- 1Department of Radiation Oncology, Cancer Hospital of Shantou University Medical College, Shantou, China
- 2Shantou University Medical College, Shantou, China
Patients with supraclavicular lymph node (SLN) metastasis from esophageal cancer encounter significant variations in treatment approaches due to differences in pathological subtypes and the lack of a unified regional staging system between East Asian and Western countries. The Tiger study aims to develop an internationally recognized staging system and to delineate the extent of regional lymph node dissection. In the context of esophageal squamous cell carcinoma (SCC) with SLN metastasis, the treatment paradigms from East Asia offer valuable insights. The Japan Esophageal Society (JES) 12th edition staging system guides a tailored comprehensive treatment strategy, emphasizing either radiotherapy and chemotherapy or surgical intervention. In contrast, esophageal adenocarcinoma (AC) predominates in Western countries, where the 8th edition of the American Joint Committee on Cancer (AJCC) staging system classifies SLN metastasis as a distant metastasis, advocating for systemic therapy as the primary treatment modality. Nonetheless, compelling evidence suggests that a multidisciplinary treatment approach, incorporating either radiotherapy and chemotherapy or surgery as the initial treatment, can yield superior outcomes for these patients compared to chemotherapy alone.
1 Introduction
In 2020, esophageal cancer was the seventh most commonly diagnosed cancer globally, and the sixth leading cause of cancer-related mortality (1). Post-surgical supraclavicular lymph node (SLN) metastasis occurs in 14.5-25% of patients with esophageal cancer (2–4). Currently, there is no unified clinical staging for esophageal cancer patients with SLN as the solitary metastasis site. The 8th edition of the American Joint Committee on Cancer (AJCC) staging system categorizes SLN metastasis as M1, equating to stage IVB (5). The National Comprehensive Cancer Network (NCCN) guidelines prioritize systemic therapy for all M1 patients. In contrast, the 12th edition of the Japan Esophageal Society (JES) staging system classifies these patients as M1a, with stages ranging from III to IVA (6). The recommended treatment approach in this system involves surgery coupled with perioperative treatment and definitive chemoradiotherapy (dCRT), emphasizing curative intent and a focus on local treatment. The Chinese Society of Clinical Oncology (CSCO) guidelines, which adopt the AJCC staging, recommend similar treatment principles to the NCCN for different stages. This leads to significant discrepancies in staging and markedly different treatment strategies for patients with esophageal cancer and SLN metastasis when different staging systems are applied. This review will compare and discuss the classification of SLNs, staging, treatment strategies, and recent advancements in the management of this patient cohort.
2 Attribution of supraclavicular lymph nodes
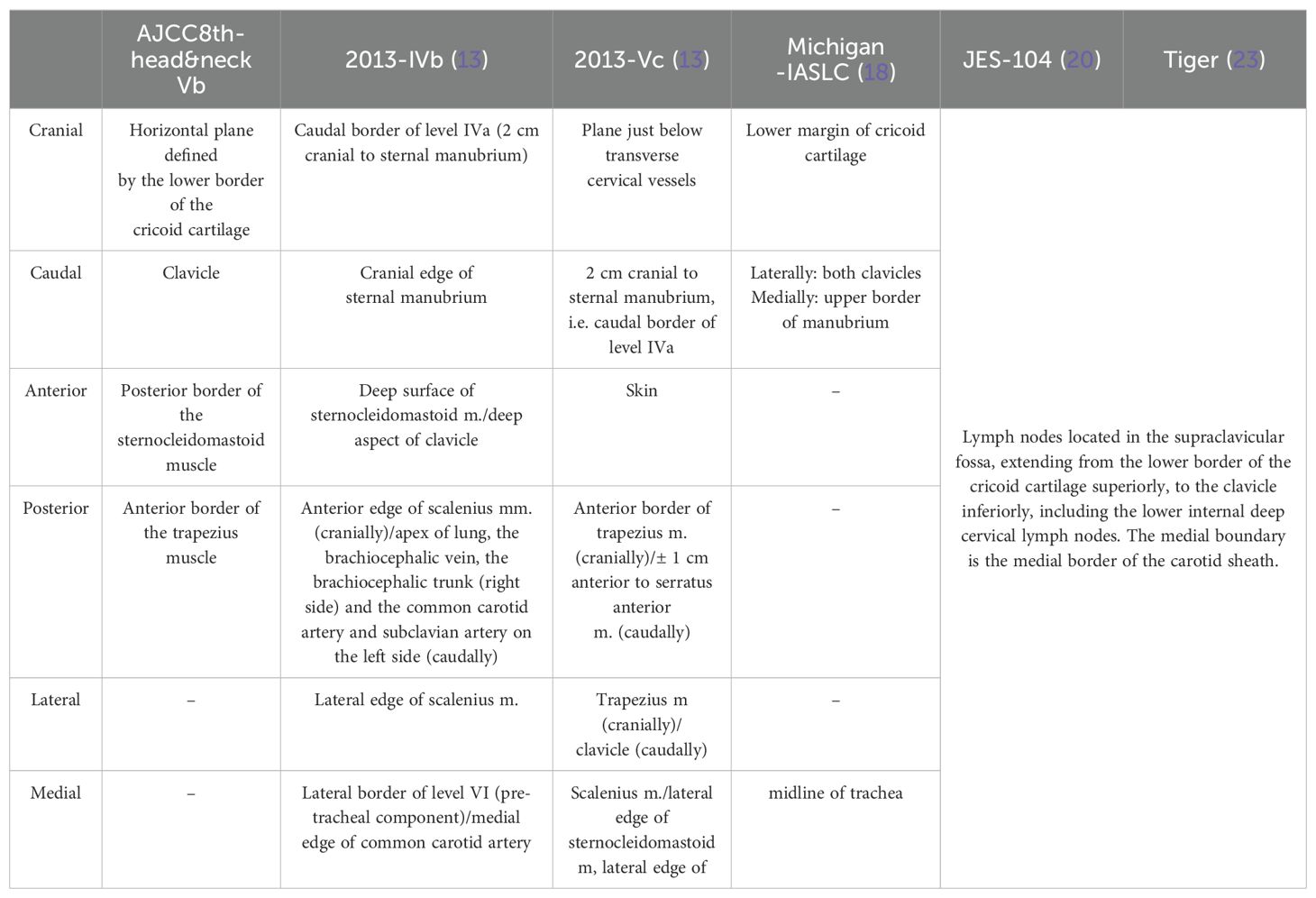
Anatomically, SLNs are part of the cervical lymph node region. However, due to frequent involvement in thoracic tumors, SLNs are often included in the definition of regional lymph nodes in thoracic tumors. There are variations in the anatomical boundaries of SLNs as defined by different staging systems, as well as differences in the regional lymph node definitions for esophageal cancer (Table 1).
2.1 Supraclavicular lymph nodes in head and neck tumors
The cervical lymph node region was initially defined by anatomist Rouvière in 1938 (7). Rouvière believed that SLNs belonged to the deep lymph nodes of the lateral and lower neck, located along the transverse cervical vessels. The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) proposed a division of cervical lymph nodes into 6 levels according to the level-based approach in 1991 (8), it was revised twice in 1998 and 2002 (9, 10), leading to the modified Robbins’ classification. The 7th edition of the AJCC staging in 2010 designated SLNs as a subset of the level Vb region. The 8th edition maintained the cervical lymph node region divisions.
With the enhanced understanding of anatomy, especially lymphatic drainage, and the advancement of imaging techniques, some researchers believe that the anatomical accuracy provided by CT and MRI can solve these problems and improve the accuracy of classification and assessment of metastatic lymph nodes. In 2000, Som first proposed an imaging-based definition of cervical lymph node regions (11), defining SLNs as lymph nodes located at or below the level of the clavicle, lateral to the carotid artery, distinct from regions I-VII. In 2003 (12), major tumor cooperative organizations in Europe and the United States jointly published an imaging-based division of cervical lymph node regions, which was updated in 2013 (13), defining SLNs as the region from the upper edge of the sternum to 2cm above it, divided into IVb (medial supraclavicular group) and Vc (lateral supraclavicular group).
2.2 Supraclavicular lymph nodes in thoracic tumors
In the context of thoracic tumors, the classification of SLNs has evolved over time. Initially, Naruke’s 1967 lymph node mapping for lung cancer did not include SLNs (14). The American Thoracic Society (ATS) later incorporated SLNs as Group 1 in their 1983 and 1988 lymph node maps (15, 16), which were not initially recognized in the AJCC’s 2nd edition. The International Association for the Study of Lung Cancer (IASLC) established a more detailed lymph node map in 2009 (17), providing detailed anatomical definitions and becoming the standard for regional lymph node classification in lung cancer. The supraclavicular region is designated as Group 1, including the lower neck, supraclavicular, and sternal lymph nodes.
As radiotherapy techniques like 3D-CRT and IMRT advanced, the University of Michigan provided a CT-based lymph node classification in 2013 to improve target delineation in lung cancer (18).
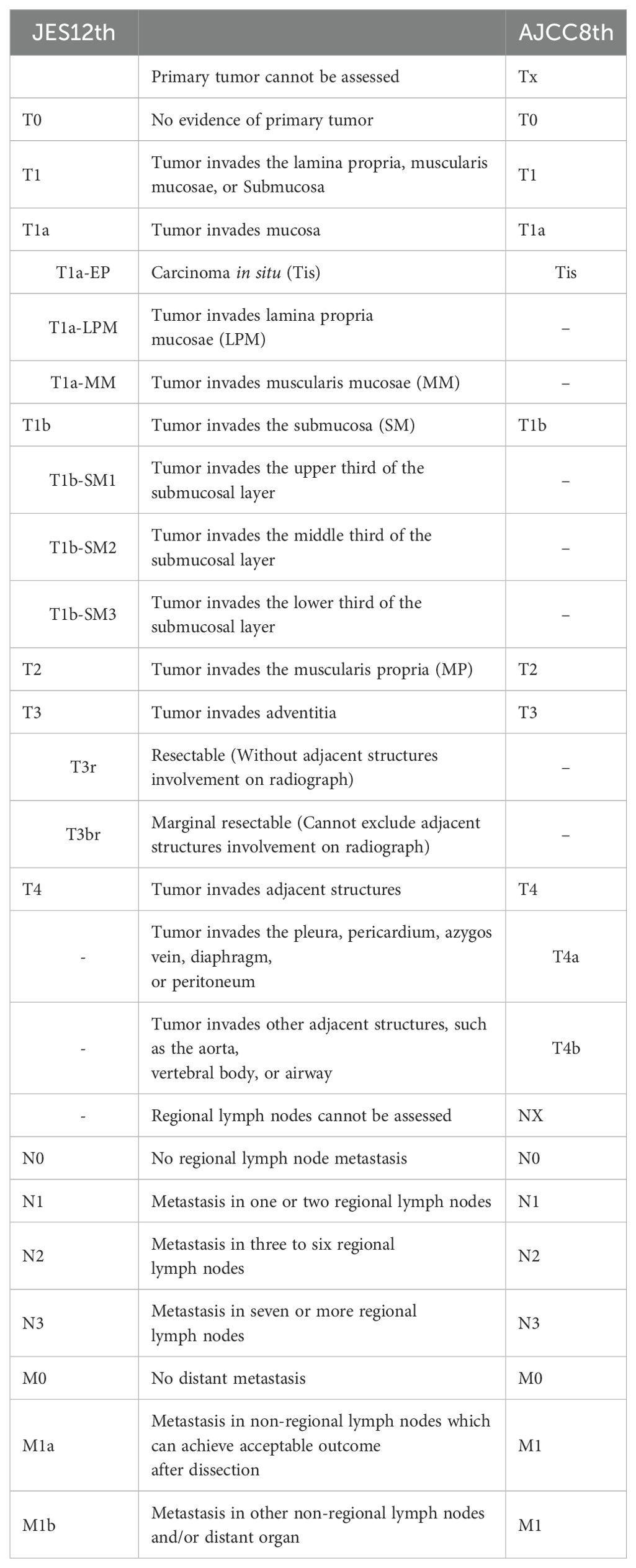
For esophageal cancer, the JES and the AJCC have different staging systems. JES’s 12th edition considers Group 104 as M1a, a non-regional lymph node, distinguishing it from other distant metastases (M1b). Prior to the 8th edition of the AJCC staging, SLNs were considered regional nodes but were reclassified out of this category in the latest edition (19).
Researchers use CT-based classifications for esophageal cancer lymph nodes (20), often relying on existing cervical or lung cancer lymph node classifications, as specific guidelines for SLNs in esophageal cancer are not fully established.
2.3 Current situation
Globally, there’s a noticeable lack of consensus regarding the involvement of SLNs in esophageal cancer research, with interpretations varying and sometimes conflicting. A primary issue is the infrequency of a precise definition for SLN metastasis within the field. The absence of a universally accepted definition often results in vague or unspecified criteria in studies, highlighting an urgent need for standardized terminology. Furthermore, even when using the same staging system, researchers interpret SLN involvement differently. Although the widely used 8th edition of the AJCC staging system for esophageal cancer has improved over the 7th edition by clearly defining lymph node stations specific to esophageal cancer and differentiating them from those in lung cancer, it still does not provide clear anatomical boundaries for lymph nodes in the esophageal cancer region. This has led to some misunderstandings. For example, Xu et al. from China mentioned in their article that SLN metastasis is considered a regional lymph node in the 8th edition of the AJCC staging system (21), and they believe that group 1 of the regional lymph nodes in the 8th edition represents the SLNs. The other two examples are retrospective studies conducted in the Netherlands and Japan, respectively (3, 22), which stated that: In the 7th edition of the AJCC staging system, SLNs are not considered regional lymph nodes in esophageal cancer.
Due to the lack of a unified standard for defining regional lymph nodes in esophageal cancer and the absence of clear anatomical boundaries for lymph nodes, researchers have not reached a consensus on the definition of SLNs and whether they should be classified as regional lymph nodes. The confusion in definitions has resulted in confusing research, which cannot yield clinically significant results. To tackle this issue at its core, the Tiger study was undertaken collaboratively by 50 institutions across multiple countries (23). The study aims to enroll 5000 patients who undergo surgery for resectable esophageal cancer or gastroesophageal junction cancer, with the goal of determining the distribution of lymph node metastasis and developing a globally standardized staging system. This will lay the foundation for establishing the optimal surgical treatment strategy for esophageal cancer patients. The definition of SLNs in this study is based on the 11th edition of the JES staging, specifically JES-104 group: lymph nodes located in the supraclavicular fossa, ranging from the upper border of the clavicle to the lower border of the cricoid cartilage, with the inner border being the inner edge of the carotid sheath.
In light of these efforts, the Tiger study’s partition scheme, endorsed by 18 countries and 50 institutions, is seen as the most rational approach to defining SLNs in esophageal cancer to date. It’s hoped that the study’s outcomes will pave the way for a unified definition of SLN involvement, enabling more consistent data comparison and guiding the development of more effective treatment strategies. Until then, clarity in defining SLNs in research papers is strongly advised.
3 Staging and treatment recommendations
3.1 Current status
There are two widely used staging systems for esophageal cancer currently, the AJCC 8th edition staging and the JES 12th edition staging (Table 2). Patients with SLN metastasis are classified as stage IVB in the AJCC 8th edition staging, but in the JES 12th edition staging, SLN as the solitary distant metastasis are classified as M1a, and the stage can be III-IVA.
Following the implementation of the AJCC 8th edition, the classification of SLN metastasis as M1 has been a subject of debate. Numerous studies, leveraging database and retrospective analyses, have been conducted to challenge this categorization. Wen et al.’s SEER database analysis suggests that the impact of SLN on prognosis is contingent upon the primary tumor’s location (24). Wang’s retrospective analysis posits that SLNs should be viewed as regional nodes for upper esophageal cancer, while for middle and lower esophageal cancer, they should be considered distant metastases (25). Numata concluded through retrospective analysis that although the cervical esophagus is close to the SLN, it should still be regarded as a non-regional lymph node for patients with cervical esophageal cancer (26). Two Japanese retrospective analyses believed that the SLN does not affect the prognosis of thoracic esophageal cancer (3, 27), and should be classified as regional lymph nodes. The ongoing lack of definitive evidence sustains the controversy over the classification of SLNs, a question that future studies like Tiger study may help resolve.
The use of different staging criteria leads to divergent treatment objectives for patients in Eastern and Western countries. The NCCN 2023 2nd Edition Esophageal Cancer Clinical Practice Guidelines recommend systemic therapy ± best supportive care for stage IVB esophageal cancer (28). China’s CSCO guidelines also use the AJCC staging, and systemic therapy is recommended for stage IVB esophageal cancer. In contrast, Japan favors a definitive treatment approach, primarily surgery or chemoradiotherapy, for patients with SLN metastasis. Based on the treatment strategies of different staging systems (Figure 1): In the 12th edition JES esophageal cancer staging, T0-3rN+M1a is stage IIIA, T3brAnyNM1a is stage IIIB, T4AnyNM1a is stage IVA. According to the 2022 Esophageal Cancer Diagnosis and Treatment Guidelines compiled by JES (32, 33), for stage III esophageal cancer patients who can tolerate surgery, preoperative chemotherapy or preoperative chemoradiotherapy plus surgical treatment is recommended. If the postoperative pathology does not achieve complete response, adjuvant treatment with nivolumab is recommended. Those who cannot tolerate surgery should choose radiotherapy and/or chemotherapy as the first choice. For stage IVA esophageal cancer, chemoradiotherapy is recommended, but the evidence level is not high, because there is currently no large clinical trial to prove that concurrent chemoradiotherapy (CCRT) is superior to simple chemotherapy for stage IVA patients.
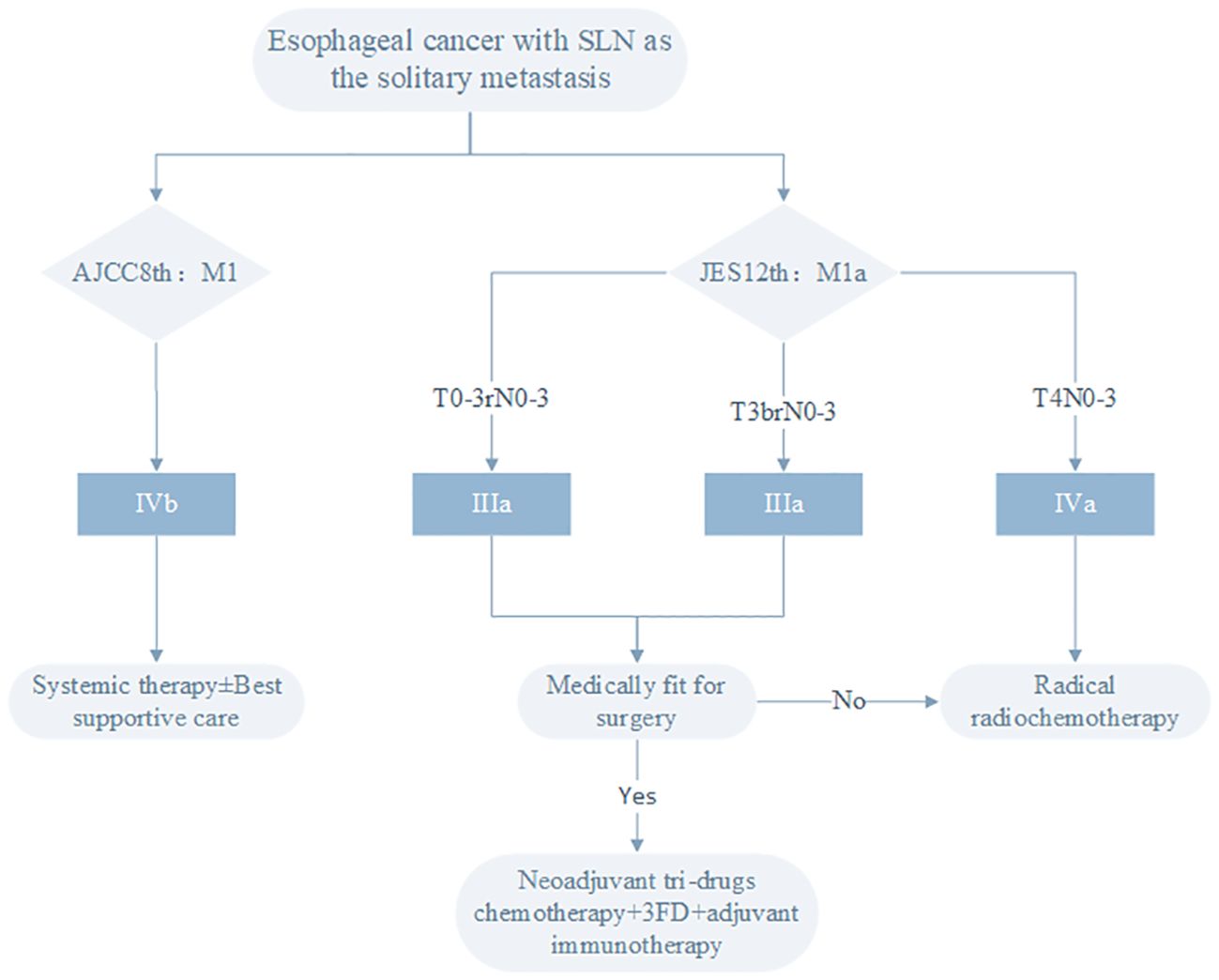
Figure 1. Treatment strategies for esophageal cancer patients with SLN as the solitary metastasis site based on different staging systems.
3.2 Progress in surgical treatment
Surgical intervention remains a cornerstone in the treatment of stage III esophageal cancer. For patients with stage III according to the JES12th staging, the JES guidelines first prioritizes a regimen of tri-drug neoadjuvant chemotherapy (NCT) followed by surgery. The surgical method is esophagectomy plus three-field lymph node dissection (3FD), the 3FD in esophagectomy is defined as neck-chest-abdominal lymph node dissection, although esophageal cancer 3FD has been controversial for a long time and has not become a world standard (29, 30). For the treatment of esophageal cancer with SLN metastasis, the 3FD is an indispensable part of definitive surgery (31). Although recent years thoracoscopic or robotic surgery were able to remove left recurrent laryngeal nerve lymph nodes (32), the JES-104 group located on the lateral of the carotid vessels still requires 3FD for complete excision.
3.2.1 Selection of surgical strategy
There are many studies that have confirmed that robot-assisted minimally invasive esophagectomy (RAMIE) and minimally invasive esophagectomy (MIE) are superior to open surgery (33–36), which have reduced the incidence of perioperative complications and shortened hospital stay. As for whether RAMIE is superior to MIE, based on the current research results, a preliminary affirmative answer can be obtained. The RAMIE trial is a prospective, multicenter, randomized controlled clinical trial (37), which randomly assigned patients to the RAMIE group or MIE group, the results showed: compared with the MIE group, the RAMIE group significantly shortened the operation time (203.8 mins vs 244.9 mins), for patients receiving neoadjuvant treatment, the RAMIE group had a higher efficiency of thoracic lymph node dissection (15 vs 12), and a higher completion rate of left recurrent laryngeal nerve lymph node dissection (79.5% vs 67.6%), there was no difference between the two groups in terms of bleeding volume, conversion rate and R0 resection rate, the 90-day mortality rate for both groups was 0.6%, and the overall incidence of complications was similar.
Western countries guided by the AJCC staging and NCCN guidelines are also exploring the role of surgery in stage IVb esophageal cancer with SLN as the solitary metastasis, a phase II clinical trial in the Netherlands enrolled 20 patients with cervical lymph node metastasis of esophageal cancer (38), all patients received preoperative radiotherapy plus RAMIE plus 3FD, and the 1-year overall survival(OS) rate reached 85%, a retrospective analysis based on the SEER database showed (24), the 1-year OS rate for patients with esophageal cancer with cervical lymph node metastasis undergoing non-surgical treatment was 31%, which was lower than the Netherlands study; Another retrospective study found (39), in patients with esophageal SCC with cervical lymph node metastasis, the 1-year OS rate of dCRT was 39%, which was also lower than the Netherlands study.
In summary, for esophageal cancer with SLN metastasis, the first-line treatment is 3FD based on MIE or RAMIE, and the treatment efficacy is better than non-surgery, but randomized controlled trials are still needed to further compare the pros and cons of MIE and RAMIE.
3.2.2 Selection of perioperative treatment strategies
3.2.2.1 Comparison of neoadjuvant tri-drugs chemotherapy and neoadjuvant chemoradiotherapy
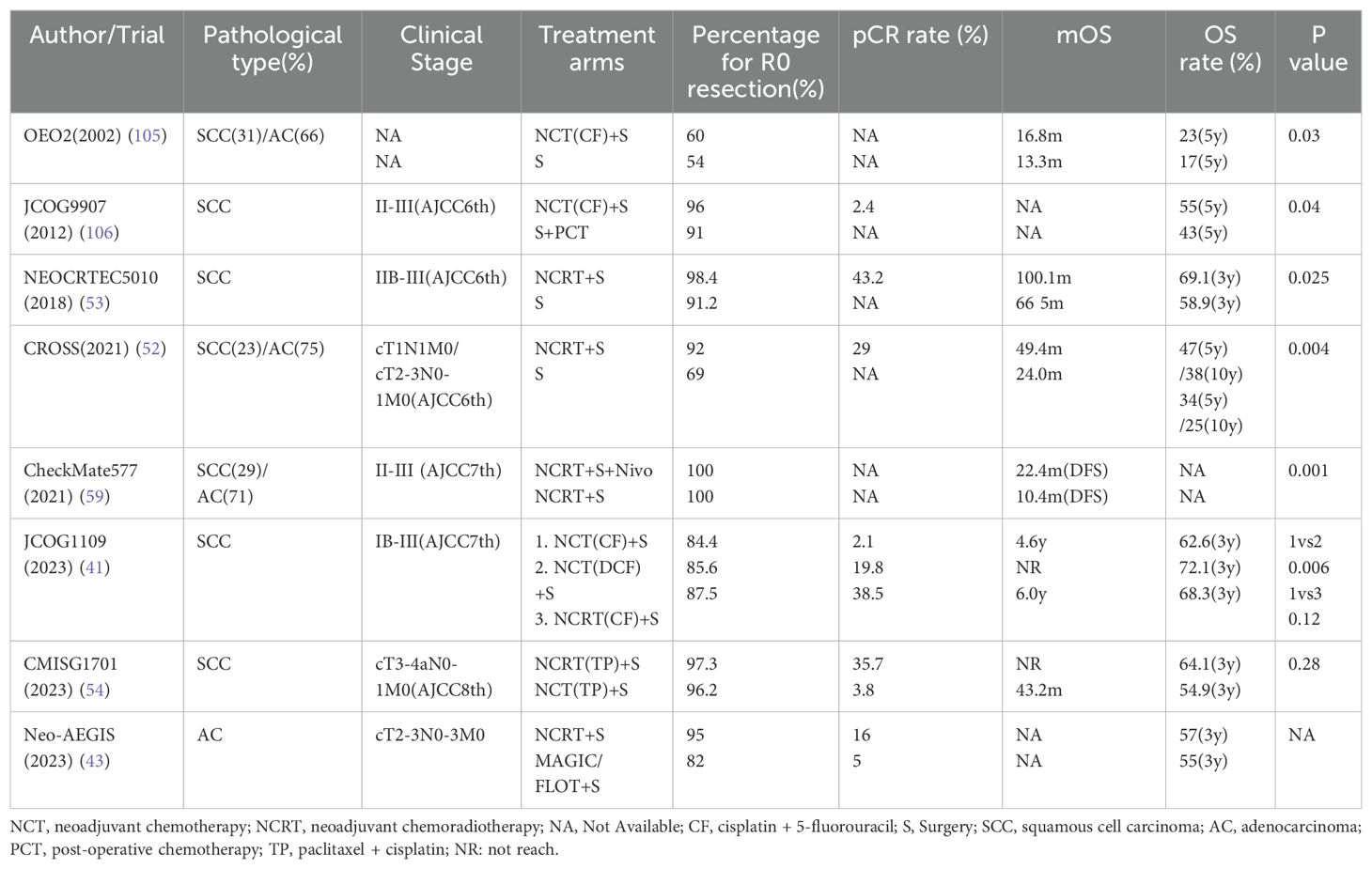
Based on the findings of JCOG1109 (40, 41), the 2022 JES guidelines prioritize a preoperative regimen of docetaxel, cisplatin, and 5-fluorouracil (DCF) in combination with surgery over neoadjuvant chemoradiotherapy for esophageal cancer. This recommendation is based on data from patients with stages IB-III, as defined by the 7th edition of the AJCC, with the understanding that SLNs are considered regional for the upper esophageal segment, making these findings relevant to stage III patients under the current JES12 staging system (Table 3).
Consequently, the JCOG1109 results may not be sufficient to persuade Western countries of the superiority of tri-drug neoadjuvant chemotherapy (NCT) over neoadjuvant chemoradiotherapy.
However, the JCOG1109 trial was limited to SCC patients, which contrasts with the prevalence of AC in Western populations. Previous trials, such as OEO5, have shown that four cycles of neoadjuvant epirubicin, cisplatin, and capecitabine (ECX) did not improve survival compared to two cycles of CF, and thus, do not establish a standard of care (42). Consequently, the JCOG1109 results may not be sufficient to persuade Western countries of the superiority of tri-drug NCT over neoadjuvant chemoradiotherapy.
Furthermore, the CROSS trial, which included SCC patients, did not demonstrate that neoadjuvant chemoradiotherapy was superior to perioperative FLOT chemotherapy for AC at the esophagogastric junction. The Neo-AEGIS trial (43), focusing solely on AC patients, compared the CORSS strategy with the FLOT/MAGIC strategy and found no significant difference in the 3-year OS rate, despite a higher pCR rate with the CROSS strategy. Additionally, for AC patients with HER2 overexpression, the concurrent use of trastuzumab in neoadjuvant chemoradiotherapy has not yet shown a definitive advantage (44).
3.2.2.2 Patients who undergo surgery after neoadjuvant therapy still need higher level evidence to add adjuvant chemotherapy
Post-neoadjuvant therapy, esophageal cancer patients who undergo surgery require robust evidence to support the use of adjuvant chemotherapy. The survival benefits of adjuvant chemotherapy following R0 resection remain a topic of debate, with some studies suggesting no significant survival advantage (45, 46), while others indicate benefits for patients with positive postoperative lymph nodes (47), or even for those with negative lymph nodes (48, 49). The phase II PIECE trial reported a 3-year survival rate of 85% for patients receiving adjuvant S-1 therapy after NCT and R0 resection (50).
3.2.2.3 Neoadjuvant radiotherapy is still the mainstream in Europe and America
The PIECE trial confirmed that R0 resection improves survival, a benefit that neoadjuvant radiotherapy can provide, translating into better outcomes. The CROSS trial (51, 52), where 75% of enrolled patients had AC and 23% had SCC. The neoadjuvant chemoradiotherapy (NCRT) group achieved a higher R0 resection rate (92% vs 69%), with a median overall survival (mOS) of 49.4 months compared to 24.0 months in the surgery group, and a 10-year survival rate of 38% and 25%, respectively. The NEOCRTEC5010 study in China (53), which included stage II-III SCC patients without supraclavicular metastasis, showed similar results, with a 3-year OS rate of 67.2%. However, the CMISG1701 trial (54), involving cT3-4aN0-1M0 ESCC patients, found no difference in the 3-year OS rate between NCT plus surgery and neoadjuvant radiotherapy plus surgery, suggesting that the CROSS strategy may not be superior to JCOG1109 in locally advanced SCC.
The evidence for the benefit of surgery in patients with SLN metastasis is limited, with neither the CROSS nor the JCOG1109 trial specifically incorporating this patient cohort. Clinical trials explicitly addressing SLN metastasis are scarce, and those that do often involve small patient numbers and are not randomized controlled trials (RCTs). A retrospective analysis in Japan revealed encouraging 5-year overall survival (OS) rates of up to 41.3% utilizing neoadjuvant radiotherapy complemented by surgery for SCC with SLN metastasis (55). In Japan’s phase II COSMOS trial (56, 57), which enrolled 48 patients with T4 or supraclavicular metastasis, the administration of DCF neoadjuvant chemotherapy (NCT) plus surgery achieved a pCR rate of 47.9% and a 1-year OS rate of 67.9%. The pCR rate and 1y-OS rate of the Dutch phase II study were 20% and 85% respectively (38), because these two phase II trials have very different pathological types and stages of research objects, they cannot be directly compared, but this suggests to us that for T4 and supraclavicular metastasis esophageal cancer patients, three-drug NCT compared to neoadjuvant radiotherapy is equally safe and effective. The JCOG1510 (58), initiated in 2019 by Japanese researchers, aims to ascertain whether three-drug NCT in conjunction with surgery or radiotherapy outperforms dCRT for esophageal cancer patients with SLN metastasis, with results pending.
In the realm of perioperative treatment strategy selection, generalizations are challenging due to regional variations in disease localization, pathological subtypes, and etiologies. In Asia and the Western world, the predominant esophageal cancer types are SCC and AC, respectively, and the clinical trial outcomes from these regions are not readily interchangeable. The absence of robust evidence leaves the optimal perioperative treatment strategy for patients with SLN metastasis undetermined, with most current treatments exploratory in nature. Future multicenter clinical trials are essential to guide more tailored and effective therapeutic approaches.
3.2.3 Perioperative treatment strategy in the era of immunotherapy
Immunotherapy checkpoint inhibitors have achieved significant results in solid tumors, including esophageal cancer, sparking interest in their potential to enhance perioperative care for this disease.
While no randomized controlled trials have yet established the survival benefits of adjuvant chemotherapy following R0 resection post-neoadjuvant therapy and surgery, the CheckMate577 trial has shifted this paradigm (59). The combination of NCRT, surgery, and adjuvant immunotherapy has emerged as a novel treatment paradigm. Prior clinical trials have consistently shown that patients achieving pCR after neoadjuvant therapy and surgery exhibit significantly improved survival compared to those without pCR (60, 61). We can only analyze the near-term effects from the perspective of pCR and speculate on the long-term effects. In Table 3, the pCR rate of patients undergoing surgery after NCT with three drugs did not exceed 20%. Nonetheless, recent Phase II clinical trials have indicated that the integration of immunotherapy with NCT is both safe and efficacious in esophageal SCC (62–67), with pCR rates ranging from 35.8% to 50%, markedly higher than the rates achieved with two-drug NCT.
Data on the combination of NCRT, immunotherapy, and surgical treatment is currently limited to Phase II clinical trials, primarily from Asia, particularly China, and involves patients exclusively with SCC. To date, no Phase III data is available. The PERFECT study, which only enrolled AC patients, reported a pCR rate of 30% (68), The US Phase Ib clinical trial yielded a pCR rate of 40% (69); The PALACE-1 trial in China had a pCR rate of 55.6% (70), The Phase II clinical trial in South Korea had a pCR rate of 46. 1% (71). For the same enrollment of SCC, the NEOCRTEC5010 study showed that the pCR rate after NCRT was 43.2%, which was lower than the data from the PALACE-1 trial. A meta-analysis showed that NCRT combined with immunotherapy has the best pCR rate among all neoadjuvant treatment plans (72), and the pCR rate of NCRT is better than that of neoadjuvant immunotherapy combined with chemotherapy. Most of the enrollment requirements for these trials exclude patients with SLN metastasis, except for Gong’s Phase II trial (63), but the final pCR rate was not ideal, only 17.6%.
Immunotherapy has demonstrated a huge potential to improve survival in Phase I/II clinical trials and has become a first-line treatment for esophageal cancer. However, as a new treatment method, there is currently no information for patients with supraclavicular metastasis of esophageal cancer, and its improvement in survival still needs to be confirmed by the long-term follow-up results of Phase III clinical trials.
3.2.4 Take home message
After the 7th edition of AJCC staging in 2009, the SLNs changed from regional lymph nodes only for cervical esophageal cancer to regional lymph nodes for all esophageal cancers. Therefore, it can be considered that clinical trials enrolled using the 7th edition of staging include patients with SLN metastasis. Based on current guidelines and clinical trial results, based on the experience in Asia, for patients with JESIII stage (SLN metastasis) esophageal SCC, neoadjuvant tri-drug chemotherapy plus three-field lymphadenectomy and adjuvant immunotherapy is currently the treatment with definite efficacy and the highest level of evidence. And based on the experience of diagnosing and treating esophageal AC in Europe and America, for AC patients, NCRT plus 3FD surgery and adjuvant immunotherapy is currently the first choice.
3.3 Progress in definitive chemoradiotherapy
For patients with JES12th stage III who cannot tolerate or refuse surgery, and patients with stage IVa at initial diagnosis of esophageal cancer, JES recommends dCRT (73).
The optimal management strategy for locally advanced esophageal cancer continues to be a subject of debate. Existing guidelines typically advise a course of NCRT followed by surgery, but ignore the possibility of tumor cure after chemoradiotherapy. Previous clinical trials have confirmed that nearly 40% of SCC patients have a pCR rate after NCRT. How to screen out these patients who can avoid surgery is a problem worthy of attention (Table 4).
Current survival data for patients with SLN metastasis treated with chemoradiotherapy are largely derived from retrospective studies with varying definitions and accuracies of SLNs. Chen et al. retrospectively analyzed 369 patients with esophageal cancer who received dCRT (74), Among them, 70 cases were combined with SLN metastasis, and 299 cases did not have SLN metastasis. The median survival of the supraclavicular metastasis group was 17.2 months, and the non-supraclavicular metastasis group was 18.4 months (p = 0.28). A similar retrospective study in the Netherlands involving 197 patients treated with chemoradiotherapy reported survival times of 23.6 months for supraclavicular metastasis and 17.1 months for non-supraclavicular metastasis (p = 0.51). These analyses suggest that SLN metastasis may not significantly impact prognosis, and that chemoradiotherapy can offer substantial survival benefits to these patients.
With the emergence of immunotherapy, research has intensified on how to optimally integrate it with chemoradiotherapy to maximize survival outcomes. Additionally, determining the optimal radiotherapy dosage, planning concurrent chemotherapy, deciding on the necessity of NCT, and evaluating the differences in adjuvant chemotherapy remain key areas of focus within the oncology community. For IVa stage patients, surgery after conversion treatment is also a hot research topic.
3.3.1 Progress in radiotherapy technology
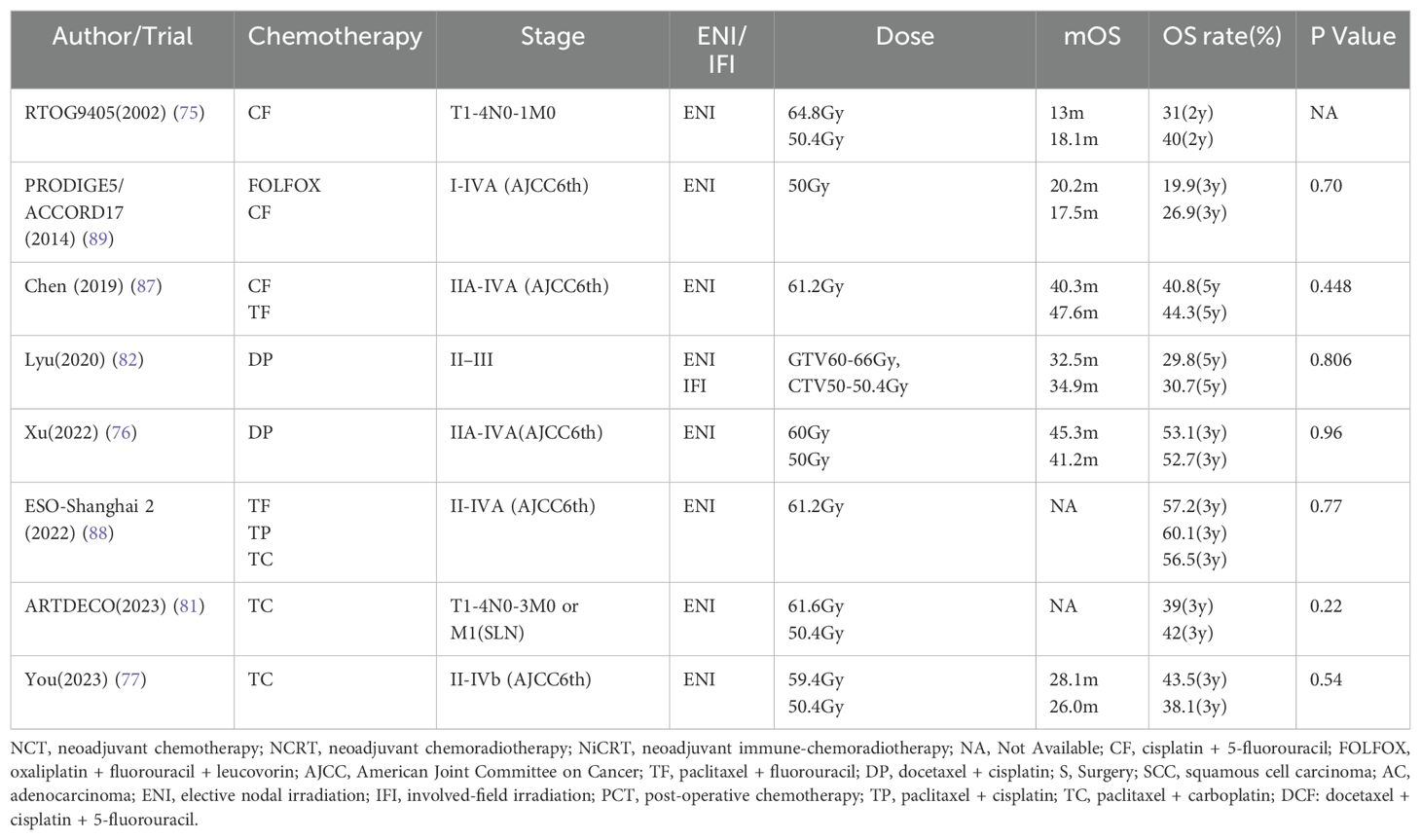
The standard radiation dose for definitive radiotherapy in esophageal cancer is currently in the range of 50-50.4Gy over 25-28 fractions, with IMRT being the preferred radiotherapy technique and Elective Nodal Irradiation (ENI) the chosen method for regional lymph node irradiation.
The relationship between escalated radiotherapy dosage and survival outcomes is a subject of ongoing debate. RTOG9405 confirmed as early as 2002 that there was no statistical difference in survival between 64.8Gy and 50.4Gy radiotherapy (75). Despite technological advancements that have reignited interest in high-dose radiotherapy, Phase III clinical trials have consistently failed to demonstrate a survival advantage, particularly for patients with SLN metastasis (76, 77).
Since increasing the dose of the target volume failed, can increasing the radiotherapy dose of the primary tumor and positive lymph nodes alone benefit patients? Can reducing the target volume still guarantee treatment efficacy? Simultaneous boost radiotherapy technology (SIB) has shown good tolerability and efficacy in some Phase I/II clinical studies. Although retrospective analysis shows that SIB tends to improve survival compared with conventional radiotherapy (78, 79), Chen’s Phase II clinical trial found that (80), locally advanced esophageal SCC, including SLN metastatic patients, received a dose of 54Gy for the subclinical lesion, and the primary tumor and lymph nodes were simultaneously boosted to 66Gy,the 5-year OS rate reached 58.4%.
However, a Phase III clinical trial published in 2021 indicated that boosting the primary lesion to 61.6Gy, as opposed to 50.4Gy, offered no additional benefit (81). It is noteworthy that this trial did not include a boost to metastatic lymph nodes. The involved-field irradiation (IFI) has been proven in Phase III clinical trials to reduce the target volume without affecting the treatment efficacy, and reduce the adverse effects of radiotherapy (82). A recent propensity score-matched study further demonstrated that for stages II-IV cervical esophageal SCC (83), IFI and ENI have equivalent therapeutic efficacy, with a reduced frequency of adverse effects in the IFI group.
The synergistic effect of SIB and IFI on survival benefits is currently under investigation in an ongoing clinical trial in China (84),which aims to provide definitive answers to these questions.
3.3.2 Radiotherapy, chemotherapy, immunotherapy, how to achieve 1 + 1 + 1>3?
Researchers have been avidly exploring the optimal chemotherapy regimen for dCRT in esophageal cancer, as well as the impact of neoadjuvant and adjuvant chemotherapy. The advent of immunotherapy has further complicated and heightened the importance of these inquiries.
The results of JCOG1109 made Japanese researchers believe that DCF tri-drugs chemotherapy is a strong and effective NCT regimen for esophageal SCC (41), which is better than CF combined with radiotherapy. Therefore, some researchers retrospectively analyzed patients with advanced esophageal cancer treated with DCF regimen CCRT and found that the 5-year OS rate of patients with SLN metastasis reached 50% (85), and the 5-year OS rate of stage III patients (AJCC 8th edition) reached 76.2%. Another study published in Japan showed that the 3-year OS rate of cervical esophageal cancer treated with DCF combined with radiotherapy reached 44.2% (86), among the 18 enrolled patients, 9 were T4b and 7 were SLN metastasis. After treatment, 15 patients were CR. Two results of Phase III clinical trials reported by Chinese researchers in 2019 and 2022 respectively showed that in dCRT (87, 88), whether it is paclitaxel plus cisplatin (TP), paclitaxel plus carboplatin (TC), or paclitaxel plus 5-fluorouracil (TF), there is no survival benefit superior to the CF regimen. Therefore, TP and CF are still the first-line chemotherapy regimens for dCRT. The DCF regimen seems to have the opportunity to become a new first-line chemotherapy regimen, but it still needs to be confirmed by Phase III clinical trials. For esophageal AC, oxaliplatin plus fluorouracil plus leucovorin (FOLFOX) regimen has no survival benefit superior to the CF regimen (89).
A retrospective study that enrolled patients with SLN metastasis analyzed the differences of three different treatment strategies and found that NCT plus CCRT plus consolidation chemotherapy and NCT plus CCRT, CCRT plus consolidation chemotherapy had no survival differences (mOS 54.0m vs 35.5m vs 45.9m) (90), A meta-analysis showed that NCT or consolidation chemotherapy compared with simple CCRT can improve the 3-year OS rate (91), but the 5-year OS rate is no different. Consolidation chemotherapy can reduce the risk of distant metastasis, but these opinions need to be confirmed by clinical trials.
The efficacy of immunotherapy in dCRT for esophageal cancer is a burgeoning area of research. A phase II study in Japan (92), the TENERGY trial showed that 105 unresectable late-stage patients, including those with SLN metastasis of esophageal cancer, had a mOS of 31.0 months after dCRT followed by sequential immunotherapy with atezolizumab. A phase Ib clinical trial of carrelizumab monotherapy and definitive radiation therapy for unresectable locally advanced esophageal SCC preliminarily explored its safety and feasibility (93), with a median follow-up time of 31.0 months, the mOS and median PFS (mPFS) were 16.7 months and 11.7 months respectively. A phase II clinical trial showed that dCRT with durvalumab and tremelimumab had promising efficacy in patients with locally advanced esophageal SCC (94), the 2-year PFS rate and OS rate were 57.5% and 75% respectively. A retrospective analysis believes that the use of neoadjuvant immunotherapy plus CCRT can achieve better PFS than CCRT alone (95), the 1-year PFS rates were 72.6% and 60.0% respectively.
Regarding whether the combination of immunotherapy and radiotherapy will increase adverse effects, especially treatment-related pneumonia, the American Food and Drug Administration (FDA) has indicated that immunotherapy performed within 90 days of the patient receiving radiotherapy may not affect the occurrence of immune adverse events (96).
The integration of immunotherapy with chemoradiotherapy, whether concurrently or sequentially, and its impact on survival and adverse effects, particularly treatment-related pneumonia, is a subject of ongoing phase III clinical trials. The optimal timing and sequence of immunotherapy in relation to radiotherapy remain subjects of active investigation, with numerous studies currently in progress.
3.3.3 Esophageal cancer with supraclavicular lymph node metastasis, surgery or chemoradiotherapy?
A propensity score-matching analysis from China showed that (97), for esophageal cancer with SLN metastasis, compared with the dCRT group, patients in the NCT combined with surgery group had a better 3-year OS rate (72.0% vs. 35.8%), PFS (24 vs. 14 months) and a lower 3-year tumor-related death rate (25.1% vs. 53.7%). In addition, compared with the simple radiotherapy group, patients in the dCRT group had a better 3-year survival rate (30.1% vs. 18.6%) and a lower 3-year tumor-related death rate (57.9% vs. 76.8%). A phase II randomized controlled trial compared the survival of locally advanced esophageal SCC after NCRT with clinical CR followed by dCRT or surgery (98), and there was no difference in survival between the two groups. A database-based analysis showed (99), esophageal cancer receiving dCRT plus salvage surgery and NCRT combined with surgery had no difference in survival. NEEDs trial is a multicenter phase III randomized controlled trial (100), planning to enroll 1200 patients with locally advanced esophageal SCC, comparing NCRT combined with surgery and dCRT followed by follow-up or salvage surgery, in the future can possibly answer which treatment strategy is the best for locally advanced esophageal SCC, but it is a pity that this trial did not enroll patients with SLN metastasis.
Based on the current limited clinical research conclusions, esophageal cancer patients with SLN metastasis can consider regular follow-up after achieving clinical CR with dCRT, and salvage surgery can be performed when the tumor is not controlled or recurs.
3.3.4 Take home message
For esophageal cancer with SLN metastasis, if surgery is not possible or refused, dCRT is the first choice, with a radiation dose of 50-50.4Gy/25-28F, the first choice of radiotherapy technology is IMRT, for lymph nodes, IFI is recommended to reduce radiation adverse effects, concurrent chemotherapy regimens including CF or TP, are all recommended, there is no obvious benefit from adjuvant chemotherapy, adjuvant immunotherapy is widely recommended, but there is currently a lack of strong evidence to confirm its ability to improve survival. It is recommended to follow up closely after chemoradiotherapy, and timely perform salvage surgery when the tumor is not controlled or recurs.
3.4 Progress in systemic therapy
For esophageal cancer patients with SLN metastasis, classified as stage IVb according to the AJCC 8th edition, NCCN recommends systemic therapy with or without best supportive care. While IVb stage in JES12th (combined with other distant metastases), if the patient is combined with esophageal obstruction symptoms, still recommend chemoradiotherapy to relieve local symptoms.
For IVb stage patients with esophageal cancer combined with SLN metastasis as the only metastatic lesion, chemotherapy plus immunotherapy maybe not sufficient. A phase II clinical trial showed (101), the mOS of metastatic esophageal SCC receiving chemoradiotherapy versus simple chemotherapy was 18.3 months and 10.2 months, respectively, and the 2-year OS rate was 43.3% and 26.7%. A recent retrospective analysis showed (102), the 5-year OS of metastatic esophageal SCC receiving chemoradiotherapy was better than simple chemotherapy, 17.6% and 8.2% respectively. Thus, whether it is SLNs or combined with other distant metastases, chemoradiotherapy is better than chemotherapy.
3.4.1 Optimal first-line chemotherapy regimens
NCCN adopts different chemotherapy regimens for different pathological types (28). JES guidelines just recommended chemotherapy regimen SCC, which is main pathological type in the East Asian population (73), and no regimen have been developed for other pathological types. JES recommends CF plus PD-1 inhibitor for all stage IVb esophageal cancers, while NCCN guidelines recommend the use of oxaliplatin instead of cisplatin to reduce chemotherapy related adverse events, and the first-line recommended chemotherapy regimen for SCC patients is 5-fluorouracil plus oxaliplatin plus PD-1 inhibitor, for AC, depending on whether HER-2 is overexpressed to decide whether to add trastuzumab or not.
Currently, there is a dearth of clinical trials or retrospective analyses specifically addressing chemotherapy for esophageal cancer patients with SLN metastasis.
According to existing guidelines, the first-line regimen for esophageal cancer chemotherapy alone involves a combination of 5-fluorouracil, cisplatin or oxaliplatin, and a PD-1 inhibitor. For AC patients with HER2 overexpression, trastuzumab may be incorporated. For patients with PD-L1 expression of 1% or more, a dual immunotherapy regimen of nivolumab plus ipilimumab may be considered.
3.4.2 Progress in targeted therapy
The 2022 guidelines for the diagnosis and treatment of esophageal cancer compiled by JES believe (73), in addition to immunotherapy, there is currently not enough evidence to show that first-line chemotherapy combined with cetuximab, panitumumab and other EGFR monoclonal antibodies can improve survival, EGFR-TKI, such as gefitinib compared with placebo also did not achieve survival benefit, the guidelines did not mention other targeted drugs. The latest version of the NCCN guidelines mentions several targets such as Her-2, VEGFR, NTRK, PD-1, CTLA-4, RET, BRAF V600E (28), the corresponding targeted drugs have all demonstrated effectiveness in clinical trials, but currently except for Her-2 targeted drug trastuzumab and immune checkpoint inhibitors:pembrolizumab, nivolumab, ipilimumab, dostarlimab-gxly can be used as first-line drugs, the rest are all second-line treatment drugs.
Zolbetuximab, a novel monoclonal antibody, targets the Claudin-18 isoform 2 (CLDN18.2) protein, which is expressed in both normal gastric cells and malignant gastric or EGJ adenocarcinoma cells. Zolbetuximab has been proven to be effective in CLDN18.2 positive EGJ adenocarcinoma (103), combining zolbetuximab with modified folinic acid, fluorouracil, and oxaliplatin regimen (mFOLFOX6), compared to using mFOLFOX6 alone, the combination treatment group had a mPFS of 10.61 months, compared to 8.67 months in the control group, which may become a new first-line treatment option for these patients. Another study targeted Her-2 negative locally advanced unresectable or metastatic gastric or EGJ adenocarcinoma patients (104), zolbetuximab combined with capecitabine and oxaliplatin as first-line treatment, significantly improved PFS and OS, 8.21 months and 14.39 months compared to 6.80 months and 12.16 months. Therefore, this treatment combination may become a new first-line treatment option for Her-2 negative advanced gastric or EGJ adenocarcinoma patients.
For esophageal SCC, EGFR monoclonal antibodies and TKIs can be considered for second-line and subsequent treatment. For esophageal AC, there are currently several new targeted drugs proven to be effective, but as first-line treatment still needs further confirmation from clinical trials, at present, immune checkpoint inhibitors and Her-2 targeted drugs are still the main force in targeted therapy for esophageal cancer.
3.4.3 Take home message
Esophageal cancer with SLN metastasis, in the AJCC8th staging, is stage IVb. It is recommended to perform second-generation sequencing to detect gene mutations, and choose different first-line treatment regimen based on pathological types and gene detection results. The first-line regimen for SCC patients is CF combined with PD-1 inhibitor. For AC patients, the regimen consists of 5-fluorouracil, oxaliplatin, and a PD-1 inhibitor, with the addition of trastuzumab for HER2-overexpressing tumors.
4 Summary and future perspectives
The treatment of esophageal cancer, particularly for patients with SLN metastasis, continues to present significant challenges. The variability in pathological types and the lack of consensus on SLN definitions and staging systems between East Asia and Western countries have led to diverse treatment strategies. The forthcoming results of the Tiger study are anticipated to provide clarity and standardization in this regard (26).
In East Asia, where ESCC is prevalent, the approach to treating patients with SLN metastasis draws from experiences that emphasize comprehensive treatment strategies. These may include radiotherapy or surgery-based treatments, with decisions informed by the staging outlined in the 12th edition of the JES guidelines. Conversely, in Western countries, where AC is more common, the 8th edition of the AJCC classifies SLN metastasis as distant metastasis, advocating for systemic therapy as the initial approach.
Emerging evidence supports the notion that a multidisciplinary approach, incorporating either radiotherapy or surgery, can yield superior outcomes compared to chemotherapy alone. As we look to the future, the integration of immune checkpoint inhibitors with existing treatment modalities is a promising avenue that warrants further exploration. The optimal sequencing and combination of these agents with other therapies remain critical issues to address, as the widespread adoption of immunotherapy has highlighted the need for strategies that maximize clinical benefit while minimizing adverse effects.
Targeted therapy for ESCC is another burgeoning research area. While the combination of chemoradiation and targeted therapy has shown potential to enhance treatment efficacy and reduce drug resistance, the current incidence of adverse events associated with such combination treatments is still concerning. The complex interplay among signaling pathways targeted by these therapies suggests a need for caution to prevent unforeseen complications. Therefore, the development of novel targeted therapies must prioritize the assessment of adverse events as a key factor in ensuring patient safety and treatment tolerability.
In summary, as we await the definitive findings of the Tiger study to guide more uniform and effective treatment strategies, the ongoing evolution of therapeutic options for esophageal cancer, including the integration of immunotherapy and targeted agents, holds great promise for the future management of this disease.
Author contributions
QC: Writing – original draft, Writing – review & editing. YH: Writing – review & editing. XH: Writing – review & editing. TC: Writing – review & editing. CC: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Yamasaki M, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, et al. Pattern of lymphatic spread of esophageal cancer at the cervicothoracic junction based on the tumor location: surgical treatment of esophageal squamous cell carcinoma of the cervicothoracic junction. Ann Surg Oncol. (2015) 22 Suppl 3:S750–757. doi: 10.1245/s10434-015-4855-y
3. Tachimori Y, Ozawa S, Numasaki H, Matsubara H, Shinoda M, Toh Y, et al. Supraclavicular node metastasis from thoracic esophageal carcinoma: A surgical series from a Japanese multi-institutional nationwide registry of esophageal cancer. J Thorac Cardiovasc Surg. (2014) 148:1224–9. doi: 10.1016/j.jtcvs.2014.02.008
4. Udagawa H, Ueno M, Shinohara H, Haruta S, Kaida S, Nakagawa M, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol. (2012) 106:742–7. doi: 10.1002/jso.v106.6
5. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol. (2017) 12(1):36–42.
6. Mine S, Tanaka K, Kawachi H, Shirakawa Y, Kitagawa Y, Toh Y, et al. Japanese Classification of Esophageal Cancer. 12th Edition: Part I. Esophagus. (2024) 21(3):179–215.
8. Robbins KT, Medina JE, Wolfe GT, Levine PA, Sessions RB, Pruet CW. Standardizing neck dissection terminology. Official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. (1991) 117:601–5. doi: 10.1001/archotol.1991.01870180037007
9. Robbins KT. Classification of neck dissection: current concepts and future considerations. Otolaryngol Clin North Am. (1998) 31:639–55. doi: 10.1016/S0030-6665(05)70077-3
10. Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. (2002) 128:751–8. doi: 10.1001/archotol.128.7.751
11. Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. (2000) 174:837–44. doi: 10.2214/ajr.174.3.1740837
12. Grégoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol. (2003) 69:227–36. doi: 10.1016/j.radonc.2003.09.011
13. Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. (2014) 110:172–81. doi: 10.1016/j.radonc.2013.10.010
14. Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg. (1978) 76:832–9. doi: 10.1016/S0022-5223(19)39559-5
15. Tisi GM, Friedman PJ, Peters RM, Pearson G, Carr D, Lee RE, et al. Clinical staging of primary lung cancer. Am Rev Respir Dis. (1983) 127:659–64. doi: 10.1164/arrd.1983.127.5.659
16. Friedman PJ. Lung cancer: update on staging classifications. AJR Am J Roentgenol. (1988) 150:261–4. doi: 10.2214/ajr.150.2.261
17. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. (2009) 4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e
18. Jawad H, Sirajuddin A, Chung JH. Review of the international association for the study of lung cancer lymph node classification system: localization of lymph node stations on CT imaging. Clin Chest Med. (2013) 34:353–63. doi: 10.1016/j.ccm.2013.04.008
19. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. (2010) 17:1721–4. doi: 10.1245/s10434-010-1024-1
20. Huang W, Huang Y, Sun J, Liu X, Zhang J, Zhou T, et al. Atlas of the thoracic lymph nodal delineation and recommendations for lymph nodal CTV of esophageal squamous cell cancer in radiation therapy from China. Radiother Oncol. (2015) 116:100–6. doi: 10.1016/j.radonc.2015.06.024
21. Xu HY, Wu SX, Luo HS, Chen CY, Lin LX, Huang HC. Analysis of definitive chemo-radiotherapy for esophageal cancer with supra-clavicular node metastasis based on CT in a single institutional retrospective study: a propensity score matching analysis. Radiat Oncol. (2018) 13:200. doi: 10.1186/s13014-018-1145-4
22. Jeene PM, Versteijne E, van Berge Henegouwen MI, Bergmann JJ, Geijsen ED, van Laarhoven HW, et al. Supraclavicular node disease is not an independent prognostic factor for survival of esophageal cancer patients treated with definitive chemoradiation. Acta Oncol. (2017) 56:33–8. doi: 10.1080/0284186X.2016.1240880
23. Hagens ERC, van Berge Henegouwen MI, van Sandick JW, Cuesta MA, van der Peet DL, Heisterkamp J, et al. Distribution of lymph node metastases in esophageal carcinoma [TIGER study]: study protocol of a multinational observational study. BMC Cancer. (2019) 19:662. doi: 10.1186/s12885-019-5761-7
24. Wen J, Chen D, Zhao T, Chen J, Zhao Y, Liu D, et al. Should the clinical significance of supraclavicular and celiac lymph node metastasis in thoracic esophageal cancer be reevaluated? Thorac Cancer. (2019) 10:1725–35. doi: 10.1111/1759-7714.13144
25. Wang F, Ge X, Wang Z, Weng Y, Yin R, You Q. Clinical significance and prognosis of supraclavicular lymph node metastasis in patients with thoracic esophageal cancer. Ann Trans Med. (2020) 8:90–0. doi: 10.21037/atm.2019.12.118
26. Numata Y, Abe T, Higaki E, Hosoi T, Fujieda H, Nagao T, et al. Should the supraclavicular lymph nodes be considered regional lymph nodes in cervical esophageal cancer? Ann Surg Oncol. (2022) 29:616–26. doi: 10.1245/s10434-021-10664-0
27. Yamasaki M, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, et al. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification: impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol. (2014) 21:2850–6. doi: 10.1245/s10434-014-3696-4
28. Ajani JA, D’Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2023) 21:393–422. doi: 10.6004/jnccn.2023.0019
29. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol. (2020) 38:2677–94. doi: 10.1200/JCO.20.00866
30. Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:992–1004. doi: 10.1016/j.annonc.2022.07.003
31. Vagliasindi A, Franco FD, Degiuli M, Papis D, Migliore M. Extension of lymph node dissection in the surgical treatment of esophageal and gastroesophageal junction cancer: seven questions and answers. Future Oncol. (2023) 19:327–39. doi: 10.2217/fon-2021-0545
32. Chao YK, Hsieh MJ, Liu YH, Liu HP. Lymph node evaluation in robot-assisted versus video-assisted thoracoscopic esophagectomy for esophageal squamous cell carcinoma: A propensity-matched analysis. World J Surg. (2018) 42:590–8. doi: 10.1007/s00268-017-4179-0
33. Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. (2012) 379:1887–92. doi: 10.1016/S0140-6736(12)60516-9
34. Luketich JD, Pennathur A, Franchetti Y, Catalano PJ, Swanson S, Sugarbaker DJ, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg. (2015) 261:702–7. doi: 10.1097/SLA.0000000000000993
35. Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. (2017) 266:232–6. doi: 10.1097/SLA.0000000000002171
36. van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: A randomized controlled trial. Ann Surg. (2019) 269:621–30. doi: 10.1097/SLA.0000000000003031
37. Yang Y, Li B, Yi J, Hua R, Chen H, Tan L, et al. Robot-assisted versus conventional minimally invasive esophagectomy for resectable esophageal squamous cell carcinoma: early results of a multicenter randomized controlled trial: the RAMIE trial. Ann Surg. (2022) 275:646–53. doi: 10.1097/SLA.0000000000005023
38. van der Horst S, Weijs TJ, Braunius WW, Mook S, Mohammed NH, Brosens L, et al. Safety and feasibility of robot-assisted minimally invasive esophagectomy (RAMIE) with three-field lymphadenectomy and neoadjuvant chemoradiotherapy in patients with resectable esophageal cancer and cervical lymph node metastasis. Ann Surg Oncol. (2023) 30:2743–52. doi: 10.1245/s10434-022-12996-x
39. Yamashita M, Takenaka HY, Nakagawa K. Semi-radical chemoradiotherapy for 53 esophageal squamous cell carcinomas with supraclavicular lymph node metastasis in a single institutional retrospective study. Hepatogastroenterology. (2014) 61:1971–8.
40. Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. (2013) 43:752–5. doi: 10.1093/jjco/hyt061
41. Kato K, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol. (2022) 40:238–8. doi: 10.1200/JCO.2022.40.4_suppl.238
42. Alderson D, Cunningham D, Nankivell M, Blazeby JM, Griffin SM, Crellin A, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol. (2017) 18:1249–60. doi: 10.1016/S1470-2045(17)30447-3
43. Reynolds JV, Preston SR, O’Neill B, Lowery MA, Baeksgaard L, Crosby T, et al. Neo-AEGIS (Neoadjuvant Trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Final primary outcome analysis. J Clin Oncol. (2023) 41:295–5. doi: 10.1200/JCO.2023.41.4_suppl.295
44. Safran HP, Winter K, Ilson DH, Wigle D, DiPetrillo T, Haddock MG, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): a multicentre, randomised, phase 3 trial. Lancet Oncol. (2022) 23:259–69. doi: 10.1016/S1470-2045(21)00718-X
45. Sisic L, Blank S, Nienhüser H, Haag GM, Jäger D, Bruckner T, et al. The postoperative part of perioperative chemotherapy fails to provide a survival benefit in completely resected esophagogastric adenocarcinoma. Surg Oncol. (2020) 33:177–88. doi: 10.1016/j.suronc.2017.06.001
46. Papaxoinis G, Kamposioras K, Weaver JMJ, Kordatou Z, Stamatopoulou S, Germetaki T, et al. The role of continuing perioperative chemotherapy post surgery in patients with esophageal or gastroesophageal junction adenocarcinoma: a multicenter cohort study. J Gastrointest Surg. (2019) 23:1729–41. doi: 10.1007/s11605-018-04087-8
47. Li J, Qiu R, Hu Y, Wang Y, Qi Z, He M, et al. Postoperative adjuvant therapy for patients with pN+ Esophageal squamous cell carcinoma. BioMed Res Int. (2021) 2021:8571438. doi: 10.1155/2021/8571438
48. Deng X, He W, Jiang Y, Deng S, Mao T, Leng X, et al. The impact of adjuvant therapy on survival for node-negative esophageal squamous cell carcinoma: a propensity score-matched analysis. Ann Transl Med. (2021) 9:998. doi: 10.21037/atm-21-2539
49. Kamarajah SK, Markar SR, Phillips AW, Kunene V, Fackrell D, Salti GI, et al. Survival benefit of adjuvant chemotherapy following neoadjuvant therapy and oesophagectomy in oesophageal adenocarcinoma. Eur J Surg Oncol. (2022) 48:1980–7. doi: 10.1016/j.ejso.2022.05.014
50. Nomura M, Goto M, Watanabe M, Hato S, Kato K, Baba E, et al. Phase II trial of perioperative chemotherapy of esophageal cancer: PIECE trial. J Clin Oncol. (2022) 40:4038–8. doi: 10.1200/JCO.2022.40.16_suppl.4038
51. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
52. Eyck BM, Van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, Van Hagen P, et al. Ten-Year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39:1995–2004. doi: 10.1200/JCO.20.03614
53. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-Label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
54. Tang H, Wang H, Fang Y, Zhu JY, Yin J, Shen YX, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol. (2023) 34:163–72. doi: 10.1016/j.annonc.2022.10.508
55. Sato Y, Motoyama S, Wada Y, Wakita A, Kawakita Y, Nagaki Y, et al. Neoadjuvant chemoradiotherapy followed by esophagectomy with three-Field lymph node dissection for thoracic esophageal squamous cell carcinoma patients with clinical stage III and with supraclavicular lymph node metastasis. Cancers (Basel). (2021) 13(5). doi: 10.3390/cancers13050983
56. Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, et al. A 3-year overall survival update from a phase 2 study of chemoselection with DCF and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Ann Surg Oncol. (2020) 27:460–7. doi: 10.1245/s10434-019-07654-8
57. Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. (2016) 115:1328–34. doi: 10.1038/bjc.2016.350
58. Terada M, Hara H, Daiko H, Mizusawa J, Kadota T, Hori K, et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol. (2019) 49:1055–60. doi: 10.1093/jjco/hyz112
59. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. New Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
60. Hipp J, Kuvendjiska J, Hillebrecht HC, Timme-Bronsert S, Fichtner-Feigl S, Hoeppner J, et al. Pathological complete response in multimodal treatment of esophageal cancer: a retrospective cohort study. Dis Esophagus. (2023) 36(7). doi: 10.1093/dote/doac095
61. Schroeder W, Ghadimi MPH, Schloesser H, Loeser H, Schiller P, Zander T, et al. Long-term outcome after histopathological complete response with and without nodal metastases following multimodal treatment of esophageal cancer. Ann Surg Oncol. (2022) 29(suppl 5). doi: 10.1245/s10434-022-11700-3
62. Qi Y, Yang Y, Li W, Yue S, Ma Y, Liu D, et al. A randomized controlled study on perioperative treatment of esophageal squamous cell carcinoma with camrelizumab combined with nab-paclitaxel and cisplatin. J Clin Oncol. (2023) 41:e16075–5. doi: 10.1200/JCO.2023.41.16_suppl.e16075
63. Gong L, Yang Y, Zhang H, Qiao Y, Wang H, Ren P, et al. Camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma (ESCC): A single-arm, phase II trial. J Clin Oncol. (2023) 41:4048–8. doi: 10.1200/JCO.2023.41.16_suppl.4048
64. Li Y, Zhou A, Liu S, He M, Chen K, Tian Z, et al. Comparing a PD-L1 inhibitor plus chemotherapy to chemotherapy alone in neoadjuvant therapy for locally advanced ESCC: a randomized Phase II clinical trial. BMC Med. (2023) 21:86. doi: 10.1186/s12916-023-02804-y
65. Zhang R, Song DS, Liu W, Yao L, Chen AG, Ge W, et al. Efficacy and safety of camrelizumab combined with chemotherapy versus chemotherapy alone as preoperative neoadjuvant therapy for resectable locally advanced esophageal squamous cell carcinoma: Preliminary results from a multicenter, prospective, randomized controlled study. J Clin Oncol. (2023) 41:4064–4. doi: 10.1200/JCO.2023.41.16_suppl.4064
66. Chen X, Xu X, Wang D, Liu J, Sun J, Lu M, et al. Neoadjuvant sintilimab and chemotherapy in patients with potentially resectable esophageal squamous cell carcinoma (KEEP-G 03): an open-label, single-arm, phase 2 trial. J Immunother Cancer. (2023) 11(2). doi: 10.1136/jitc-2022-005830
67. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg. (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
68. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res. (2021) 27:3351–9. doi: 10.1158/1078-0432.CCR-20-4443
69. Kelly RJ, Smith KN, Anagnostou V, Thompson E, Hales RK, Battafarano RJJ, et al. Neoadjuvant nivolumab plus concurrent chemoradiation in stage II/III esophageal/gastroesophageal junction cancer. J Clin Oncol. (2019) 37:142–2. doi: 10.1200/JCO.2019.37.4_suppl.142
70. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
71. Lee S, Ahn BC, Park SY, Kim DJ, Lee CG, Cho J, et al. 1859P - A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). Ann Oncol. (2019) 30:v754. doi: 10.1093/annonc/mdz065.004
72. Wang T, Cai L, Guo Z, You P, Liu S, Qiu H, et al. Comparison of efficacy and safety of neoadjuvant regimens for resectable esophageal squamous cell carcinoma: A meta-analysis. J Clin Oncol. (2023) 41:e16098–8. doi: 10.1200/JCO.2023.41.16_suppl.e16098
73. Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. (2023) 20:343–72. doi: 10.1007/s10388-023-00993-2
74. Chen YH, Lu HI, Lo CM, Wang YM, Chou SY, Huang CH, et al. The clinical impact of supraclavicular lymph node metastasis in patients with locally advanced esophageal squamous cell carcinoma receiving curative concurrent chemoradiotherapy. PloS One. (2018) 13:e0198800. doi: 10.1371/journal.pone.0198800
75. Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. (2002) 20:1167–74. doi: 10.1200/JCO.2002.20.5.1167
76. Xu Y, Dong B, Zhu W, Li J, Huang R, Sun Z, et al. A phase III multicenter randomized clinical trial of 60 gy versus 50 gy radiation dose in concurrent chemoradiotherapy for inoperable esophageal squamous cell carcinoma. Clin Cancer Res. (2022) 28:1792–9. doi: 10.1158/1078-0432.CCR-21-3843
77. You J, Zhu S, Li J, Li J, Shen J, Zhao Y, et al. High-dose versus standard-dose intensity-modulated radiotherapy with concurrent paclitaxel plus carboplatin for patients with thoracic esophageal squamous cell carcinoma: A randomized, multicenter, open-label, phase 3 superiority trial. Int J Radiat Oncol Biol Phys. (2023) 115:1129–37. doi: 10.1016/j.ijrobp.2022.11.006
78. Lan W, Lihong L, Chun H, Shutang L, Qi W, Liang X, et al. Comparison of efficacy and safety between simultaneous integrated boost intensity-modulated radiotherapy and standard-dose intensity-modulated radiotherapy in locally advanced esophageal squamous cell carcinoma: a retrospective study. Strahlenther Onkol. (2022) 198:802–11. doi: 10.1007/s00066-021-01894-y
79. Li C, Tan L, Liu X, Wang X, Zhou Z, Chen D, et al. Definitive simultaneous integrated boost versus conventional-Fractionated intensity modulated radiotherapy for patients with advanced esophageal squamous cell carcinoma: A propensity score-Matched analysis. Front Oncol. (2021) 11:618776. doi: 10.3389/fonc.2021.618776
80. Chen C, Chen J, Luo T, Wang S, Guo H, Zeng C, et al. Late toxicities, failure patterns, local tumor control, and survival of esophageal squamous cell carcinoma patients after chemoradiotherapy with a simultaneous integrated boost: A 5-Year phase II study. Front Oncol. (2021) 11:738936. doi: 10.3389/fonc.2021.738936
81. Hulshof M, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO study). J Clin Oncol. (2021) 39:2816–24. doi: 10.1200/JCO.20.03697
82. Lyu J, Yisikandaer A, Li T, Zhang X, Wang X, Tian Z, et al. Comparison between the effects of elective nodal irradiation and involved-field irradiation on long-term survival in thoracic esophageal squamous cell carcinoma patients: A prospective, multicenter, randomized, controlled study in China. Cancer Med. (2020) 9:7460–8. doi: 10.1002/cam4.v9.20
83. Wang J, Wu Y, Zhang W, Chen Y, Liu Q, Jing S, et al. Elective nodal irradiation versus involved-field irradiation for stage II-IV cervical esophageal squamous cell carcinoma patients undergoing definitive concurrent chemoradiotherapy: a retrospective propensity study with 8-year survival outcomes. Radiat Oncol. (2023) 18:142. doi: 10.1186/s13014-023-02332-2
84. Gao LR, Wang X, Han W, Deng W, Li C, Wang X, et al. A multicenter prospective phase III clinical randomized study of simultaneous integrated boost intensity-modulated radiotherapy with or without concurrent chemotherapy in patients with esophageal cancer: 3JECROG P-02 study protocol. BMC Cancer. (2020) 20:901. doi: 10.1186/s12885-020-07387-y
85. Sakai M, Sohda M, Uchida S, Yamaguchi A, Watanabe T, Saito H, et al. Concurrent chemoradiotherapy with docetaxel, cisplatin, and 5-Fluorouracil (DCF-RT) for patients with potentially resectable esophageal cancer. Anticancer Res. (2022) 42:4929–35. doi: 10.21873/anticanres.15999
86. Okamoto H, Taniyama Y, Sato C, Fukutomi T, Ozawa Y, Ando R, et al. Definitive chemoradiotherapy with docetaxel, cisplatin, and 5-Fluorouracil for advanced cervical esophageal cancer: A medium-Term outcome. Asian Pac J Cancer Prev. (2022) 23:495–9. doi: 10.31557/APJCP.2022.23.2.495
87. Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, et al. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: A randomized, multicenter, phase III clinical trial. J Clin Oncol. (2019) 37:1695–703. doi: 10.1200/JCO.18.02122
88. Ai D, Ye J, Wei S, Li Y, Luo H, Cao J, et al. Comparison of 3 paclitaxel-Based chemoradiotherapy regimens for patients with locally advanced esophageal squamous cell cancer: A randomized clinical trial. JAMA Netw Open. (2022) 5:e220120. doi: 10.1001/jamanetworkopen.2022.0120
89. Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. (2014) 15:305–14. doi: 10.1016/S1470-2045(14)70028-2
90. Xiang M, Liu B, Zhang G, Gong H, Han D, Ma C. Induction chemotherapy followed by chemoradiotherapy with or without consolidation chemotherapy versus chemoradiotherapy followed by consolidation chemotherapy for esophageal squamous cell carcinoma. Front Oncol. (2022) 12:813021. doi: 10.3389/fonc.2022.813021
91. Wang J, Xiao L, Wang S, Pang Q, Wang J. Addition of induction or consolidation chemotherapy in definitive concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone for patients with unresectable esophageal cancer: A systematic review and meta-analysis. Front Oncol. (2021) 11:665231. doi: 10.3389/fonc.2021.665231
92. Bando H, Kumagai S, Kotani D, Saori M, Habu T, Tsushima T, et al. 1211P A multicenter phase II study of atezolizumab monotherapy following definitive chemoradiotherapy for unresectable locally advanced esophageal squamous cell carcinoma (EPOC1802). Ann Oncol. (2022) 33:S1102–3. doi: 10.1016/j.annonc.2022.07.1329
93. Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncol. (2021) 26:e1110–24. doi: 10.1002/onco.13797
94. Park S, Oh D, Choi Y-L, Chi SA, Kim K, Ahn M-J, et al. Durvalumab and tremelimumab with definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancer. (2022) 128:2148–58. doi: 10.1002/cncr.v128.11
95. Peng F, Lian HM, Niu SQ, Liufu WJ, Yu TT, Bao Y. Induction anti-PD-1 immunotherapy plus chemotherapy followed by definitive chemoradiation therapy in locally advanced esophageal squamous cell carcinoma: A real-world retrospective study. Int J Radiat OncologyBiologyPhysics. (2022) 114:e165. doi: 10.1016/j.ijrobp.2022.07.1041
96. Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: A pooled analysis of trials in the US food and drug administration database. JAMA Oncol. (2022) 8:232–40. doi: 10.1001/jamaoncol.2021.6439
97. Yu Y, Xu L, Chen X, Li H, Liu Q, Zhang R, et al. Neoadjuvant therapy combined with surgery is superior to chemoradiotherapy in esophageal squamous cell cancer patients with resectable supraclavicular lymph node metastasis: a propensity score-matched analysis. Ann Transl Med. (2022) 10:349. doi: 10.21037/atm-22-577
98. Qian D, Chen X, Shang X, Wang Y, Tang P, Han D, et al. Definitive chemoradiotherapy versus neoadjuvant chemoradiotherapy followed by surgery in patients with locally advanced esophageal squamous cell carcinoma who achieved clinical complete response when induction chemoradiation finished: A phase II random. Radiother Oncol. (2022) 174:1–7. doi: 10.1016/j.radonc.2022.06.015
99. Kamarajah SK, Phillips AW, Hanna GB, Low D, Markar SR. Definitive chemoradiotherapy compared to neoadjuvant chemoradiotherapy with esophagectomy for locoregional esophageal cancer: national population-based cohort study. Ann Surg. (2022) 275:526–33. doi: 10.1097/SLA.0000000000003941
100. Nilsson M, Olafsdottir H, Alexandersson von Döbeln G, Villegas F, Gagliardi G, Hellström M, et al. Neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma versus definitive chemoradiotherapy with salvage surgery as needed: the study protocol for the randomized controlled NEEDS trial. Front Oncol. (2022) 12:917961. doi: 10.3389/fonc.2022.917961
101. Li T, Lv J, Li F, Diao P, Wang J, Li C, et al. Prospective randomized phase 2 study of concurrent chemoradiation therapy (CCRT) versus chemotherapy alone in stage IV esophageal squamous cell carcinoma (ESCC). Int J Radiat Oncol Biol Phys. (2016) 96:S1. doi: 10.1016/j.ijrobp.2016.06.020
102. Li LQ, Fu QG, Zhao WD, Wang YD, Meng WW, Su TS. Chemoradiotherapy versus chemotherapy alone for advanced esophageal squamous cell carcinoma: the role of definitive radiotherapy for primary tumor in the metastatic setting. Front Oncol. (2022) 12:824206. doi: 10.3389/fonc.2022.824206
103. Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. (2023) 401:1655–68. doi: 10.1016/S0140-6736(23)00620-7
104. Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. (2023) 29:2133–41. doi: 10.1038/s41591-023-02465-7
105. Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. (2002) 359:1727–33. doi: 10.1016/S0140-6736(02)08651-8
106. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. (2012) 19:68–74. doi: 10.1245/s10434-011-2049-9
Keywords: esophageal cancer, supraclavicular lymph node, American joint committee on cancer (AJCC) 8th staging systems, Japan esophageal society (JES) 12th edition classification, chemoradiotherapy, surgery
Citation: Cai Q, Hong Y, Huang X, Chen T and Chen C (2024) Current status and prospects of diagnosis and treatment for esophageal cancer with supraclavicular lymph node metastasis. Front. Oncol. 14:1431507. doi: 10.3389/fonc.2024.1431507
Received: 12 May 2024; Accepted: 25 September 2024;
Published: 11 October 2024.
Edited by:
Qingyuan Huang, Fudan University, ChinaReviewed by:
Alessio Vagliasindi, Oncological Center of Basilicata (IRCCS), ItalyRen Luo, Sichuan University, China
Copyright © 2024 Cai, Hong, Huang, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuangzhen Chen, Y3pjaGVuMkBzdHUuZWR1LmNu
†These authors have contributed equally to this work
 Qingxin Cai
Qingxin Cai Yingji Hong1†
Yingji Hong1† Xuehan Huang
Xuehan Huang Chuangzhen Chen
Chuangzhen Chen