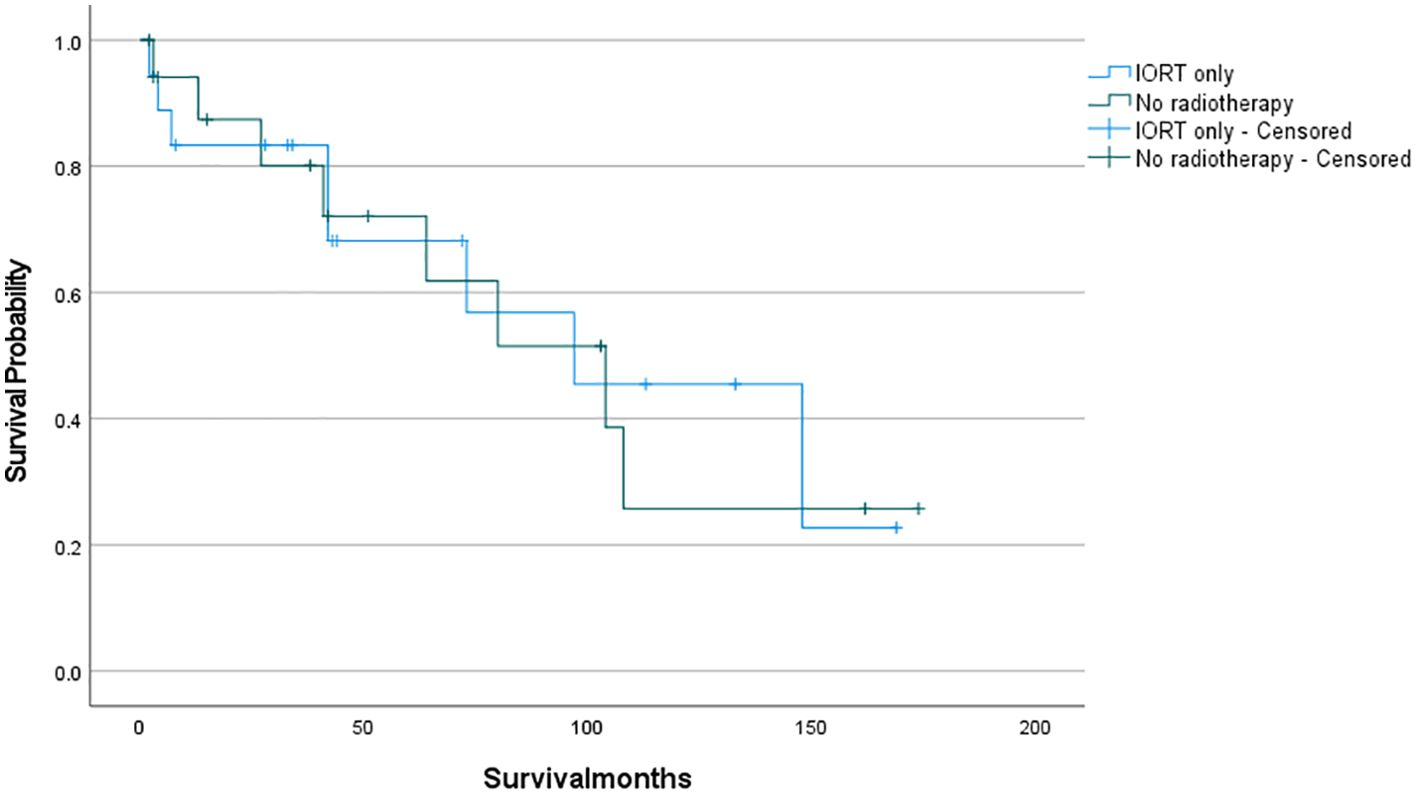
Figure 4. Patients who only received IORT compared to those who did not received radiotherapy (P= 0.899).
Retroperitoneal sarcomas (RPS) frequently lead to local recurrences, which are the primary cause of mortality in affected patients, complete resection is the only means of radical cure, but the effect is not satisfactory (3, 9, 10). There is also a lack of substantial evidence supporting the efficacy of adjunctive chemotherapy (6). And the prognosis of radiotherapy for these patients is also controversial (11–15). In response to these challenges, our study aimed to evaluate the effectiveness of IORT in the treatment of RPL. Our findings revealed that IORT did not significantly improve treatment outcomes.
The effectiveness of preoperative radiotherapy was first demonstrated in the RPL subgroup in the recently published randomized STRASS trial (EORTC 62092/STRASS). This trial reported a notable improvement in 3-year abdominal relapse-free survival in patients who received preoperative radiotherapy (71.6% vs. 60.4%). However, patients diagnosed with leiomyosarcoma (LMS) and high-grade sarcoma were not found to have an increased LR rate when administered preoperative radiotherapy. The findings suggest that preoperative radiotherapy provides a favorable prognosis for patients with this RPL subtype (8).
Callegaro et al. then compared the abdominal relapse-free survival (ARFS) of patients with primary RPS. The study compared the outcomes of patients who were treated with RT in EORTC-STBSG-62092 (STRASS) Phase 3 randomized controlled trial (STRASS cohort) with those who were treated with RT off-trial (STREXIT cohort). The results indicated that the use of radiotherapy improved ARFS in patients with liposarcoma, especially well-differentiated liposarcoma and G1-G2 dedifferentiated liposarcoma. However, radiotherapy did not benefit patients with leiomyosarcoma or G3 dedifferentiated liposarcoma (16).
IORT is a procedure that allows for the administration of high doses of radiation during surgery while the surgeon is removing vital organs and exposing the tumor site (5). The application of IORT has demonstrated prognostic benefits for patients in cases of breast cancer (17). It is a reasonable option to increase the dose and improve local control (LC). The risk of wound healing disorder or gastrointestinal toxicity is minimal. Delivering sufficient radiation over a significant retroperitoneal area without harming other organs poses a considerable challenge regarding RPL. Radiation treatment can have adverse effects on overall survival. Nonetheless, the impact of IORT on the prognosis of patients with RPL remains unknown.
The initial prospective randomized trial aimed to evaluate IORT for retroperitoneal soft tissue sarcoma. A total of 35 patients were enrolled in the trial, with 15 patients received IORT at 20Gy in combination with postoperative radiotherapy at 35-40Gy, and with 20 patients received postoperative radiotherapy at 50-55Gy alone. The results indicated a decrease in local relapses and radiation-related abdominal complications among patients who underwent IORT together with postoperative radiotherapy (18). Their findings is similar to ours, however, there was no significant improvement in overall survival (OS) or cancer-specific survival (CSS) among the patients. Although their study only compared the efficacy of intraoperative radiotherapy (IORT) combined with postoperative radiotherapy versus postoperative radiotherapy alone, and failed to distinguish between the various histologic types. Nonetheless, their research presents novel strategies for implementing IORT in RPS patients.
There has been ongoing debate regarding the efficacy of IORT for sarcoma treatment in previous studies. Wang L. B. et al. found that IORT benefits the OS of liposarcoma patients, which is contrary to our results. This discrepancy may stem from differences in baseline characteristics such as tumor size, race, and grade among patients receiving different treatments in their cohort, as well as a smaller sample size. Our study increased comparability by balancing baseline characteristics between different groups through 1:1 PSM and included a larger sample size, which may explain the differing results (19). Similarly, Gieschen H. L. et al. reported that IORT benefits OS in patients with retroperitoneal sarcoma, but their inclusion of various histological types and a smaller sample of patients receiving IORT (only 16 cases) may have led to different outcomes. Although they found that IORT benefits disease-free survival (DFS) and local control (LC), the differences did not reach statistical significance, possibly due to the small sample size (20). J.-P.E.N. Pierie et al. compared preoperative radiotherapy with combined preoperative and intraoperative radiotherapy and found that the combined treatment improved disease-specific survival and recurrence. Their cohort included 103 patients but did not analyze the effects of using IORT alone, so the benefits of IORT alone in their cohort were still unknown (21). Timothy M. Pawlik et al. analyzed the results of preoperative radiotherapy combined with either IORT or other radiotherapy, finding a 5-year LC rate of 60% and a 5-year OS of 61%, higher than many studies, but similarly did not clearly evaluate the pros and cons of using IORT alone (22). Robert Krempien et al. also found benefits of IORT for retroperitoneal sarcoma patients but noted a higher risk of complications. They included various histological types and had a small sample size, and did not clearly balance baseline characteristics between different treatment groups, which may have led to different results from ours (23). Falk Roeder et al. demonstrated the benefits of IORT, and although their sample size was larger (156 patients), they only compared IORT with combined treatment (IORT combined with additional radiotherapy), without analyzing the group of patients not receiving IORT, leaving the benefits of IORT unclear (24).
Previous studies often included multiple histological types such as liposarcoma, leiomyosarcoma, malignant fibrous histiocytoma, etc. and did not specifically analyze liposarcoma. Most studies had limitations such as small sample sizes and unbalanced baseline characteristics. Additionally, some studies had design flaws, making it difficult to clearly distinguish between the effects of using IORT and not using IORT. Our study used a larger cohort specifically focusing on liposarcoma and balanced baseline characteristics through 1:1 PSM, making the cohorts more comparable. Additionally, we conducted a detailed analysis of the effects of IORT by comparing patients who received IORT to those who did not, those who received only IORT to those who received no radiotherapy at all, and those who received IORT in combination with other adjuvant radiotherapies to those who received only other adjuvant radiotherapies, eliminating interference from preoperative/postoperative adjuvant radiotherapy. However, the results indicated that receiving intraoperative radiotherapy had no impact on patient prognosis, regardless of whether adjuvant radiotherapy was administered.
This investigation aimed to examine the effects of IORT on the prognosis of RPL patients and suggest novel approaches for managing the entire process for these patients. Regrettably, our findings imply that IORT does not hold significant value for the prognosis of these patients. Based on the outcomes of numerous retrospective studies and the STRASS experiment, it is not recommended to use IORT as a routine treatment for RPL patients, because the effect of combined or single use of IORT is the same as that of other radiotherapy modalities. What’s more, preoperative radiotherapy has the advantage that the target (tumor volume [GTV]) is clearly visible and can be more precisely defined to ensure repeatability and accuracy of the radiotherapy plan, and lower and, therefore, safer radiation doses are used before surgery (5). In conclusion, preoperative radiation might be the best option.
Moreover, previous studies have mainly reported LC as the primary endpoint for the benefit of IORT. While it is possible that IORT enhances LC, it may not translate into better OS due to some factors, leading our results to contradict some previous results. It is important to note, however, that our study results were influenced by a selection bias in the patients chosen for IORT. Our study is a retrospective exploratory research, and the results offer insight into future radiotherapy methods for RPL patients. Further prospective experiments could provide a better analysis of IORT’s efficacy.
The subsequent generation of STRASS 2 trials will assess the influence of neoadjuvant chemotherapy in leiomyosarcoma and high-risk liposarcoma patients, thereby promoting the integrated treatment of RPS (25).
Our study has several limitations. The SEER database only includes U.S. data, potentially introducing selection bias by excluding patients treated elsewhere. It also lacks key treatment details such as surgical margins, resection extent, and radiotherapy doses, limiting our analysis, especially given the importance of surgical margins. Additionally, SEER’s limited survival data prevents assessment of local control and recurrence rates, and the absence of TNM staging restricts patient staging analysis. As a retrospective study, inherent selection biases exist despite using 1:1 PSM to mitigate these. Nevertheless, SEER remains valuable for studying rare tumors and overcoming sample size limitations.
Our results suggest that IORT alone or combined with pre - or post-operative radiotherapy does not improve patients’ OS. As an exploratory study, although the sample size is small and public data is used, we have conducted the first global exploration of the efficacy of IORT in RPL, which is expected to provide references for further prospective clinical research. Future studies should include prospective and randomized controlled trials. Additionally, further multicenter studies could not only increase the sample size but also enhance the representativeness and external validation of the research.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
XZ: Writing – original draft. AZ: Writing – original draft. XL: Writing – original draft. ZX: Writing – original draft. YC: Writing – original draft. GY: Writing – original draft. YW: Writing – original draft. GZ: Writing – original draft. YH: Writing – original draft. CZ: Writing – original draft. FX: Writing – original draft. XM: Writing – review & editing. TW: Writing – review & editing. WL: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. (1987) 205(4):349–59. doi: 10.1097/00000658-198704000-00003
2. Hsiegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72(1):7–33. doi: 10.3322/caac.21708doi: 10.3322/caac.21708
3. Porter GA, Baxter NN, Pisters PW. Retroperitoneal sarcoma: a population-based analysis of epidemiology, surgery, and radiotherapy. Cancer. (2006) 106(7):1610–6. doi: 10.1002/cncr.21761
4. Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and molecular spectrum of liposarcoma. J Clin Oncol. (2018) 36(2):151–9. doi: 10.1200/JCO.2017.74.9598
5. Farooqi AS, Guadagnolo BA, Mitra D, Bishop AJ. Radiation therapy for retroperitoneal sarcomas: A strass-ful situation. Curr Oncol. (2023) 30(1):598–609. doi: 10.3390/curroncol30010047
6. Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Desai A, Gladdy RA, et al. Management of primary retroperitoneal sarcoma (RPS) in the adult: an updated consensus approach from the transatlantic australasian RPS working group. Ann Surg Oncol. (2021) 28(12):7873–88. doi: 10.1245/s10434-021-09654-z
7. Seidensaal K, Dostal M, Kudak A, Jaekel C, Meixner E, Liermann J, et al. Preoperative dose-escalated intensity-modulated radiotherapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft-tissue sarcoma: final results of a clinical phase I/II trial. Cancers (Basel). (2023) 15(10):2747. doi: 10.3390/cancers15102747
8. Bonvalot S, Gronchi A, Le Péchoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2020) 21(10):1366–77. doi: 10.1016/s1470-2045(20)30446-0
9. Zhuang A, Yue X, Tong H, Zhang Y, He F, Lu W. Nomogram predicting overall survival after surgical resection for retroperitoneal leiomyosarcoma patients. Front Endocrinol (Lausanne). (2023) 14:1160817. doi: 10.3389/fendo.2023.1160817
10. Zhuang A, Zhuang A, Wu Q, Lu W, Tong H, Zhang Y. Prognostic factor analysis and nomogram construction of primary retroperitoneal liposarcoma: A review of 10 years of treatment experience in a single Asian cohort of 211 cases. Front Oncol. (2022) 11:777647. doi: 10.3389/fonc.2021.777647
11. Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. (2009) 27(1):31–7. doi: 10.1200/jco.2008.18.0802
12. Le Péchoux C, Musat E, Baey C, Al Mokhles H, Terrier P, Domont J, et al. Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (FAS) in patients with primary retroperitoneal sarcoma? Ann Oncol. (2013) 24(3):832–7. doi: 10.1093/annonc/mds516
13. Kelly KJ, Yoon SS, Kuk D, Qin LX, Dukleska K, Chang KK, et al. Comparison of perioperative radiation therapy and surgery versus surgery alone in 204 patients with primary retroperitoneal sarcoma: A retrospective 2-institution study. Ann Surg. (2015) 262(1):156–62. doi: 10.1097/SLA.0000000000001063doi: 10.1097/SLA.0000000000001063
14. Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, Collini P, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. (2009) 27(1):24–30. doi: 10.1200/jco.2008.17.8871
15. Gronchi A, Miceli R, Colombo C, Stacchiotti S, Collini P, Mariani L, et al. Frontline extended surgery is associated with improved survival in retroperitoneal low- to intermediate-grade soft tissue sarcomas. Ann Oncol. (2012) 23(4):1067–73. doi: 10.1093/annonc/mdr323
16. Callegaro D, Raut CP, Ajayi T, Strauss D, Bonvalot S, Ng D, et al. Preoperative radiotherapy in patients with primary retroperitoneal sarcoma: EORTC-62092 trial (STRASS) versus off-trial (STREXIT) results. Ann Surg. (2023) 278(1):127–34. doi: 10.1097/SLA.0000000000005492
17. Liu J, Shi X, Niu Z, Qian C. Comparative efficacy of intraoperative radiotherapy and external boost irradiation in early-stage breast cancer: a systematic review and meta-analysis. PeerJ. (2023) 11:e15949. doi: 10.7717/peerj.15949
18. Sindelar WF, Kinsella TJ, Chen PW, DeLaney TF, Tepper JE, Rosenberg SA, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. (1993) 128(4):402–10. doi: 10.1001/archsurg.1993.01420160040005
19. Wang LB, Mcaneny D, Doherty G, Sachs T. Effect of intraoperative radiotherapy in the treatment of retroperitoneal sarcoma. Int J Clin Oncol. (2017) 22(3):563–8. doi: 10.1007/s10147-016-1086-6
20. Gieschen HL, Spiro IJ, Suit HD, Ott MJ, Rattner DW, Ancukiewicz M, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. (2001) 50(1):127–31. doi: 10.1016/s0360-3016(00)01589-3
21. Pierie JP, Betensky RA, Choudry U, Willett CG, Souba WW, Ott MJ. Outcomes in a series of 103 retroperitoneal sarcomas. Eur J Surg Oncol. (2006) 32(10):1235–41. doi: 10.1016/j.ejso.2006.07.002
22. Pawlik TM, Pisters PW, Mikula L, Feig BW, Hunt KK, Cormier JN, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. (2006) 13(4):508–17. doi: 10.1245/aso.2006.05.035
23. Krempien R, Roeder F, Oertel S, Weitz J, Hensley FW, Timke C, et al. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. (2006) 65(3):773–9. doi: 10.1016/j.ijrobp.2006.01.028
24. Roeder F, Alldinger I, Uhl M, Saleh-Ebrahimi L, Schimmack S, Mechtersheimer G, et al. Intraoperative electron radiation therapy in retroperitoneal sarcoma. Int J Radiat Oncol Biol Phys. (2018) 100(2):516–27. doi: 10.1016/j.ijrobp.2017.10.034
Keywords: retroperitoneal liposarcoma, intraoperative radiotherapy, SEER, propensity score matching, overall survival
Citation: Zhou X, Zhuang A, Li X, Xi Z, Cheng Y, Yan G, Wang Y, Zhang G, Huang Y, Zhang C, Xie F, Ma X, Wu T and Li W (2024) Intraoperative radiotherapy might not serve as a standard therapy for retroperitoneal liposarcoma: insights from a population-based propensity score-matched study. Front. Oncol. 14:1431920. doi: 10.3389/fonc.2024.1431920
Received: 13 May 2024; Accepted: 07 October 2024;
Published: 25 October 2024.
Edited by:
Hanxing Tong, Fudan University, ChinaReviewed by:
Dorian Yarih Garcia-Ortega, National Institute of Cancerology (INCAN), MexicoCopyright © 2024 Zhou, Zhuang, Li, Xi, Cheng, Yan, Wang, Zhang, Huang, Zhang, Xie, Ma, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Ma, bWExNTIxNDY2ODIwMEAxNjMuY29t; Ting Wu, d3V0aW5nNzhAMTg5LmNu; Wengang Li, bHdnYW5nQHhtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.