- 1Department of Thoracic Oncology, Aichi Cancer Center, Nagoya, Japan
- 2Department of Pharmacy, Aichi Cancer Center, Nagoya, Japan
- 3Biostatistics Center, Kurume University, Kurume, Japan
- 4School of Medical Technology, Kurume University, Kurume, Japan
Interstitial lung disease (ILD) or pneumonitis caused by epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) or immune checkpoint inhibitors (ICI) is a major concern in the treatment of non-small cell lung cancer (NSCLC). Whether the addition of vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) inhibitors can reduce the incidence of drug-induced ILD remains unclear. We conducted a systematic review to assess the incidence of ILD induced by EGFR-TKIs or ICIs in the presence or absence of VEGF/VEGFR inhibitors in relevant randomized trials between January 2009 and October 2023. The primary outcome was the odds ratio for the incidence of ILD in all patients worldwide and Asians. Secondary outcomes were the odds ratios (ORs) of the incidence at grade-3 or higher ILD in all patients worldwide and Asians. We identified 13 randomized studies, one sub-analysis in the EGFR-TKI group, and three randomized studies in the ICI group. In the EGFR-TKI group, the OR of ILD incidence at any grade with VEGF/VEGFR inhibitors was 0.54 (95% CI, 0.32–0.90; p = 0.02), which represented a significantly lower incidence than that without VEGF/VEGFR inhibitors. Contrarily, the OR of ILD incidence at grade ≥ 3 with VEGF/VEGFR inhibitors was 1.00 (95% CI, 0.43–2.36; p = 0.99). In all subjects in the ICI group, the OR of ILD incidence at any grade with VEGF/VEGFR inhibitors was 0.78 (95% CI, 0.51–1.21; p = 0.27). The systematic review demonstrated that the addition of VEGF/VEGFR inhibitors could reduce the incidence of drug-induced ILD at any grade caused by EGFR-TKI in patients with NSCLC but could not reduce that at grade ≥ 3. The ILD induced by ICIs remains undetermined owing to the limited number of randomized trials for which ILD data are available.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=409534, identifier CRD42023409534.
Introduction
The discovery of molecular-targeted agents for driver gene alterations and immune checkpoint inhibitors (ICI) has led to a dramatic paradigm shift in therapeutic strategies for advanced non-small-cell lung cancer (NSCLC) (1–3). Currently, standard chemotherapy for advanced NSCLC is administered based on the molecular subtype of the tumor and the programmed death-ligand 1 (PD-L1) status in tumor cells. The most common druggable gene alteration in patients with NSCLC is epidermal growth factor receptor (EGFR) mutation, occurring in 30–50% of East Asians and 10–15% of Westerners (4). First- and second-generation EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib and afatinib, have demonstrated impressive clinical activity and superiority over chemotherapy in patients with EGFR-mutated NSCLC. The third-generation EGFR TKI; osimertinib, potently and selectively inhibits both EGFR-TKI sensitizing and EGFR T790M resistance mutations. Randomized phase 3 studies of AURA3 and FLAURA demonstrated that osimertinib is indicated as the first-line treatment for metastatic patients with NSCLC having EGFR sensitizing mutations and for patients with metastatic NSCLC having EGFR T790M mutations whose disease has progressed or after the treatment with first- or second-generation EGFR TKI (5, 6). However, notable toxicities related to EGFR TKIs includes acneiform rash, diarrhea, paronychia, and interstitial lung disease (ILD)/pneumonitis which can lead to fatal outcomes. The incidence of ILD with the first- and second-generation EGFR-TKI has been reported to occur in 3–5% of East Asians, which is substantially higher than in Caucasians (7, 8). Osimertinib-induced ILD in Japanese patients has been reported to occur in 12–18% for any grades and 2–4.6% for grade 3 or higher (9, 10).
ICIs, such as cytotoxic T-lymphocyte antigen-4, programmed cell death protein-1 (PD-1), and its ligand PD-L1, target down-regulators of the anticancer immune response, unleashing the host immune reaction against tumor cells by T-cell activation. ICIs generate unique immune-mediated side-effects, called immune-related adverse events (irAEs), in almost all organs (11). ICI-induced ILD is a major concern, particularly in patients with NSCLC. Its incidence is 3–5%, regardless of race and may lead to fatal outcomes; therefore, careful attention is warranted (12–14).
Tumor angiogenesis is indispensable for tumor proliferation and metastasis. Abnormalities in the vascular endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR) pathway have been recognized as key factors in tumor angiogenesis (15, 16). In the treatment of NSCLC, several monoclonal antibodies, such as bevacizumab and ramucirumab, and small-molecule inhibitors, such as vandetanib, sunitinib, nintedanib, and anlotinib, have been developed to interrupt the interaction between VEGF and its receptors in order to achieve a therapeutic effect (17, 18). Furthermore, VEGF and EGF share common downstream signaling pathways and may function exclusively with each other during tumor progression and acquired therapeutic resistance (19, 20). Therefore, the approaches toward dual blockade of these molecular targets have been developed to demonstrate promising results in the treatment of advanced NSCLC. Several randomized studies have demonstrated the combination therapy with EGFR and VEGF/VEGFR inhibitors has superior progression-free survival than that with EGFR inhibitors in patients with untreated EGFR-mutated metastatic NSCLC (21–24). In cancer immunotherapy, VEGF is not only an important angiogenic factor but also an immunomodulator of the tumor microenvironment (TME). VEGFs can suppress antigen presentation and stimulate the activity of regulatory T (Treg) cells, and tumor-associated macrophages, which in turn promote an immunosuppressive microenvironment in NSCLC (25–27). Therefore, blockade of the VEGF/VEGFR pathway may overcome therapeutic resistance to ICIs. Several randomized studies have demonstrated that combination therapy with ICIs and VEGF/VEGFR inhibitors resulted in superior progression-free survival than that with ICIs alone in patients with metastatic NSCLC (28, 29).
Combination therapy with VEGF/VEGFR inhibitors generally causes adverse events more frequently, and of higher grade, than monotherapy. However, some randomized studies have demonstrated a lower incidence and grade of ILD caused by EGFR TKI in combination therapy with VEGF/VEGFR inhibitors than in monotherapy, although the underlying reasons are still unknown (24, 30). Since the key endpoints in these studies were the efficacy of progression-free survival and overall survival, statistical power of the incidence of ILD was low. In this review, we reported that the addition of VEGF/VEGFR inhibitors can reduce the incidence of drug-induced ILD caused by EGFR TKI or ICI in patients with NSCLC.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (31) and was prospectively registered at PROSPERO (CRD42023409534).
Primary and secondary objectives
The primary objective was to understand the odds ratio (OR) of the incidence of ILD induced by EGFR-TKIs and ICIs in all patients worldwide and Asians. Secondary objectives to explore the odds ratios of the incidence of ILD at grade 3 or higher induced by EGFR-TKIs and ICIs in all patients worldwide and Asians.
Eligibility criteria
Potentially eligible studies had to meet the following criteria: 1) randomized phase 2 or 3 studies in advanced or metastatic (stage IIIB, IIIC, IV, and postoperative recurrence) NSCLC treated with either EGFR-TKI or ICI; 2) studies published in English from January 1st, 2009, to October 31st, 2023; 3) availability of data on the incidence and grade of drug-induced ILD such as pneumonitis, pneumonia, and acute lung injury (The data on lung infection and respiratory tract infection were excluded from drug-induced ILD); 4-a) In EGFR-TKI group, the control arm was an EGFR TKI (± placebo) and the experimental (VEGF/VEGFR) arm was EGFR TKI plus VEGF/VEGFR inhibitors; 4-b) In ICI group, the control arm was an ICI ± cytotoxic chemotherapy (± placebo) and the experimental (VEGF/VEGFR) arm was an ICI ± cytotoxic chemotherapy plus VEGF/VEGFR inhibitors.
Studies were excluded if they met the following criteria: 1) studies of combination therapy with EGFR-TKIs and ICIs; and 2) retrospective studies, reviews, case reports, meta-analyses, or non-English publications.
Institutional review board approval and written informed consent are not required for database analysis.
Search strategy
A systematic review was conducted using medical subject heading terms and text words related to EGFR inhibitors, ICI, and VEGF inhibitors in lung cancer while searching for relevant publications or presentations between January 2009 and October 2023 across PubMed, EMBASE, and the Cochrane Controlled Trials Register. Additionally, we analyzed abstracts and presentation contents from the meetings of the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), the World Conference on Lung Cancer (WCLC), and other pivotal annual meetings for lung cancer.
The search terms are listed in Supplementary Table S1. Publications and abstracts were extracted based on the eligibility criteria, and the data were reviewed thereafter for discrepancies and inconsistencies. In case of duplicate publications, the most recent version of the publication was included, with older publications referred to only if the primary or secondary outcomes were not reported in the most recent study.
Data collection process
From all the included studies, we collected the basic information, including the authors’ names, date of publication, name of publication journal or meeting, phase of the study, therapeutic regimen, treatment line, subject size (number), participants’ characteristics (race, age, sex, smoking status, ECOG-PS, EGFR mutation type, PD-L1 TPS, etc.), and the incidence and grade of ILD/pneumonitis.
Quality analysis
A risk-of-bias table was generated for the included trials using the Cochrane risk-of-bias domains of random sequence generation, allocation concealment, blinding of participants/physicians, blinding of outcome assessment, incomplete outcome data, and selective reporting (32). Quality of the method, the risk of bias in the study, and the discrepancies and inconsistencies were independently reviewed in this study.
Statistical analysis
We calculated the odds ratios (ORs) and 95% confidence intervals (CIs) for ILD from all studies and synthesized the data. We used a random-effects model to perform the meta-analysis. Heterogeneity among studies was assessed using chi-square-based Q statistics and I² statistics. We used funnel plot to investigate potential publication bias, when there were 10 or more research articles. All statistical analyses were performed using Review Manager Software, version 5.4 (The Cochrane Collaboration, Copenhagen: The Nordic Cochrane Centre, 2020). A P value of < 0.05 indicated statistically significant difference.
Results
Study selection
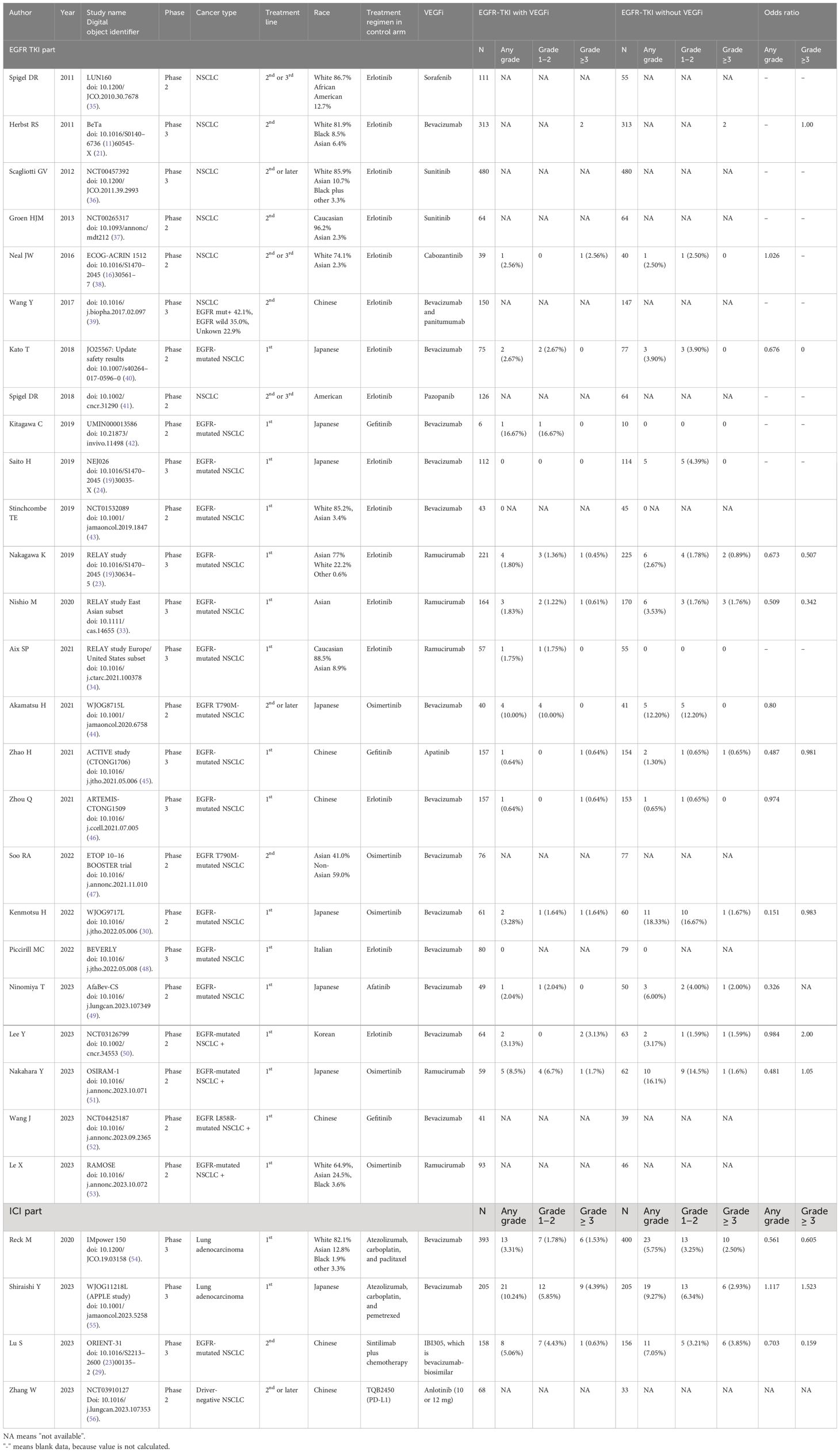
Our search extracted 119 publications in the EGFR-TKI group and 26 publications in the ICI group between January 2009 and October 2023. After removing duplicates and studies that did not meet the inclusion criteria, we identified 23 randomized studies in the EGFR-TKI group and four randomized studies in the ICI group, based on the eligibility criteria. In a randomized phase III RELAY study of ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced NSCLC, the results were published after primary analysis, performed separately in two sub-analyses for the East Asian and Europe/United States subsets (23, 33, 34). A total of 29 publications including these sub-analyses, are listed in Table 1. Thirteen studies and one sub-analysis in the EGFR-TKI group and three in the ICI group were extracted, owing to the selection of studies for which ILD data were available in the publications (Figure 1). Among the studies, BeTa study did not include ILD data for any grade, except for grade ≥ 3 (21). Therefore, we performed a meta-analysis of the EGFR-TKI group to evaluate the OR of ILD incidence for any grade across 12 studies and one sub-analysis, and to evaluate the OR of ILD incidence for grade ≥ 3 in 13 studies and one sub-analysis.
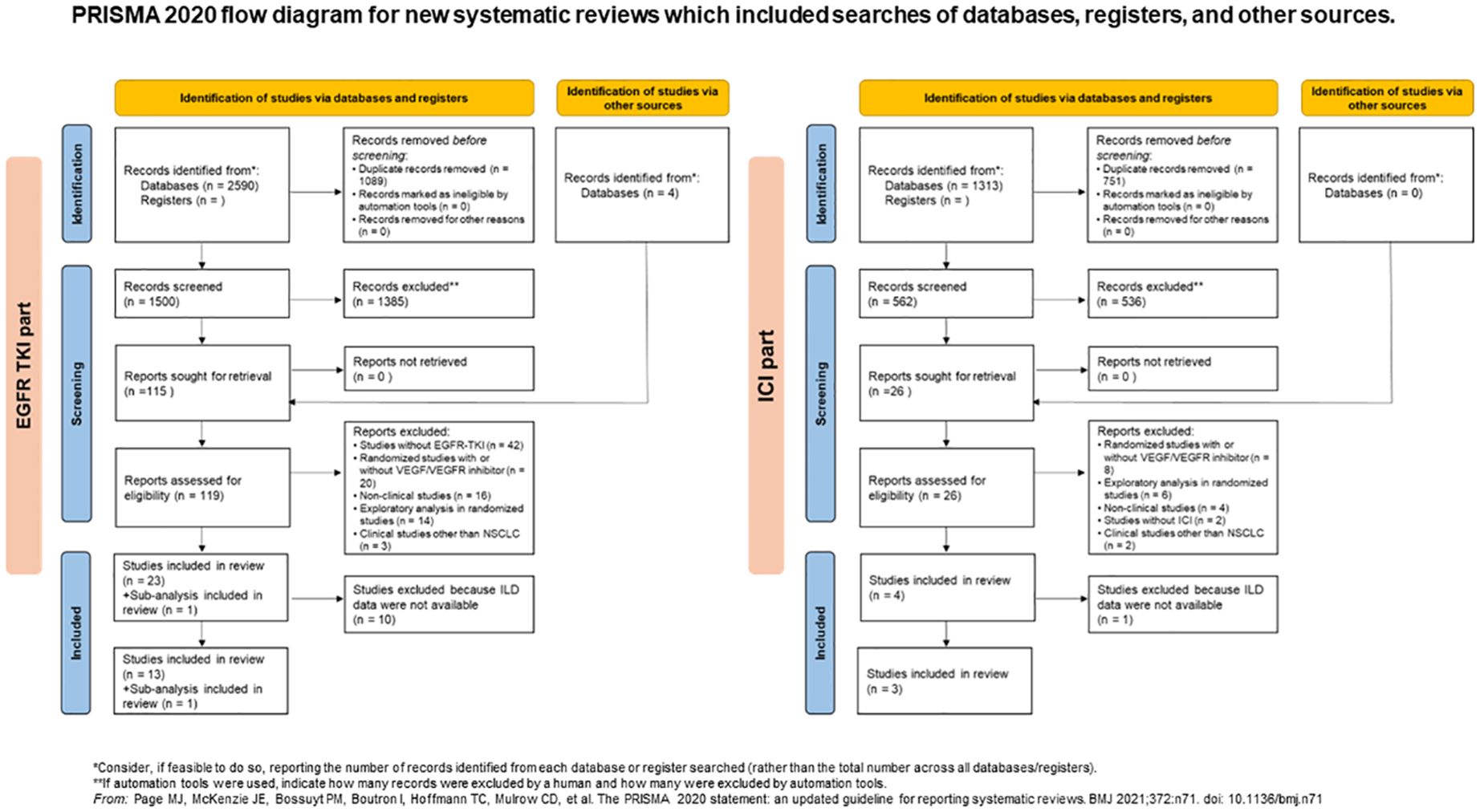
Figure 1 PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers, and other sources. EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor, interstitial lung disease; ILD, interstitial lung disease; NSCLC, non-small lung cancer; TKI, tyrosine kinase inhibitor.
Study characteristics
The breakdown of EGFR-TKIs was as follows: erlotinib in seven of 13 studies, osimertinib in three studies, gefitinib in two studies, afatinib in one study, VEGF/VEGFR inhibitors in nine studies, ramucirumab in two studies, and VEGF TKIs in two studies. In the ICI group, the ICIs in combination with cytotoxic chemotherapy were atezolizumab in two studies and sintilimab in one study whereas all VEGF/VEGFR inhibitors were bevacizumab or its biosimilar antibody.
Thirteen studies in the EGFR-TKI group included 2,715 patients (1,353 in the VEGF/VEGFR arm and 1,362 in the control arm), with individual study sizes varying from 16 to 626 patients. Three studies in the ICI group included 1,517 patients (756 in the VEGF/VEGFR arm and 761 in the control arm), with individual study sizes ranging from 314 to 793 patients. Among the 13 studies in the EGFR-TKI group, ten studies and one sub-analysis were conducted only in Asian countries (7 in Japan, 2 in China, one in Korea, and one in an East Asian country). Eleven Asian studies, along with one sub-analysis, included 1,898 patients (944 in the VEGF/VEGFR arm and 954 in the control arm), with individual study sizes varying from 16 to 334 patients. Among the three studies in the ICI group, two were conducted in Asian countries (one each in China and Japan). The studies included 724 patients (363 and 361 in the VEGF/VEGFR and control arms, respectively).
Risk of bias in studies
Since the 13 studies were extracted from the EGFR-TKI group, funnel plots were used to evaluate the publication bias. However, since only three studies were extracted from the ICI group, further analyses were performed to evaluate the potential risk. Visual inspection of the funnel plots for the ORs in the EGFR-TKI group revealed no asymmetry (Figure 2). Our understanding about each risk-of-bias item is presented as a percentage across all included studies that showed a low risk of bias.
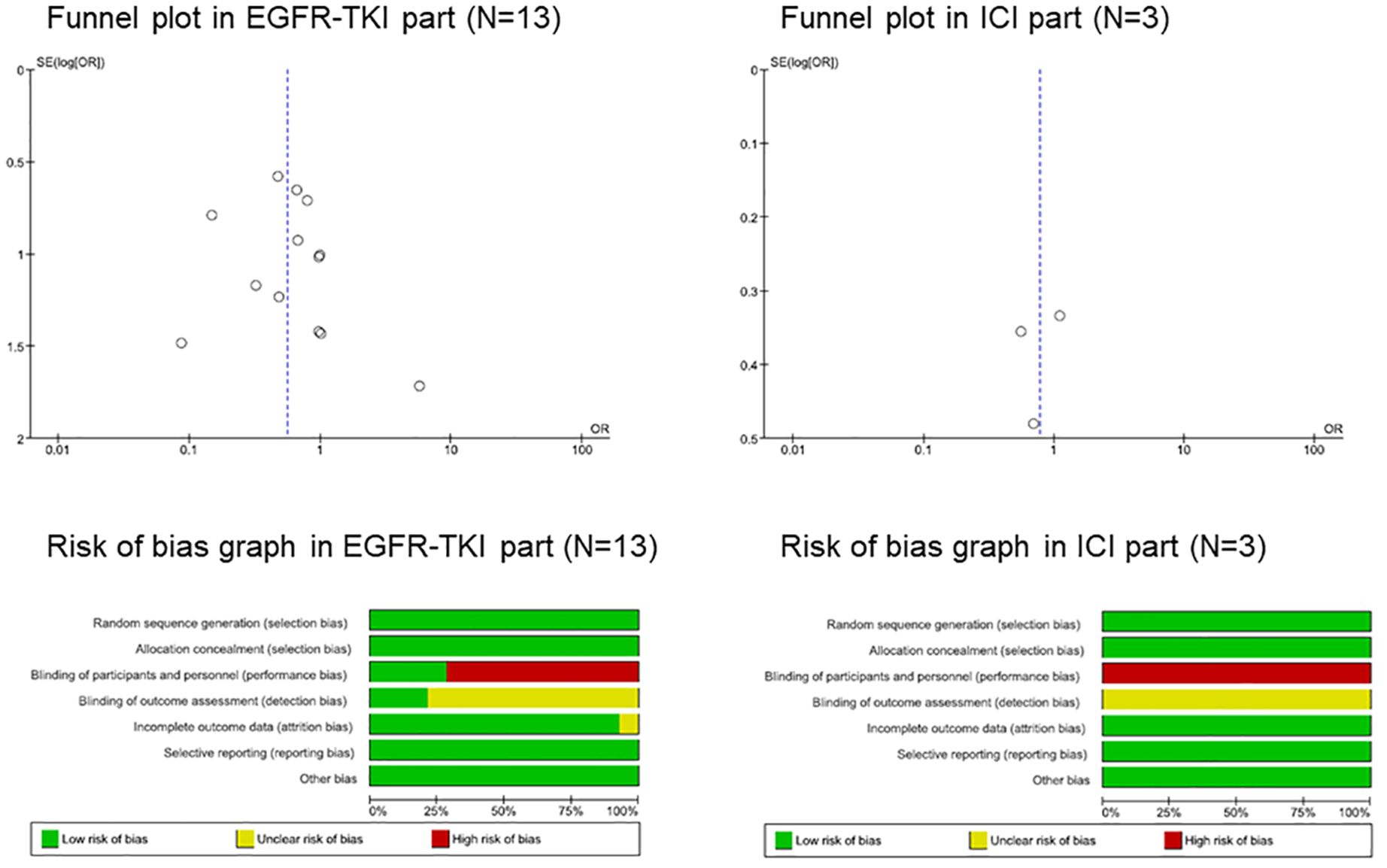
Figure 2 Funnel plot and risk of bias graph. EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor, interstitial lung disease; TKI, tyrosine kinase inhibitor.
Randomized trials can be divided into double-blind, placebo-controlled, and open-label. We classified the blinding of participants and personnel (performance bias) as low-risk in double-blind placebo-controlled trials and as high-risk in open-label trials, and the blinding of outcome assessment (detection bias) as low-risk in double-blind placebo-controlled trials and uncertainty risk in open-label trials (Figure 2; Supplementary Figure S1).
Results of individual studies
Meta-analysis was performed to evaluate the OR of ILD incidence with or without VEGF/VEGFR inhibitors using a random-effects model (Figure 3A). In all subjects in the EGFR-TKI group, the OR of ILD incidence at any grade with VEGF/VEGFR inhibitors was 0.54 (95% CI, 0.32–0.90; p = 0.02), which represented a significantly lower incidence than that without VEGF/VEGFR inhibitors. On the other hand, the OR of ILD incidence at grade ≥ 3 with VEGF/VEGFR inhibitors was 1.00 (95% CI, 0.43–2.36; p = 0.99), which did not represent a significant incidence compared to that without VEGF/VEGFR inhibitors (Figure 3B).
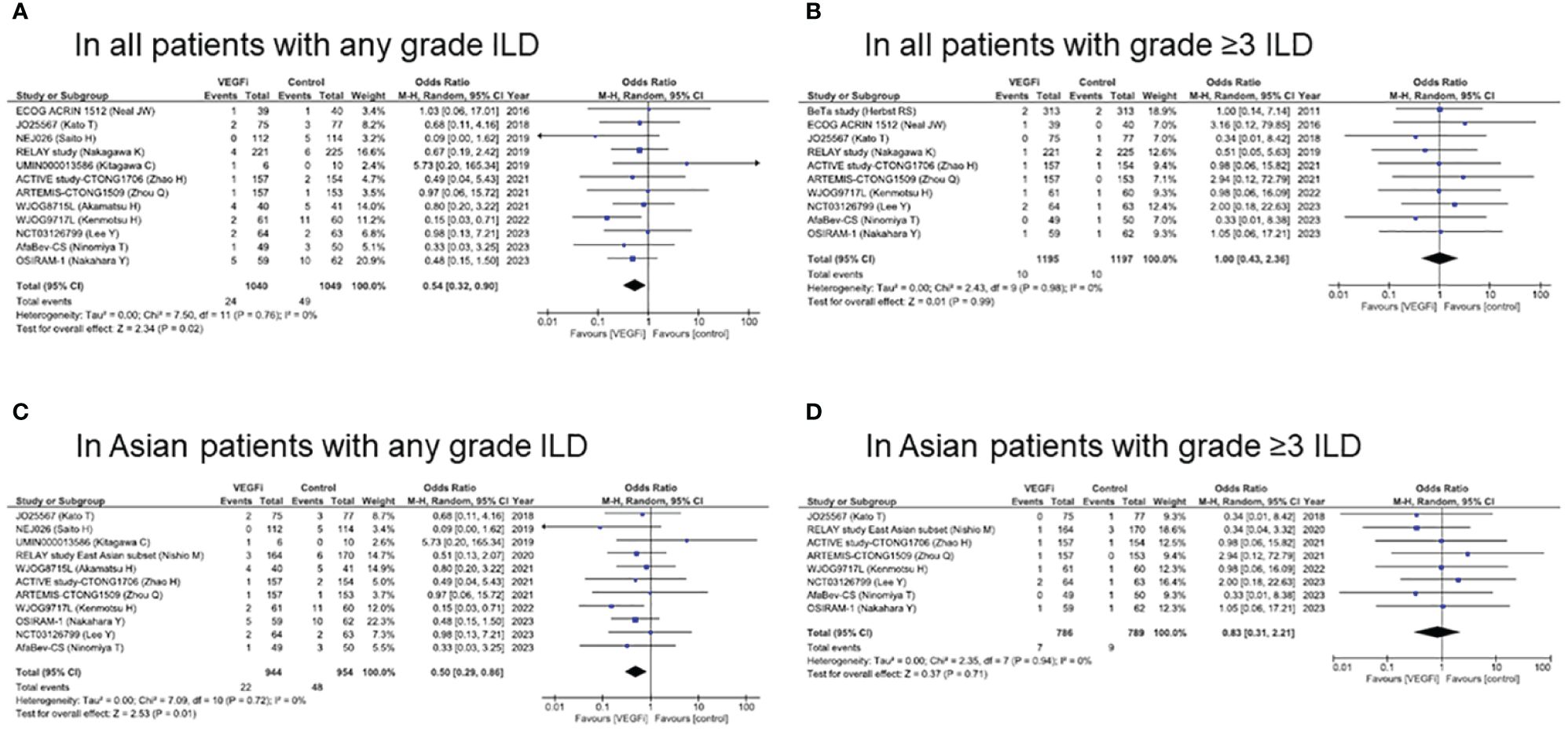
Figure 3 Forest plot and pooled odds ratio of ILD caused by EGFR-TKI in all patients (A, B) and in Asian patients (C, D) with/without VEGF/VEGFR inhibitors. EGFR, epidermal growth factor receptor; ILD, interstitial lung disease; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
In Asian subjects in the EGFR-TKI group, the OR of ILD incidence at any grade with VEGF/VEGFR inhibitors was 0.50 (95% CI, 0.29–0.86; p = 0.01), which indicated a significantly lower incidence than that without VEGF/VEGFR inhibitors (Figure 3C). On the other hand, the OR of ILD incidence at grade ≥ 3 with VEGF/VEGFR inhibitors was 0.83 (95% CI, 0.31–2.21; p = 0.71) (Figure 3D). Forest plots by each EGFR-TKI and by each VEGF/VEGFR inhibitors were presented in Supplementary Figures S2, S3, respectively. In all subjects treated with erlotinib (n=7), the OR of ILD incidence at any grade with VEGF/VEGFR inhibitors was 0.70 (95% CI, 0.33–1.47; p = 0.34). In all subjects treated with EGFR-TKI, the OR of ILD incidence at any grade with bevacizumab (n=8) was 0.51 (95% CI, 0.23–1.03; p = 0.06).
In all subjects in the ICI group, the OR of ILD incidence at any grade with VEGF/VEGFR inhibitors was 0.78 (95% CI, 0.51–1.21; p = 0.27) and that at grade ≥ 3 with VEGF/VEGFR inhibitors was 0.69 (95% CI, 0.24–1.98; p = 0.49), which was not a significant incidence (Supplementary Figure S4). Additionally, in all subjects with advanced or metastatic NSCLC treated with either EGFR-TKI or ICI, the OR of ILD incidence at any grade with VEGF/VEGFR inhibitors was 0.67 (95% CI, 0.49–0.94; p = 0.02), which showed a significantly lower incidence than that without VEGF/VEGFR inhibitors. On the other hand, the OR of ILD incidence at grade ≥ 3 with VEGF/VEGFR inhibitors was 0.87 (95% CI, 0.51–1.48; p = 0.60), which did not indicate a significant incidence.
Discussion
This systematic review reported that the addition of VEGF/VEGFR inhibitors could reduce the incidence of drug-induced ILD at any grade caused by EGFR-TKI in patients with NSCLC, but not of those at grade ≥ 3, although odds ratio varied across clinical studies. Although the tendencies were maintained in an additional integrated analysis of drug-induced ILD caused by either EGFR-TKI or ICI, whether the incidence of drug-induced ILD caused by ICI could be reduced by VEGF/VEGFR inhibitors is yet to be determined due to the limited number of randomized trials with ILD data available.
A previous systematic review of the efficacy and toxicity in patients with EGFR-mutated NSCLC had shown that combined inhibition of the EGFR and VEGF pathways significantly increased any grade 3–4 toxicity compared to EGFR inhibition alone (57). However, the current systematic review did not refer to drug-induced ILD/pneumonitis at grade 1–2 toxicities. Another systematic review had investigated whether the addition of bevacizumab could reduce the incidence of drug-induced ILD at any grade caused by cancer drug therapies, including cytotoxic agents, antibodies, TKI, and ICI in patients with malignant solid tumors. The systematic review demonstrated that the odds ratio for ILD in the bevacizumab group was 0.62 (95% CI 0.42–0.92; p = 0.02), which showed a significantly lower incidence than in the control. This tendency was observed in the targeted therapy groups but not in the cytotoxic agent groups. The three systematic reviews mentioned above, including ours, consisted of many overlapping clinical trials identified by their respective database searches. However, the clinical questions addressed in each systematic review differed. ILD induced by anti-cancer agents is known to occur more frequently in lung cancer than in other solid tumors (23, 58–62). Therefore, we concluded that the addition of VEGF/VEGFR inhibitors could reduce the incidence of drug-induced ILD caused by EGFR-TKI or ICI in patients with NSCLC.
VEGF is considered to play an important role in pathogenesis of acute exacerbation of interstitial pulmonary fibrosis (63). In preclinical model, VEGF expression is associated with angiogenesis and positive remodeling of damaged tissues, which increase vascular permeability and pulmonary edema, resulting in acute lung injury (64). The VEGF inhibitor CBO-P11 suppressed the expression of key mediators of pro- and antifibrotic responses in a bleomycin-induced pulmonary fibrosis model (65). On the other hand, an imbalance in VEGF splice isoforms has been reported to be important in the development of pulmonary fibrosis (66). Clinical studies has shown that nintedanib, a multi-targeted TKI of VEGF, PDGF, and FGF, is effective in reducing the decline of forced vital capacity in patients with progressive fibrosing ILD and decreasing the events of ILD progression (67–69). However, in the J-SONIC study for advanced NSCLC with idiopathic pulmonary fibrosis, nintedanib plus chemotherapy did not improve the exacerbation-free survival compared with chemotherapy alone (HR 0.89, 90% CI 0.67–1.17; p=0.24) (70). The mechanisms by which VEGF inhibitors reduce the incidence of drug-induced ILD remains to be elucidated.
The prognosis of NSCLC has improved dramatically with the advent of molecular-targeted agents for patients with driver gene alterations and with the use of immune checkpoint inhibitors for patients without driver gene alterations. However, since severe adverse events can lead to fatal outcomes, drug-induced ILD or pneumonitis is a major concern in cancer treatment. In the clinical management of patients receiving molecular targeting agents and ICIs, the diagnosis of drug-induced ILD is performed using high-resolution CT and is usually achieved by excluding other potential known causes, such as infection or disease progression of primary cancer (71). When drug-induced ILD develops, the severity is assessed according to CTCAE, and careful monitoring and treatment, including corticosteroids and other immunosuppressive therapies, is initiated along with supportive measures, including supplemental oxygen and intensive care, based on its severity, suspected agent, and risk factors. The incidence of EGFR-TKI-induced ILD is genetically different between East Asians and Caucasians (7, 72–74). Therefore, drug-induced ILD is a major concern, particularly in East Asian patients with NSCLC.
Our current systematic review had a few limitations. First, the primary endpoint in almost all randomized trials was PFS or OS, not an adverse event, including drug-induced ILD. We analyzed 75 ILD events in the EGFR-TKI group and 94 ILD events in the ICI group included in our systematic review, which in general does not represent a large number of events. Second, the clinical characteristics were not the same in all comparison groups. Our systematic review included a variety of NSCLC types, including EGFR-mutated and wild-type, and treatment with EGFR inhibitors, ICI, and VEGF inhibitors. The incidence of ILD with the first- and second-generation EGFR-TKI has been reported to occur in 3–5% of East Asians and the incidence of ILD with osimertinib in 12–18% of East Asians (7–10). Such heterogeneity could lead to the possibility of bias from one trial to another. It is the limitation in this systematic review. Third, there are many differences between East Asian and Western countries in the reporting criteria for ILD and in the concerns among medical professionals regarding drug-induced ILD. In the phase 3 PACIFIC study of durvalumab in patients with locally advanced NSCLC after concurrent chemoradiotherapy, the incidence of any-grade pneumonitis was 33.9% in all patients and 73.6% in the Japanese subgroup, although the incidence of pneumonitis at grade ≥ 3 was 3.4% in all patients and 5.6% in the Japanese subgroup (75). In Japan, ILDs at grade 1 may have been reported more frequently owing to concerns about adverse effects. However, drug-induced ILD has not yet been assessed in many Western countries.
In conclusion, this systematic review demonstrated that the addition of VEGF/VEGFR inhibitors could reduce the incidence of drug-induced ILD at any grade caused by EGFR-TKI in patients with NSCLC, but not in grade ≥ 3. In ICI-induced ILD, whether the incidence could be reduced remains to be determined owing to the limited number of randomized trials for which ILD data are available.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. KS: Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TY: Conceptualization, Methodology, Writing – review & editing. JS: Writing – review & editing. NW: Writing – review & editing. RM: Writing – review & editing. KM: Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. YH: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.com) for the English language editing.
Conflict of interest
YF reports receiving personal fees for honoraria for lectures from AstraZeneca, Amgen, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Eli Lilly, Merck Biopharma, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, Pfizer, Takeda, and Taiho Pharmaceutical, outside the submitted work; reports receiving personal fees for being on the advisory board from AstraZeneca, Chiome Bioscience, Daiichi-Sankyo, Micron, Otsuka Pharmaceutical, and Ono Pharmaceutical, outside the submitted work; reports receiving research grants from Abbvie, Amgen, AnHeart, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Eli Lilly, Incyte, Merck KGaA, Merck Sharp & Dohme, and Taiho Pharmaceutical, outside the submitted work. TY reports receiving personal fees for honoraria for lectures from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck Biopharma, Merck Sharp & Dohme, Ono Pharmaceutical, and Taiho Pharmaceutical, outside the submitted work; reports receiving personal fees for being on the advisory board from Daiichi-Sankyo. JS reports receiving personal fees for honoraria for lectures from AstraZeneca, Amgen, Chugai, Merck Biopharma, Merck Sharp & Dohme, Novartis, Pfizer, Taiho Pharmaceutical and Takeda Pharmaceutical, outside the submitted work. RM reports receiving personal fees for honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Chugai, Eli Lilly, Merck Sharp & Dohme, Ono Pharmaceutical, Pfizer and Taiho Pharmaceutical, outside the submitted work. KM reports receiving personal fees for honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi-Sankyo, Merck Sharp & Dohme, Pfizer and Taiho Pharmaceutical, outside the submitted work; reports receiving personal fees for consulting fees from Otsuka Pharmaceutical. YH reports receiving research grants from Eli Lilly.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1419256/full#supplementary-material
Supplementary Table 1 | Literature search strategy. EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor, interstitial lung disease; TKI, tyrosine kinase inhibitor.
Supplementary Figure 2 | Forest plot and pooled odds ratio of ILD by EGFR-TKI with/without VEGF/VEGFR inhibitors. (A) erlotinib, (B) osimertinib, (C) gefitinib, and (D) afatinib.
Supplementary Figure 3 | Forest plot and pooled odds ratio of ILD by EGFR-TKI with/without VEGF/VEGFR inhibitors. (A) bevacizumab and (B) ramucirumab.
References
1. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:358–76. doi: 10.1016/j.annonc.2022.12.013
2. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:339–57. doi: 10.1016/j.annonc.2022.12.009
3. Singh N, Temin S, Baker S Jr., Blanchard E, Brahmer JR, Celano P, et al. Therapy for stage iv non-small-cell lung cancer without driver alterations: asco living guideline. J Clin Oncol. (2022) 40:3323–43. doi: 10.1200/JCO.22.00825
4. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. (2022) 40:611–25. doi: 10.1200/JCO.21.01626
5. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in egfr T790m-positive lung cancer. N Engl J Med. (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
6. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, egfr-mutated advanced nsclc. N Engl J Med. (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
7. Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, et al. Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case-control study. Am J Respir Crit Care Med. (2008) 177:1348–57. doi: 10.1164/rccm.200710–1501OC
8. Gemma A, Kudoh S, Ando M, Ohe Y, Nakagawa K, Johkoh T, et al. Final safety and efficacy of erlotinib in the phase 4 polarstar surveillance study of 10 708 Japanese patients with non-small-cell lung cancer. Cancer Sci. (2014) 105:1584–90. doi: 10.1111/cas.12550
9. Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T, et al. Osimertinib versus standard-of-care egfr-tki as first-line treatment for egfrm advanced nsclc: flaura Japanese subset. Jpn J Clin Oncol. (2019) 49:29–36. doi: 10.1093/jjco/hyy179
10. Sato Y, Sumikawa H, Shibaki R, Morimoto T, Sakata Y, Oya Y, et al. Drug-related pneumonitis induced by osimertinib as first-line treatment for epidermal growth factor receptor mutation-positive non-small cell lung cancer: A real-world setting. Chest. (2022) 162:1188–98. doi: 10.1016/j.chest.2022.05.035
11. Sharma P, Allison JP. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. (2020) 20:75–6. doi: 10.1038/s41577–020-0275–8
12. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
13. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
14. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: asco guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
15. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
16. Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discovery. (2016) 15:385–403. doi: 10.1038/nrd.2015.17
17. Manzo A, Montanino A, Carillio G, Costanzo R, Sandomenico C, Normanno N, et al. Angiogenesis inhibitors in nsclc. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18102021
18. Malapelle U, Rossi A. Emerging angiogenesis inhibitors for non-small cell lung cancer. Expert Opin Emerg Drugs. (2019) 24:71–81. doi: 10.1080/14728214.2019.1619696
19. Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, et al. Dual egfr-vegf pathway inhibition: A promising strategy for patients with egfr-mutant nsclc. J Thorac Oncol. (2021) 16:205–15. doi: 10.1016/j.jtho.2020.10.006
20. Wang Q, Zeng A, Zhu M, Song L. Dual inhibition of egfr−Vegf: an effective approach to the treatment of advanced non−Small cell lung cancer with egfr mutation (Review). Int J Oncol. (2023) 62. doi: 10.3892/ijo.2023.5474
21. Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (Beta): A double-blind, placebo-controlled, phase 3 trial. Lancet. (2011) 377:1846–54. doi: 10.1016/s0140–6736(11)60545-x
22. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring egfr mutations (Jo25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. (2014) 15:1236–44. doi: 10.1016/s1470–2045(14)70381-x
23. Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, et al. Ramucirumab plus erlotinib in patients with untreated, egfr-mutated, advanced non-small-cell lung cancer (Relay): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:1655–69. doi: 10.1016/s1470–2045(19)30634–5
24. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with egfr-positive advanced non-squamous non-small-cell lung cancer (Nej026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. (2019) 20:625–35. doi: 10.1016/s1470–2045(19)30035-x
25. Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and pd-1/pd-L1 pathways: challenges and opportunities. Front Immunol. (2020) 11:598877. doi: 10.3389/fimmu.2020.598877
26. Lazzari C, Bulotta A, Damiano G, Mirabile A, Vigano M, Veronesi G, et al. Angiogenesis inhibition in lung cancer: emerging novel strategies. Curr Opin Oncol. (2022) 34:107–14. doi: 10.1097/CCO.0000000000000807
27. Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X, et al. Vegf/vegfr-targeted therapy and immunotherapy in non-small cell lung cancer: targeting the tumor microenvironment. Int J Biol Sci. (2022) 18:3845–58. doi: 10.7150/ijbs.70958
28. Reck M, Wehler T, Orlandi F, Nogami N, Barone C, Moro-Sibilot D, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without bevacizumab versus bevacizumab plus chemotherapy in non-small-cell lung cancer. J Clin Oncol. (2020) 38:2530–42. doi: 10.1200/JCO.19.03158
29. Lu S, Wu L, Jian H, Chen Y, Wang Q, Fang J, et al. Sintilimab plus bevacizumab biosimilar ibi305 and chemotherapy for patients with egfr-mutated non-squamous non-small-cell lung cancer who progressed on egfr tyrosine-kinase inhibitor therapy (Orient-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:1167–79. doi: 10.1016/s1470–2045(22)00382–5
30. Kenmotsu H, Wakuda K, Mori K, Kato T, Sugawara S, Kirita K, et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous nsclc harboring egfr mutations: wjog9717l study. J Thorac Oncol. (2022) 17:1098–108. doi: 10.1016/j.jtho.2022.05.006
31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
32. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
33. Nishio M, Seto T, Reck M, Garon EB, Chiu CH, Yoh K, et al. Ramucirumab or placebo plus erlotinib in egfr-mutated, metastatic non-small-cell lung cancer: east asian subset of relay. Cancer Sci. (2020) 111:4510–25. doi: 10.1111/cas.14655
34. Ponce Aix S, Novello S, Garon EB, Nakagawa K, Nadal E, Moro-Sibilot D, et al. Relay, ramucirumab plus erlotinib versus placebo plus erlotinib in patients with untreated, egfr-mutated, metastatic non-small cell lung cancer: europe/United States subset analysis. Cancer Treat Res Commun. (2021) 27:100378. doi: 10.1016/j.ctarc.2021.100378
35. Spigel DR, Burris HA 3rd, Greco FA, Shipley DL, Friedman EK, Waterhouse DM, et al. Randomized, double-blind, placebo-controlled, phase ii trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol. (2011) 29:2582–9. doi: 10.1200/jco.2010.30.7678
36. Scagliotti GV, Krzakowski M, Szczesna A, Strausz J, Makhson A, Reck M, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: A phase iii trial. J Clin Oncol. (2012) 30:2070–8. doi: 10.1200/jco.2011.39.2993
37. Groen HJ, Socinski MA, Grossi F, Juhasz E, Gridelli C, Baas P, et al. A randomized, double-blind, phase ii study of erlotinib with or without sunitinib for the second-line treatment of metastatic non-small-cell lung cancer (Nsclc). Ann Oncol. (2013) 24:2382–9. doi: 10.1093/annonc/mdt212
38. Neal JW, Dahlberg SE, Wakelee HA, Aisner SC, Bowden M, Huang Y, et al. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with egfr wild-type advanced non-small-cell lung cancer (Ecog-acrin 1512): A randomised, controlled, open-label, multicentre, phase 2 trial. Lancet Oncol. (2016) 17:1661–71. doi: 10.1016/S1470–2045(16)30561–7
39. Wang Y, Wang H, Jiang Y, Zhang Y, Wang X. A randomized phase iii study of combining erlotinib with bevacizumab and panitumumab versus erlotinib alone as second-line therapy for chinese patients with non-small-cell lung cancer. BioMed Pharmacother. (2017) 89:875–9. doi: 10.1016/j.biopha.2017.02.097
40. Kato T, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non-small-cell lung cancer (Jo25567): updated safety results. Drug Saf. (2018) 41:229–37. doi: 10.1007/s40264–017-0596–0
41. Spigel DR, Burris HA 3rd, Greco FA, Shih KC, Gian VG, Lipman AJ, et al. Erlotinib plus either pazopanib or placebo in patients with previously treated advanced non-small cell lung cancer: A randomized, placebo-controlled phase 2 trial with correlated serum proteomic signatures. Cancer. (2018) 124:2355–64. doi: 10.1002/cncr.31290
42. Kitagawa C, Mori M, Ichiki M, Sukoh N, Kada A, Saito AM, et al. Gefitinib plus bevacizumab vs. Gefitinib alone for egfr mutant non-squamous non-small cell lung cancer. In Vivo. (2019) 33:477–82. doi: 10.21873/invivo.11498
43. Stinchcombe TE, Janne PA, Wang X, Bertino EM, Weiss J, Bazhenova L, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced egfr-mutant non-small cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. (2019) 5:1448–55. doi: 10.1001/jamaoncol.2019.1847
44. Akamatsu H, Toi Y, Hayashi H, Fujimoto D, Tachihara M, Furuya N, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with egfr T790m-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: west Japan oncology group 8715l phase 2 randomized clinical trial. JAMA Oncol. (2021) 7:386–94. doi: 10.1001/jamaoncol.2020.6758
45. Zhao H, Yao W, Min X, Gu K, Yu G, Zhang Z, et al. Apatinib plus gefitinib as first-line treatment in advanced egfr-mutant nsclc: the phase iii active study (Ctong1706). J Thorac Oncol. (2021) 16:1533–46. doi: 10.1016/j.jtho.2021.05.006
46. Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, et al. Bevacizumab plus erlotinib in chinese patients with untreated, egfr-mutated, advanced nsclc (Artemis-ctong1509): A multicenter phase 3 study. Cancer Cell. (2021) 39:1279–91.e3. doi: 10.1016/j.ccell.2021.07.005
47. Soo RA, Han JY, Dafni U, Cho BC, Yeo CM, Nadal E, et al. A randomised phase ii study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced nsclc with confirmed egfr and acquired T790m mutations: the european thoracic oncology platform (Etop 10–16) booster trial. Ann Oncol. (2022) 33:181–92. doi: 10.1016/j.annonc.2021.11.010
48. Piccirillo MC, Bonanno L, Garassino MC, Esposito G, Dazzi C, Cavanna L, et al. Addition of bevacizumab to erlotinib as first-line treatment of patients with egfr-mutated advanced nonsquamous nsclc: the beverly multicenter randomized phase 3 trial. J Thorac Oncol. (2022) 17:1086–97. doi: 10.1016/j.jtho.2022.05.008
49. Ninomiya T, Ishikawa N, Inoue K, Kubo T, Yasugi M, Shibayama T, et al. Phase 2 study of afatinib alone or combined with bevacizumab in chemonaive patients with advanced non-small-cell lung cancer harboring egfr mutations: afabev-cs study protocol. Clin Lung Cancer. (2019) 20:134–8. doi: 10.1016/j.cllc.2018.10.008
50. Lee Y, Kim HR, Hong MH, Lee KH, Park KU, Lee GK, et al. A randomized phase 2 study to compare erlotinib with or without bevacizumab in previously untreated patients with advanced non-small cell lung cancer with egfr mutation. Cancer. (2023) 129:405–14. doi: 10.1002/cncr.34553
51. Nakahara Y, Kato T, Isomura R, Misumi T, Tamiya M, Yoh K, et al. Osiram-1: A multicenter, open label, randomized phase ii study of osimertinib plus ramucirumab versus osimertinib alone as initial chemotherapy for egfr mutation-positive non-squamous non-small cell lung cancer (Torg1833). Ann Oncol. (2023) 34:S1313. doi: 10.1016/j.annonc.2023.10.071
52. Wang J, Zhao X, Xiong K, Liu C, Wu X, Wang H, et al. Efficacy and safety of gefitinib plus bevacizumab versus gefitinib monotherapy in patients with egfr L858r mutant non-small cell lung cancer (Nsclc): A randomized, open-controlled, single-center trial. Ann Oncol. (2023) 34:S768. doi: 10.1016/j.annonc.2023.09.2365
53. Le X, Patel J, Shum E, Sanborn RE, Baik CS, Shu CA, et al. A multi-centre open-label randomized phase ii study of osimertinib with and without ramucirumab in tki-naïve egfr-mutant metastatic nsclc (Ramose trial interim analysis). Ann Oncol. (2023) 34:S1313–S4. doi: 10.1016/j.annonc.2023.10.072
54. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (Impower150): key subgroup analyses of patients with egfr mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. (2019) 7:387–401. doi: 10.1016/s2213–2600(19)30084–0
55. Shiraishi Y, Kishimoto J, Sugawara S, Mizutani H, Daga H, Azuma K, et al. Atezolizumab and platinum plus pemetrexed with or without bevacizumab for metastatic nonsquamous non-small cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2023) 10(3):315–24. doi: 10.1001/jamaoncol.2023.5258
56. Zhang W, Wang J, Wang Q, Cheng Y, Yang L, Li Y, et al. A randomized double-blind trial of tqb2450 with or without anlotinib in pretreated driver-negative non-small cell lung cancer. Lung Cancer. (2023) 184:107353. doi: 10.1016/j.lungcan.2023.107353
57. Deluce J, Maj D, Verma S, Breadner D, Boldt G, Raphael J. Efficacy and toxicity of combined inhibition of egfr and vegf in patients with advanced non-small cell lung cancer harboring activating egfr mutations: A systematic review and meta-analysis. Am J Clin Oncol. (2023) 46:87–93. doi: 10.1097/COC.0000000000000976
58. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. (2005) 353:123–32. doi: 10.1056/NEJMoa050753
59. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase iii trial of the national cancer institute of Canada clinical trials group. J Clin Oncol. (2007) 25:1960–6. doi: 10.1200/JCO.2006.07.9525
60. Long K, Suresh K. Pulmonary toxicity of systemic lung cancer therapy. Respirology. (2020) 25 Suppl 2:72–9. doi: 10.1111/resp.13915
61. Spagnolo P, Chaudhuri N, Bernardinello N, Karampitsakos T, Sampsonas F, Tzouvelekis A. Pulmonary adverse events following immune checkpoint inhibitors. Curr Opin Pulm Med. (2022) 28:391–8. doi: 10.1097/MCP.0000000000000895
62. Kaku S, Horinouchi H, Watanabe H, Yonemori K, Okusaka T, Boku N, et al. Incidence and prognostic factors in severe drug-induced interstitial lung disease caused by antineoplastic drug therapy in the real world. J Cancer Res Clin Oncol. (2022) 148:1737–46. doi: 10.1007/s00432–022-03932–3
63. McKeown S, Richter AG, O'Kane C, McAuley DF, Thickett DR. Mmp expression and abnormal lung permeability are important determinants of outcome in ipf. Eur Respir J. (2009) 33:77–84. doi: 10.1183/09031936.00060708
64. Barratt S, Medford AR, Millar AB. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration. (2014) 87:329–42. doi: 10.1159/000356034
65. Kulkarni YM, Dutta S, Iyer AK, Venkatadri R, Kaushik V, Ramesh V, et al. A proteomics approach to identifying key protein targets involved in vegf inhibitor mediated attenuation of bleomycin-induced pulmonary fibrosis. Proteomics. (2016) 16:33–46. doi: 10.1002/pmic.201500171
66. Barratt SL, Blythe T, Jarrett C, Ourradi K, Shelley-Fraser G, Day MJ, et al. Differential expression of vegf-a(Xxx) isoforms is critical for development of pulmonary fibrosis. Am J Respir Crit Care Med. (2017) 196:479–93. doi: 10.1164/rccm.201603–0568OC
67. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2071–82. doi: 10.1056/NEJMoa1402584
68. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. (2019) 381:1718–27. doi: 10.1056/NEJMoa1908681
69. Flaherty KR, Wells AU, Cottin V, Devaraj A, Inoue Y, Richeldi L, et al. Nintedanib in progressive interstitial lung diseases: data from the whole inbuild trial. Eur Respir J. (2022) 59. doi: 10.1183/13993003.04538–2020
70. Otsubo K, Kishimoto J, Ando M, Kenmotsu H, Minegishi Y, Horinouchi H, et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: A randomised phase 3 trial. Eur Respir J. (2022) 60. doi: 10.1183/13993003.00380–2022
71. Johkoh T, Lee KS, Nishino M, Travis WD, Ryu JH, Lee HY, et al. Chest ct diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: A position paper from the fleischner society. Chest. (2021) 159:1107–25. doi: 10.1016/j.chest.2020.11.027
72. Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. (2004) 91 Suppl 2:S3–10. doi: 10.1038/sj.bjc.6602061
73. Koo LC, Clark JA, Quesenberry CP, Higenbottam T, Nyberg F, Wolf MK, et al. National differences in reporting 'Pneumonia' and 'Pneumonia interstitial': an analysis of the who international drug monitoring database on 15 drugs in nine countries for seven pulmonary conditions. Pharmacoepidemiol Drug Saf. (2005) 14:775–87. doi: 10.1002/pds.1071
74. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of egfr-tki treatment for egfr mutation-positive non-small cell lung cancer. Lung Cancer. (2015) 88:74–9. doi: 10.1016/j.lungcan.2015.01.026
Keywords: epidermal growth factor receptor, immune checkpoint inhibitor, interstitial lung disease, non-small cell lung cancer, pneumonitis, tyrosine kinase inhibitor, vascular endothelial growth factor
Citation: Fujiwara Y, Shimomura K, Yamaguchi T, Shimizu J, Watanabe N, Matsuzawa R, Murotani K and Horio Y (2024) The incidence of drug-induced interstitial lung disease caused by epidermal growth factor receptor tyrosine kinase inhibitors or immune checkpoint inhibitors in patients with non-small cell lung cancer in presence and absence of vascular endothelial growth factor inhibitors: a systematic review. Front. Oncol. 14:1419256. doi: 10.3389/fonc.2024.1419256
Received: 17 April 2024; Accepted: 29 May 2024;
Published: 11 June 2024.
Edited by:
James C. M. Ho, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Wang Chun Kwok, The University of Hong Kong, Hong Kong SAR, ChinaRoland Leung, Queen Mary Hospital, Hong Kong SAR, China
Copyright © 2024 Fujiwara, Shimomura, Yamaguchi, Shimizu, Watanabe, Matsuzawa, Murotani and Horio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yutaka Fujiwara, eS5mdWppd2FyYUBhaWNoaS1jYy5qcA==
 Yutaka Fujiwara
Yutaka Fujiwara Kazuhiro Shimomura
Kazuhiro Shimomura Teppei Yamaguchi
Teppei Yamaguchi Junichi Shimizu
Junichi Shimizu Naohiro Watanabe1
Naohiro Watanabe1 Kenta Murotani
Kenta Murotani