- 1Department of Translational Biomedicine and Neuroscience, University of Bari Medical School, Bari, Italy
- 2Department of Precision and Regenerative Medicine and Ionian Area, University of Bari Medical School, Bari, Italy
- 3Department of Medicine and Surgery, Libera Università del Mediterraneo (LUM) Giuseppe Degennaro University, Bari, Italy
Non-Hodgkin lymphomas (NHLs) encompass a diverse group of malignancies arising from B cells, T cells, and natural killer (NK) cells at various stages of differentiation. Conversely, classical Hodgkin lymphomas (cHLs) primarily feature Reed-Sternberg cells (RSCs) amid a background of reactive immune cells. Immunomodulatory pathways, notably the PD-1/PD-L1 axis, play pivotal roles in tumor immune evasion across both NHLs and cHLs. Elevated expression of PD-1 and PD-L1 is observed in a spectrum of lymphomas, influencing prognosis and treatment response. Therapeutically, immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 have revolutionized lymphoma management, particularly in relapsed/refractory cases. Nivolumab and pembrolizumab, among others, have demonstrated efficacy in various B-cell lymphomas, with promising outcomes in cHL. Combination strategies incorporating ICIs with conventional chemotherapy or targeted agents show enhanced efficacy and are being explored extensively. In this review we discuss the most important features of the tumor microenvironment of NHLs and cHLs, address the therapeutic approaches with ICIs and try to outline future perspectives.
1 Introduction
1.1 Histopathological features of human lymphomas and their microenvironment
Non Hodgkin lymphomas (NHLs) derive from B cells, T cells, and natural killer (NK) cells in different stages of their differentiation. NHLs have been classified into more than 30 different histopathologic types, including indolent lymphomas, aggressive lymphomas, and highly aggressive lymphomas. In classical Hodgkin lymphomas (cHLs), Reed-Sternberg cells (RSCs) constitute a small portion of the involved lymph nodes, while most cells are represented by reactive T cells and other immune cells. cHL are approximately 95% of cases and nodular lymphocyte predominant HL (NLPHL) are approximately 5% of cases of HLs. Hodgkin cells are defined as having a single nucleus and they have proliferative potential while RSCs are multinucleated due to incomplete cytokinesis and lack proliferative potential.
Tumor growth, progression, and metastatic capability in NHLs are influenced by different cellular component of tumor microenvironment, including tumor associated macrophages (TAMs), mast cells, T and B cells, myeloid-derived suppressor cells (MDSCs), tumor-associated neutrophils (TANs), natural killer (NK) cells, dendritic cells (DCs). Over 95% of the cells in the tumor microenvironment of HL are non-malignant cells. RSCs are a minority of cells in tumors (approximately 1%) and recruit monocytes and TAMs, regulatory T cells (Treg), helper T cells (Th), mast cells, fibroblasts, eosinophils, neutrophils, and NK cells, which are all involved in immune evasion.
2 Immune checkpoint inhibitors
PD-1 is an inhibitory co-receptor expressed on CD8+ and CD4+ T cells, NK and B cells, and tumor-infiltrating lymphocytes (TILs) (1). On B-cells, PD-1 is markedly regulated by B-cell receptor (BCR) signaling, lipopolysaccharide (LPS), CpG oligodeoxynucleotides, and several proinflammatory cytokines.
PD-1 interacts with PD-L1, expressed on the cell surfaces of activated T, B, and NK cells (2), peripheral tissues and organs, and tumor cells (3), and PD-L2, expressed by macrophages and DCs (4). PD-L1 is expressed on endothelium in different tumors and correlated with activation of immune cells and a poor prognosis (5). Increased T-cell infiltration observed after anti-angiogenic treatment is associated with enhanced tumor PD-L1 expression (6), epigenetic modifications, and drug resistance (7–9). Overexpression of PD-1 and its ligands, PD-L1 and PD-L2, by malignant neoplastic cells, allows the ligation of PD-1 on T-cells and the consequent induction of T-cell “exhaustion”. In this context, the malignant cells escape from the antitumor immune response (10). Other molecules have been investigated in the microenvironment of HLs, such as lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin and mucin-domain containing proteins 3 (TIM-3) and it was demonstrated that in all cases of HLs there is immune-expression of these pathways, that could be targeted in future clinical trials, particularly, in cases of relapsed/refractory HL. (11).
Immune checkpoint inhibitors (ICIs) have been approved for use in different solid tumors, including metastatic melanoma, advanced non-small cell lung cancer, metastatic renal cell carcinoma, metastatic bladder cancer, advanced head and neck cancer, and hematological tumors, including lymphomas.
To date, 12 antibodies targeting PD-1 and 5 antibodies targeting PD-L1 have been approved by regulatory agencies worldwide. The approved anti-PD-1 and anti-PD-L1 antibodies blocking the axis PD-1 and PD-L1/PD-L2 have confirmed the immune system’s role in mediating the antitumor response, leading T cells to kill tumor cells (12). Different strategies of combining anti-PD1/PDL1 and other immunological agents, chemotherapy, or target molecules have been investigated. The combination of different immunotherapies, targeting distinctive immune checkpoints might be more effective than monotherapy. Indeed, the anti-PD-1/PD-L1 in combination with anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4), improved objective response rates despite a high rate of toxicities. Thus, ipilimumab, the first new-generation immune checkpoint inhibitor agent approved in monotherapy by the Food and Drug Administration (FDA) in 2011 for the treatment of patients with advanced or metastatic melanoma (13), can be used in combination with nivolumab in advanced melanoma, renal cell carcinoma (RCC), microsatellite instable colorectal cancer, hepatocellular carcinoma (HCC), malignant pleural mesothelioma (14).
Different therapeutic strategies to block PD-1/PD-L1 interaction are under clinical development to prevent PD-1-mediated attenuation of T cell receptor (TCR) signaling, allowing for activity restoration of exhausted CD8+ T-cells. In addition, a co-stimulatory signal through B7 protein is required for target-cell lysis and effector cell responses. B7 protein on activated antigen-presenting cells (APCs) can pair with either a CD28 on the surface of a T cell to produce a co-stimulatory signal to enhance the activity of TCR signal and T cell activation, or it can pair with T lymphocyte-associated protein-4 to produce an inhibitory signal to keep the T cell in the inactive state. CTLA-4 inhibition by monoclonal antibodies may induce tumor rejection through direct blockade of CTLA-4 competition for CD-80 (B7-1) and CD-86 (B7-2) ligands, which enhances CD28 co-stimulation. Alternative immune checkpoint molecules expressed on tumor cells or immune cells in the tumor microenvironment can be simultaneously modulated to restore an effective anti-lymphoma immune response.
3 PD-1/PDL-1 expression in human lymphomas
PD-1 and PDL-1 are expresses in both NHLs and cHLs (Figures 1–4). In NHLs B cell lymphomas, the highest level of PD-L1 expression has been observed in diffuse large B cell lymphoma (DLBCL), followed by small lymphocytic lymphoma (SLL), mantle cell lymphoma (MLC), while follicular lymphoma (FL) had the lowest PD-L1 expression level (15, 16). In DLBCL, PD-L1 is expressed by the nonmalignant compartment in 26% to 75% of the cases (17). PD-L1 was expressed in about 60% of cases with an average of 20% of patients having a PD-L1/PD-L2 genetic expression in Epstein Barr Virus (EBV)-positive lymphoma (18). EBV infection has been correlated with a much higher PD-L1 expression in DLBCL tumors and a poorer outcome has been reported in cases with PD-L1+ macrophages (19). PD-1 expression was detected in 39.5–68.6% of DLBCL cases (20), supporting the notion that a high number of PD-1+ TILs are associated with favorable clinical features and prognosis (21). 10.5% of DLBCL samples expression of PD-L1 and PD-1 is associated with poor overall survival (22). Approximately 30-80% of patients with primary mediastinal B cell lymphoma (PMBCL) have PD-L1 overexpression (23). In primary central nervous system lymphoma (PCNSL) and testicular lymphoma it has been demonstrated a high PD-L1 and PD-L2 expression through amplification 9p24.1 (24). In SLL/chronic lymphocytic leukemia (CLL), PD-L1/PD-1 expression ranges between 10-90% (25, 26). FL tumor cells are largely negative for PD-L1 and PD-L2 (27), and the TILs are characterized by high PD-1 expression and suppressed cytokine signaling (28). Several studies have shown that PD-L1 expression is low or absent in MCL (17). In contrast, others have shown a constitutive expression of PD-L1 on tumor cells in both cell lines and primary patient samples (29).
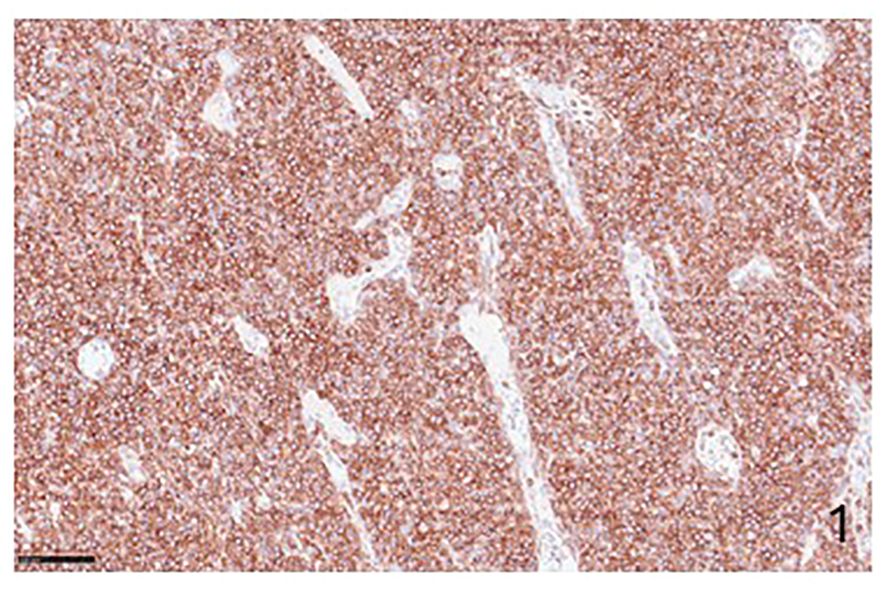
Figure 1 Immunohistochemical preparation showing an example of NHLs with PD-1-positive cells (Immunohistochemistry for anti-PD1, Original Magnification 20x).
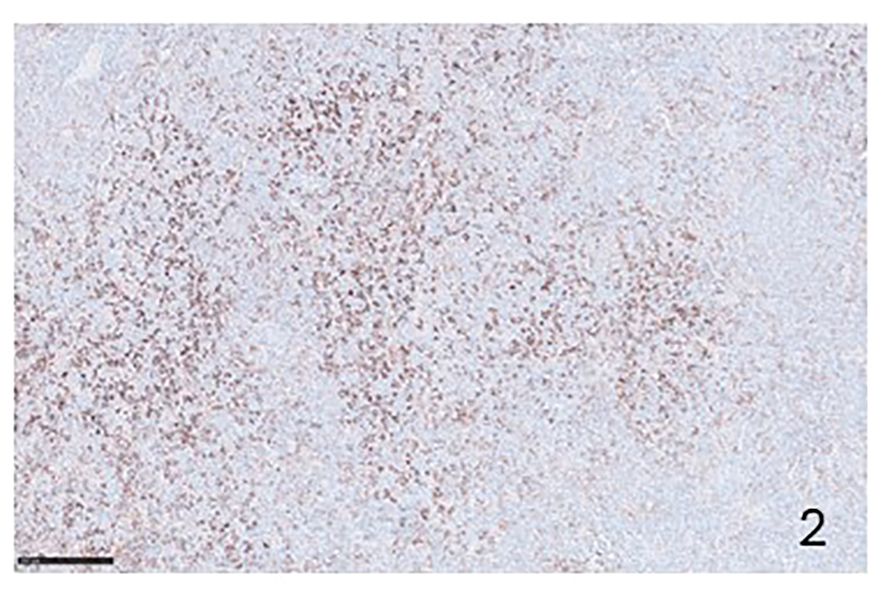
Figure 2 Immunohistochemical preparation showing PD-L1 positive lymphocytes in a case of T-peripheral NHL (Immunohistochemistry for anti PD-L1, Original Magnification 20x).
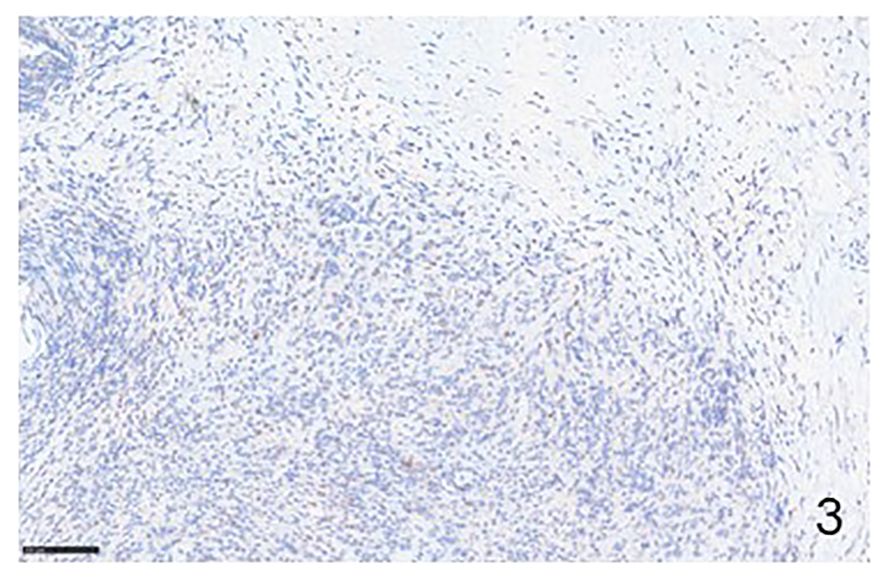
Figure 3 Immunohistochemical example of HLs, classical-type, sclero-nodular, showing some lymphocytes positive for PD-1 (Immunohistochemistry for anti-PD1, Original Magnification 20x).
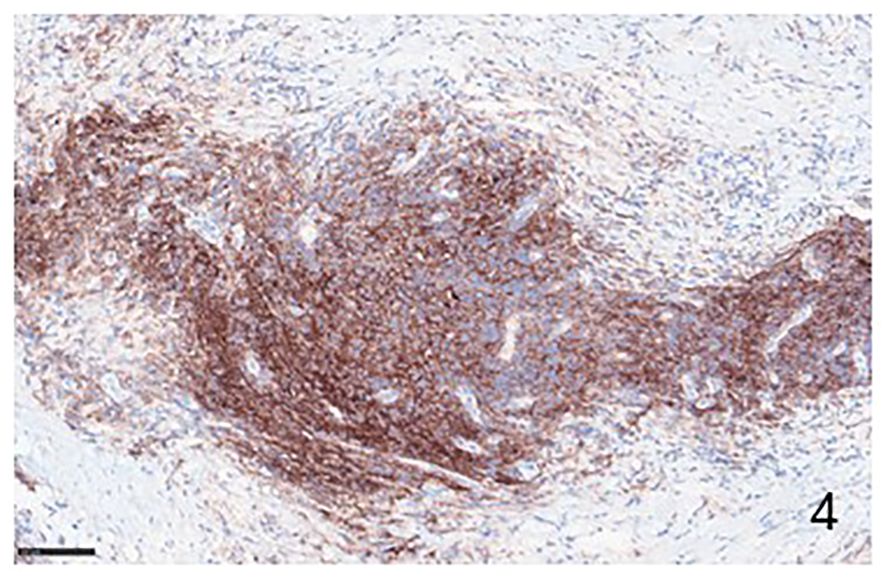
Figure 4 Immunohistochemical example of HLs, classical-type, sclero-nodular, immunolabeled with anti-PD-L1 antibody, showing many positive PD-L1 lymphocytes (Immunohistochemistry for anti PD-L1, Original Magnification 20x).
Upregulation and activation of the PD-1/PD-L1 and PD-2/PD-L2 pathways is a typical characteristic of HL (30). In 70–87% of cHL patients, PD-L1 is detected on the surface of both RSCs cells and TAMs (17). It is associated with worse event-free survival (EFS) and shorter progression-free survival (PFS) (31). This overexpression can be consequent to EBV infection (32). EBV infection in cHL increases PD-L1 expression (33). Increased PDL-1 expression by TAMs following interferon (IFN)-γ signaling is relevant in cHL clinical outcomes due to the close relationship between HRS and PD-1+ CD4+ T-cells (18, 34).
4 Immune checkpoint inhibitors in the treatment of lymphomas
Two anti-PD-1 antibodies (nivolumab (Opdivo®) and pembrolizumab (Keytruda®) and three anti-PD-L1 antibodies (durvalumab, atezolizumab, and avelumab) have been approved for the treatment of B cell lymphomas (35). Nivolumab and pembrolizumab, two fully humanized IgG4-kappa-blocking monoclonal antibodies target the PD-1 receptor on human T-cells. The blockade of the PD-1 signaling pathway by nivolumab induces the proliferation of lymphocytes and the release of IFN-γ. Pembrolizumab binds with high affinity to human PD-1, blocking receptor ligation by both PD-L1 and PD-L2 and leading to enhanced T-lymphocyte immune responses in preclinical models of cancer, with the modulation of key interleukin (IL)-2, tumor necrosis factor-alpha (TNF)-α, and IFN-γ (36).
FL and DLBCL presented the highest objective response to therapy with nivolumab, while MCL lacked a response to treatment (37). In DLBCL treatment with nivolumab, a phase I study demonstrated an ORR of 36% (37), and a phase II study with the same treatment an objective response rate (ORR) of 3% (38). Durvalumab administration in combination with rituximab and bendamustine was associated with an ORR of 88.9% in FL, and of 30% in DLBCL (39). Combination therapy with durvalumab and ibrutinib, a BKT inhibitor, was associated with an ORR in MCL (39).
Ansell et al. investigated the efficacy and safety of nivolumab in relapsed/refractory cHL and demonstrated an overall response rate of 87% and a progression-free survival of 86% at 24 weeks (33). In a further study, the combination of nivolumab with doxorubicin, vinblastine, and dacarbazine (AVD) was evaluated in patients with stage III and IV cHL (40). This study again showed that with the use of nivolumab in the frontline setting an objective response rate of 84% with complete remission in 67% of patients was obtained (40). Another trial evaluated nivolumab-AVD versus brentuximab-AVD in the frontline setting in patients 12 years and older who had stage III-IV disease, demonstrating an increase in progression-free survival with nivolumab-AVD group compared to the brentuximab-AVD group (41). Pembrolizumab, a human immunoglobulin G4 monoclonal antibody that blocks the PD-1/PD-L1 and PD-2/PD-L2 pathway, showed positive survival outcomes for patients with refractory/relapsed cHL (42). A further work compared pembrolizumab and brentuximab vedotin in patients with refractory/relapsed cHL, demonstrating that the two-year progression-free survival and safety outcomes favored the use of pembrolizumab (43). Atezolizumab is a humanized immunoglobulin G1 monoclonal antibody that targets PD-L1 and has previously shown antitumor activity in several tumor types. Younes et al. (44) evaluated the safety and efficacy of atezolizumab in combination with R-CHOP in patients with previously untreated DLBCL and demonstrated that the combination improves complete remission rates compared with controls. Tislelizumab, a humanized anti-PD-1 monoclonal antibody, blocks PD-1 with a high specificity and affinity and is another a promising treatment option in cHL. Song et al. (45) showed that tislelizumab demonstrated a favorable safety profile for patients with relapsed/refractory cHL. Otherwise, a favorable clinical activity has been reported when a PD-1 inhibitor was co-administrated with an anti-CD20 monoclonal antibodies (46).
Low efficacy of ICIs monotherapy in most lymphomas includes defects in antigen presentation, non-inflamed tumor microenvironment, immunosuppressive metabolites, and genetic factors. Clinical trials have investigated the efficacy of ICIs in combined-modal strategies with contrasting results. For example, dual checkpoint blockade with anti-PD-1 and CTLA-4 monoclonal antibodies, with or without STAT3 inhibitors, did not show promising clinical activity in DLBCL and FL (47). Major et al. (48) performed a large, retrospective, multicenter study across 15 US cancer centers of patients with aggressive B-cell lymphomas relapsing after or refractory to chimeric antigen receptor (CAR)-T and subsequently received ICI therapy. The results of this study showed that poor outcomes, particularly among patients relapsing early after CAR-T. The Authors concluded that ICI therapy is not an effective salvage strategy for most patients after CAR-T.
5 Concluding remarks
The conventional therapies for human lymphomas are chemotherapy, radiotherapy. More recently, immunotherapy has been successfully proposed as a new therapeutic approach for the treatment of these tumors. The tumor microenvironment allows the discovery of targeted therapies and provides data to improve the prediction of tumor progression. PD-1/PD-L1 controls excessive immunity of cytotoxic T cells leading to the failure of T cell immunity. Tumor cells express PD-L1, and as an effect of PD-1/PD-L1 pathway inhibition, T cells become active and exert more pronounced antitumor effects by rescuing exhausted T cells. Antibodies blocking the interaction PD-1/PD-L1 restore the T cell-mediated antitumor immune response. In B-cell lymphoma, the PD-1/PD-L1 blockade therapy exerts positive effects promoting the formation of a “hot” immune-inflamed tumor microenvironment (49). Combination treatment with CAR-T and nivolumab, or PD-1 inhibition with concomitant radiotherapy can then be used (50).
Author contributions
DR: Writing – original draft, Writing – review & editing. GC: Data curation, Supervision, Writing – review & editing. RT: Supervision, Validation, Writing – review & editing. TA: Supervision, Validation, Writing – review & editing. GI: Supervision, Validation, Writing – review & editing. GS: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Associazione “Il Sorriso di Antonio,” Corato, Italy, and Associazione Italiana Contro le Leucemie, Linfomi e Mielomi (AIL), Bari, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
2. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. (2016) 16:275–87. doi: 10.1038/nrc.2016.36
3. Naboush A, Roman CAJ, Shapira I. Immune checkpoint inhibitors in Malignancies with mismatch repair deficiency: a review of the state of the current knowledge. J Investig Med Off Publ Am Fed Clin Res. (2017) 65:754–8. doi: 10.1136/jim-2016-000342
4. Reiss KA, Forde PM, Brahmer JR. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy. (2014) 6:459–75. doi: 10.2217/imt.14.9
5. Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. (2020) 11:309. doi: 10.1038/s41419-020-2511-3
6. Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. (2017) 9:eaak9679. doi: 10.1126/scitranslmed.aak9679
7. Liu X-D, Hoang A, Zhou L, Kalra S, Yetil A, Sun M, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res. (2015) 3:1017–29. doi: 10.1158/2326-6066.CIR-14-0244
8. Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. (2017) 9:eaak9670. doi: 10.1126/scitranslmed.aak9670
9. Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: A therapeutic challenge for immunotherapy. Front Immunol. (2019) 10:168. doi: 10.3389/fimmu.2019.00168
10. Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. (2014) 153:145–52. doi: 10.1016/j.clim.2014.04.010
11. El Halabi L, Adam J, Gravelle P, Marty V, Danu A, Lazarovici J, et al. Expression of the immune checkpoint regulators LAG-3 and TIM-3 in classical hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. (2021) 21:257–66. doi: 10.1016/j.clml.2020.11.009
12. Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. (2021) 23:39. doi: 10.1208/s12248-021-00574-0
13. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
14. Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol J Hematol Oncol. (2021) 14:156. doi: 10.1186/s13045-021-01164-5
15. Yang J, Hu G. Significance of PD-L1 in the diagnosis and treatment of B-cell Malignant lymphoma. Oncol Lett. (2019) 17:3382–6. doi: 10.3892/ol
16. Fereshteh Ameli F, Elham Shajareh E, Mokhtari M, Kosari F. Expression of PD1 and PDL1 as immune−checkpoint inhibitors in mantle cell lymphoma. BMC Cancer. (2022) 22:848. doi: 10.1186/s12885-022-09803-x
17. Menter T, Bodmer-Haecki A, Dirnhofer S, Tzankov A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. (2016) 54:17–24. doi: 10.1016/j.humpath.2016.03.005
18. Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MGM, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated Malignancies. Clin Cancer Res. (2013) 19:3462–73. doi: 10.1158/1078-0432.CCR-13-0855
19. Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik JH, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumor microenvironments of diffuse large B cell lymphomas. Histopathology. (2016) 68:1079–89. doi: 10.1111/his.12882
20. Song MK, Park BB, Uhm J. Understanding immune evasion and therapeutic targeting associated with PD-1/PD-L1 pathway in diffuse large B-cell lymphoma. Int J Mol Sci. (2019) 20:1326. doi: 10.3390/ijms20061326
21. Fang X, Xiu B, Yang Z, Qiu W, Zhang L, Zhang S, et al. The expression and clinical relevance of PD-1, PD-L1, and TP63 in patients with diffuse large B-cell lymphoma. Medicine. (2017) 96:e6398. doi: 10.1097/MD.0000000000006398
22. Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. (2015) 126:2193–201. doi: 10.1182/blood-2015-02-629600
23. Tomassetti S, Chen R, Dandapani S. The role of pembrolizumab in relapsed/refractory primary mediastinal large B-cell lymphoma. Ther Adv Hematol. (2019) 10:2040620719841591. doi: 10.1177/2040620719841591
24. Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. (2016) 127:869–81. doi: 10.1182/blood-2015-10-673236
25. Muenst S, Hoeller S, Willi N, Dirnhofera F, Tzankof A. Diagnostic and prognostic utility of PD-1 in B cell lymphomas. Dis Markers. (2010) 29:47–53. doi: 10.1155/2010/404069
26. Brusa D, Serra S, Coscia M, Rossi D, D'Arena G, Laurenti L, et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. (2013) 98:953–63. doi: 10.3324/haematol.2012.077537
27. Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. (2014) 15:69–77. doi: 10.1016/S1470-2045(13)70551-5
28. Myklebust JH, Irish JM, Brody J, Czerwinski DK, Houot R, Kohrt HE, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. (2013) 121:1367–76. doi: 10.1182/blood-2012-04-421826
29. Harrington BK, Wheeler E, Hornbuckle K, Smith L, Klamer B, Zhang X, et al. Modulation of immune checkpoint molecule expression in mantle cell lymphoma. HHS Public Access. (2019) 60:2498–507. doi: 10.1080/10428194.2019.1569231
30. Weniger MA, Kuppers R. Molecular biology of Hodgkin lymphoma. Leukemia. (2021) 35:968–81. doi: 10.1038/s41375-021-01204-6
31. Roemer MGM, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. (2016) 34:2690–7. doi: 10.1200/JCO.2016.66.4482
32. Green MR, Rodig S, Juszczynski P, Ouyang J, Donnell EO, Neuberg D, et al. Constitutive AP-1 activity and EBVInfection induce PD-L1 in hodgkin lymphomas and post-transplant lymphoproliferative disorders: Implications for targeted therapy. Clin Cancer Res. (2012) 18:1611–8. doi: 10.1158/1078-0432.CCR-11-1942
33. Ansell SM, Lesokhin AM, Borrello I, Alwani A, Scott EC, Gutierrezz M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. (2015) 372:311–9. doi: 10.1056/NEJMoa1411087
34. Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. (2017) 130:2420–30. doi: 10.1182/blood-2017-03-770719
35. Sokołowski M, Sokołowska A, Mazur G, Butrym A. Programmed cell death protein receptor and ligands in haematological Malignancies–Current status. Crit Rev Oncol Hematol. (2019) 135:47–58. doi: 10.1016/j.critrevonc.2019.01.003
36. Xu-Monette Z, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. (2018) 131:68–83. doi: 10.1182/blood-2017-07-740993
37. Lesokhin AM, Ansell SM, Armand P, Scott EC, Alwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic Malignancy: Preliminary results of a phase ib study. J Clin Oncol. (2016) 34:2698–704. doi: 10.1200/JCO.2015.65.9789
38. Ansell SM, Minnema MC, Johnson P, Timmermann JM, Armand P, Shipp MA, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. J Clin Oncol. (2019) 37:481–9. doi: 10.1200/JCO.18.00766
39. Armengol M, Santos JC, Fernandez-Serrano M, Profitos-Peleja N, Ribeiro ML, Roue G. Immune-checkpoint inhibitors in B-cell lymphoma. Cancers. (2021) 13:214. doi: 10.3390/cancers13020214
40. Ramchandren R, Domingo-Domenech E, Rueda A, Trneny M, Feldman TA, Lee HJ, et al. Nivolumab for newly diagnosed advanced-stage classic hodgkin lymphoma: safety and efficacy in the phase II CheckMate 205 study. J Clin Oncol. (2019) 37:1997–2007. doi: 10.1200/JCO.19.00315
41. Herrera A, Castellino SM, Li H, Rutherford SC, Evens AM, Davison K, et al. SWOG S1826, a randomized study of nivolumab(N)-AVD versus brentuximab vedotin(BV)-AVD in advanced stage (AS) classic Hodgkin lymphoma (HL). J Clin Oncol. (2023) 41(suppl 17; abstr LBA4). doi: 10.1200/JCO.2023.41.17_suppl.LBA4
42. Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE- 087. Blood. (2019) 134:1144–53. doi: 10.1182/blood.2019000324
43. Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicenter, randomised, open-label, phase 3 study. Lancet Oncol. (2021) 22:512–24. doi: 10.1016/S1470-2045(21)00005-X
44. Younes A, Burke JM, Cheson BD, Diefenbach CS, Ferrari S, Hahn UH, et al. Safety and efficacy of atezolizumab with rituximab and CHOP in previously untreated diffuse large B-cell lymphoma. Blood Adv. (2023) 7:1488–95. doi: 10.1182/bloodadvances.2022008344
45. Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, et al. Tislelizumab for relapsed/refractory Classical Hodgkin Lymphoma: 3-year follow-up and correlative biomarker analysis. Clin Cancer Res. (2022) 28:1147–56. doi: 10.1158/1078-0432.CCR-21-2023
46. Tuscano JM, Maverakis E, Groshen S, Tsao-Wei D, Luxardi G, Merleev AA, et al. A phase I study of the combination of rituximab and ipilimumab in patients with relapsed/refractory B-cell lymphoma. Clin Cancer Res. (2019) 25:7004–13. doi: 10.1158/1078-0432.CCR-19-0438
47. Ansell S, Gutierrez ME, Shipp MA, Gladstone D, Moskowitz A, Borello I, et al. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic Malignancies (CheckMate 039). Blood. (2016) 128:183. doi: 10.1182/blood.V128.22.183.183
48. Major A, Yu J, Shukla N, Che Y, Karrison TG, Treitman R, et al. Efficacy of checkpoint inhibition after CAR-T failure in aggressive B-cell lymphomas: outcomes from 15 US institution. Blood Adv. (2023) 7:4528–38. doi: 10.1182/bloodadvances.2023010016
49. Chu Y, Zhou X, Wang X. Antibody-drug conjugates for the treatment of lymphoma: Clinical advances and latest progress. J Hematol Oncol. (2021) 14:88. doi: 10.1186/s13045-021-01097-z
Keywords: Hodgkin lymphoma, immune checkpoint inhibitors, non-Hodgkin lymphoma, therapy, PD-1/PD-L1
Citation: Ribatti D, Cazzato G, Tamma R, Annese T, Ingravallo G and Specchia G (2024) Immune checkpoint inhibitors targeting PD-1/PD-L1 in the treatment of human lymphomas. Front. Oncol. 14:1420920. doi: 10.3389/fonc.2024.1420920
Received: 21 April 2024; Accepted: 05 July 2024;
Published: 18 July 2024.
Edited by:
Roberto Crocchiolo, Niguarda Ca’ Granda Hospital, ItalyReviewed by:
Li Qinlu, Huazhong University of Science and Technology, ChinaCopyright © 2024 Ribatti, Cazzato, Tamma, Annese, Ingravallo and Specchia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico Ribatti, domenico.ribatti@uniba.it
 Domenico Ribatti
Domenico Ribatti Gerardo Cazzato
Gerardo Cazzato Roberto Tamma
Roberto Tamma Tiziana Annese1,3
Tiziana Annese1,3 Giuseppe Ingravallo
Giuseppe Ingravallo