- 1Medical Oncology Unit, Department of Systems Medicine, University Tor Vergata, Rome, Italy
- 2Phase 1 Unit, Fondazione Policlinico Universitario A. Gemelli, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
The treatment of patients affected by a teratoma with somatic-type malignancy (STM) is challenging, since they are characterized by a poor prognosis, due to chemoresistance to standard cisplatin-based regimens. Only five more case reports were described for melanomatous STM and for which there are no data available for efficacy evidences of immune checkpoint inhibitors in this setting. Here we report the case of a patient with an initial diagnosis of mediastinal pure seminoma at the first biopsy. After four cycles of a standard cisplatin-based regimen and a partial response, a radical surgery was performed, revealing a mediastinal teratoma with triple STM component (melanoma, leiomyoarcoma and primitive neuroectodermal tumor). However, during post-surgical follow-up, he developed distant metastases from the melanomatous component and a first-line treatment with immune checkpoint inhibitors (ICI) was started.
1 Introduction
Teratomas are defined as germ cell tumors (GCTs) consisting of tissues derived from more than one primitive germ cell layers. They can be distinguished in prepubertal- or postpubertal-type based on the presence of germ cell neoplasia in situ. In addition, the WHO 2016 Classification of Tumors of the Urinary System and Male Genital Organs recognizes a specific entity defined as “teratoma with somatic type malignancy” (1).
The phenomenon of a somatic-type malignancy (STM) in teratomas refers the occurrence of a secondary malignant component in the context of the tumor. It is a rare phenomenon and it has been described under a variety of names, including “secondary malignancy” and teratoma with “malignant transformation”. The main criterion for diagnosing a secondary malignancy from teratoma is represented by the overgrowth of a particular element, to the extent that others are excluded (a low-power magnification or field, 5 mm in diameter) (2). Besides, multiple somatic malignant components can be contemporary found in the same teratoma, despite this represents an even rarer event (3).
GCTs with STM represent a group of neoplasms made of various histological subtypes with different clinical and prognostic characteristics. Gonads are the most common primary site of GCTs, despite a small proportion of tumors arise in extragonadal sites presenting as retroperitoneal, mediastinal or pineal masses, in order of descending frequency. Mediastinal GCTs account for 7-12% of primary mediastinal malignancies and are frequently diagnosed in children or young men in their 3rd to 4th decade (4). Among the different histotypes of mediastinal GCTs, teratomas account for approximately 8% (4). STM may assume various histologies including sarcoma (commonly embryonal rhabdomyosarcomaan, less often leiomyosarcoma or angiosarcoma), primitive neuroectodermal tumor (PNET), carcinoma, glial or meningeal neoplasms, hematological neoplasms, and nephroblastoma-like tumors (2, 3, 5–12). Regarding the occurrence of a secondary malignant component attributable to melanoma, only four case reports are available in the literature (13–16).
The treatment of patients affected by a teratoma with STM is challenging. They are characterized by a poor prognosis and the radical surgery represents the milestone of the therapy. Despite a higher development of chemoresistance to standard cisplatin-based regimens in comparison to traditional GCT (11), several reports described improved outcomes in patients who were treated with chemotherapeutic regimens tailored to the somatic component (8). In contrast, to our knowledge no data are available for immune checkpoint inhibitors in this setting.
Here we report of a patient diagnosed with a mediastinal teratoma with multiple STM (melanoma, leiomyosarcoma, and PNET) treated with neoadjuvant standard cisplatin-based regimen, who underwent radical surgery. After three months of follow-up the patient developed distant metastases from the melanomatous component and, thus, started a first-line treatment with immune checkpoint inhibitors (ICI). This case report was described according to the CARE guidelines.
2 Case description
In September 2019, a non-smoker 61-year-old man, without relevant medical history except for mild hypertension, referred to his general practitioner complaining of a dry cough for the past two weeks. A chest X-ray showed a radio-opacity attributable to a mediastinal mass. A 18F-fluorodeoxyglucose positron emission tomography with integrated computed tomography (FDG-PET/CT) scan showed a 14x15x18 cm right anterior mediastinal solid mass associated with an inhomogeneous contrast-enhanced internal necrotic area, superior vena cava compression, left brachiocephalic vein compression and right pleural and pericardial effusion (Figures 1A–D).
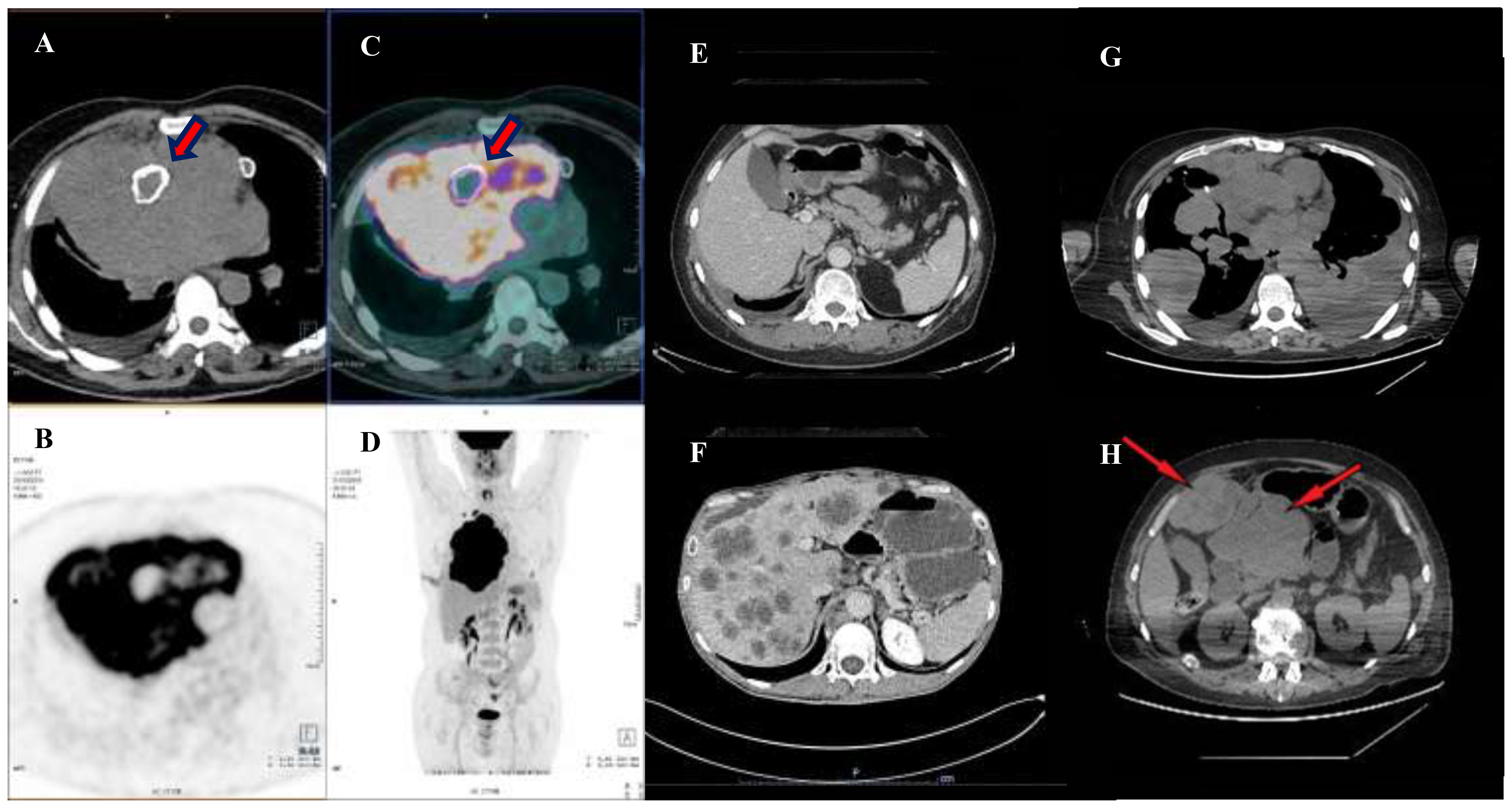
Figure 1. Preoperative and pre-chemotherapy PET-CT scan. (A–D) shows a PET-CT scan performed in October 2019, evidencing a bulky expansive lesion occupying the large part of mediastinum and dislocating the right lung peripherally. Note thick red arrows pointing to a calcified necrotic center, englobed in the neoplatic mass. Thereafter, an induction chemotherapy was performed with the aim of reducing the mass and leading the patient to the surgical excision. Image in (E) shows a post-surgical CT scan performed in April 2020, during outpatient clinic follow up, note that the abdomen window was free from disease. However, an early relapse occurred in August 2020 with development of hepatic metastases (F). A first line of therapy SMT-oriented, with a sequential use of Nivolumab and Ipilimumab was used. However, after four months of therapy, a restaging with a CT scan was performed highlighting a progressive disease. The disease spread in the lung bilaterally (G) and in the abdomen (H) with a disease extension of the II and III hepatic segments over the epigastrium (thin red arrows).
In October 2019, a video-assisted thoracoscopic guided biopsy of the mediastinal mass was performed and histological examination of the samples revealed the presence of a seminoma (PLAP+, cKit+, PanCK+/-, S100, CK 5/6-, CD30-, TTF1-, p40-, CD20-, CD3-, LCA-). In addition, thoracentesis and pericardiocentesis were carried out, but cytological analysis of pleural and pericardial effusions did not reveal cancer cells. Testicular ultrasonography was negative. Serum β-human chorionic gonadotropin (β-HCG), alpha-fetoprotein (αFP) and lactate dehydrogenase (LDH) levels were 13.94 mIU/ml (normal range 0 - 5 mIU/ml), 7301 IU/ml (normal range 0 – 6.72 IU/ml) and 6835 U/L (normal range 125 – 220 U/L), respectively.
Based on those data, a locally-advanced mediastinal seminoma was diagnosed. The patient was not candidate to an upfront surgical removal of the mediastinal mass and, thus, in November 2019 he underwent cytotoxic chemotherapy with the combination BEP (bleomycin 30 units IV weekly on days 2,9 and 16; etoposide 100 mg/m2 on days 1-5; cisplatin 20 mg/m2 on days 1-5, cycles repeated every 21 days) for four cycles. At the end of treatment, the patient repeated a 18F-fluorodeoxyglucose positron emission tomography with integrated computed tomography (FDG-PET/CT) scan that showed a reduction of the mediastinal mass (13 x 9 x 10 cm) with the resolution of pleuro-pericardia effusions and the mediastinal vessels’ compression (Figures 1E, F).
Considering the partial response, as per RECIST criteria, detected with FDG-PET/CT, the patient was re-evaluated in a multidisciplinary meeting and, shortly thereafter, in May 2020 he underwent the removal of the mediastinal mass together with a right pulmonary resection. The histological examination showed a teratoma with both immature and mature components associated with a minimal presence of seminoma. Besides, the presence of multiple malignant somatic components attributable to melanoma (SOX10+, MART1+, HMB45+, VIM+, S100+/-), leiomyosarcoma (alpha-actin+,desmin+), and PNET (CD99+, αFP+) was documented. Surgical margins were clear. A week after surgery procedure, β-HCG, αFP, and LDH levels were <1 mUI/ml, 101.4 UI/ml, and 160 U/L, respectively. Thus, the patient started the follow-up in June 2020.
In August 2020, a new CT scan revealed pulmonary, hepatic, and bone metastases. In September 2020, a CT-guided biopsy of pulmonary and bone metastases was performed. Histopathology showed the presence of melanoma cells (HMB45+, SOX10+, desmin-, panCK-) with a negative immunohistochemistry for V600E BRAF mutation and positive for c-Kit. A dermatological physical examination excluded any primary cutaneous or mucosal melanoma. Therefore, the patient started a first-line treatment for advanced melanoma with the PD-1 ICI, Nivolumab in October 2020, without associating cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitor, Ipilimumab, according to the Italian Drug Regulatory Agency (AIFA) directives.
The patient referred to a tertiary Center to continue the ongoing treatment with ICI. After 6 cycles with Nivolumab, the instrumental re-assessment found a widely extended, progressive disease, and a therapy with anti-CTLA-4 Ipilimumab was promptly started. At the same time, the mutational analysis of c-Kit was requested, revealing the D816Y mutation on exon 17 and disclosing Imatinib mesylate, for a hypothetical fourth metastatic line of treatment. Unluckily, in the intervening time, the patient’s general conditions worsened and he referred to the Emergency Room of our hospital with a diagnosis of a strangulated hernia. Eventually, he died for septic shock. Table 1 reports a timeline with relevant data from the episode of care.
3 Discussion
The occurrence of a triple histology of STMs is a rare event hardly described in case series and retrospective cohort analysis (3, 12, 17, 18) and the development of a melanomatous component is a unique feature described only in other 4 case reports in literature. Our case report showed both this characteristics, moreover an STM-oriented therapy was chosen and Nivolumab was administered for the first time in literature in a male GCT with STM.
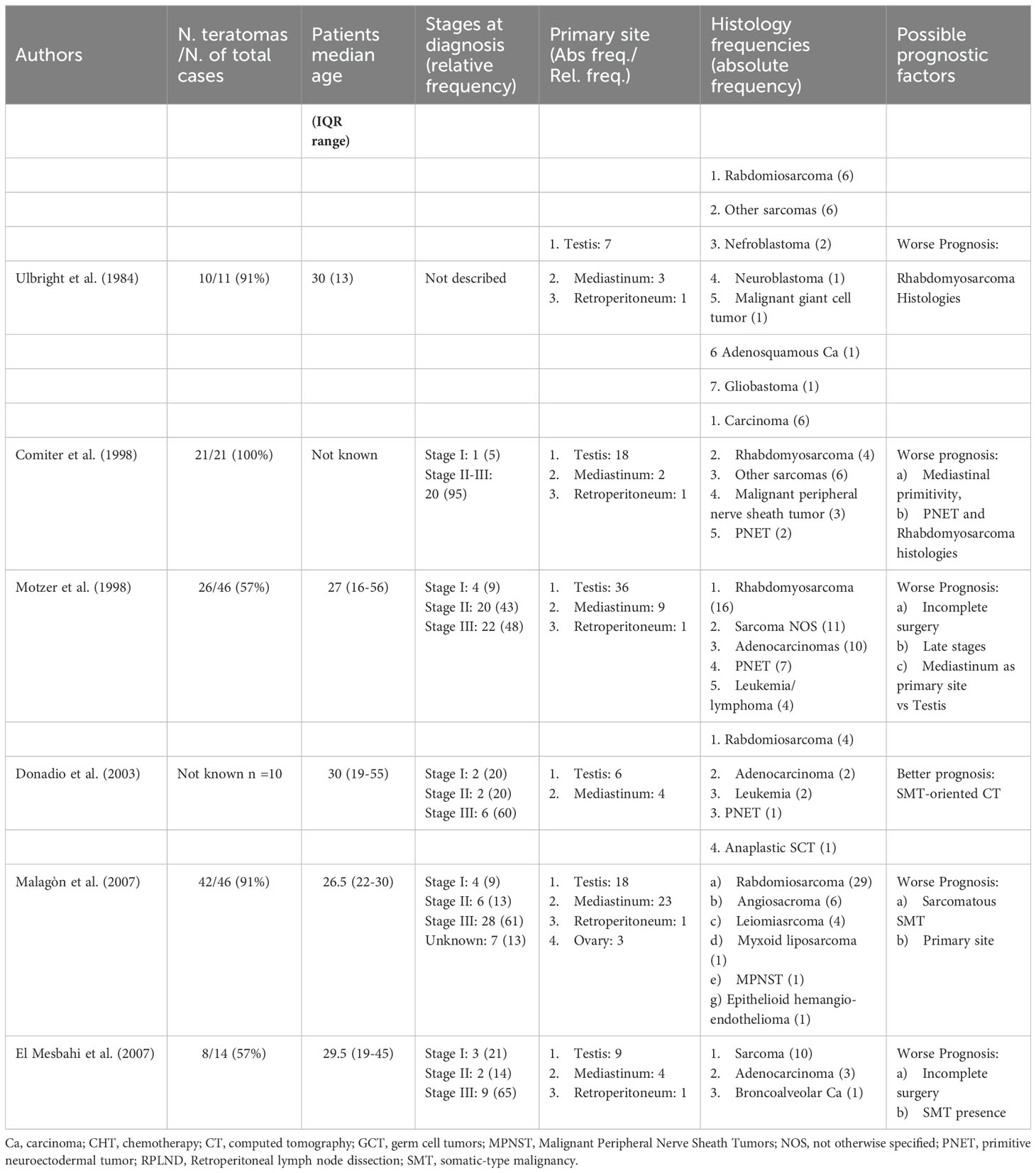
The phenomenon of STM in teratomas refers to the occurrence of a secondary malignant component that may be found in the late stages of various GCT histotypes, but in large part of cases is associated with teratomas (2). Although rare, several case series or retrospective studies on this pathology available in the literature (3, 5–12) (Table 2), described that the commonest histologies were sarcoma, PNET and carcinoma (3, 12, 18, 19), whereas no mention was made of the occurrence of a somatic malignant component attributable to melanoma. Indeed, only other four reports were found querying Pubmed database, assessing this feature as an even rarer event (3, 12).
There are two main debated theories about the origin of a somatic malignant component and the old distinction between mature and immature teratoma could still be useful. According to a first hypothesis, a somatic-type malignancy could derive from the differentiation of a totipotent tumoral cell, a common progenitor, which could develop two different parallel lines, the teratoma and the STM, phylogenetically related (20, 21). Conversely, a second hypothesis points to a malignant transformation of an existing teratomatous component (5, 22) through a process of de-differentiation (3) (Figure 1). However, the old hypothesis that the GCT-oriented therapy could induce a differentiation from the teratomatous component seems to be only apparent as suggested by some Authors (5, 23). Teratomas with STM represent a therapeutic challenge. They are associated with poorer cancer specific survival characterized by development of chemoresistance to standard cisplatin-based regimens and a strong resilience to radiotherapy, in comparison to traditional GCTs (12, 19). In this direction, prognostic factors used for GCT lose their ability in the presence of secondary malignant components. Classically, the therapeutic options have been divided into either GCT- or STM-oriented therapy. The former is older and relies on chemotherapy schemes based on the treatment of germinal lines with contrasting results (7, 8, 10, 12). The latter has promising premises by targeting therapy to the STM component; indeed, several reports have described improved patient outcomes in those presenting with single histology a STM when treatment regimens were tailored to the somatic component (8, 12).
Concerning the detection of a malignant somatic component attributable to melanoma in mediastinal teratomas, only four case reports are available in the literature (Table 3). The first case report, published by McNab et al. in 2012, described a 32 years-old patient affected by a malignant melanoma arising from respiratory epithelium in a mediastinal malignant teratomatous GCT. Thirteen months after the initial diagnosis, a CT scan revealed multiple hepatic lesions attributable to distant metastases from melanoma. The Authors hypothesized that melanomatous somatic-type malignancy occurred in the teratomatous bronchial mucosa, along with other neuroendocrine cell types, could be explained by the “dispersed neuroendocrine system” (DNES) theory. This suggested a derivation from neural crest cells that, in turn, arose out of the neuroectodermal layer of the developing embryo. Notably, in accordance with McNab theory, in our case we observed the association with PNET and melanoma as somatic- type malignant transformation, suggesting either a common origin or the evolution of the second tumor from the former one (13). The second case report published by Mustafa et al. in 2016, described a 21 years-old man who was diagnosed with an immature mediastinal teratoma with multiple malignant components attributable to sarcoma, carcinoma, and melanoma. Unfortunately, after the surgical removal of the tumor, the patient developed multiple liver and bone metastases from melanoma, which, despite the beginning of a first-line treatment with Temozolomide, brought the patient to exitus a few months later (14). Analogously, our patient was affected by multiple co-existing somatic malignancies with the main aggressive component being a melanoma. Of note, Mustafa et al. observed the co- existence of immature neuroectodermal cells with melanoma, whereas we found an association between PNET and melanoma and this could, intriguingly, reinforce McNab’s theory. The third case report published by Nozaki et al. in 2018, described a 14-year-old affected by a malignant teratoma with areas of yolk sac tumor, who developed distant metastases attributable to melanoma after surgical removal of the mediastinal mass. A retrospective revision of the histopathological slide detected a melanomatous component (15). Lastly, Lee et al. published in 2020 the story of a 32-year-old patient diagnosed with a mediastinal teratoma with a somatic malignant component attributable to melanoma, without evidence of distant metastases. Although the patient was disease-free at the time of publication, we don’t know if there has been any evolution of the patient’s health status nor of possible therapies (16). Eventually, our case report differed to all those previously published for the evident older age (13–16), furthermore similarly only to the Mustafa’s case report and confirmed by several case series (3, 12, 19), the finding of a triple somatic malignant component is a very rare event, as well as a poor prognostic factor (12, 19). Moreover, our clinical case in agreement with the previous ones highlighted that STM and the development of metastases from the melanomatous component determined a poorer prognosis for the patients. Finally, a common development by neural crest and/or PNET might suggest different mutational drivers than classical melanoma oncogenesis, in addition to the abundant evidence that PNET acts as a worse independent prognostic factor (12). From the available evidences, we expected a poor prognosis for which an effective and innovative therapy was needed, leading our choice toward a therapy oriented to the somatic malignant component. Nowadays ICIs have changed the landscape of the treatment of advanced melanoma and the administration of these drugs in primary metastatic melanoma is recommended. To our best knowledge, we proposed the first case of teratoma with multiple distant metastases derived from the melanomatous somatic component treated with immune checkpoint inhibitors. In literature, two similar cases were described, which used immunotherapy as first line treatment. The first one, described a 63-year-old woman affected by an ovarian teratoma, who developed distant metastases from a melanomatous component, after which a sequential treatment with Ipilimumab, radiotherapy, Nivolumab and Pembrolizumab was chosen. However, the benefit was poor and a slow progression until death was observed (24). The second case reported a patient affected by a mediastinal germ cell tumor with a large teratomatous component with melanocytic neuroectodermal tumor, likely deriving from thymic tissue (25). The lesion was surgically removed but the patient developed bone metastases from melanocytic neuroectodermal tumor, ten years later. Interestingly, the gene profiling performed resulted to be similar to a melanoma profile. This 39 year-old patient was treated with an off-label combination of Ipilimumab and Nivolumab and he was still alive at the time of publication (25). Our case report shared similarities with both these case reports. Similar to the latter case, a neuroectodermal tumor was found as SMT (a common feature in teratoma with SMT). Interestingly, the tumor shared a common genomic profiling with melanomas, whereas our case reported a melanoma within a context of neuroectodermal tumor suggesting a common pattern of differentiation. On the other hand, the former case report showed a poor prognosis similar to our patient, thus suggesting a scarce responsivity of melanomatous somatic malignant component to ICIs.
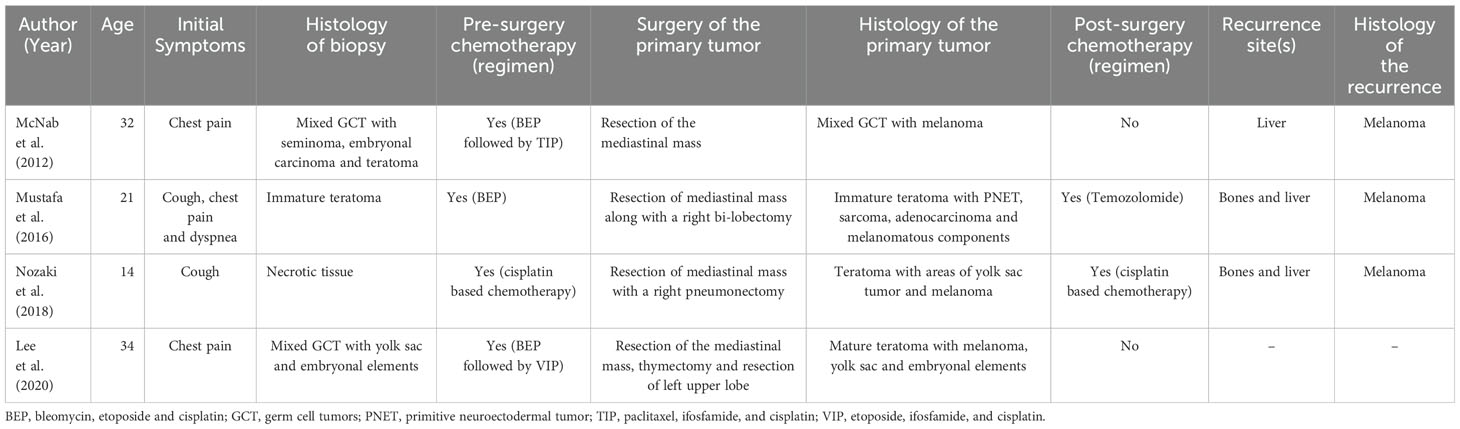
Table 3. Case reports available in the literature concerning the detection of a malignant somatic component attributable to melanoma in mediastinal teratomas.
4 Conclusions and perspectives
This case report describes the first patient affected by a mediastinal teratoma with multiple somatic- type malignancies and distant metastases of melanomatous SMT, who was treated with ICIs. There is large disagreement in literature on the best treatment to be administered for SMTs, whether a GCT- oriented therapy or an SMT-oriented one. In our case report after choosing an SMT-oriented therapy, based on ICI, a progressive disease was observed, confirming the poor prognosis of this disease. Still, before advocating the possible scarce response to ICIs, it is important to underline that our patient developed a very important disease burden before starting Nivolumab and it is largely known that ICIs have a slow response, determining the fatal outcome. For this reason, further researches are needed on the topic to assess definitive information on the usefulness of a therapy tailored to the somatic malignant component, especially in patients who develop distant metastases. Furthermore, the peculiar biology of this type of melanoma, derived from a GCT, should be taken in account for establishing if similar prognostic results would be comparable to classical cutaneous-primitive melanoma.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RR: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. SR: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. GP: Data curation, Writing – review & editing. RA: Data curation, Writing – review & editing. GI: Data curation, Writing – review & editing. FT: Data curation, Writing – review & editing. SM: Data curation, Writing – review & editing. MR: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumors of the urinary system and male genital organs—Part A: renal.; penile.; and testicular tumors. Eur Urol. (2016) 70:93–105. doi: 10.1016/j.eururo.2016.02.029
2. Williamson SR, Delahunt B, Magi-Galluzzi C, Algaba F, Egevad L, Ulbright TM, et al. The World Health Organization 2016 classification of testicular germ cell tumors: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology. (2017) 70:335–46. doi: 10.1111/his.13102
3. Necchi A, Colecchia M, Nicolai N, Piva L, Catanzaro M, Biasoni D, et al. Towards the definition of the best management and prognostic factors of teratoma with Malignant transformation: a single-institution case series and new proposal. BJU Int. (2011) 107:1088–94. doi: 10.1111/j.1464-410X.2010.09705.x
4. Dulmet EM, Macchiarini P, Suc B, Verley JM. Germ cell tumors of the mediastinum. A 30-year experience. Cancer. (1993) 72:1894–901. doi: 10.1002/1097-0142(19930915)72:6<1894::AID-CNCR2820720617>3.0.CO;2-6
5. Ulbright TM, Loehrer PJ, Roth LM, Einhorn LH, Williams SD, Clark SA. The development of non-germ cell Malignancies within germ cell tumors. A clinicopathologic study of 11 cases. Cancer. (1984) 54:1824–33. doi: 10.1002/1097-0142(19841101)54:9<1824::AID-CNCR2820540910>3.0.CO;2-J
6. Comiter CV, Kibel AS, Richie JP, Nucci MR, Renshaw AA. Prognostic features of teratomas with Malignant transformation: a clinicopathological study of 21 cases. J Urol. (1998) 159:859–63. doi: 10.1016/S0022-5347(01)63754-6
7. Motzer RJ, Amsterdam A, Prieto V, Sheinfeld J, Murty VV, Mazumdar M, et al. Teratoma with Malignant transformation: diverse Malignant histologies arising in men with germ cell tumors. J Urol. (1998) 159:133–8. doi: 10.1016/s0022-5347(01)64035-7
8. Donadio AC, Motzer RJ, Bajorin DF, Kantoff PW, Sheinfeld J, Houldsworth J, et al. Chemotherapy for teratoma with Malignant transformation. J Clin Oncol. (2003) 21:4285–91. doi: 10.1200/JCO.2003.01.019
9. Malagón HD, Valdez AMC, Moran CA, Suster S. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol. (2007) 31:1356–62. doi: 10.1097/PAS.0b013e318033c7c4
10. El Mesbahi O, Terrier-Lacombe MJ, Rebischung C, Theodore C, Vanel D, Fizazi K. Chemotherapy in patients with teratoma with Malignant transformation. Eur Urol. (2007) 51:1306–11. doi: 10.1016/j.eururo.2006.10.021
11. Scheckel CJ, Kosiorek HE, Butterfield R, Ho TH, Hilal T. Germ cell tumors with Malignant somatic transformation: a Mayo Clinic experience. Oncol Res Treat. (2019) 42:95–100. doi: 10.1159/000495802
12. Giannatempo P, Pond GR, Sonpavde G, Albany C, Loriot Y, Sweeney CJ, et al. Treatment and clinical outcomes of patients with teratoma with somatic-type Malignant transformation: an International Collaboration. J Urol. (2016) 196:95–100. doi: 10.1016/j.juro.2015.12.082
13. McNab P, Quigley B, Mendoza T, Hakam A, Khalil F, Fishman M, et al. The histogenic origin of melanoma arising in respiratory epithelium of a teratomatous germ cell tumor of the mediastinum: an enigma unraveled from an unlikely source. Int J Clin Exp Pathol. (2012) 5:982–90.
14. Mustafa OM, Mohammed SF, Aljubran A, Saleh WN. Immature mediastinal teratoma with unusual histopathology: a case report of multi-lineage.; somatic-type Malignant transformation and a review of the literature. Med (Baltimore). (2016) 95:e3378. doi: 10.1097/MD.0000000000003378
15. Nozaki I, Tone Y, Yamanaka J, Uryu H, Shimizu-Motohashi Y, Sato N, et al. A case of Malignant melanoma arising in mediastinal Malignant teratoma. Case Rep Pediatr. (2018) 2018:2661306824. doi: 10.1155/2018/1306824
16. Lee S, Chornenkyy Y, Swete MT, Kim SS, Bharat A, Yang XJ, et al. Malignant melanoma arising in a primary mediastinal germ cell tumor. Pathol Res Pract. (2020) 216:269153210. doi: 10.1016/j.prp.2020.153210
17. Colecchia M, Necchi A, Paolini B, Nicolai N, Salvioni R. Teratoma with somatic-type Malignant components in germ cell tumors of the testis: a clinicopathologic analysis of 40 cases with outcome correlation. Int J Surg Pathol. (2011) 19:321–7. doi: 10.1177/1066896910390680
18. Lobo J, Rodrigues Â, Henrique R, Christiansen A, Beyer J, Moch H, et al. Morphological spectrum and molecular features of somatic Malignant transformation in germ cell tumours. Histopathology. (2022) 81:84–98. doi: 10.1111/his.14667
19. Konneh B, Leonard AJ, Lafin JT, Jia L, Bagrodia A. Management of testicular germ cell tumor with somatic-type Malignancy. Oncol Williston Park. (2022) 36:375–7. doi: 10.46883/2022.25920962
20. Kum JB, Ulbright TM, Williamson SR, Wang M, Zhang S, Foster RS, et al. Molecular genetic evidence supporting the origin of somatic-type Malignancy and teratoma from the same progenitor cell. Am J Surg Pathol. (2012) 36:1849–56. doi: 10.1097/PAS.0b013e31826df1ab
21. Umbreit EC, Siddiqui BA, Hwang MJ, Joon AY, Maity T, Westerman ME, et al. Origin of subsequent Malignant neoplasms in patients with history of testicular germ cell tumor. Cancers. (2020) 12:E3755. doi: 10.3390/cancers12123755
22. Hwang MJ, Hamza A, Zhang M, Joon AY, Maity T, Westerman ME, et al. Somatic-type Malignancies in testicular germ cell tumors: a clinicopathologic study of 63 Cases. Am J Surg Pathol. (2022) 46:11–7. doi: 10.1097/PAS.0000000000001789
23. Oosterhui SJW, Suurmeyer AJH, Sleyfer DT, Koops HS, Oldhoff J, Fleuren G. Effects of multiple-drug chemotherapy (cis-diammine-dichloroplatinum.; bleomycin.; andvinblastine) on the maturation of retroperitoneal lymph node metastases of nonseminomatous germcell tumors of the testis: No evidence for de novo induction of differentiation. Cancer. (1983) 51:408–16. doi: 10.1002/1097-0142(19830201)51:3<408::aid-cncr2820510309>3.0.co;2-4
24. Yano M, Asami Y, Nishikawa T, Yoshida S, Kamada K, Katoh T, et al. Immune checkpoint inhibitors of CTLA4 and PD-1 for Malignant melanoma arising in ovarian cystic teratoma: A case report. Med (Baltimore). (2018) 97:e12937. doi: 10.1097/MD.0000000000012937
25. Mayeur S, Lhermitte B, Gantzer J, Molitor A, Stemmelen T, Meyer S, et al. Genomic profiling of a metastatic anaplastic melanocytic neuroectodermal tumor arising from a mature thymic teratoma as part of a mediastinal germ cell tumor. Cold Spring Harb Mol Case Stud. (2023) 9:a006257. doi: 10.1101/mcs.a006257
Keywords: teratoma, germ cell tumors, somatic-type malignancy, immune checkpoint inhibitors, case report
Citation: Rosenfeld R, Riondino S, Parisi G, Iannantuono GM, Ajdhoni R, Torino F, Mariotti S and Roselli M (2024) Case report: Metastatic melanoma derived from a somatic-type malignant transformation of a mediastinal teratoma treated with immune checkpoint inhibitors. Front. Oncol. 14:1417776. doi: 10.3389/fonc.2024.1417776
Received: 15 April 2024; Accepted: 10 October 2024;
Published: 13 November 2024.
Edited by:
Mirella Marino, Hospital Physiotherapy Institutes (IRCCS), ItalyReviewed by:
Zhi Hu, The Affiliated Hospital of Southwest Medical University, ChinaKortnye Smith, Peter MacCallum Cancer Center, Australia
Marco Russano, Campus Bio-Medico University, Italy
Copyright © 2024 Rosenfeld, Riondino, Parisi, Iannantuono, Ajdhoni, Torino, Mariotti and Roselli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Riondino, c2lsdmlhLnJpb25kaW5vQHVuaXJvbWEyLml0
†These authors share first authorship
 Roberto Rosenfeld
Roberto Rosenfeld Silvia Riondino
Silvia Riondino Giusy Parisi1
Giusy Parisi1 Francesco Torino
Francesco Torino Mario Roselli
Mario Roselli