- 1Department of Hepatobiliary Surgery, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
- 2Department of Oncology, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
Objective: To investigate the pathogenesis, clinical manifestations, imaging and pathological features, and treatment methods of primary hepatic lymphoma (PHL).
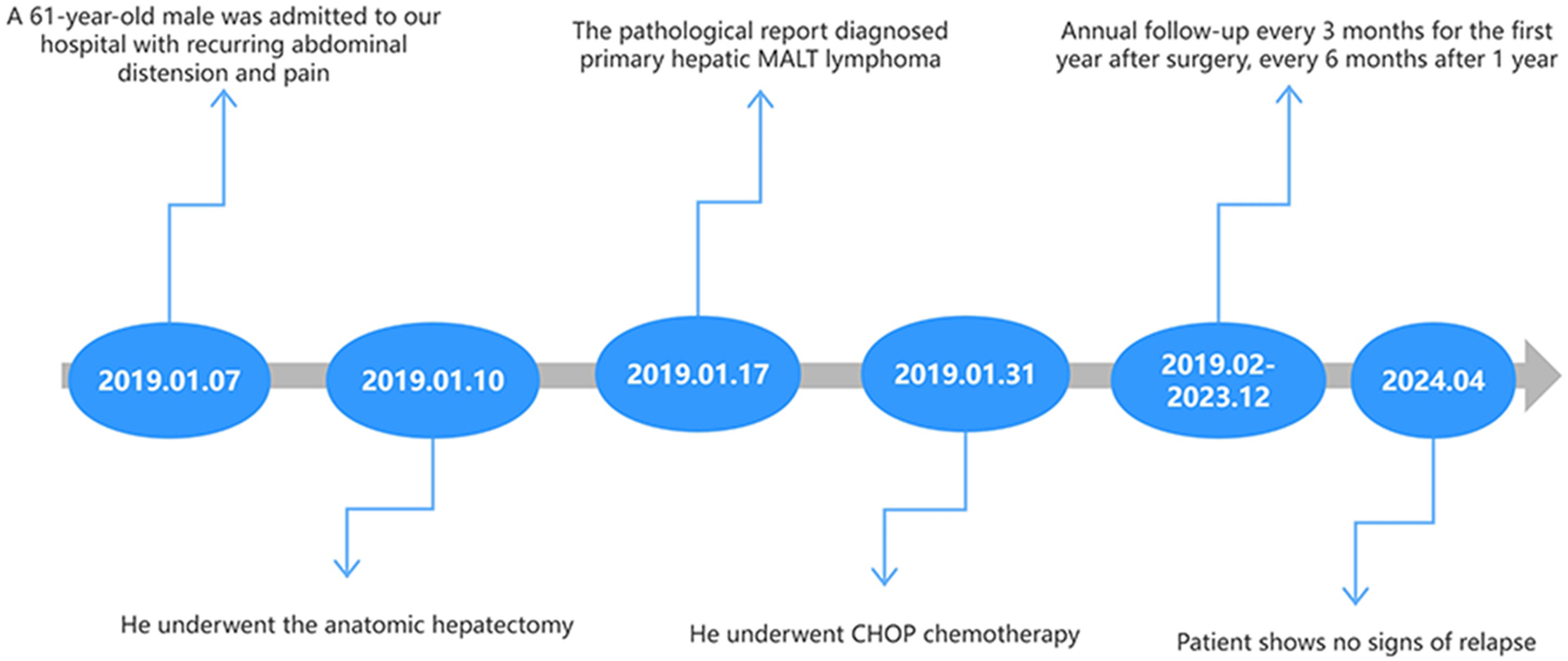
Case presentation: A 61-year-old male with a history of hepatitis B virus (HBV) infection presented to the hospital complaining of abdominal pain. Preoperative abdominal computed tomography (CT) revealed a mass in the right lobe of the liver, accompanied by an elevated alpha-fetoprotein (AFP) level. Consequently, hepatocellular carcinoma (HCC) was initially suspected. Following a comprehensive multidisciplinary consultation, the patient underwent an anatomical hepatectomy. Histopathological examination post-surgery confirmed the diagnosis of primary hepatic mucosa-associated lymphoid tissue (MALT) lymphoma. The patient received chemotherapy as an adjunct to surgical treatment. During the five-year follow-up period, there was no evidence of tumor recurrence.
Conclusion: Primary hepatic MALT lymphoma is infrequently encountered in clinical practice. Its clinical and radiological presentations are often nonspecific, making the pathological evaluation the definitive diagnostic tool. Surgical resection, in conjunction with chemotherapy, remains the cornerstone of management for this condition. The prognosis for most patients is favorable.
Introduction
Primary hepatic lymphoma (PHL) is an uncommon lymphoproliferative disorder confined to the liver, without extrahepatic lymph node involvement, and typically associated with a normal leukocyte count in the peripheral blood smear (1). A specific subtype of PHL, mucosal-associated lymphoid tissue (MALT) lymphoma, accounts for less than 0.4% of all non-Hodgkin’s lymphomas (NHL) (2). Due to its rarity, most current knowledge about PHL is derived from case reports and case series. This lack of extensive data contributes to limited understanding of the etiology, pathogenesis, and distinct imaging features of PHL, leading to a high probability of misdiagnosis (3). In this manuscript, we present an intriguing case of PHL that was initially suspected to be hepatocellular carcinoma (HCC) preoperatively. We aim to delve into the various aspects of PHL, including its pathogenesis, epidemiology, clinical presentation, imaging features, pathological findings, and treatment. Our goal is to enhance clinicians’ awareness of PHL and provide a comprehensive diagnostic and management framework for this rare condition.
Case presentation
A 61-year-old male was admitted to our hospital with a two-year history of recurring abdominal distension and pain. The patient was a long-term smoker, consuming a pack of cigarettes daily for 40 years, but did not consume alcohol. He reported a history of hepatitis B virus (HBV) infection lasting over 30 years, with numerous unsuccessful treatment attempts. He denied any familial history of malignancy. Physical examination revealed deep pressure pain and abdominal distension in the right upper quadrant without jaundice or pyrexia. No superficial lymphadenopathy was observed.
Laboratory results are summarized in Table 1. The patient tested positive for hepatitis B surface Antigen (HBsAg), hepatitis B e antigen (HBeAg), and hepatitis B core antibody (HBcAb). Tests for hepatitis B surface antibody (HBsAb) and hepatitis B e antibody (HBeAb) returned negative results. Importantly, the patient tested negative for Helicobacter pylori(HP) infection, other hepatitis viruses, human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), and all autoimmune antibodies. Notably, the laboratory report indicated significantly elevated levels of alpha-fetoprotein (AFP) and HBV-DNA, at 185ng/ml and 3.78x106IU/ml, respectively. Other serum analyses, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, lactic dehydrogenase (LDH), and tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 199 (CA199), were within normal limits. Abdominal CT scans with intravenous contrast revealed a low-density mass (4.7x4.6cm) in the right hepatic lobe, exhibiting mild arterial enhancement margins (Figure 1A). The patient showed no signs of mediastinal, hilar, or axillary lymphadenopathy, pulmonary nodules, or effusions, and there was no evidence of metastatic disease in CT scans. The contrast-enhanced ultrasonography(CEUS) showed a hypoechoic tumor near the hepatic portal vein (Figure 1B). Besides, we scheduled the patient for an abdominal enhanced MRI, but he was forced to cancel due to the metal material after surgery for a fracture of the right lower limb.
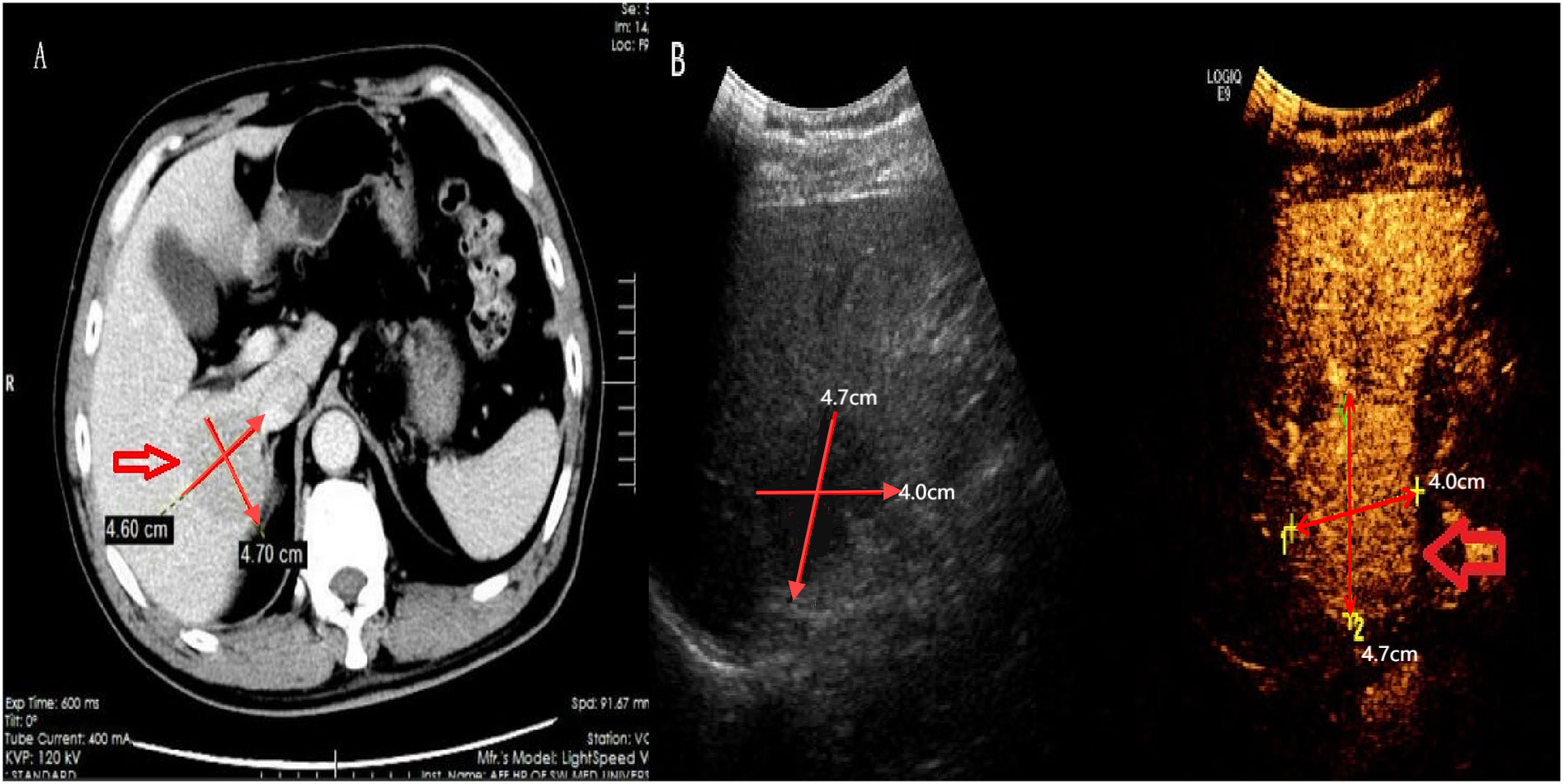
Figure 1. Computed tomography and contrast ultrasound results. Shown here the lump with hypodense lesion on CT (A) and low echo on contrast ultrasound and contrast-enhanced ultrasonography (B).
Given the patient’s history of HBV, elevated AFP, and the laboratory and imaging findings, a hepatic malignancy, specifically HCC, was suspected. However, the patient declined a biopsy. After a multidisciplinary discussion, it was agreed that the patient had no clear surgical contraindications, and surgical resection was indicated. An anatomical hepatectomy was performed on the third day following admission. During surgery, no metastatic lesions were found, and the liver showed no obvious signs of cirrhosis or ascites. A solid tumor was located in segment VII of the liver, and frozen sections revealed negative hepatic margins. Postoperatively, the patient received hepatoprotective medications, anti-infective therapy, and nutritional support, recovering well without complications.
However, postoperative pathology revealed hepatic lymphoma, with an abundance of lymphocytes present in the tumor tissue. Hematoxylin and eosin staining highlighted the representative cytological feature of small, round tumor cells against a hepatic cell background (Figure 2A). Immunohistochemical staining showed diffuse reactivity of lymphocytes with CD20 (Figure 2B). Moreover, lymphocytes within the tumor were positive for CD19, CD21, CD79a (Figure 2C), and Ki67 (+3%), but negative for Bcl-6, CD3, CD10, and cyclin D1. The resected specimen revealed a partially necrotic lump with a clear boundary and a complete capsule (Figure 2D). These findings confirmed the diagnosis of B-cell NHL (B-NHL) of the MALT type. Following the diagnosis, the patient underwent CHOP (Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone) chemotherapy in the department of hematology. Furthermore, t(14;18)(q32;q21) and mucosa-associated lymphoid tissue 1 (MALT1) were observed by cytogenetic analysis in this case. Analysis of the clonal amplification peaks revealed a rearrangement of the IGK gene Vk-Kde+intron-Kde (Tube B) and a rearrangement of the IGH gene FR1-JH (Tube A), FR2-JH (Tube B), FR3-JH (Tube C) and DH-JH (Tube D). At a 5-year follow-up (as shown in Figure 3), the patient remained well without signs of recurrence, as determined by physical examination and out-of-hospital imaging, including enhanced CT and tumor markers. The lack of postoperative imaging follow-up in our hospital might be a limitation, but out-of-hospital imaging confirmed the patient’s continued remission.
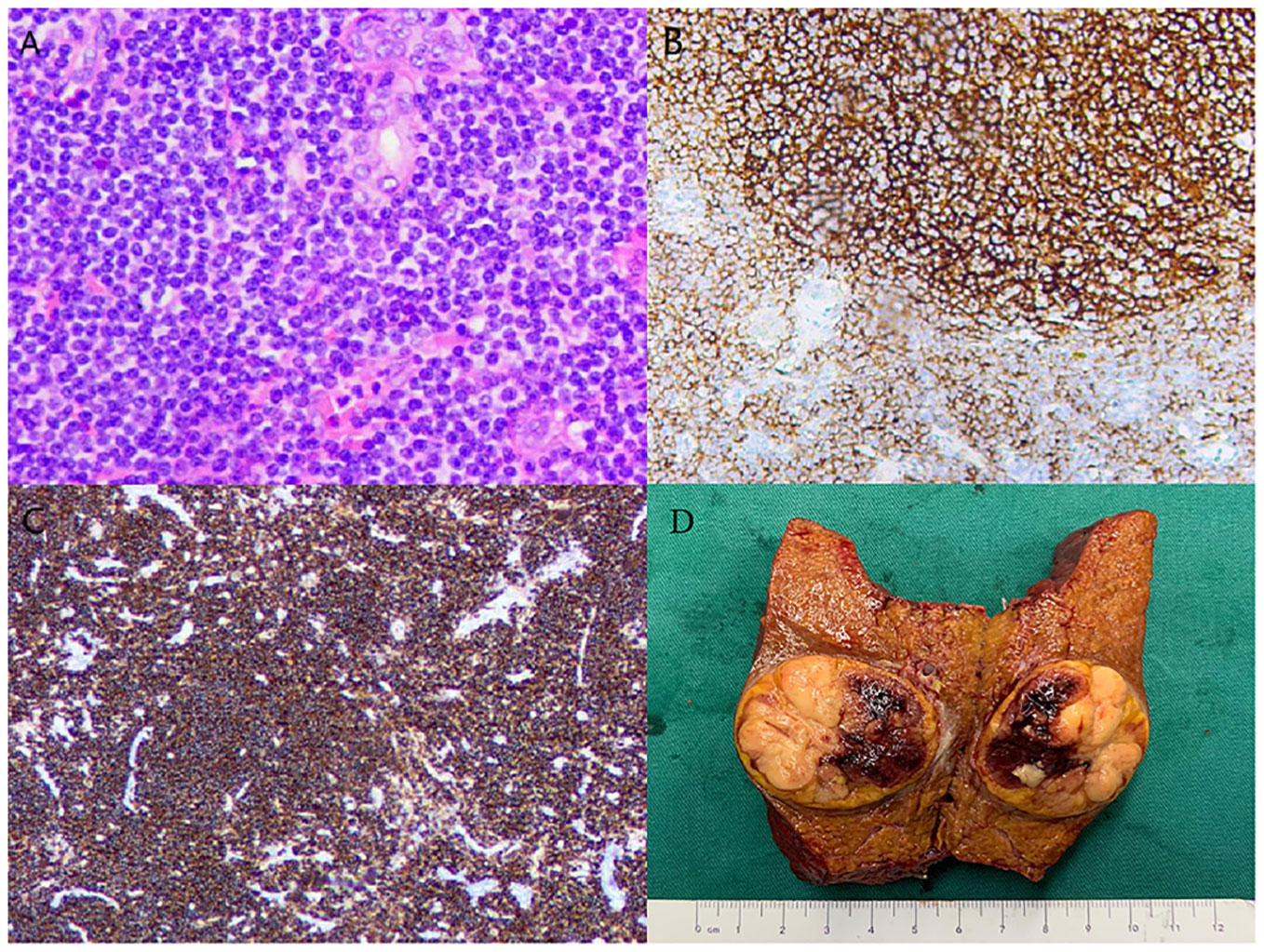
Figure 2. Histologic characteristics of the resected tumor. Hematoxylin-eosin staining shows (A) x100) small round cell tumor. Immunohistochemical analysis (B, C) showed that the lymphocytes were positive for CD20 and CD79a. Resected tumor specimen (D).
Discussion
Primary hepatic MALT lymphoma is a type of NHL confined to the liver, without involvement of extrahepatic lymph nodes. It typically manifests as an indolent lymphoma (4). PHL predominantly affects middle-aged individuals, with a marked male predominance and a median age of 63.5 years (5). The specific risk factors for PHL remain unclear; however, multiple studies suggest that chronic liver diseases, such as EBV, HBV, HCV, HP infection, cirrhosis, and primary biliary cirrhosis, may contribute to its development (6–10). Yang et al. (9) reported HBV infection in 33.3% of cases and HCV infection in 11.1%. Many researchers propose that chronic liver inflammation is a common factor in PHL pathogenesis, promoting lymphocyte migration to the liver and chronic proliferation of B lymphocytes, ultimately leading to hepatic lymphoma (11, 12). These findings provide a strong theoretical foundation for PHL treatment strategies. In our case, the patient had a history of HBV infection with a high viral load and had undergone anti-HBV therapy. PHL has also been observed in patients with immune disorders such as systemic lupus erythematosus, AIDS, and Buerger’s disease (13–15), as well as in patients with tumors including gastric cancer, rectal cancer, and hepatic hemangioma (16–18). Additionally, the most common translocation in primary hepatic MALT lymphoma is t(14;18)(q32;q21), which leads to overexpression of the MALT1 gene and activation of the NF-κB pathway, along with overexpression of BCL-2, an anti-apoptotic factor, and rearrangement of monoclonal IgH (10, 19).
Most cases of PHL are incidental findings during postoperative pathology, and their clinical presentation is not distinctive. Symptoms may include fatigue, anorexia, fever, weight loss, and jaundice (11, 20). The majority of laboratory results are within normal ranges. However, LDH, a diagnostic and prognostic marker, is elevated in 30%-80% of cases (11). Furthermore, tumor markers such as AFP, CEA, and CA199 often have limited clinical value in PHL diagnosis, as they are frequently negative, except in cases of HCC with slightly elevated AFP levels (9, 21, 22). Previous research (23) indicated that the proportion of HBsAg-positive patients in indolent B-NHL is significantly higher compared with aggressive B-NHL. Moreover, HBV-DNA levels are significantly higher in patients with indolent B-NHL compared to aggressive B-NHL. In the present case, all laboratory tests, including complete blood count and liver function tests, were negative. Notably, a high viral load of HBV and positive AFP were unique manifestations in this case.
In the absence of biopsy or pathological findings, imaging plays a crucial role in the diagnosis and differential diagnosis of PHL. Ultrasound typically reveals homogeneous hypoechoic lesions confined to the liver, with dilation of intrahepatic and extrahepatic bile ducts when the mass is located in the hilum (20). CEUS generally shows mild inhomogeneous enhancement in the arterial phase, which disappears in the portal and late stages (24). CEUS evaluation of intratumoral hemodynamics in real time may reveal the presence of blood vessels penetrating the tumor, which is useful for diagnosing malignant lymphoma (25). On CT scans, the most common manifestation is a solitary hypoattenuating lesion with a core area of low intensity. Enhanced CT usually shows low-density masses with slight or no enhancement in the arterial phase and progressive enhancement in the venous phase (17, 26). MRI of PHL lesions typically exhibits hypointensity on T1-weighted images and hyperintensity on T2-weighted images (5, 9). Furthermore, significant signal enhancement on diffusion-weighted imaging is commonly seen in PHL patients, with a lower apparent diffusion coefficient value compared to other malignant liver diseases (27). MALT lymphomas may show arterial phase enhancement, restricted diffusion, vessel penetration signs, and ‘speckled enhancement,’ a term used to describe punctate positive enhancement within the low-signal intensity lesions on the hepatobiliary phase of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI (28). Positron emission tomography/computed tomography (PET/CT) is often used to detect metastatic tumors through differential 18F-fluorodeoxyglucose (FDG) uptake. In PHL, PET/CT typically shows an abnormal ring-like metabolic focus in the mass lobe, exhibiting less FDG uptake at the center and negativity in other body sites (28, 29).
The clinical presentation and imaging manifestations of primary hepatic MALT lymphoma are nonspecific, necessitating pathological confirmation for a definitive diagnosis. Diagnosing PHL can be challenging until pathological results are available. A detailed clinical history and thorough examination, including flow cytometry and immunohistochemistry, are essential for accurate diagnosis and appropriate treatment. Moreover, differential diagnosis plays a crucial role in therapeutic decision-making for clinicians. Firstly, PHL needs to be distinguished from HCC. HCC is often associated with a history of hepatitis, elevated AFP, and unique imaging characteristics. On dynamic enhanced CT and MRI, HCC typically shows homogeneous or heterogeneous marked enhancement in the arterial phase (mainly late arterial stage) and less enhancement in the portal and delayed phases compared to liver parenchyma (30). These characteristics may help differentiate HCC from other liver tumors. However, since PHL can show slight enhancement in the arterial and venous phases (17, 26), many PHL cases are preoperatively misdiagnosed as HCC in clinical practice. For example, Xu et al. (5) reported a case of a hepatic tumor in segment 6 (S6) that showed enhancement in the arterial phase and washout in the portal phase with low signal intensity in the hepatocyte-specific phase on enhanced MRI. Despite the absence of risk factors such as elevated HBV, HCV, HIV, or AFP, this mass was considered HCC based on imaging and underwent radiofrequency ablation (RFA). Similarly, Fu et al. (31) described a left hepatic tumor with a history of hepatitis B and elevated HBV DNA but no elevated AFP. Abdominal MRI revealed a long T1 and long or iso-T2 signal nodule measuring 10 x 6 mm in segment 2 (S2) of the liver. The tumor was preoperatively diagnosed as tiny HCC and subsequently underwent hepatectomy, with postoperative pathology confirming MALT lymphoma. In our case, due to the patient’s refusal of liver biopsy, mildly elevated AFP, high HBV load, and slight arterial phase enhancement, the tumor was suspected to be HCC.
Secondly, hepatic adenoma (HCA) cannot be ignored as a benign solid tumor, usually affecting women of childbearing age and solitary in 80% of cases. It is soft and well-defined, with almost no fibrous capsule. Clinically, HCA is often asymptomatic and associated with elevated gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP) (32). HCA is typically isointense to mildly hyperintense on T1- and T2-weighted images, with moderate enhancement in the arterial phase and no sustained enhancement in the portal venous and delayed phases (33). For instance, Wang et al. (21) detected a hepatic lesion without positive clinical manifestations or liver function abnormalities. The lesion was significantly enhanced in the arterial phase and decreased in the portal and delayed phases, similar to HCA. The patient underwent hepatectomy, and postoperative pathology revealed PHL.
Additionally, hepatic pseudolymphoma (HPL), also known as reactive lymphoid hyperplasia or nodular lymphoid lesion, is a rare disease characterized by the proliferation of non-neoplastic, polyclonal lymphocytes forming follicles with an active germinal center. Although HPL has benign behavior, it is clinicopathologically similar to MALT lymphoma and indistinguishable by conventional means. Zen et al. (34) claimed that HPL can be challenging to diagnose but can be differentiated from PHL by different infiltration patterns. Scientists have shown that HPL presents a portal distribution of atypical lymphoid cells without nodule formation, suggesting that pseudolymphoma originates from lymphoid tissue related to a portal tract and can enlarge by involving nearby portal tracts. Furthermore, simple observation has proven to be adequate, as spontaneous diminution or regression of the tumor has been reported (34, 35).
In terms of clinicopathological features, the atypical lymphoid cells characteristic of primary hepatic MALT lymphoma are small, with mildly irregular nucleoli, dense chromatin, and scant cytoplasm, notably lacking germinal center differentiation (36). A hallmark feature of hepatic MALT lymphoma is the presence of lymphoepithelial lesions within the bile ducts (37). Immunohistochemical analysis, essential for lymphoma classification and differentiation, typically reveals B-cell lineage with positivity for markers such as CD19, CD20, and CD79a, and negativity for CD3 (5, 6, 13). Furthermore, MALT lymphomas distinctively express CD21 and are negative for CD5, CD10, and cyclin D1, along with a low Ki-67 proliferation index (5, 37). In the present case, immunohistochemistry results showed positivity for CD19, CD20, and CD79a, and a low Ki-67 proliferation index of 3%. Therefore, histopathological evaluation of the tissue samples confirmed the diagnosis of MALT lymphoma in this case.
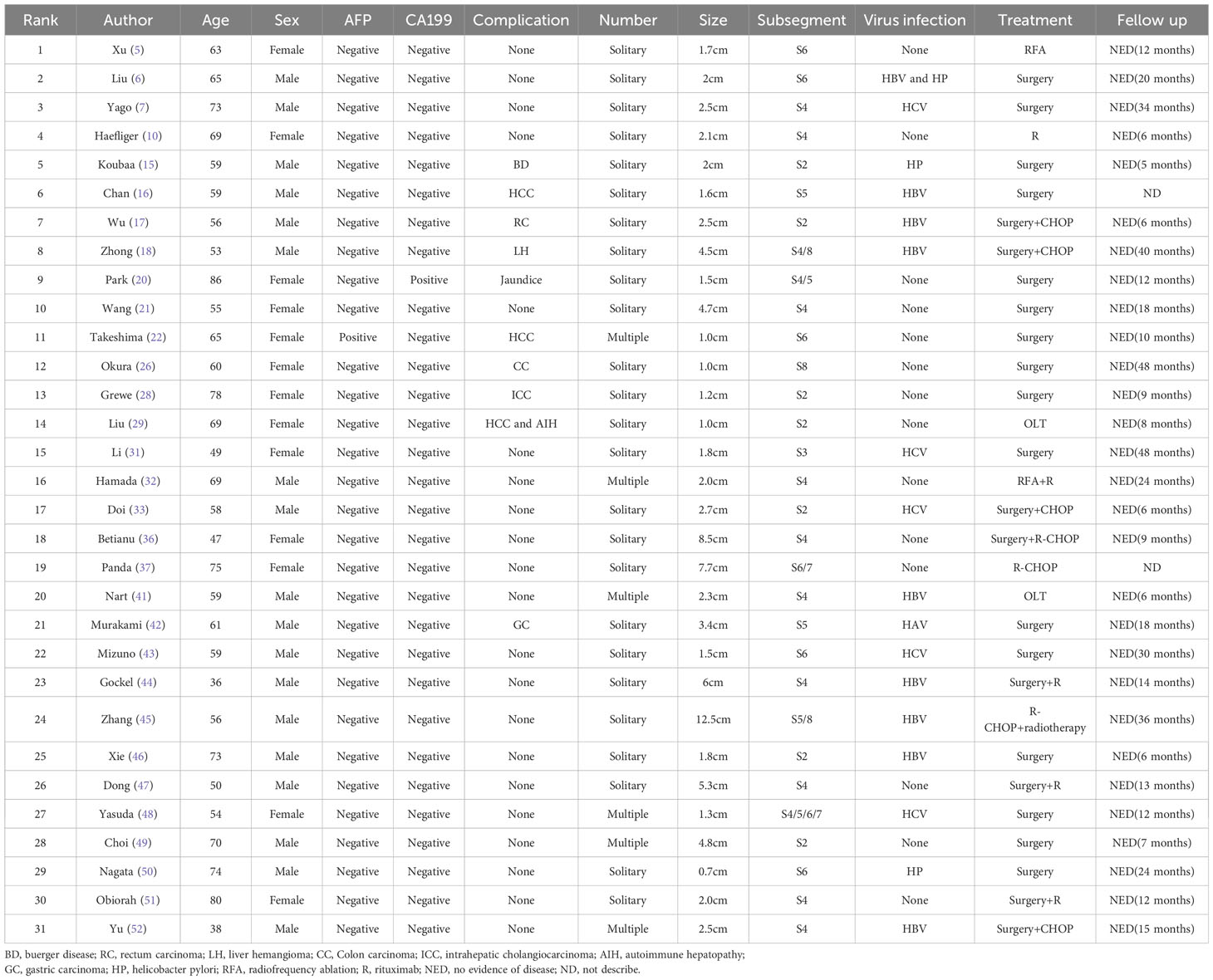
Current therapeutic approaches for primary hepatic MALT lymphoma encompass surgery, radiotherapy, chemotherapy, and integrative treatments. We summarized the treatment methods of PHLs in recent years (Table 2). An analysis of recent PHL cases reveals the employment of various treatment methods, including partial liver resection and liver transplantation (17, 37). Historical data underscores the significance of surgical resection for localized liver tumors in enhancing prognosis (21, 38, 39). For instance, a 55-year-old woman admitted to the hospital with upper abdominal pain was pathologically diagnosed with MALT lymphoma after surgery and achieved 18 months of tumor-free survival (21). Similarly, Li et al. (39) declared that local resection is beneficial due to the oncological indolence of the disease. Additionally, instances of employing radiofrequency ablation (RFA) for treating hepatic MALT lymphoma have reported favorable outcomes (5, 40).
Chemotherapy, especially the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone), is commonly adopted as a first-line treatment for PHL, attributed to its high sensitivity and regarded as a pivotal prognostic factor (9, 17, 18). For example, a report found a solitary mass 27 mm in size in the left lobe of the liver of a 58-year-old man with a history of hepatitis-C infection who received surgical resection and three courses of the CHOP regimen after hepatectomy and remained without any evidence of disease for 2 years (41). A cohort study of 24 PHL patients undergoing chemotherapy showed an 83.3% complete response rate, with 5-year cause-specific and failure-free survival rates of 87.1% and 70.1%, respectively (42). The addition of rituximab, an anti-CD20 monoclonal antibody, to the CHOP regimen enhances the complete response rate and extends both event-free and overall survival among elderly patients with diffuse large B-cell lymphoma, without markedly increasing clinical toxicity (41, 43, 44). Rituximab is also effective in relapsed hepatic MALT lymphoma. Gockel et al. (45) reported a case of recurred MALT lymphoma in the porta hepatis that disappeared after only rituximab treatment for 26 months. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase (PI3K), is a possible effective therapy for those following treatment failure with rituximab (46).
Radiotherapy has also shown promise in treating PHL. Shin et al. (47) achieved long-term remission of primary MALT lymphoma by radiotherapy alone. Recent studies have demonstrated the efficacy of adjuvant radiotherapy or the combination of chemotherapy and radiotherapy in PHL treatment (48, 49). Avlonitis et al. (50) substantiated improved prognoses in PHL cases treated with both surgery and chemotherapy compared to chemotherapy alone. Additionally, HBV/HCV infection plays a prominent role in PHL, and antiviral therapy should be highlighted throughout the therapeutic process.Some cases have also been reported in recent years, there are several differences between this patient and another study, such as Wang et al. (21) case. The first difference is the preoperative diagnosis. The patient we described was diagnosed with HCC rather than HCA, before liver tumor surgery due to his unique features (elevated AFP and higher HBV-DNA load). Moreover, our patient received adjuvant chemotherapy after surgery and obtained a longer tumor-free survival of up to 5 years (5 years vs 18 months). Besides, our review of recent reports of MALT lymphoma (51–60) revealed that our case had the longest tumour-free survival time.
In addition, since MALT lymphomas present inertly with less aggressive features, most of them have a good prognosis. Relapse may occur several years after treatment, with a median recurrence time of 5 years, and these relapses usually involve the same organ or other extranodal sites.
In conclusion
Primary hepatic MALT lymphoma represents an exceptionally uncommon malignancy, characterized by a lack of distinctive clinical and imaging features, which renders preoperative diagnosis exceedingly challenging and usually misdiagnosis. Since the accurate diagnosis of this entity is difficult, the laparoscopic approach would provide a reasonable diagnostic and therapeutic advantage with minimal invasiveness for patients. Furthermore, we advised that in hepatic MALT lymphoma patients with a localized tumor lesion, hepatectomy followed by chemotherapy or radiotherapy should be considered to achieve better outcomes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethics Committee of Chengdu Second People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TH: Data curation, Formal analysis, Investigation, Writing – original draft. JZ: Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lei KI. Primary non-Hodgkin's lymphoma of the liver. Leuk Lymphoma. (1998) 29:293–9. doi: 10.3109/10428199809068566
2. Padhan RK, Das P, Shalimar. Primary hepatic lymphoma. Trop Gastroenterol. (2015) 36:14–20. doi: 10.7869/tg.239
3. Qiu MJ, Fang XF, Huang ZZ, Li QT, Wang MM, Jiang X, et al. Prognosis of primary hepatic lymphoma: A US population-based analysis. Transl Oncol. (2021) 14:100931. doi: 10.1016/j.tranon.2020.100931
4. Salar A. Gastric MALT lymphoma and helicobacter pylori. linfoma MALT gástrico y helicobacter pylori. Med Clin (Barc). (2019) 152:65–71. doi: 10.1016/j.medcli.2018.09.006
5. Xu Z, Pang C, Sui J, Gao Z. A case of primary hepatic extranodal marginal zone B-cell mucosa-associated lymphoid tissue (MALT) lymphoma treated by radiofrequency ablation (RFA), and a literature review. J Int Med Res. (2021) 49:300060521999539. doi: 10.1177/0300060521999539
6. Liu X, Cao X, Pang Y, Min F. Primary hepatic mucosa-associated lymphoid tissue lymphoma with HP and previous HBV infection: A case report and literature review. J Infect Chemother. (2022) 28:1182–8. doi: 10.1016/j.jiac.2022.04.014
7. Yago K, Shimada H, Itoh M, Ooba N, Itoh K, Suzuki M, et al. Primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT)-type of the liver in a patient with hepatitis C virus infection. Leuk Lymphoma. (2002) 43:1497–500. doi: 10.1080/1042819022386734
8. Panjala C, Talwalkar JA, Lindor KD. Risk of lymphoma in primary biliary cirrhosis. Clin Gastroenterol Hepatol. (2007) 5:761–4. doi: 10.1016/j.cgh.2007.02.020
9. Yang XW, Tan WF, Yu WL, Shi S, Wang Y, Zhang YL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. (2010) 16:6016–9. doi: 10.3748/wjg.v16.i47.6016
10. Haefliger S, Milowich D, Sciarra A, Trimeche M, Bouilly J, Kaiser J, et al. Primary hepatic marginal B cell lymphoma of mucosa-associated lymphoid tissue (MALT) and non-alcoholic steatohepatitis (NASH): more than a coincidence? Ann Hematol. (2019) 98:1513–6. doi: 10.1007/s00277-018-3565-5
11. Noronha V, Shafi NQ, Obando JA, Kummar S. Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol Hematol. (2005) 53:199–207. doi: 10.1016/j.critrevonc.2004.10.010
12. Shetty S, Bruns T, Weston CJ, Stamataki Z, Oo YH, Long HM, et al. Recruitment mechanisms of primary and Malignant B cells to the human liver. Hepatology. (2012) 56:1521–31. doi: 10.1002/hep.25790
13. Park JE, Lee KM, Choi HY, Ahn SE, You MW. Methotrexate-associated primary hepatic lymphoma and cranial neuropathy in a patient with rheumatoid arthritis: A case report with clinical follow-up over a 7-year period. Med (Baltimore). (2019) 98:e14997. doi: 10.1097/MD.0000000000014997
14. Jacobs SL, Rozenblit A. HIV-associated hypervascular primary Burkitt's lymphoma of the liver. Clin Radiol. (2006) 61:453–5. doi: 10.1016/j.crad.2005.12.007
15. Koubaa Mahjoub W, Chaumette-Planckaert MT, Murga Penas EM, Dierlamm J, Leroy K, Delfau MH, et al. Primary hepatic lymphoma of mucosa-associated lymphoid tissue type: a case report with cytogenetic study. Int J Surg Pathol. (2008) 16:301–7. doi: 10.1177/1066896907312671
16. Chan RC, Chu CM, Chow C, Chan SL, Chan AW. A concurrent primary hepatic MALT lymphoma and hepatocellular carcinoma. Pathology. (2015) 47:178–81. doi: 10.1097/PAT.0000000000000220
17. Wu GB, Huang CY, Huang S, Ru HM, Xiang BD, Yuan WP, et al. Primary hepatic non-Hodgkin's lymphoma with rectal cancer: A case report. Oncol Lett. (2015) 9:324–6. doi: 10.3892/ol.2014.2673
18. Zhong Y, Wang X, Deng M, Fang H, Xu R. Primary hepatic mucosa-associated lymphoid tissue lymphoma and hemangioma with chronic hepatitis B virus infection as an underlying condition. Biosci Trends. (2014) 8:185–8. doi: 10.5582/bst.2014.01057
19. Streubel B, Lamprecht A, Dierlamm J, Cerroni L, Stolte M, Ott G, et al. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood. (2003) 101:2335–9. doi: 10.1182/blood-2002-09-2963
20. Park YK, Choi JE, Jung WY, Song SK, Lee JI, Chung CW., et al. Mucosa-associated lymphoid tissue (MALT) lymphoma as an unusual cause of Malignant hilar biliary stricture: a case report with literature review. World J Surg Oncol. (2016) 14:167. doi: 10.1186/s12957-016-0928-z
21. Wang RL, Wang J, Li YS, Wang Y, Su Q. Primary hepatic lymphoma of MALT type mimicking hepatic adenoma treated by hepatectomy: a case report and literature review. Front Surg. (2023) 10:1169455. doi: 10.3389/fsurg.2023.1169455
22. Takeshima F, Kunisaki M, Aritomi T, Osabe M, Akama F, Nakasone T. Hepatic mucosa-associated lymphoid tissue lymphoma and hepatocellular carcinoma in a patient with hepatitis B virus infection. J Clin Gastroenterol. (2004) 38:823–6. doi: 10.1097/01.mcg.0000139058.43414.a1
23. Zhou X, Wuchter P, Egerer G, Kriegsmann M, Kommoss FKF, Witzens-Harig M, et al. Serological hepatitis B virus (HBV) activity in patients with HBV infection and B-cell non-Hodgkin's lymphoma. Eur J Haematol. (2020) 104:469–75. doi: 10.1111/ejh.13388
24. Foschi FG, Dall'Aglio AC, Marano G, Lanzi A, Savini P, Piscaglia F, et al. Role of contrast-enhanced ultrasonography in primary hepatic lymphoma. J Ultrasound Med. (2010) 29:1353–6. doi: 10.7863/jum.2010.29.9.1353
25. Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kikuchi Y, Kaneko H, et al. A case of contiguous primary hepatic marginal zone B-cell lymphoma and hemangioma ultimately diagnosed using contrast-enhanced ultrasonography. Case Rep Oncol. (2015) 8:50–6. doi: 10.1159/000375118
26. Lu Q, Zhang H, Wang WP, Jin YJ, Ji ZB. Primary non-Hodgkin's lymphoma of the liver: sonographic and CT findings. Hepatobiliary Pancreat Dis Int. (2015) 14:75–81. doi: 10.1016/s1499-3872(14)60285-x
27. Colagrande S, Calistri L, Grazzini G, Nardi C, Busoni S, Morana G, et al. MRI features of primary hepatic lymphoma. Abdom Radiol (NY). (2018) 43:2277–87. doi: 10.1007/s00261-018-1476-5
28. Okura K, Seo S, Shimizu H, Nishino H, Yoh T, Fukumitsu K, et al. Primary hepatic extranodal marginal zone B-cell mucosa-associated lymphoid tissue lymphoma treated by laparoscopic partial hepatectomy: a case report. Surg Case Rep. (2023) 9:29. doi: 10.1186/s40792-023-01613-y
29. Wang L, Dong P, Hu W, Tian B. 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in the diagnosis and follow-up of primary hepatic diffuse large B-cell Lymphoma: A clinical case report. Med (Baltimore). (2020) 99:e18980. doi: 10.1097/MD.0000000000018980
30. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for thediagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. (2020) 9:682–720. doi: 10.1159/000509424
31. Fu Z, Wu L, Chen J, Zheng Q, Li P, Zhang L, et al. Primary hepatic mucosa-associated lymphoid tissue lymphoma: case report and literature review. Int J Clin Exp Pathol. (2021) 14:375–82.
32. Szor DJ, Ursoline M, Herman P. Hepatic adenoma. Arq Bras Cir Dig. (2013) 26:219–22. doi: 10.1590/s0102-67202013000300012
33. Wong VK, Fung AW, Elsayes KM. Magnetic resonance imaging of hepatic adenoma subtypes. Clin Liver Dis (Hoboken). (2021) 17:113–8. doi: 10.1002/cld.996
34. Zen Y, Fujii T, Nakanuma Y. Hepatic pseudolymphoma: a clinicopathological study of five cases and review of the literature. Mod Pathol. (2010) 23:244–50. doi: 10.1038/modpathol.2009.165
35. Ota H, Isoda N, Sunada F, Kita H, Higashisawa T, Ono K, et al. A case of hepatic pseudolymphoma observed without surgical intervention. Hepatol Res. (2006) 35:296–301. doi: 10.1016/j.hepres.2006.04.012
36. Grewe S, Shahid M, Zhang L, Jiang K. Clinically unsuspected primary hepatic mucosa-associated lymphoid tissue lymphoma collision with an intrahepatic cholangiocarcinoma: A case report and literature review. SAGE Open Med Case Rep. (2021) 9:2050313X211041838. doi: 10.1177/2050313X211041838
37. Liu J, Guo RR, Fang JC, Zhong L. Primary hepatic mucosa-associated lymphoid tissue lymphoma with hepatocellular carcinoma: A case report and literature review. J Dig Dis. (2020) 21:526–8. doi: 10.1111/1751-2980.12917
38. Ugurluer G, Miller RC, Li YX, Thariat J, Ghadjar P, Schick U, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. (2016) 8:6502. doi: 10.4081/rt.2016.6502
39. Li LX, Zhou ST, Ji X, Ren H, Sun YL, Zhang JB, et al. Misdiagnosis of primary hepatic marginal zone B cell lymphoma of mucosa-associated lymphoid tissue type, a case report. World J Surg Oncol. (2016) 14:69. doi: 10.1186/s12957-016-0817-5
40. Hamada M, Tanaka Y, Kobayashi Y, Takeshita E, Joko K. A case of MALT lymphoma of the liver treated by RFA and Rituximab. Nihon Shokakibyo Gakkai Zasshi. (2006) 103:655–60.
41. Doi H, Horiike N, Hiraoka A, Koizumi Y, Yamamoto Y, Hasebe A, et al. Primary hepatic marginal zone B cell lymphoma of mucosa-associated lymphoid tissue type: case report and review of the literature. Int J Hematol. (2008) 88:418–23. doi: 10.1007/s12185-008-0153-9
42. Page RD, Romaguera JE, Osborne B, Medeiros LJ, Rodriguez J, North L, et al. Primary hepatic lymphoma: favorable outcome after combination chemotherapy. Cancer. (2001) 92:2023–9. doi: 10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
43. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. (2002) 346:235–42. doi: 10.1056/NEJMoa011795
44. Betianu CI, Dima A, Pavaloiu G. Primary hepatic mucosa-associated lymphoid tissue lymphoma in a patient with no chronic liver disease: Case report. Radiol Case Rep. (2017) 12:715–9. doi: 10.1016/j.radcr.2017.08.004
45. Gockel HR, Heidemann J, Lugering A, Mesters R, Parwaresch RM, Domschke W, et al. Stable remission after administration of rituximab in a patient with primary hepatic marginal zone B-cell lymphoma. Eur J Haematol. (2005) 74:445–7. doi: 10.1111/j.1600-0609.2005.00419.x
46. Obiorah IE, Johnson L, Ozdemirli M. Primary mucosa-associated lymphoid tissue lymphoma of the liver: A report of two cases and review of the literature. World J Hepatol. (2017) 9:155–60. doi: 10.4254/wjh.v9.i3.155
47. Shin SY, Kim JS, Lim JK, Hahn JS, Yang WI, Suh CO. Longlasting remission of primary hepatic mucosa-associated lymphoid tissue (MALT) lymphoma achieved by radiotherapy alone. Korean J Intern Med. (2006) 21:127–31. doi: 10.3904/kjim.2006.21.2.127
48. Chowla A, Malhi-Chowla N, Chidambaram A, Surick B. Primary hepatic lymphoma in hepatitis C: case report and review of the literature. Am Surg. (1999) 65:881–3. doi: 10.1177/000313489906500916
49. Ozaki K, Ikeno H, Koneri K, Higuchi S, Hosono N, Kosaka N, et al. Primary hepatic diffuse large B-cell lymphoma presenting unusual imaging features. Clin J Gastroenterol. (2020) 13:1265–72. doi: 10.1007/s12328-020-01203-7
50. Avlonitis VS, Linos D. Primary hepatic lymphoma: a review. Eur J Surg. (1999) 165:725–9. doi: 10.1080/11024159950189474
51. Nart D, Ertan Y, Yilmaz F, Yüce G, Zeytunlu M, Kilic M, et al. Primary hepatic marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type in a liver transplant patient with hepatitis B cirrhosis. Transplant Proc. (2005) 37:4408–12. doi: 10.1016/j.transproceed.2005.10.109
52. Murakami J, Fukushima N, Ueno H, Saito T, Watanabe T, Tanosaki R, et al. Primary hepatic low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue type: a case report and review of the literature. Int J Hematol. (2002) 75:85–90. doi: 10.1007/BF02981985
53. Mizuno S, Isaji S, Tabata M, Uemoto S, Imai H, Shiraki K. Hepatic mucosa-associated lymphoid tissue (MALT) lymphoma associated with hepatitis C. J Hepatol. (2002) 37:872–3. doi: 10.1016/s0168-8278(02)00316-1
54. Zhang KJ, Chen S, Chen JL, Dong LH. Complete response to comprehensive treatment of a primary hepatic diffuse large B cell lymphoma: A case report. Oncol Lett. (2015) 9:1557–60. doi: 10.3892/ol.2015.2920
55. Xie H, Lv J, Ji Y, Du X, Yang X. Primary hepatic mucosa-associated lymphoid tissue lymphoma: A case report and literature review. Med (Baltimore). (2019) 98:e15034. doi: 10.1097/MD.0000000000015034
56. Dong S, Chen L, Chen Y, Chen X. Primary hepatic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type: A case report and literature review. Med (Baltimore). (2017) 96:e6305. doi: 10.1097/MD.0000000000006305
57. Yasuda T, Nakagawa S, Imai K, Okabe H, Hayashi H, Yamashita YI, et al. A case of primary hepatic mucosa-associated lymphoid tissue lymphoma incidentally found in the sustained virological response state of chronic hepatitis C: review of the literature of this rare disease. Int Cancer Conf J. (2020) 9:59–65. doi: 10.1007/s13691-019-00397-z
58. Choi S, Kim JH, Kim K, Kim M, Choi HJ, Kim YM, et al. Primary hepatic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue. J Pathol Transl Med. (2020) 54:340–5. doi: 10.4132/jptm.2020.03.18
59. Nagata S, Harimoto N, Kajiyama K. Primary hepatic mucosa-associated lymphoid tissue lymphoma: a case report and literature review. Surg Case Rep. (2015) 1:87. doi: 10.1186/s40792-015-0091-8
Keywords: PHL, MALT lymphoma, hepatectomy, chemotherapy, prognosis
Citation: He T and Zou J (2024) Primary hepatic mucosa-associated lymphoid tissue lymphoma: a case report and literature review. Front. Oncol. 14:1430714. doi: 10.3389/fonc.2024.1430714
Received: 10 May 2024; Accepted: 16 September 2024;
Published: 01 October 2024.
Edited by:
Vincent Donckier, Université libre de Bruxelles, BelgiumReviewed by:
Corrado Tagliati, Azienda Usl Teramo, ItalyChiara De Molo, Sant’Orsola-Malpighi Polyclinic, Italy
Copyright © 2024 He and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieyu Zou, em91ank5NjE4QG91dGxvb2suY29t
 Tao He
Tao He Jieyu Zou
Jieyu Zou