- 1Affiliated Hospital of Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu, China
- 2School of No. 1 Clinical Medical, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Background: In China, Atractylodes-containing Chinese medicines are widely used as adjuvant therapy to neoadjuvant chemotherapy (NAC) in individuals diagnosed with advanced gastric cancer (AGC). Nevertheless, the findings concerning its effectiveness are still restricted. The aim from this research was to examine the efficiency and security Atractylodes macrocephala-containing traditional Chinese medicine together with NAC in the management of AGC.
Methods: Literature was systematically searched across 8 electronic databases until September 20, 2023. Two researchers conducted a thorough review of the selected studies. The primary outcome measures included the objective response rate (ORR), disease control rate (DCR), quality of life (QOL), adverse drug reactions (ADRs), and levels of peripheral blood lymphocytes. The relevant effect estimates are as follows as risk ratios (RR) or mean differences (MD) with corresponding 95% confidence intervals (CI). Credibility of information was evaluated using the GRADE analyzer.
Results: The results showed that solely on the basis of the accessible literature examined in NAC patients, individuals who received the therapeutic regimen containing Atractylodis Macrocephalae Chinese herbal preparations demonstrated a superior overall response rate (Relative Risk: 1.41, 95% confidence interval: 1.27-1.57, P < 0.001); DCR (RR: 1.20, 95% confidence interval: 1.13-1.27, P < 0.001), as compared to QOL (RR: 1.43, 95% confidence interval: 1.30-1.57, P < 0.001, MD: 8.47, 95% confidence interval: 7.16 - 9.77, P < 0.001); the proportions of CD3+ T-cells, CD4+ T-cells, CD8+ T-cells, CD4+CD8+ T-cells were increased; and the incidence of adverse reactions was decreased. Subgroup analyses showed that oral administration of all the traditional Chinese medicines containing Atractylodes macrocephala could improve tumor efficacy. Regardless of the duration of therapy of ≥8 weeks or <8 weeks, Atractylodes macrocephala-containing traditional Chinese medicine increased the tumor response in AGC patients. Combination of Atractylodes macrocephala-containing TCM with neoadjuvant chemotherapy increased ORR and DCR; when used in conjunction with cisplatin, only ORR was increased.
Conclusion: The combination of Atractylodes macrocephala-containing herbs with NAC in the treatment of AGC improves efficacy, improves prognosis, and reduces adverse effects. Nevertheless, additional high-quality randomized trials are required.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023461079.
1 Introduction
Gastric cancer, among the most prevalent tumors in humans, continues to be the third primary reason for cancer-related deaths globally, it ranks 5th with regard to prevalence and 4th with regard to mortality (1, 2). The key symptoms of gastric cancer include weight loss, indigestion, nausea, vomiting, loss of appetite, and difficulty swallowing (3). Nonetheless, early-stage gastric cancer often presents with subtle clinical manifestations, leading to many patients being diagnosed at an advanced stage (4). While surgical intervention continues to be the main approach option for advanced gastric cancer, research findings indicate that over 50% of patients experience recurrence within five years post-surgery (5).
According to the Cancer Guidelines of the Chinese Clinical Society (version 2023) (6), neoadjuvant chemotherapy is generally used for advanced gastric cancer, including the combination of fluorouracil (capecitabine [CAP], tegor), platinum (cisplatin [DDP],oxaliplatin [OXA]), taxoid drugs, etc. Neoadjuvant chemotherapy can reduce the mortality of advanced gastric cancer and esophageal gastric cancer, reduce the total recurrence rate of esophageal gastric cancer, and improve the pathologic complete response (pathCR) rate of patients with advanced gastric cancer (7, 8). Perioperative chemotherapy of Epirubicin, cisplatin and fluorouracil can reduce the size and stage of tumors in patients with operable gastric or lower esophageal adenocarcinomas, the patients’ progression-free survival and overall survival were significantly improved (9). However, neoadjuvant chemotherapy may also cause certain damage to the body function of patients and produce drug resistance (10), which limits the clinical efficacy, thus prompting researchers and clinicians to pay more attention to the study of alternative and complementary therapies.
Atractylodes macrocephala, the root of the chrysanthemum plant, has been considered one of the main tonic herbs in traditional Chinese medicine for more than 2,000 years and is widely used in China to treat malignant tumors. By conducting network pharmacology studies and in vitro and in vivo experiments on Atractylodes macrocephala, it is demonstrated that palmitic acid, atractylenolide I, beta-caryophyllene, D-Serine, alpha-humulene, atractylenolide III and other Atractylenolide extracts can be used as active substances to specifically target genes associated with gastric cancer (11). A basic experimental study (12), showed that Chinese traditional medicine compound containing Atractylodes macrocephala combined with oxaliplatin could affect the apoptosis of gastric cancer cells in tumor-bearing mice through the PI3K/Akt/caspase-9 signaling pathway.
In recent decades, a large number of trials containing Atractylodes macrocephala Chinese medicine combined with NAC have been published in the treatment of AGC. The purpose of this study was to conduct a systematic analysis of these findings to evaluate the safety and efficacy of Atractylodes macrocephala Chinese medicine combined with NAC in the treatment of AGC to provide a more solid evidence base for further clinical studies, and further explore the application potential of Atractylodes macrocephala in the treatment of gastric cancer.
2 Methods
This research has been carried out adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The project is incorporated with PROSPERO under registration number CRD42023461079.
2.1 Search strategy
We conducted a systematic search in Web of Science, PubMed, EMBASE, Cochrane Central Register of Controlled Trials (Central), clinicaltrials.gov, China National Knowledge Infrastructure (CNKI), China Academic Journals (CQVIP), SINOMED and Wanfang databases were systematically searched to find articles published up to September 20, 2023: (gastric* or stomach* or digestive* or gastrointestinal*]. A systematic search of the China Academic Journals (CD-ROM Version) (CQVIP) and Wanfang databases was conducted to find articles published until September 20, 2023: (gastric* or stomach* or digestive* or gastrointestinal*] and [carcinoma* or cancer* or neoplasm* or tumour* or tumor* or tumor* or growth* or adenocarcinoma* or malignant*]) as well as (Atractylodes macrocephalus or Cangzhu). All queries used MeSH terms and non-restrictive vocabulary (detailed search strategies are provided in the Supplementary Material). Only Chinese and English were included.
2.2 Inclusion and exclusion criteria
Inclusion criteria: (1) All research included in the study were randomized controlled trials (RCT) or quasi-RCTs. (2) Patients diagnosed with TNM stage II-IV AGC using histopathological and cytological criteria. (3) Patients in the experimental group were given a combination of white-herb-based traditional Chinese medicine and neoadjuvant chemotherapy. Individuals in the control group were given neoadjuvant chemotherapy treatment alone. (4) The outcomes assessed in this study included tumor response, quality of life, adverse events, and peripheral blood lymphocyte levels, with each study reporting at least one of these outcomes. (5) In cases where multiple publications of the same study were available, we chose the dataset that provided the most exhaustive information and had the longest follow-up time.(6)The papers included in this study were published before September 23, 2023.
Exclusion criteria: (1) Exclusion of individuals with serious infections, other malignancies, and severe medical conditions; (2) Avoidance of inconsistent prescriptions of traditional Chinese medicines based on Atractylodes macrocephala;(3) Studies from which it was not possible to extract data were excluded; (4) Baseline data were not comparable between the two groups of patients.
2.3 Study selection and data extraction
Two reviewers independently selected studies based on the specified inclusion and exclusion criteria. Data extraction was also carried out independently by two reviewers. Whatever discrepancies there are will be resolved through discussion the third reviewer. The data collected shall include the first author and publication year, sample size, gender distribution, age range, study group details, dosage regimen, treatment duration, outcomes, and assessment criteria.
2.4 Risk of bias assessment
Two reviewers independently assessed the quality of the included studies utilizing the Cochrane Collaboration’s Tool for Randomized Controlled Trials.
These studies will be categorized as low, unclear, or high risk of bias. Assessment criteria included random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and staff (performance bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential biases. The results of these assessments have all been summarized and analyzed through Review Manager 5.3.
2.5 Outcome definition
Tumor response is the main prognostic indicator. Tumor response includes objective remission rate (ORR) and disease control rate (DCR) based on World Health Organization (WHO) criteria (13) and solid tumor criteria (RECIST) (14). Complete remission (CR), partial remission (PR), stable disease (SD) and progression (PD) were used as indicators.
The remaining observations were quality of life (QOL), adverse drug reactions (ADRs) and peripheral blood lymphocyte levels.
Adverse reactions were obtained by measuring hematological toxicity (leukocyte abnormalities, thrombocytopenia, anemia), gastrointestinal toxicity (nausea, vomiting, and diarrhea), hepatic and renal dysfunction, neurotoxicity, alopecia, and stomatitis, and were assessed according to World Health Organization (WHO) criteria (13) or the National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE) (15). Peripheral blood lymphocyte levels were assessed by measuring T lymphocyte subsets such as CD3+ T cells, CD4+ T cells, and CD8+ T cells, as well as the CD4+/CD8+ T cell ratio.
2.6 Data analysis
Review Manager 5.3 and Stata 17.0 software were utilized for data analysis in this research. Risk ratios (RR) and 95% confidence intervals (CI) were employed to evaluate binary variables, while mean difference (MD) with 95% CI was used for assessment of continuous variables.
Statistical heterogeneity was assessed using the I2 statistic and chi-square test. Total risk ratios (RR), mean differences (MD), and their respective 95% confidence intervals were estimated through random-effects modeling. The significance of the results was determined by P values; statistical significance was defined when P < 0.05. Funnel plots and Egger’s tests were employed to examine possible publication bias when I2 ≥ 50%. Subgroup analyses were conducted based on first treatment, NAC regimen, and treatment duration to explore clinical heterogeneity and its effect on tumor response.
2.7 Evidence quality assessment
Independent review by two reviewers evaluated the level of evidence for each result utilizing the GRADE method (16). Any discrepancies were discussed and resolved with the involvement of a third reviewer. The quality of evidence is categorized as high, moderate, low, and very low. The evidence was only reduced by a single level if most trials exhibited unclear or elevated risk of bias but had robust results; a two-level downgrade occurred if the results lacked robustness. Other factors for downgrading included inconsistency (presence of statistical heterogeneity and poor robustness of the results of the sensitivity analyses), indirectness (the study’s participants, interventions, outcomes, or comparisons did not align with the study objectives), imprecision (sample size fewer than 300 cases for each outcome), and reporting bias (publication bias). Apart from the risk of bias, the evidence was downgraded by one level.
3 Results
3.1 Search results
In total 2716 records identified via comprehensive database searches. Total records underwent screening comments from two reviewers through a process consisting of three steps. Firstly, We reviewed titles and eliminated duplicate records 2155 records. Secondly, we included 289 full-text articles by reading the titles or abstracts. Thirdly, we evaluated the full text was assessed based on the incorporation of and exclusion criteria and excluded 257 trials. Finally, 32 eligible studies involving 2157 patients with stage II-IV AGC were incorporated into our study (Figure 1).
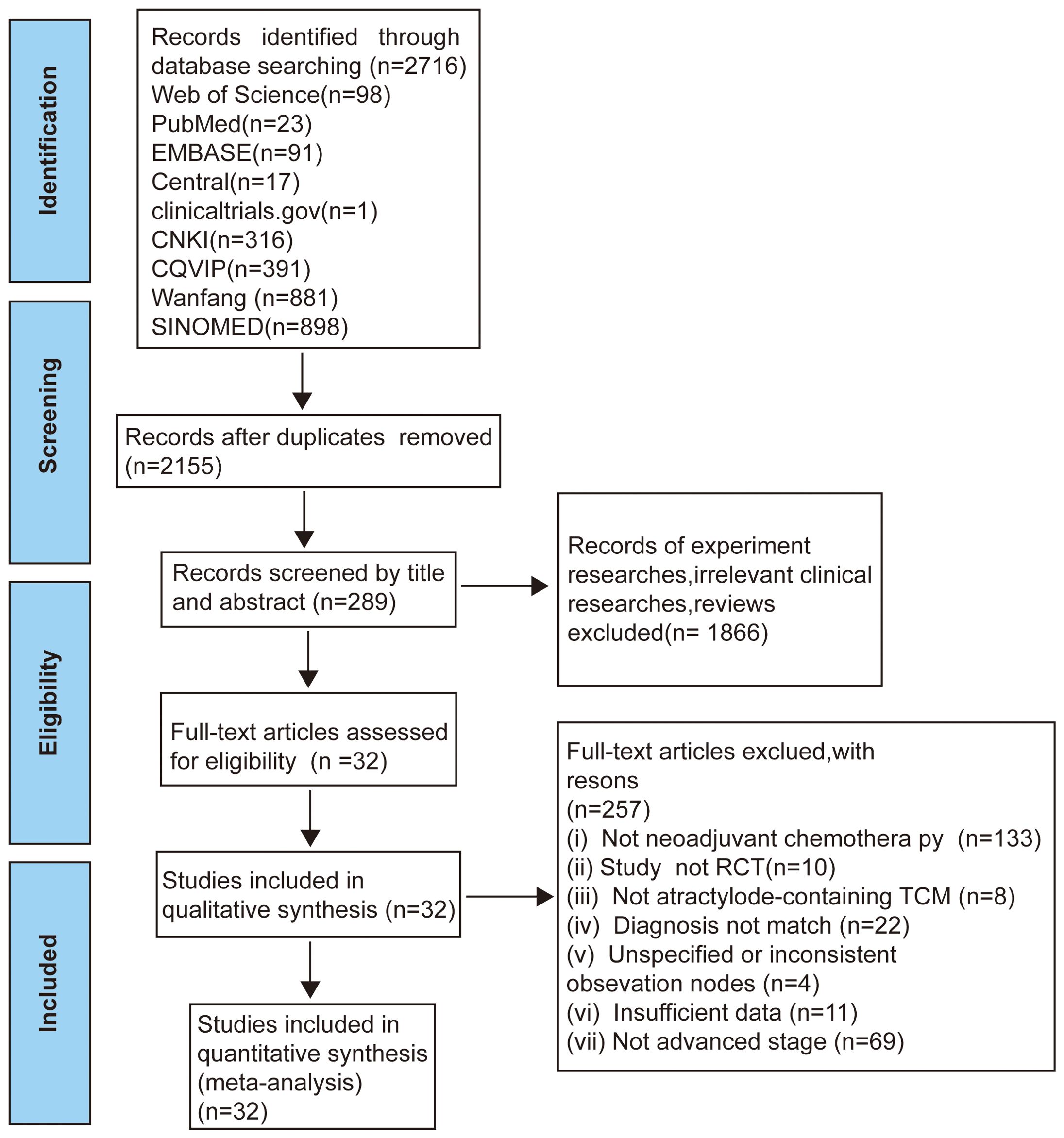
Figure 1. Flow diagram of selection process (17).
3.2 Characteristics of the included studies
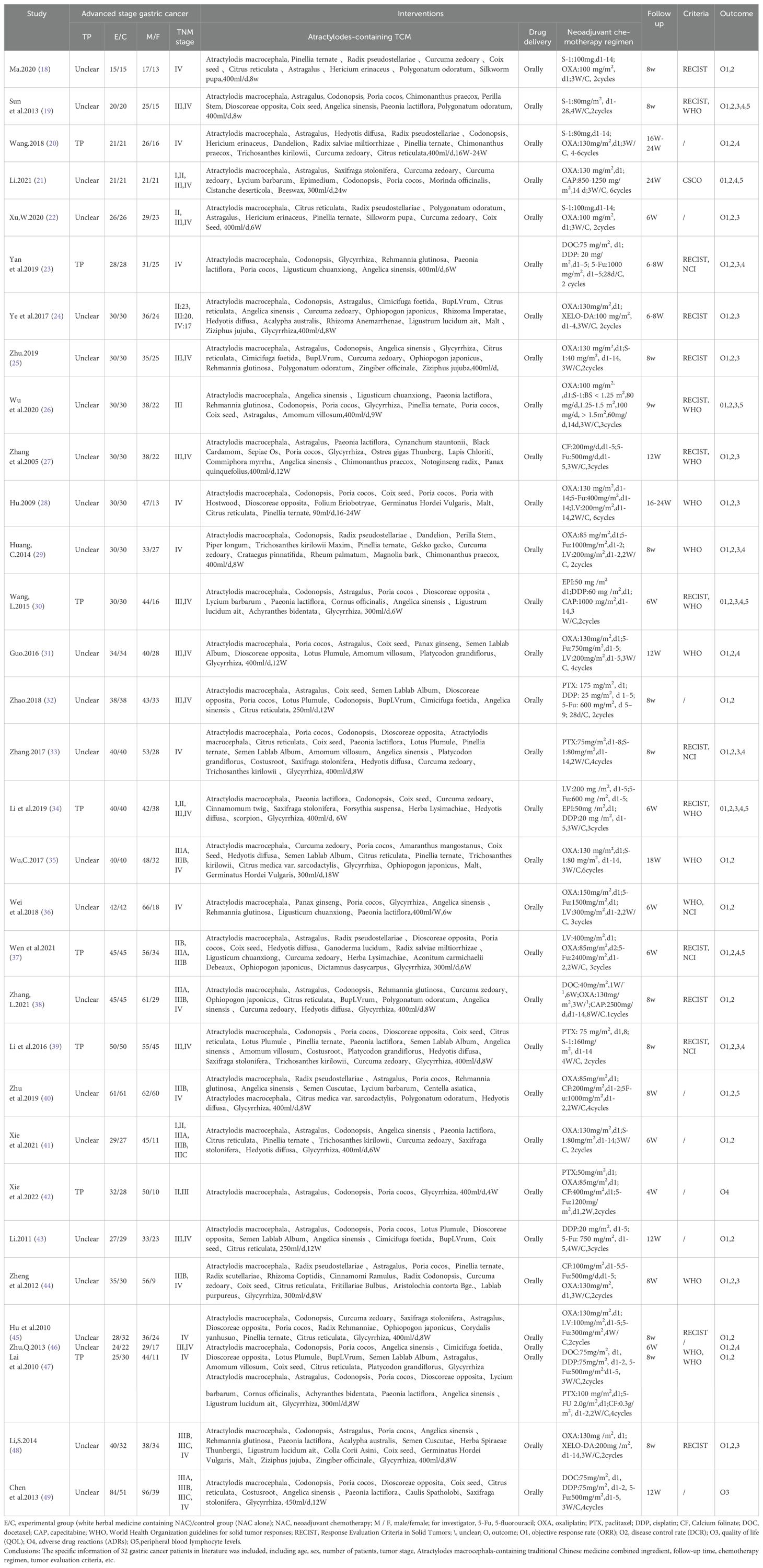
Details regarding the features included in the study are presented in Table 1. All 32 studies were conducted in China. A total of 1383 male patients and 774 female patients, with sample size ranging from 30 to 135. All clinical trials were founded on the main drug of Atractylodes macrocephala-containing the traditional Chinese medicine in combination with neoadjuvant chemotherapy.
Follow-up ranged from 4 W to 24 W, tumor responses were reported in 7 trials according to the WHO guidelines and in 15 trials according to the RECIST guidelines. Adverse effects were reported in 6 trials using the WHO.NCI CTCAE criteria were used in 5 cases, and unknown criteria were used in 9 cases.
3.3 Risk of bias
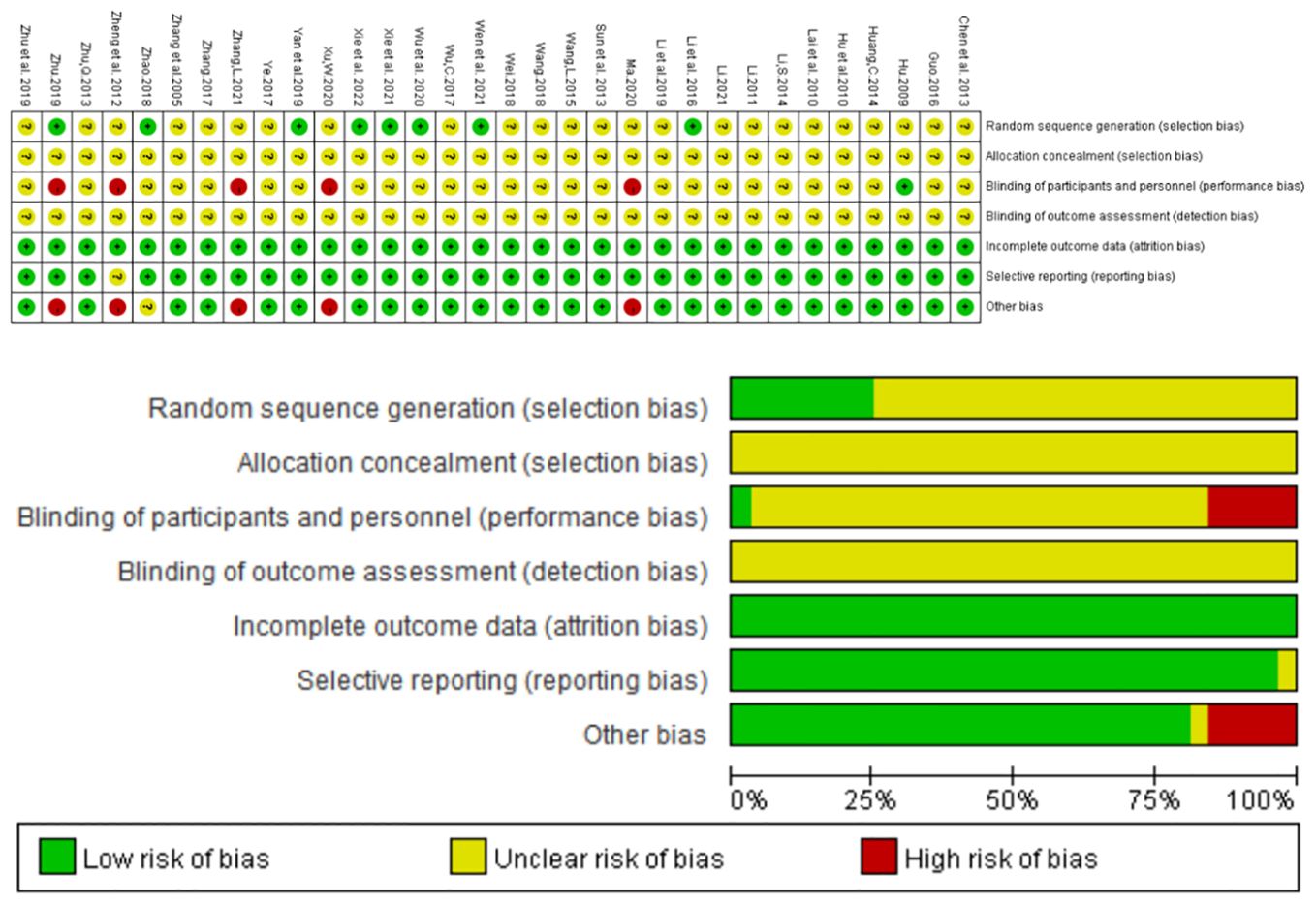
32 studies involved randomization; Of these, eight studies (24, 26, 32, 34, 37, 40–42) used random number tables to generate random sequences, three studies used admission order (18, 25, 44), and two studies (22, 38) used treatment modalities, none of which adequately described masking for investigators, patients, and outcome evaluators. The exposure bias for each individual trial is summarized in Figure 2 below.
3.4 Tumor response
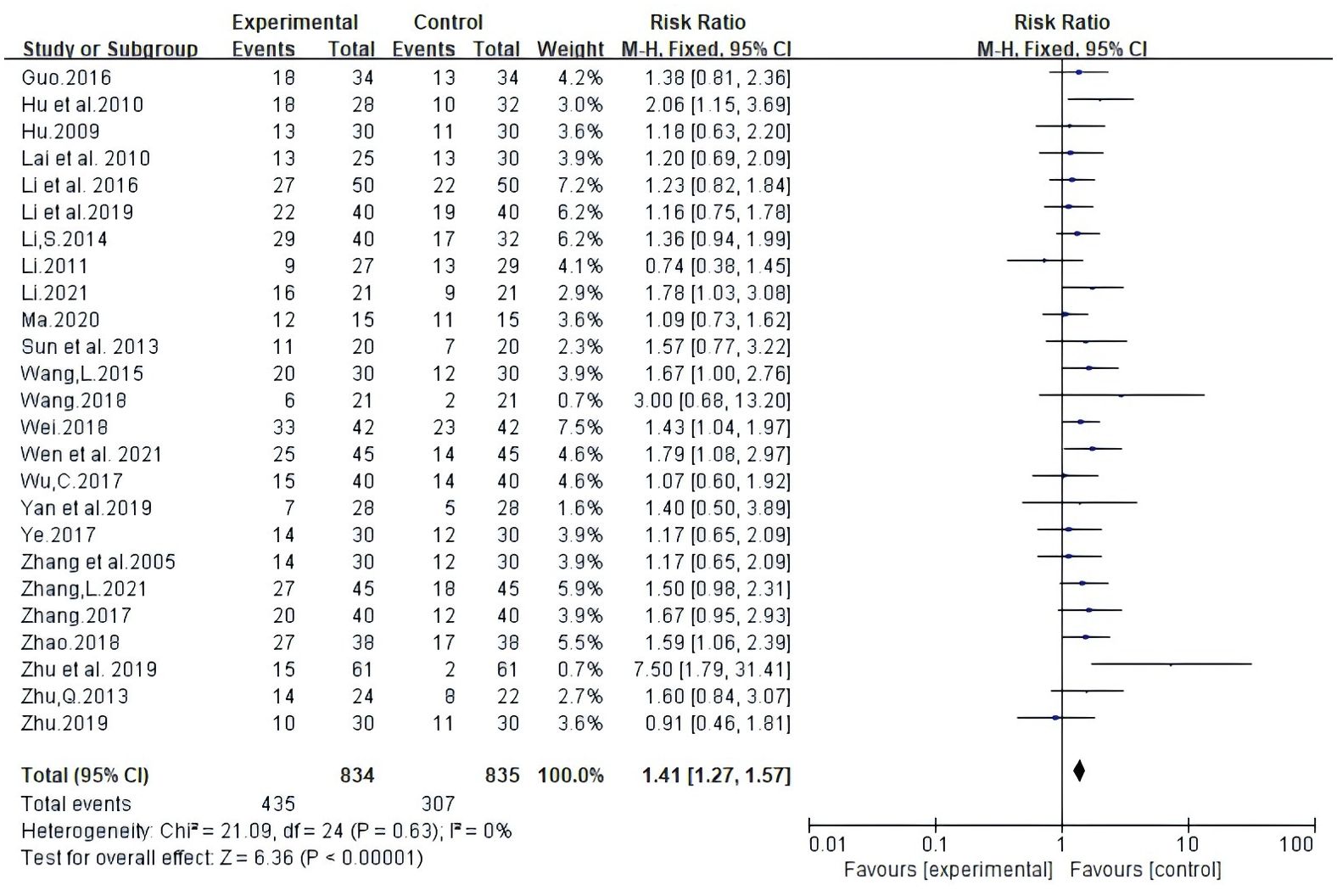
Twenty-five trials involving 1669 patients reported ORR according to Guidelines from the World Health Organization (WHO) or Response Evaluation Criteria In Solid Tumors (RECIST). Random-effects meta-analysis demonstrated that White Atractylodes Chinese herbal medicine combined with NAC improved ORR; the difference was statistically significant (RR: 1.41, 95% CI: 1.27 ~ 1.57, P < 0.00001, I2 = 0%, Figure 3).
Twenty-four test reports involved 1,587 DCR. Meta-analysis using random-effects models demonstrated that the combination of Atractylodes macrocephala and NAC enhanced Disease Control Rate DCR; statistically significant differences (RR: 1.20, 95% CI: 1.13 ~ 1.27, P < 0.00001, I2 = 62%, Figure 4).
3.5 Quality of life
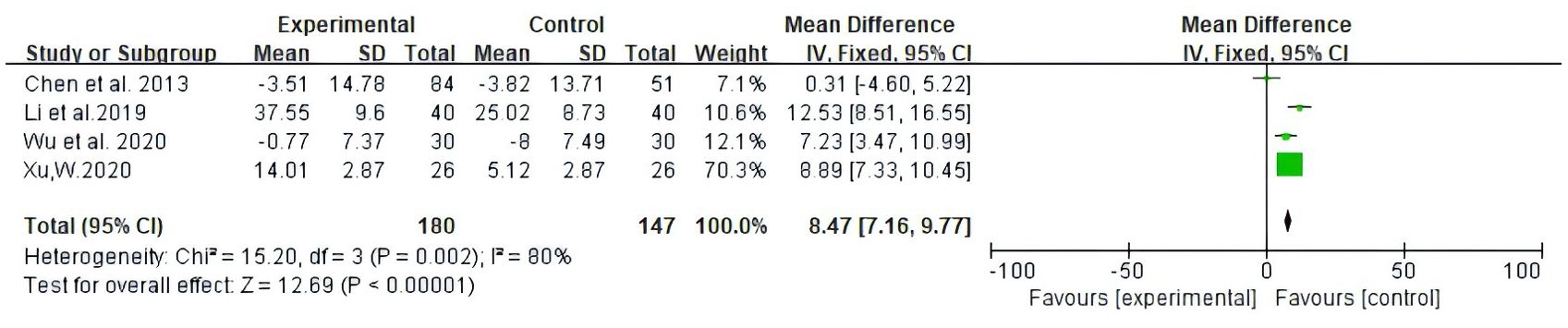
Among the studies covered, two forms of data were used to document improvements in quality of life based on the assessment of KPS scores. The first is number of patients indicating improvement in quality of life. Secondly, the mean ± standard deviation (SD) of KPS scores pre- and post-treatment were reported. As illustrated in Figure 5, 13 studies reported patient count with improved quality of life after the KPS scores (RR: 1.43, 95% CI: 1.30-1.57, P = 0.25, I2 = 19%); the other four studies all reported the mean ± SD of KPS scores (MD: 8.47, 95% CI: 7.16 - 9.77, P = 0.002, I2 = 80%) (Figure 6). In conclusion, compared with NAC, Atractylodes macrocephala-containing with NAC substantially enhanced the patients’ quality of life.
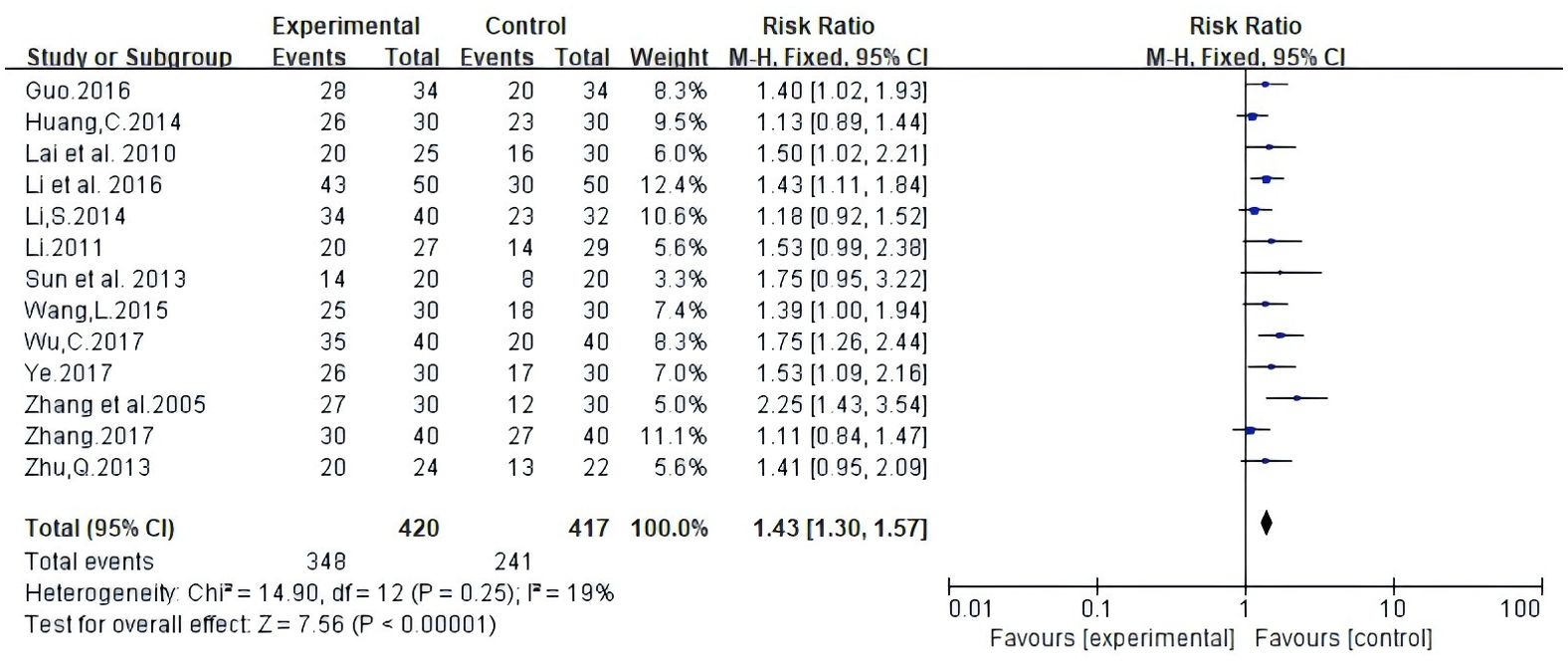
Figure 5. Meta-analysis results of quality of life (QOL) according to the number of KPS improved patients.
3.6 Adverse drug reactions
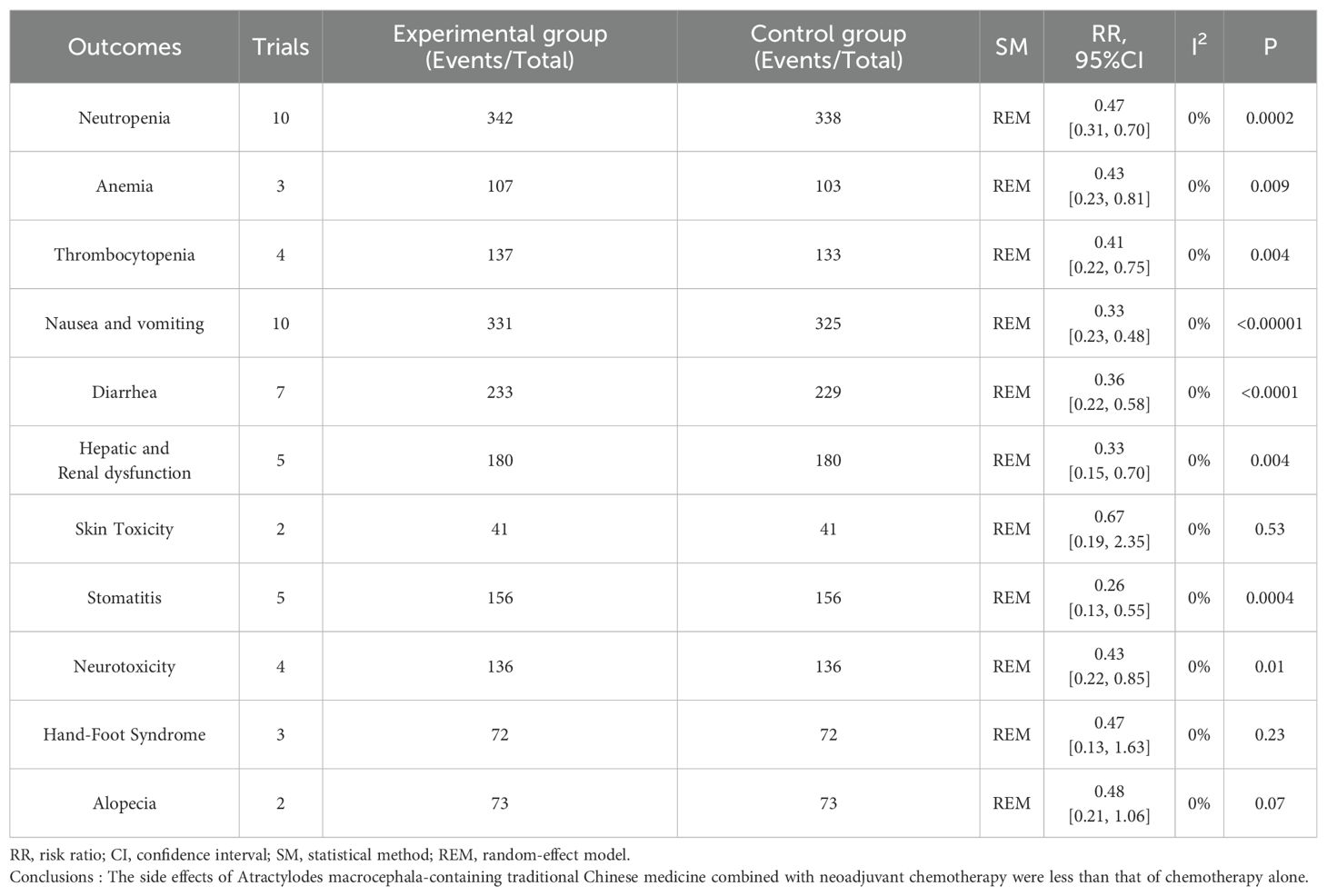
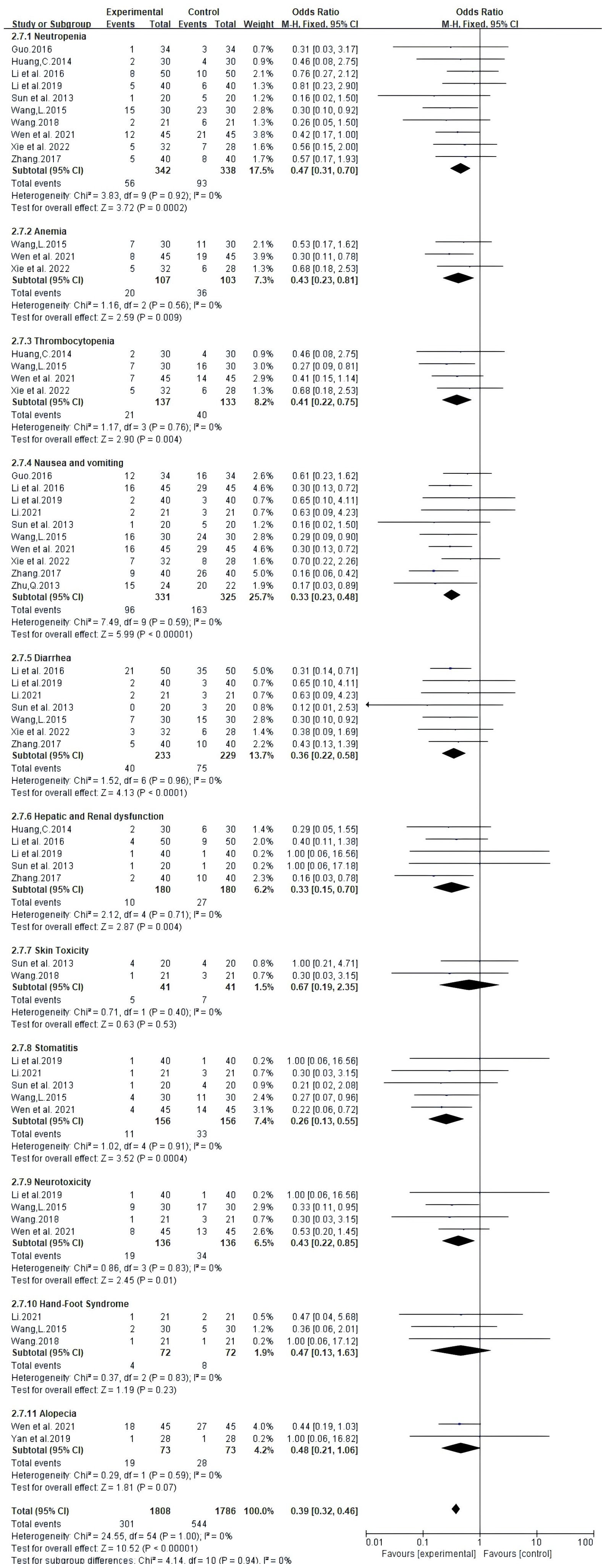
Adverse effects reported in 13 trials that included 914 patients mainly met the WHO criteria (Table 2, Figure 7). The findings from Meta-analysis with random effects models indicated that when compared to NAC used independently, the combination of Atractylodes macrocephala-containing herbs with NAC significantly reduced leukocytosis (RR: 0.60, 95% CI: 0.45-0.79, P = 0.0002), anemia (RR: -0.2, 95% CI: -0.34–0.06, P = 0.006), thrombocytopenia (RR. 0.51, 95% CI: 0.32-0.81, P = 0.005), nausea and vomiting (RR: 0.33, 95% CI: 0.23-0.48, P < 0.00001), diarrhea (RR: 0.53, 95% CI: 0.39 - 0.72, P < 0.0001), and liver and renal dysfunction (RR: 0.37, 95% CI. 0.19 - 0.74,P=0.005), danger of neurotoxicity (RR: 0.43, 95% CI: 0.22 ~ 0.85, P = 0.01), stomatitis (RR: 0.26, 95% CI: 0.13 ~ 0.55, P = 0.0004), but incidence of alopecia (RR: 0.68, 95% CI: 0.44 ~ 1.04, P = 0.08), hand-foot syndrome (RR: 0.68, 95% CI: 0.13 ~ 1.63, P = 0.23) were not associated.
3.7 Peripheral blood lymphocyte levels
Seven trials, involving 494 patients, documented the levels of peripheral blood lymphocytes. Our results showed that Atractylodes macrocephala-containing herbs plus NAC raise significantly CD3+ T cells (MD: 17.75, 95% CI: 16.2-19.31, P < 0.00001), CD4+ T cells (MD: 0.13, 95% CI: 0.08-0.19, P < 0.00001), CD8+ T cells (MD:-0.55, 95% CI: -1.39-0.29, P < 0.00001) and the proportion of CD4+CD8+ T cells (MD: 0.13, 95% CI: 0.08-0.19, P < 0.00001) (Figure 8).
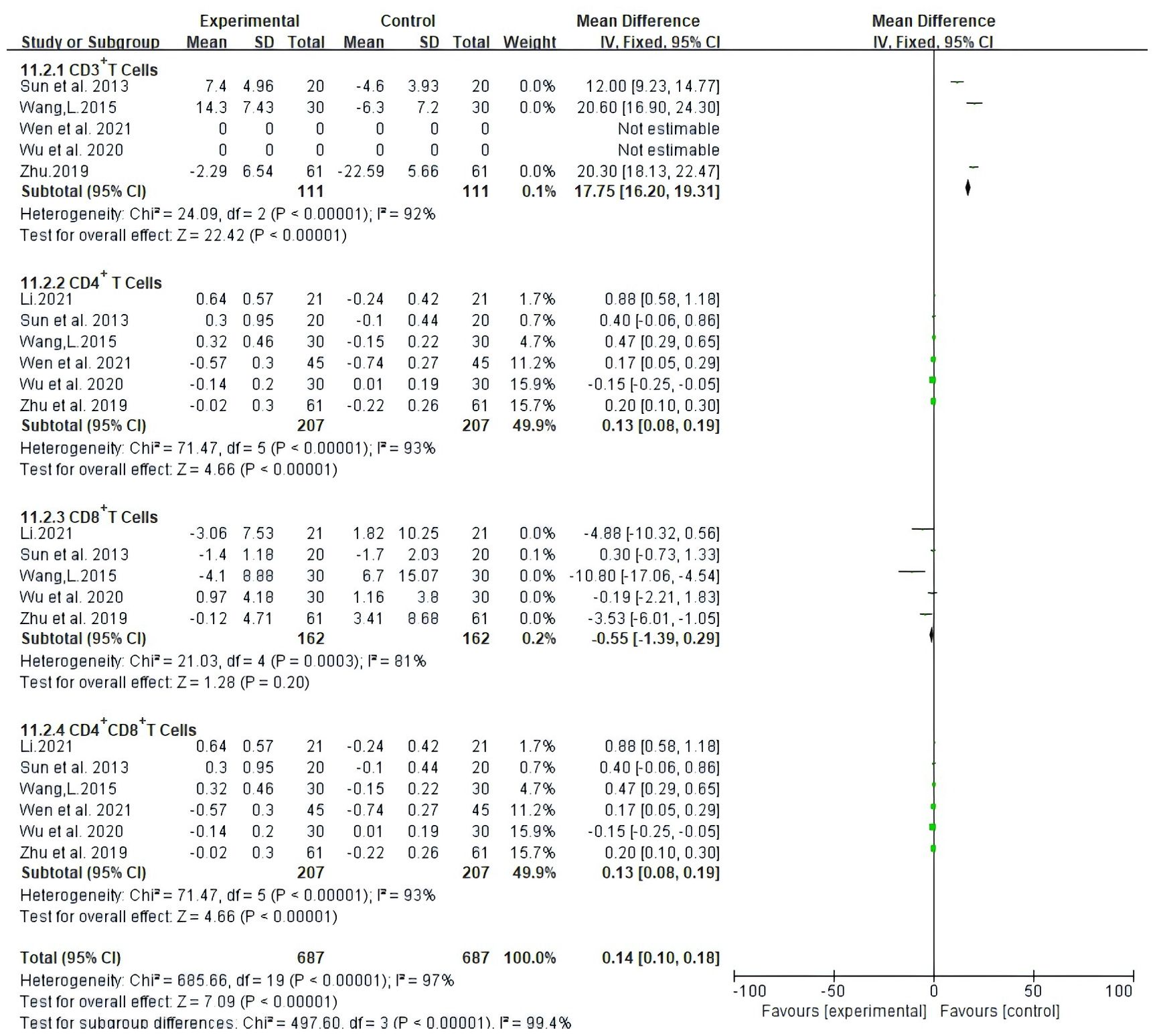
Figure 8. Meta-analysis results of Meta-analysis results of CD3+ T cells,CD4+ T cells, CD4+ CD8+ Tcells between the two groups.
3.8 Subgroup analysis
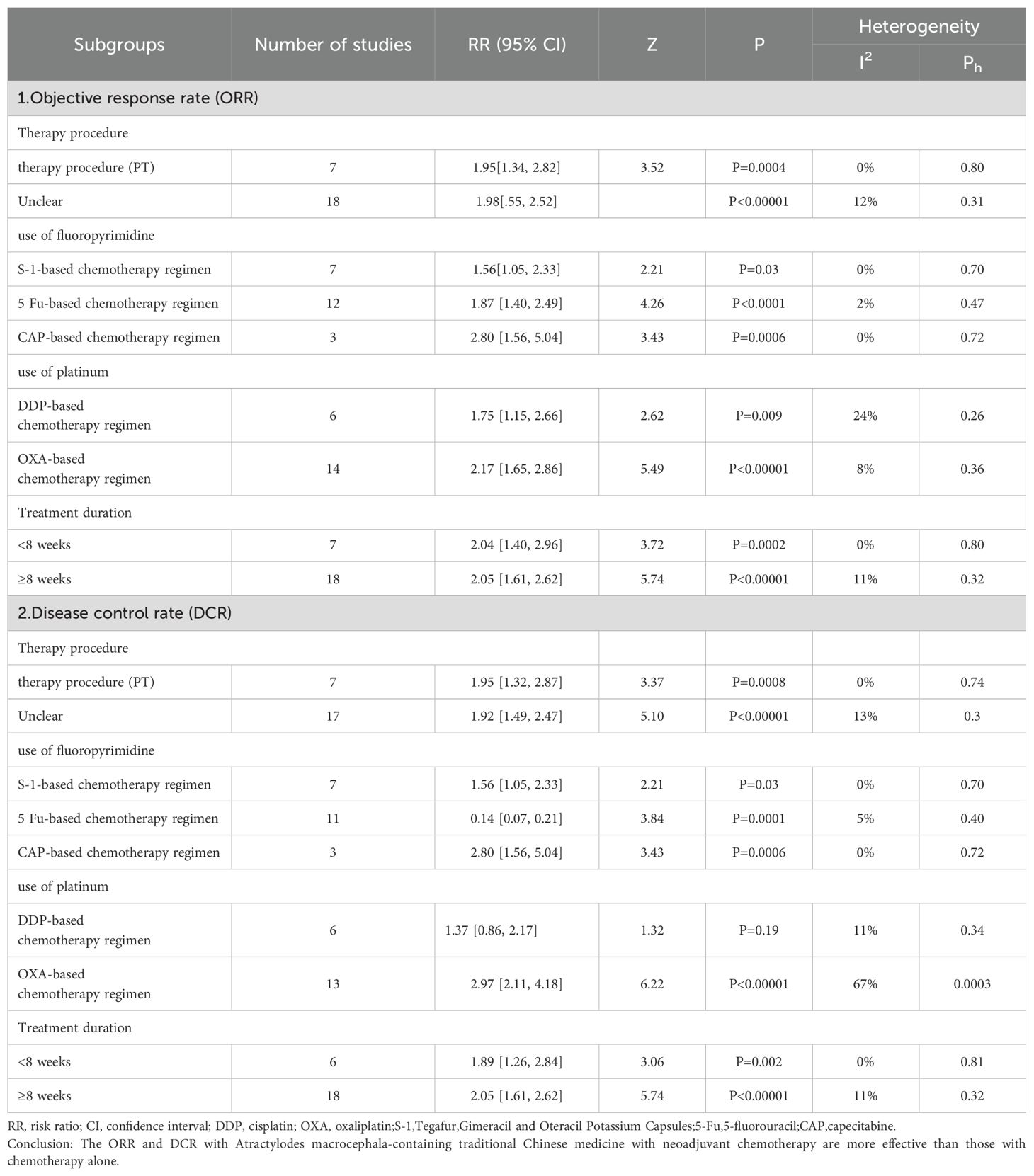
Our subgroup analyses were performed according to the treatment procedures, the NAC regimen, treatment duration, and inclusion of Atractylodes macrocephala-containing herbs were examined to assess their impact on the objective response rate ORR and disease control rate DCR. Individuals were categorized into primary treatment (PT) and unspecified treatment according to treatment procedures. Subgroup analyses showed that atractylodes-containing herbs improved ORR and DCR in both primary and indeterminate treatment (Table 3).The NAC regimen was divided into two subgroups, fluorouracil-based and platinum-based. The fluorouracil subgroup was further divided into three chemotherapy regimens based on 5-Fu, S-1,CAP. The platinum-based subgroup was further divided into two chemotherapy regimens based on DDP and OXA.The subgroup findings suggested that regardless of the chemotherapy regimen with 5-Fu, S-1,CAP, OXA, and DDP, the Atractylodes macrocephala-containing herbs improved ORR and DCR, except for the use of cisplatin, which was not associated with DCR. two independent duration of treatment were established: <8 weeks and ≥8 weeks. According to the subgroup analysis, regardless of duration of treatment of <8 weeks or ≥8 weeks, leucovorin-containing herbal medicines improved ORR and DCR.
As previously mentioned (50, 51), we performed a subgroup analysis of specific ingredients containing Atractylodes macrocephala preparations in each literature to determine the contribution of the combination of Atractylodes macrocephala and other herbs to advanced gastric cancer. Tables 4 and 5 include only combinations of Chinese medicines with low heterogeneity (I2 < 30%). In 32 literatures, a total of 90 TCM were used, with an average of 13 TCM. Among the most commonly used traditional Chinese medicines in combination with Baizhou in the treatment of advanced gastric cancer, the following are Codonopsis(n=24),Astragalus(n=23),Poria cocos(n=23),Glycyrrhiza(n=23) and Angelica sinensis(n=19),Curcuma zedoary(n=18),Coix seed(n=17). When these plants were present in combinations of pairs, triples, and higher levels in the TM intervention, their effects on ORR and DCR were evaluated, and when TMs combinations showed higher RRs than TMs alone, these were considered possible examples of synergies. Table 4 shows that at Level 1, when Baichu is combined with other single Chinese medicine, the most commonly used combination is Atractylodis macrocephala+Poria cocos (n=19; RR=1.98 [1.57, 2.49],I2 = 0), Atractylodis macrocephala+Malt (n=4; RR=1.45 [0.89, 2.36], I2 = 0) was the lowest, and RR of the 10 combinations were significantly higher than that of the total pool (1.41). Level 2, combinations of 3 TMs, the most common combinations are Atractylodis macrocephala+Poria cocos+Glycyrrhiza (n=14; RR=2.18 [1.68, 2.84], I2 = 0), the lowest RR of Atractylodis macrocephala+Panax ginseng+Astragalus(n=2) was 1.56 [0.77, 3.14]. RR of 19 TCM combinations was significantly higher than that of total pool (1.41). Level 3, combinations of 4 TMs, the most common combinations are Atractylodis macrocephala+Codonopsis+Astragalus+Angelica sinensis (n=10; RR=1.77 [1.29, 2.45], I2 = 2),Atractylodis macrocephala+Poria cocos+Glycyrrhiza+Angelica sinensis (n=10; RR=2.22 [1.61, 3.06], I2 = 0), while Atractylodis macrocephala+Codonopsis+Astragalus+Angelica sinensis(n=10) is also the combination with the lowest Level 3RR. RR of 13 TCM combinations was significantly higher than that of total pool (1.41). Level 4, combinations of 5 TMs, the most common combinations are Atractylodis macrocephala+Codonopsis+Astragalus+Poria cocos+Angelica sinensis (n=10; RR=1.95 [1.31, 2.90], I2 = 7), Atractylodis macrocephala+Poria cocos+Glycyrrhiza+Angelica sinensis +Curcuma zedoary (n=2) had the lowest RR value, 1.81 [1.00, 3.28]. The RR of the 7 TCM combinations was significantly higher than that of the total pool (1.41). Table 5 shows that at Level 1, the most commonly used combination of macrocephala and Codonopsis was Atractylodis macrocephala (n=18; RR=1.86 [1.46, 2.37], I2 = 0), Atractylodis macrocephala+Poria cocos (n=18; RR=1.93 [1.52, 2.44], I2 = 0), the lowest RR of Atractylodis macrocephala+Malt (n=4) was 1.45 [0.89, 2.36], and the RR of 9 combined TCM combinations was significantly higher than that of the total pool (1.20). Level 2, combinations of 3 TMs, the most common combinations are: Atractylodis macrocephala+Codonopsis+Astragalus (n=13; RR=2.02 [1.51, 2.71], I2 = 7), Atractylodis macrocephala+Codonopsis+Poria cocos (n=13; RR=2.02 [1.51, 2.71], I2 = 7), Atractylodis macrocephala+Codonopsis+Angelica sinensis (n=13; RR=1.77 [1.34, 2.33], I2 = 0), Atractylodis macrocephala+Astragalus+Poria cocos (n=13; RR=2.34 [1.75, 3.12], I2 = 13), Atractylodis macrocephala+Poria cocos+Glycyrrhiza (n=13; RR=2.12 [1.61, 2.79], I2 = 0), the lowest RR of Atractylodis macrocephala+Panax ginseng+Astragalus (n=2) was 1.56 [0.77, 3.14]. RR of 19 TCM combinations was significantly higher than that of total pool (1.20).Level 3, combinations of 4 TMs, the most commonly used combination with the lowest RR is Atractylodis macrocephala+Codonopsis+Astragalus+Angelica sinensis (n=10; RR=2.12 [1.61, 2.79], I2 = 2), RR of 13 combinations was significantly higher than that of total pool RR (1.20).Level 4, combinations of 5 TMs, The most common combination is Atractylodis macrocephala+Codonopsis+Astragalus+Poria cocos+Angelica sinensis (n=7; RR=1.95 [1.31, 2.90], I2 = 7), RR of the 7 TCM combinations were significantly higher than that of the total pool (1.20). For Level 5 and Level 6, the combinations of Chinese medicines in Table 4 and Table 5 are the same. Atractylodis macrocephala+Codonopsis+Astragalus+Poria cocos+Glycyrrhiza+Angelica sinensis (n=4) and Atractylodis macrocephala+Codonopsis+Astragalus+Poria cocos+Glycyrrhiza+Angelica sinensis+Coix seed(n=2), RR values were greater than total pool RR.
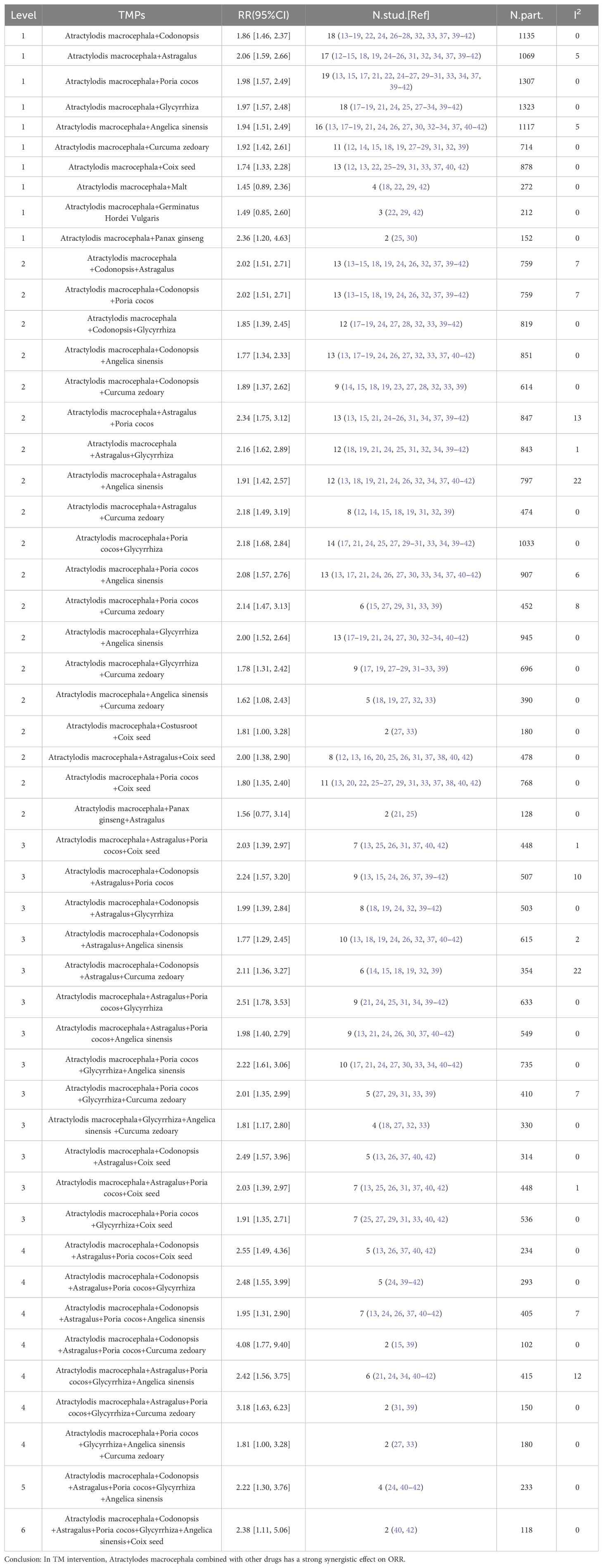
Table 4. Effect of oral conventional drugs (TMs) on tumor response: Atractylodes macrocephala and combined TMs on ORR.
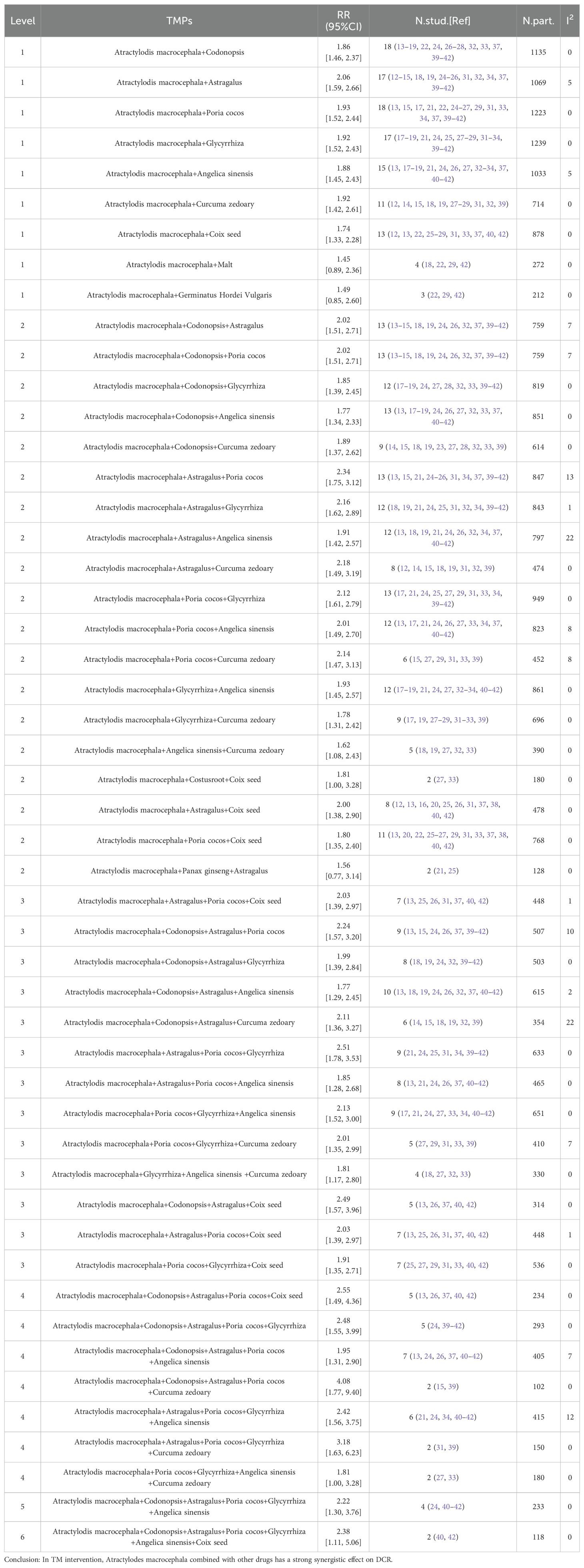
Table 5. Effect of oral conventional drugs (TMs) on tumor response:Atractylodes macrocephala and combined TMs on DCR.
3.9 Sensitivity analysis
We conducted analyses to evaluate the robustness by systematically excluding each investigation to evaluate the reliability pertaining to the primary outcomes, which included ORR, DCR, QOL, and ADRs. The ORR, and the combined RR values of the ADRs were stable (Table 6). Considering the DCR dichotomous variable extracted from Zhu et al., 2019 (40), which this study does not include, I2 declined from 62% to 0%. Considering the QOL continuous class variable data extracted from Li et al., 2019 (34), Chen et al., 2013 (49), we excluded these 2 studies and I2 decreased from 80% to 0%.
3.10 Publication bias
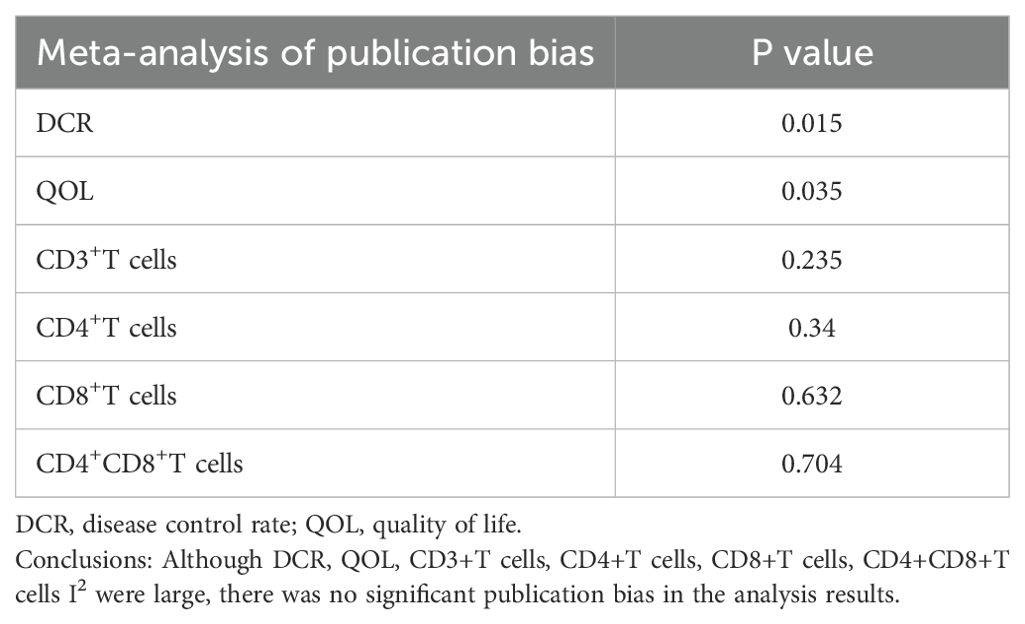
The funnel plots showed some asymmetry and were not perfectly symmetrical in the meta-analysis of DCR, QOL, CD3+T cells, CD4+T cells, CD8+T cells, and CD4+CD8+T cells ratios(S1-S6). However, Egger’s test (Table 6) showed that there was no significant publication bias in the meta-analysis of CD3+T cells (P= 0.235), CD4+T cells (P=0.34), CD8+T cells (P= 0.632), and CD4+CD8+T cells (P= 0.704.) The meta-analysis of DCR (P= 0.015) (P= 0.015), and QOL (P= 0.035) meta-analysis all had significant publication bias.
3.11 Quality of evidence
In conclusion, regarding ORR, QOL dichotomous variables, as well as thrombocytopenia, nausea and vomiting, diarrhea, and hepatorenal dysfunction, CD3+T Cells, CD4+T Cells, CD8+T Cells, CD4+CD8+T Cells, the quality was moderate; DCR, Neutropenia, Anemia, Skin Toxicity, Stomatitis, Neurotoxicity, Hand-Foot Syndrome, Alopecia, and KPS, according to mean ± SD had very low results (Table 7).
4 Discussion
Cancer is not only a worldwide health problem, but it also poses a serious danger to both individuals and society, serving as a major threat to public health. Cancer is the primary reason for most deaths in every country around the world, with millions of individuals losing their lives to cancer every year, and a huge obstacle in increasing life expectancy of mankind (52). According to the U.S. Cancer Report 2023, gastric cancer occupies the top 6 places in terms of incidence and mortality of highly prevalent malignant tumors in various countries and regions around the world (53). In China, in 2022, the number of incidence cases of malignant tumors in China was about 4,824,700 cases, and the number of death cases was about 2,574,200 cases, and gastric cancer ranked 5th among the cancer types with the number of death cases of malignant tumors in China (54). Early gastric cancer patients often develop AGC because of nonspecific symptoms such as belching and epigastric discomfort, or even no obvious symptoms. neoadjuvant chemotherapy is a short-term chemotherapy intervention before surgical treatment, which can promote tumor shrinkage and improve the effect of surgical treatment (55). Nevertheless, the survival advantage for patients treatment of patients with advanced gastric cancer remains constrained. Therefore, there is a need for developing an effective portfolio program therapy regimen to improve the effectiveness of NAC, reducing adverse impacts, and improve patient compliance and elapsed time for survival, thereby enhancing the quality of patients’ lives.
Chinese medicine compounding, being a crucial component of a comprehensive gastric cancer treatment, is commonly employed in gastric cancer patients in China (56).
In order to assess whether oral Atractylodes macrocephala-containing Chinese herbal preparations combined with NAC could improve the clinical efficacy and safety, 32 randomized controlled trials involving 2157 AGC practitioner participated in this meta-analysis. Our findings demonstrated that the herbal combination containing Atractylodes enhanced the ORR and DCR of NAC, suggesting that the intervention group exhibited superior short-term effectiveness. Additionally, we conducted subgroup analyses according to Atractylodes macrocephala-containing herbal treatment procedures, NAC regimens, and length of therapy. The results showed that ORR and DCR could be improved in AGC patients with or without initial treatment. Oral administration of Atractylodes macrocephala-containing herbs had better tumor efficacy in patients with AGC compared to NAC alone. A chemotherapy regimen in which Atractylodes macrocephala-containing herbs were given in combination with fluorouracil-based chemotherapy and oxaliplatin in patients with AGC improved the ORR and the DCR; however, DCR did not improve in those who took aloe-containing herbs plus cisplatin. The subgroup analysis results also showed that the ORR and DCR could be improved by Atractylodes macrocephala-containing herbs plus NAC in patients with AGC, regardless of the duration of administration. Additionally, researchers have isolated a variety of chemicals from Atractylodes macrocephala. Among them, atractylodes macrocephalae polysaccharides consist of various groups of sugars, including arabinose (Ara), glucose (Glc), mannose (Man), rhamnose (Rha), galacturonic acid (GalA), galactose (Gal), and xylose (Xyl), among others (57).Its anticancer activity is mediated in several ways: modulation of the tumor microenvironment and immune function, inhibition of tumor cell migration and invasion and metastasis, induction of apoptosis and inhibition of tumor cell proliferation (58), and it has a wide range of antitumor activity against a variety of tumor cell types like esophageal cancer, hepatocellular carcinoma, malignant glioma, colorectal cancer, lung cancer cells, etc (59, 60). The volatile oil of Atractylodes macrocephala exemplifies good antitumor effects through its suppressing effects on a range of tumor cells (61). Both Atractylenolide I and Atractylenolide II have antitumor effects. In addition to the antitumor effects of atractylenolide I on lung cancer cells, ovarian cancer cells, and leukemia cells in vitro experiments (62–64), in vivo the results of the study show that Atractylenolide 1 significantly inhibited the tumor body of SGC-7901 gastric cancer cells in BALB/C nude mice with loaded mice by increasing the expression of Bax, cleaved caspase-3, and p53, and decreasing the manifestation of Bcl-2 proliferation (65). Atractylenolide II significantly induces apoptosis in HGC-27 and AGS gastric cancer cells by deactivating Pathways of the Ras/ERK and PI3K/AKT signaling pathways (66, 67). These findings provide indirect evidence for the basis and principle of the anticancer mechanism of AGC containing Atractylodes macrocephala components.
NAC can lead to multiple adverse effects in AGC patients, which can seriously affect quality of life and survival expectations. Therefore, it is essential to maintain efficacy while minimizing adverse effects. In one study, AM(Atractylodes macrocephala) extract significantly promotes the migration of intestinal epithelial cells by modulating the polyamine Kv1 signaling pathway.1.This suggests that atractylodes helps promote the repair of intestinal damage (68). AM polysaccharides are beneficial to functions of the digestive tract, as the presence of chitin is associated with recovery from intestinal damage (69). In a rat experimental model, atractylenolide I and atractylenolide III significantly reduced muscle contraction in isolated ileum (70). Atractylenolide I improves the malignant quality of tumor patients, and atractylenolide I treatment increases body weight, appetite, KPS score, and TNF-α level in gastric cancer patients (71, 72). In serum-deficiency-induced PC12 cells, atractylenolide III inhibited apoptosis by increasing cellular activity, suggesting that Atractylenolide III has significant neuroprotective effects (73).
According to our findings, Atractylodis Macrocephalae-containing herbs combined with NAC reduced ADRs in AGC patients (Neutropenia, Anemia, Thrombocytopenia, Nausea and vomiting, Diarrhea, Hepatic and Renal dysfunction, Stomatitis, Neurotoxicity, Skin Toxicity, Hand-Foot Syndrome, Alopecia) (P < 0.05) and significantly improved KPS-based QOL.
T lymphocytes are important immunoregulatory cells and effector cells of human cellular immunity, which play a significant role in inhibiting the proliferation of tumor cells and maintaining the immune function of the human body, therefore, T lymphocyte subpopulations can be used to assess the cellular immune system function the organism. Some studies have pointed out that the function of the human immune system can partly reflect the prognosis and development of gastric cancer (74).
Therefore, improving immunity is important for human health anti-tumor therapy. An animal study showed that AM polysaccharides increased peripheral blood lymphoid levels and their conversion rate, as well as the expression of IL-6, TNF-α, IFN-γ, and nuclear factor-activated transcription factor of activated T-cells (NF-AT) in splenic tissues of piglets. These effects suggest that AM polysaccharides may enhance immune responses by promoting lymphocyte proliferation (75). A study using a chicken spleen tissue model system found that HS increased the expression of pro-apoptosis-related genes such as IL-1 and TNF-α, and decreased the expression of mitochondrial and anti-apoptosis-related genes such as IL-2 and IFN-γ. Studies have shown that AM polysaccharides mitigate these adverse effects by reducing oxidative stress, enhancing mitochondrial function, and inhibiting apoptosis; this finding suggests that AM polysaccharides may play a role in protecting immune organs (76). In a mouse airbag model system, extracts from AM inhibited the production of NO, TNF-α, IL-1β, IL-6, VEGF and PlGF. This suggests a strong inhibitory effect of Atractylodes macrocephala in the treatment of chronic inflammation (77). In RAW264.7 cells, the addition of AM extracts resulted in a dose-dependent decrease in NO production after LPS stimulation. This suggests that AM extracts have the same anti-inflammatory activity (78).
There are several limitations to this study. Firstly, we only searched for literature in Chinese and English databases, which may have caused us to miss some relevant studies in Japanese, Korean, or other language databases. All randomized controlled trials in our research was conducted in China, and the results from the funnel plot and Egger’s test suggest that there may be possible publication bias. In addition, only eight studies explicitly described the method of randomized allocation. In most of the included trials, hiding the mandate and blindness were labeled as “unclear,” which could lead to possible implementation bias and selectivity bias. Third, all of the randomized controlled trials in our study did not have tumor markers as a key indicator; therefore, we look forward to conducting more studies on tumor markers containing Atractylodes macrocephala. Fourth, many outcomes, such as DCR, QOL, and peripheral blood lymphocyte levels, showed significant differences (I2>50%) between studies, which may affect the reliability of the meta-analysis results. Therefore, it is necessary to conduct more rigorously designed randomized controlled trials to validate our findings. Fifth, Atractylodes macrocephala is rarely used as a monotherapy in the treatment of AGC; it is usually used in combination with other different herbs. The mechanism of specific immunity and cytotoxicity of white atractylodes in adjuvant chemotherapy for AGC needs to be further investigated. Sixth, in all the included RCTs, only oral treatments containing Atractylodes macrocephala herbal medicine were available; injections and other preparations were not found; more preparations containing Atractylodes macrocephala herbal medicine need to be further investigated. Finally, based on the GRADE approach, the quality of the results ranged from moderate to exceedingly low; most of the trials incorporated in the original manuscript may not have been reported strictly according to the CONSORT reporting criteria. All these limitations may have led to an inadequate assessment of the results. However, we aspire that our manuscript will encourage an increasing number of physicians to acknowledge the potential advantages of herbal medicines containing white herbs in AGC. We also hope to inspire the undertaking of more meticulously planned clinical trials in the future to validate the effectiveness of herbal medicines containing white herbs in alignment with the CONSORT guidelines.
5 Conclusion
Our study showed that the use of herbal medicine containing Atractylodis Macrocephalae in combination with NAC had improved outcomes and safety for patients with gastric adenocarcinoma. Additional endeavors are required to advocate for the utilization of Atractylodis Macrocephalae-containing herbal medicines in clinical environments.
Furthermore, the lasting effectiveness of Atractylodis Macrocephalae-containing herbal remedies in conjunction with NAC for treating AGC should be confirmed in upcoming meticulously planned clinical studies that adhere to the CONSORT guidelines.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XN: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. HG: Investigation, Methodology, Writing – review & editing. JL: Investigation, Methodology, Writing – review & editing. JZ: Data curation, Methodology, Writing – review & editing. WR: Data curation, Methodology, Writing – review & editing. YH: Data curation, Methodology, Writing – review & editing. XS: Data curation, Methodology, Writing – review & editing. PS: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing. CJ: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by the National Natural Science Foundation of China (82374539), the Key Project of Jiangsu Provincial Science and Technology Development Program of Traditional Chinese Medicine (ZD202214), Jiangsu Provincial Science, Technology Development Program of Traditional Chinese Medicine (YB201916) and Jiangsu Province Graduate Student Practice and Innovation Program (KYCX24_2230).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1431381/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Wanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, Osteen R. Cancer of the stomach. A patient Care study by Am Coll Surgeons. Ann Surg. (1993) 218:583–92. doi: 10.1097/00000658-199321850-00002
4. Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. (2016) 22:2403–14. doi: 10.3748/wjg.v22.i8.2403
5. Wang W, Sun Z, Deng J, Wang Z, Zhou Z, Liang H, et al. Integration and analysis of surgical treatment-related data for gastric cancer based on a large multi-center database. Chin J Gastrointestinal Surg. (2016) 19:179–85. doi: 10.3760/cma.j.issn.1671-0274.2016.02.014
6. Wang FH, Zhang XT, Tang L, Wu Q, Cai MY, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun (Lond). (2024) 44:127–72. doi: 10.1002/cac2.12516
7. Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis randomized trials. Int J Surg. (2018) 51:120–7. doi: 10.1016/j.ijsu.2018.01.008
8. Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. (2006) 24:3953–8. doi: 10.1200/JCO.2006.06.4840
9. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. (2006) 355:11–20. doi: 10.1056/NEJMoa055531
10. Zachariae R, Paulsen K, Mehlsen M, Jensen AB, Johansson A, von der Maase H. Chemotherapy-induced nausea, vomiting, and fatigue–the role of individual differences related to sensory perception and autonomic reactivity. Psychother Psychosom. (2007) 76:376–84. doi: 10.1159/000107566
11. Choi NR, Choi WG, Zhu A, Park J, Kim YT, Hong J, et al. Exploring the Therapeutic Effects of Atractylodes macrocephala Koidz against Human Gastric Cancer. Nutrients. (2024) 16:965. doi: 10.3390/nu16070965
12. Huang Y, Zhang H, Gong H, Liu Y, Dong H, Chen H, et al. Effect of Guiqi Baizhu Recipe combined with oxaliplatin on apoptosis of gastric cancer cells in mice bearing gastric cancer based on PI3K/Akt/caspase-9 signaling pathway. Chin Pharmacol Bull. (2019) 39:1781–6. doi: 10.12360/CPB202207050
13. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. (1981) 47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
14. Watanabe H, Yamamoto S, Kunitoh H, Sekine I, Yamamoto N, Ohe Y, et al. Tumor response to chemotherapy: the validity and reproducibility of RECIST guidelines in NSCLC patients. Cancer Sci. (2003) 94:1015–20. doi: 10.1111/j.1349-7006.2003.tb01394.x
15. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. (2003) 13:176–81. doi: 10.1016/S1053-4296(03)00031-6
16. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. (2009) 3:e123–30. doi: 10.1371/journal.pmed.1000097
18. Ma Y. Analysis of the effect of spleen-strengthening and knot-dissolving soup combined with SOX chemotherapy regimen in treating advanced gastric cancer. Contemp Med Symposium. (2020) 18:218–9.
19. Shi-Ling S, Hong-Rui Z, Fa-Xiang Z. Clinical analysis of modified sijunzi decoction combined with tegafur gimeracil oteracil potassium capsule in treating 20 cases of late gastric cancer. Chin J Exp Traditional Med Formulae. (2013) 13:7–8. doi: 10.1515/pubhef-2005-2185
20. Lu W. Effect of jianpi yiqi sanjie decoction combined with chemotherapy in treating stage IV of gastric cancer with syndrome of spleen deficiency phlegm and blood stasis. Doctor. (2018) 3:49–50. doi: 10.19604/j.cnki.dys.2018.10.024
21. Li. The self-made Fuzheng Quxie Jiedu decoction plus neoadjuvant chemotherapy on gastric cancer and its influence on the immune function. Clin J Chin Med. (2021) 16:112–4. doi: 10.3969/j.issn.1674-7860.2021.16.036
22. Xu Y, Wang JB. Clinical study of spleen-strengthening and eliminating stagnation soup combined with SOX regimen in the treatment of patients with stage IIB-IV gastric cancer. Capital Food Med. (2020) 27:196.
23. Xiang-Yong Y, Jun LI, Cai-Juan L, Shuzheng S, Zhongsheng Y. Effects of the modified bazhen tang in the patients with advanced gastric cancer during chemotherapy. World J Integrated Traditional Western Med. (2019) 14:540–3. doi: 10.13935/j.cnki.sjzx.190423
24. Ye Q, Liu HH. Clinical observation on 30 cases of advanced gastric cancer treated with chemotherapy combined with tonifying the middle and benefiting qi soup and benefiting stomach soup. Guiding J Of Traditional Chin Med Pharm. (2017) 23(02):47–8. doi: 10.13862/j.cnki.cn43-1446/r.2017.02.011
25. Zhu. Observation on the clinical efficacy of tonifying zhong yi qi soup combined with yi gastric soup combined with chemotherapy in the treatment of middle and advanced gastric cancer. Electronic J Clin Med Literature. (2019) 6(96):163. doi: 10.16281/j.cnki.jocml.2019.96.133
26. Wu d, Zheng JJ, Luo DS, Xu HT. The effect and significance of bazhen decoction in neoadjuvant chemotherapy for the elderly advanced gastric. Cancer. (2020) 26(04):706–10. doi: 10.3969/j.issn.1007-6948.2020.04.027
27. Zhang YM, Wang XS, Gao YM. Treatment of 30 cases of middle and advanced gastric cancer with Qing Yu Fu Zheng Tang. Shaanxi J Traditional Chin Med. (2005) 09:889–91.
28. Hu PP. 30 cases of advanced gastric cancer treated with Fu Zheng He Gastric Compound combined with OLF regimen. Shaanxi J Traditional Chin Med. (2009) 30(01):6–7.
29. Huang JQ, Cao JX. Therapeutic effect of spleen-strengthening and stasis-removing soup combined with chemotherapy in treating 30 cases of advanced gastric cancer with spleen deficiency and phlegm stasis. HUNAN J Of Traditional Chin Med. (2014) 04:53–4. doi: 10.16808/j.cnki.issn1003-7705.2014.04.027
30. Wang BQ, Liu XD. Clinical efficacy and safety observation of spleen and kidney strengthening soup combined with chemotherapy in the treatment of advanced gastric cancer in elderly people. Acta Chin Med Pharmacol. (2015) 06:97–9. doi: 10.19664/j.cnki.1002-2392.2015.06.032
31. Guo XP. Therapeutic efficacy of combining chemotherapy with the addition and subtraction of ginseng-ling-white-juju-san in the treatment of middle and advanced gastric cancer. World Latest Med Inf. (2016) 40:96–9. doi: 10.3969/j.issn.1671-3141.2016.40.070
32. Zhao YH. Study on the efficacy of Ginseng Ling Baiyue San plus and minus and chemotherapy in the treatment of middle and late gastric cancer. China’s Rural Health. (2018) 2:90.
33. Zhang YY. Clinical efficacy of ginseng lingbai juju san combined with chemotherapy in treating patients with gastric cancer. China J Of Pharm Economics. (2017) 09:39–41. doi: 10.12010/j.issn.1673-5846.2017.09.011
34. Li BX, Zhang XQ, Zheng K. Clinical efficacy and safety of jianpi jiedu decoction combined with chemotherapy in patients with gastric cancer. Chin Arch Traditional Chin Med. (2019) 37:2833–6. doi: 10.13193/j.issn.1673-7717.2019.12.004
35. Wu XP, Chen ZL. Observation on the clinical effect of oxaliplatin and tigio combined with ginseng-lingbaijusan in the treatment of advanced gastric cancer. Henan J Surg. (2017) 23:93–4. doi: 10.16193/j.cnki.hnwk.2017.03.052.2017,(03):93-94
36. Wei HL, Li JT, Yan SG, Xiao G, Li C, Si MM, et al. Clinical observation of Bazhen Tang plus reduction combined with FOLFOX6 regimen for preoperative chemotherapy of progressive gastric cancer. Modern Trad Chinese Med. (2018) 38:64–7. doi: 10.13424/j.cnki.mtcm.2018.06.022
37. Wen ZW, Wan CX, He JH, Tao HF. Effect to fJianpiFuzheng prescription on short-term efficacy and immune function of gastric cancer patients receiving neoadjuvant chemotherapy. Chin J Of Integrated Traditional And Western Med On Digestion. (2021) 29:14–8. doi: 10.3969/j.issn.1671-038X.2021.01.03
38. Zhang QS, Li L. Effect of buzhong yiqi decoction combined with yiwei decoction on the efficacy and quality of life for patients with advanced gastric cancer Receiving palliative chemotherapy. J Sichuan Traditional Chin Med. (2021) 03:93–6. doi: 10.1155/2021/3685213
39. Li ZP, Xu L, Xu T, Liu SL, Li X. Clinical observation on 50 cases of progressive gastric cancer treated with Ginseng Ling Bai Zhu San combined with TS chemotherapy regimen. J Traditional Chin Med. (2016) 16:1393–6. doi: 10.13288/j.11-2166/r.2016.16.012
40. Zhu LM, Guo LJ, Mao ZJ, Zhang QJ, Du DF, Shen KP. The effect of jianpiyishen detoxification prescription combined with chemotherapyon cancer-related fatigue and immune function of patients with advanced gastric cancer. Chin J Of Microcirculation. (2019) 03:39–44,48. doi: 10.3969/j.issn.1005-1740.2019.03.009
41. Xie XD, Qiang YH, Liu SL, Chen YZ, Wu W, Zhang XX. Study on clinical efficacy and mechanism of J ianpiYangwei formula combined with neoadjuvant chemotherapy for patients with gastric cancer. J NanJing Univ Traditional Chin Med. (2021) 37:198–204. doi: 10.14148/j.issn.1672-0482.2021.0198
42. Xie GP, He YN, Zhai J, Yao XQ, Shen LZ. Clinical study of Huangqi-Sijunzi Decoction in enhancing the sensitivity of preoperative adjuvant chemotherapy for advanced gastric cancer. China J Traditional Chin Med Pharm. (2022) 37:1810–4.
43. Li ZQ. Twenty-seven cases of middle and advanced gastric cancer treated with chemotherapy by adding and subtracting ginseng-ling-white atractylodes powder. Shaanxi J Traditional Chin Med. (2011) 01:4–6.
44. Zheng QH, Chen WG, Wang FL, Wang SP, Sun F, Wang YH, et al. Observation on the adjuvant therapeutic effect of spleen-enhancing and kidney-benefiting traditional Chinese medicine on chemotherapy patients with advanced gastric cancer. J SIchuan Traditional Chin Med. (2012) 03:73–4.
45. Hu PP, Wang WW, Xue Q. Efficacy observation on 28 cases of advanced gastric cancer treated with Strengthening the Spleen and Dispersing Jie Tang combined with FOLFOX regimen. J New Chin Med. (2010) 42:76–7. doi: 10.13457/j.cnki.jncm.2010.06.080
46. Zhu DY, Qiu WB. Twenty-four cases of middle and advanced gastric cancer treated with chemotherapy by addition and subtraction of Ginseng Ling Bai Zhu San. Modern Diagnosis Treat. (2013) 14:3183–4.
47. Lai YQ, Chen NJ, Wu DH, Yu JP. Clinical observation on 25 cases of advanced gastric cancer treated with chemotherapy by self-proposed spleen and kidney strengthening soup. Fujian J Of Traditional Chin Med. 41:18–9. doi: 10.13260/j.cnki.jfjtcm.010041
48. Li ZJ, Shi L. Clinical observation on 40 cases of advanced gastric cancer treated with Bazhen Tang plus reduction combined with chemotherapy. Chin J Of Ethnomedicine And Ethnopharmacy. (2014) 22:70–1.
49. Chen YC, Liu SL, Wang RP, Zhou X. Clinical study on 84 cases of “Strengthening the spleen, nourishing the body and eliminating the symptoms” to improve the quality of life of advanced gastric cancer patients after chemotherapy. Jiangsu J Traditional Chin Med. (2013) 06:18–20.
50. Hu J, Cheng M, Li Y, Shi B, He S, Yao Z, et al. Ginseng-containing traditional medicine preparations in combination with fluoropyrimidine-based chemotherapy for advanced gastric cancer: A systematic review and meta-analysis. PloS One. (2023) 18:e0284398. doi: 10.1371/journal.pone.0284398
51. Chen M, May BH, Zhou IW, Xue CC, Zhang AL. Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integr Cancer Ther. (2016) 15:40–59. doi: 10.1177/1534735415596424
52. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. (2021) 127:3029–30. doi: 10.1002/cncr.33587
53. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
54. Wang SM, Zheng RS, Han BF, Li L, Chen R, Sun KX, et al. Age distribution of cancer incidence and mortality in China in 2022. China Cancer. (2024) 33:165–17. doi: 10.11735/j.issn.1004-0242.2024.03.A001
55. Li CL, Bai XF, Wei YZ. Application of radical gastrectomy combined with neoadjuvant chemotherapy in patients with advanced gastric cancer and its effect on T lymphocyte level. The Pract J Cancer. (2020) 35:2035–7. doi: 10.3969/j.issn.1001-5930.2020.12.029
56. Liu SL, Liu BR. Guidelines for combined Chinese and Western medicine diagnosis and treatment of gastric cancer. Chin J Integrated Traditional Chin Med. (2023) 44:261–72. doi: 10.7661/j.cjim.20240131.008
57. Liu C, Wang S, Xiang Z, Xu T, He M, Xue Q, et al. The chemistry and efficacy benefits of polysaccharides from Atractylodes macrocephala Koidz. Front Pharmacol. (2022) 13:952061. doi: 10.3389/fphar.2022.952061
58. Chen L, Huang G. Antitumor activity of polysaccharides: an overview. Curr Drug Targets. (2018) 19:89–96. doi: 10.2174/1389450118666170704143018
59. Kou N, Cho H, Kim HE, Sun Q, Ahn K, Ji H, et al. Anti-cancer effect of Atractylodes macrocephala extract by double induction of apoptotic and autophagic cell death in head and neck cancer cells. Bangladesh J Pharmacol. (2017) 12:25. doi: 10.3329/bjp.v12i2.31238
60. Zhu B, Zhang QL, Hua JW, Cheng WL, Qin LP. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J Ethnopharmacol. (2018) 226:143–67. doi: 10.1016/j.jep.2018.08.023
61. Gu S, Li L, Huang H, Wang B, Zhang T. Antitumor, antiviral, and anti-inflammatory efficacy of essential oils from atractylodes macrocephala koidz. Produced Different Process Methods Molecules. (2019) 24:2956. doi: 10.3390/molecules24162956
62. Huang HL, Lin TW, Huang YL, Huang RL. Induction of apoptosis and differentiation by atractylenolide-1 isolated from Atractylodes macrocephala in human leukemia cells. Bioorg Med Chem Lett. (2016) 26:1905–9. doi: 10.1016/j.bmcl.2016.03.021
63. Liu H, Zhu Y, Zhang T, Zhao Z, Zhao Y, Cheng P, et al. Anti-tumor effects of atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules. (2013) 18:13357–68. doi: 10.3390/molecules181113357
64. Long F, Wang T, Jia P, Wang H, Qing Y, Xiong T, et al. Anti-tumor effects of atractylenolide-I on human ovarian cancer cells. Med Sci Monit. (2017) 23:571–9. doi: 10.12659/msm.902886
65. Li X, Zhang D, Song DQ, Song S, Liu M. Effects of atractylenolide I on tumor growth and apoptosis of human gastric cancer cell line SGC-7901 xenografts in nude mice. Chin J Hosp Pharm. (2018) 38:1921–5. doi:10.13286/j.cnki.chinhosppharmacyj.2018.18.09
66. Gao X, Wang B, Yin S, Zhang Z, Chen Y. Research of atractylenolide on proliferation ability of IEC-6 cells and esophageal cancer ECA9706 cells. China J Traditional Chin Med Pharm. (2015) 30:921–3.
67. Tian S, Yu H. Atractylenolide II inhibits proliferation, motility and induces apoptosis in human gastric carcinoma cell lines HGC-27 and AGS. Molecules. (2017) 22:1886. doi: 10.3390/molecules22111886
68. Song HP, Li RL, Chen X, Wang YY, Cai JZ, Liu J, et al. Atractylodes macrocephala Koidz promotes intestinal epithelial restitution via the polyamine–voltage-gated K+ channel pathway. J Ethnopharmacol. (2014) 152:163–72. doi: 10.1016/j.jep.2013.12.049
69. Wang Z, Li RL, Xu SF, Chen WW. Effects of Atractylodes macrocephala monosaccharide composition on cytodifferentiation and villin expression of IEC-6 cells in vitro. Zhong Yao Cai. (2010) 33:938–44. doi: 10.13863/j.issn1001-4454.2010.06.037
70. Zhang Y, Xu S, Lin Y. Gastrointestinal inhibitory effects of sesquiterpene lactones from Atractylodes macrocephala. Zhong Yao Cai. (1999) 22:636–40. doi: 10.13863/j.issn1001-4454.1999.12.012
71. Liu Y, Ye F, Qiu GQ, Zhang M, Wang R, He QY, et al. Effects of lactone I from Atractylodes macrocephala Koidz on cytokines and proteolysis-inducing factors in cachectic cancer patients. Di Yi Jun Yi Da Xue Bao. (2005) 25:1308–11.
72. Liu Y, Jia Z, Dong L, Wang R, Qiu G. A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid Based Complement Alternat Med. (2008) 5:337–44. doi: 10.1093/ecam/nem031
73. Chi D, Sun Y, Pan S. The protective effect and its mechanism of Antrodia camphorata enzyme on the cytotoxicity injury of PC12 cells induced by amphetamine. Chin J Clin Anat. (2017) 35:397–401+408. doi: 10.13418/j.issn.1001-165x.2017.04.009
74. Li H, Dai S, Zhang W, Zhang M, Yin X. Analysis of the relationship between peripheral blood T lymphocyte subsets,PD-1 and prognosis in patients with gastric cancer. Modern Med J. (2018) 46:1119–22.
75. Li LL, Yin FG, Zhang B. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livest Sci. (2011) 142:33–41. doi: 10.1016/j.livsci.2011.06.013
76. Xu D, Li B, Cao N, Li W, Tian Y, Huang Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget. (2017) 8:70394–405. doi: 10.18632/oncotarget.19709
77. Wang C, Duan H, He L. Inhibitory effect of atractylenolide I on angiogenesis in chronic inflammation in vivo and in vitro. Eur J Pharmacol. (2009) 612:143–52. doi: 10.1016/j.ejphar.2009.04.001
Keywords: Atractylodes macrocephala, neoadjuvant chemotherapy, progressive gastric cancer, traditional Chinese medicine, meta-analysis
Citation: Niu X, Gu H, Li J, Zuo J, Ren W, Huang Y, Shu X, Jiang C and Shu P (2024) Efficacy and safety of Atractylodes macrocephala-containing traditional Chinese medicine combined with neoadjuvant chemotherapy in the treatment of advanced gastric cancer: a systematic evaluation and meta-analysis. Front. Oncol. 14:1431381. doi: 10.3389/fonc.2024.1431381
Received: 11 May 2024; Accepted: 18 September 2024;
Published: 16 October 2024.
Edited by:
Ka Wu, Nanning Second People’s Hospital, ChinaReviewed by:
Yang Li, Baylor College of Medicine, United StatesYilin Tong, China Medical University, China
Zhuofei Bi, Sun Yat-sen University, China
Copyright © 2024 Niu, Gu, Li, Zuo, Ren, Huang, Shu, Jiang and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Jiang, ZG9jdG9yamNAMTYzLmNvbQ==; Peng Shu, c2h1cGVuZ25qdWNtQDE2My5jb20=
 Xiaotao Niu
Xiaotao Niu Haoqing Gu1,2
Haoqing Gu1,2 Jingzhan Li
Jingzhan Li Jiaqian Zuo
Jiaqian Zuo Chao Jiang
Chao Jiang