- 1Center for Study of Heredo-Familial Tumors, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari, Italy
- 2Molecular Diagnostics and Pharmacogenetics Unit, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari, Italy
- 3Department of Oncology, “F. Miulli” General Regional Hospital, Acquaviva Delle Fonti, Italy
The gene protection of telomere 1 (POT1) is involved in telomere maintenance and stability and plays a crucial role in the preservation of genomic stability. POT1 is considered a high-penetrance melanoma susceptibility gene; however, the number of cancer types associated with the pathogenic germline variants of POT1 is gradually increasing, including chronic lymphocytic leukemia (CLL), angiosarcomas, and gliomas, even though many associations are still elusive. Here, we reported a case of a 60-year-old man who showed early-onset multiple neoplasms, including multiple melanomas, gastrointestinal stromal tumor (GIST), and lung adenocarcinoma. Next-generation sequencing (NGS) analyses revealed a germline heterozygous pathogenic variant in the POT1 gene. Notably, GIST and lung adenocarcinoma were not previously reported in association with the POT1 germline variant. Lung cancer susceptibility syndrome is very rare and the actual knowledge is limited to a few genes although major genetic factors are unidentified. Recently, genome-wide association studies (GWAS) have pointed out an association between POT1 variants and lung cancer. This case report highlights the clinical relevance of POT1 alterations, particularly their potential involvement in lung cancer. It also suggests that POT1 testing may be warranted in patients with familial cancer syndrome, particularly those with a history of melanoma and other solid tumors.
1 Introduction
The gene protection of telomere 1 (POT1) encodes a crucial component of the human shelterin complex, involved in the regulation and preservation of telomeric ends (1). Deleterious genetic variants in POT1 are involved in telomere biology disorders (TBD), characterized by abnormal telomeric length (2). Indeed, POT1 mutations may generate two opposite phenotypes of telomere elongation or telomere shortening: the first one is associated with neoplastic predisposition, and the second one is involved in telomeropathies (3). Germline loss-of-function (LOF) variants in POT1 are associated with a rare oncological predisposition syndrome, known as POT1-tumor predisposition syndrome (POT1-TPD), with no more than 100 affected families currently reported (4). POT1-TPD is inherited with an autosomal dominant pattern, typically manifesting in adulthood. However, genetic anticipation and gradual lowering of the age of onset across successive generations have been observed (5). POT1-TPD is associated with an increased lifetime risk of multiple melanomas (6), chronic lymphocytic leukemia (CLL), angiosarcoma (7, 8), and glioma (9). Despite its rarity, POT1 mutations have been also reported in various kinds of neoplasms including colorectal cancer (10), thyroid cancer (11), uveal melanoma (12), and myeloma (13). Definitive associations, however, remain elusive due to the lack of consistent data. Here, we report a unique case of a male patient diagnosed with POT1-TPD, subsequently found to be manifesting gastrointestinal stromal tumor (GIST) and lung adenocarcinoma. To the best of our knowledge, this is the first reported case of GIST and lung adenocarcinoma associated with a POT1 germline variant. This case represents an example suggesting the clinical relevance of POT1 alterations and their involvement in a broad range of cancer types and indicates the possibility that management approaches should be evaluated for mutation carriers.
2 Case description
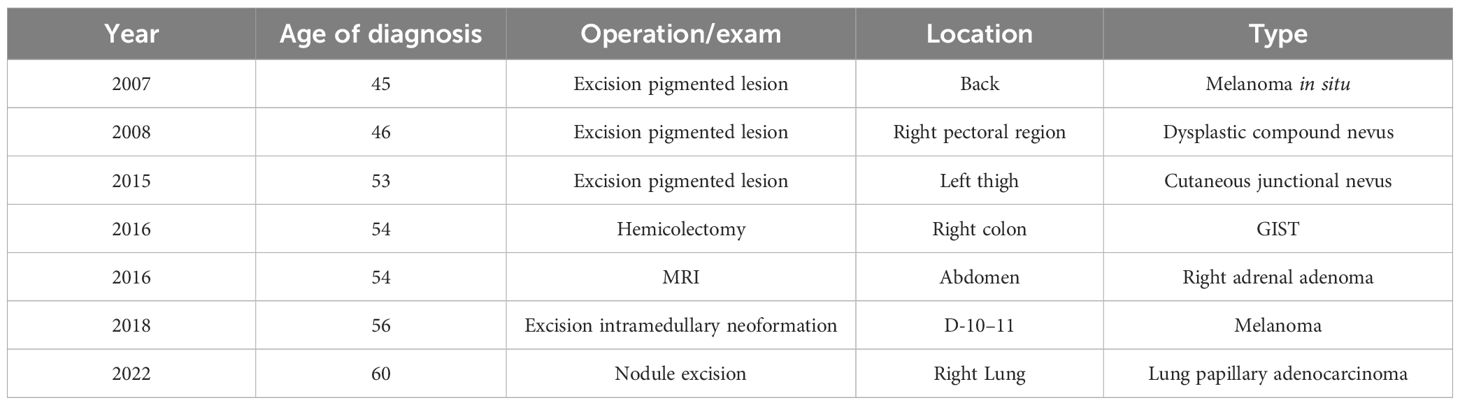
A 60-year-old man, who is a non-smoker, was referred by an oncologist to our unit for oncogenic counseling, prompted by a suspicion of an oncological predisposition syndrome. His medical history (Table 1) outlined a series of neoplastic events, beginning with surgical excision of different suspected pigmented lesions, one of which was classified as melanoma in situ. He also underwent a hemicolectomy for a mesenchymal neoplasm identified as GIST in the left colon, and at that time, a right adrenal adenoma was identified during an MRI exam. Another surgical intervention involved the removal of a metastatic intramedullary neoplasm at D-10 and D-11 vertebral levels. The metastasis originated from melanoma even though the primitive lesion was not identified. The patient had recently been diagnosed with papillary lung adenocarcinoma. Examination of familial history also pointed to a possible genetic syndrome of oncological predisposition (Figure 1), given the cases of melanoma in the patient’s sister (III-11), paternal aunt (II-2), and father (II-5), coupled with an indistinct skin condition in the mother (II-6), referred to as “skin tumors” that had not been well characterized. Given the clinical suspicion of the presence of a genetic risk factor predisposing to melanoma, genetic analysis was indicated. Genomic DNA was extracted from the proband’s peripheral blood, and analysis was conducted using next-generation sequencing (NGS). The targeted custom panel was used to examine six hereditary melanoma susceptibility genes (CDKN2A, MITF, MC1R, CDK4, POT1, and BAP1). Libraries were prepared with the Ion AmpliSeq Library Kit Plus and were sequenced with Ion Torrent S5 (Thermo Fisher, Waltham, USA). Variant calling was performed through the Torrent Variant Caller after alignment using GRCh37 as a reference. The analysis revealed the presence of the heterozygous pathogenic variant c.1087 C>T; p. (Arg363*)(rs756198077), in the POT1 gene (NM_015450.3), with the GRCh37 coordinate chr7:124482937. The alteration was confirmed by Sanger sequencing (Figure 2). This variant is present on the gnomAD database, with a maximum subpopulation frequency of 0.0047% in the European (non-Finnish) population (7–124482937-G-A, gnomAD v2.1.1, gnomad.broadinstitute.com). Loss of function is an established mechanism of the disease in the POT1 gene. Fifty-two nonsense variants are reported on the ClinVar database (ncbi.nlm.gov/clinvar/), most of which (51/52) were classified as likely pathogenic/pathogenic. Indeed, ClinVar contains an entry for the identified variant (Variation ID: 475019). This finding led to the diagnosis of POT1-TPD. In the absence of official guidelines for POT1-TPD, the only surveillance plan suggested is very similar to that for the Li–Fraumeni-like syndrome (14), due to the comparable associated phenotypical spectra. The recommended surveillance, indicated for the patient, includes dermatological checkup every 6 months, routine complete blood count (CBC) with differential analysis, lymph node palpation, and annual brain and whole-body MRI. Since beginning at age 18 years old. We recommended expanding the genetic analysis to the family (Figure 3), and relatives were tested by Sanger sequencing. Segregation analysis was performed on the father, daughter, and son. The daughter (IV-3), 32 years old, is the sole family member who tested positive for the POT1 variant (Figure 3) and had two suspected lesions that were removed from the right side and the back after both were identified as basal cell carcinoma. The same surveillance protocol was recommended, given the oncological clinical history of her father.
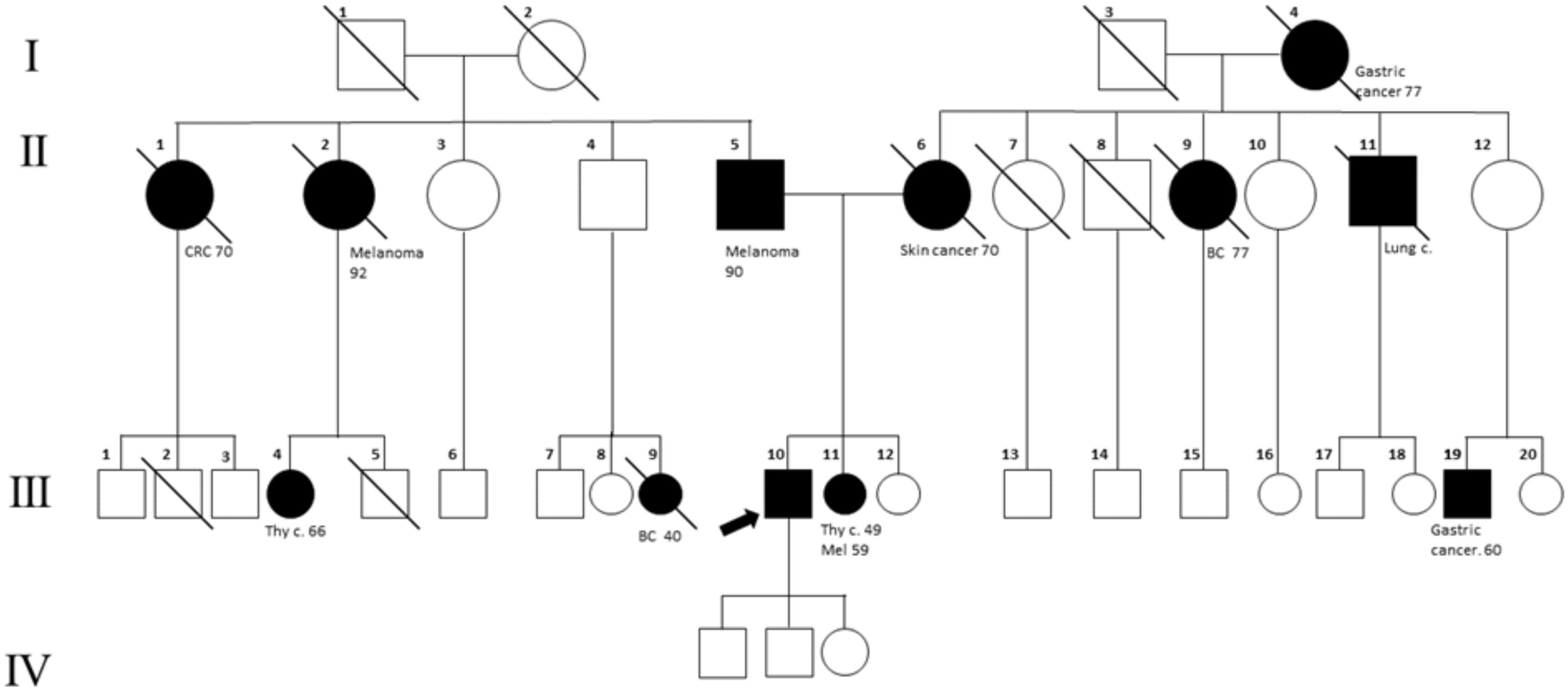
Figure 1 Family tree. The proband is indicated with an arrow (III_10). Melanoma cases are reported in the father (II-5), sister (III-11), and paternal aunt (II-2). The mother (II-6) has been reported to have a not well-defined “skin cancer.” In the family, other cancer types are present: breast cancer (BC; II-9, III-9), lung cancer (III-11), gastric cancer (III-19, I-4), colorectal cancer (CRC, II-1), and thyroid cancer (III-11, III-4).

Figure 2 IGV screenshot and Sanger sequencing. On the top, the IGV screenshot is depicted (A), showing the variant R363* in POT1, in a heterozygous state, identified in the NGS analyses. On the bottom, an electropherogram of Sanger sequencing confirms the NGS results (B).
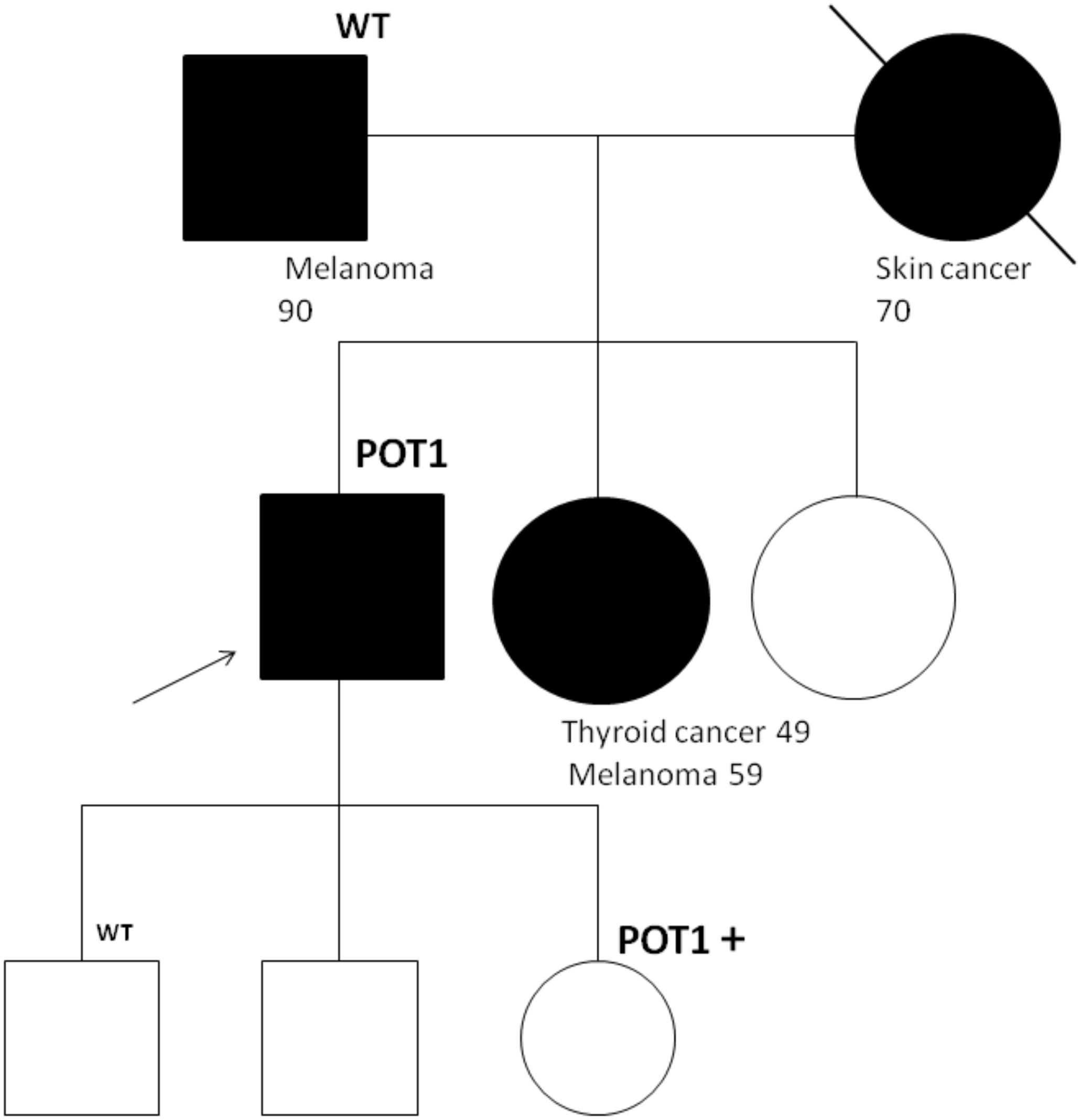
Figure 3 Segregation analysis for the POT1 variant R363*. The proband is indicated with an arrow. In the father, daughter, and son, segregation analysis by Sanger sequencing was performed. The daughter, who was positive for the POT1 variant, underwent exportation of two basal cell carcinoma after the test.
3 Discussion
In this paper, we report a 60-year-old male patient affected by a series of malignancies including multiple melanomas, GIST, adrenal adenoma, and lung adenocarcinoma, with a familial history marked by numerous cases of melanoma. Genetic analysis identified the pathogenic variant p.R363* in the POT1 gene. The variant R363* was previously identified in cases of colorectal cancer (9). LOF mutations in POT1 are associated with telomeric lengthening (5), increased longevity, and the promotion of carcinogenesis (4, 15). Our case, notably featuring GIST in association with a POT1 mutation, contributes additional insights to the existing literature. Furthermore, given that major genetic factors for lung cancer predisposition are still to be identified, our study provides the first reported clinical evidence supporting previous genome-wide association studies (16, 17) that indicate a potential association between POT1 variants and lung cancer (18). Notably, a pan-cancer study on 62,368 tumors revealed that deleterious POT1 variants are present in approximately 5% of non-small cell lung cancers (19). Furthermore, an observational study on 95,568 individuals of a general population underscored the correlation between longer telomeres and increased risk of both melanoma and lung cancer (18). In light of current knowledge, the Li–Fraumeni-like surveillance protocol, described by Henry et al. (14), is the only available proposal at this time. However, the discussion about the suitability of this protocol is still open, considering the burden of oversurveillance in POT1 variant carriers. It is important to specify that surveillance should be modulated on the emerging phenotypic spectrum of POT1-TPD and the individual’s personal family history (14). However, in this case, we decided to recommend the Li–Fraumeni-like surveillance protocol to POT1 variant carriers, especially for the proband’s daughter given the personal clinical history of the proband and the lack of known environmental risk factors. Indeed, MRI is reliable in the detection of both GIST lesion (20) and early lung cancer (21), and despite its limitations, it is considered a safe alternative to TC, especially in the context of oncological screening, in healthy subjects, which should be reiterated for a long period of time. In exploring this unique case, we aim to contribute valuable insights into the clinical relevance of POT1 mutations, particularly given the scarcity of available data and the current lack of a definition of the complete phenotypical spectrum associated with POT1. Our findings also prompt considerations for tailored management approaches for individuals carrying POT1 mutations, suggesting the need for vigilant surveillance and genetic counseling.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The description of a case report does not require ethical approval. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SM: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. SD: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. BP: Investigation, Validation, Visualization, Writing – review & editing. MD: Visualization, Writing – review & editing. LL: Investigation, Visualization, Writing – review & editing. ST: Visualization, Writing – review & editing. MP: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Funding 5X1000 Program, year 2020, provided by the Ministry of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aramburu T, Kelich J, Rice C, Skordalakes E. POT1-TPP1 binding stabilizes POT1, promoting efficient telomere maintenance. Comput Struct Biotechnol J. (2022) 20:675–84. doi: 10.1016/j.csbj.2022.01.005
2. Byrjalsen A, Brainin AE, Lund TK, Andersen MK, Jelsig AM. Size matters in telomere biology disorders − expanding phenotypic spectrum in patients with long or short telomeres. Hered Cancer Clin Pract. (2023) 21:7. doi: 10.1186/s13053-023-00251-7
3. Zade NH, Khattar E. POT1 mutations cause differential effects on telomere length leading to opposing disease phenotypes. J Cell Physiol. (2023) 238:1237–55. doi: 10.1002/jcp.31034
4. Wu Y, Poulos RC, Reddel RR. Role of POT1 in human cancer. Cancers. (2020) 12:2739. doi: 10.3390/cancers12102739
5. DeBoy EA, Tassia MG, Schratz KE, Yan SM, Cosner ZL, McNally EJ, et al. Familial clonal hematopoiesis in a long telomere syndrome. N Engl J Med. (2023) 388:2422–33. doi: 10.1056/NEJMoa2300503
6. Goldstein AM, Xiao Y, Sampson J, Zhu B, Rotunno M, Bennett H, et al. Rare germline variants in known melanoma susceptibility genes in familial melanoma. Hum Mol Genet. (2017) 26:4886–95. doi: 10.1093/hmg/ddx368
7. Li Y, Xie Y, Wang D, Xu H, Ye J, Yin JC, et al. Whole exome sequencing identified a novel POT1 variant as a candidate pathogenic allele underlying a Li-Fraumeni-like family. Front Oncol. (2022) 12:963364. doi: 10.3389/fonc.2022.963364
8. Calvete O, Garcia-Pavia P, Domínguez F, Mosteiro L, Pérez-Cabornero L, Cantalapiedra D, et al. POT1 and damage response malfunction trigger acquisition of somatic activating mutations in the VEGF pathway in cardiac angiosarcomas. J Am Heart Assoc. (2019) 8:e012875. doi: 10.1161/JAHA.119.012875
9. Bainbridge MN, Armstrong GN, Gramatges MM, Bertuch AA, Jhangiani SN, Doddapaneni H, et al. Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst. (2015) 107:384. doi: 10.1093/jnci/dju384
10. Chubb D, Broderick P, Dobbins SE, Frampton M, Kinnersley B, Penegar S, et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat Commun. (2016) 7:11883. doi: 10.1038/ncomms11883
11. Srivastava A, Miao B, Skopelitou D, Kumar V, Kumar A, Paramasivam N, et al. A germline mutation in the POT1 gene is a candidate for familial non-medullary thyroid cancer. Cancers. (2020) 12:1441. doi: 10.3390/cancers12061441
12. Nathan V, Palmer JM, Johansson PA, Hamilton HR, Warrier SK, Glasson W, et al. Loss-of-function variants in POT1 predispose to uveal melanoma. J Med Genet. (2021) 58:234–6. doi: 10.1136/jmedgenet-2020-107098
13. Hakkarainen M, Koski JR, Heckman CA, Anttila P, Silvennoinen R, Lievonen J, et al. A germline exome analysis reveals harmful POT1 variants in multiple myeloma patients and families. EJHaem. (2022) 3:1352–7. doi: 10.1002/jha2.557
14. Henry ML, Osborne J, Else T, Adam MP, Feldman J, Mirzaa GM, et al. POT1 Tumor Predisposition. Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al, editors. Seattle (WA: University of Washington, Seattle (1993). Available at: http://www.ncbi.nlm.nih.gov/books/NBK563529/. curatori. GeneReviews®.
15. McNally EJ, Luncsford PJ, Armanios M. Long telomeres and cancer risk: The price of cellular immortality. J Clin Invest. (2019) 129:3474–81. doi: 10.1172/JCI120851
16. Wang C, Dai J, Qin N, Fan J, Ma H, Chen C, et al. Analyses of rare predisposing variants of lung cancer in 6,004 whole genomes in Chinese. Cancer Cell. (2022) 40:1223–1239.e6. doi: 10.1016/j.ccell.2022.08.013
17. Bhat GR, Jamwal RS, Sethi I, Bhat A, Shah R, Verma S, et al. Associations between telomere attrition, genetic variants in telomere maintenance genes, and non-small cell lung cancer risk in the Jammu and Kashmir population of North India. BMC Cancer. (2023) 23:874. doi: 10.1186/s12885-023-11387-z
18. Rode L, Nordestgaard BG, Bojesen SE. Long telomeres and cancer risk among 95 568 individuals from the general population. Int J Epidemiol. (2016) 45:1634–43. doi: 10.1093/ije/dyw179
19. Shen E, Xiu J, Lopez GY, Bentley R, Jalali A, Heimberger AB, et al. POT1 mutation spectrum in tumour types commonly diagnosed among POT1-associated hereditary cancer syndrome families. J Med Genet. (2020) 57:664–70. doi: 10.1136/jmedgenet-2019-106657
20. Kalkmann J, Zeile M, Antoch G, Berger F, Diederich S, Dinter D, et al. Consensus report on the radiological management of patients with gastrointestinal stromal tumours (GIST): recommendations of the German GIST Imaging Working Group. Cancer Imaging. (2012) 12:126–35. doi: 10.1102/1470-7330.2012.0013
Keywords: tumor predisposition syndrome, lung adenocarcinoma, GIST, POT1, case report
Citation: Martino S, De Summa S, Pilato B, Digennaro M, Laera L, Tommasi S and Patruno M (2024) Case report: Germline POT1 mutation in a patient with GIST and lung adenocarcinoma. Front. Oncol. 14:1419739. doi: 10.3389/fonc.2024.1419739
Received: 24 April 2024; Accepted: 16 July 2024;
Published: 02 August 2024.
Edited by:
Elisa Frullanti, University of Siena, ItalyReviewed by:
Valentina Calò, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyEmma M. Rath, Victor Chang Cardiac Research Institute, Australia
Copyright © 2024 Martino, De Summa, Pilato, Digennaro, Laera, Tommasi and Patruno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Martino, cy5tYXJ0aW5vQG9uY29sb2dpY28uYmFyaS5pdA==
 Stefania Martino
Stefania Martino Simona De Summa
Simona De Summa Brunella Pilato
Brunella Pilato Maria Digennaro1
Maria Digennaro1 Letizia Laera
Letizia Laera Stefania Tommasi
Stefania Tommasi