- 1Department of Diagnostic radiology, King Hussein Cancer Center, Amman, Jordan
- 2Department of Pharmacy, King Hussein Cancer Center, Amman, Jordan
- 3Department of Pathology, King Hussein Cancer Center, Amman, Jordan
- 4Department of Surgery, King Hussein Cancer Center, Amman, Jordan
- 5Department of Nursing, King Hussein Cancer Center, Amman, Jordan
- 6Department of Radiation Oncology, King Hussein Cancer Center, Amman, Jordan
- 7Division of Hematology/Oncology, The Hospital for Sick Children, Toronto, ON, Canada
- 8Department of Pediatrics, King Hussein Cancer Center, Amman, Jordan
Introduction: Most pediatric low-grade-gliomas (LGG) and some high-grade-gliomas (HGG) have alterations in the RAS/MAPK pathway. Promising high tumor response rates were achieved using BRAF/MEK inhibitors, however data on their use in low-middle-income-countries (LMICs) are limited.
Methods: We retrospectively reviewed our Jordanian experience of using compassionate BRAF/MEK inhibitors in treating children with gliomas. We reviewed patients’ clinical characteristics, tumor response, and side effects.
Results: Twenty patients (13 males, 7 females) were identified. Median age at diagnosis was 8.3 years (0.3-18.9years). There were fifteen LGGs, three HGGs and two grade-2 pleomorphic xanthoastrocytoma (PXA-2). Fifteen tumors were supratentorial, three posterior fossa/brainstem, one diffuse-glioneuronal tumor (DLGNT) and one spinal. Five tumors were metastatic. Except for one patient with neurofibromatosis, ten patients underwent partial resection and nine had biopsy. All patients, except three, received BRAF/MEK inhibitors after initial standard chemo/radiotherapy. Seven LGGs had BRAF-mutation, six had BRAF-fusion, and two were empirically treated (one neurofibromatosis and one DLGNT). Fourteen LGGs were treated with 1-4 chemotherapy regimens before BRAF/MEK inhibitors’ use; all had partial/stable response on targeted therapy at a median of 1.9 years (0.5-5.4years). Two patients with BRAFv600E-mutated/CDKN2A deleted PXA-2, had progression following resection, and experienced stable/partial response at 9 months of dabrafenib use. Two patients with HGGs had BRAFv600E-mutation, and one had an FGFR-mutation. All three patients with HGG had temporary stable/partial response, two with significant clinical improvement. At a median of 2.7 years (1.3-3.2years), all patients experienced tumor progression, and two died. Eight patients (40%) developed acneiform rash, three (15%) paronychia, and one had significant panniculitis and fatigue. Six patients (30%) needed dose-reduction. Nine patients had temporary drug interruptions [due to side effects (5) and drug shortage (4)]. Two patients who stopped trametinib due to side effects (significant acneiform rash/paronychia and intracranial bleeding) did not experience progression.
Conclusions: Our experience with BRAF/MEK inhibitors’ use was positive achieving response in all LGGs and provided sustained response with good quality of life for patients with HGG. Cost effectiveness analyses and patients’ satisfaction comparisons with chemotherapy are needed to evaluate the routine use of these drugs in LMICs.
Introduction
Gliomas are the most common pediatric CNS tumors with low-grade glioma (LGG) being more prevalent than high-grade glioma (HGG). LGGs are usually cured with gross tumor resection (GTR), however this is not achievable at every neuroaxis location, nor it is enough when the tumor is metastatic. The decision to treat or not incompletely resected or unresectable LGGs and with what modality depends on many factors including the child’s age, neurofibromatosis (NF1) status, size of the residual tumor, the anticipated neurological compromise with further tumor progression, and the availability of treatment modalities (chemotherapeutic agents or radiotherapy) (1). Several chemotherapeutic protocols (vincristine/carboplatin, vinblastine, TPCV) are considered as first, second and third lines of treatments for unresectable or progressing LGGs achieving a 5-year progression free survival (PFS) of 30-50% (2–4). While radiotherapy achieves higher PFS rates >70% (5, 6), its long-term neurocognitive and neuroendocrine side effects preclude its use as a frontline therapy in young children. While overall survival (OS) of patients with LGG is high (>80%) (2–4), PFS is low (<50%) highlighting the importance of preserving the best quality of life (QoL) for these children who may require multiple lines of treatment. In comparison, HGGs have poor prognosis (3year-OS < 30%) (7)despite surgery, radiotherapy, and chemotherapy, therefore maintaining a decent QoL during this short survival is integral.
Most LGG (>80%) harbor a driver alteration in the RAS/MAPK pathway signaling which makes this a plausible target for medical intervention (8). The type of this alteration plays a major role in the tumor trajectory, response to therapy and the risk of transformation to HGG. The presence of BRAFv600E mutation in a LGG (which occurs in 15-20%) was associated with a worse PFS and a higher risk of transformation to HGG even in the absence of radiotherapy (9). On the other hand, BRAF mutations are uncommon in pediatric HGGs (5-10%) (10) where the most frequent alteration is the H3K27M mutation (11). Integration of the molecular diagnosis with the histologic features is now required for several tumor types according to the WHO-CNS-5 classification (11). While this approach provides a more accurate diagnosis and a better understanding of the tumor’s behavior, it also helps in utilizing some targeted drugs for treatment. Several publications have demonstrated the efficacy of BRAF/MEK inhibitors in treating progressive LGGs (12–15) and HGGs (16–18) leading recently to the FDA approval of the dabrafenib and trametinib combination for the first line treatment of BRAFv600E mutated LGGs (19).
In a resource-limited setting, access to “new drugs” is challenging. These countries barely participate in international clinical trials and most families are not able to afford the high cost of these new drugs. On occasions, temporary access through off-label and compassionate drug access programs may be available to some institutions. This is not an ideal situation, however increasingly, off-label and compassionate use prescriptions are becoming common in the pediatric oncology world with the limited approved treatments for children and the scarce number of pediatric clinical trials (20, 21). There are very few publications on the use of compassionate targeted drugs in treating pediatric CNS gliomas in low-middle-income countries (LMICs) (22, 23).
Jordan is a LMIC according to the World Bank classification (24) with an estimated population of 10.3 million (including 37.7% are children aged 0-17 years old) (25). King Hussein Cancer center (KHCC), is the only cancer-dedicated hospital in Jordan to treat children and adults. Most children (> 80%) with CNS tumors are treated at KHCC. All Jordanians are insured through the Jordanian government for cancer therapy, while most non-Jordanians are covered through charities or self-paid.
In this study, we report on the compassionate use of dabrafenib and/or trametinib in pediatric patients with gliomas at KHCC. We demonstrated its feasibility, efficacy, and plausibility for the patients. In addition, this experience displayed the challenges encountered particularly in relation to the sustainability of access to these drugs.
Methods
We retrospectively reviewed the medical charts of all children <18 years old at the time of diagnosis of gliomas at KHCC who received dabrafenib and/or trametinib before December 2023. The earliest child received therapy was in 2015. Targeted therapies were provided through a compassionate drug access program from Novartis. The decision to request and start the drugs was made by the multidisciplinary pediatric neuro-oncology team (MDT) and approved by the pharmacy and therapeutics committee at KHCC. We reviewed our patients’ clinical characteristics, tumor pathology and molecular alterations. We assessed the indication behind using dabrafenib/trametinib, drugs’ side effects and any clinical or radiological responses achieved.
Tumor diagnosis was extracted from the pathology reports issued by the KHCC neuropathologists. BRAF mutation was confirmed by immunohistochemistry (IHC), mutation analysis or TruSight next generation sequencing (NGS) (26). BRAF fusion was tested either by nanoString or NGS testing; both were performed at the laboratory of the Hospital for Sick Children (Sickkids) in Toronto. Not all gliomas were tested for molecular alterations. The decision to do so was based on the MDT discussions after weighing the likelihood of finding an alteration, the clinical condition of the patient, response of tumor to previous therapies (if previous treatment was given) and the expectations to have access to the targeted therapy. Once an alteration was found and compassionate access was available, the case was discussed again in the MDT to review if targeted therapy was needed immediately. This would be mostly in the context of tumor growth/progression despite previously administered chemotherapy and/or radiotherapy.
Tumor characteristics on MRI were reviewed for tumor location, presence or absence of metastasis, and response. GTR was considered if no residual tumor could be appreciated on the postoperative MRI, subtotal resection (STR) when a residual tumor is present, and a biopsy was considered if reported as such by the neurosurgeon. MRI scans just before and after the use of dabrafenib/trametinib were reviewed by the KHCC radiologist (D.A) according to the RANO criteria (27). These were reported as complete response (CR) in the absence of a residual tumor, partial response (PR) if the sum of the perpendicular diameter of the mass improved by 50% or more, stable disease (SD) if sum of the perpendicular diameter of the mass remained unchanged, improved by < 50% or increased by <25%. Progression was considered if the perpendicular diameter of the mass increased >25% or if new lesions appeared.
Drugs’ side effects that were suspected to be related to the use of dabrafenib/trametinib were extracted from the medical charts. A need for drug dose reduction, steroids use, or interruption/discontinuation of therapy was documented. For this study, parents, and children (older than 12-year-old) were asked to fill a one-time short questionnaire (Supplementary Table S1) on their opinion on the use of dabrafenib/trametinib; what they like, and dislike of this treatment option compared to chemotherapy (if it was previously prescribed). The questionnaire was administered between June and December 2023.
This study was approved by the Institutional Review Board at KHCC.
Results
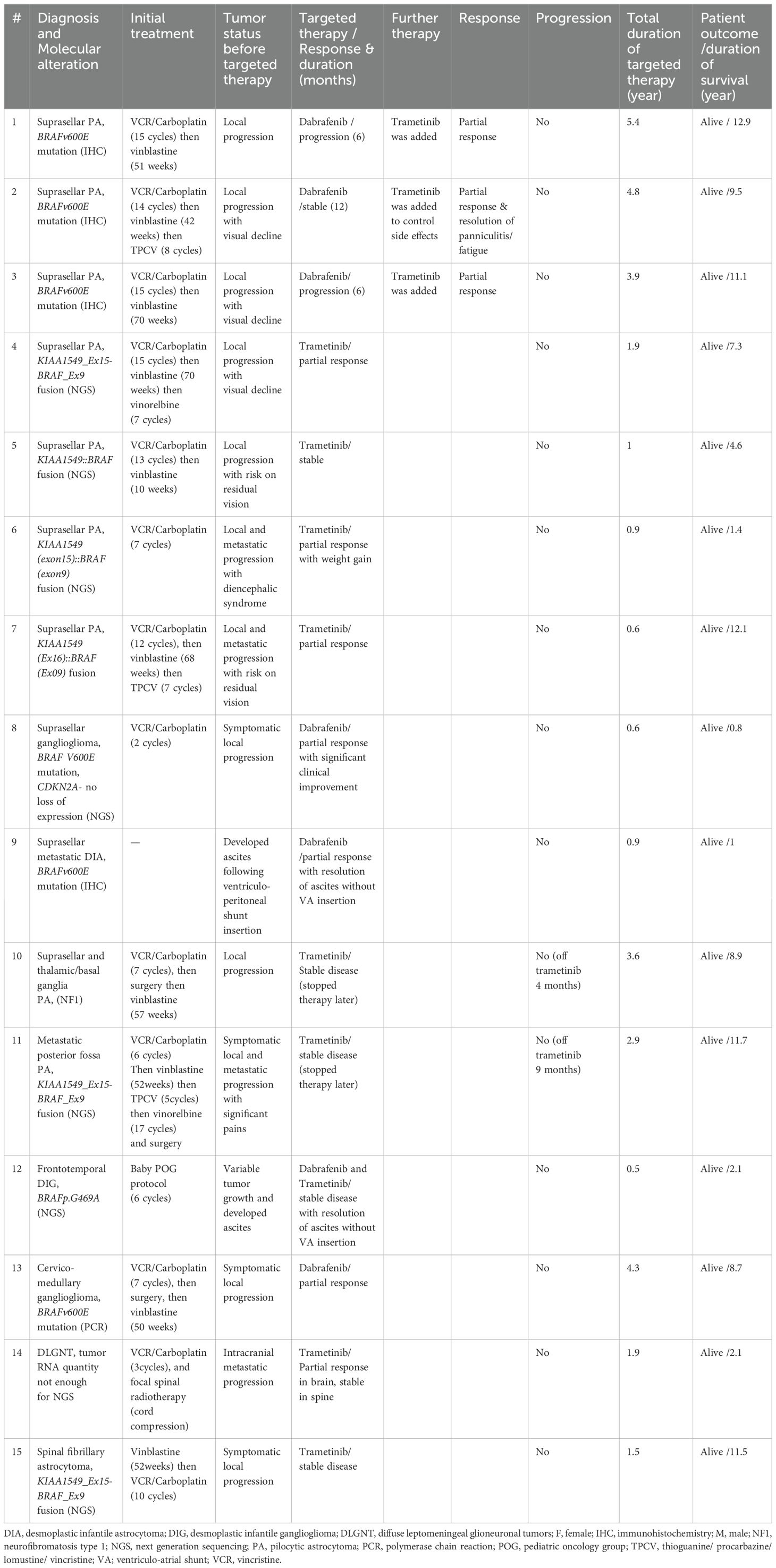
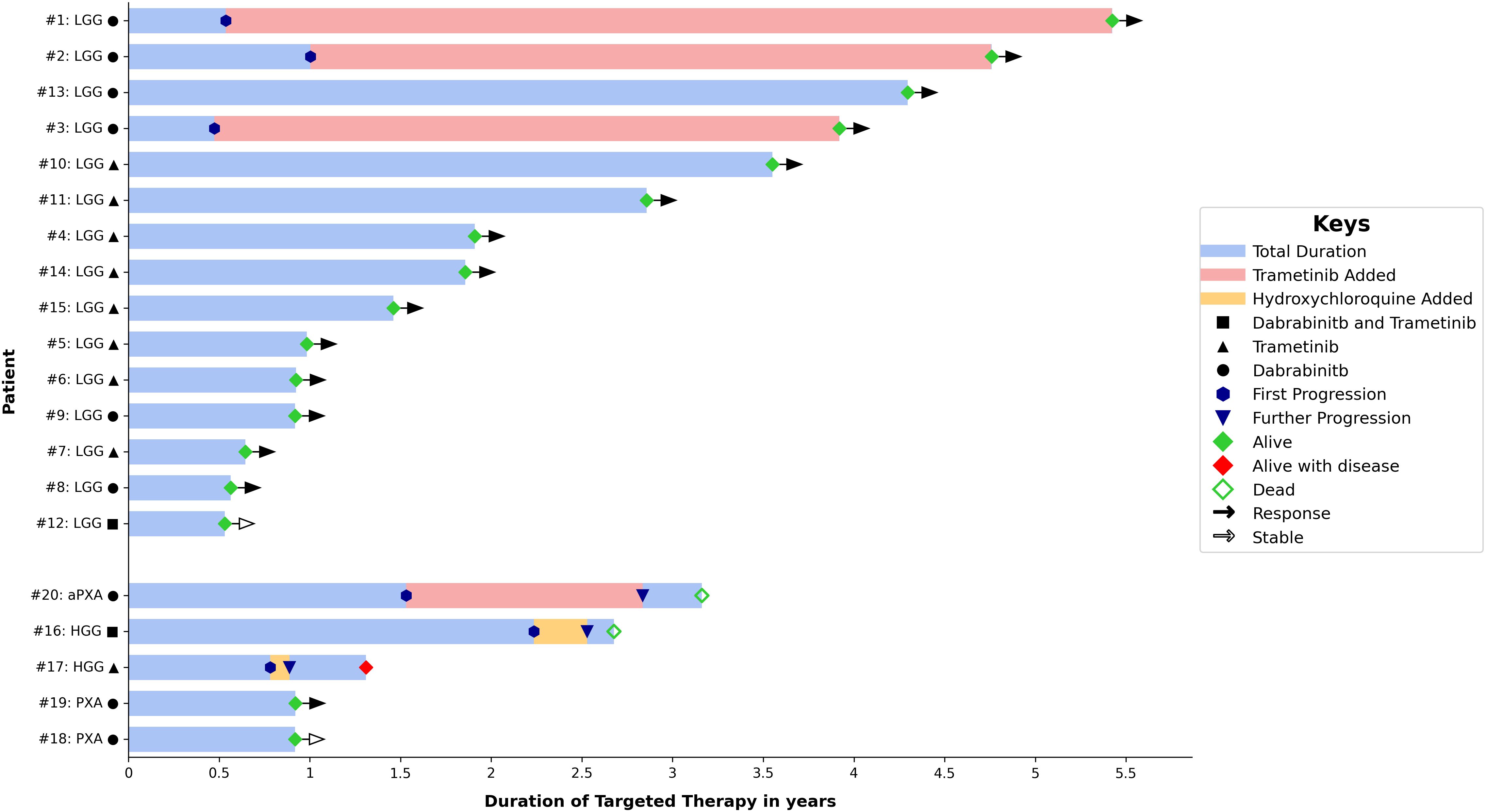
Twenty patients were identified, 13 males and 7 females (Table 1). The median age at diagnosis was 8.3 years (range, 0.3-18.9 years). The oldest patient (Table 2, #4) was originally treated for posterior fossa pilocytic astrocytoma (PA) with partial resection followed by vincristine and carboplatin. Then he was observed regularly with a stable residual tumor for 7 years. At 18.9 years, significant tumor progression upon transformation to glioblastoma was noted (Supplementary Figure S1). The retrospective analysis of the initial tumor identified a BRAF V600E mutation associated with CDKN2A deletion. There were 3 patients with HGG, two with pleomorphic xanthoastrocytoma (PXA, WHO grade 2) and 15 with LGG. Fifteen tumors were supratentorial, three were in the posterior fossa/brainstem, one diffuse leptomeningeal glioneuronal tumor (DLGNT) and one primary spinal LGG. Five tumors were metastatic at time of initiation of the targeted therapy: two HGG, one DLGNT, one posterior fossa PA and one suprasellar desmoplastic infantile astrocytoma (DIA). Except for one patient with NF1, all patients had tissue proven diagnosis. Ten patients underwent STR and nine had tumor biopsy. All patients, except three, received dabrafenib and/or trametinib after the standard treatment protocol (chemotherapy with/without radiotherapy). Summary of patients’ and tumors’ characteristics, treatment received, response to targeted therapy and duration are demonstrated in Table 1 and Figure 1.

Table 1. Summary of patients’ and tumors’ characteristics, treatment received and response to targeted therapy.
Patients with LGG
We identified 15 patients with LGG (Table 2); nine males and six females at a median age of 5.4 years (range, 0.3- 13.1 years) at diagnosis. Ten patients had optic hypothalamic pathway gliomas (OPG). Three tumors were metastatic. Ten patients underwent tumor biopsy and five had STR. Nine tumors were PA, two DIA/DIG, two gangliogliomas, one fibrillary astrocytoma, and one DLGNT. Seven tumors had BRAF mutation (one was a rare mutation: BRAFp.G469A), six had BRAF fusion, and two were empirically treated; one (#10) had NF1 and one (#14) with DLGNT had small tumor biopsy insufficient for NGS testing. Tumors with BRAF mutation were treated with dabrafenib and trametinib was added after tumor progression, while tumors with BRAF fusion, NF1 or DLGNT were treated with trametinib. Six patients were started on dabrafenib alone and later trametinib was added in three of them; two due to tumor progression and one to help control the side effects. After adding trametinib, this patient (#2) could be weaned off opioids and steroids that were used to control his panniculitis and fatigue. Eight patients were initially started on trametinib, and one patient (#12) was started on the combination of dabrafenib and trametinib due to his rare mutation (BRAFp.G469A). All patients except one used dabrafenib/trametinib after tumor progression following chemotherapy use. This one patient (#9), who was previously reported, underwent a ventriculoperitoneal shunt insertion and biopsy of his metastatic DIA, and later developed ascites. Dabrafenib achieved significant tumor response and ascites resolved without a need for permanent shunt diversion. All patients, except two (#10 & #11), are continuing treatment. All tumors showed SD or PR at a median follow up of 1.9 years (range, 0.5-5.4 years) from starting dabrafenib/trametinib. Figure 2 demonstrates the tumor response to targeted therapy in two patients with LGG. The two patients who stopped trametinib (#10 & #11) had no tumor progression on follow-up MRI scans at 4 and 9 months, respectively.
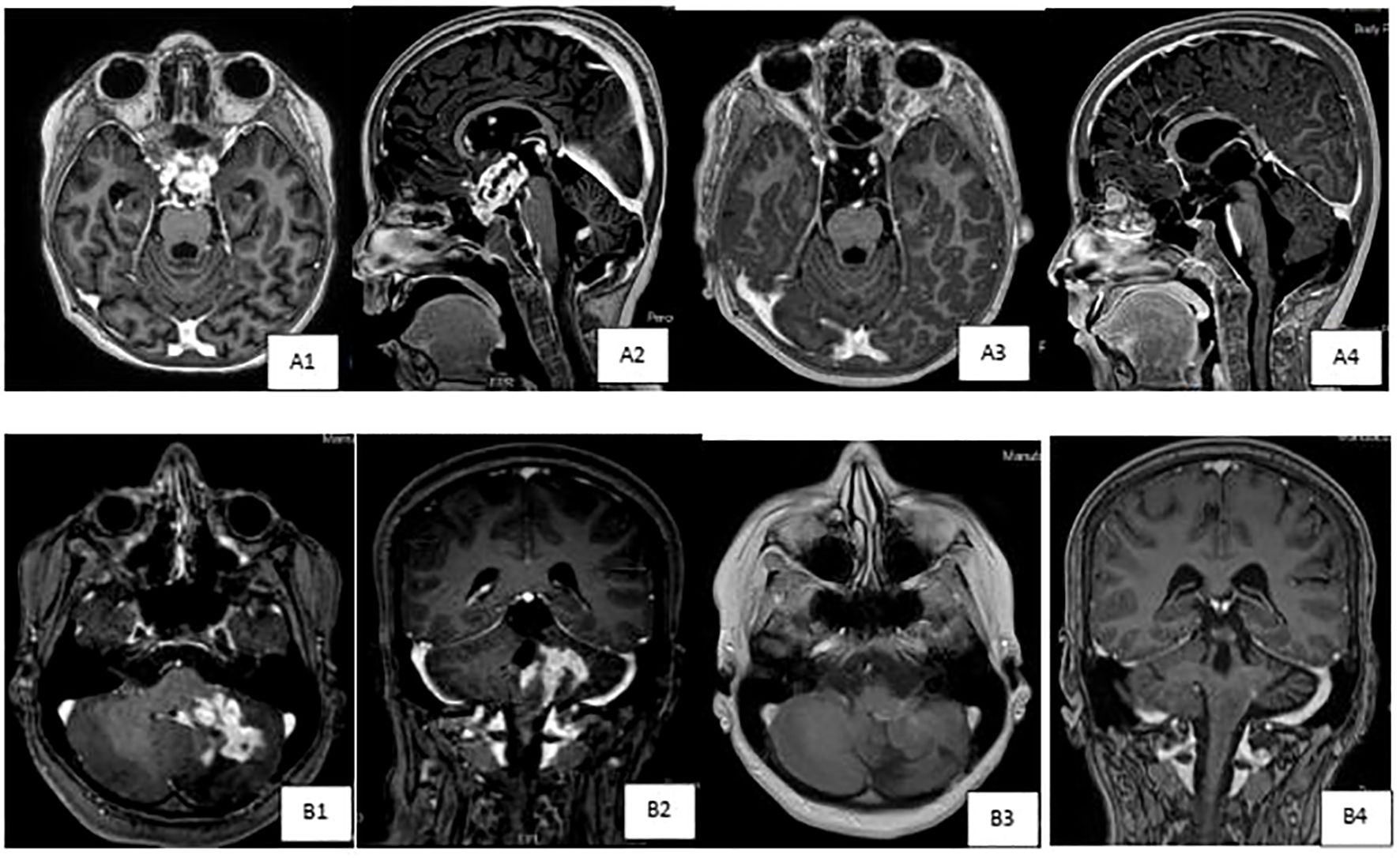
Figure 2. Brain MRI scans demonstrating tumor response to targeted therapy in two patients with low grade gliomas. (A) Axialand sagittal T1-weighted post IV contrast brain images of patient # 14 with diffuse leptomeningeal glioneuronal tumor (DLGNT),demonstrating pretreatment (A1& A2) large contrast enhancing mass in the suprasellar cistern,inseparable from the optic chiasm,extending to the floor of the third ventricle. Subependymal,intraventricular enhancing nodules are also noted, seen onlower row images. Marked interval tumor response {A3 & A4) with resolution of cortrast enhancement with almost resolution of previously seen subependymal enhancing nodules,currently much smaller and nonenhancing, as seen in upper row images. (B) Axialand coronal T1-weighted post IV contrast brain images of patient # 13 with cervico-medullary gangiloglioma,demonstrating pretreatment (B1 & B2) heterogeneous contrast enhancingmass in the left cerebellar hemisphere,with the involvement of the brain stem,particularly l eft hemi medulla,and leptomeningealenhancement extending to the left foramen of Luschka.Marked interval tumor response (B3 & B4) in the tumoral component within theleft cerebellar hemisphere, with almost resolution of mass like contrast enhancement,development of leukomalacia, improvement in the expansion of the left hemi medulla and contrast enhancement.
Patients with PXA and HGG
Two patients with supratentorial PXA-2 underwent STR and GTR, respectively (Table 3). On histology, their tumor exhibited high risk features. Both patients had tumor progression within 3 months. Because further surgical resection was felt to achieve less than GTR, and to avoid giving radiotherapy, a trial of medical therapy was felt reasonable. Both had BRAFv600E mutation and CDKN2A deletion. Dabrafenib was started and during the first 9 months SD and PR were achieved respectively.
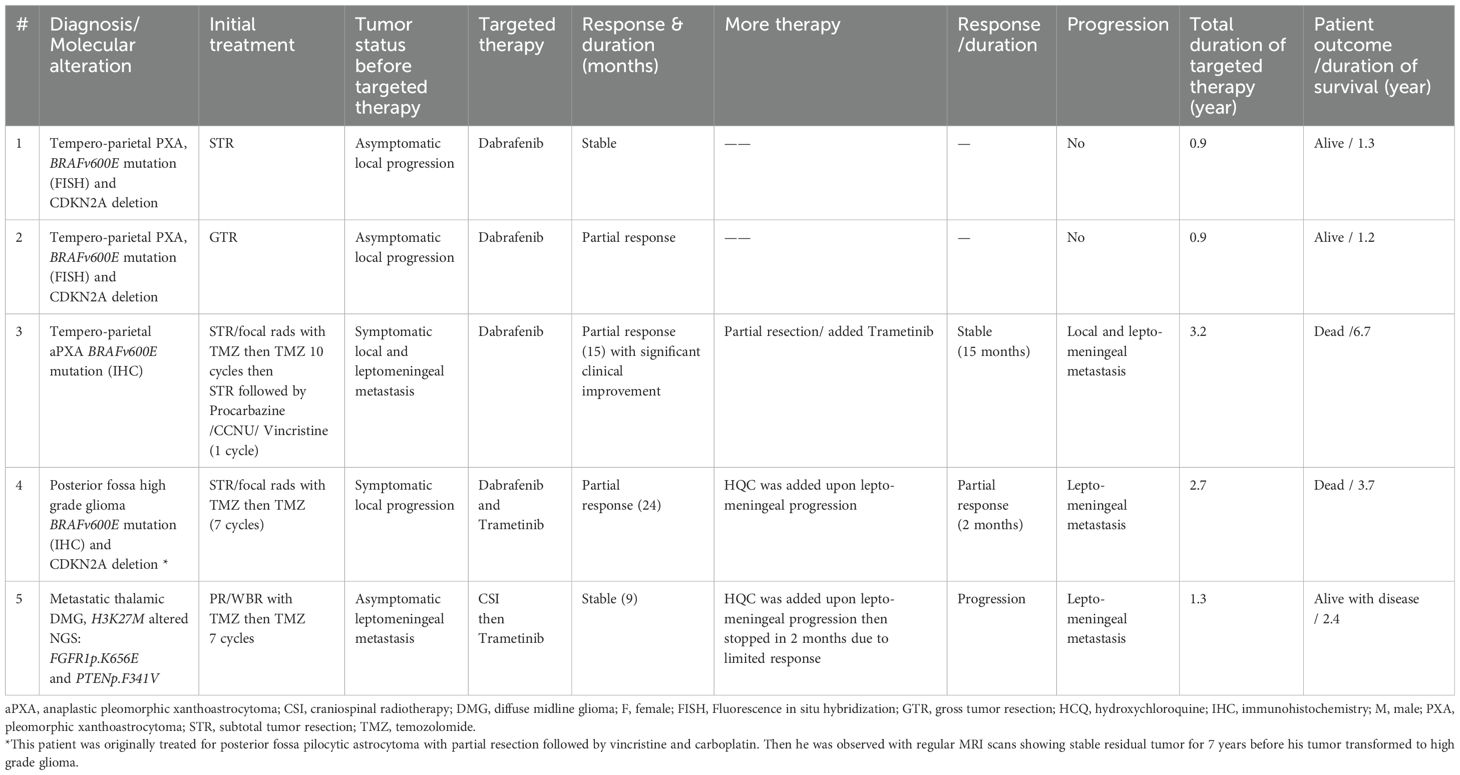
Table 3. Characteristics of patients with pleomorphic xanthoastrocytoma and high grade glioma and their treatment.
Three patients had HGG (Table 3); one had multiple recurrent BRAF mutated aPXA [#3, previously published (23)] was treated with dabrafenib then trametinib was added upon progression, one had posterior fossa BRAF mutant PA transformed to HGG after 7 years without prior radiotherapy use and was started on combined dabrafenib and trametinib, and the third had K27M altered HGG with FGFR1p.K656E and ependymal metastatic lesions who received trametinib and still alive with disease. All tumors underwent STR followed by radiotherapy and temozolomide, then upon further tumor progression they received dabrafenib and/or trametinib. In addition to the radiological response, two patients (#3 & 4) had significant symptomatic improvement. In two patients, hydroxychloroquine was tried to overcome the drug resistance; this was temporarily successful in one patient. With a median of 2.7 years (range, 1.3-3.2 years) from starting dabrafenib and/or trametinib, all tumors progressed, and two patients died.
BRAF/MEK inhibitors side effects
Eight patients (40%) developed acneiform rash; six were on trametinib alone. Three patients (15%) developed paronychia, and one had panniculitis (needing opioids and systemic steroid use) with fatigue. Six patients (30%) needed dose reduction in addition to the supportive measures. Panniculitis and fatigue resolved with addition of trametinib in patient (#2 in Table 2). Ophthalmic and cardiac toxicities were not reported on our regular assessments. One patient (#11 in Table 2) with a difficult to control metastatic LGG, stopped trametinib after 2.2 years despite significant clinical response (became off multiple analgesics including opioids). She had repeated acneiform rash and significant paronychia needing multiple surgical debridement despite the medical care and drug interruptions. Nine months off trametinib, she was asymptomatic with no evidence of radiological tumor progression. One patient (#10 in Table 2) developed significant intracranial bleeding and trametinib was held. Four months later, his tumor did not re-grow. Nine patients had temporary drug interruptions: five due to drug-related side-effects and four due to periods of drug shortage. Three patients developed significant neurological symptoms coinciding with radiological tumor progression within 3 weeks of drug interruption.
Parents and children’s opinions on using BRAF/MEK inhibitors
Eleven of 17 parents of patients with PXA or LGG answered the questionnaire (Supplementary Table S1) in addition to 5 of their children. Children had similar responses to their parents. Except for the patient who stopped trametinib due to side effects (#11 in Table 2), all others were very satisfied with the drugs and felt they were better than chemotherapy. They mainly liked the oral route of these drugs, less frequent hospital visits, the minimal hematological toxicity and lack of hair loss. They disliked the dermatological side effects, particularly those patients who had severe symptoms, and the drugs’ risks on the heart and retina. The risk of tumor progression with drug interruptions and the need to continue these drugs for long time was of a significant concern to the families.
Discussion
We report for the first time on a series of children with gliomas treated with BRAF/MEK inhibitors in a resource-limited country. The compassionate drug access program allowed us to prescribe these drugs and achieve an excellent tumor control in LGGs and a temporary prolonged control in HGGs. Though most families were very satisfied using these new drugs, there are several challenges encountered.
Treating pediatric LGGs is an art that requires to balance tumor control with the treatment’s side effects. The discovery of the molecular landscape of pediatric LGGs and the integral role of the RAS/MAPK pathway signaling in tumorigenesis led to the introduction of BRAF/MEK inhibitors in their management. Many case series demonstrated their efficacy in the recurrent setting achieving reasonable tumor control with a favorable side effects’ profile. This triggered a still ongoing debate as whether targeted therapies should replace chemotherapy (28). A recently published phase II trial (29) on 110 children with BRAFv600E-mutated LGG randomized in a 2:1 ratio to receive dabrafenib and trametinib or standard chemotherapy (carboplatin and vincristine), led to the FDA approval of this combination as a frontline therapy (19). In this trial, and at a median follow-up of 18.9 months, overall tumor response occurred in 47% of children treated with targeted therapy compared to 11% for those given chemotherapy, with observed clinical benefit of 86% and 46% respectively. This resulted in a significantly longer median PFS in the dabrafenib/trametinib arm (20.1 months) compared to 7.4 months in the chemotherapy arm. Currently, the type II RAF inhibitor tovorafenib, is being investigated in a randomized phase 3 trial (30) as a frontline therapy compared to standard chemotherapy in children with BRAF-altered LGG. Type II RAF inhibitors result in tumor response regardless of the BRAF alteration type (mutation or fusion) without a risk of paradoxical activation.
In comparison, the outcome of pediatric HGG is significantly lower despite surgery, radiotherapy, and chemotherapy. BRAFv600E-mutated HGGs are a clinically distinct subtype, and most are secondary to transformed LGGs (10). Nobre et al (31) reported on eleven HGGs previously received radio-chemotherapy; four responded to targeted therapy (36%) with all but one tumor progressed in 18 months. Forty-one children with relapsed/refractory BRAFV600E-mutated HGG received combined dabrafenib and trametinib in a phase II trial (17) had overall response rate of 56% with a median duration of response of 22.2 months. At a median follow-up of 25.1 months, 51% of patients remained on treatment. This is exceptional in recurrent HGGs which rarely respond to chemotherapy resulting in OS of only few months. This raises the question of whether upfront use of BRAF/MEK inhibitors (32–34) is superior in children with HGGs to optimize their management and try to delay radiotherapy use with its deleterious neurocognitive side effects. One of our patients (#5, Table 3) had the unique entity of K27M altered HGG with FGFR1 mutation. His tumor response to trametinib and prolonged survival despite disease progression was previously described in the literature (35).
The use of dabrafenib/trametinib in our setting was encouraging. All gliomas showed tumor control, and though it was temporary in HGGs the duration was of the longest reported (1.3-3.2 years). Importantly, many patients experienced significant control of their symptoms; two children experienced dramatic improvement in their neurological function and were able to practice normal daily activities (Table 1 patient # 8 & Table 3 patient # 3), two patients were spared from a CSF diversion procedure for their ascites (Table 1 patients # 9 &12) (36), one patient with significant sleep apnea became off night BiPap (Table 1 patient #13), one patient became off pain control medications including opioids (Table 1 patient #11), and one child with diencephalic syndrome gained weight (Table 1 patient #6). These symptoms were not previously controlled despite the use of multiple lines of chemotherapy. We would argue whether the earlier introduction of dabrafenib/trametinib, with their rapid tumor response, would have saved some patients from the morbidities of recurrent tumor progressions, particularly on vision, and resulted in a better overall functional outcome. None of our patients with LGG had visual decline while using dabrafenib/trametinib, but several patients had dropping vision with previous tumor progressions. While we did not easily have the option of upfront use of dabrafenib/trametinib through the compassionate drug access program, it is clearly an FDA approved indication now for BRAF-mutated LGGs. This further supports the opinion that every CNS tumor should be tested molecularly as this can make a huge impact on the child’s management and outcome.
Our experience echoes the published data on the side effects’ profile of dabrafenib/trametinib. While most side effects are dermatological, mild, and manageable (17, 29) they can be very distressing to the patients particularly the adolescents. Meticulous skin care is needed to help control these side effects which can be very demanding and challenging to the patients. Emollients and sunscreens were regularly prescribed to our patients and most reported compliance using them. One patient (Table 1, #11), and despite the great control of her neuropathic pains, she could not tolerate the recurrent paronychia and acneiform rash. She eventually stopped trametinib despite her awareness of the risk of rebound and the possible need for radiotherapy. This is a well reported risk when stopping the targeted therapies (37). Fortunately, her tumor is still under control 9 months after discontinuation of treatment. Recently, experts from Canada developed a consensus algorithm for discontinuation of targeted therapies in children with BRAFV600E gliomas (38). One patient (Table 1, #10) developed significant intracranial bleeding while on trametinib. This rare event was previously reported in the literature (39). We did not notice cardiac dysfunctions or ophthalmic side effects in our cohort despite regular assessments. These risks were one of major drawbacks of using targeted therapies according to the families. In addition, the uncertainty on the duration of using these drugs, and the high risk of rebound tumor growth with drug interruptions were stressful to the families. This is still a medical challenge. There are anecdotal data on successful rechallenge after stopping BRAF inhibitors (31), or shifting to a selective BRAF inhibitor (40), or combining it with chemotherapy. Despite these risks, most of our patients preferred the use of targeted therapies over chemotherapy.
With the use of the compassionate drug access program, we provided new targeted drugs to our patients however this is not without a challenge. We had times with drugs interruptions related to drug importation and during the COVID era. This route of drug access is used globally particularly in children with cancer where there are limited drug approvals or clinical trials access (20). It may be more “justified” in a LMIC setting where access to new drugs will take long time, if ever. The high cost of the targeted drugs is a challenge for routine clinical use even after the accumulating evidence of efficacy in the literature. We are now working on a cost effectiveness analysis and specific indications to use dabrafenib/trametinib at KHCC after closure of the compassionate drug access program in Jordan following the FDA approval of the combination of trametinib and dabrafenib for pediatric patients with BRAF mutated LGGs in March 2023. It is important as well to consider the participation of LMICs in international clinical trials of new targeted medications. Most of these drugs are orally administered and need less frequent monitoring which makes the idea of using them is more plausible in a resource-limited setting. This hopefully would result in less abandonment of therapy or a need to use alternative choices with shorter duration of therapy, like radiotherapy, with its detrimental neurocognitive side-effects particularly on young children. In addition, most targeted drugs act rapidly which help decrease the morbidities associated with tumor growth (e.g. visual loss or neurological deficits) which are more difficult to “tolerate” in a resource-limited setting. On the other hand, inclusion of LMICs in the international clinical trials will help advance the whole health system in these countries.
The present study is limited by the fact it is a retrospective review of a single center experience in a resource-limited setting. KHCC is a relatively advanced center for a LMIC and has excellent infrastructure and trained staff. Furthermore, KHCC has a long-standing twinning program with SickKids hospital. This has contributed to facilitate the interaction with the team involved in the Novartis compassionate program, to build a strong relationship with this team and to be granted approvals for compassionate use for this entire cohort of patients. This makes our experience unique, as reports on targeted treatment in children with brain tumors in LMICs remains anecdotal (22). The response rate observed in our experience appears to be higher than in clinical trials of targeted therapies (29). This may be related to a selection bias in our MDT. However, discrepancies between institutional evaluation and central reviews were noted in several trials (29, 41), with higher response rates reported by investigators. Capturing toxicity data was limited by the retrospective nature of this review and the toxicity may appear lower than in prospective trials of targeted treatments. However, only significant side effects were captured particularly those resulted in dose reductions or interruptions. The positive insight provided by the parents and children on using dabrafenib/trametinib is encouraging and rarely documented in LMICs.
In conclusion, our experience demonstrates the feasibility of using new targeted drugs in a resource-limited setting and the effectiveness in achieving good tumor control with excellent patients’ satisfaction. Questions remain to be answered regarding the duration of using these drugs and their long-term toxicity in children. The current ethical challenge facing LMICs is to balance the affordability of using these drugs in routine clinical practice. Moving targeted drugs to the frontline can save children several morbidities and be more cost effective on the long-term even in a resource-limited setting. Well-designed global studies that combine patients’ reported outcome, families’ perspective, tumor response and cost effectiveness are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Institutional Review Board at KHCC. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it is a retrospective data review.
Author contributions
DA: Data curation, Writing – review & editing. AA: Writing – review & editing. MA-H: Writing – review & editing. MO: Writing – review & editing. BM: Writing – review & editing. QA: Writing – review & editing. AM: Writing – review & editing. SJ: Writing – review & editing. RR: Writing – review & editing. KK: Writing – review & editing. AI: Writing – review & editing. NS: Writing – review & editing. EB: Writing – review & editing. NA: Conceptualization, Data curation, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Part of this data was accepted for presentation in the 55th congress of the International Society of Pediatric Oncology (SIOP 2023). We would like to thank Novartis for providing compassionate access to Dabrafenib/Trametinib to our patients. We also than Mr. Mohammad O. Al-Bssol and Mrs. Ayat Taqash for their statistical help.
Conflict of interest
EB is a member of the advisory board of Novartis and Alexion.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1417484/full#supplementary-material
References
1. Hessissen L, Parkes J, Amayiri N, Mushtaq N, Sirachainan N, Anacak Y, et al. SIOP PODC Adapted treatment guidelines for low grade gliomas in low and middle income settings. Pediatr Blood Cancer. (2017) 64 Suppl. doi: 10.1002/pbc.26737
2. Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. (2012) 30:2641–7. doi: 10.1200/JCO.2011.36.6054
3. Gnekow AK, Walker DA, Kandels D, Picton S, Perilongo G, Grill J, et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (</=16 years) low grade glioma - A final report. Eur J Cancer. (2017) 81:206–25. doi: 10.1016/j.ejca.2017.04.019
4. Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, et al. Phase II weekly vinblastine for chemotherapy-naive children with progressive low-grade glioma: A canadian pediatric brain tumor consortium study. J Clin Oncol. (2016) 34:3537–43. doi: 10.1200/JCO.2016.68.1585
5. Cherlow JM, Shaw DWW, Margraf LR, Bowers DC, Huang J, Fouladi M, et al. Conformal radiation therapy for pediatric patients with low-grade glioma: results from the children’s oncology group phase 2 study ACNS0221. Int J Radiat Oncol Biol Phys. (2019) 103:861–8. doi: 10.1016/j.ijrobp.2018.11.004
6. Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. (2009) 27:3598–604. doi: 10.1200/JCO.2008.20.9494
7. Jakacki RI, Cohen KJ, Buxton A, Krailo MD, Burger PC, Rosenblum MK, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol. (2016) 18:1442–50. doi: 10.1093/neuonc/now038
8. Ryall S, Zapotocky M, Fukuoka K, Nobre L, Stucklin AG, Bennett J, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. (2020) 37:569–583 e5. doi: 10.1016/j.ccell.2020.03.011
9. Lassaletta A, Zapotocky M, Mistry M, Ramaswamy V, Honnorat M, Krishnatry R, et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol. (2017) 35:2934–41. doi: 10.1200/JCO.2016.71.8726
10. Mistry M, Zhukova N, Merico D, Rakopoulos P, Krishnatry R, Shago M, et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol. (2015) 33:1015–22. doi: 10.1200/JCO.2014.58.3922
11. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
12. Drobysheva A, Klesse LJ, Bowers DC, Rajaram V, Rakheja D, Timmons CF, et al. Targeted MAPK pathway inhibitors in patients with disseminated pilocytic astrocytomas. J Natl Compr Canc Netw. (2017) 15:978–82. doi: 10.6004/jnccn.2017.0139
13. Kondyli M, Larouche V, Saint-Martin C, Ellezam B, Pouliot L, Sinnett D, et al. Trametinib for progressive pediatric low-grade gliomas. J Neurooncol. (2018) 140:435–44. doi: 10.1007/s11060-018-2971-9
14. Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, Zapotocky M, McKeown T, Hawkins C, et al. Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer. (2016) 63:2038–41. doi: 10.1002/pbc.26086
15. Miller C, Guillaume D, Dusenbery K, Clark HB, Moertel C. Report of effective trametinib therapy in 2 children with progressive hypothalamic optic pathway pilocytic astrocytoma: documentation of volumetric response. J Neurosurg Pediatr. (2017) 19:319–24. doi: 10.3171/2016.9.PEDS16328
16. Brown NF, Carter T, Kitchen N, Mulholland P. Dabrafenib and trametinib in BRAFV600E mutated glioma. CNS Oncol. (2017) 6:291–6. doi: 10.2217/cns-2017-0006
17. Hargrave DR, Terashima K, Hara J, Kordes UR, Upadhyaya SA, Sahm F, et al. Phase II trial of dabrafenib plus trametinib in relapsed/refractory BRAF V600-mutant pediatric high-grade glioma. J Clin Oncol. (2023) 41:5174–83. doi: 10.1200/JCO.23.00558
18. Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. (2014) 14:258. doi: 10.1186/1471-2407-14-258
19. Barbato MI, Nashed J, Bradford D, Ren Y, Khasar S, Miller CP, et al. FDA approval summary: dabrafenib in combination with trametinib for BRAFV600E mutation-positive low-grade glioma. Clin Cancer Res. (2024) 30:263–8. doi: 10.1158/1078-0432.CCR-23-1503
20. Lerose R, Musto P, Aieta M, Papa C, Tartarone A. Off-label use of anti-cancer drugs between clinical practice and research: the Italian experience. Eur J Clin Pharmacol. (2012) 68:505–12. doi: 10.1007/s00228-011-1173-6
21. Lim M, Shulman DS, Roberts H, Li A, Clymer J, Bona K, et al. Off-label prescribing of targeted anticancer therapy at a large pediatric cancer center. Cancer Med. (2020) 9:6658–66. doi: 10.1002/cam4.3349
22. Mustansir F, Mushtaq N, Darbar A. Dabrafenib in BRAFV600E mutant pilocytic astrocytoma in a pediatric patient. Childs Nerv Syst. (2020) 36:203–7. doi: 10.1007/s00381-019-04346-2
23. Amayiri N, Swaidan M, Al-Hussaini M, Halalsheh H, Al-Nassan A, Musharbash A, et al. Sustained response to targeted therapy in a patient with disseminated anaplastic pleomorphic xanthoastrocytoma. J Pediatr Hematol Oncol. (2018) 40:478–82. doi: 10.1097/MPH.0000000000001032
24. World Bank Group Jordan data . Available online at: https://data.worldbank.org/country/jordan. Accessed July 1,2024.
25. UNICEF Jordan country profile. Available online at: https://www.unicef.org/mena/media/19921/file. Accessed July 1,2024.
26. TruSight sequencing panels . Available online at: https://www.illumina.com/products/trusight-panels.html. Accessed February 1,2024.
27. Wen PY, van den Bent M, Youssef G, Cloughesy TF, Ellingson BM, Weller M, et al. RANO 2.0: update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol. (2023) 41:5187–99. doi: 10.1200/JCO.23.01059
28. Cooney T, Yeo KK, Kline C, Prados M, Haas-Kogan D, Chi S, et al. Neuro-Oncology Practice Clinical Debate: targeted therapy vs conventional chemotherapy in pediatric low-grade glioma. Neurooncol Pract. (2020) 7:4–10. doi: 10.1093/nop/npz033
29. Bouffet E, Hansford JR, Garre ML, Hara J, Plant-Fox A, Aerts I, et al. Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N Engl J Med. (2023) 389:1108–20. doi: 10.1056/NEJMoa2303815
30. van Tilburg CM, Kilburn LB, Perreault S, Schmidt R, Azizi AA, Cruz-Martínez O, et al. LOGGIC/FIREFLY-2: a phase 3, randomized trial of tovorafenib vs. chemotherapy in pediatric and young adult patients with newly diagnosed low-grade glioma harboring an activating RAF alteration. BMC Cancer. (2024) 24:147. doi: 10.1186/s12885-024-11820-x
31. Nobre L, Zapotocky M, Ramaswamy V, Ryall S, Bennett J, Alderete D, et al. Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis Oncol. (2020) 4:PO.19.00298. doi: 10.1200/PO.19.00298
32. Nobre L, Bouffet E. BRAF inhibitors in BRAFV600E-mutated pediatric high-grade gliomas: Upfront or at recurrence? Neuro Oncol. (2022) 24:1976–7. doi: 10.1093/neuonc/noac160
33. Arbour G, Ellezam B, Weil AG, Cayrol R, Vanan MV, Coltin H, et al. Upfront BRAF/MEK inhibitors for treatment of high-grade glioma: A case report and review of the literature. Neurooncol Adv. (2022) 4:vdac174. doi: 10.1093/noajnl/vdac174
34. Rosenberg T, Yeo KK, Mauguen A, Alexandrescu S, Prabhu SP, Tsai JW, et al. Upfront molecular targeted therapy for the treatment of BRAF-mutant pediatric high-grade glioma. Neuro Oncol. (2022) 24:1964–75. doi: 10.1093/neuonc/noac096
35. Roberts HJ, Ji S, Picca A, Sanson M, Garcia M, Snuderl M, et al. Clinical, genomic, and epigenomic analyses of H3K27M-mutant diffuse midline glioma long-term survivors reveal a distinct group of tumors with MAPK pathway alterations. Acta Neuropathol. (2023) 146:849–52. doi: 10.1007/s00401-023-02640-7
36. Amayiri N, Obeidat M, Laban DA, Musharbash A, Al-Hussaini M, Maraqa B, et al. BRAF/MEK inhibitors use to treat ventriculoperitoneal shunt-associated ascites in pediatric low-grade gliomas. Pediatr Blood Cancer. (2024) 71:e31058. doi: 10.1002/pbc.31058
37. O’Hare P, Cooney T, de Blank P, Gutmann DH, Kieran M, Milde , et al. Resistance, rebound, and recurrence regrowth patterns in pediatric low-grade glioma treated by MAPK inhibition: A modified Delphi approach to build international consensus-based definitions-International Pediatric Low-Grade Glioma Coalition. Neuro Oncol. (2024) 26(8):1357–66. doi: 10.1093/neuonc/noae074
38. Canadian consensus for treatment of BRAF V600E mutated pediatric and AYA gliomas . Available online at: https://mdpi-res.com/d_attachment/curroncol/curroncol-31-00299/article_deploy/curroncol-31-00299.pdf?version=1721115119.
39. Manoharan N, Choi J, Chordas C, Zimmerman MA, Scully J, Clymer J, et al. Trametinib for the treatment of recurrent/progressive pediatric low-grade glioma. J Neurooncol. (2020) 149:253–62. doi: 10.1007/s11060-020-03592-8
40. Seghers AC, Wilgenhof S, Lebbe C, Neyns B. Successful rechallenge in two patients with BRAF-V600-mutant melanoma who experienced previous progression during treatment with a selective BRAF inhibitor. Melanoma Res. (2012) 22:466–72. doi: 10.1097/CMR.0b013e3283541541
Keywords: BRAF/MEK inhibitors, dabrafenib, trametinib, low-middle-income countries (LMIC), targeted therapy, glioma, off-label/compassionate
Citation: Abu Laban D, Alsharif A, Al-Hussaini M, Obeidat M, Maraqa B, Alzoubi Q, Musharbash A, Jaddoua S, Ramlawi R, Khaleifeh K, Ibrahimi AK, Sarhan N, Bouffet E and Amayiri N (2024) BRAF/MEK inhibitors use for pediatric gliomas; real world experience from a resource-limited country. Front. Oncol. 14:1417484. doi: 10.3389/fonc.2024.1417484
Received: 15 April 2024; Accepted: 27 August 2024;
Published: 27 September 2024.
Edited by:
Diana Osorio, The University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Ahmed Gilani, University of Colorado Hospital, United StatesJordan Robert Hansford, South Australian Health and Medical Research Institute (SAHMRI), Australia
Copyright © 2024 Abu Laban, Alsharif, Al-Hussaini, Obeidat, Maraqa, Alzoubi, Musharbash, Jaddoua, Ramlawi, Khaleifeh, Ibrahimi, Sarhan, Bouffet and Amayiri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nisreen Amayiri, bmFtYXlpcmlAa2hjYy5qbw==
 Dima Abu Laban1
Dima Abu Laban1 Abeer Alsharif
Abeer Alsharif Maysa Al-Hussaini
Maysa Al-Hussaini Mouness Obeidat
Mouness Obeidat Bayan Maraqa
Bayan Maraqa Qasem Alzoubi
Qasem Alzoubi Nasim Sarhan
Nasim Sarhan Eric Bouffet
Eric Bouffet Nisreen Amayiri
Nisreen Amayiri