
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 13 August 2024
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1419338
 Haeseong Park1*
Haeseong Park1* Samuel J. Klempner2
Samuel J. Klempner2 Joseph Chao3
Joseph Chao3 Zev A. Wainberg4
Zev A. Wainberg4 Mariusz Lukanowski5
Mariusz Lukanowski5 Suresh Chenji6
Suresh Chenji6 Shannon Bourke7
Shannon Bourke7 Anindya Chatterjee8
Anindya Chatterjee8 Sylvie Lorenzen9
Sylvie Lorenzen9Introduction: The aim of this study was to provide a review of the clinical evidence for use of ramucirumab (RAM) plus folinic acid (leucovorin), fluorouracil (5-FU), and irinotecan (FOLFIRI) or irinotecan as second-line treatment in gastroesophageal adenocarcinoma (GEA).
Methods: A systematic and comprehensive search of PubMed was performed to identify phase 2 clinical trials or retrospective studies using RAM plus FOLFIRI or irinotecan in GEA, including abstracts from major congresses, in addition to published manuscripts. An aggregated review and meta-analysis was performed to assess the effectiveness (overall response rate [ORR] as primary outcome) and safety data of RAM plus FOLFIRI or irinotecan. ORR for each study was calculated with 95% confidence interval estimated from normal approximation. To generate the combined ORR with 95% confidence interval, random-effects meta-analysis was conducted to synthesize response data from available studies.
Results: Six studies were identified with non-overlapping populations, 3 phase 2 clinical trials and 3 retrospective studies. Across these studies the ORR ranged from 22% to 38%, and pooled ORR was 25.4%. Two of the 3 studies reported better ORR in patients pretreated with taxanes followed by RAM plus FOLFIRI. Treatment with RAM plus FOLFIRI or irinotecan was well tolerated. Neutropenia and diarrhea were the most common adverse events reported across studies.
Conclusion: The studies examined in this review suggest that RAM plus FOLFIRI or irinotecan have activity in previously treated GEA irrespective of prior-taxane use. Overall, RAM plus FOLFIRI or irinotecan was well tolerated with no new safety concerns identified beyond established profiles for these regimens.
Gastroesophageal adenocarcinomas (GEA) account for over 1.3 million annual deaths, representing nearly 13.2% of global cancer deaths (1). In the past few years, clinical advances including upfront use of programmed cell death protein 1 (PD-1) inhibitors and the addition of docetaxel in the perioperative treatment may impact survival benefits across lines of therapy in advanced GEA (2). The current standard first-line treatment for advanced GEA is platinum and fluoropyrimidine-based doublet with or without the addition of a PD-1 inhibitor and with trastuzumab in human epidermal growth factor receptor 2-positive disease (3, 4). Taxane-containing regimens used in localized and advanced disease is increasing, and perioperative chemotherapy with fluorouracil, leucovorin, oxaliplatin, and docetaxel became standard in many regions (2). In Japan, S1 (a novel oral fluoropyrimidine derivative) plus docetaxel is the new standard of care for the adjuvant therapy of stage III gastric cancer (5).
Despite such advances in first-line and perioperative treatments, there is no randomized phase 3 trial that improves upon ramucirumab (RAM) plus paclitaxel in the later lines of treatment. RAM with paclitaxel has been established as second-line standard after platinum and fluoropyrimidine-containing treatment on the basis of positive results of the phase 3 RAINBOW trial (6). RAM is a recombinant human immunoglobulin G1 monoclonal antibody receptor antagonist designed to bind to the extracellular domain of vascular endothelial growth factor receptor 2, thereby blocking the binding of multiple vascular endothelial growth factor (VEGF) ligands and inhibiting receptor activation (7). Also, ramucirumab inhibits all VEGFs thus enabling inhibition of downstream receptor activation of VEGF signaling pathways resulting in reduced tumor neovascularization and growth (8). Chemotherapy in combination with anti-vascular endothelial growth factor receptors (VGFR) such as ramucirumab has shown to significantly improve overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) in patients with advanced gastric cancer (8). Furthermore, ramucirumab shows a better favorable risk profile compared to other anti-angiogenic agents and exhibits anti-angiogenic effects beyond progression (9).
Patients receiving first-line oxaliplatin regimens often develop neuropathy that may limit taxane tolerance or eligibility (10, 11). Because of concerns for taxane-related neuropathy as well as earlier exposure to taxane during the disease course in many patients, the identification of a taxane-free second-line therapy is of critical importance. A phase 3 clinical trial in colorectal cancer has shown safety and activity of RAM plus folinic acid (leucovorin), fluorouracil (5-FU), irinotecan and bevacizumab (FOLFIRI) as a second-line therapy after progression on folinic acid (leucovorin), 5-FU, and oxaliplatin with bevacizumab (12), thus providing a scientific basis for studying this combination in other cancers of the gastrointestinal tract. Despite the lack of large-scale randomized phase 3 trials, RAM plus FOLFIRI or irinotecan has emerged as a second-line option for patients with advanced GEA. The National Comprehensive Cancer Network (NCCN) guideline recommendations also support the use of RAM in combination with FOLFIRI or irinotecan as second-line therapy for patients with GEA.
In this literature review and meta-analysis, we aimed to identify publications, both clinical trials and retrospective studies, to review the data supporting the inclusion of RAM in combination with FOLFIRI or irinotecan as second-line therapy for patients with GEA and prior-taxane use as per the NCCN guideline recommendations; as well as reviewing safety data and performing an aggregated review to assess the efficacy of these combinations.
A systematic and comprehensive search of PubMed was performed to identify phase 2 clinical trials or retrospective studies using RAM plus FOLFIRI or irinotecan, including abstracts from major congresses, in addition to published manuscripts. The search was restricted to human studies, with no restrictions placed on language and all studies published before August 2022. The following search terms were combined: 1) gastric cancer OR gastric adenocarcinoma OR gastroesophageal junction cancer; 2) ramucirumab OR Cyramza; 3) FOLFIRI OR irinotecan, and 4) phase 2 clinical trial OR phase II clinical trial OR phase two clinical trial OR retrospective study. All results were reviewed and verified by the study team. All of the original publications were checked and reviewed; studies which were retrospective analyses or phase 2 clinical trials which examined the effectiveness and safety of RAM plus FOLFIRI or irinotecan were included for review. All manuscripts and publications that did not include the use of RAM plus FOLFIRI or irinotecan were excluded.
The ORR for each study was calculated with 95% confidence interval (CI) estimated from normal approximation. To generate the combined ORR with 95% CI, random-effects meta-analysis was conducted to synthesize response data from the available studies mentioned above. Logistic regression was used to model the binary response data with random effect accounting for across-study variability in the analysis. ORR in patients pretreated with taxane and patients who were taxane-naïve across studies were summarized with proportions and their 95% CI using an exact binomial approach.
In this review, only ORR data was pooled as the studies identified and reviewed had small sample sizes including both clinical trial studies and retrospective studies. Also, all studies reported ORR as the primary endpoint and not all studies reported PFS or OS data consistently which could provide clinically meaningful results.
Six studies were identified, with nonoverlapping populations, 3 phase 2 clinical trials: Lorenzen et al. (13) (NCT03081143), Park et al. (14) (NCT03141034), and Kawamoto et al. (15) (UMIN000030372); and 3 retrospective studies: Klempner et al. (16), Vogl et al. (17), and Schlintl et al. (18). An overview of the studies is provided in Table 1. The baseline characteristics of patients in the studies identified are outlined in Table 2.
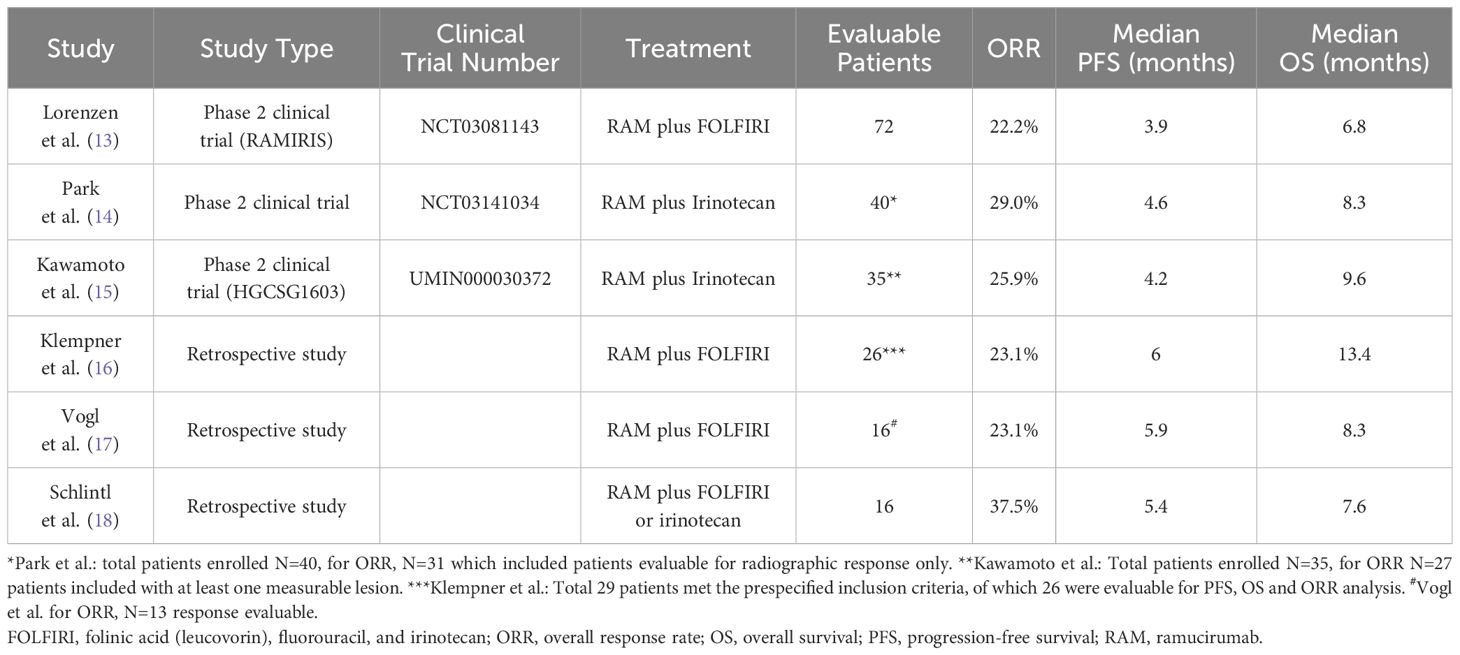
Table 1. Overview of studies included in review, including study type, treatment overview, overall response rate (ORR), median progression-free survival (PFS), and median overall survival (OS).
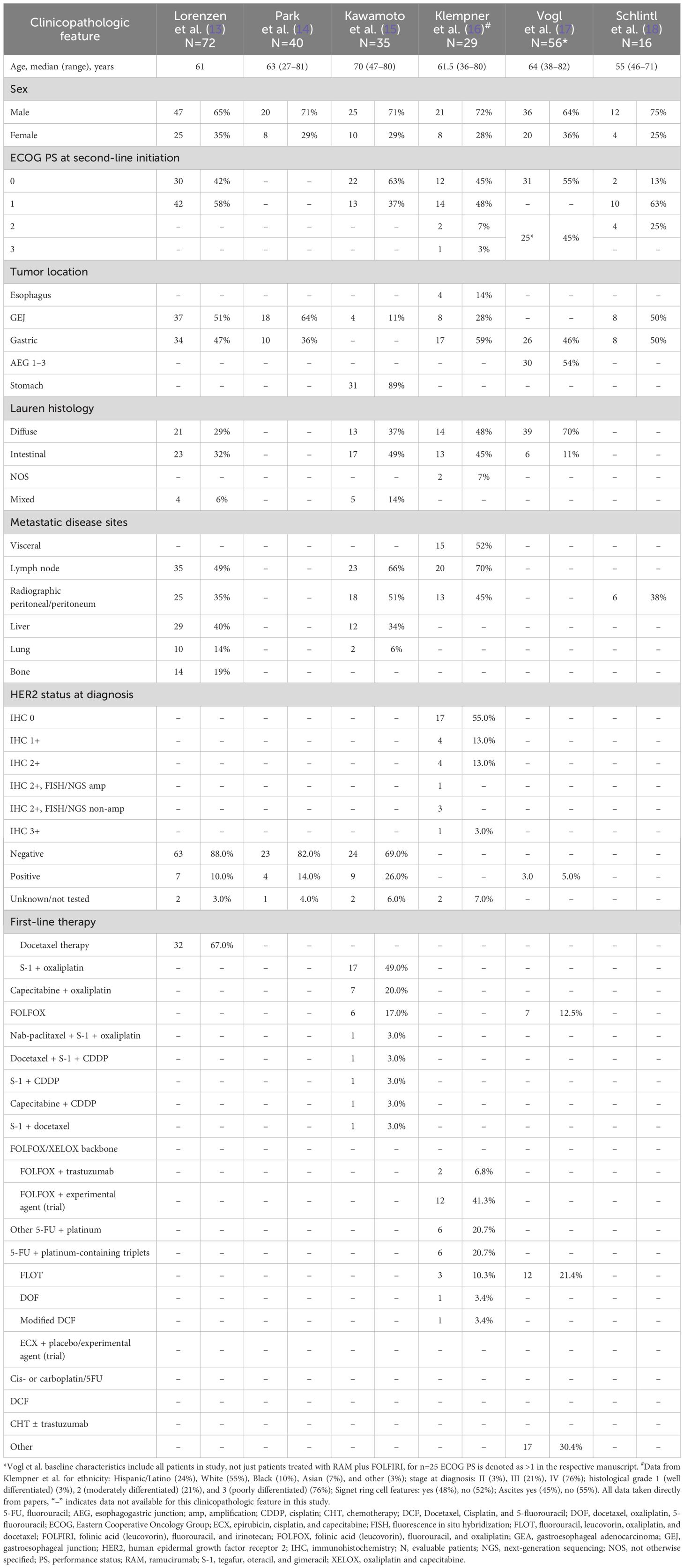
Table 2. Baseline characteristics of patient cohorts receiving RAM plus FOLFIRI or RAM plus irinotecan.
In the multicenter, randomized, phase 2 clinical trial by Lorenzen et al. (13) (RAMIRIS, NCT03081143) patients with GEA who progressed on 5-FU or platinum first-line treatment were randomized 2:1 to FOLFIRI plus RAM (Arm A, N=72) or RAM plus paclitaxel (Arm B, N=38). Patients treated with RAM plus FOLFIRI had a median OS of 6.8 months and a median PFS of 3.9 months. The 6-month OS rate in the FOLFIRI plus RAM arm was 54% (95% CI 44–67) and the study did not meet the primary endpoint for the comparison with historical control. There were 48 evaluable taxane-pretreated patients, with 12 responders (ORR, 25.0%) and 24 evaluable taxane-naïve patients, with 4 responders (ORR, 16.7%). Patients treated with RAM plus paclitaxel had a median OS of 7.6 months and a median PFS of 3.7 months.
In the single-arm, phase 2 study by Park et al. (14), (NCT03141034) 40 patients were enrolled. All patients received platinum-based chemotherapy prior to enrollment, 8 patients had human epidermal growth factor receptor 2-positive disease, and 6 patients had received an immune checkpoint inhibitor. Median PFS was 4.6 months (95% CI, 2.7–5.4). Of the 31 patients evaluable for response, 9 out of 30 patients evaluable for radiographic response only (29%) had objective responses (1 complete response, 8 partial responses) and 5 patients (16%) had stable disease greater than 6 months. There were 7 evaluable taxane-pretreated patients with 3 responders (ORR, 42.9%) and 26 evaluable taxane-naïve patients with 6 responders (ORR, 23.1%).
In the multi-institutional nonrandomized, single-arm, phase 2 clinical trial by Kawamoto et al. (15) (HGCSG1603; jRCTs011180029), 35 patients with advanced GEA who were refractory or intolerant to first-line chemotherapy were enrolled and treated with RAM plus irinotecan. Median PFS and OS were 4.2 months (95% CI, 2.5–5.4) and 9.6 months (95% CI, 6.4–16.6), respectively. Data from 27 patients with measurable disease (ORR, 25.9%) was used in the review and meta-analysis.
Klempner et al. (16) performed a retrospective study of 29 patients who had received second-line RAM plus FOLFIRI. In the 26 evaluable patients, median PFS was 6.0 months, with a range of 2 to 24 months, and median OS was 13.4 months.
Vogl et al. (17) performed a retrospective study of 56 patients treated with RAM plus paclitaxel (N=38, as second-line [75%] or beyond second-line [25%]) or RAM plus FOLFIRI (N=16). This study found a significant increase in the median PFS and OS of patients treated with RAM plus FOLFIRI compared with patients treated with RAM plus paclitaxel (P=0.05). The median PFS and OS for patients RAM plus paclitaxel was 2.9 months (95% CI, 2.3–3.6) and 4.4 months (95% CI, 4.1–4.7), respectively; for those treated with RAM plus FOLFIRI, the median PFS and OS was 5.9 months (95% CI, 0.4–11.4) and 8.3 months (95% CI, 6.6–10), respectively.
Schlintl et al. (18) performed a retrospective analysis of 16 patients with advanced or metastatic gastric cancer, who received treatment with RAM plus FOLFIRI or irinotecan. The median PFS and OS of all patients was 5.4 months (95% CI, 3.7–7.1) and 7.6 months (95% CI, 6.1–9.1), respectively. Patients receiving RAM plus FOLFIRI displayed a statistically significant longer OS compared with patients receiving RAM plus irinotecan, with a median of 15.2 months (95% CI, 4.7–25.7) versus 6.9 months (95% CI, 1.0–12.8; P=0.01), respectively. However, there was no statistically significant difference in the median PFS (5.4 versus 4.6 months, P=0.19).
An aggregated review of ORR was performed using random-effects meta-analyses. The pooled ORR was 25.4% (95% CI, 18.0–34.5) (Figure 1).
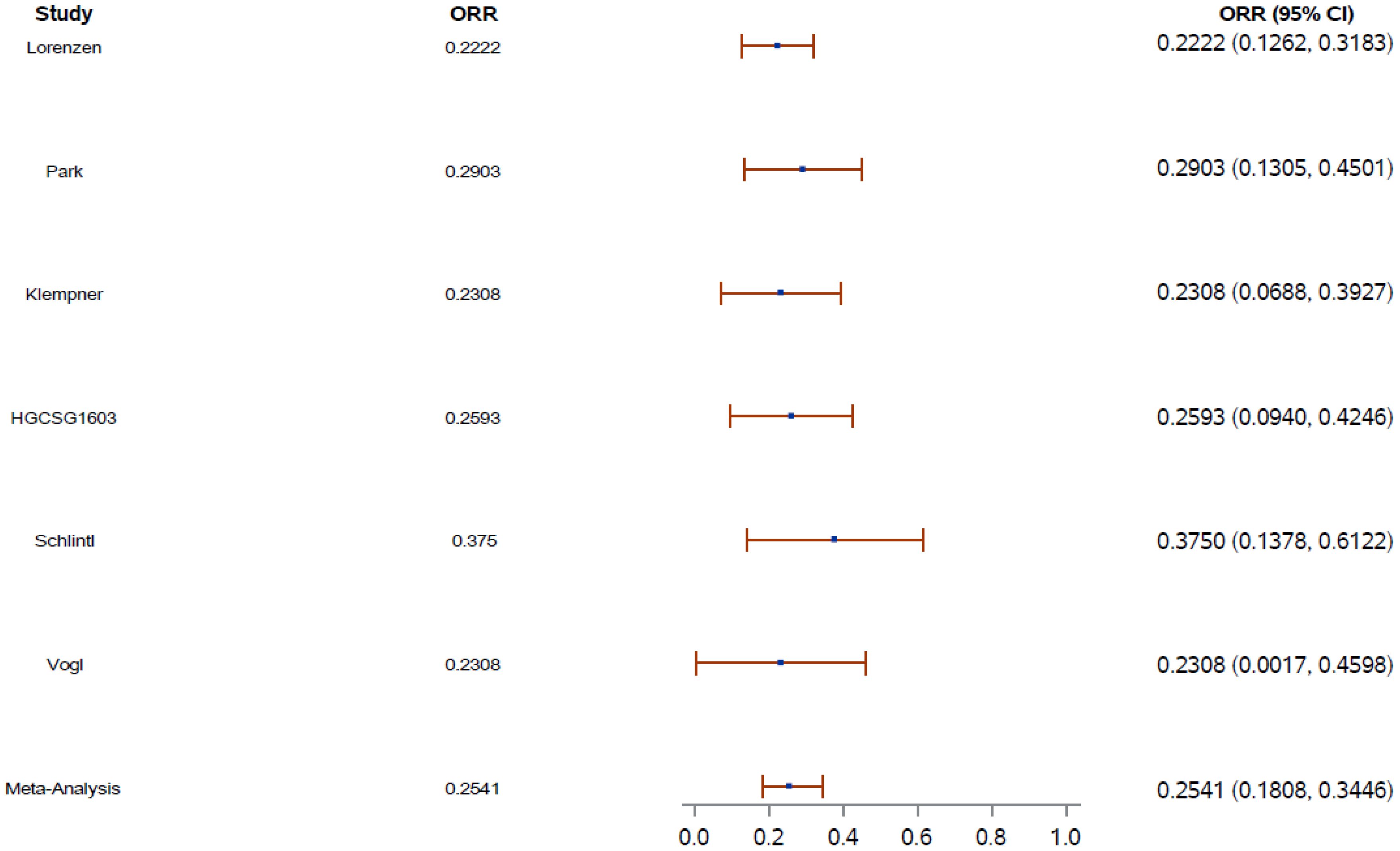
Figure 1. Overall response rate (ORR) aggregate weight (blue dot) on the basis of the number of patients in each study. Error shown as red bar. CI, confidence interval; ORR, overall response rate.
Three studies were identified which included patients with prior-taxane use. These studies evaluated ORR and PFS in patients treated with RAM plus FOLFIRI.
In the studies by Lorenzen et al. (13), and Park et al. (14) there was a numerical increase in the ORR with RAM plus FOLFIRI in patients pretreated with taxane versus patients who were taxane-naïve (Table 3). The study by Klempner (16) observed an improved ORR in patients who were taxane-naïve versus patients who were pretreated with taxane.
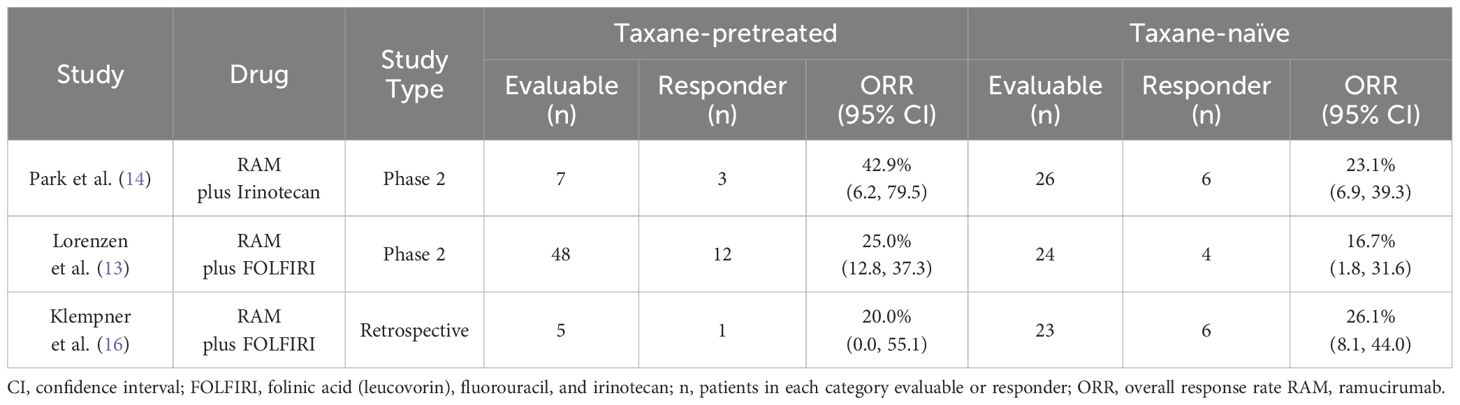
Table 3. Table comparing the overall response rate (ORR) of patients pretreated with taxane versus patients who were taxane-naïve in the studies by Park et al., Lorenzen et al., and Klempner et al.
In the study by Lorenzen et al. (13), for patients with prior docetaxel treatment (72/110), the median PFS was 4.6 months for patients treated with RAM plus FOLFIRI versus 2.1 months for patients treated with RAM plus paclitaxel, and the median OS was 7.5 months versus 6.6 months, respectively. Sixty-seven patients were evaluable for response and were pretreated with docetaxel. ORR was 25% in patients treated with RAM plus FOLFIRI and 8% in patients treated with RAM plus paclitaxel. Disease control rate was 65% and 38% for RAM plus FOLFIRI and RAM plus paclitaxel, respectively.
Vogl et al. (17) observed a trend towards prolonged PFS after perioperative taxane-based 5-FU, leucovorin, oxaliplatin, and docetaxel chemotherapy (N=12) with RAM plus FOLFIRI compared with RAM plus paclitaxel, with a median PFS of 5.6 months (95% CI, 4–7.8) and 2.9 months (95% CI, 1.6–4.3), respectively. In data from the study by Klempner et al. (16), there was an improved ORR (maximum partial responses) for patients who were taxane-naïve (partial response, 44.8%) versus patients who were pretreated with taxane (partial response, 20.7%). This may be because of the low number of patients pretreated with taxane included in the retrospective study.
The safety profile reported across all reviewed studies showed that with RAM plus FOLFIRI or irinotecan, the most common adverse event (AE) at any grade was neutropenia. In the clinical trial by Lorenzen et al. (13), the most common grade ≥3 AEs in patients treated with RAM plus FOLFIRI were neutropenia (N=12, 17%), leukopenia (N=3, 4%), diarrhea (N=7, 10%), and stomatitis (N=7, 10%). Of the patients treated with RAM plus FOLFIRI, 56% had at least 1 serious AE. In the study by Kawamoto et al. (15), the most common grade ≥3 AEs were neutropenia (N=18, 51%), leukopenia (N=15, 43%), anemia (N=7, 20%), anorexia (N=5, 14%), and febrile neutropenia (N=4, 11%). No deaths or new safety signals with a causal relation to the study treatment were observed. In the study by Park et al. (14), diarrhea (N=27, 68%), nausea (N=24, 60%), vomiting (N=18, 45%), and neutropenia (N=15, 38%) were common AEs; no grade 3 or 4 neuropathy was reported.
In the retrospective studies, Klempner et al. (16) found toxicities were largely grade 1 or 2, with only 6.9% developing grade 3 or 4 AEs (all fatigue, grade 3). Fatigue (76%), diarrhea (31%), anemia (24%), and neutropenia (14%) were the most common AEs, and there were no toxic deaths. Vogl et al. (17) found the most common grade 3 toxicity for patients treated with RAM plus FOLFIRI was neutropenia (44%), followed by diarrhea, fatigue, and polyneuropathy. Safety data were not available for the study by Schlintl et al. (18), however, only 1 patient discontinued RAM-based therapy because of toxicity.
As per NCCN guidelines, oxaliplatin-based regimens are generally preferred over cisplatin-based regimens as first-line therapy for locally advanced, recurrent, or metastatic gastric cancer. The preferred second-line therapy regimens include ramucirumab and paclitaxel, fam-trastuzumab deruxtecan-nxki for HER2 over expressive positive adenocarcinoma, docetaxel, paclitaxel, irinotecan, fluorouracil and irinotecan, and trifluridine and tipiracil for third-line or subsequent therapy. Careful consideration must be given when selecting a second-line therapy, particularly for safety, efficacy, and treatment compliance.
Ramucirumab plus FOLFIRI or irinotecan is a non-neurotoxic regimen comparing favorably with the combination of RAM plus paclitaxel used in the seminal RAINBOW trial (6). In this review, we examined multiple prospective phase 2 clinical trials and retrospective studies to analyze the data supporting RAM plus FOLFIRI or irinotecan as second-line therapy for patients with GEA. While the number of evaluable patients varied across these studies, ORR ranged from 22% to 38%, median PFS ranged from 3.9 to 6.0 months, and median OS ranged from 6.8 to 13.4 months.
The initial results from the phase 2 clinical trial by Lorenzen et al. provided a rationale for continuation of the trial as phase 3, which enrolls patients who were pretreated with taxane only and is currently recruiting (13, 19). Data reported by Park et al. and Kawamoto et al. demonstrated comparable efficacy outcomes as observed by Lorenzen et al. (13–15).
Vogl et al. (17) found that RAM plus FOLFIRI-treated patients showed favorable results with a better median PFS than RAM plus paclitaxel-treated patients (P=0.05). This highlights the potential for RAM plus FOLFIRI or irinotecan combinations as an alternative to treatment with taxanes, fulfilling a huge unmet clinical need for GEA patients. However, studies by both Lorenzen et al. and Vogl et al. showed that patients pretreated with taxanes had better outcomes when treated with RAM plus FOLFIRI combination than when treated with paclitaxel (13, 17). Overall, 2 of the 3 studies reported better ORR in patients pretreated with taxanes followed by RAM plus FOLFIRI. However, given the small sample sizes, the retrospective design and overlapping confidence intervals, no conclusions can be drawn from these results.
Further support for safety of RAM plus irinotecan as second-line therapy was also shown in a small phase 1b (N=6) Japanese trial (20). The authors found this regimen was well tolerated by patients with advanced gastric cancer. In addition, the RAISE trial with a large sample size of over 500 patients with metastatic colorectal cancer (progressed on or after first-line oxaliplatin-based therapy) treated with second-line RAM plus FOLFIRI showed that RAM plus FOLFIRI resulted in improved OS and was well tolerated with no new safety findings (12). Overall, treatment with RAM plus FOLFIRI or irinotecan was well tolerated by patients. The most common AE of any grade observed was netropenia, which is in line with RAM toxicity profiles known from FOLFIRI or irinotecan regimens (Table 4).
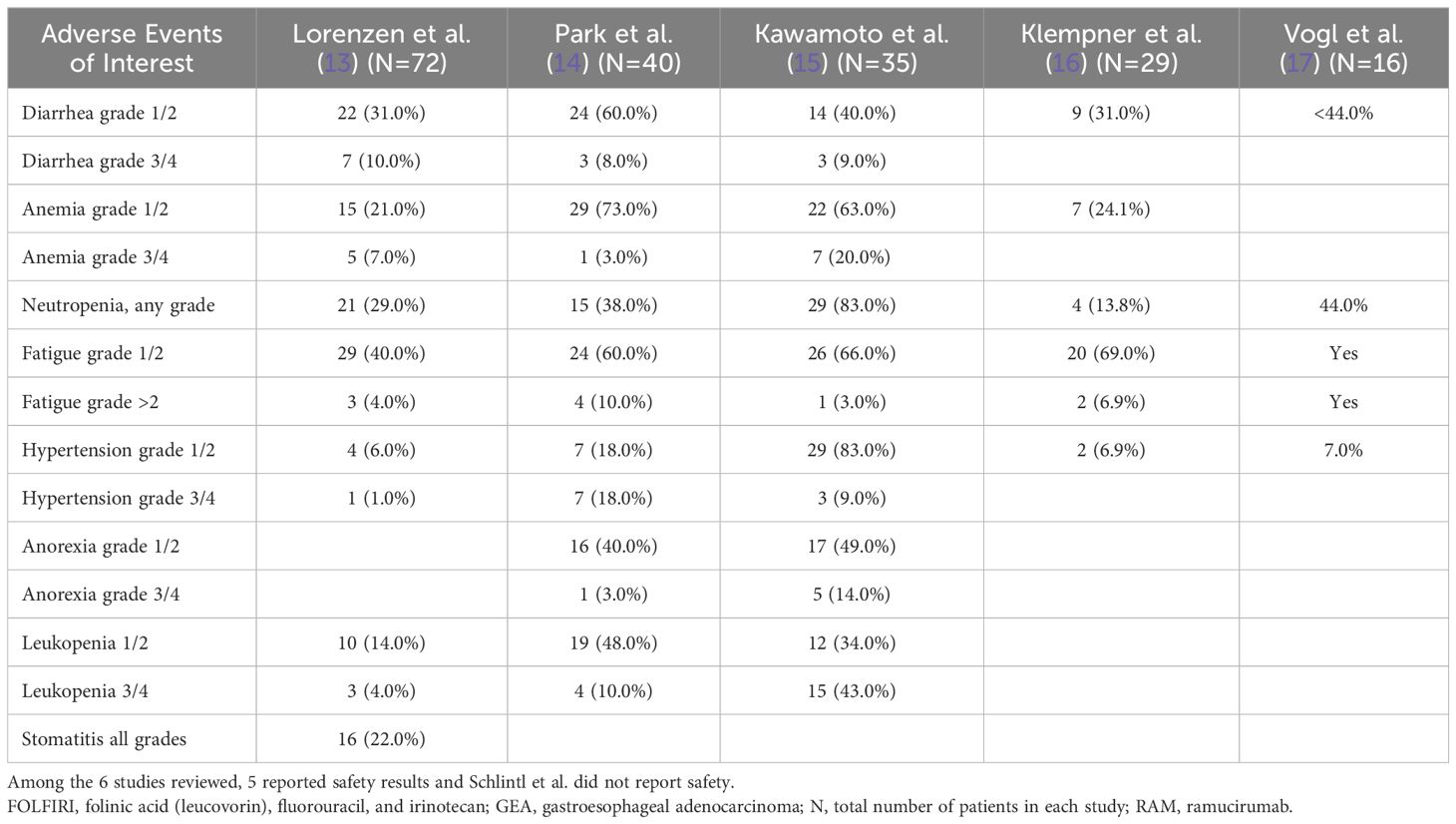
Table 4. Available toxicity profiles of cohorts of patients with advanced GEA receiving second-line RAM plus FOLFIRI or irinotecan, N (%).
The culmination of the available data to date, including work published by Klempner et al. (16), has resulted in the inclusion of RAM plus FOLFIRI or irinotecan in the NCCN Clinical Practice Guidelines for second-line treatment of GEA (3).
When analyzing these data, a number of additional factors should be considered, including duration of neuropathy, grade, resolution, and other comorbidities that can affect second-line efficacy outcomes. Additionally, the time between prior treatment (both taxane-pretreated and taxane-naïve) and FOLFIRI or irinotecan with RAM should be considered when determining the differences in effectiveness (ORR).
Given this, it is not possible to establish why differences in ORR are observed without speculation. In the study by Lorenzen et al. (13), a numerical increase in ORR was observed for patients who were pretreated with a taxane, however, these results are inconclusive given the small sample size.
Limitations of this study include the small sample sizes in the studies reviewed, and limited availability of data presented at congresses for some of the reports. The reviewed studies differed with respect to study design, eligibility, and response criteria. In addition, the studies were not designed to determine statistical differences in efficacy endpoints on the basis of prior-taxane versus naïve-taxane patient groups. Also, the studies reviewed did not have consistent RAM plus FOLFIRI or RAM plus irinotecan as comparator arms. A few studies had paclitaxel plus RAM as the comparator. To determine the benefit of the alternative strategies and make a definitive conclusion on RAM-based treatment regimens, the ideal comparator arm would be RAM plus FOLFIRI or RAM plus irinotecan. Despite the limitations, there are noteworthy strengths of this review such as the patients across the reviewed studies include a more representative patient sample, the baseline characteristics were generally consistent across all studies, and the patients across the reviewed studies were inclusive of multiple geographies. The first-line treatment landscape has evolved with recent approvals of CheckMate-649, KEYNOTE-590, and KEYNOTE-811 involving PD-1 inhibitor therapeutic options. With the utilization of frontline immune checkpoint inhibition regimens, the efficacy of subsequent RAM combinations remains an important consideration in treatment sequencing strategies. In a retrospective analysis, Sasaki et al. reported better efficacy in patients receiving RAM plus taxanes when exposed to prior anti-PD-1 treatments as compared with the reversed sequence (21). Similar data were presented by Kankeu Fonkoua et al. (22, 23) demonstrating predefined serial immunotherapy combinations followed by RAM plus taxanes provides efficacy benefits and may overcome resistance to PD-1 inhibitor therapy. An ongoing study is expected to further analyze these findings in a prospective setting (SEQUEL [NCT04069273]). Also, as noted earlier, there is an ongoing phase 2 RAMIRIS clinical trial, assessing the efficacy and safety of RAM plus FOLFIRI versus RAM plus paclitaxel in patients with previous taxane therapy (NCT03081143) which will provide additional data and further evidence.
The studies identified in this review suggest that patients previously treated with systemic therapy maintains benefits with RAM-based treatment regimens irrespective of prior-taxane use. This treatment strategy will especially benefit patients who become ineligible to receive RAM plus paclitaxel. Also, RAM-based treatment regimens are included in NCCN category 2A (lower levels of evidence, uniform expert opinion) recommendations.
While this review supports the safety and clinical benefit of RAM plus FOLFIRI or irinotecan combination on the basis of small clinical trials and retrospective analyses, a randomized phase 3 study would provide stronger evidence. Results from phase 3 trials and additional data are needed to provide additional evidence.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
HP: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. SK: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JC: Data curation, Writing – review & editing. ZW: Data curation, Formal analysis, Writing – review & editing. ML: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SB: Data curation, Methodology, Writing – original draft. AC: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
All authors were involved in the writing of the manuscript and its revisions. James Hegarty from Eli Lilly and Company and Deepika Kajarekar from Syneos Health provided medical writing assistance and editorial support.
HP received support for present manuscript writing support, provision of study materials, article processing charges from Eli Lilly and company; grants or contracts from Ambrx, Merck, GlaxoSmithKline, Bristol-Myers Squibb, Array BioPharma, Genentech, Xencor, PsiOxus Therapeutics, Oncologie, Turning Point Therapeutics, Gossamer Bio, MacroGenics, TopAlliance Biosciences, BJ Bioscience, Vedanta Biosciences, Daiichi Pharmaceutical, Novartis Pharmaceuticals, Synermore Biologics, Mirati Therapeutics, ImmuneOncia Therapeutics, Aprea Therapeutics AB, Seattle Genetics, Mabspace Biosciences, Elicio Therapeutics, Adlai Nortye USA, RePare Therapeutics, Exelixis, AstraZeneca, ImmunoGen, Fate Therapeutics, Tizona, Mersana, Bolt Biotherapeutics, StrataPATH, Chugai, Huaota, D3Bio and Amgen. SK received payment for advisory board from Eli Lilly and company; consulting fees from Amgen, Astellas, Merck, BMS, AstraZeneca, Daiichi-Sankyo, Taiho, Mersana, Pieris, Natera, Novartis, Sanofi-Aventis, Pfizer, Coherus, Exact Sciences; payment for lecture from Merck; advisory board unpaid member at Debbies Dream Foundation, Hope for Stomach Cancer; and panel unpaid member for NCCN Guidelines. JC medical writing support for current manuscript from Eli Lilly and company; received consulting fees from Bristol-Myers Squibb; payment for speakers’ bureau honoraria from Merck and Bristol-Myers Squibb; received DSMB member fees from Yiviva and Daiichi-Sankyo; employment and holds stocks from Amgen. ZW received consulting fees from Amgen, Astellas, Lilly, Novartis, Arcus, Pfizer, Seagen, Astra Zeneca, Daiichi, Merck, EMD Serono, and Alligator; support for attending meetings and/or travel from Amgen, and Merck; participation on a data safety monitoring board or advisory board from Pfizer, Daiichi, and Astra Zeneca. ML was an employee of Eli Lilly and Company during analysis and drafting of this manuscript. SC is an employee of Eli Lilly and Company and holds Eli Lilly stocks. SB is an employee of Eli Lilly and Company and holds restricted stock units from the company. AC is an employee of Eli Lilly and Company and holds Eli Lilly stocks.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Eli Lilly and Company. The funder had the following involvement in the study: analysis, interpretation of data, and the writing of this article (Syneos).
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AE, adverse event; 5-FU, fluorouracil; CI, confidence interval; FOLFIRI, folinic acid (leucovorin), fluorouracil (5-FU), and irinotecan; GEA, gastroesophageal adenocarcinoma; NCCN, National Comprehensive Cancer Network; ORR, overall response rate; OS, overall survival; PD-1, programmed cell death protein 1; PFS, progression-free survival; RAM, ramucirumab.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Al-Batran S-E, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. (2019) 393:1948–57. doi: 10.1016/S0140-6736(18)3255-1
3. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1005–20. doi: 10.1016/j.annonc.2022.07.004
4. National Comprehensive Cancer Network (NCCN). Gastric Cancer (Version 2.2022) (2022). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
5. Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. (2019) 37:1296–304. doi: 10.1200/JCO.18.01138
6. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. (2014) 15:1224–35. doi: 10.1016/S1470-2045(14)70420-6
7. Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. (2010) 28:780–7. doi: 10.1200/JCO.2009.23.7537
8. Mawalla B, Yuan X, Luo X, Chalya PL. Treatment outcome of anti-angiogenesis through VEGF-pathway in the management of gastric cancer: a systematic review of phase II and III clinical trials. BMC Res Notes. (2018) 11:21. doi: 10.1186/s13104-018-3137-8
9. Arnold D, Fuchs CS, Tabernero J, Ohtsu A, Zhu AX, Garon EB, et al. Meta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumab. Ann Oncol. (2017) 28:2932–42. doi: 10.1093/annonc/mdx514
10. Yoon HH, Bendell JC, Braiteh FS, Firdaus I, Philip PA, Cohn AL, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol. (2016) 27:2196–203. doi: 10.1093/annonc/mdw423
11. Lorenzen S, Stahl M, Hofheinz RD, Al-Batran SE, Lordick F. Influence of taxanes on treatment sequence in gastric cancer. Oncol Res Treat. (2020) 43:42–7. doi: 10.1159/000503428
12. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. (2015) 16:499–508. doi: 10.1016/S1470-2045(15)70127
13. Lorenzen S, Thuss-Patience P, Pauligk C, Gökkurt E, Ettrich T, Lordick F, et al. FOLFIRI plus ramucirumab versus paclitaxel plus ramucirumab as second-line therapy for patients with advanced or metastatic gastroesophageal adenocarcinoma with or without prior docetaxel - results from the phase II RAMIRIS Study of the German Gastric Cancer Study Group at AIO. Eur J Cancer. (2022) 165:48–57. doi: 10.1016/j.ejca.2022.01.015
14. Park H, Sanjeevaiah A, Hosein PJ, Mehta R, Jin R, Grierson P, et al. Ramucirumab and irinotecan in patients with previously treated gastroesophageal adenocarcinoma: final analysis of a phase II trial. J Clin Oncol. (2022) 40. doi: 10.1200/JCO.2022.40.4_supple.284
15. Kawamoto Y, Yuki S, Sawada K, Nakamura M, Muto O, Sogabe S, et al. Phase II study of ramucirumab plus irinotecan combination therapy as second-line treatment in patients with advanced gastric cancer: HGCSG1603. Oncologist. (2022) 27:e642–9. doi: 10.1093/oncolo/oyac086
16. Klempner SJ, Maron SB, Chase L, Lomnicki S, Wainberg ZA, Catenacci DVT. Initial report of second-line FOLFIRI in combination with ramucirumab in advanced gastroesophageal adenocarcinomas: a multi-institutional retrospective analysis. Oncologist. (2019) 24:475–82. doi: 10.1634/theoncologist.2018-0602
17. Vogl UM, Vormittag L, Winkler T, Kafka A, Weiser-Jasch O, Heinrich B, et al. Ramucirumab plus paclitaxel or FOLFIRI in platinum-refractory advanced or metastatic gastric or gastroesophageal junction adenocarcinoma—experience at two centres. J Gastrointest Oncol. (2020) 11:366–75. doi: 10.21037/jgo.2020.03.10
18. Schlintl V, Huemer F, Greil R, Weiss L. Ramucirumab plus FOLFIRI or irinotecan as second-line therapy in advanced or metastatic gastric or gastroesophageal junction adenocarcinoma. J Gastrointest Oncol. (2020) 12:906–9. doi: 10.21037/jgo-20-230
19. Al-Batran S-E, Götze TO, Dechow T, Goekkurt E, Algül H, Decker T, et al. 1499TiP FOLFIRI plus ramucirumab versus paclitaxel plus ramucirumab for taxane-pretreated patients with advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction as second-line therapy – The phase II/III RAMIRIS study. Ann Oncol. (2020) 31. doi: 10.1016/j.annonc.2020.08.2005
20. Satake H, Sagawa T, Fujikawa K, Hatachi Y, Yasui H, Koqtaka M, et al. Phase Ib study of irinotecan and ramucirumab for advanced gastric cancer previously treated with fluoropyrimidine with/without platinum and taxane. Cancer Chemother Pharmacol. (2018) 82:839–45. doi: 10.1007/s00280-018-3678-5
21. Sasaki A, Kawazoe A, Eto T, Okunaka M, Mishima S, Sawada K, et al. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open. (2020) 4:e000775. doi: 10.1136/esmoopen-2020-000775
22. Kankeu Fonkoua LA, Chakrabarti S, Sonbol MB, Kasi PM, Starr JS, Liu AJ, et al. Enhanced efficacy of anti-VEGFR2/taxane therapy after progression on immune checkpoint inhibition (ICI) in patients (pts) with metastatic gastroesophageal adenocarcinoma (mGEA). J Clin Oncol. (2020) 38:v. doi: 10.1200/JCO.2020.38.15_suppl.4541
Keywords: ramucirumab, gastroesophageal adenocarcinoma, second-line, irinotecan, FOLFIRI
Citation: Park H, Klempner SJ, Chao J, Wainberg ZA, Lukanowski M, Chenji S, Bourke S, Chatterjee A and Lorenzen S (2024) Ramucirumab plus FOLFIRI or irinotecan as second-line treatment for patients with gastroesophageal adenocarcinoma: a review and meta-analysis of an emerging option. Front. Oncol. 14:1419338. doi: 10.3389/fonc.2024.1419338
Received: 18 April 2024; Accepted: 22 July 2024;
Published: 13 August 2024.
Edited by:
René Thieme, Leipzig University, GermanyReviewed by:
Hossein Taghizadeh, Medical University of Vienna, AustriaCopyright © 2024 Park, Klempner, Chao, Wainberg, Lukanowski, Chenji, Bourke, Chatterjee and Lorenzen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haeseong Park, aGFlc2VvbmdfcGFya0BkZmNpLmhhcnZhcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.