
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 21 August 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1416888
Introduction: Patients with renal insufficiency are more prone to postoperative complications (PCs). Studies have shown that minor changes in serum creatinine (SCr), immediately post-surgery, can aid in assessing patients’ renal function. This study aimed to explore the relationship between the changes in SCr and PCs in patients with gastric cancer (GC).
Materials and methods: We prospectively collected data regarding the SCr of 530 GC patients, within 2 weeks before surgery and within 24 hours after surgery in our hospital (2014–2016). The patients were divided into three groups according to the level of SCr change after surgery: reduced (<10%), normal (10%), and elevated (>10%) creatinine groups. Univariate and multivariate logistic analysis were performed to evaluate its correlation with short-term PCs in the patients. The R language was used to construct a nomogram.
Results: 83, 217, and 230 patients were assigned to the elevated, reduced, and normal SCr groups, respectively. Multivariate analysis showed that the reduced and elevated SCr groups were independently associated with the occurrence of PCs and severe postoperative complications (SPCs), respectively. Additionally, postsurgical SCr change, age, hypoalbuminemia, total gastrectomy, combined resection, and laparoscopy, were independently related to PCs. Combining the above influential factors, the predictive model can distinguish patients with PCs more reliably (c-index is 0.715).
Conclusion: Post-surgery, reduced SCr is a protective factor for PCs, while elevated serum creatinine is an independent risk factor for SPCs. Our nomogram can identify GC patients with high risks of PCs.
Gastric cancer (GC) is one of the most commonly diagnosed malignancies and the third leading cause of cancer-related deaths worldwide (1). More than 20% of global new cancer cases and cancer-related deaths occur in China (2). Radical gastrectomy with D2 lymph node dissection is considered the standard treatment for GC but carries a 12.8–14% incidence of postoperative complications (PCs) (3–7). Postoperative complications (PCs) not only increase costs and prolong hospital stays, but also affect subsequent treatment, and may lead to poor long-term prognosis for GC patients (8, 9). Hence, early identification and intervention for high-risk GC are important.
The continuous improvement of medical standards has given patients with chronic health problems, such as renal insufficiency, the opportunity to be successfully treated through surgical operations (10, 11). As an indicator of the risk in perioperative surgery, preoperative renal insufficiency has also been reported to affect the outcome of patients after surgery (12–14). Recent literature states that the measurement of changes in the level of serum creatinine (SCr) immediately after surgery is also related to the patient’s renal function (15, 16). In our clinical work, we have observed that some patients with gastric cancer have different degrees of change in SCr after surgery. In the follow-up clinical diagnosis and treatment process, we found that the clinical prognosis of patients with varying degrees change in SCr, undergoing radical gastric cancer surgery during the same period, is not the same.
Currently, there are no reports on the influence of SCr level changes on the clinical prognosis of GC patients. Thus, the purpose of this study was to explore the correlation between different degrees of postoperative SCr changes and the short-term outcomes of patients after radical gastrectomy.
Overall, 595 GC patients were recruited at the First Affiliated Hospital of Wenzhou Medical University from 2014 to 2016. The exclusion criteria included: (1) history of uremia and severe preoperative kidney function damage; (2) history of emergency surgery; (3) incomplete medical records; (4) underwent palliative gastrectomy; or (5) difference in pre- and postoperative diagnosis. After screening, 65 patients were excluded and 530 patients who underwent radical gastrectomy were included and analyzed in this study. The experimental flow chart is shown in Figure 1. All eligible participants were requested to provide written informed consent before participating. All GC patients in this study received conventional therapy complies with the Japanese Gastric Cancer Treatment Guidelines (17).
The relevant data were prospectively acquired and analyzed: (1) age; sex; body mass index (BMI); concentration of albumin, hemoglobin, and SCr; neutrophil/lymphocyte ratio (NLR); platelet/lymphocyte ratio (PLR); diabetes mellitus; hypertension; abdominal surgery; comorbidities; American Society of Anesthesiologists (ASA) grade; Nutritional Risk Screening 2002 (NRS 2002) score; tumor location, size, pathological type and tumor-node-metastasis (TNM) stage (preoperative patients and disease characteristics aspect); (2) type of resection, combined resection, surgical method, and type of reconstruction (operative details aspect); and (3) PCs, length of postoperative hospital stay, and hospital costs (postoperative outcomes aspect).
Two researchers recorded the grade of complications within one month after surgery according to the Clavien–Dindo classification (18), and PCs (≥ grade II) were analyzed in this study. Severe postoperative complications (SPCs) were defined as Clavien–Dindo classification ≥ grade III.
The SCr levels were prospectively collected within two weeks before surgery and within 24 hours after surgery in 530 patients who underwent curative gastrectomy in our hospital. All preoperative and postoperative SCr measurements were obtained from the same biochemical laboratory. The primary independent variable of interest was percent change in ΔSCr, calculated as [(postoperative SCr − preoperative SCr)/preoperative SCr] × 100. %ΔSCr was classified into three levels for purposes of analysis: %ΔSCr decrease<10% (defined as the reduced SCr group), %ΔSCr within 10% (defined as the normal SCr group), and %ΔSCr increase >10% (defined as the elevated SCr group).
Categorical variables were expressed in terms of quantity (percentage), while continuous variables conforming to normal distribution are described by the mean (standard deviation); otherwise, they are described by the median (interquartile range). The Kolmogorov–Smirnov test was used to assess the normality of continuous variables. In univariate analysis, Chi-square test and Fisher’s exact test were used to analyze the differences in categorical variables, while the independent t-test and Mann–Whitney U test were used for continuous variables. In the subsequent multivariate analysis, the variables with P <0.10 were included in the univariate analysis into the multivariate logistic regression analysis to calculate the odds ratio (OR) and 95% confidence interval (CI) of the independent variables. A nomogram composed of independent risk factors obtained by multivariate analysis is used to construct a predictive model and calculate the probability of PCs (19).
Values of P < 0.05 were considered statistically significant and all tests were two-tailed. All data analyses were performed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA). The prediction model was constructed using the R statistical software (The R Foundation, Vienna, Austria). The generated graphical displays were analyzed using the rms package.
According to the percent change in ΔSCr, the remaining 530 patients were divided into three groups: 230 (43.4%) into the normal SCr group, 83(15.7%) into the elevated SCr group, and 217 (40.9%) into the reduced SCr group. The detailed clinical characteristics of the patients are shown in Table 1. There were significant intergroup differences in age (P = 0.004), Charlson comorbidity index (P = 0.017), intraoperative bleeding (P = 0.047), postoperative hospital stays (P = 0.011), and hospitalization expense (P = 0.027). Furthermore, the reduced SCr group had a lower mean hospitalization expense than the normal group and elevated SCr groups (P < 0.05).
As seen in Table 2, 193 postoperative events occurred in 116 patients (21.9%). Among them, 55 (10.4%) patients had grade IIIa or higher PCs. Abdominal complications (mainly intra-abdominal infections, postoperative bleeding, and bowel obstruction) and pulmonary complications (mainly including pulmonary infections and pleural effusions) were the most frequent postoperative events.
The number of patients with PCs and the incidence of complications between the three groups are shown in Figure 2. Patients with PCs had longer hospital stays (PCs vs. non-PCs: 25 vs. 12 days, P <0.001) and incurred higher hospital costs (PCs vs. non-PCs: 78,781.4 ¥ vs. 54,790.1 ¥, P <0.001) than those who did not.
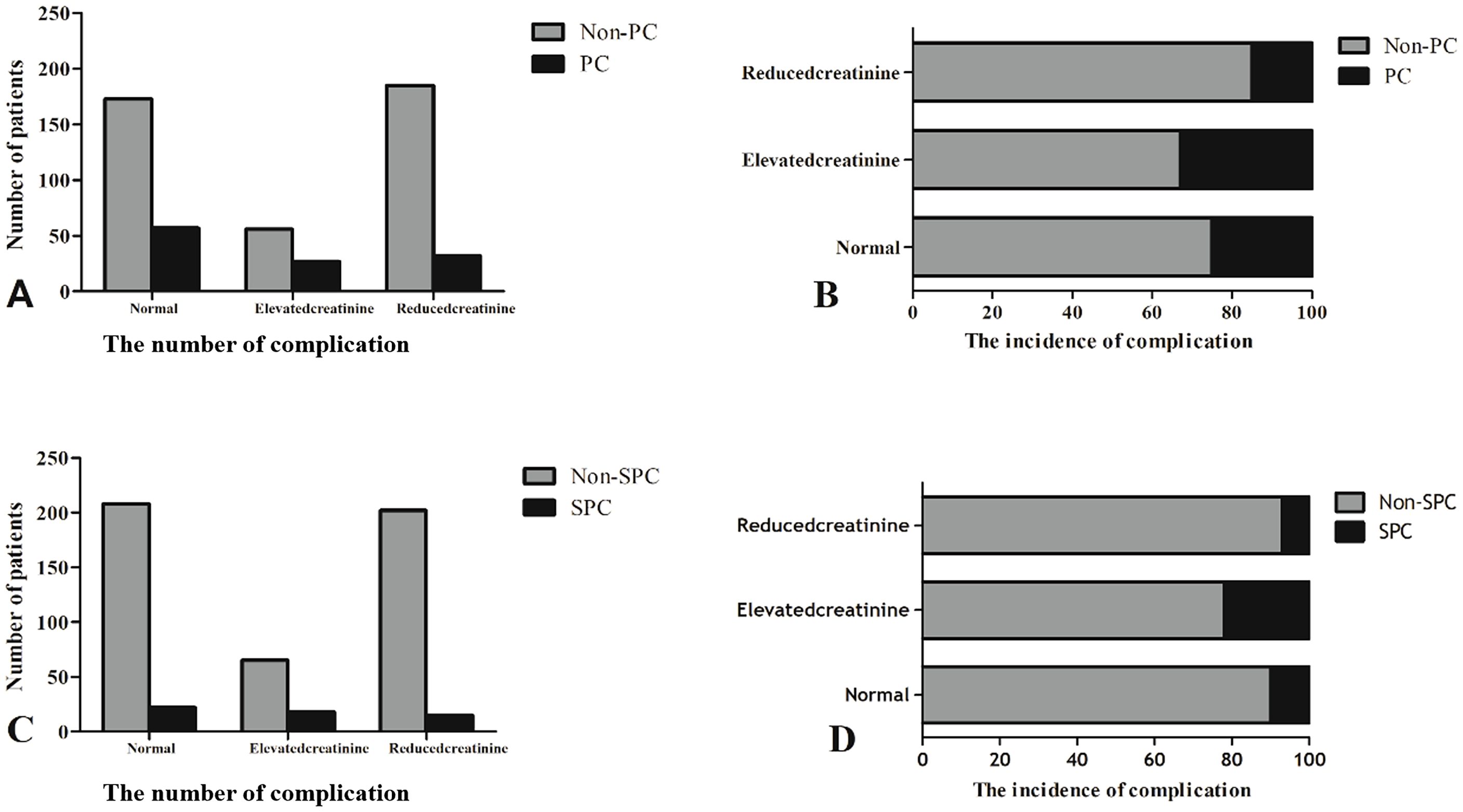
Figure 2. The number of patients with PCs and the incidence of complications between the three groups. (A, C) The number of complication. (B, D) The incidence of complication.
The chi-square test was used to assess the relationship between clinical characteristics of GC patients and the occurrence of PCs. The univariate analysis results showed that age (P < 0.001), NRS score (P = 0.01), hypoalbuminemia (P < 0.001), anemia (P = 0.086), NLR (P = 0.029), Charlson comorbidity index (P = 0.001), ASA score (P = 0.017), Charlson comorbidity index (P < 0.0001), ASA (P = 0.008), tumor size (P = 0.001), TNM stages (P = 0.001), total gastrectomy (P < 0.001), combined resection (P < 0.001), surgical method (P < 0.001), reconstruction methods (P = 0.005), intraoperative bleeding (P = 0.028), and postsurgical SCr change (P = 0.011) differed significantly (Table 3). Variables with a P value < 0.1 on univariate analysis were included in the multivariate logistic regression analysis. Age (OR = 1.785, P = 0.012), hypoalbuminemia (OR = 4.506, P = 0.004), total gastrectomy (OR = 1.89 1, P = 0.005), combined resection (OR = 2.231, P = 0.020), laparoscopic surgery (OR = 0.453, P = 0.013), and postsurgical SCr change were independently associated with PCs.
A nomogram based on independent risk factors was constructed to predict the probability of PCs (≥ grade IIa) (Figure 3). A discrimination concordance index of 0.715 indicated a 71.5% correct ordering of risk across pairs of patients and a good calibration of observed versus predicted PCs.
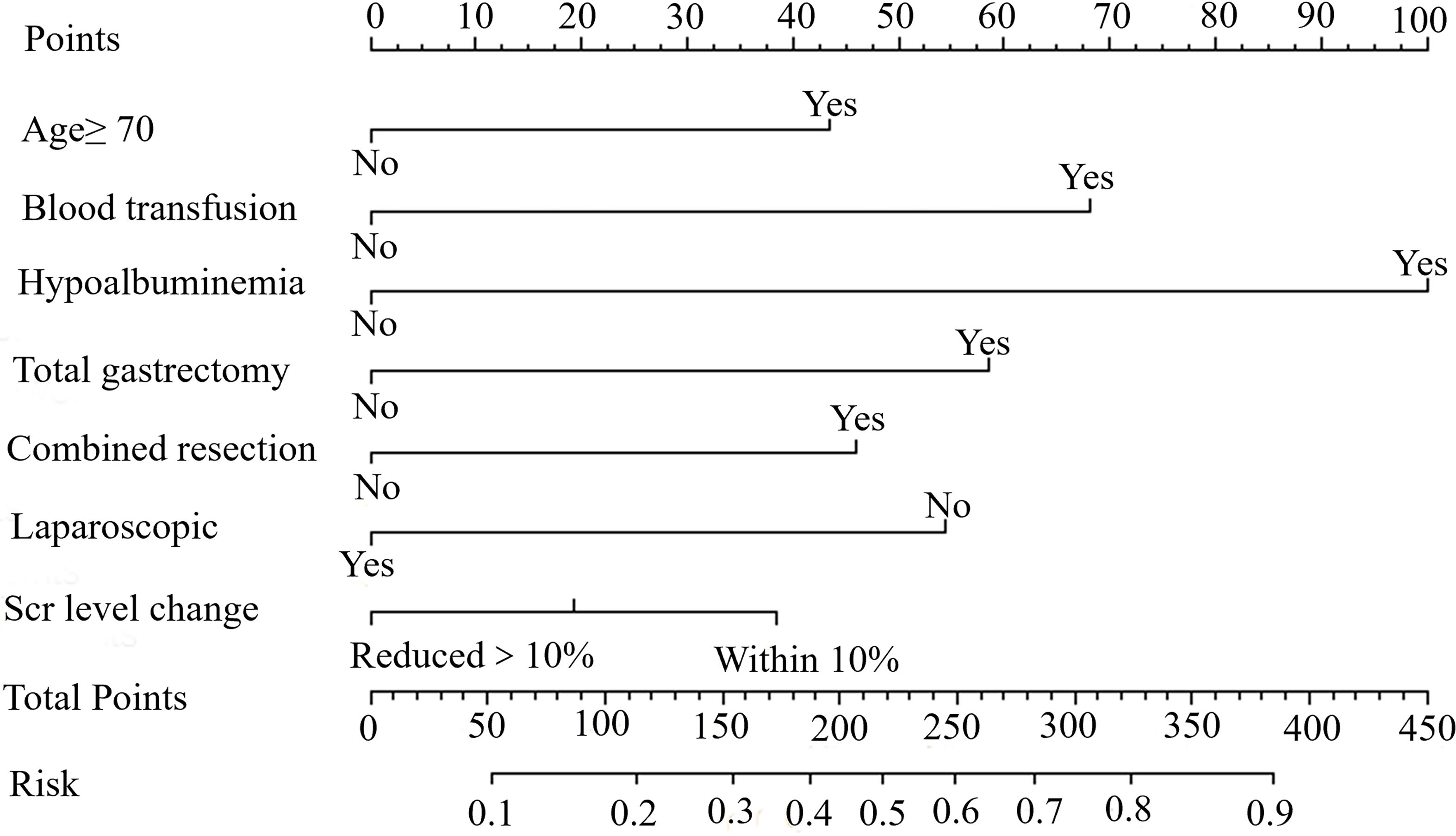
Figure 3. A nomogram indicating the probability of PCs for young GC patients. An example of a nomogram-estimating PCs risk in resected gastric cancer. Draw an upward vertical line from the covariate to the points bar to calculate points. Based on the sum of the covariate points, draw a downward vertical line from the total points line to calculate risk of PCs.
The relationship between postsurgical SCr change and SPCs was also evaluated in this study. The univariate analysis results showed that age (P = 0.001), NRS score (P = 0.030), Charlson comorbidity index (P = 0.070), ASA score (P = 0.061), TNM stage (P = 0.037), total gastrectomy (P = 0.007), combined resection (P < 0.001), laparoscopic surgery (P = 0.003), reconstruction methods (P = 0.031), and intraoperative bleeding (P = 0.023) differed significantly (Table 4). Variables with a P value < 0.1 on univariate analysis were included in the multivariate logistic regression analysis. In multivariate analysis, age, combined resection, laparoscopic surgery, and postsurgical SCr change, were independently associated with SPCs (all P < 0.05).
The incidence of PCs was 21.9% in this study. The median hospital stays in the PC group vs. the non-PC group was 25 vs. 12 days. Additionally, patients with PCs spent significantly more during their hospitalization, which is consistent with the conclusions of previous studies (4–9). The emergence of PCs will prolong the hospital stay, increase personal burden, reduce the trust between doctors and patients, and make it more likely to have conflicts between doctors and patients (20, 21). Therefore, it is particularly important to identify and intervene in patients with complications after radical GC surgery to reduce the occurrence of PCs.
The clinical indicators used to predict the short-term prognosis of patients with GC have their own shortcomings, such as sarcopenia, in assessing the physical function of patients through imaging data, which require physicians to have a certain degree of imaging knowledge (22–24). In contrast, calculating postoperative changes in SCr levels is easier and more efficient, which can be mastered only by short-term learning and more suitable for heavy clinical work. Moreover, the novel predictive model, based on postoperative creatinine changes, age, hypoalbuminemia, total gastrectomy, combined resection, and laparoscopic surgery can be used to assist surgeons in distinguishing low-risk and high-risk patients, ensuring successful perioperative management, and postoperative rehabilitation to reduce the incidence of PCs in high-risk patients (the discriminant consistency index is 0.715).
This is the first time we reported that elevated SCr is an independent risk factor for SPCs (OR = 2.475, P = 0.013) and reduced SCr is an independent protective factor for PCs (OR = 0.559, P = 0.024), which is maintained after including multiple risk indicators in the multivariate analysis. As a large part of the population suffering from GC, elderly patients are more likely to have hemodynamic instability after surgery, which can increase the incidence of renal insufficiency and make patients more susceptible to PCs (25, 26). Latest research shows that minor changes in SCr after surgery are also closely related to the poor prognosis of patients (15, 16). However, most of the research in this area focuses on cardiothoracic surgery research (27–29). No study to our knowledge has found that slight changes in postoperative SCr is related to the short-term prognosis of GC patients underwent curative gastrectomy. Renal insufficiency will trigger the continuous release of systemic pro-inflammatory factors and an immunosuppressive state, making patients more prone to bone loss and malnutrition (30), eventually leading to PCs. Meanwhile, postoperative SCr decrease is likely related to hemodilution, which is considered to be one of the protective factors of kidney function (31, 32), and further related to the decrease in the incidence of PCs in our experiment. However, it is worth noting that this study did not find that elevated SCr is independently associated with PCs of GC. This may be due to our insufficient sample size. In subsequent studies, we should further expand the research volume to explore whether the increase in SCr is related to PCs.
The results in Table 1 show that there were significant differences in the postoperative hospital stay between the three groups, and the reduced SCr group had significantly lower hospital costs than the other two groups (P < 0.05). The previous data show that the deterioration of renal function is associated with a significantly longer hospital stay, an higher in hospital costs, and an increased risk of death in the hospital (33). Compared with the other two groups, the reduced creatinine group had a lower incidence of pulmonary complications, thrombosis, and intestinal obstruction (Table 2). Interventions for these complications will inevitably increase patient costs, but they can return to normal status quickly after appropriate treatment, which may partly explain why there is small difference in postoperative hospital stay between the three groups of patients. We should strengthen early postoperative monitoring of small changes in SCr levels in clinical work, and initiate renal protective interventions to prevent complications after radical GC surgery.
In this study, we also found that age, combined resection, and non-laparoscopic surgery are not only independently related to the occurrence of PCs in patients after radical GC surgery, but also independent risk factors for severe PCs. Elderly patients have poor tolerance to surgery (34), greater surgical trauma after combined organ resection (35), and better exposure to laparoscopic surgery compared to open surgery for surgeon’s operations (36, 37), resulting in differences in the incidence of surgery-related complications. Therefore, for elderly patients with GC, we recommend that under the premise of achieving radical resection, the preoperative choice of endoscopic surgery is performed, and intraoperative resection is as non-combined as possible to reduce the occurrence of PCs. Previous studies have confirmed that GC patients with preoperative hypoalbuminemia are more likely to develop infections and are related to poor prognosis (38). In this study, we found that preoperative hypoalbuminemia was related to PCs, and not an independent risk factor for severe PCs. We speculate that this may be related to the incidence of hypoproteinemia in this experiment is only 3.5%, which is much lower than the 6% reported in previous studies (23).
Several potential limitations still exist in our study and careful consideration is required. This study only explored the relationship between postoperative SCr changes and the short-term prognosis of patients with GC, without the long-term prognosis. In the follow-up work, we will supplement this part of the research. Moreover, the research conclusions of this study come from a single-center subject and lack the support of multi-center data. We will further improve this in the follow-up research.
This is the first time we reported that minor changes in serum creatinine is an independent risk factor for PCs. Post-surgery, reduced SCr is a protective factor for PCs, while elevated serum creatinine is an independent risk factor for SPCs. Calculating postoperative changes in SCr levels is easier and more efficient, which can be mastered only by short-term learning and more suitable for heavy clinical work. Our nomogram is a simple and practical instrument to identify patients at high risk of surgical complications.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethical Review Meeting of the First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
WC: Data curation, Writing – original draft. XZ: Formal analysis, Writing – review & editing. YH: Methodology, Writing – review & editing. WC: Investigation, Writing – review & editing. XC: Supervision, Writing – review & editing. XX: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The protocol for this research was authorized by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University. All GC patients have signed their written informed consent.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
3. Choi YY, Cho M, Kwon IG, Son T, Kim H-I, Choi SH, et al. Ten thousand consecutive gastrectomies for gastric cancer: perspectives of a master surgeon. Yonsei Med J. (2019) 60:235–42. doi: 10.3349/ymj.2019.60.3.235
4. Yasunaga H, Horiguchi H, Kuwabara K, Matsuda S, Fushimi K, Hashimoto H, et al. Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: a propensity-matched analysis. Ann Surg. (2013) 257:640–6. doi: 10.1097/SLA.0b013e31826fd541
5. Yu J, Hu J, Huang C, Ying M, Peng X, Wei H, et al. The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: results from the Chinese laparoscropic gastrointestinal surgery study (CLASS) group. Eur J Surg Oncol. (2013) 39:1144–9. doi: 10.1016/j.ejso.2013.06.021
6. Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. (2014) 32:627–33. doi: 10.1200/JCO.2013.48.8551
7. Goglia. M, Pepe. S, Pace. M, Fattori. L, Minervini. A, Giulitti. D, et al. Complication of gastric cancer surgery: A single centre experience. In Vivo (Athens Greece). (2023) 37:2166–72. doi: 10.21873/invivo.13315
8. Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. (2013) 20:1575–83. doi: 10.1245/s10434-012-2720-9
9. Zhang WT, Lin J, Chen WS, Huang YS, Wu RS, Chen XD, et al. Sarcopenic obesity is associated with severe postoperative complications in gastric cancer patients undergoing gastrectomy: a prospective study. J Gastrointest Surg. (2018) 22:1861–9. doi: 10.1007/s11605-018-3835-5
10. Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol. (2016) 29:413–20. doi: 10.1097/ACO.0000000000000329
11. Mooney JF, Chow CK, Hillis GS. Perioperative renal function and surgical outcome. Curr Opin Anaesthesiol. (2014) 27:195–200. doi: 10.1097/ACO.0000000000000054
12. Squires MH 3rd, Lad NL, Fisher SB, Kooby DA, Weber SM, Brinkman A, et al. The effect of preoperative renal insufficiency on postoperative outcomes after major hepatectomy: a multi-institutional analysis of 1,170 patients. J Am Coll Surg. (2014) 219:914–22. doi: 10.1016/j.jamcollsurg.2014.05.015
13. Matsumoto A, Nakagawa T, Kanatani A, Ikeda M, Kawai T, Miyakawa J, et al. Preoperative chronic kidney disease is predictive of oncological outcome of radical cystectomy for bladder cancer. World J Urol. (2018) 36:249–56. doi: 10.1007/s00345-017-2141-2
14. Wang W, Wang Y, Xu R, Chai J, Zhou W, Chen H, et al. Outcomes following coronary artery bypass graft surgery in patients with mild preoperative renal insufficiency. Braz J Cardiovasc Surg. (2018) 33:155–61. doi: 10.21470/1678-9741-2017-0148
15. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. (2004) 15:1597–605. doi: 10.1097/01.ASN.0000130340.93930.DD
16. Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens. (2005) 14:265–70. doi: 10.1097/01.mnh.0000165894.90748.72
17. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. (2017) 20:1–19. doi: 10.1007/s10120-016-0622-4
18. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
19. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
20. Wang S, Xu L, Wang Q, Li J, Bai B, Li Z, et al. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: a systematic review and meta-analysis of observational studies. World J Surg Oncol. (2019) 17:52. doi: 10.1186/s12957-019-1593-9
21. Blitz JD, Shoham MH, Fang Y, Narine V, Mehta N, Sharma BS, et al. Preoperative renal insufficiency: underreporting and association with readmission and major postoperative morbidity in an academic medical center. Anesth analgesia. (2016) 123:1500–15. doi: 10.1213/ANE.0000000000001573
22. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Med (Baltimore). (2016) 95:e3164. doi: 10.1097/MD.0000000000003164
23. Shi B, Liu S, Chen J, Liu J, Luo Y, Long L, et al. Sarcopenia is associated with perioperative outcomes in gastric cancer patients undergoing gastrectomy. Ann Nutr Metab. (2019) 75:213–22. doi: 10.1159/000504283
24. Ongaro E, Buoro V, Cinausero M, Caccialanza R, Turri A, Fanotto V, et al. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer. (2017) 20:563–72. doi: 10.1007/s10120-017-0722-9
25. Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg. (2016) 103:1316–25. doi: 10.1002/bjs.10186
26. Weiss R, Meersch M, Pavenstädt HJ, Zarbock A. Acute kidney injury: A frequently underestimated problem in perioperative medicine. Deutsches Arzteblatt Int. (2019) 116:833–42. doi: 10.3238/arztebl.2019.0833
27. Husain-Syed F, Ferrari F, Sharma A, Hinna Danesi T, Bezerra P, Lopez-Giacoman S, et al. Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrol Dial Transplant. (2019) 34:308–17. doi: 10.1093/ndt/gfy227
28. Xu J, Yu J, Xu X, Shen B, Wang Y, Jiang W, et al. Preoperative hidden renal dysfunction add an age dependent risk of progressive chronic kidney disease after cardiac surgery. J cardiothoracic Surg. (2019) 14:151. doi: 10.1186/s13019-019-0977-9
29. Dardashti A, Ederoth P, Algotsson L, Brondén B, Bjursten H. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg. (2014) 147:800–7. doi: 10.1016/j.jtcvs.2013.07.073
30. Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. (2013) 13:738–53. doi: 10.1038/nri3523
31. Rajagopalan PR, Reines HD, Pulliam C, Fitts CT, LeVeen HH. Reversal of acute renal failure using hemodilution with hydroxyethyl starch. J Trauma. (1983) 23:795–800. doi: 10.1097/00005373-198309000-00004
32. Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg. (2003) 76:784–91;discussion 792. doi: 10.1016/S0003-4975(03)00558-7
33. Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, Bradford WD, et al. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol. (2000) 85:1110–3. doi: 10.1016/S0002-9149(00)00705-0
34. Fujisaki M, Shinohara T, Hanyu N, Kawano S, Tanaka Y, Watanabe A, et al. Laparoscopic gastrectomy for gastric cancer in the elderly patients. Surg Endosc. (2016) 30:1380–7. doi: 10.1007/s00464-015-4340-5
35. Huang YS, Chen XD, Shi MM, Xu LB, Wang SJ, Chen WS, et al. Diffuse reduction of spleen density is an independent predictor of post-operative outcomes after curative gastrectomy in gastric cancer: A multi-center study. Front Oncol. (2020) 10:1050. doi: 10.3389/fonc.2020.01050
36. Tsekrekos A, Klevebro F, Hayami M, Kamiya S, Lindblad M, Nilsson M, et al. Laparoscopic versus open gastrectomy for cancer: A western center cohort study. J Surg Res. (2020) 247:372–9. doi: 10.1016/j.jss.2019.10.006
37. Honda M, Kumamaru H, Etoh T, Miyata H, Yamashita Y, Yoshida K, et al. Surgical risk and benefits of laparoscopic surgery for elderly patients with gastric cancer: a multicenter prospective cohort study. Gastric Cancer. (2019) 22:845–52. doi: 10.1007/s10120-018-0898-7
Keywords: gastric cancer, serum creatinine changes, postoperative outcomes, nomogram, prospective study
Citation: Cai W-T, Zeng X-Y, Huang Y-S, Chen W-S, Chen X-J and Xie X-H (2024) The clinical outcome of minor changes in serum creatinine for patients after curative gastrectomy: a prospective study. Front. Oncol. 14:1416888. doi: 10.3389/fonc.2024.1416888
Received: 25 April 2024; Accepted: 29 July 2024;
Published: 21 August 2024.
Edited by:
Marta Goglia, Sapienza University of Rome, ItalyReviewed by:
Marco Pace, Sapienza University of Rome, ItalyCopyright © 2024 Cai, Zeng, Huang, Chen, Chen and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Hai Xie, MzQ4Mzg3MjVAcXEuY29t; Xiang-Jian Chen, d3oxMzcwQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.