
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Oncol., 31 July 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1428498
This article is part of the Research TopicMultidisciplinary and Personalized Approach in the Treatment of Advanced Breast CancerView all 20 articles
Introduction: 68Ga labeled DOTA-Ibandronate (68Ga-DOTA-IBA) positron emission tomography/computed tomography (PET/CT), is a novel bone-targeting imaging tracer and promising diagnostic method for bone metastases detection. Therefore, this study aimed to compare 68Ga-DOTA-IBA PET/CT to the 99mTc-MDP whole-body bone scan (WBBS) for detecting bone metastases in breast cancer (BC).
Materials and methods: In this prospective study, 45 women with BC underwent imaging via 68Ga-DOTA-IBA PET/CT and 99mTc-MDP WBBS. Clinical and demographic information as well as BC imaging features were recorded. The two methods were compared in terms of their detection rate for bone metastases and the number of lesions.
Results: The 45 women were aged 53.5 ± 11.0 years. The bone metastases detection rate with 68Ga-DOTA-IBA PET/CT was 100% (45/45) and with 99mTc-MDP WBBS was 95.6% (43/45). A total of 546 bone metastases lesions were detected. The lesion detection rate using 68Ga-DOTA-IBA PET/CT was 100% (546/546) and using 99mTc-MDP WBBS was 67.8% (370/546). More lesions were found at each site via 68Ga-DOTA-IBA than via 99mTc-MDP WBBS.
Conclusions: 68Ga-DOTA-IBA PET/CT is a more sensitive method than 99mTc-MDP WBBS for assessing bone metastases in BC and may therefore represent a useful imaging technique for bone metastases, while offering a visual basis for 177Lu-DOTA-IBA diagnosis and therapy response assessments for BC. Further validation using a broader study cohort is warranted to confirm these findings.
Clinical trial registration: https://www.chictr.org.cn/showproj.html?proj=170163, identifier ChiCTR2200064487.
Breast cancer (BC) is the primary cause of cancer-related illness, impairment, and mortality in women worldwide (1). Bones are the most common sites of distant metastases in BC and impact prognosis, quality of life, and therapy, which may affect approximately 65–90% of patients with advanced illnesses (2). Patients with less metastatic diseases have better prognoses, and those who have primarily bone-related metastases have higher survival rates than those with visceral metastases—suggesting that prognosis may be influenced by early detection. Therefore, early diagnosis and therapy response monitoring are essential in these patients (3).
Conventional imaging techniques such as radiography, computed tomography (CT), and magnetic resonance imaging (MRI) are considered to be inadequate for detecting and accurately assessing the effectiveness of BC treatment. While the 99mTc-MDP whole-body bone scan (WBBS) is commonly recommended, its sensitivity and specificity are not ideal (4, 5). Therefore, it is advisable to perform 18F-fludeoxyglucose (FDG) positron emission tomography (PET)/CT after conducting CT and WBBS of the chest, abdomen, and pelvis for stage IIA–IIIC BC (6). Compared with other methods, single-photon emission computed tomography (SPECT)/CT and PET/CT exhibit greater accuracy for bone staging diagnosis and provide the possibility for early individualized treatment. However, SPECT/CT may lead to false positive diagnoses or missed diagnoses. Moreover, the 18F-FDG PET/CT method has a limited ability to detect skull metastases and lacks specificity in identifying bone lesions (7). Nevertheless, treatments for BC bone metastases have become possible with the development of integrated probes for molecular-targeted diagnosis and treatment in nuclear medicine. Thus, accurate detection and monitoring of the response to bone metastases has gained importance (8).
The 68Ga or 177Lu labeled DOTA-Ibandronate (68Ga/177Lu-DOTA-IBA) approach, an integrated probe for both diagnosis and treatment exhibits strong targeting of hydroxyapatite, low uptake in background organs, and long retention time in bone metastases (9, 10). Preliminary clinical studies have shown that 68Ga-DOTA-IBA PET/CT detects more bone metastases in various solid tumors compared with WBBS or Sodium fluoride PET/CT (18F-NaF). However, the previous studies only evaluated several cases of BC (11, 12). 177Lu-DOTA-IBA treated bone metastases without significant liver and kidney function damage and bone marrow suppression; patient symptoms significantly improved and the short-term curative effect was definite (11). Thus, the accurate detection of more bone metastases by 68Ga-DOTA-IBA may also contribute to the early treatment of 177Lu-DOTA-IBA. Therefore, this study aimed to compare 68Ga-DOTA-IBA PET/CT with WBBS for detecting BC bone metastases and provide evidence for the use of 177Lu-DOTA-IBA as a BC treatment.
The Affiliated Hospital of Southwest Medical University served as the site of this prospective, single-center study. Participants were consecutively enrolled between January 2022 and October 2023 (clinical trial registration no. ChiCTR2200064487; Ethics Committee Approval No. KY2022114). All participants provided written informed consent before undergoing 68Ga-DOTA-IBA PET/CT imaging. Following registration, the individuals underwent a PET/CT scan using 68Ga-DOTA-IBA and a WBBS within one week. All participants were followed-up for a minimum period of 3 months.
Eligible participants were required to meet the following criteria: (a) recently diagnosed, recurring, or spreading BC; (b) BC confirmed through histological examination; and (c) willingness to undergo 68Ga-DOTA-IBA PET/CT and WBBS scans. Patients who had additional primary malignancies during the examination, severe hepatic, or renal insufficiency, or declined to undergo 68Ga-DOTA-IBA PET/CT were excluded. Biopsies and histopathological examinations were used to diagnose both initial and recurring cases of BC. Diagnoses of bone metastases were established using multiple imaging modalities, including brain MRI, chest and abdominal CT, WBBS, and PET/CT. Owing to the advanced stage of the participants’ illnesses, only a limited number of biopsies were performed to investigate potential metastatic lesions.
Imaging was performed according to a previously described protocol (11). No specific preparations were required before the examinations and 1.85 MBq (0.05 mCi) per kilogram body weight of 68Ga-DOTA-IBA was administered through intravenous injection. A PET/CT scan was performed 40–60 min after the tracer was injected, covering the entire body from head to toe, with 3 min per position. The resulting images underwent attenuation correction and iterative reconstruction to obtain transverse, coronal, and sagittal PET/CT scan views. The WBBS was performed 3–4 h following the intravenous administration of 740–925 MBq (20–25 mCi) of 99mTc-MDP.
Two trained and board-certified nuclear medicine doctors independently analyzed the obtained images to compare the detection of bone metastases between the two methods. Any disagreements were resolved through discussion. Individual skeletal metastases were classified into nine regions: the cervical spine, thoracic spine, lumbosacral spine, pelvis, long bone and clavicle, craniofacial bone, scapula, rib, and sternum. We recorded the number of osteoarticular lesions with abnormal tracer uptakes on PET/CT or WBBS, along with their sites, any abnormal CT findings, and the maximum standardized uptake value (SUVmax). Metastases to the same vertebral body or appendage were identified as a single lesion. The imaging features of PET/CT and bone scans were analyzed, and the detection rates of the bone metastases and the number of lesions using the two methods were calculated.
SPSS Statistics version 26.0 (IBM) was used for the data analysis. Descriptive statistics are shown as either means ± standard deviations, medians (ranges), or numbers (%). The paired Chi-square test (McNemar test) was used to compare the detection rates of 68Ga-DOTA-IBA PET/CT and WBBS. P < 0.05 was considered statistically significant.
Between January 2022 and October 2023, 45 patients diagnosed with BC, with an average age of 53.5 ± 11.0 years, were included in this study. Histopathological analyses revealed six cases of triple-negative BC. Surgery, chemotherapy, radiotherapy, targeted therapy, and endocrine therapy were performed at least 3 months before each examination (Table 1).
Bone metastases were detected in all 45 patients. WBBS did not detect bone metastasis in two patients, resulting in a detection rate of 95.6% (43/45). Whereas 68Ga-DOTA-IBA PET/CT detected bone metastases in all patients, exhibiting a detection rate of 100% (45/45) (P > 0.05). The number of patients detected as having scapular lesions was similar across the two methods (16/16), whereas 68Ga-DOTA-IBA PET/CT detected more patients with other lesions than WBBS. A significantly higher number of patients were detected with lesions in the cervical (24/16) and thoracic vertebrae (25/20) using 68Ga-DOTA-IBA PET/CT than using WBBS (Table 2). The diagnosis of bone metastasis was confirmed by pathological examination in only five patients (five lesions) who visited the orthopedic department due to bone-related events.
A total of 546 bone metastases were detected in 45 patients, 370 of which were detected using WBBS. The lesion detection rate was 67.8% (370/546) via WBBS and 100% (546/546) via 68Ga-DOTA-IBA (P < 0.001).
The lesion detection rate of 68Ga-DOTA-IBA in the central bone was significantly higher than that of WBBS. For all lesions, the median SUVmax of 68Ga-DOTA-IBA was 6.57 (range: 4.76–10.30; Table 2, Figure 1). Notably, 68Ga-DOTA-IBA PET/CT showed increased uptake of DOTA-IBA at the primary BC site in two patients (Figure 2). The 68Ga-DOTA-IBA PET/CT imaging results of one out of the two patients were previously reported (13).
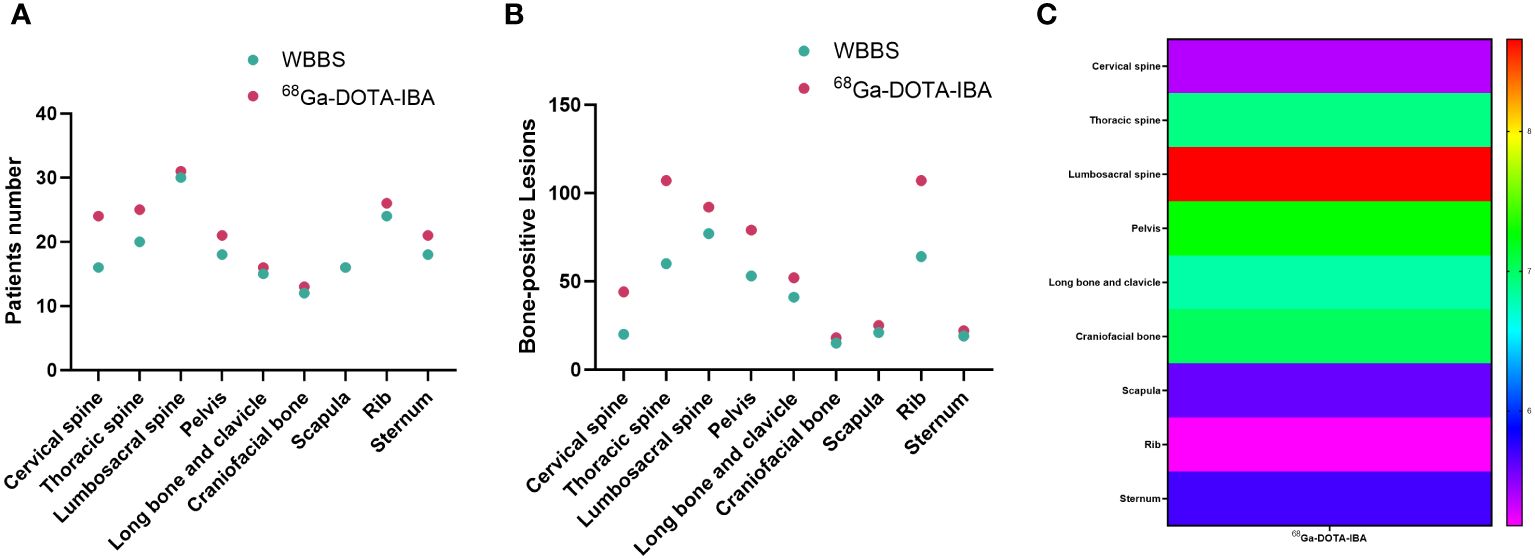
Figure 1 Comparison of 68Ga-DOTA-ibandronate (IBA) PET/CT and 99mTc-MDP bone scan (WBBS) in detecting bone metastases in breast cancer. (A) Number of patients testing positive; (B) Number of positive lesions; (C) Heat maps showing the SUVmax of 68Ga-DOTA-IBA in bone metastases at various anatomical sites.
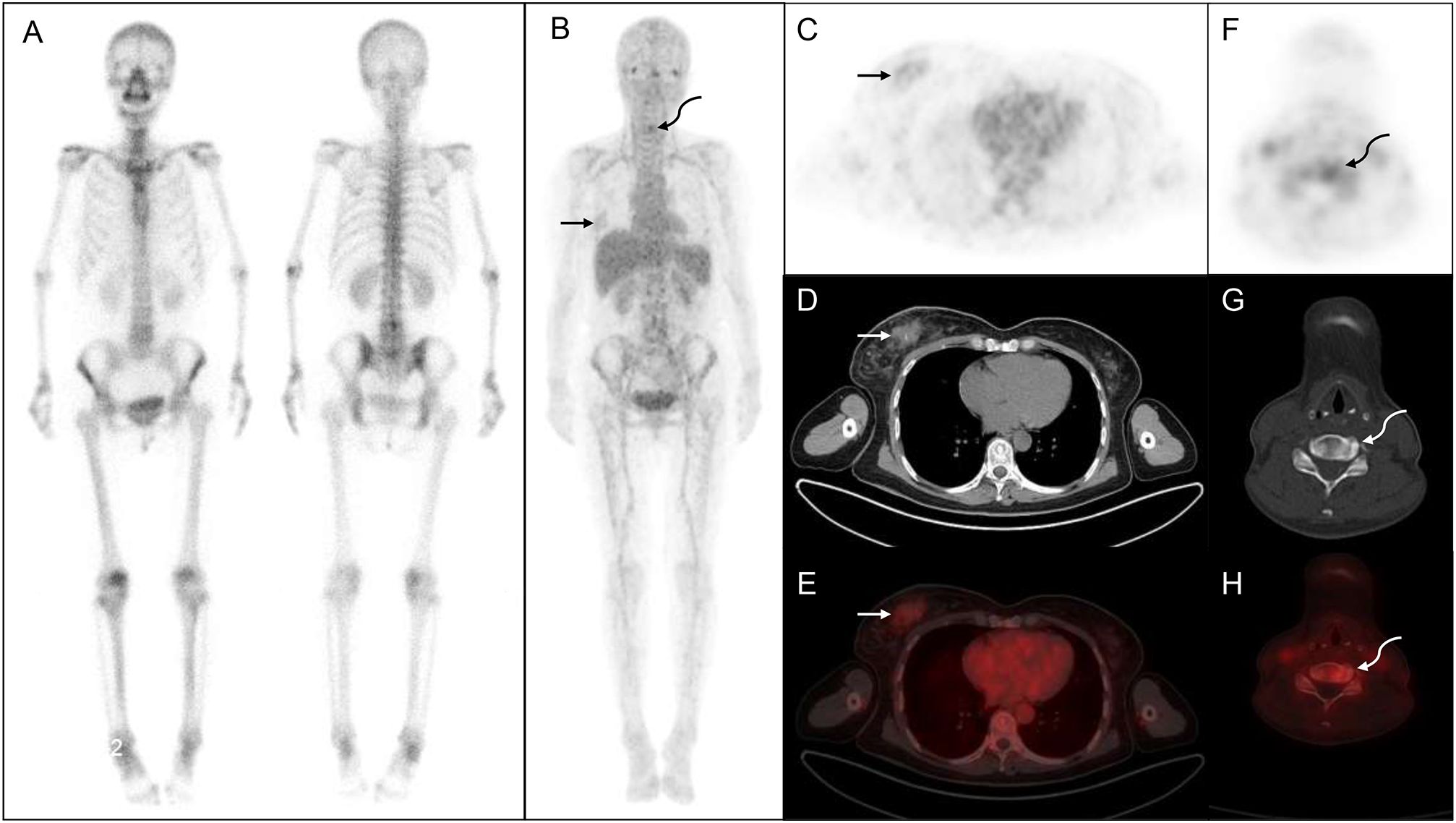
Figure 2 68Ga-DOTA- ibandronate (IBA) PET/CT performed on a 54-year-old woman with breast cancer. A 99mTc-MDP bone scan (A) shows no significant abnormalities, whereas a 68Ga-DOTA-IBA PET/CT (B) shows increased tracer uptake in the right mammary gland (straight arrow) and cervical spine (curved arrow). (C–E) shows right breast nodules with abnormal tracer uptake (SUVmax, 1.84; straight arrow). (F–H) shows a cervical bone change with increased metabolism (SUVmax, 3.46, curved arrow) that is subsequently confirmed as invasive cancer via biopsy.
This study prospectively evaluated the diagnostic accuracy of 68Ga-DOTA-IBA PET/CT and WBBS for detecting bone metastases in BC. The bone metastases were divided into nine groups for individual assessment. The overall detection rates between 68Ga-DOTA-IBA PET/CT and WBBS were not significantly different, at 100% and 95.6%, respectively, P > 0.05. However, in terms of the lesion detection rate, 68Ga-DOTA-IBA PET/CT was superior to WBBS (100% vs. 67.8%, respectively, P < 0.05). Overall, 68Ga-DOTA-IBA PET/CT detected more bone metastases than WBBS.
The 68Ga-DOTA-IBA PET/CT approach revealed multiple significant bone metastases in the vertebral body in cases where WBBS only showed suspicious metastases in the area; this could potentially affect the clinical treatment plans (Figure 3). Moreover, when bone metastases were detected by WBBS, 68Ga-DOTA-IBA accurately displayed multiple bone metastases with high SUVmax uptake levels, which is beneficial for radionuclide-targeted therapy and post-treatment evaluation (Figure 4). In recent years, 18F-FDG PET/CT has become a valuable technique for staging BC (14–16). However, there is ongoing debate regarding the accuracy and sensitivity of PET/CT for identifying bone metastases when compared with WBBS (17). Some scholars believe that its accuracy and sensitivity for diagnosing skull metastases are higher than those of PET/CT (18). In this study, 68Ga-DOTA-IBA PET/CT was superior to bone scans for the diagnosis of skull metastases due to the type of molecular probe used. The high uptake of FDG in brain tissues may mask skull metastases and interfere with diagnoses (19, 20), whereas 68Ga-DOTA-IBA avoids this shortcoming (Figure 4). Additionally, there is a higher uptake of 68Ga-DOTA-IBA PET/CT in non-calcified BC tissues, which may be caused by localized increase in blood pool, calcium metabolism, or interstitial volume in the breast tissue (21).
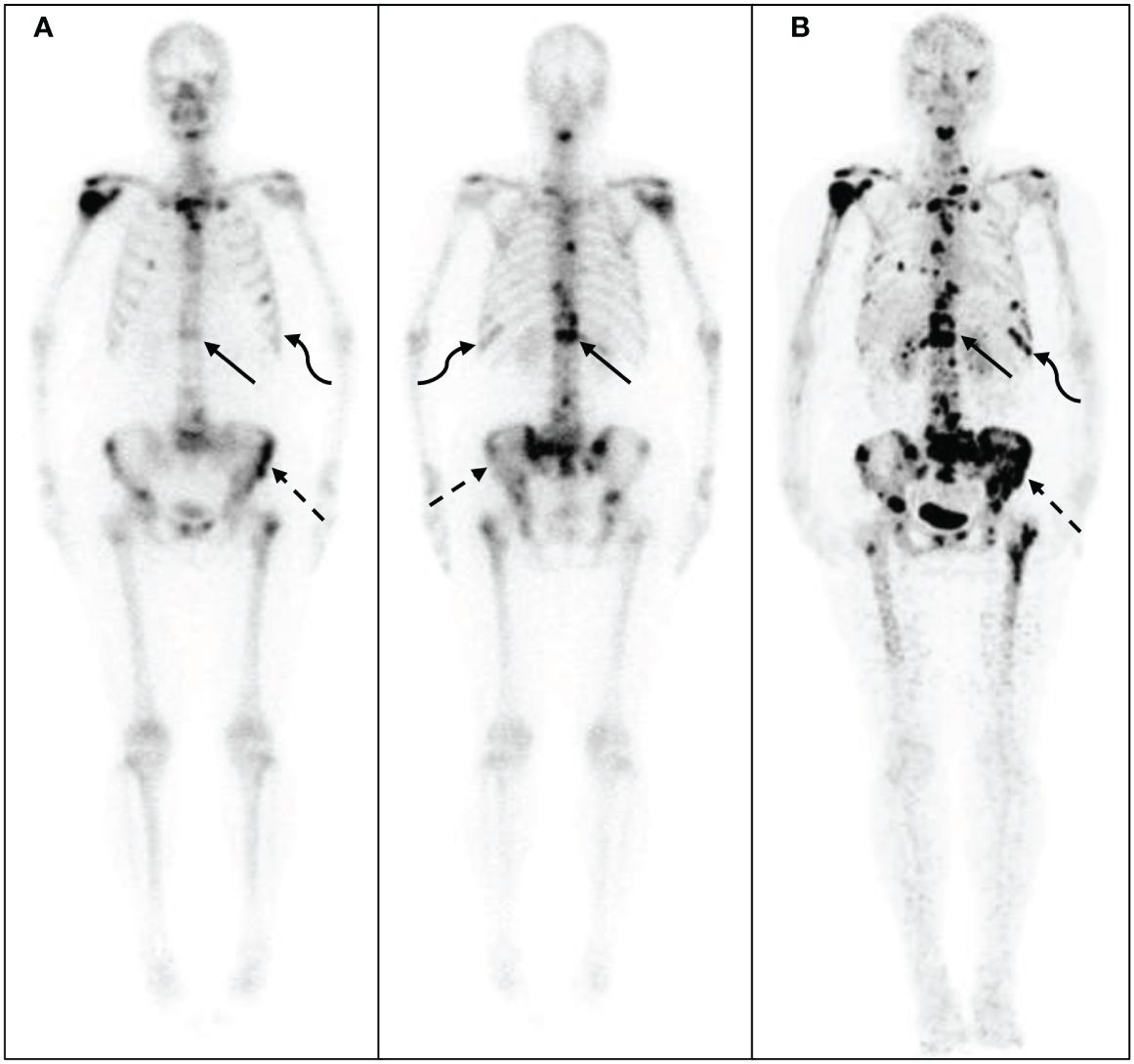
Figure 3 68Ga-DOTA- ibandronate (IBA) PET/CT performed on a 58-year-old woman with breast cancer 10 years following surgical treatment. A 99mTc-MDP bone scan (A) shows no significant increase in bone metabolism, whereas a 68Ga-DOTA-IBA PET/CT shows (B) multiple bone metastases throughout the body and significantly increased bone metabolism in the ribs (straight arrow), spine (dotted arrow), and sternum (curved arrow).
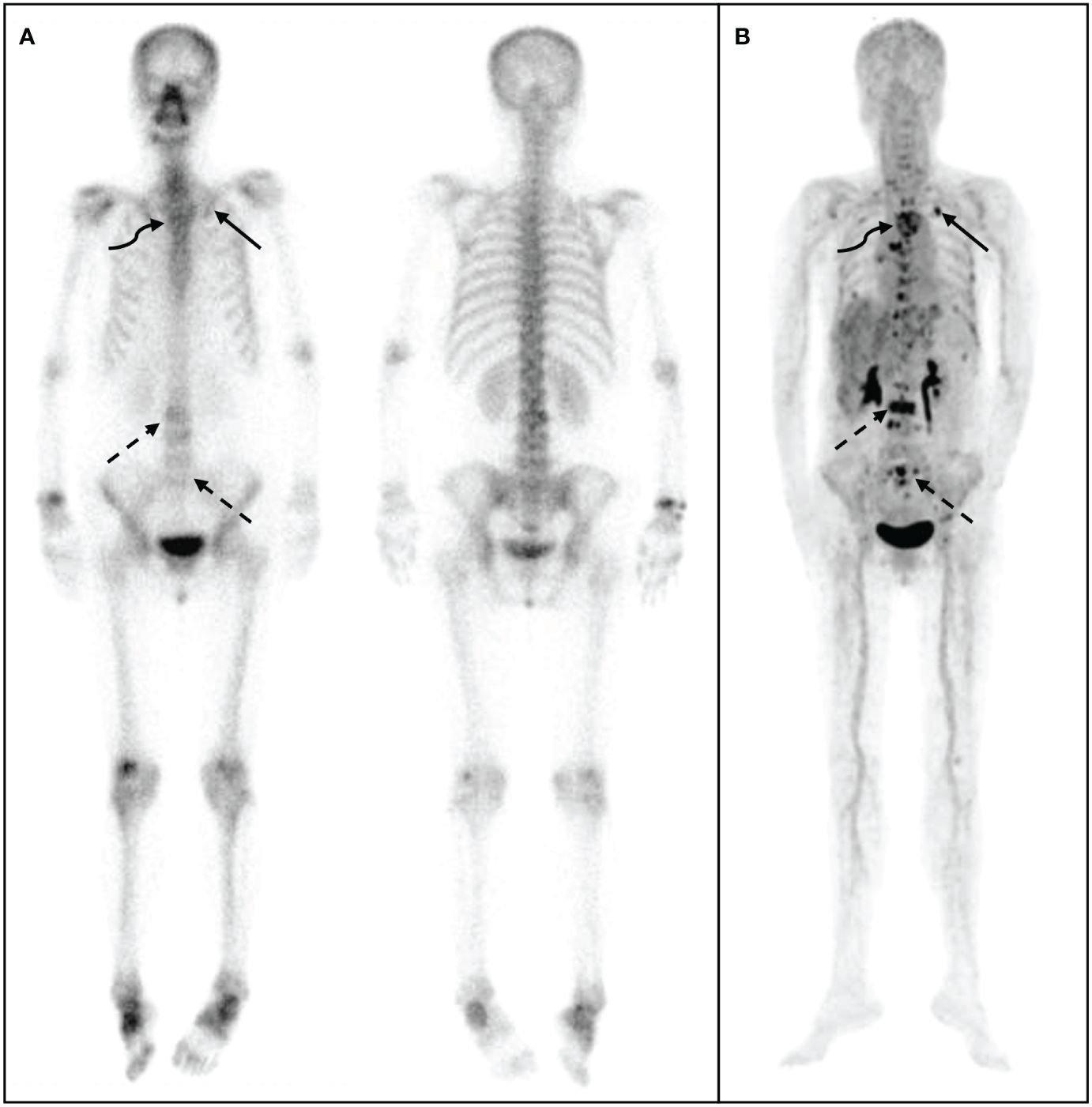
Figure 4 68Ga-DOTA- ibandronate (IBA) PET/CT performed on a 48-year-old woman with invasive cancer 4 years following surgical treatment for BC. A 99mTc-MDP bone scan (A) shows multiple bone metastases in sites such as the vertebral body, ribs, ilium, and humerus. A 68Ga-DOTA-IBA PET/CT (B) reveals more vertebral (straight arrow) and rib (curved arrow) metastases, particularly in the pelvis (dotted arrow).
Thus, compared with WBBS, 68Ga-DOTA-IBA PET/CT can be used to obtain an earlier diagnosis of bone metastases in BC. The 68Ga-DOTA-IBA targets hydroxyapatite, which has a higher uptake at bone metastasis sites and is more sensitive to osteolysis, making it beneficial for the early diagnosis of bone metastases (9). In contrast, PET/CT has the advantages of providing full imaging and anatomical localization that can be used to accurately locate the sites of bone metastases (22, 23). However, WBBS, which lacks anatomical localization, may lead to errors in diagnosis (24). 18F-FDG PET/CT is routinely used for systemic staging, monitoring treatment response and recurrence of advanced BC (stage IIB-IIIC). A previous study compared the 18F-FDG alternative with WBBS for evaluating bone metastases in patients with recently detected metastatic BC; WBBS provided insufficient information and warranted an additional evaluation via 18F-FDG PET/CT in > 25% of the patients (25). However, 18F-FDG does not target the bone, and the sensitivity and accuracy of its diagnoses vary because of the tumor heterogeneity (26). Additionally, 18F-NaF PET/CT is more sensitive to bone metastases than 99mTc-MDP or CT in patients with metastatic BC (27). A prospective comparison showed that 18F-NaF PET/CT was more accurate than SPECT for the diagnosis of BC bone metastases (28). Consistent with previous findings, PET/CT showed advantages over WBBS for the diagnosis of BC bone metastases in this study. However, different molecular probes have different diagnostic accuracies and sensitivities for PET/CT, and most can only diagnose bone metastases, lacking diagnosis and treatment integration (29, 30). Therefore, the diagnostic efficacy of 68Ga-DOTA-IBA PET/CT compared with 18F-NaF PET/CT or 18F-FDG PET/CT, which are more sensitive to detect BC bone metastases, requires further investigation.
The previously developed 68Ga/177Lu-DOTA-IBA approach is an integrated probe for both diagnosis and treatment. The SUVmax of 68Ga-DOTA-IBA for detecting bone metastases was found to be higher than that of WBBS in our patient cohort; the whole-body SUVmax of bone metastases was 6.57, and the highest value was found in the lumbosacral vertebrae (8.66; Table 2). Thus, 68Ga-DOTA-IBA can be used to diagnose and evaluate bone metastases in patients with BC who have bone metastases. Additionally, 177Lu-DOTA-IBA has a high therapeutic effect that can rapidly relieve bone pain due to cancerous lesions (11, 31). We found that 68Ga-DOTA-IBA tracer uptake was also present in the primary lesion of two patients, suggesting that 177Lu-DOTA-IBA may have a therapeutic effect on the primary lesion in addition to targeting the bone metastases. While some radiopharmaceuticals like 89Sr, 223Ra, 188Re/186Re-HEDP, 153Sm-EDMTP, 177Lu-EDTMP, etc, are currently used for treating bone metastases and have demonstrated high analgesic potential, they are not suitable for therapeutic use due to the absence of corresponding diagnostic analogs (11). Nevertheless, this integrated diagnosis and treatment probe (68Ga/177Lu-DOTA-IBA) has potentially broad applications for the diagnosis and treatment of bone metastases resulting from solid tumors.
Despite the promising results, this study had some limitations worth noting. First, the number of enrolled patients was small, and their cancer stages were not discussed. Due to the limited number of different stages of the patients included in this study, whether 68Ga-DOTA-IBA can impact patient management requires further evaluation with a larger sample cohort of patients at different stages. Second, most of the patients did not have pathological examination results, which were mainly evaluated using imaging and comprehensive treatment responses. Third, 68Ga-DOTA-IBA PET/CT was not compared to 18F-NaF PET/CT or 18F-FDG PET/CT. Finally, the follow-up period was relatively short. Therefore, future multi-center and longer-term studies addressing these limitations are warranted to validate our results.
68Ga-DOTA-IBA PET/CT, compared to the WBBS, represents a more precise method for the detection of bone metastases in BC. It offers a potential imaging method and establishes a basis for diagnoses and treatment response evaluations using 177Lu-DOTA-IBA. However, further validation using a broader study cohort is warranted.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Affiliated Hospital of Southwest Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
FX: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. YZ: Methodology, Writing – review & editing, Writing – original draft, Investigation. XT: Writing – review & editing, Formal analysis, Data curation. YY: Writing – review & editing, Software, Formal analysis. HL: Validation, Data curation, Writing – review & editing. WM: Writing – review & editing, Validation, Supervision, Project administration. YC: Writing – review & editing, Resources, Project administration, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (Grant No. U20A20384), the Health Commission of Sichuan Province, China (Grant No.21ZD005), the Foundation of Southwest Medical University (Grant No.2023QN003).
Thanks for the guidance of the Statistics Teaching and Research Department of our school.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Trapani D, Ginsburg O, Fadelu T, Lin NU, Hassett M, Ilbawi AM, et al. Global challenges and policy solutions in breast cancer control. Cancer Treat Rev. (2022) 104:102339. doi: 10.1016/j.ctrv.2022.102339
2. D’Oronzo S, Coleman R, Brown J, Silvestris F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J Bone Oncol. (2019) 15:4. doi: 10.1016/j.jbo.2018.10.004
3. Jokar N, Velikyan I, Ahmadzadehfar H, Rekabpour SJ, Jafari E, Ting HH, et al. Theranostic approach in breast cancer: A treasured tailor for future oncology. Clin Nucl Med. (2021) 46:e410–20. doi: 10.1097/RLU.0000000000003678
4. Cook GJR, Goh V. Molecular imaging of bone metastases and their response to therapy. J Nucl Med. (2020) 61:799–806. doi: 10.2967/jnumed.119.234260
5. Mohseninia N, Zamani-Siahkali N, Harsini S, Divband G, Pirich C, Beheshti M. Bone metastasis in prostate cancer: Bone scan versus PET imaging. Semin Nucl Med. (2024) 54:97–118. doi: 10.1053/j.semnuclmed.2023.07.004
6. Ko H, Baghdadi Y, Love C, Sparano JA. Clinical utility of 18F-FDG PET/CT in staging localized breast cancer before initiating preoperative systemic therapy. J Natl Compr Canc Netw. (2020) 18:1240–6. doi: 10.6004/jnccn.2020.7592
7. Zheng S, Chen Y, Zhu Y, Yao S, Miao W. Both [68Ga]Ga-FAPI and [18F]FDG PET/CT missed bone metastasis in a patient with breast cancer. Eur J Nucl Med Mol Imaging. (2021) 48:4519–20. doi: 10.1007/s00259-021-05453-6
8. Bodei L, Herrmann K, Schöder H, Scott AM, Lewis JS. Radiotheranostics in oncology: Current challenges and emerging opportunities. Nat Rev Clin Oncol. (2022) 19:534–50. doi: 10.1038/s41571-022-00652-y
9. Wang Y, Wang Q, Chen Z, Yang J, Liu H, Peng D, et al. Preparation, biological characterization and preliminary human imaging studies of 68Ga-DOTA-IBA. Front Oncol. (2022) 12:1027792. doi: 10.3389/fonc.2022.1027792
10. Wang Q, Yang J, Wang Y, Liu H, Feng Y, Qiu L, et al. Lutetium177-labeled DOTA-ibandronate: A novel radiopharmaceutical for targeted treatment of bone metastases. Mol Pharm. (2023) 20:1788–95. doi: 10.1021/acs.molpharmaceut.2c00978
11. Qiu L, Wang Y, Liu H, Wang Q, Chen L, Liu L, et al. Safety and efficacy of 68 Ga- or 177 Lu-labeled DOTA-IBA as a novel theranostic radiopharmaceutical for bone metastases: A Phase 0/I study. Clin Nucl Med. (2023) 48:489–96. doi: 10.1097/RLU.0000000000004634
12. Deng J, Yang J, Wang Y, Liu G, Chen Y. Comparison of the relative diagnostic performance of 68Ga-DOTA-IBA and 18F-NaF for the detection of bone metastasis. Front Oncol. (2024) 14:1364311. doi: 10.3389/fonc.2024.1364311
13. Xiang F, Liu H, Tan X, Chen Y. 68Ga-DOTA-IBA uptake in breast cancer. Clin Nucl Med. (2024) 49:574–5. doi: 10.1097/RLU.0000000000005200
14. Dayes IS, Metser U, Hodgson N, Parpia S, Eisen AF, George R, et al. Impact of (18)F-labeled fluorodeoxyglucose positron emission tomography-computed tomography versus conventional staging in patients with locally advanced breast cancer. J Clin Oncol. (2023) 41:3909–16. doi: 10.1200/JCO.23.00249
15. Hyland CJ, Varghese F, Yau C, Beckwith H, Khoury K, Varnado W, et al. Use of 18F-FDG PET/CT as an initial staging procedure for Stage II–III breast cancer: A multicenter value analysis. J Natl Compr Canc Netw. (2020) 18:1510–7. doi: 10.6004/jnccn.2020.7598
16. Hildebrandt MG, Naghavi-Behzad M, Vogsen M. A role of FDG-PET/CT for response evaluation in metastatic breast cancer? Semin Nucl Med. (2022) 52:520–30. doi: 10.1053/j.semnuclmed.2022.03.004
17. Minamimoto R, Loening A, Jamali M, Barkhodari A, Mosci C, Jackson T, et al. Prospective comparison of 99mTc-MDP scintigraphy, combined 18F-NaF and 18F-FDG PET/CT, and whole-body MRI in patients with breast and prostate cancer. J Nucl Med. (2015) 56:1862–8. doi: 10.2967/jnumed.115.162610
18. Cristo Santos J, Henriques Abreu M, Seoane Santos M, Duarte H, Alpoim T, Próspero I, et al. Bone metastases detection in patients with breast cancer: Does bone scintigraphy add information to PET/CT? Oncologist. (2023) 28:e600–5. doi: 10.1093/oncolo/oyad087
19. Pang Y, Zhao L, Chen H. 68Ga-FAPI outperforms 18F-FDG PET/CT in identifying bone metastasis and peritoneal carcinomatosis in a patient with metastatic breast cancer. Clin Nucl Med. (2020) 45:913–5. doi: 10.1097/RLU.0000000000003263
20. Magarik MA, Walker RC, Gilbert J, Manning HC, Massion PP. Intracardiac metastases detected by 18F-FSPG PET/CT. Clin Nucl Med. (2018) 43:28–30. doi: 10.1097/RLU.0000000000001883
21. Liu H, Liu L, Fu W, Chen Y. 18F-NaF uptake in breast cancer. Clin Nucl Med. (2020) 45:878–9. doi: 10.1097/RLU.0000000000003114
22. Shankar LK, Schöder H, Sharon E, Wolchok J, Knopp MV, Wahl RL, et al. Harnessing imaging tools to guide immunotherapy trials: Summary from the National Cancer Institute Cancer Imaging Steering Committee workshop. Lancet Oncol. (2023) 24:e133–43. doi: 10.1016/S1470-2045(22)00742-2
23. Van Baelen K, Geukens T, Maetens M, Tjan-Heijnen V, Lord CJ, Linn S, et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol. (2022) 33:769–85. doi: 10.1016/j.annonc.2022.05.006
24. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet. (2020) 395:1208–16. doi: 10.1016/S0140-6736(20)30314-7
25. van Es SC, Velleman T, Elias SG, Bensch F, Brouwers AH, Glaudemans AWJM, et al. Assessment of bone lesions with 18F-FDG PET compared with 99mTc bone scintigraphy leads to clinically relevant differences in metastatic breast cancer management. J Nucl Med. (2021) 62:177–83. doi: 10.2967/jnumed.120.244640
26. Alçın G, Arslan E, Aksoy T, Cin M, Erol Fenercioğlu Ö, Beyhan E, et al. 68 ga-FAPI-04 PET/CT in selected breast cancer patients with low FDG affinity: A head-to-head comparative study. Clin Nucl Med. (2023) 48:e420–30. doi: 10.1097/RLU.0000000000004751
27. Ulaner GA, Vaz SC. Women's health update: growing role of PET for patients with breast cancer. Semin Nucl Med. (2024) 54:247–55. doi: 10.1053/j.semnuclmed.2024.01.007
28. Bénard F, Harsini S, Wilson D, Zukotynski K, Abikhzer G, Turcotte E, et al. Intra-individual comparison of 18F-sodium fluoride PET-CT and 99mTc bone scintigraphy with SPECT in patients with prostate cancer or breast cancer at high risk for skeletal metastases (MITNEC-A1): A multicentre, phase 3 trial. Lancet Oncol. (2022) 23:1499–507. doi: 10.1016/S1470-2045(22)00642-8
29. Hadebe B, Harry L, Ebrahim T, Pillay V, Vorster M. The role of PET/CT in breast cancer. Diagnostics (Basel). (2023) 13(4):597. doi: 10.3390/diagnostics13040597
30. Ulaner GA, Castillo R, Goldman DA, Wills J, Riedl CC, Pinker-Domenig K, et al. (18)F-FDG-PET/CT for systemic staging of newly diagnosed triple-negative breast cancer. Eur J Nucl Med Mol Imaging. (2016) 43:1937–44. doi: 10.1007/s00259-016-3402-9
Keywords: breast cancer, positron emission tomography, whole-body bone scan, bone metastases, computed tomography
Citation: Xiang F, Zhang Y, Tan X, Yan Y, Liu H, Ma W and Chen Y (2024) Prospective comparison of 68Ga-DOTA-ibandronate and bone scans for detecting bone metastases in breast cancer. Front. Oncol. 14:1428498. doi: 10.3389/fonc.2024.1428498
Received: 06 May 2024; Accepted: 15 July 2024;
Published: 31 July 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesCopyright © 2024 Xiang, Zhang, Tan, Yan, Liu, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Chen, Y2hlbnl1ZTU1MjNAMTI2LmNvbQ==; Wenzhe Ma, d3ptYUBtdXN0LmVkdS5tbw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.