
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 05 August 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1419145
This case report describes a 16-year-old patient with refractory Hodgkin’s lymphoma who developed bilateral anterior and intermediate uveitis as an adverse reaction to Brentuximab vedotin (BV). This is a rare case of an ocular adverse reaction potentially related to BV, with symptoms like blurred vision, decreased visual acuity, photophobia, and redness. Potential mechanisms include BV targeting CD30+ T cells in the uveal tissue or an immune response triggered by the microtubule-disrupting agent MMAE within BV. This highlights the need for vigilant monitoring of ocular adverse events in BV-treated patients and further research into their mechanisms and management.
BV is an antibody-drug conjugate (ADC) that targets CD30, a protein expressed on the surface of cancer cells in Hodgkin’s lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL). It consists of a monoclonal antibody (cAC10), a microtubule-disrupting agent (MMAE) (1), and a protease-cleavable linker. Approved by the FDA in August 2011, BV treats HL and systemic ALCL (2), and since March 2018, it is also approved for use with chemotherapy in stage III or IV classical Hodgkin’s lymphoma (cHL) (3).
The most frequently observed adverse reactions (≥20%) associated with this drug include neutropenia, anemia, peripheral sensory neuropathy, nausea, malaise, constipation, diarrhea, vomiting, and fever. While the safety analysis of BV did not report uveitis and the FDA labeling does not specifically address ocular adverse events, only a few case reports have documented in the literature (4–8).
A 16-year-old male presented with a painless neck mass in the left neck without B symptoms (fever, night sweats, or weight loss), in September 2022 at the outpatient clinic of West China Hospital, Sichuan University. PET/CT revealed multiple enlarged lymph nodes in the bilateral neck, posterior cervical triangle, supraclavicular fossa, and mediastinum (SUVmax 13.89). It also showed two hypodense lesions in the spleen (SUVmax 6.81) and bone density reduction in three cervical vertebrae (SUVmax 8.99) (Figure 1). Immunohistochemistry of the neck mass biopsy indicated classical Hodgkin’s lymphoma: LCA (–), CD20 (–), CD3 (–), CD30 (+), CD15 (+, individual), PAX-5 (+, weak), MUM1 (+), PD-1 (–), EBV (–), ALK (OTI1H7) (–), CD68PG-M1 (–). Bone marrow aspiration flow cytometry (FCM) showed no significant abnormalities. The disease was classified as Advanced Stage (AS) according to the GHSG staging system (Lugano stage IVS, group A, IPS score 2) (9–11). “Group A” refers to the absence of systemic symptoms such as fever, night sweats, and weight loss, which are collectively known as B symptoms (12). Due to the patient’s age and reproductive toxicity of bleomycin, bleomycin was excluded from treatment after consultation with the patient’s guardian. The patient was treated with Brentuximab vedotin, pirubicin, vincristine, and dacarbazine (A+AVD). One month after the first cycle of BV, the patient experienced photophobia and conjunctival redness. Visual acuity was 0.8 in the right eye and 0.6 in the left eye. Slit-lamp examination showed mixed hyperemia and corneal dust KP+ in both eyes. Bilateral uveitis was diagnosed and managed with Tobramycin Dexamethasone Eye Drops (Tobradex) and Diclofenac Sodium Eye Drops. Although the symptoms were initially relieved, the patient returned to the clinic the day after the second cycle of BV treatment with complaints of photophobia and redness in both eyes. Local treatment was administered, and the symptoms were alleviated. Complete response was not achieved after two cycles of treatment (Figure 2). Patients receiving BV+Nivo which are used in salvage therapy for cHL and then proceeding to Allogeneic Hematopoietic Cell Transplantation (AHCT) have a 91% 3-year PFS rate (13). Therapy was switched to the PD-1 inhibitor tislelizumab combined with BV for further salvage treatment.
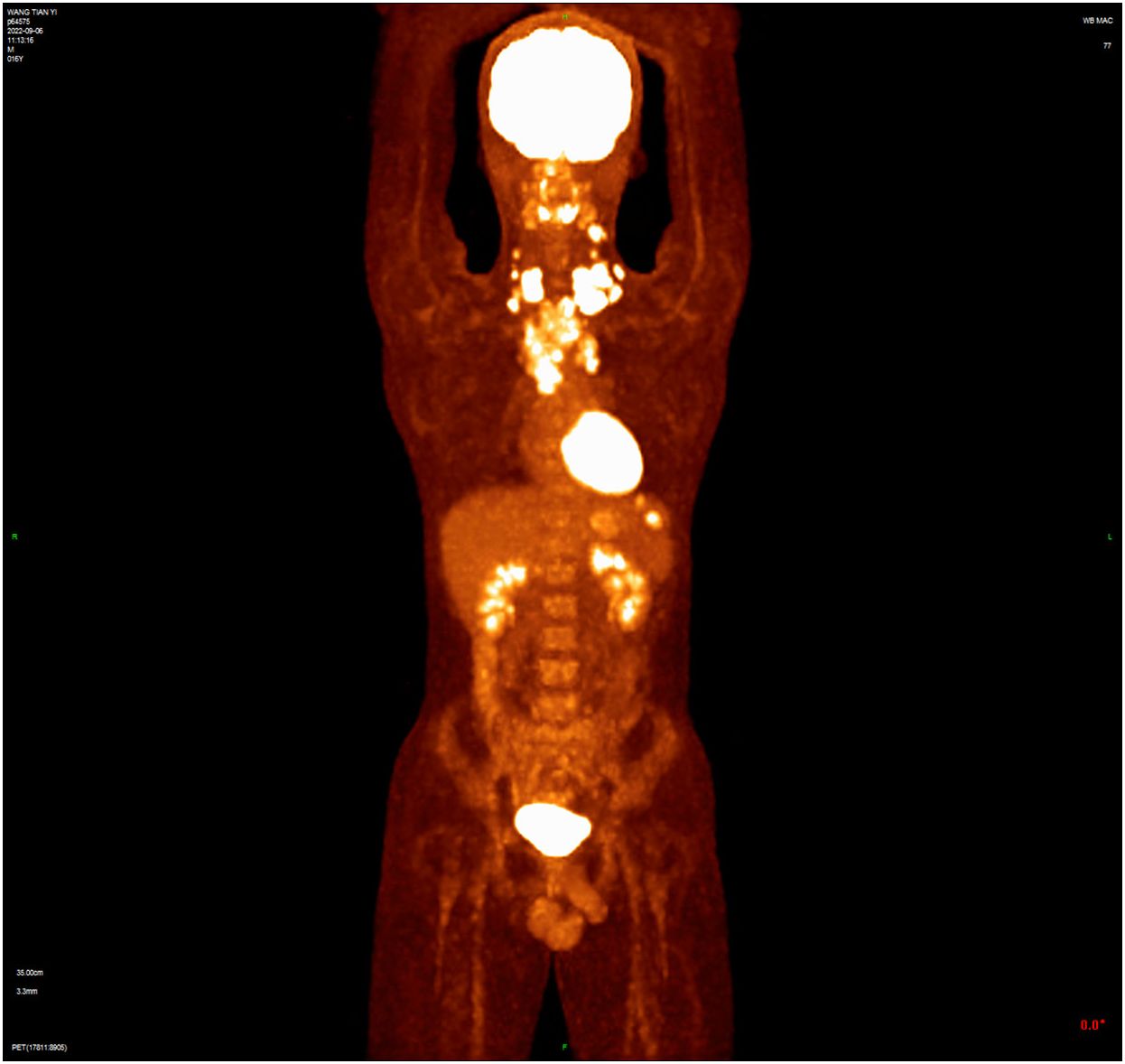
Figure 1 The baseline PET/CT shows that tumor had invaded the cervical and thoracic lymph nodes, spleen, and three cervical vertebrae.
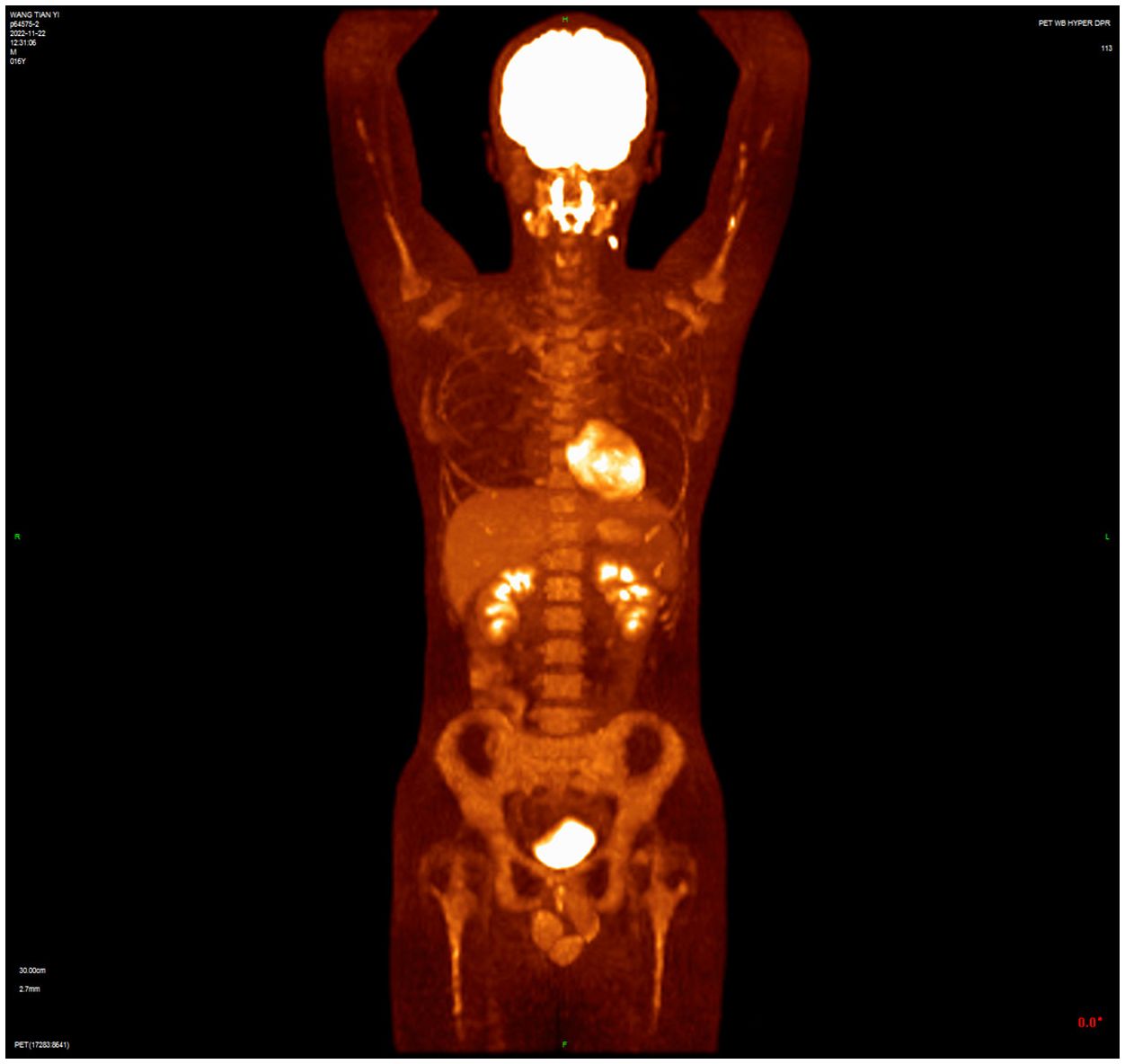
Figure 2 PET/CT after two cycles of treatment showed incomplete resolution of the lesion, with increased lymph node uptake of 18F-FDG in the left posterior cervical triangle.
On the second day after the third cycle of BV and the first use of Tirelizumab, the patient experienced significant vision loss, conjunctival congestion, blurred vision, and photophobia. Visual acuity was severely impaired, and bilateral uveitis was diagnosed (Figure 3). The cerebrospinal fluid analysis showed regular reminders of small lymphocytes, with no prominent abnormal lymphoid cells observed via flow cytometry, thereby ruling out intracranial metastasis of lymphoma for the time being. Tobashi eye drops, Diclofenac Sodium Eye Drops, and atropine eye ointment were topically applied to manage the uveitis. After the discontinuation of BV, the symptoms gradually improved over a period of four weeks with the use of topical corticosteroids and mydriatic agents, leading to significant reduction in inflammation and discomfort. Follow-up examinations at two and four weeks post-discontinuation showed a marked decrease in anterior chamber cells and flare. Considering the potential for uveitis as a drug-related adverse effect, the patient’s treatment was then switched to Tislelizumab combined with the Gemox regimen (gemcitabine and oxaliplatin). After two and four cycles, PET/CT confirmed CR. However, seven months later, the patient noticed swollen lymph nodes in the left neck. PET/CT showed enlarged left supraclavicular lymph nodes with increased glucose metabolism (SUV max=6.48), indicating tumor recurrence. A cervical lymph node biopsy confirmed relapse of classical Hodgkin’s lymphoma, nodular sclerosis subtype.
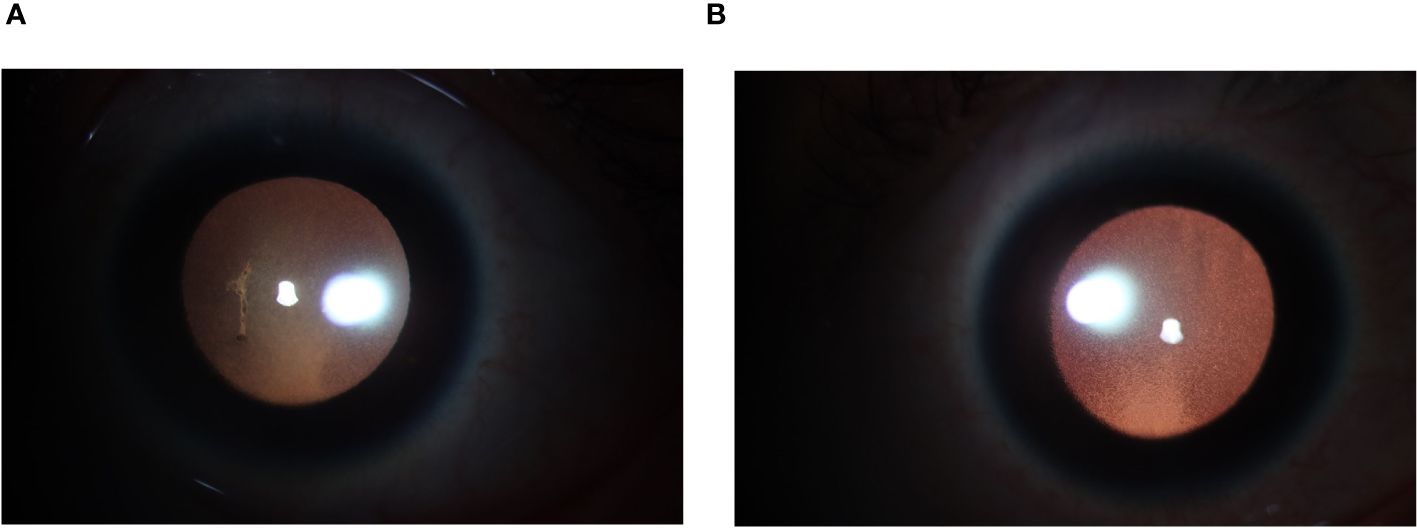
Figure 3 (A) Anterior segment photograph (L): Pupil dilated, diameter approximately 6mm. KP(+++), with anterior chamber exudation. (B) Anterior segment photograph (R): Pupil dilated, diameter approximately 6mm, KP (+++).
The patient then received four cycles of Sindellizumab combined with the ICE regimen (ifosfamide, carboplatin, etoposide), achieving CR. On May 10, 2024, the patient underwent autologous peripheral blood stem cell transplantation and is currently scheduled for outpatient follow-up.
In this report, we present another important and rare case of bilateral anterior and intermediate acute uveitis in an adolescent with refractory Hodgkin’s lymphoma undergoing BV therapy. Possible causes for the uveitis included BV reuse, PD-1 inhibitors, or intraocular metastasis. From a temporal perspective, the patient experienced recurrent ocular symptoms following BV treatment, which were alleviated with local therapy. Since the removal of BV from the treatment regimen, the patient has not experienced any further episodes of uveitis. Additionally, routine examinations, including cerebrospinal fluid analysis, biochemistry, exfoliative cytology, and flow cytometry, did not indicate lymphoma metastasis. A retinal biopsy was not performed due to its invasive nature. Hou-Ting Kuo et al. reported that ICI therapies can lead to ocular immune-related adverse events (IRAEs), such as uveitis, due to the reprogramming of immune checkpoints, which can alter immune responses and affect various tissues, including ocular structures (14). During ongoing PD-1 therapy after discontinuation of BV, the patient did not experience any further episodes of uveitis. This suggests that BV was the main factor responsible for the uveitis.
In previous 7 cases of BV-induced ocular adverse events (Table 1) (4–8), similar to previous case reports, symptoms in our patient appeared more than two weeks after the first administration of BV, with photophobia often being the initial sign. Treatment involved discontinuing BV and using topical or systemic steroids. Vogt-Koyanagi-Harada syndrome (VKH syndrome) is a rare autoimmune disorder with bilateral granulomatous panuveitis and additional neurological, auditory, and skin symptoms (15). Therssen S et al. reported VKH-like uveitis that recurred upon reapplication of BV (8). Phylactic antiviral therapy should be considered for at-risk patients, as demonstrated by Tudesq JJ et al. who reported four cases of retinitis during BV treatment, with three of these cases linked to cytomegalovirus (CMV) infection (6). These cases underscore the potential link between BV and ocular side effects and the need for careful monitoring and management.
The mechanisms behind BV-induced uveitis may involve two main factors. One is the disruption of the balance between regulatory and inflammatory T cells due to BV’s cytotoxic effect on CD30+ T cells. Therssen et al. proposed that BV’s direct cytotoxic effect on CD30+ T cells in the uveal tissue could disrupt the balance between regulatory and inflammatory T cells, leading to increased inflammation (8). Additionally, the MMAE component disrupts microtubule dynamics in ocular cells, leading to inflammation through immune responses. Similar to BV, other MMAE-based ADCs such as Enfortumab Vedotin and Tisotumab Vedotin have also been reported to cause uveitis, suggesting that the MMAE component might be responsible for ocular adverse effects. Enfortumab Vedotin (Padcev) targets Nectin-4 and is linked to blurry vision, dry eye, and the first case of bilateral anterior subcapsular cataract (16). Tisotumab Vedotin (Tivdak) targets Tissue Factor and has been associated with conjunctivitis and dry eye in Japanese patients (17). BV cause uveitis and retinitis, with a total of eight reported cases of ocular adverse effects, including this one. These findings indicate that the ocular adverse events are likely related to the MMAE payload rather than the specific target antigen. These potential mechanisms are based on limited reports, and more research is needed to fully understand and develop preventive strategies.
In conclusion, this case highlights the occurrence of acute uveitis as a rare adverse reaction associated with BV in a patient with refractory Hodgkin lymphoma. Previous reports have summarized the clinical features and potential mechanisms of BV-induced ocular adverse events. According to the ASCO guidelines (18), it is essential for clinicians to remain vigilant in detecting and managing these reactions during BV therapy. Timely evaluation, appropriate discontinuation of the drug, and treatment adjustments are crucial for optimizing patient management and prognosis. Continued case reporting is necessary to further elucidate the mechanisms of BV-induced uveitis and improve clinical management practices.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
ML: Writing – original draft. KR: Writing – review & editing. PA: Writing – review & editing. LZ: Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. (2012) 30:631–7. doi: 10.1038/nbt.2289
2. (FDA) USFaDA. FDA approves Adcetris to treat two types of lymphoma (2011). Available online at: https://www.prnewswire.com/news-releases/fda-approves-adcetris-to-treat-two-types-of-lymphoma-128086178.html.
3. (FDA) USFaDA. FDA expands approval of Adcetris for first-line treatment of Stage III or IV classical Hodgkin lymphoma. Silver Spring, MD, USA: U.S. Food and Drug Administration (FDA) (2018).
4. Antman GG, Abrahami G, Berliner O, Erlich R, Reich E, Kramer M, et al. Uveitis induced by brentuximab vedotin-a novel adverse response to antibody-drug conjugate therapy. Clin Oncol Case Rep. (2021) 4:12. doi: 10.1007/s12345-021-00012-3
5. Costa PA, Espejo-Freire AP, Fan KC, Albini TA, Pongas G. Panuveitis induced by brentuximab vedotin: a possible novel adverse event of an antibody-drug conjugate. Leuk Lymphoma. (2021) 63:239–42. doi: 10.1080/10428194.2021.1978090
6. Tudesq JJ, Vincent L, Lebrun J, Hicheri Y, Gabellier L, Busetto T, et al. Cytomegalovirus infection with retinitis after brentuximab vedotin treatment for CD30(+) lymphoma. Open Forum Infect Dis. (2017) 4:ofx091. doi: 10.1093/ofid/ofx091
7. Ayhan Z, Kaya SY, Ozcan MA, Saatci AO. Brentuximab vedotin related bilateral Purtscher-like retinopathy unresponsive to pulse steroid therapy and intravitreal aflibercept injection.pdf. GMS Ophthalmol Cases. (2017) 7:Doc29. doi: 10.3205/oc000080
8. Therssen S, Meers S, Jacob J, Schauwvlieghe PP. Brentuximab vedotin induced uveitis. Am J Ophthalmol Case Rep. (2022) 26:101440. doi: 10.1016/j.ajoc.2022.101440
9. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. (2014) 32:3059–68. doi: 10.1200/jco.2013.54.8800
10. Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. (2003) 348:2386–95. doi: 10.1056/NEJMoa022473
11. Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. (1998) 339:1506–14. doi: 10.1056/nejm199811193392104
12. Gobbi PG, Cavalli C, Gendarini A, Crema A, Ricevuti G, Federico M, et al. Reevaluation of prognostic significance of symptoms in Hodgkin’s disease. Cancer. (1985) 56:2874–80. doi: 10.1002/1097-0142(19851215)56:12<2874::aid-cncr2820561227>3.0.co;2-2
13. Mei MG, Lee HJ, Palmer JM, Chen R, Tsai NC, Chen L, et al. Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood. (2022) 139:3605–16. doi: 10.1182/blood.2022015423
14. Kuo HT, Chen CY, Hsu AY, Wang YH, Lin CJ, Hsia NY, et al. Association between immune checkpoint inhibitor medication and uveitis: a population-based cohort study utilizing TriNetX database. Front Immunol. (2023) 14:1302293. doi: 10.3389/fimmu.2023.1302293
15. Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. (2001) 131:647–52. doi: 10.1016/s0002-9394(01)00925-4
16. Thibodeau A, Nallasamy N. Bilateral anterior subcapsular cataract development following initiation of enfortumab vedotin. Int Med Case Rep J. (2021) 14:707–9. doi: 10.2147/imcrj.S324394
17. Yonemori K, Kuboki Y, Hasegawa K, Iwata T, Kato H, Takehara K, et al. Tisotumab vedotin in Japanese patients with recurrent/metastatic cervical cancer: Results from the innovaTV 206 study. Cancer Sci. (2022) 113:2788–97. doi: 10.1111/cas.15443
Keywords: Brentuximab vedotin, Hodgkin’s lymphoma, uveitis, MMAE, antibody-drug conjugate, case report
Citation: Liu M, Ren K, Ai P and Zou L (2024) Insights into uveitis from Brentuximab vedotin in refractory Hodgkin’s lymphoma: a case report and brief review. Front. Oncol. 14:1419145. doi: 10.3389/fonc.2024.1419145
Received: 17 April 2024; Accepted: 22 July 2024;
Published: 05 August 2024.
Edited by:
Jean El Cheikh, American University of Beirut Medical Center, LebanonReviewed by:
Ayushi Chauhan, Augusta University, United StatesCopyright © 2024 Liu, Ren, Ai and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Zou, aHhsY3l4eUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.