- Department of Gynecology, Dalian Women and Children’s Medical Center (Group), Dalian, Liaoning, China
Objectives: To assess the comparative efficacy of neoadjuvant chemotherapy followed by surgery (NACT+S) versus concurrent chemoradiotherapy (CCRT) for patients with cervical cancer stages IB2 to IIB.
Method: An exhaustive literature search was conducted up to November 2023 in databases including PubMed, Embase, Web of Science, and the Cochrane Library, focusing on disease-free survival (DFS) and overall survival (OS). Data were analyzed using STATA version 15.
Results: The meta-analysis included data from two randomized controlled trials and eight retrospective cohort studies, totaling 2,879 patients with stages IB2 to IIB cervical cancer. Pooled data showed no significant difference in OS [hazard ratio (HR) 0.71, 95% confidence interval (CI): 0.51 to 1.00, p = 0.052] and DFS (HR 0.65, 95% CI: 0.38 to 1.14, p = 0.132) between NACT+S and CCRT. Subgroup analysis revealed that NACT+S provided a better OS in Asian populations, retrospective cohort studies, TP regimen chemotherapy, and multivariate analysis.
Conclusion: The findings indicate that CCRT and NACT+S are comparably effective for treating cervical cancer stages IB2 to IIB. Notably, in specific subgroups such as Asian patients and those receiving the TP regimen, NACT+S appears to enhance OS.
1 Introduction
Cervical cancer ranks as the fourth most common and fatal cancer among women globally (1), with approximately 604,127 new cases and 341,831 deaths in 2020. The age-standardized incidence and mortality rates were reported at 13.3 and 7.2 per 100,000 women-years, respectively (95% CI for both ranges slightly) (2). In response, the World Health Organization launched the Cervical Cancer Elimination Initiative in 2020, aimed at accelerating the eradication of this disease. Prominent risk factors include smoking, oral contraceptive use, early sexual activity, multiple sexual partners, sexually transmitted diseases, certain autoimmune diseases, and chronic immunosuppression (3, 4).
The 2018 update to the International Federation of Gynecology and Obstetrics (FIGO) system refined cervical cancer staging to include subdivisions of Stage IB (IB1, IB2, IB3), with Stage IB3 (FIGO 2018) now corresponding to the earlier Stage IB2 (FIGO 2009), ensuring clinical staging consistency. For women with (FIGO 2018) Stage IB2 or higher, the standard treatment is chemoradiotherapy, combining cisplatin-based chemotherapy with concurrent radiotherapy over seven weeks to enhance the cancer cells’ sensitivity to radiation, thus improving outcomes. However, surgery may be considered under specific conditions in locally advanced cervical cancer (LACC) (Stages IB2 to III): if there is no complete remission within two to three months post-chemoradiotherapy and the tumor remains operable; if prior treatments reduce the tumor to a surgically removable size; or following neoadjuvant chemotherapy (NACT), especially in regions with limited radiotherapy access. The efficacy of NACT over surgery alone or combined with chemoradiotherapy continues to be evaluated (5, 6). Despite a five-year overall survival (OR) rate of around 70% for these stages, patients’ quality of life is often affected (7), with radiotherapy notably impacting sexual health in younger, premenopausal women (8).
In locally advanced or Stage IIB cervical cancer, neoadjuvant chemotherapy prior to surgery (NACT-S) has shown potential in reducing tumor size and addressing subclinical lesions (9). However, consensus on the efficacy of this treatment is not fully established, with studies yielding mixed results. Chang et al. reported a 2-year survival rate of 81% (95% CI: 71%-91%) and an estimated 5-year survival rate of 70% for patients undergoing NACT-S (10). Uegaki et al. suggested that NACT-S followed by radical hysterectomy might cure approximately 70% of patients with Stages IB2 to IIB cervical cancer (11). Despite these findings, the definitive benefits of NACT-S are still under investigation. Comparative studies, such as those by Yoshida et al., have noted significantly longer progression-free and OS in patients receiving surgery post-neoadjuvant chemoradiation compared to those receiving chemoradiation alone, with p-values of 0.027 and 0.017 respectively (12).
2 Objectives
The ongoing debate underscores the need for further research to clarify the comparative prognosis of NACT-S and concurrent chemoradiotherapy (CCRT). This study aims to assess the comparative efficacy of NACT followed by surgical intervention (NACT+S) versus CCRT in patients with cervical carcinoma at stages IB2 to IIB.
3 Methods
3.1 Search strategy
The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD 42023482991). A comprehensive literature search up to November 2023 was conducted across databases such as PubMed, Embase, Web of Science, and the Cochrane Library to identify relevant studies. Additional sources including references in found articles and related reviews were examined to ensure the capture of all pertinent publications. Search terms included “cervical cancer” or “uterine cervical neoplasms”, and “chemoradiotherapy” or “chemoradiation”, without language restrictions. The detailed search strategy is available in Appendix 1.
3.2 Eligibility criteria
Inclusion criteria were: (1) patients with FIGO stage IB2-IIB cervical cancer (the year of FIGO staging used in each article is marked in Table 1); (2) study groups undergoing CCRT; (3) control groups receiving NACT+RS; (4) outcomes measured included OS and/or DFS; (5) study designs were either cohort studies or RCTs. Exclusion criteria included: (1) animal studies; (2) studies without available full texts; (3) non-empirical articles such as reviews, meta-analyses, conference abstracts, case reports, and guidelines.
3.3 Data collection and quality assessment
Initial screenings of titles and abstracts were followed by full-text reviews for articles meeting the inclusion criteria. Two researchers independently extracted data using predefined forms, focusing on OS, DFS, and essential study characteristics including authorship, publication year, geographical location, study design, FIGO stage, NACT methodology, and follow-up period. Data on OS and DFS not directly available were extracted from Kaplan-Meier survival curves using Engauge Digitizer 4.1. The methodological quality of studies was assessed using a modified 7-point Jadad Score for randomization, allocation concealment, double-blinding, and withdrawals/dropouts, with scores 1–3 indicating high risk of bias and 4–7 indicating low risk. The Newcastle-Ottawa Scale (NOS) further evaluated the selection, comparability, and outcome of studies, categorizing quality as low (0–3), moderate (4–6), or high (7–9).
3.4 Statistical analysis
Statistical analyses were performed using Stata (version 15.0) and Review Manager (version 5.3.3) for literature quality evaluation. Pooled data provided summary hazard ratios (HRs) and 95% CIs for OS and DFS. Risk ratios (RRs) and corresponding 95% CIs compared adverse events between the NACT+S and CCRT groups. Cochran’s Q test and the I2 statistic assessed study heterogeneity. An I2 value above 50% prompted the use of a random-effects model, while values below 50% utilized a fixed-effects model. Subgroup analyses were based on study design, region, average follow-up period, NACT regimens, and data sources. Egger’s test evaluated publication bias, and sensitivity analyses applied the “remove one study” method to determine the impact of individual studies on overall outcomes. A p-value less than 0.05 was considered statistically significant.
4 Results
4.1 Study selection and quality assessment
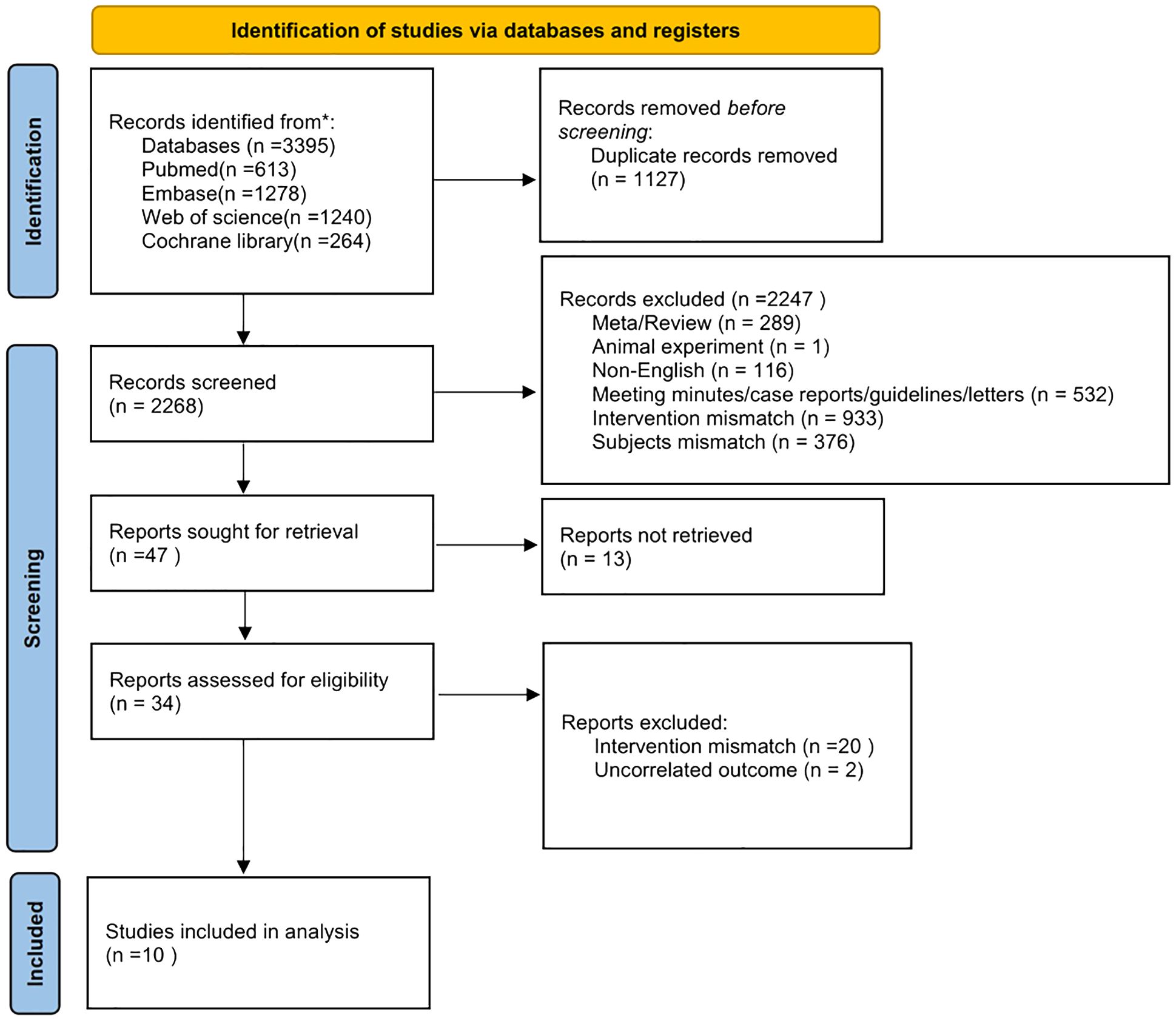
The literature search and screening, summarized in Figure 1, identified 3,395 articles from databases including PubMed, Embase, Web of Science, and the Cochrane Library. After removing 1,127 duplicates, 2,268 articles were screened, leading to the exclusion of 2,247 articles for various reasons: meta-analyses/reviews (289), animal studies (1), non-English articles (116), meeting minutes/case reports/guidelines/letters (532), intervention mismatch (953), subject mismatch (376), and unrelated outcomes (2). Ultimately, ten articles met the inclusion criteria for this study.
4.2 Study characteristics and quality assessment
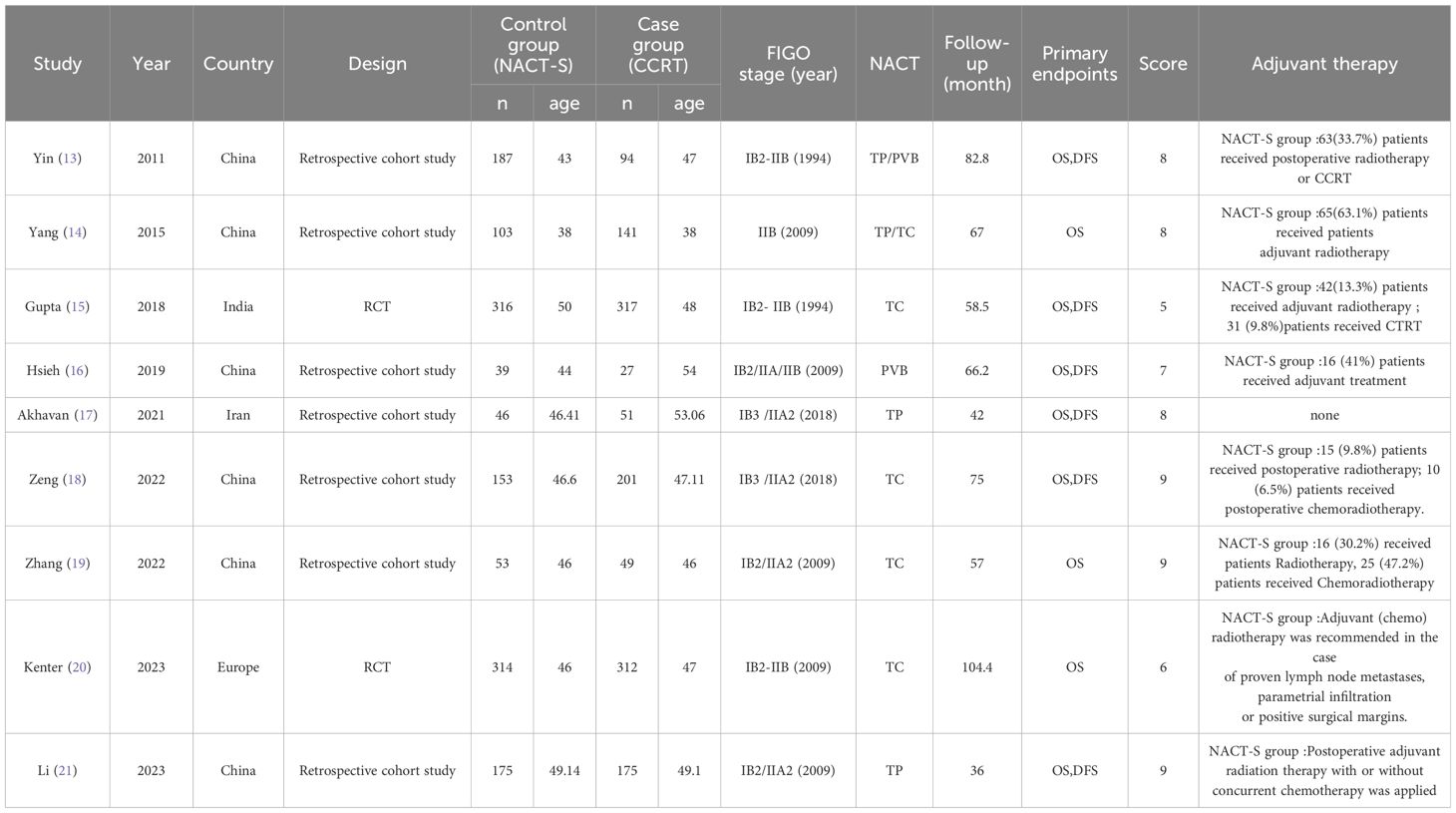
The ten selected studies (12–21), comprising eight retrospective cohort studies and two RCTs, involved a total of 2,879 patients aged 38 to 56.9 years, diagnosed with cervical cancer stages IB2 to IIB. These studies originated from Asia and Europe and were assessed for quality using the Cochrane Collaboration’s risk of bias tool. Studies scoring ≥7 for cohort studies and ≥5 for RCTs were classified as high quality, as detailed in Table 1.
4.3 Pooled analysis for OS
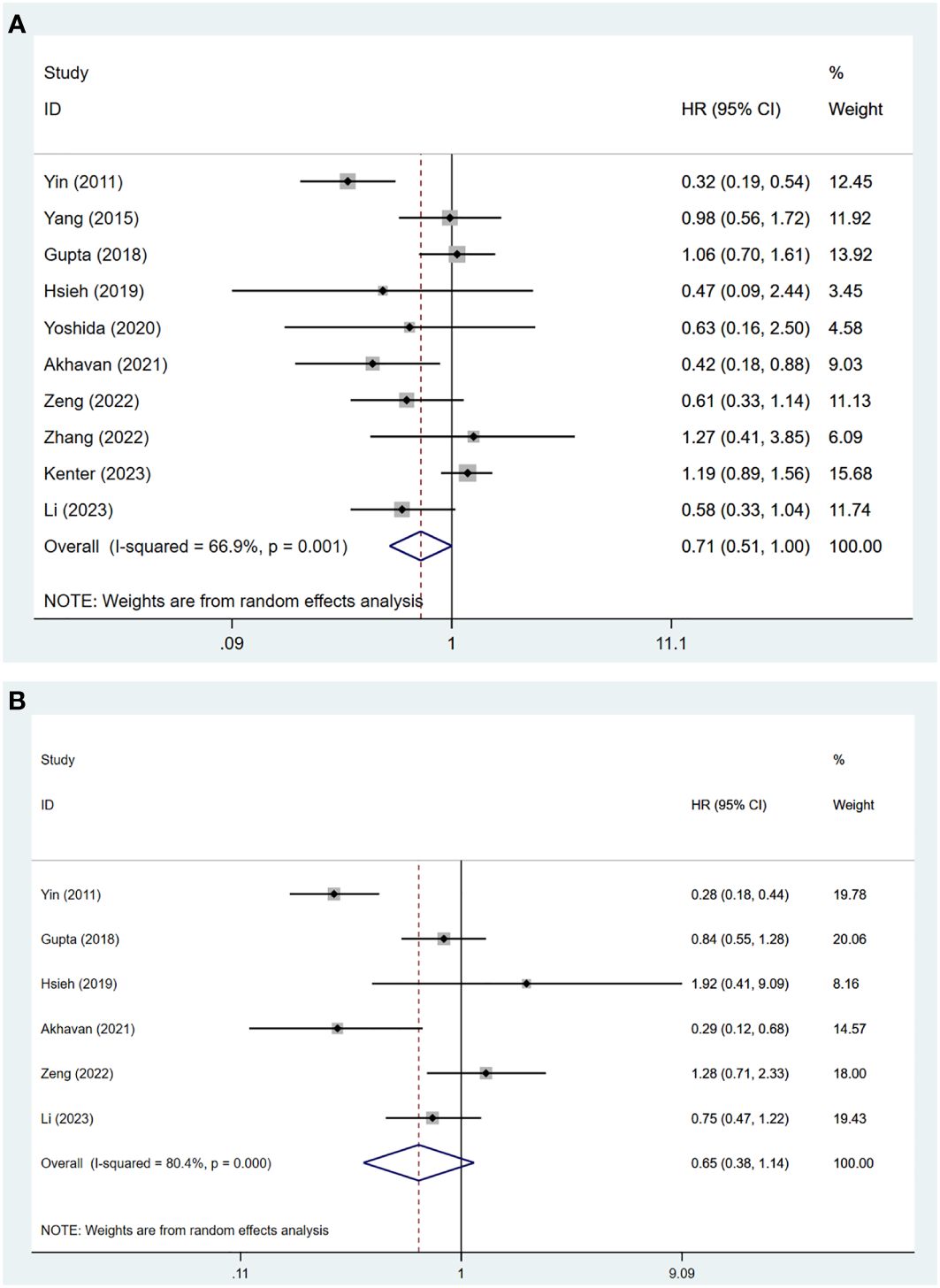
The meta-analysis of these ten studies showed substantial heterogeneity (I2 = 66.90%, p = 0.001), necessitating a random-effects model. The results indicated no significant difference in OS between patients treated with NACT+S and those undergoing CCRT (HR = 0.71, 95% CI: 0.51 to 1.00, p = 0.052) (Figure 2A).
4.4 Subgroup analysis for OS
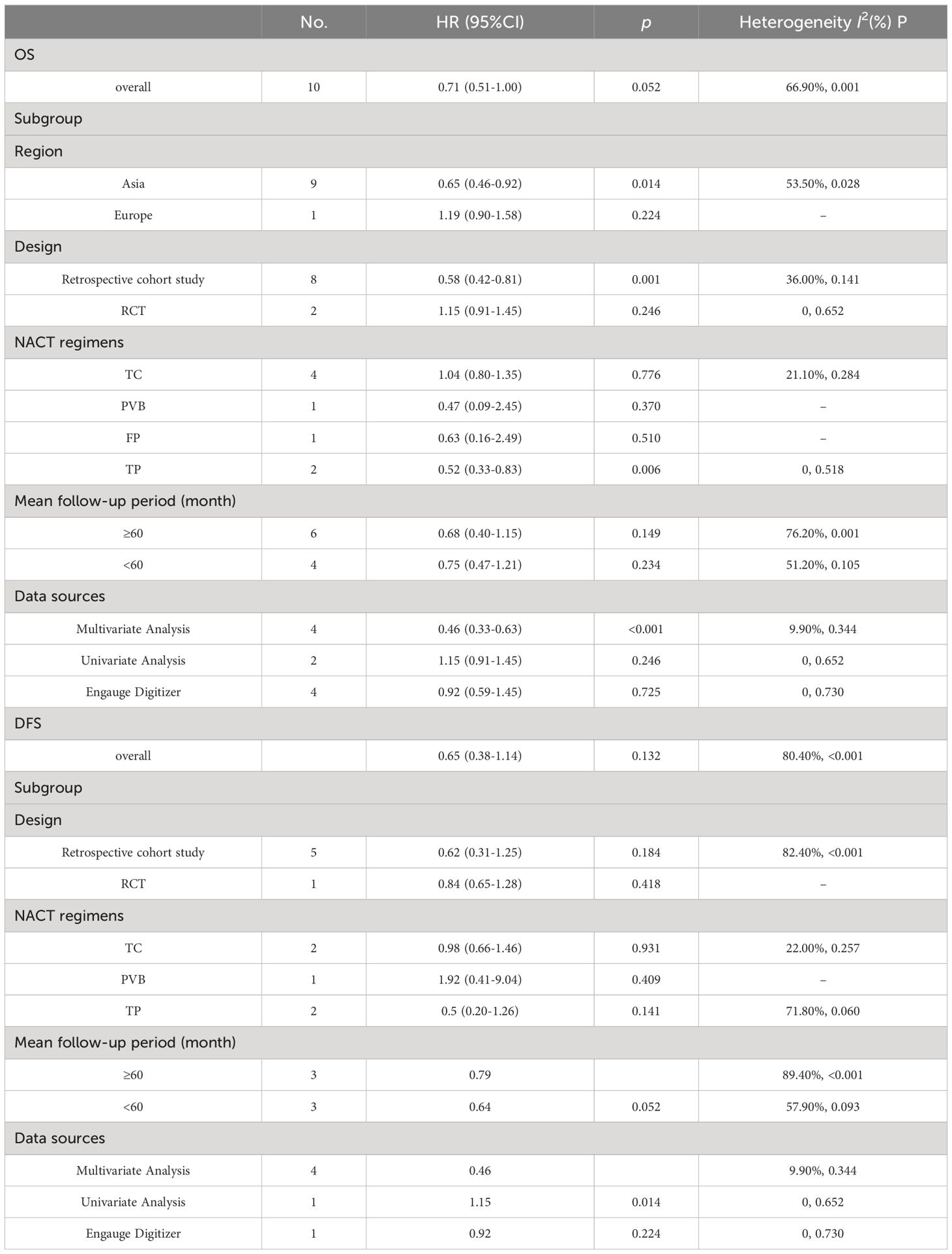
Subgroup analyses explored sources of heterogeneity and yielded several findings: In the Asian population, OS was significantly higher with NACT+S compared to CCRT (HR = 0.65, 95% CI: 0.46 to 0.92, p = 0.014). Retrospective cohort studies also showed improved OS with NACT+S (HR = 0.58, 95% CI: 0.42 to 0.81, p = 0.001), a result not seen in RCTs. NACT using the TP regimen was associated with better OS outcomes (HR = 0.52, 95% CI: 0.33 to 0.83, p = 0.006). Follow-ups longer than 60 months did not show significant variation in OS. Multivariate analysis further confirmed superior OS with NACT+S (HR = 0.46, 95% CI: 0.33 to 0.63, p < 0.001), whereas univariate analysis and data extracted via software showed no significant differences. These detailed findings are elaborated in Table 2; Supplementary Figures in the Appendix.
4.5 Pooled analysis for DFS
A pooled analysis was conducted on data from six studies involving 1,781 patients with cervical cancer stages IB2 to IIB. Due to significant heterogeneity (I2 = 80.40%, p < 0.001), a random-effects model was applied. The results showed no significant difference in DFS between patients undergoing NACT+S and those receiving CCRT (HR = 0.65, 95% CI: 0.38 to 1.14, p = 0.132), as shown in Figure 2B.
4.6 Subgroup analysis for DFS
Subgroup analyses, considering variables such as study design, NACT regimens, follow-up periods, and data sources, revealed no statistically significant differences in DFS between the NACT+S and CCRT groups. These findings are detailed in Table 2.
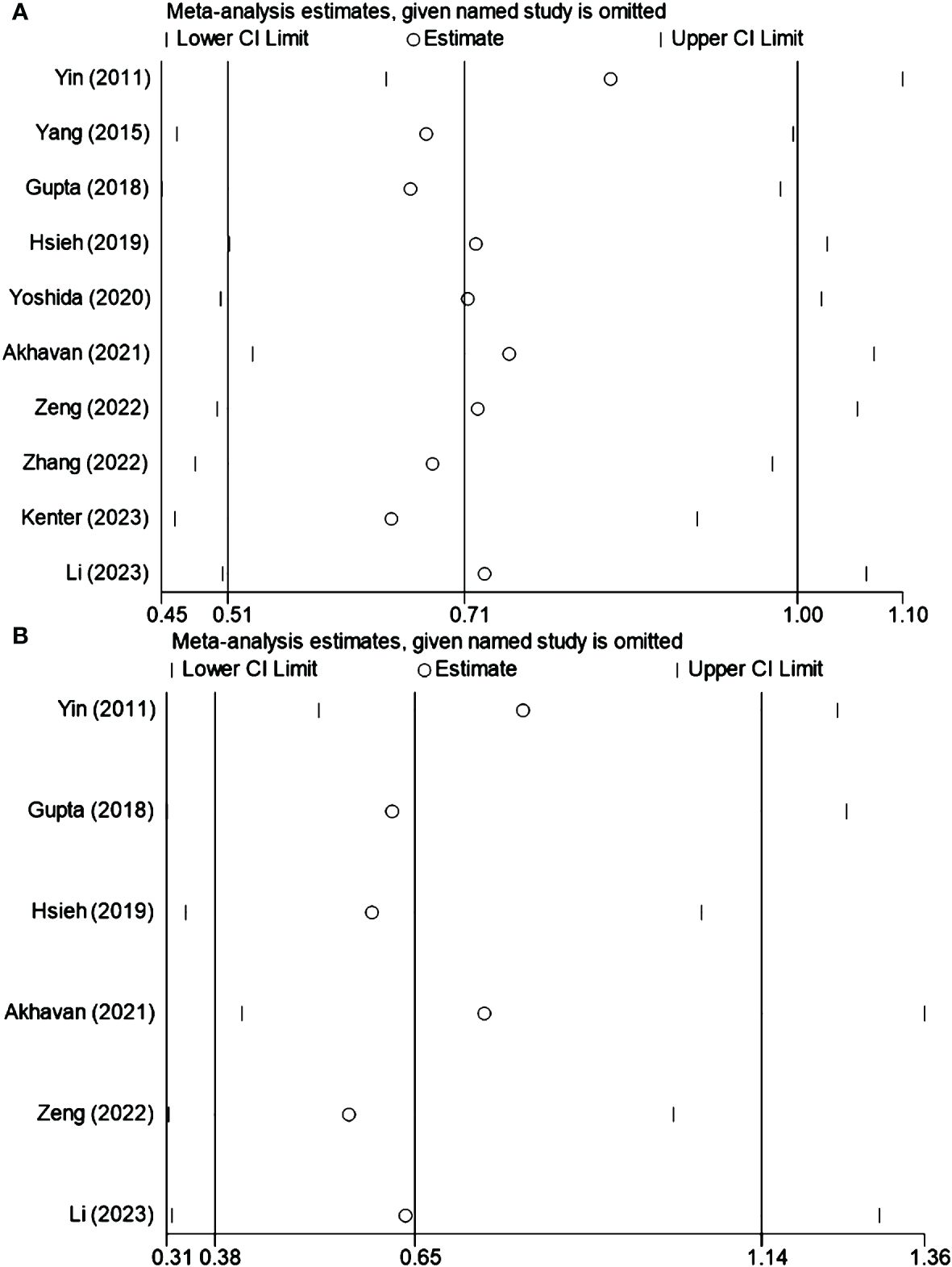
4.7 Sensitivity analysis
Sensitivity analysis was conducted to assess the influence of individual studies on the overall outcomes. This analysis affirmed that no single study markedly affected the composite results, confirming the robustness of the findings. The results are presented in Figures 3A, B for OS and DFS, respectively.
4.8 Publication bias
With ten studies included in the OS analysis, potential publication bias was evaluated using the Egger test. The results indicated no significant publication bias (p = 0.154), reinforcing the reliability of the OS findings.
5 Discussion
5.1 Summary of main results
This meta-analysis, incorporating ten studies, compared the effectiveness of NACT+S versus CCRT in patients with cervical cancer stages IB2 to IIB. The overall findings revealed no significant differences in OS and DFS between the two treatments. However, subgroup analyses indicated that NACT+S might improve OS in Asian populations, in retrospective cohort studies, with the TP chemotherapy regimen, and in multivariate analyses. Conversely, other subgroup analyses showed no disparities in OS or DFS across treatment groups.
5.2 Results in the context of published literature
The results contrast with those of Cheng et al., who suggested that CCRT might be more effective for cervical cancer at FIGO (2009) stage IB2/IIA2 (22). This discrepancy may be due to this analysis including only two studies with the CCRT regimen and a recent large-scale RCT published in 2023, which may enhance the reliability of our findings. This is consistent with Kenter et al., whose RCT reported no significant difference in 5-year OS between the NACT+S (72%; 95% CI: 66%-77%) and CCRT groups (76%; 95% CI: 70%-80%) (20).
Subgroup analyses from nine Asian studies showed higher OS for the NACT+S group compared to CCRT, a finding not replicated in a single European study. This might reflect limited European data or inherent differences in treatment response due to racial or regional factors. The techniques and strategies of administering CCRT and NACT might also differ across regions. Retrospective cohort studies indicated poorer OS outcomes for the CCRT group, contrasting with findings from RCTs, which benefit from randomized grouping to enhance comparability and minimize bias.
Patients treated with the TP regimen in the NACT+S group exhibited higher OS compared to those undergoing CCRT, suggesting a potential preference for the TP regimen in NACT+S planning. Caution is warranted in interpreting these results, however, due to the limited number of studies focused on specific regimens and the use of multiple regimens within some studies. Finally, while substantial differences were observed in multivariate analysis, univariate analysis and data extracted using Engauge Digitizer did not show similar disparities, possibly due to the limited scope of studies included in these analyses.
This research suggests that NACT+S and CCRT may have comparable therapeutic outcomes. NACT+S can reduce tumor volume, facilitate surgical resection, and potentially decrease subclinical metastasis. It also enables assessment of tumor responsiveness to chemotherapy and prognosis prediction. Considering the risk of vaginal dysfunction associated with CCRT, NACT+S might be preferable for younger patients concerned about sexual health. However, it is critical to acknowledge that NACT+S could obscure high-risk factors, potentially leading to increased recurrence, alongside added side effects and treatment costs. For patients exhibiting high-risk factors post-NACT+S, adjuvant radiochemotherapy remains necessary. Despite these considerations, our analysis of ten studies, nine of which included cases of adjuvant treatment post-NACT+S (Table 1), found no differences in DFS and OS between the groups. Given the increased side effects and economic burden associated with overlapping treatments, CCRT may sometimes be more suitable. The role of NACT+S in managing LACC remains controversial but is supported by Li et al.’s findings, which show promising antitumor activity and a tolerable adverse event profile from combining neoadjuvant chemo-immunotherapy with radical surgery, suggesting a novel approach for LACC treatment (23). Continued research is essential to determine the optimal treatment strategy for LACC.
5.3 Strengths and weaknesses
This study offers methodological strengths as the first to compare the effectiveness and safety of NACT+S versus CCRT in this patient group, providing clinically significant insights. The inclusion of quality assessments, comprehensive evaluations for publication bias, and meticulous sensitivity analyses enhance the reliability of our findings. Limitations include a predominance of retrospective studies and a regional focus on Asia, which may restrict the generalizability of results. The limited number of RCTs and the regional skew also necessitate cautious interpretation of our findings.
5.4 Implications for practice and future research
In summary, while findings suggest comparable therapeutic efficacies of NACT+S and CCRT for cervical cancer stages IB2 to IIB, the predominance of retrospective cohort studies highlights the need for more prospective, high-quality RCTs to confirm these outcomes.
Author contributions
YG: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. HW: Conceptualization, Methodology, Resources, Writing – review & editing. MJ: Investigation, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1426002/full#supplementary-material
References
1. Cibula D, Raspollini MR, Planchamp F, Centeno C, Chargari C, Felix A, et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer - Update 2023. Int J Gynecol Cancer. (2023) 33:649–66. doi: 10.1136/ijgc-2023–004429
2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. (2023) 11:e197–206. doi: 10.1016/s2214–109x(22)00501–0
3. Bouvard V, Wentzensen N, Mackie A, Berkhof J, Brotherton J, Giorgi-Rossi P, et al. The IARC perspective on cervical cancer screening. N Engl J Med. (2021) 385:1908–18. doi: 10.1056/NEJMsr2030640
4. Yang M, Du J, Lu H, Xiang F, Mei H, Xiao H. Global trends and age-specific incidence and mortality of cervical cancer from 1990 to 2019: an international comparative study based on the Global Burden of Disease. BMJ Open. (2022) 12:e055470. doi: 10.1136/bmjopen-2021–055470
5. Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev. (2010) 2010:Cd008285. doi: 10.1002/14651858.Cd008285
6. Rydzewska L, Tierney J, Vale CL, Symonds PR. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev. (2010) 1):Cd007406. doi: 10.1002/14651858.CD007406.pub2
7. Güth U, Hadwin RJ, Schötzau A, McCormack M. Clinical outcomes and patterns of severe late toxicity in the era of chemo-radiation for cervical cancer. Arch Gynecol Obstet. (2012) 285:1703–11. doi: 10.1007/s00404–011-2193–2
8. Pfaendler KS, Wenzel L, Mechanic MB, Penner KR. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther. (2015) 37:39–48. doi: 10.1016/j.clinthera.2014.11.013
9. de la Torre M. Neoadjuvant chemotherapy in woman with early or locally advanced cervical cancer. Rep Pract Oncol Radiother. (2018) 23:528–32. doi: 10.1016/j.rpor.2018.09.005
10. Chang TC, Lai CH, Hong JH, Hsueh S, Huang KG, Chou HH, et al. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. J Clin Oncol. (2000) 18:1740–7. doi: 10.1200/jco.2000.18.8.1740
11. Uegaki K, Shimada M, Sato S, Deura I, Naniwa J, Sato S, et al. Outcome of stage IB2-IIB patients with bulky uterine cervical cancer who underwent neoadjuvant chemotherapy followed by radical hysterectomy. Int J Clin Oncol. (2014) 19:348–53. doi: 10.1007/s10147–013-0559–0
12. Yoshida K, Kajiyama H, Yoshihara M, Tamauchi S, Ikeda Y, Yoshikawa N, et al. The role of additional hysterectomy after concurrent chemoradiation for patients with locally advanced cervical cancer. Int J Clin Oncol. (2020) 25:384–90. doi: 10.1007/s10147–019-01551–6
13. Yin M, Zhao F, Lou G, Zhang H, Sun M, Li C, et al. The long-term efficacy of neoadjuvant chemotherapy followed by radical hysterectomy compared with radical surgery alone or concurrent chemoradiotherapy on locally advanced-stage cervical cancer. Int J Gynecol Cancer. (2011) 21:92–9. doi: 10.1111/IGC.0b013e3181fe8b6e
14. Yang S, Gao Y, Sun J, Xia B, Liu T, Zhang H, et al. Neoadjuvant chemotherapy followed by radical surgery as an alternative treatment to concurrent chemoradiotherapy for young premenopausal patients with FIGO stage IIB squamous cervical carcinoma. Tumour Biol. (2015) 36:4349–56. doi: 10.1007/s13277–015-3074–2
15. Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: A randomized controlled trial. J Clin Oncol. (2018) 36:1548–55. doi: 10.1200/jco.2017.75.9985
16. Hsieh HY, Huang JW, Lu CH, Lin JC, Wang L. Definite chemoradiotherapy is a competent treatment option in FIGO stage IB2 cervical cancer compared with radical surgery +/- neoadjuvant chemotherapy. J Formos Med Assoc. (2019) 118:99–108. doi: 10.1016/j.jfma.2018.01.015
17. Akhavan S, Alibakhshi A, Parsapoor M, Alipour A, Rezayof E. Comparison of therapeutic effects of chemo-radiotherapy with neoadjuvant chemotherapy before radical surgery in patients with bulky cervical carcinoma (stage IB3 & IIA2). BMC Cancer. (2021) 21:667. doi: 10.1186/s12885–021-08416–0
18. Zeng J, Sun P, Ping Q, Jiang S, Hu Y. Clinical outcome of FIGO 2018 stage IB3/IIA2 cervical cancer treated by neoadjuvant chemotherapy followed by radical surgery due to lack of radiotherapy equipment: A retrospective comparison with concurrent chemoradiotherapy. PloS One. (2022) 17:e0266001. doi: 10.1371/journal.pone.0266001
19. Zhang Y, Tang X, Ma S, Shen M, Jiang L, Yuan W, et al. Association between three therapeutic strategies and clinical outcomes of 2009 FIGO stage IB2/IIA2 cervical cancer. J Oncol. (2022) 2022:9497798. doi: 10.1155/2022/9497798
20. Kenter GG, Greggi S, Vergote I, Katsaros D, Kobierski J, van Doorn H, et al. Randomized phase III study comparing neoadjuvant chemotherapy followed by surgery versus chemoradiation in stage IB2-IIB cervical cancer: EORTC-55994. J Clin Oncol. (2023) 41:5035–43. doi: 10.1200/jco.22.02852
21. Li W, Liu P, He F, Sun L, Zhao H, Wang L, et al. The long-term outcomes of clinical responders to neoadjuvant chemotherapy followed by radical surgery in locally advanced cervical cancer. J Cancer Res Clin Oncol. (2023) 149:4867–76. doi: 10.1007/s00432–022-04401–7
22. Cheng J, Liu B, Wang B, Long X, Li Z, Chen R, et al. Effectiveness comparisons of various therapies for FIGO stage IB2/IIA2 cervical cancer: a Bayesian network meta-analysis. BMC Cancer. (2021) 21:1078. doi: 10.1186/s12885–021-08685–9
Keywords: cervical cancer, neoadjuvant chemotherapy, chemoradiotherapy, surgery, meta-analysis
Citation: Gao Y, Wang H and Jiang M (2024) Comparative effectiveness of neoadjuvant chemotherapy plus surgery versus concurrent chemoradiotherapy in stages IB2 to IIB of cervical cancer: a meta-analysis. Front. Oncol. 14:1426002. doi: 10.3389/fonc.2024.1426002
Received: 30 April 2024; Accepted: 11 June 2024;
Published: 24 June 2024.
Edited by:
Annamaria Ferrero, Mauriziano Hospital, ItalyReviewed by:
Mikel Gorostidi, University of the Basque Country, SpainStefano Restaino, Ospedale Santa Maria della Misericordia di Udine, Italy
Copyright © 2024 Gao, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huali Wang, d2FuZ2hsX2RsQDEyNi5jb20=
 Yue Gao
Yue Gao Huali Wang
Huali Wang Meng Jiang
Meng Jiang