- Radiology Department, Jingzhou Hospital Affiliated to Yangtze University, Jingzhou, Hubei, China
Background: There remains a pressing need to identify biomarkers capable of reliably predicting prognostic outcomes for colorectal cancer (CRC) patients. As several body composition parameters have recently been reported to exhibit varying levels of prognostic significance in particular cancers, the present study was devised to assess the ability of body composition to predict long-term outcomes for CRC patients with different stages of disease.
Methods: In total, this retrospective analysis enrolled 327 stage I-III CRC patients whose medical records were accessed for baseline demographic and clinical data. Primary outcomes for these patients included disease-free and overall survival (DFS and OS). The prognostic performance of different musculature, visceral, and subcutaneous fat measurements from preoperative computed tomography (CT) scans was assessed.
Results: Over the course of follow-up, 93 of the enrolled patients experienced recurrent disease and 39 died. Through multivariate Cox regression analyses, the visceral/subcutaneous fat area (V/S) ratio was found to be independently associated with patient DFS (HR=1.93, 95% CI: 1.24–3.01, P=0.004), and the skeletal muscle index (SMI) as an independent predictor for OS (HR=0.43, 95% CI: 0.21–0.89, P=0.023). Through subgroup analyses, higher V/S ratios were found to be correlated with reduced DFS among patients with stage T3/4 (P=0.011), lymph node metastasis-positive (P=0.002), and TNM stage III (P=0.002) disease, whereas a higher SMI was associated with better OS in all T stages (P=0.034, P=0.015), lymph node metastasis-positive cases (P=0.020), and in patients with TNM stage III disease (P=0.020).
Conclusion: Both the V/S ratio and SMI offer potential utility as clinical biomarkers associated with long-term CRC patient prognosis. A higher V/S ratio and a lower SMI are closely related to poorer outcomes in patients with more advanced disease.
1 Introduction
Colorectal cancer (CRC) is characterized by high, steadily rising rates of global incidence and mortality (1, 2), yet biomarkers capable of predicting the long-term outcomes of affected patients are still lacking. Quantitative measurements of skeletal muscle mass and adipose tissue distributions have recently been demonstrated to offer significant prognostic utility in CRC and many other forms of cancer, leading to a growing body of research focused on how body composition relates to oncological outcomes.
Chronic inflammation is a major driver of carcinogenesis (3). Sarcopenia is a condition defined by declines in the function and mass of skeletal muscle. Cancer-associated muscle loss primarily results from systemic inflammatory responses induced by host-tumor interactions (4), as inflammatory cytokines derived from tumor cells such as IL-6 and TNF-α can impair the differentiation of skeletal muscle cells (5), interfere with insulin signaling, and thereby drive more severe insulin resistance and the degradation of muscle tissue (6). Insulin resistance can be further exacerbated by sarcopenia as well (7, 8), and the low-grade tumor-induced inflammatory activity can provoke inflammatory activity within the muscles, contributing to systemic inflammatory activity and additional muscle degradation (9). Adipose tissue serves as an endocrine organ that is vital for the control of metabolic and energy homeostasis such that when it accumulates at excessively high levels, this can trigger the release of inflammatory cytokines and adipokines related to CRC progression (10). Given its proximity to nearby organs, visceral adipose tissue can transfer lipids to these nearby tissues, leading to altered metabolic activity that can be conducive to oncogenic growth (10, 11).
Abdominopelvic computed tomography (CT) is an imaging strategy that is routinely employed for the management and initial staging of patients with CRC, and it can provide accurate information regarding muscle mass and the presence of adipose tissue (12). Quantitative measures of adipose tissue distribution and skeletal muscle mass have both been linked to poor prognostic outcomes in patients with CRC (7, 8, 13–24). Efforts to evaluate and improve body composition either before tumor development or after disease onset may provide opportunities for novel therapeutic interventions.
Both exercise (25) and nutritional support (26, 27) have previously been demonstrated to have positive effects on body composition and other physiological changes that may protect against the development of CRC. While there have been some prior efforts to examine the relevance of body composition to CRC patient outcomes, the results of these studies have been inconsistent and relatively little is known with respect to how these parameters vary as a function of tumor staging. The present study was conducted based on the hypothesis that body composition measurements can provide valuable insights into prognostic outcomes across different stages of CRC, particularly in patients with more advanced disease. The specific goal of these analyses was to probe the relationship between a range of body composition parameters and both overall and disease-free survival (OS and DFS) in CRC patients with stage I-III disease, validating these analyses in different tumor stages.
2 Materials and methods
2.1 Study population
This was a retrospective analysis of patients with stage I-III CRC who underwent surgical treatment in the Colorectal Surgery Department of Jingzhou Hospital affiliated with Yangtze University between January 2017 and December 2021 (Figure 1A). To be eligible for inclusion, patients needed to (1) have a pathological diagnosis of primary CRC; (2) have undergone an abdominal CT scan within a 30-day period prior to surgery; and (3) have a complete set of clinical and follow-up data. Patients were excluded if they (1) had been diagnosed with other malignancies; (2) had preoperatively undergone chemotherapy or radiotherapy; (3) exhibited any preoperative metastases; (4) were missing any clinicopathological or follow-up data; (5) had undergone palliative surgery. Tumor staging was assessed by pathologists as per the 8th edition AJCC TNM staging guidelines.
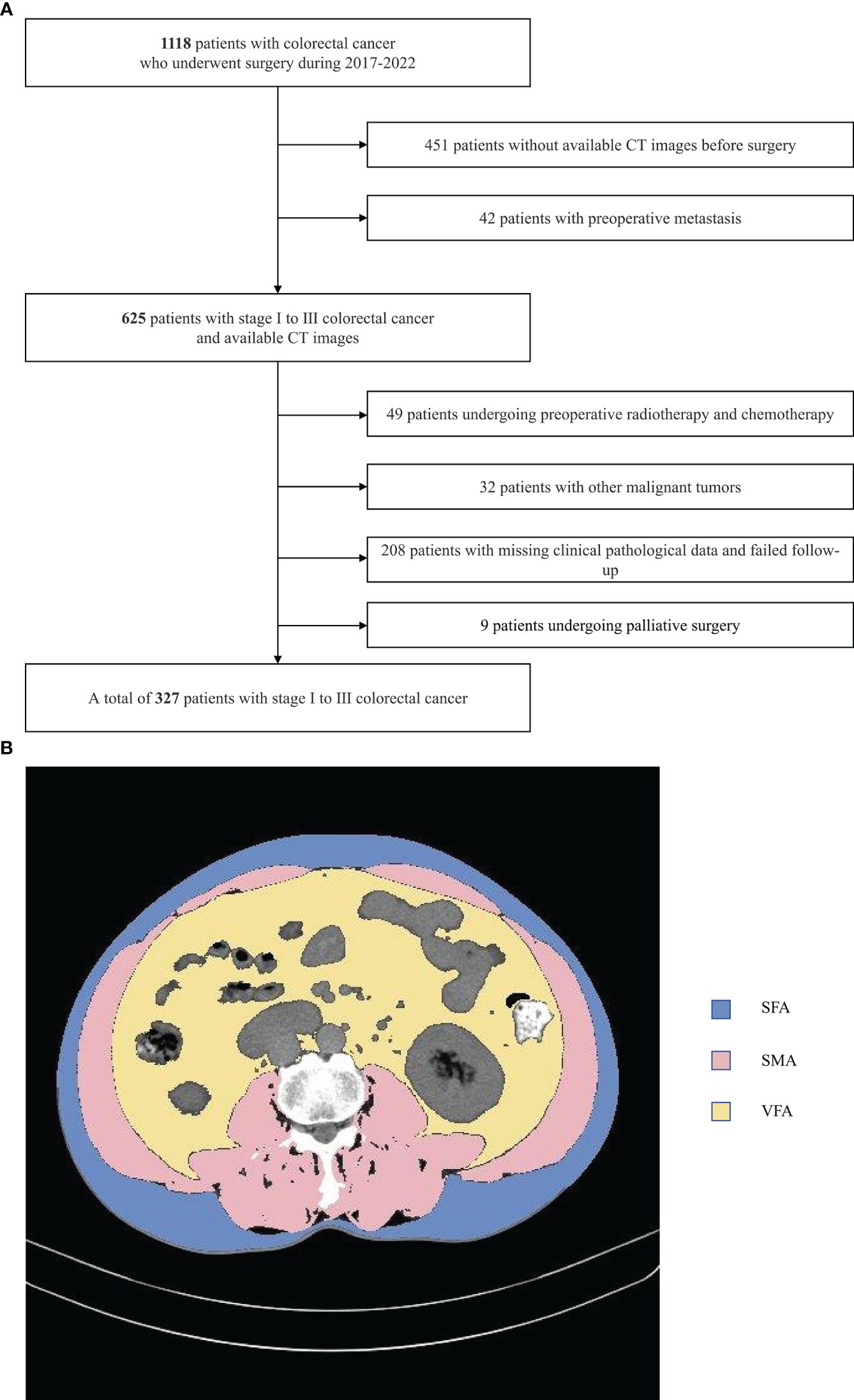
Figure 1 Study flow chart (A) and CT-based measures of body composition (B) subcutaneous fat area (SFA),visceral fat area (VFA) and skeletal muscle area(SMA).
The studies involving human participants were reviewed and approved by [Ethics Committee of Jingzhou Hospital affiliated with Yangtze University]. As this study was retrospective in nature, the need for informed consent was waived.
2.2 Clinical data
Medical records were accessed to obtain patient clinical and demographic characteristics including age, gender, BMI, American Society of Anesthesiologists (ASA) classification, diabetes, hypertension, surgical approach, and postoperative chemotherapy. In addition, preoperative blood test results for serum CEA and CA199 levels were also analyzed, as were clinicopathological features such as tumor location, tumor differentiation, pathological tumor type, and tumor T stage, N stage, and TNM staging.
2.3 Clinical outcomes
Primary endpoints for this study were OS and DFS. OS was measured from tumor resection to death or most recent follow-up, while DFS was measured from tumor resection to metastasis, recurrence, death, or most recent follow-up. Follow-up was primarily conducted through surveys administered in an outpatient setting or phone-based contact with patients.
2.4 Body composition measurements
ImageJ v1.52 (NIH, USA) was used to analyze all images, conducting semi-automated tissue segmentation with the following HU thresholds: adipose tissue (-190 to -30), skeletal muscle (-29 to 150) (Figure 1B). This approach allowed for the measurement of skeletal muscle, visceral fat, and subcutaneous fat area values (in cm²), together with the calculation of the visceral to subcutaneous fat area (V/S) ratio. The measured area values were normalized to patient height (m²), enabling the calculation of the subcutaneous fat index (SFI), visceral fat index (VFI), and skeletal muscle index (SMI) values (in cm²/m²). The average radiodensity values for subcutaneous fat (SFD), visceral fat (VFD), and skeletal muscle (SMD) were measured using equally sized regions of interest.
2.5 Statistical analysis
Given the lack of standardized cutoff values for various body composition metrics, particularly in Asian populations, we derived the cutoff values used in this study from the median values of our sample data to divide patients into high and low groups. A radiologist blinded to patient characteristics conducted all measurements.
Continuous data are reported as means ± SD or medians and interquartile ranges (IQRs), while categorical variables are presented as numbers and percentages. Continuous data were compared with Mann-Whitney U-tests or Student’s t-tests, while categorical variables were compared using Fisher’s exact test or chi-square tests. Nonlinear associations between body composition and patient survival were assessed with restricted cubic spline models. Variables related to the duration of patient survival were identified through univariate and multivariate analyses, with those variables that exhibited a P < 0.1 being incorporated into the multivariate analysis, calculating hazard ratios (HRs) with 95% confidence intervals (CIs). ROC curves were used for model evaluation, while survival analyses for the overall patient cohort and particular subgroups were performed with Kaplan-Meier curves and log-rank tests. Data were analyzed in R 4.3.0 (2023–04-21) and Zstats (www.medsta.cn/software). P<0.05 served as the cutoff for significance.
3 Results
3.1 Baseline characteristics
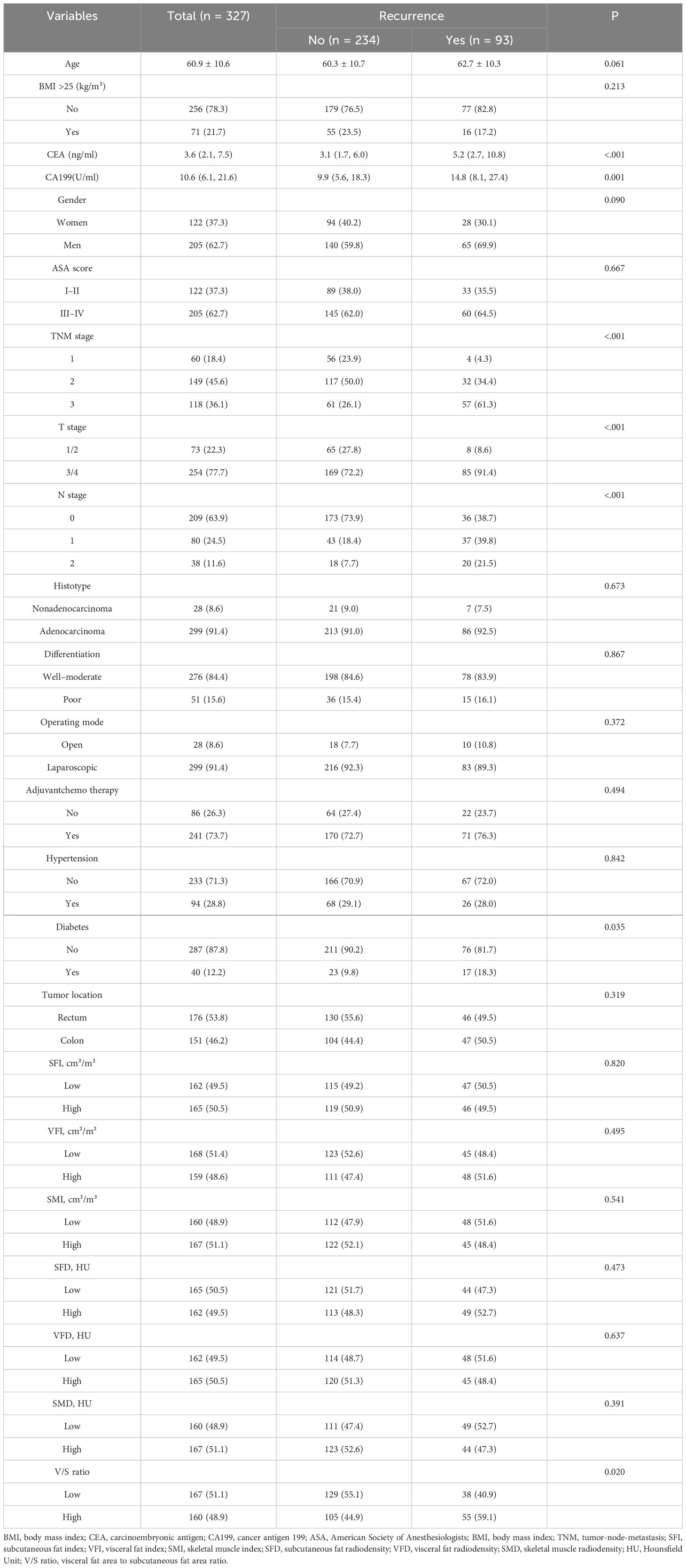
In total, 1118 patients were evaluated for study inclusion, of whom 327 met with the criteria for enrollment in these analyses. These 327 patients exhibited an average age of 60.9 ± 10.6 years, 62.7% were male, and the median duration of follow-up was 48.7 (40.2–60.2) months. Over the course of follow-up, 93 of these 327 patients developed recurrent disease at a median recurrence time of 45.1 (36.9–59.1) months, while 39 died with a median of 30.0 (21.0–40.0) months to death. CA199 and CEA levels in the recurrence group were significantly higher than those for patients who did not experience recurrent disease (P<0.001, P=0.001). The distributions of diabetes (P=0.035), T stage (P<0.001), N stage (P<0.001), and TNM stage (P<0.001) differed significantly between patients who did and did not develop recurrent CRC. Of the analyzed body composition parameters, only the V/S ratio differed significantly between these two groups of patients (P=0.020). For further details, see Table 1.
3.2 Correlation between BMI and body composition
Among the fat-related indicators, BMI was found to be correlated with VFA and SFA. Specifically, BMI and VFA showed a positive correlation (rs=0.666, P < 0.001) (Supplementary Figure 1A), while BMI and SFA exhibited a moderate positive correlation (rs=0.539,P < 0.001) (Supplementary Figure 1B). In contrast, no correlation was found between BMI and the V/S ratio (rs=0.237, P < 0.001) (Supplementary Figure 1C). Furthermore, SMI and the V/S ratio showed a weak positive correlation (rs=0.331, P < 0.001) (Supplementary Figure 1D).
3.3 Testing for non-linear associations between body composition parameters and long-term outcomes
Using restricted cubic spline (RCS) curve analyses, body composition parameters were found to be linearly associated with the OS and DFS of CRC patients. In particular, an increase in SMD was associated with corresponding reductions in the DFS hazard ratio (Figure 2B), whereas as the V/S ratio rose, a linear increase in the hazard ratio for DFS was observed (Figure 2D).
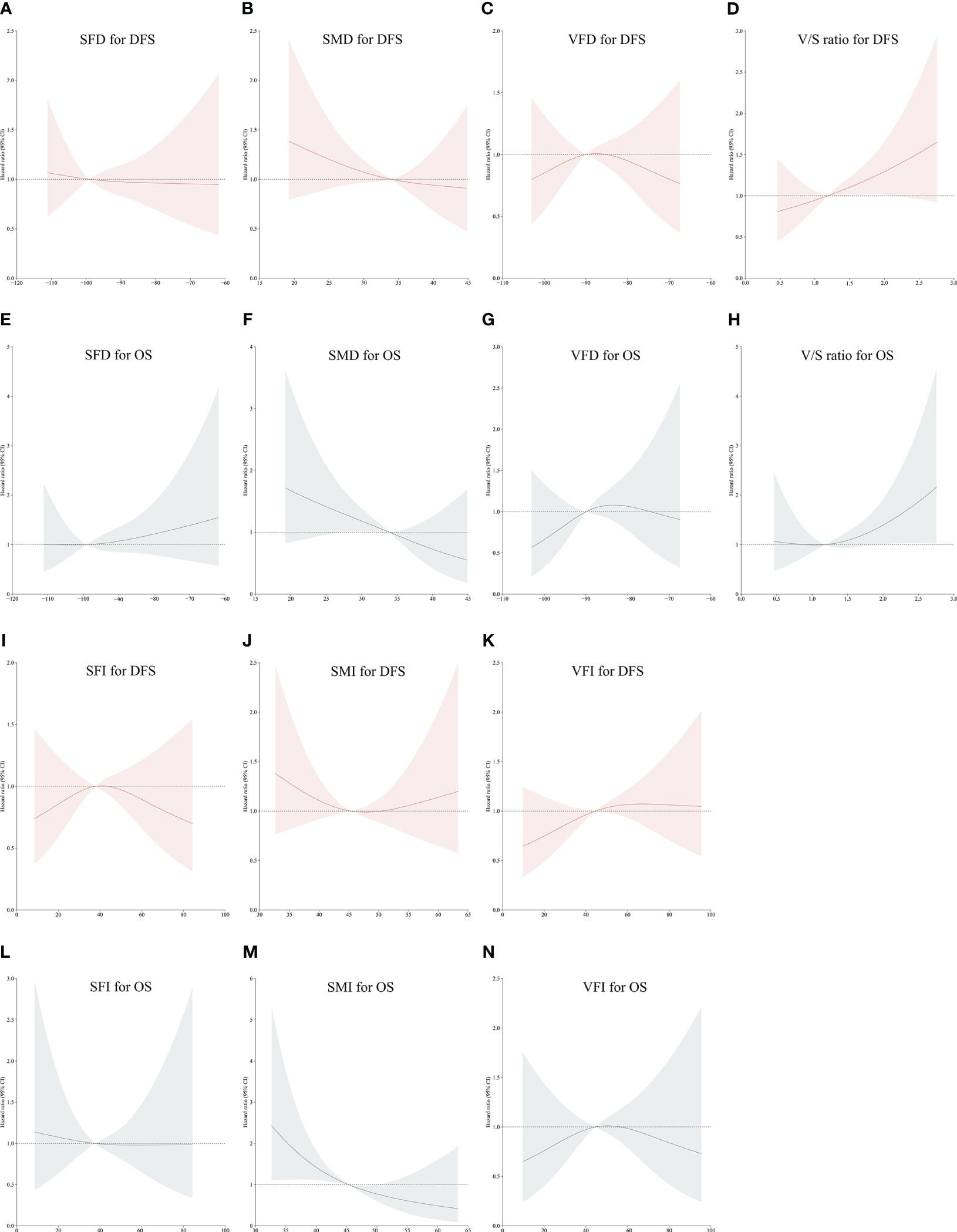
Figure 2 Restricted Cubic Spline curves corresponding to disease-free or overall survival (DFS or OS) based on patient SFD (A, E), SMD (B, F), VFD (C, G), V/S ratio values (D, H), SFI (I, L), SMI (J, M) and VFI (K, N). Red portions of the curves correspond to DFS, while gray portions correspond to OS.
A linear decrease in the hazard ratio for OS was observed with increasing SMI (Figure 2M) and SMD (Figure 2F). A linear increase in the hazard ratio for OS was also observed with increasing V/S ratio values (Figure 2H). However, no significant trends were found in other body compositions concerning the long-term prognosis of colorectal cancer (Figures 2A, C, E, G, I–L, N).
3.4 The relationship between body composition parameters and prognostic outcomes in the overall patient cohort
To clarify which body composition parameters were significantly related to certain clinical outcomes, univariate and multivariate Cox regression analyses of OS and DFS were conducted (Table 2). Of the 327 patients with stage I-III disease in this study, recurrence affected 93 (28.4%), with a median time to recurrence of 29.9 (21.0–39.5) months. In multivariate analyses, the V/S ratio was identified as the only measure of body composition that was independently associated with patient DFS. Specifically, the risk of recurrent disease was higher among patients with higher V/S ratios as compared to patients with lower V/S ratios (HR=1.93, 95% CI: 1.24–3.01, P=0.004). Kaplan-Meier curves confirmed that the DFS of patients with a high V/S ratio was significantly reduced as compared to patients exhibiting lower V/S ratio values (44.9 (30.4–58.0) vs 45.4 (38.8–59.4), P=0.022) (Figure 3A). ROC curves additionally suggested the potential for the utility of the V/S ratio as a supplementary component of the overall model, providing for superior predictive accuracy (Figure 3C).
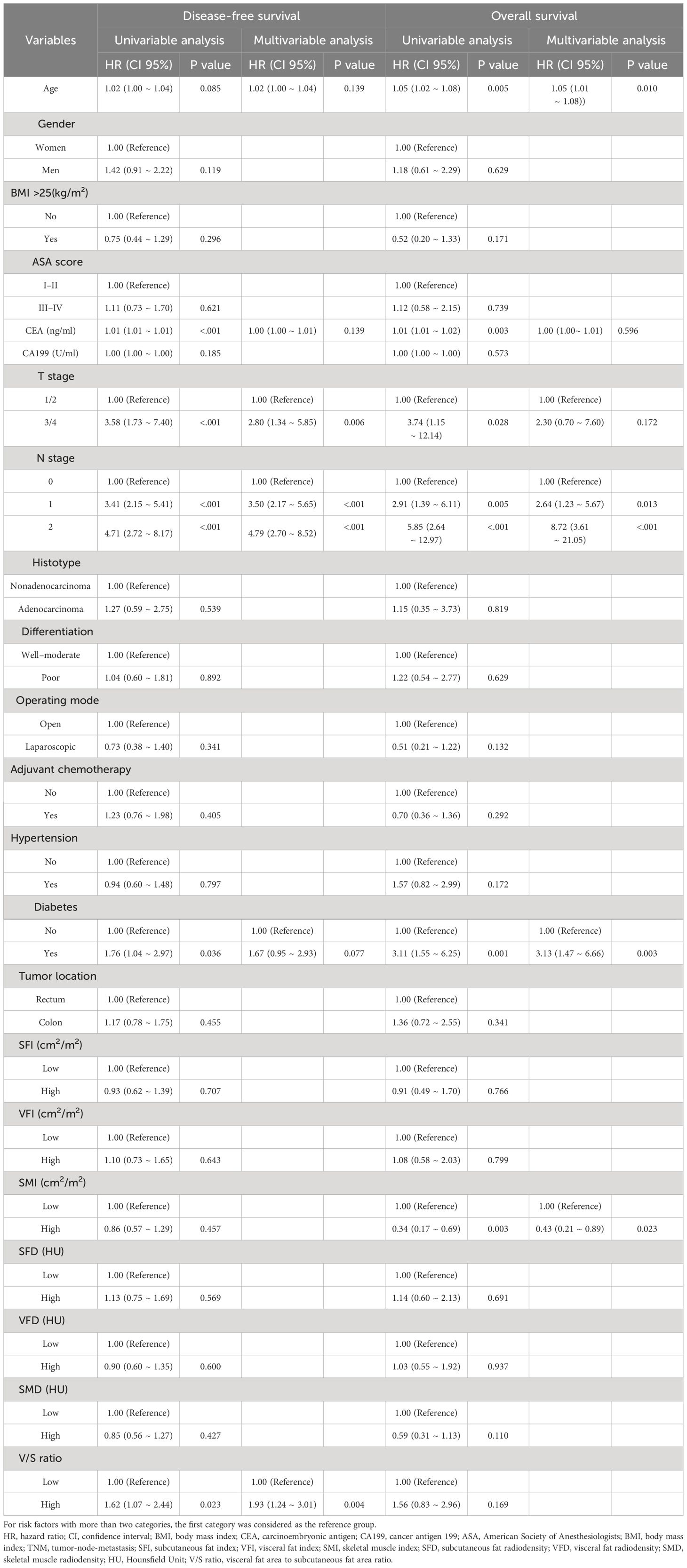
Table 2 Univariable and multivariable Cox regression analyses for disease-free survival and overall survival.
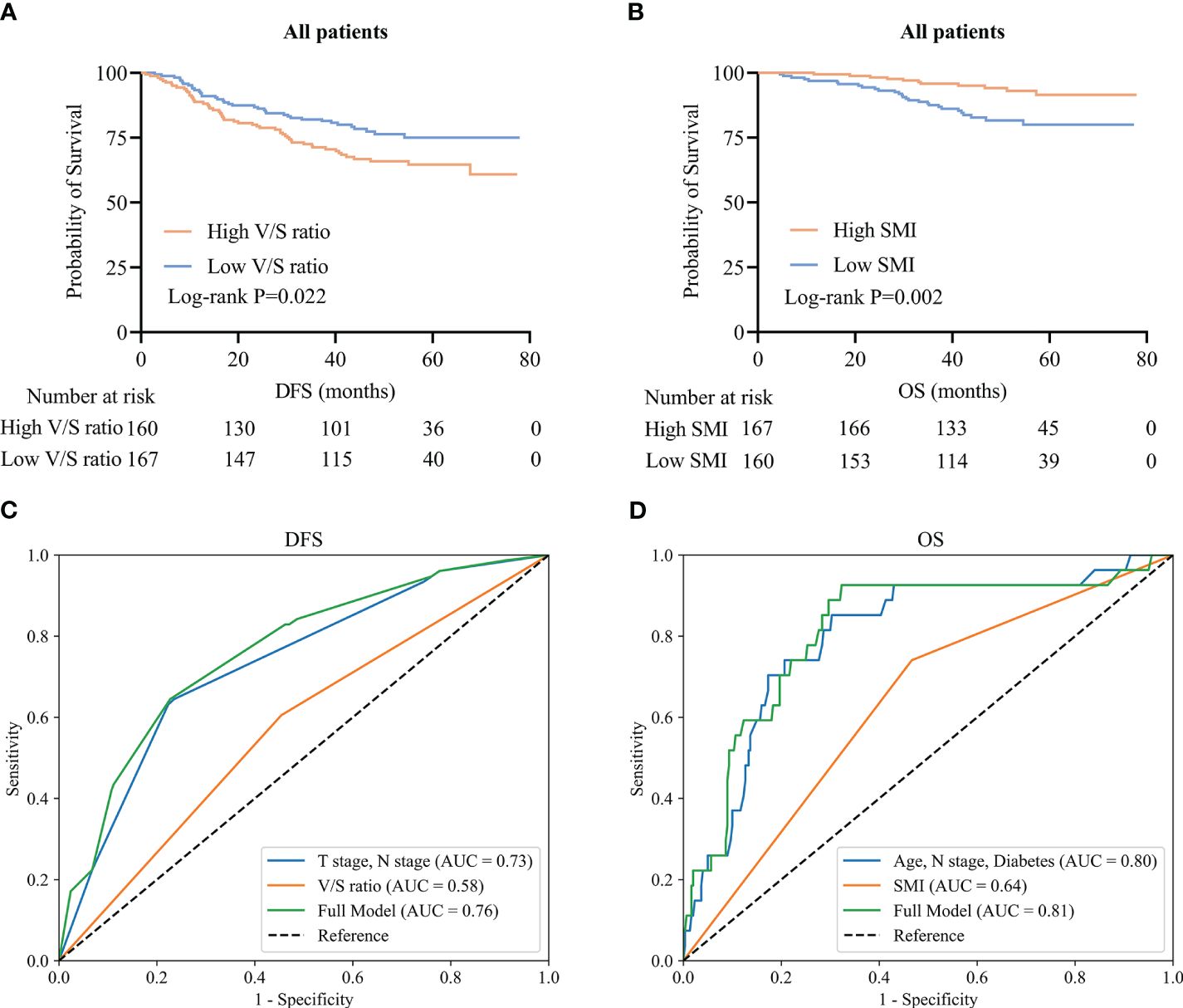
Figure 3 CRC patient survival and time-dependent ROC curves. (A, B) Kaplan-Meier DFS (A) and OS (B) curves for the overall CRC patient population. (C, D) Time-dependent ROC curves for patient DFS (C) and OS (D).
Of the included patients, 39 had died as of the follow-up cut-off with a median of 30.0 (21.0–40.0) months to death. Following adjustment for clinical factors, SMI was identified as an independent predictor of OS (HR=0.43, 95% CI: 0.21–0.89, P=0.023). Kaplan-Meier curves indicated that patients with low SMI values tended to exhibit shorter OS than patients with high SMI values (30.0 (20.0–37.0) vs 33.0 (25.0–44.0); P=0.002) (Figure 3B). ROC curves indicated that the incorporation of SMI into comprehensive clinical analyses provided improved model accuracy when seeking to predict OS (Figure 3D).
3.5 The relationship between body composition parameters and prognostic outcomes across T staging subgroups
Among patients with T1/2 disease, DFS did not differ significantly as a function of V/S ratio (P=0.321) (Figure 4A), whereas among patients with more advanced T staging (T3/4), a high V/S ratio was associated with significantly worse DFS as compared to that of individuals with a low V/S ratio (41.5 (24.4–57.3) vs 44.4 (38.2–59.4), P=0.011) (Figure 4C). Irrespective of T staging (T1/2 or T3/4), patients with a high SMI presented with significantly better OS as compared to that of patients with a low SMI (P=0.034; 33.0 (25.0–44.0) vs 30.0 (17.0–37.0), P=0.015) (Figures 4B, D).
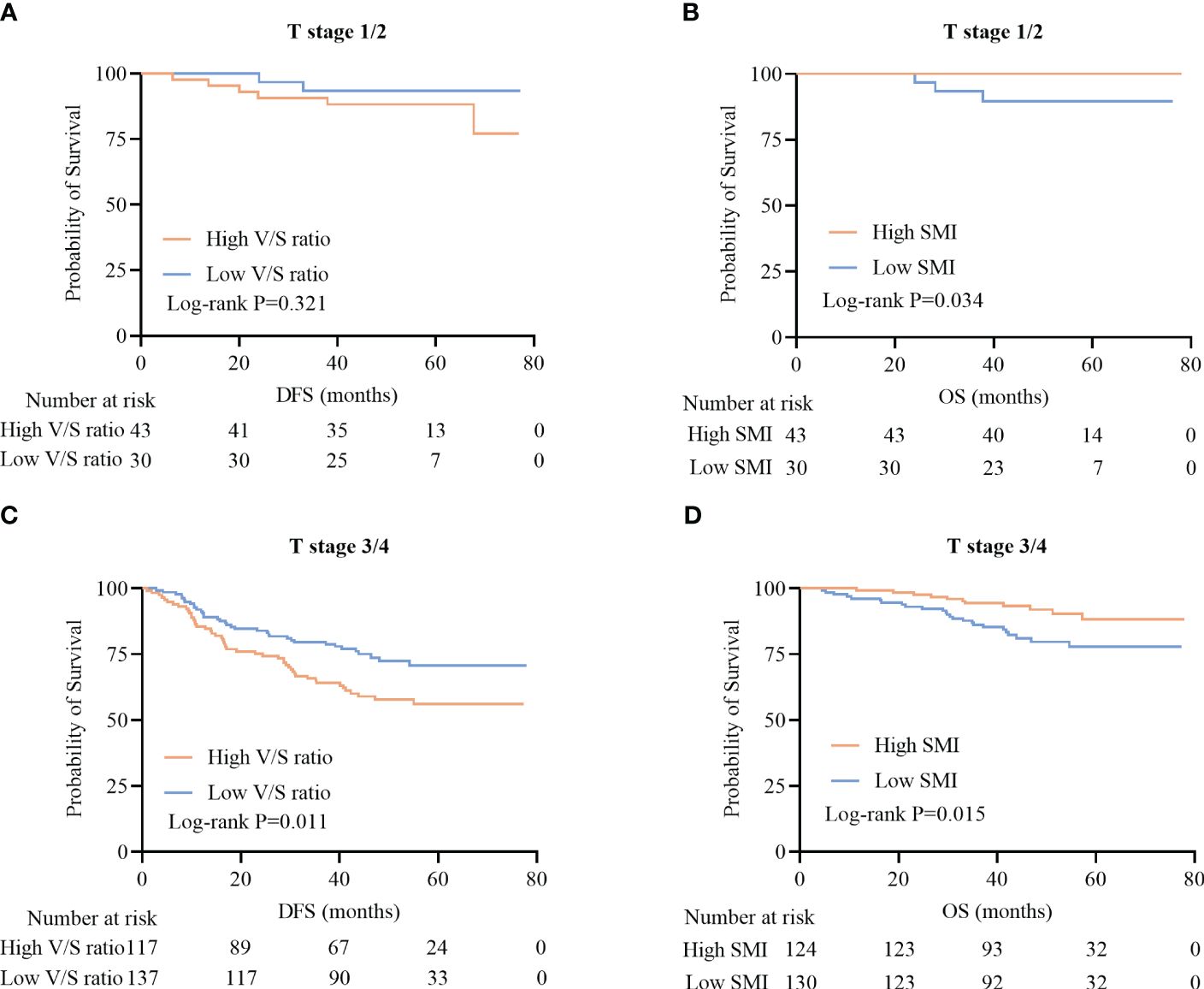
Figure 4 Survival curves for CRC patients stratified according to T stage. (A, B) Kaplan-Meier DFS (A) and OS (B) curves for patients with stage T1/2 disease. (C, D) Kaplan-Meier DFS (C) and OS (D) curves for patients with stage T3/4 disease.
3.6 The relationship between body composition parameters and prognostic outcomes across N staging subgroups
Among patients positive for lymph node metastasis (LNM), high V/S ratio values were associated with significantly worse DFS as compared to that of patients with low V/S ratio values (28.7 (13.3–41.7) vs 41.3 (25.6–49.9), P=0.002) (Figure 5C). LNM-positive patients with lower SMI values presented with significantly worse OS as compared to patients with higher SMI values (31.0 (23.0–36.0) vs 32.0 (22.0–40.0), P=0.020) (Figure 5D). In contrast, DFS and OS did not differ significantly between LNM-negative patients based on these body composition parameters (P=0.142, P=0.118) (Figures 5A, B).
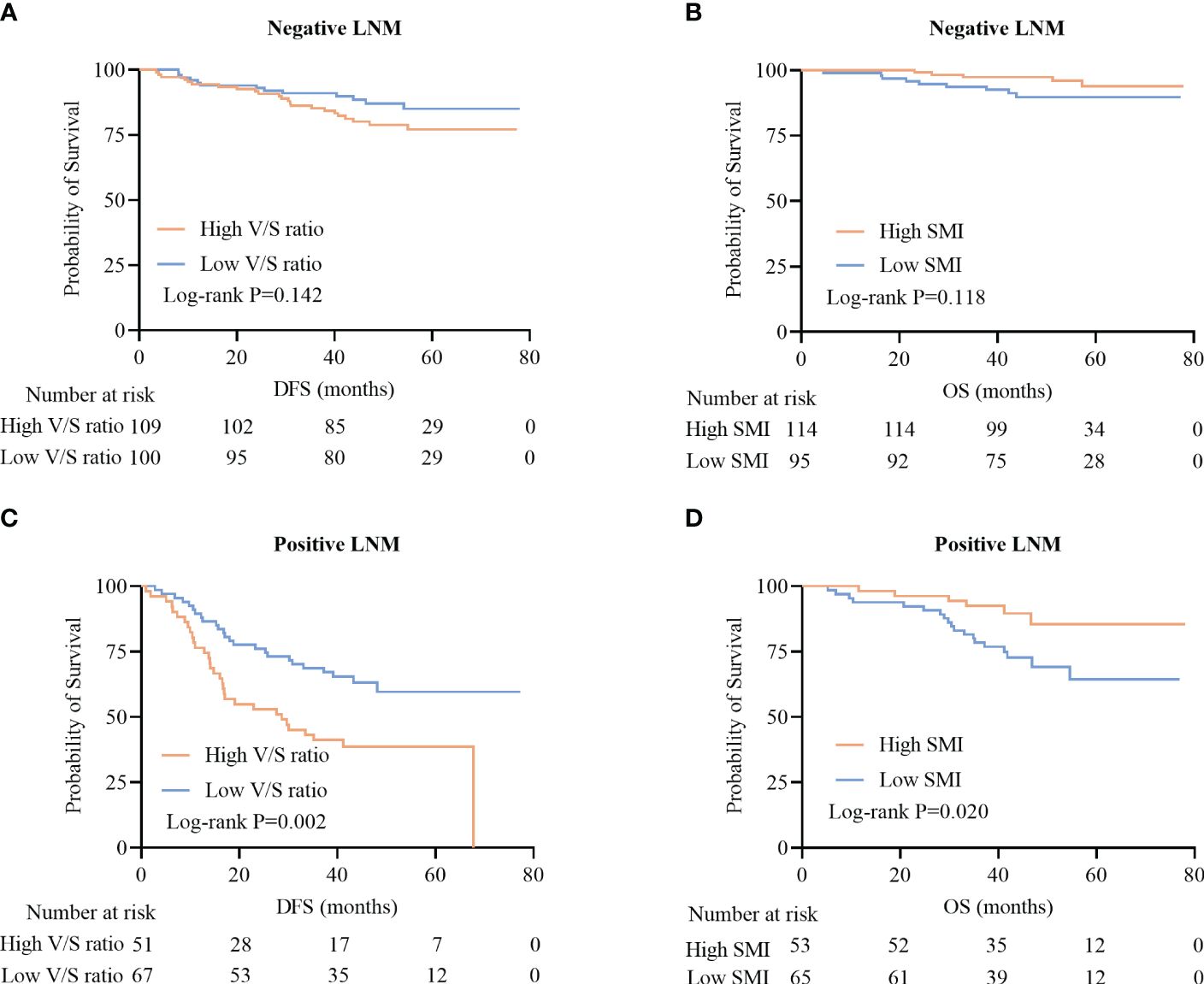
Figure 5 Survival curves for CRC patients stratified according to lymph node metastasis (LNM). (A, B) Kaplan-Meier DFS curves for LNM-negative patients (A), OS curves for LNM-negative patients (B), (C, D) DFS curves for LNM-positive patients (C), and OS curves for LNM-positive patients (D).
3.7 The relationship between body composition parameters and prognostic outcomes across TNM-stage subgroups
When patients were analyzed according to their TNM staging, no significant differences in the DFS of patients with TNM stage I and II disease were observed when comparing individuals with high and low V/S ratio values (P=0.482 and P=0.137) (Figures 6A, C). However, among TNM stage III patients, individuals with high V/S ratios exhibited a significant reduction in DFS relative to those with low V/S ratios (28.7 (13.3–41.7) vs 41.3 (25.6–49.9), P=0.002) (Figure 6E).
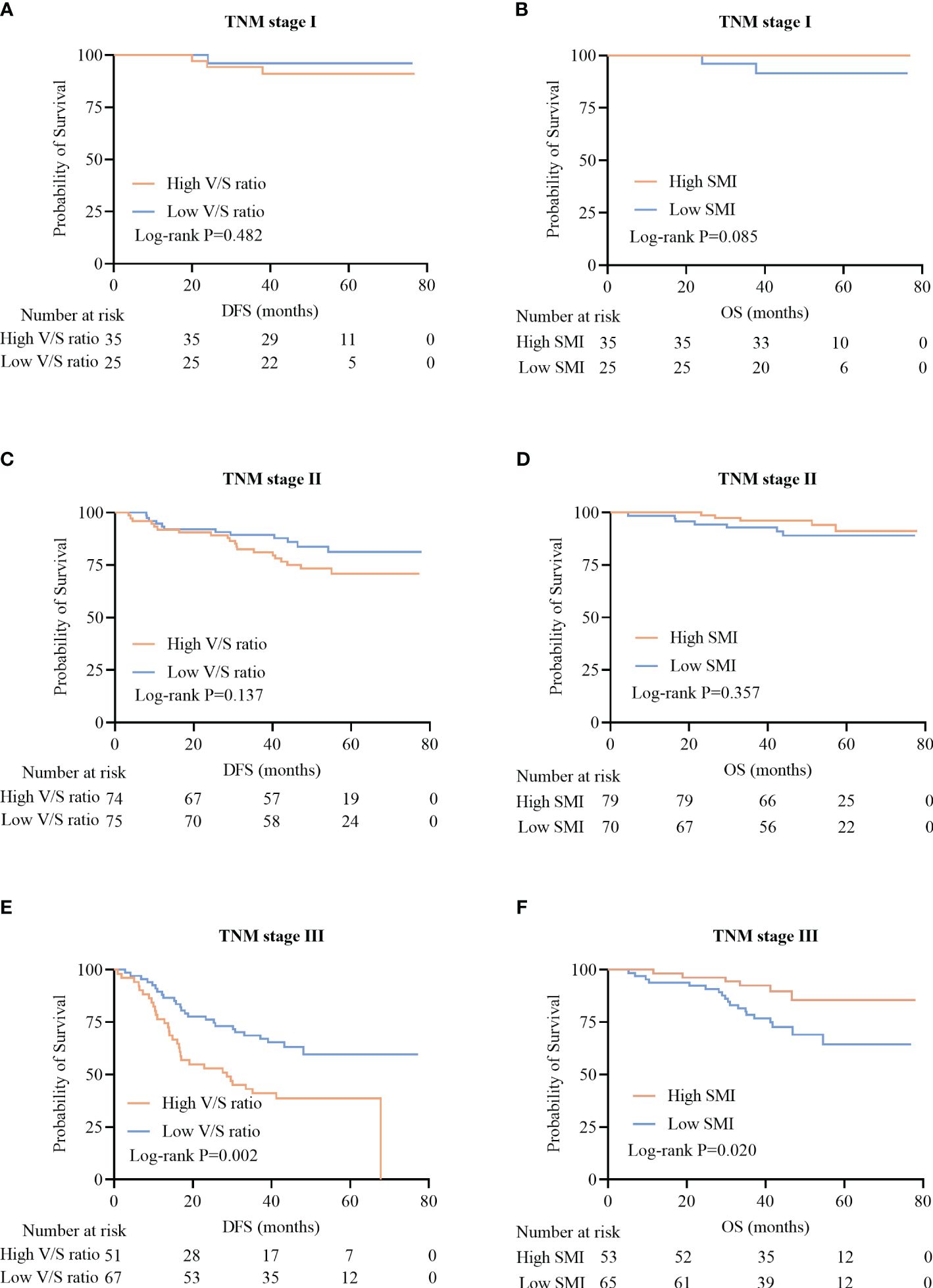
Figure 6 Kaplan-Meier survival curves for CRC patients with different TNM stages of disease. (A-F) Kaplan-Meier DFS (A, C, E) and OS (B, D, F) curves for patients with TNM stage I (A, B), stage II (C, D), and stage III (E, F) disease.
In line with these results, no significant relationship between SMI and OS was observed among patients with TNM stage I and II disease (P=0.085, P=0.357) (Figures 6B, D), whereas among TNM stage III patients, those individuals with a low SMI exhibited significantly worse OS than that of patients with a high SMI (31.0 (23.0–36.0) vs 32.0 (21.6–39.3), P=0.020)(Figure 6F).
4 Discussion
The present results suggest that among CRC patients with stage I-III disease, a low SMI is associated with worse OS but is unrelated to DFS, whereas a high V/S ratio is independently associated with DFS but unrelated to OS. Subgroup analyses further suggested that the prognostic benefits of SMI and V/S ratio values are more apparent in individuals with more advanced disease, including TNM stage III, T3/4, and LNM-positive patients. These results emphasize the need to assess the characteristics of skeletal muscle quality and adipose tissue distributions on diagnosis, especially in CRC patients with more advanced forms of these, thus providing an opportunity to achieve better long-term oncological outcomes.
This study included both rectal cancer and colon cancer in the analysis, primarily based on their significant similarities in biological characteristics, clinical features, and treatment strategies, with an important reason being that most rectal cancer patients in this study did not receive standard neoadjuvant therapy. This commonality provided a homogeneous basis without treatment interference, facilitating objective comparison. Moreover, the study strictly ensured the completeness of the monitoring protocol, guaranteeing accurate and reliable data. All recurrence cases (93 patients) were confirmed through outpatient records, which included regular clinical examinations and diagnostic results, accurately identifying recurrence events. For the death cases (39 patients), we employed various confirmation methods to enhance data completeness and reliability. Specifically, 30 cases were confirmed via telephone follow-up, and the remaining 9 were verified through affiliated hospital medical records. By integrating outpatient records, telephone follow-ups, and hospital records, we constructed a multi-dimensional monitoring network, reducing information omissions and misreporting.
In patients with CRC, sarcopenia has been inconsistently linked to survival outcomes, with some studies suggesting that it is significantly related to DFS and OS (13–17), whereas others have failed to ascribe any prognostic relevance to sarcopenia (18–20). These conflicting reports may partially be related to differences in ethnicity among cohorts, as sarcopenia incidences and associated threshold levels differ among ethnic groups (28). Here, SMI was found to be independently associated with CRC patient outcomes, with lower SMI values corresponding to greater mortality risk, providing support for certain past reports (29–31). Subgroup analyses specifically indicated that SMI was a more reliable predictor for CRC patients with advanced disease. This may be because patients with lower SMI values exhibit poorer chemotherapy and radiotherapy tolerance, reductions in overall treatment efficacy (32, 33), or higher complication rates while undergoing treatment (34, 35), thereby affecting their long-term prognostic outcomes. A lower SMI also tends to coincide with worse nutritional status (36), and this can substantially impact overall patient health, including their immune function and capacity for recovery, especially in individuals with advanced forms of cancer.
The degree to which fat distributions impact prognostic outcomes in CRC patients remains a matter of some debate. Higher levels of visceral fat have been linked to a greater risk of disease recurrence and death in some reports (7, 8, 21, 22), but other studies have failed to observe any relationship between these parameters, and some have even identified subcutaneous fat as a protective factor associated with a lower risk of recurrence (23, 24). Here, a high V/S ratio was found to be associated with a greater risk of recurrence, in line with what has been reported previously (37, 38). Past reports have demonstrated that subcutaneous fat exhibits a high degree of metabolic stability and greater lipolysis resistance as compared to visceral fat (39, 40), with its leptin production having a favorable impact on insulin sensitivity and overall levels of energy metabolism (41, 42). The protective effects of subcutaneous fat may thus partially overcome the adverse effects of visceral fat, thus explaining the ability of the V/S ratio, rather than visceral or subcutaneous fat tissue alone, to serve as an accurate predictor of recurrence.
The majority of adverse changes in body composition can be reversed such that there are opportunities to develop targeted interventional strategies. Prior studies have confirmed that it is possible to improve body composition, alleviate inflammation, and reduce the incidence of metabolic disorders through the use of anti-obesity medications including metformin and orlistat (43), together with lifestyle interventions such as routine physical activity and a balanced diet, thus contributing to a lower risk of cancer. In the future, studies should focus on defining new targets for therapeutic intervention so that the relationship between body composition and CRC patient prognosis can be translated into effective clinical strategies for the individualized treatment of patients.
There are some limitations to this study. For one, this was a single-center retrospective analysis that is thus susceptible to the potential for selection bias, and validation in future large-scale multi-center cohort studies will be important. In addition, only muscle area and density were evaluated in this study, without any corresponding interrogation of muscle function or strength, underscoring the need for additional assessment efforts in future prospective studies. Moreover, patients who had received neoadjuvant therapy were excluded, which may have introduced some selection bias; thus, future studies should include a more comprehensive patient population. There is also no accepted threshold for the standardized assessment of skeletal muscle or fat at present. Even so, the present results offer new evidence in support of the relevance of these parameters to oncological outcomes. While no efforts to assess intra- or inter-observer variability with respect to analyses of CT images were implemented in this study, the analytical approach employed herein has previously been shown to be objective and reliable (44, 45).
5 Conclusion
In summary, these data demonstrate that both the V/S ratio and SMI offer significant clinical utility as biomarkers capable of predicting long-term CRC patient outcomes. Strikingly, higher V/S ratios and lower SMI values were associated with significantly worse prognostic outcomes among CRC patients with more advanced disease. These data emphasize the need for further studies focused on the prognostic implications of different body composition parameters in CRC and their potential to guide individualized treatment efforts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Jingzhou Hospital affiliated with Yangtze University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because, as this study was retrospective in nature, the need for informed consent was waived.
Author contributions
HZ: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. LT: Methodology, Visualization, Writing – review & editing. YW: Investigation, Methodology, Writing – review & editing. SL: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1420917/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. (2022) 2:1–9. doi: 10.1016/j.jncc.2022.02.002
3. Lee M-J, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. (2013) 34:1–11. doi: 10.1016/j.mam.2012.10.001
4. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. (2009) 30:1073–81. doi: 10.1093/carcin/bgp127
5. Fearon KCH, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. (2012) 16:153–66. doi: 10.1016/j.cmet.2012.06.011
6. Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. (1999) 245:621–5. doi: 10.1046/j.1365-2796.1999.00490.x
7. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the national health and nutrition examination survey III. PloS One. (2010) 5:e10805. doi: 10.1371/journal.pone.0010805
8. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. (2008) 9:629–35. doi: 10.1016/S1470-2045(08)70153-0
9. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
10. Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. (2014) 20:5177–90. doi: 10.3748/wjg.v20.i18.5177
11. Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. (2010) 16:4773–83. doi: 10.3748/wjg.v16.i38.4773
12. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. (1985) 2000) 89:104–10. doi: 10.1152/jappl.2000.89.1.104
13. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. (2016) 103:572–80. doi: 10.1002/bjs.10075
14. Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The impact of muscle and adipose tissue -term son long urvival in patients with stage I to III colorectal cancer. Dis Colon Rectum. (2019) 62:549–60. doi: 10.1097/DCR.0000000000001352
15. Sueda T, Takahasi H, Nishimura J, Hata T, Matsuda C, Mizushima T, et al. Impact of low muscularity and myosteatosis on long-term outcome after curative colorectal cancer surgery: A propensity score-matched analysis. Dis Colon Rectum. (2018) 61:364–74. doi: 10.1097/DCR.0000000000000958
16. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. (2017) 3:e172319. doi: 10.1001/jamaoncol.2017.2319
17. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
18. van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, Vrijland WW, Dekker JWT, Zimmerman DDE, et al. Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol. (2018) 44:1354–60. doi: 10.1016/j.ejso.2018.05.029
19. Black D, Mackay C, Ramsay G, Hamoodi Z, Nanthakumaran S, Park KGM, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. (2017) 24:2241–51. doi: 10.1245/s10434-017-5829-z
20. Nakanishi R, Oki E, Sasaki S, Hirose K, Jogo T, Edahiro K, et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today. (2018) 48:151–7. doi: 10.1007/s00595-017-1564-0
21. Rickles AS, Iannuzzi JC, Mironov O, Deeb A-P, Sharma A, Fleming FJ, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointestinal Surg. (2013) 17:133–143; discussion p.143. doi: 10.1007/s11605-012-2045-9
22. Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil J-P, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. (2010) 59:341–7. doi: 10.1136/gut.2009.188946
23. Perrin T, Lenfant M, Boisson C, Bert M, Rat P, Facy O. Effects of body composition profiles on oncological outcomes and postoperative intraabdominal infection following colorectal cancer surgery. Surg Obes Related Dis. (2021) 17:575–84. doi: 10.1016/j.soard.2020.10.022
24. Kim J-M, Chung E, Cho E-S, Lee J-H, Shin S-J, Lee HS, et al. Impact of subcutaneous and visceral fat adiposity in patients with colorectal cancer. Clin Nutr. (2021) 40:5631–8. doi: 10.1016/j.clnu.2021.10.001
25. Orange ST, Jordan AR, Odell A, Kavanagh O, Hicks KM, Eaglen T, et al. Acute aerobic exercise-conditioned serum reduces colon cancer cell proliferation in vitro through interleukin-6-induced regulation of DNA damage. Int J Cancer. (2022) 151:265–74. doi: 10.1002/ijc.33982
26. Ten Haaf DSM, Eijsvogels TMH, Bongers CCWG, Horstman AMH, Timmers S, de Groot LCPGM, et al. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo-controlled trial. J Cachexia Sarcopenia Muscle. (2019) 10:298–310. doi: 10.1002/jcsm.12394
27. Fuchs CJ, Hermans WJH, Holwerda AM, Smeets JSJ, Senden JM, van Kranenburg J, et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial. Am J Clin Nutr. (2019) 110:862–72. doi: 10.1093/ajcn/nqz120
28. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
29. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle. (2018) 9:53–9. doi: 10.1002/jcsm.12234
30. Liu J, Yu X, Huang X, Lai Q, Chen J. Associations of muscle and adipose tissue parameters with long-term outcomes in middle and low rectal cancer: a retrospective cohort study. Cancer Imaging. (2023) 23:5. doi: 10.1186/s40644-022-00514-x
31. Han JS, Ryu H, Park IJ, Kim KW, Shin Y, Kim SO, et al. Association of body composition with long-term survival in non-metastatic rectal cancer patients. Cancer Res Treat. (2020) 52:563–72. doi: 10.4143/crt.2019.249
32. Vega MCMD, Laviano A, Pimentel GD. Sarcopenia and chemotherapy-mediated toxicity. Einstein (Sao Paulo). (2016) 14:580–4. doi: 10.1590/s1679-45082016md3740
33. Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH, et al. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C-SCANS study. Cancer. (2017) 123:4868–77. doi: 10.1002/cncr.30950
34. Feng Z, Pang K, Tian M, Gu X, Lin H, Yang X, et al. Sarcobesity, but not visceral fat, is an independent risk factor for complications after radical resection of colorectal cancer. Front Nutr. (2023) 10:1126127. doi: 10.3389/fnut.2023.1126127
35. Xiao J, Caan BJ, Cespedes Feliciano EM, Meyerhardt JA, Peng PD, Baracos VE, et al. Association of low muscle mass and low muscle radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surg. (2020) 155:942. doi: 10.1001/jamasurg.2020.2497
36. Wang S, Xie H, Gong Y, Kuang J, Yan L, Ruan G, et al. The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci Rep. (2020) 10:8153. doi: 10.1038/s41598-020-65091-0
37. Park JW, Chang SY, Lim JS, Park SJ, Park JJ, Cheon JH, et al. Impact of visceral fat on survival and metastasis of stage III colorectal cancer. Falk Symp. (2022) 16:53–61. doi: 10.5009/gnl20266
38. Moon H-G, Ju Y-T, Jeong C-Y, Jung E-J, Lee Y-J, Hong S-C, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. (2008) 15:1918–22. doi: 10.1245/s10434-008-9891-4
39. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. (2010) 11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x
40. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. (2000) 21:697–738. doi: 10.1210/edrv.21.6.0415
41. Van Harmelen V, Reynisdottir S, Eriksson P, Thörne A, Hoffstedt J, Lönnqvist F, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. (1998) 47:913–7. doi: 10.2337/diabetes.47.6.913
42. Minocci A, Savia G, Lucantoni R, Berselli ME, Tagliaferri M, Calò G, et al. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord. (2000) 24:1139–44. doi: 10.1038/sj.ijo.0801385
43. Yang H, Yue GGL, Leung PC, Wong CK, Lau CBS. A review on the molecular mechanisms, the therapeutic treatment including the potential of herbs and natural products, and target prediction of obesity-associated colorectal cancer. Pharmacol Res. (2022) 175:106031. doi: 10.1016/j.phrs.2021.106031
44. Cruz RJ, Dew MA, Myaskovsky L, Goodpaster B, Fox K, Fontes P, et al. Objective radiologic assessment of body composition in patients with end-stage liver disease: going beyond the BMI. Transplantation. (2013) 95:617–22. doi: 10.1097/TP.0b013e31827a0f27
Keywords: colorectal cancer, CT, biomarkers, body composition, prognosis
Citation: Zhou H, Tian L, Wu Y and Liu S (2024) Computed tomography-measured body composition can predict long-term outcomes for stage I-III colorectal cancer patients. Front. Oncol. 14:1420917. doi: 10.3389/fonc.2024.1420917
Received: 21 April 2024; Accepted: 20 June 2024;
Published: 08 July 2024.
Edited by:
Antonio Mario Scanu, University of Sassari, ItalyReviewed by:
Robert J. Canter, University of California, Davis, United StatesJenny Georgina Turcott, National Institute of Cancerology (INCAN), Mexico
Copyright © 2024 Zhou, Tian, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sibin Liu, bGl1c2liOTE1OUBxcS5jb20=
 Han Zhou
Han Zhou Lei Tian
Lei Tian Yiting Wu
Yiting Wu