- Shenzhen Traditional Chinese Medicine Anorectal Hospital (Futian), Shenzhen, China
Background: Inflammation plays a pivotal role in tumor growth, with the platelet-to-lymphocyte ratio (PLR) emerging as a promising serum biomarker for prognostic assessment in patients with cancer. However, its specific role in rectal cancer remains controversial.
Methods: A comprehensive literature review encompassing PubMed, EMBASE, and the Cochrane Library, spanning from their inception to March 2024, was conducted. The systematic review and meta-analysis strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (PRISMA). Quality assessment was conducted using the Newcastle–Ottawa scale (NOS). This study aimed to assess the available literature on the association of PLR with both overall survival (OS) and disease-free survival (DFS) in patients with rectal cancer undergoing resection.
Results: Twenty-three observational studies, encompassing 7577 patients, were included in the analysis. These comprised 20 retrospective and 3 prospective cohort studies, with NOS scores ranging from 5 to 8. A significant association was found between high PLR and worse OS (hazard ratio [HR] 1.00; 95% confidence interval [CI] 1.00–1.01; P = 0.01). Conversely, no significant association was observed between PLR and DFS (HR 1.14; 95% CI 0.98–1.32; P = 0.09).
Conclusions: PLR serves as an independent clinical predictor of OS in patients with rectal cancer treated with curative surgery, but not of DFS. This easily accessible biomarker appears to be an optimal prognostic index and may aid clinicians in predicting the prognosis of rectal cancer, facilitating the development of individualized treatment strategies.
1 Introduction
Rectal cancer is one of the most common tumors worldwide. At present, it is treated using a multimodal approach that combines neoadjuvant chemoradiotherapy (nCRT), total mesorectal resection, and adjuvant chemotherapy (1), which reduces the recurrence rate and increases the survival rate of patients with rectal cancer (2, 3). However, predicting treatment outcomes is a complex issue involving TNM staging, tumor grading, patient age, and laboratory parameters (4). Therefore, reliable prognostic factors for treatment outcomes must be determined to improve treatment strategies and subsequent monitoring.
The tumor microenvironment, particularly the inflammatory response, may play a crucial role in cancer development and progression and may be associated with systemic inflammation (5). The platelet-to-lymphocyte ratio (PLR) is an inflammation score recently identified as a valuable predictor in various solid tumors (6–10). Such predictive factors are both inexpensive and easy to implement in the daily management of patients with cancer.
Some studies have also reported on the relationship between PLR and prognosis in patients with rectal cancer; however, the results are inconsistent. In a meta-analysis, Hamid et al. showed that the PLR does not correlate with diagnosis after curative intent surgery for rectal cancer (11), whereas a meta-analysis by Portale et al. showed that PLR is an independent clinical predictor of overall survival (OS), but not of disease-free survival (DFS), in patients with rectal cancer undergoing surgery (12).
Therefore, we conducted a systematic review and meta-analysis to evaluate the predictive role of the PLR in the prognosis of patients with rectal cancer undergoing surgery.
2 Methods
2.1 Protocol and guidance
This study adhered strictly to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA) (13). Given the nature of the study, ethical approval or informed consent was deemed unnecessary.
2.2 Search strategy
A comprehensive literature search was conducted in PubMed, EMBASE, and the Cochrane Library, targeting English articles published from database inception to March 2024. The following search keywords were used: (“Rectal Cancer” or “rectal carcinoma” or “Rectal”) and (“Platelet-to-Lymphocyte Ratio” or “Platelet to Lymphocyte Ratio” or “Platelet Lymphocyte Ratio” or “PLR”) and (“prognosis” or “outcome” or “survival” or “mortality” or “recurrence”). Additional studies were identified by reviewing the reference lists and qualified publications of potentially eligible studies. Both searches were independently conducted by two authors, and any differences were resolved through discussion.
2.3 Criteria for considering studies for this review
For inclusion in this review, studies must have investigated the association between PLR and OS or DFS in patients with rectal cancer who had undergone surgery with or without nCRT. Studies lacking a defined cutoff value for PLR classification or insufficient data for hazard ratio (HR) estimation were excluded. In cases of duplicate publications reporting on the same patient population, only the most recent and complete data were considered. Nonhuman studies were also excluded.
2.4 Data extraction and quality assessment
Data were extracted independently by two reviewers. The extracted information encompassed the first author’s name, publication year, country of origin, study type, number of participants, age, neoadjuvant therapy details, tumor staging, PLR cutoff values, primary research outcomes, and follow-up duration.
2.5 Quality assessment
The quality of all selected articles was rigorously examined using the Newcastle–Ottawa Scale (NOS) for cohort studies (14). This quantitative scale uses a star-rating system to assess the quality of eight items across three domains: selection (four items, awarded one star each), comparability (one item, eligible for up to two stars), and exposure (three items, each awarded a star). For this meta-analysis, articles were categorized as having excellent (≥7 stars), moderate (4–6 stars), or poor (<4 stars) quality. Any disparities between the two reviewers were resolved through deliberation with a third reviewer.
2.6 Data analysis
The primary endpoints were the OS and DFS, evaluated based on high versus low PLR. This approach was based on the HRs obtained from each study, accompanied by a 95% confidence interval (CI). If multiple HR estimates were reported in a single article, the results from multivariate analyses were preferred. Additionally, subgroup analysis was conducted, stratified by population (Eastern and Western) and cutoff values (≥150 and <150).
2.7 Statistical analysis
For data analysis, Review Manager version 5.4, a software tool developed by the Nordic Cochrane Center of the Cochrane Collaboration in London, UK, was used. HR with a 95% CI was employed as a measure of effectiveness. To quantify heterogeneity among studies, we relied on I2 values, which were categorized into four distinct levels: no (I2 < 25%), low (25% ≤ I2 < 50%), moderate (50% ≤I2 <75%), and high (I2 ≥ 75%) heterogeneity. When the I2 value was <50%, indicating a relatively low heterogeneity level, a fixed-effects model was used for analysis. Conversely, when the I2 value was >50%, signifying a higher degree of heterogeneity, a random-effects model was used. This approach allowed us to account for the varying degrees of heterogeneity across studies and provide more robust and reliable estimates of the treatment effect.
3 Results
3.1 Study identification and characteristics
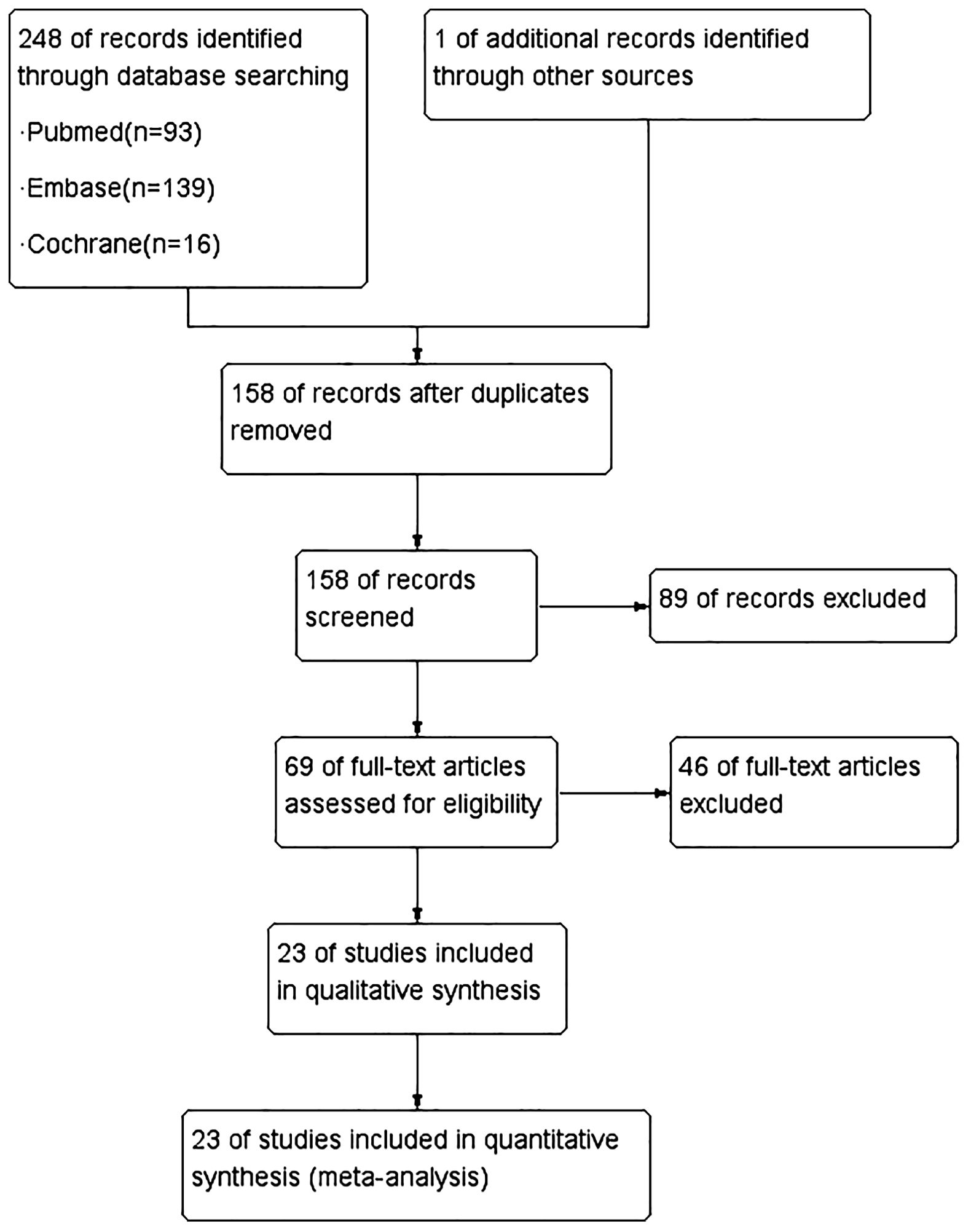
The initial search yielded a total of 249 citations. After a thorough review of the titles and abstracts, 69 articles were deemed potentially relevant and subjected to further scrutiny. Ultimately, 23 studies (15–37), published between 2012 and 2023, were selected for evidence synthesis (Figure 1).
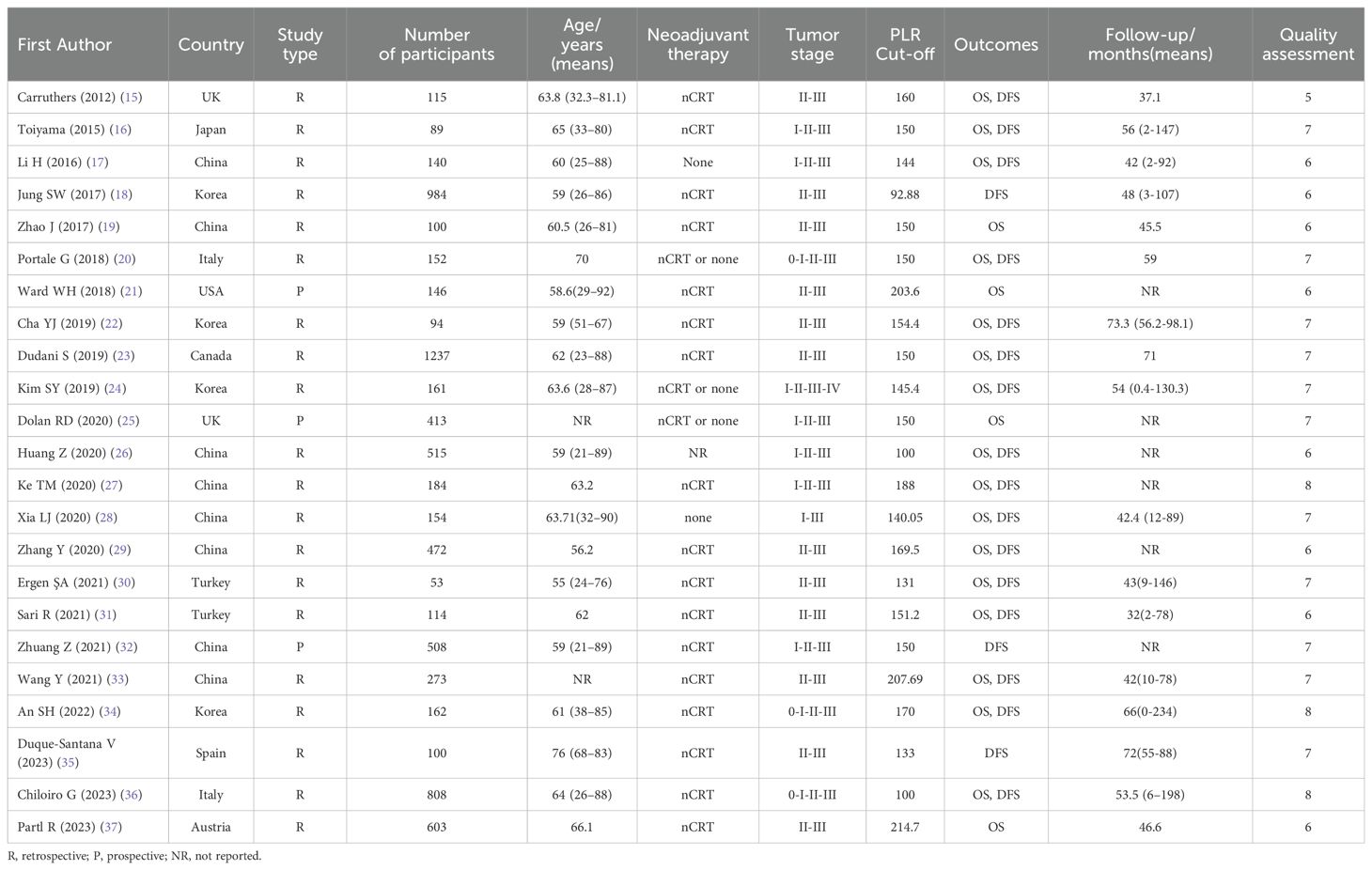
Table 1 summarizes the key characteristics of the included studies. A cumulative total of 7577 patients with cancer were enrolled in these studies, with sample sizes varying from 53 to 1237 patients. Notably, 20 and 3 studies were retrospective and prospective studies, respectively. The primary outcome measures focused on OS and DFS. Geographically, 10 and 13 studies originated from the Western and Eastern countries, respectively. Based on rigorous quality evaluation criteria, the quality scores for observational studies ranged from 5 to 8 points on the NOS, indicating that the quality of the entire cohort was relatively high.
3.2 Disease-free survival
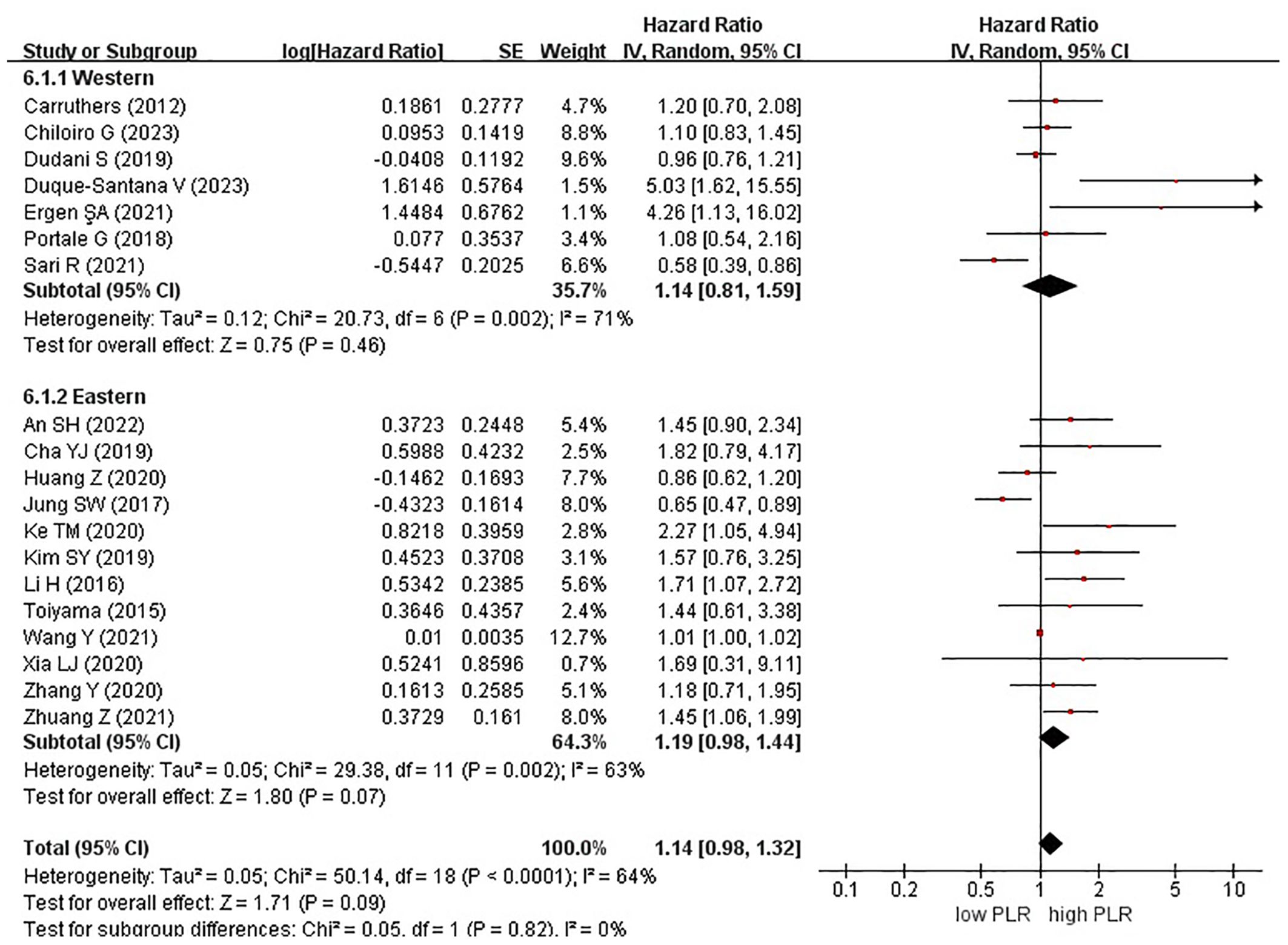
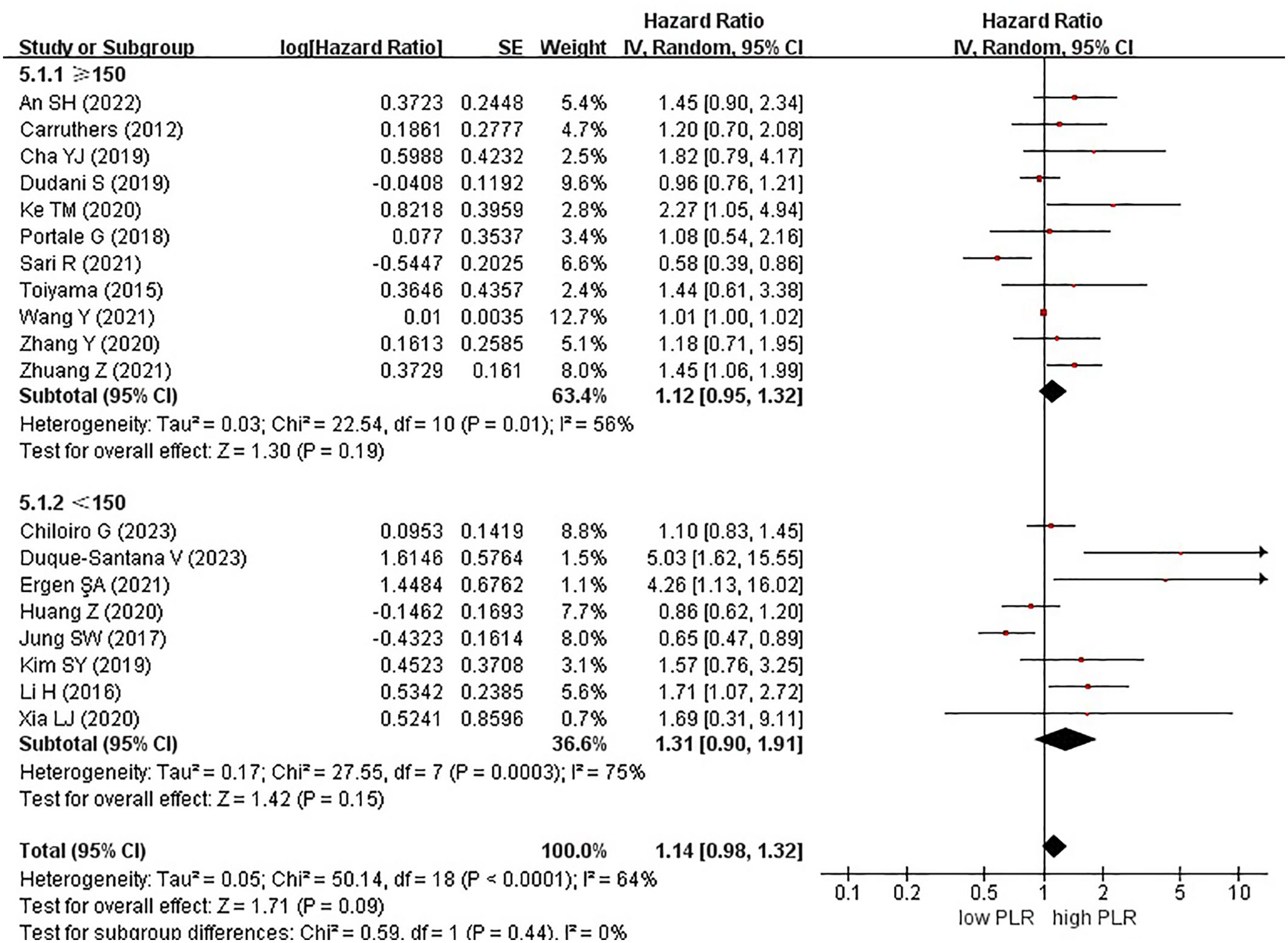
Nineteen studies examined the effect of high and low PLR on DFS among patients with rectal cancer. A total of 6315 patients were included in these studies. Notably, when comparing high PLR with low PLR, no significant association was observed with DFS in patients with rectal cancer (HR, 1.14; 95% CI, 0.98–1.32; P = 0.09) (Figure 2).
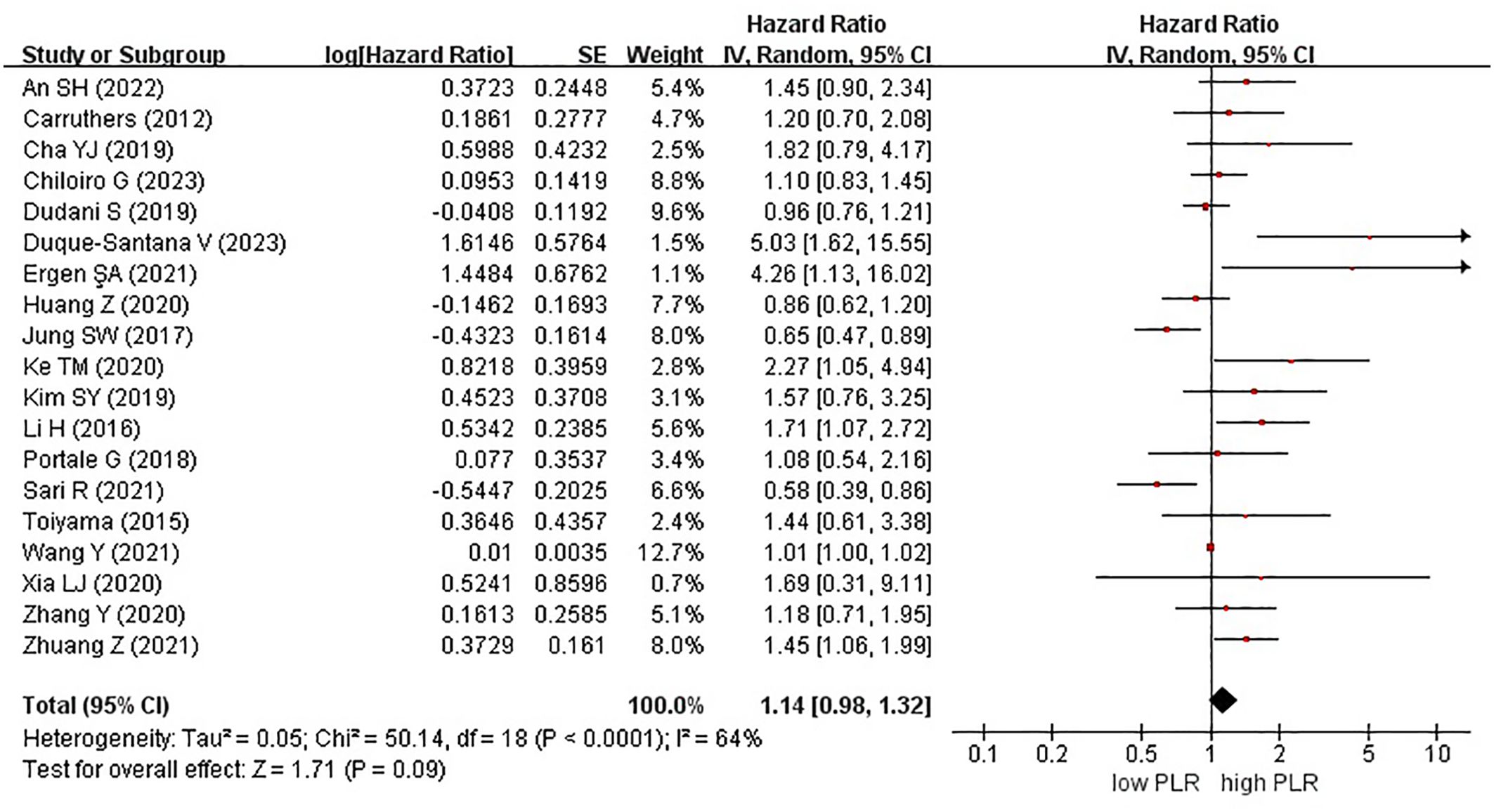
Figure 2. Forest plots of disease-free survival in patients with high versus low platelet-to-lymphocyte ratio.
Among the 19 studies, 7 and 12 originated from Western and Eastern countries, respectively. Additionally, 11 studies employed a cutoff value of ≥150, whereas 8 studies utilized a cutoff value of <150. A subgroup analysis was subsequently conducted, stratifying the data based on both the country of origin and cutoff value. No significant correlation was found between patients from Western (HR, 1.14; 95% CI, 0.81–1.59; P = 0.46) or Eastern (HR, 1.19; 95% CI, 0.98–1.44; P = 0.07) countries (Figure 3). Similarly, in the subgroup analysis based on cutoff values, no significant associations were observed for the ≥150 group (HR, 1.12; 95% CI, 0.95–1.32; P = 0.19) or the <150 group (HR, 1.31; 95% CI, 0.90–1.91; P = 0.15) (Figure 4).
3.3 Overall survival
Twenty studies, encompassing a total of 5985 patients, examined the effect of high and low PLR on OS in rectal cancer. Notably, a high PLR was associated with poorer OS than a low PLR, as indicated by an HR of 1.00 (95% CI, 1.00–1.01; P = 0.01) (Figure 5).
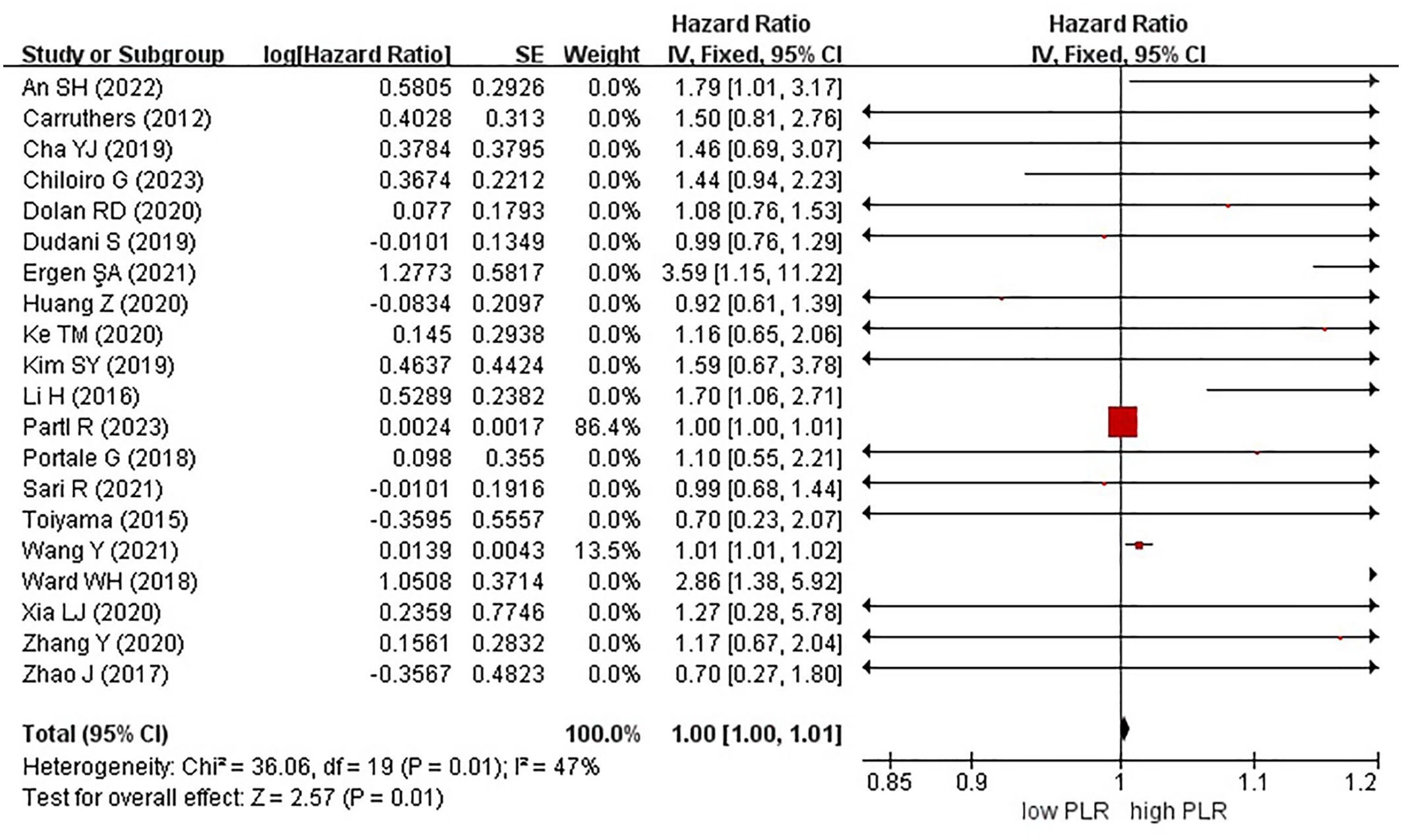
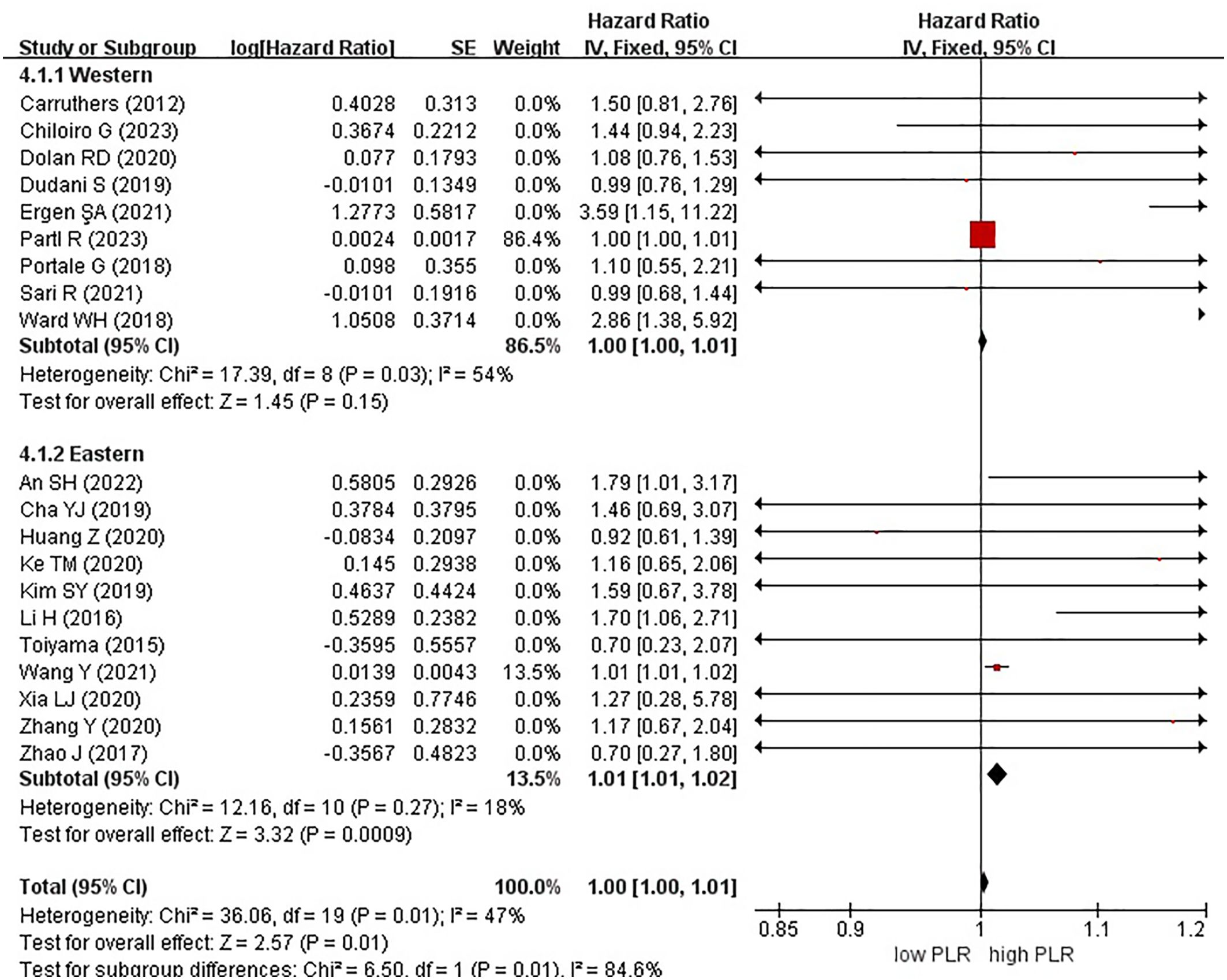
Figure 5. Forest plots of overall survival in patients with high versus low platelet-to-lymphocyte ratio.
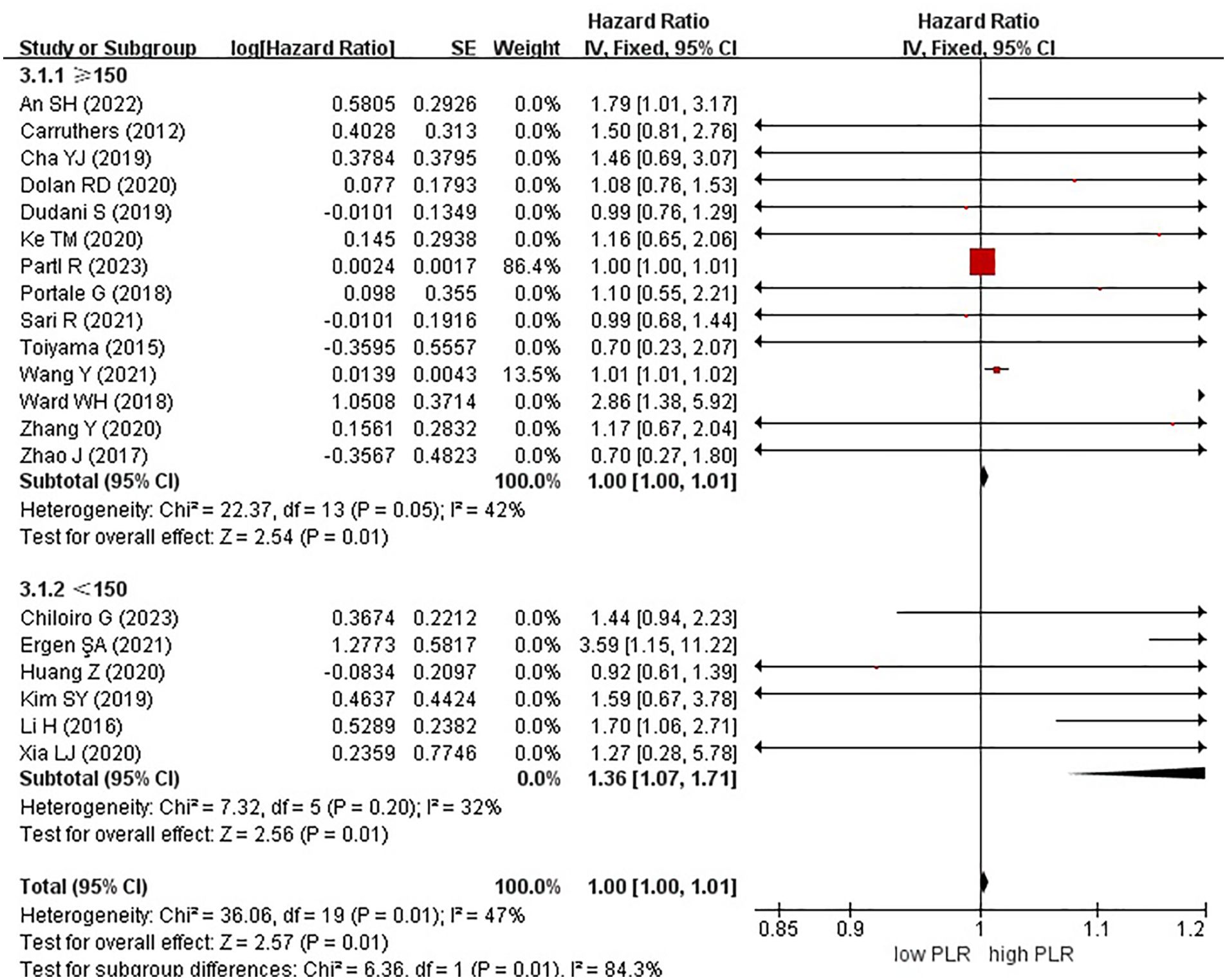
In these analyses, 9 studies originated from Western countries, whereas the remaining 11 studies originated from Eastern countries. Fourteen studies employed a cutoff value of ≥150, whereas six studies used a cutoff value of <150. A subgroup analysis was subsequently conducted, stratifying the data based on both the country of origin and cutoff value. The results revealed a significant correlation between patients from Eastern countries and OS, with an HR of 1.01 (95% CI, 1.01–1.02; P = 0.0009). However, no correlation was observed among patients from Western countries (HR, 1.00; 95% CI, 1.00–1.01; P = 0.15) (Figure 6). In the subgroup analysis based on cutoff values, significant associations were observed in both groups with ≥150 (HR, 1.00; 95% CI, 1.00–1.01; P = 0.01) and <150 (HR, 1.36; 95% CI, 1.07–1.71; P = 0.01), exhibiting significant effects on the OS (Figure 7).
3.4 Publication bias
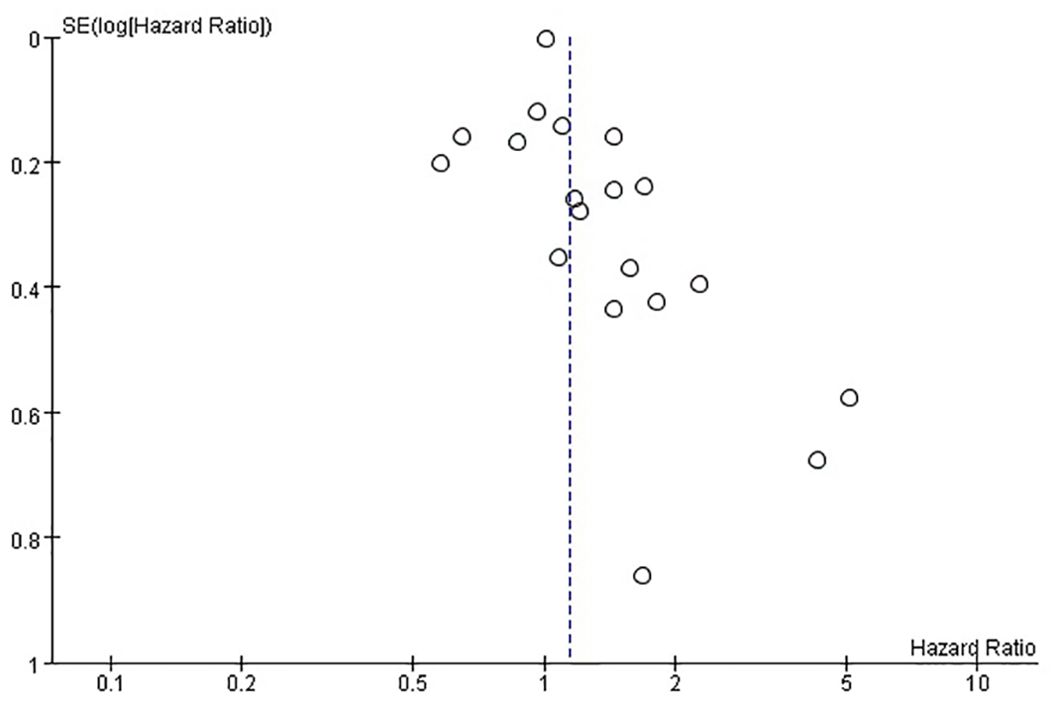
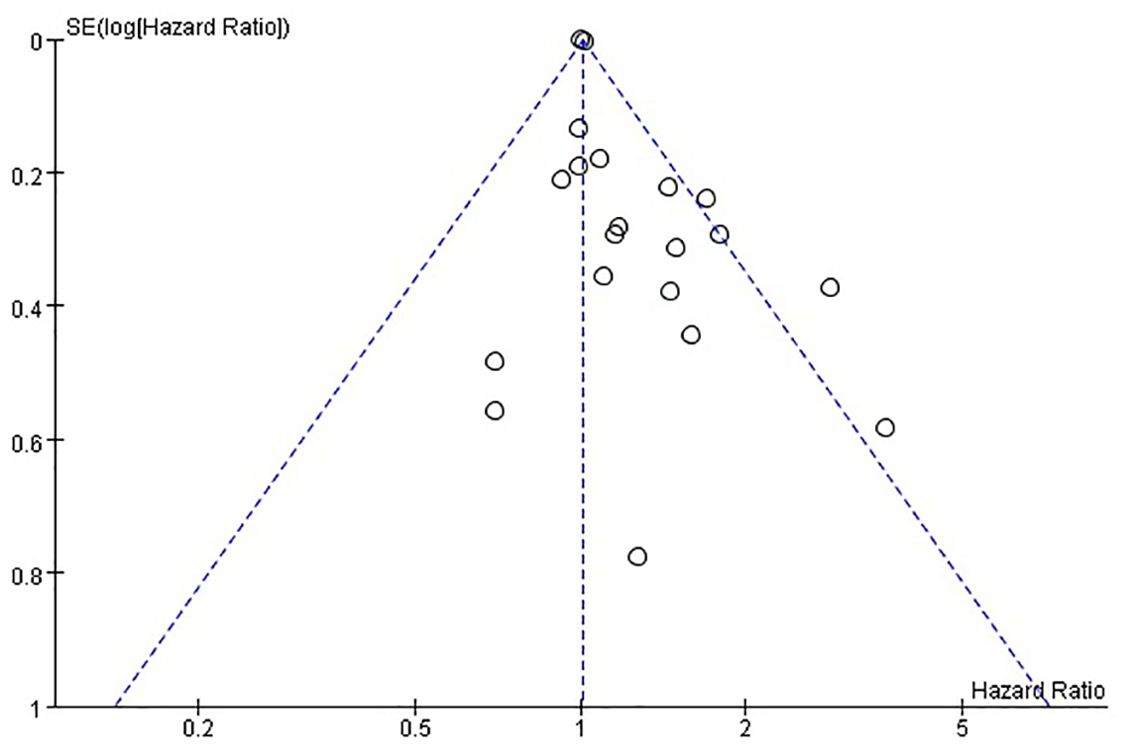
The funnel plots provided in Figure 8 of disease-free survival and Figure 9 of overall survival demonstrate that the scatter points were generally symmetrical within the CI, indicating the absence of notable publication bias (Figures 8, 9).
4 Discussion
This meta-analysis encompassed 23 studies to compare the effect of high versus low PLR on postoperative prognosis following rectal cancer resection. Notably, most studies were published in 2012 or later, reflecting the recent surge of interest in exploring the potential role of these biomarkers in predicting survival outcomes among patients with rectal cancer. Our results revealed a statistically significant difference in the OS between patients with high and low PLR, whereas no significant difference was observed in DFS. While earlier meta-analyses have yielded different results, our meta-analysis, which includes the largest number of studies to date, supports the findings of Portale et al. (12). Thus, we consider PLR to be a valuable laboratory parameter.
In the subgroup analysis of OS, a statistically significant difference between patients with high and low PLR in Eastern countries was discovered, whereas no such difference was evident in patients from Western countries. Furthermore, in terms of cutoff values, we reviewed previous relevant studies and statistically analyzed the distribution characteristics of cutoff values across the 23 included studies. A cutoff value of 150 was selected for subgroup analysis. A significant association was observed between high and low PLR in patients with cutoff values of ≥150 and <150. However, in the subgroup analysis of DFS stratified by both countries and cutoff values, no significant associations were found.
“Inflammation” and “genomic instability and mutation” were considered the pathophysiological basis for promoting tumorigenesis and development (38). The tumor microenvironment consists of normal tissue, tumor, inflammatory, and stromal cells and other components (39), which regulate the tumor through interactions between signaling pathways and cytokines (39, 40).
The underlying mechanisms of the relationship between systemic inflammation and tumor biology are not fully understood. Chronic inflammation can lead to tissue damage, and repeated regenerative processes can result in permanent genetic mutations such as point mutations, deletions, or rearrangements. Activated inflammatory cells produce numerous chemokines and cytokines, which affect tumor growth, migration, and differentiation by releasing growth factors. Platelets play a crucial role in hemostasis by adhering and aggregating in the injured tissue and are important in the host inflammatory and immune systems (41, 42). Activated platelets release growth factors that promote tumor growth and invasion and facilitate tumor metastasis by assisting cancer cells in adhesion and extravasation (43). A high platelet count is associated with long-term prognosis in patients with colorectal cancer (44, 45). Lymphocytes, a subtype of white blood cells (WBCs), are responsible for innate immunity. Lymphocytes play a pivotal role in counteracting tumor progression, and a high density of lymphocyte infiltration in the tumors is a known prognostic factor for improved survival in many malignancies (46). Neutrophils, the most abundant WBCs, play a crucial role in acute inflammatory responses. Additionally, neutrophils are implicated in carcinogenic processes, such as tumor growth and proliferation, and tumor angiogenesis through the release of reactive oxygen and nitrogen species or proteases. Neutrophils can also contribute to metastatic spread by suppressing natural killer cell function and promoting tumor cell extravasation (47).
A high preoperative PLR is often associated with increased platelet count or decrease lymphocyte count, indicating an activated inflammatory state and suppressed immune response in patients. PLR, a marker representing the balance between two inflammatory states, has demonstrated prognostic value in multiple studies (26, 48, 49). Additionally, an index that combines inflammation, nutrition, and immune system status (CALLY) is used to predict the long-term prognosis of colorectal cancer patients, and the research conclusion suggests that it is an independent biomarker (50). A high platelet count tends to induce an aggregation of tumor cells by releasing biological factors; assist in stimulating the development of new blood vessels through interactions with PDGF, VEGF, and PF4; and activate DNA damage promoters, which may contribute to carcinogenesis (43).
The current meta-analysis is limited by the retrospective design of most included studies. To validate the PLR as a prognostic indicator, prospective assessment of the clinical significance of this marker, considering factors such as clinical tumor staging, tumor grade, and the type of nCRT protocol, is necessary. The critical threshold must be established in a large patient cohort and independently validated in another cohort. Although most included studies excluded patients with inflammatory diseases or infections, some excluded patients with immune deficiencies, rheumatoid arthritis, or those receiving glucocorticoids or nonsteroidal anti-inflammatory drugs, and the reported PLR may still be influenced by comorbid noncancerous conditions.
In conclusion, a high pretreatment PLR is associated with poorer OS in patients with rectal cancer undergoing curative resection but not with DFS. This easily accessible and cost-effective serum biomarker could be a valuable tool in guiding more personalized treatment decisions.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Author contributions
LM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. FY: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. WG: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft. ST: Formal analysis, Investigation, Software, Writing – original draft. YL: Data curation, Software, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol. (2020) 17:414–29. doi: 10.1038/s41575-020-0275-y
2. Lee JL, Yu CS, Kim CW, Yoon YS, Lim SB, Kim JC. Chronological improvement in survival following rectal cancer surgery: a large-scale, single-center study. World J Surg. (2013) 37:2693–9. doi: 10.1007/s00268-013-2168-5
3. Wiegering A, Isbert C, Dietz UA, Kunzmann V, Ackermann S, Kerscher A, et al. Multimodal therapy in treatment of rectal cancer is associated with improved survival and reduced local recurrence - a retrospective analysis over two decades. BMC Cancer. (2014) 14:816. doi: 10.1186/1471-2407-14-816
4. Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:1291–305. doi: 10.1016/j.annonc.2020.06.022
5. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
6. Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Ann Surg Oncol. (2016) 23:646–54. doi: 10.1245/s10434-015-4869-5
7. Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. (2009) 197:466–72. doi: 10.1016/j.amjsurg.2007.12.057
8. Zheng J, Cai J, Li H, Zeng K, He L, Fu H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. (2017) 44:967–81. doi: 10.1159/000485396
9. Zhou Y, Cheng S, Fathy AH, Qian H, Zhao Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther. (2018) 11:1899–908. doi: 10.2147/OTT.S154162
10. Gunaldi M, Goksu S, Erdem D, Gunduz S, Okuturlar Y, Tiken E, et al. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med. (2015) 8:5937–42.
11. Hamid HKS, Emile SH, Davis GN. Prognostic significance of lymphocyte-to-monocyte and platelet-to-lymphocyte ratio in rectal cancer: A systematic review, meta-analysis, and meta-regression. Dis Colon Rectum. (2022) 65:178–87. doi: 10.1097/DCR.0000000000002291
12. Portale G, Bartolotta P, Azzolina D, Gregori D, Fiscon V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: systematic review and meta-analysis. Langenbecks Arch Surg. (2023) 408:85. doi: 10.1007/s00423-023-02786-8
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
15. Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. (2012) 14:e701–7. doi: 10.1111/j.1463-1318.2012.03147.x
16. Toiyama Y, Inoue Y, Kawamura M, Kawamoto A, Okugawa Y, Hiro J, et al. Elevated platelet count as predictor of recurrence in rectal cancer patients undergoing preoperative chemoradiotherapy followed by surgery. Int Surg. (2015) 100:199–207. doi: 10.9738/INTSURG-D-13-00178.1
17. Li H, Song J, Cao M, Wang G, Li L, Zhang B, et al. Preoperative neutrophil-to-lymphocyte ratio is a more valuable prognostic factor than platelet-to-lymphocyte ratio for nonmetastatic rectal cancer. Int Immunopharmacol. (2016) 40:327–31. doi: 10.1016/j.intimp.2016.09.014
18. Jung SW, Park IJ, Oh SH, Yeom SS, Lee JL, Yoon YS, et al. Association of immunologic markers from complete blood counts with the response to preoperative chemoradiotherapy and prognosis in locally advanced rectal cancer. Oncotarget. (2017) 8:59757–65. doi: 10.18632/oncotarget.15760
19. Zhao J, Xu J, Zhang R. Clinical and prognostic significance of pathological and inflammatory markers in mucinous rectal cancer patients receiving neoadjuvant chemoradiotherapy and curative surgery. Med Sci Monit. (2017) 23:4826–33. doi: 10.12659/msm.904116
20. Portale G, Cavallin F, Valdegamberi A, Frigo F, Fiscon V. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio are not prognostic biomarkers in rectal cancer patients with curative resection. J Gastrointest Surg. (2018) 22:1611–8. doi: 10.1007/s11605-018-3781-2
21. Ward WH, Goel N, Ruth KJ, Esposito AC, Lambreton F, Sigurdson ER, et al. Predictive value of leukocyte- and platelet-derived ratios in rectal adenocarcinoma. J Surg Res. (2018) 232:275–82. doi: 10.1016/j.jss.2018.06.060
22. Cha YJ, Park EJ, Baik SH, Lee KY, Kang J. Prognostic impact of persistent lower neutrophil-to-lymphocyte ratio during preoperative chemoradiotherapy in locally advanced rectal cancer patients: A propensity score matching analysis. PloS One. (2019) 14:e0214415. doi: 10.1371/journal.pone.0214415
23. Dudani S, Marginean H, Tang PA, Monzon JG, Raissouni S, Asmis TR, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer. (2019) 19:664. doi: 10.1186/s12885-019-5892-x
24. Kim SY, Moon CM, Yoon HJ, Kim BS, Lim JY, Kim TO, et al. Diffuse splenic FDG uptake is predictive of clinical outcomes in patients with rectal cancer. Sci Rep. (2019) 9:1313. doi: 10.1038/s41598-018-35912-4
25. Dolan RD, Alwahid M, McSorley ST, Park JH, Stevenson RP, Roxburgh CS, et al. A comparison of the prognostic value of composite ratios and cumulative scores in patients with operable rectal cancer. Sci Rep. (2020) 10:17965. doi: 10.1038/s41598-020-73909-0
26. Huang Z, Wang X, Zou Q, Zhuang Z, Xie Y, Cai D, et al. High platelet-to-lymphocyte ratio predicts improved survival outcome for perioperative NSAID use in patients with rectal cancer. Int J Colorectal Dis. (2020) 35:695–704. doi: 10.1007/s00384-020-03528-8
27. Ke TM, Lin LC, Huang CC, Chien YW, Ting WC, Yang CC. High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict poor survival in rectal cancer patients receiving neoadjuvant concurrent chemoradiotherapy. Med (Baltimore). (2020) 99:e19877. doi: 10.1097/MD.0000000000019877
28. Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. (2020) 20:208. doi: 10.1186/s12885-020-6698-6
29. Zhang Y, Liu X, Xu M, Chen K, Li S, Guan G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep. (2020) 10:8017. doi: 10.1038/s41598-020-64684-z
30. Ergen ŞA, Barlas C, Yıldırım C, Öksüz DÇ. Prognostic role of peripheral neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy. J Gastrointest Cancer. (2022) 53:151–60. doi: 10.1007/s12029-020-00578-7
31. Sari R, Kaya S, Altn O, Tuzun S, Altuntaş YE, Küçük HF. Prognostic significance of systemic inflammatory markers in rectal cancer. Ann Med Res. (2021). doi: 10.5455/ANNALSMEDRES.2020.06.596
32. Zhuang Z, Wang X, Huang M, Luo Y, Yu H. Serum calcium improved systemic inflammation marker for predicting survival outcome in rectal cancer. J Gastrointest Oncol. (2021) 12:568–79. doi: 10.21037/jgo-20-479
33. Wang Y, Chen L, Zhang B, Song W, Zhou G, Xie L, et al. Pretreatment inflammatory-nutritional biomarkers predict responses to neoadjuvant chemoradiotherapy and survival in locally advanced rectal cancer. Front Oncol. (2021) 11:639909. doi: 10.3389/fonc.2021.639909
34. An SH, Kim IY. Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer? Ann Coloproctol. (2022) 38:253–61. doi: 10.3393/ac.2021.00633.0090
35. Duque-Santana V, López-Campos F, Martin-Martin M, Valero M, Zafra-Martín J, Couñago F, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as prognostic factors in locally advanced rectal cancer. Oncology. (2023) 101:349–57. doi: 10.1159/000526450
36. Chiloiro G, Romano A, Mariani S, Macchia G, Giannarelli D, Caravatta L, et al. Predictive and prognostic value of inflammatory markers in locally advanced rectal cancer (PILLAR) - A multicentric analysis by the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Gastrointestinal Study Group. Clin Transl Radiat Oncol. (2023) 39:100579. doi: 10.1016/j.ctro.2023.100579
37. Partl R, Paal K, Stranz B, Hassler E, Magyar M, Brunner TB, et al. The pre-treatment platelet-to-lymphocyte ratio as a prognostic factor for loco-regional control in locally advanced rectal cancer. Diagnostics (Basel). (2023) 13:679. doi: 10.3390/diagnostics13040679
39. Witz IP. Yin-yang activities and vicious cycles in the tumor microenvironment. Cancer Res. (2008) 68:9–13. doi: 10.1158/0008-5472.CAN-07-2917
40. Liu SQ, Ma YJ, Yan HL, et al. Expression and clinical significance of RhoGDI2 in colorectal cancer. Chin J Immunol. (2017) 33:108–11.
41. Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. (2007) 100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab
42. Jenne CN, Kubes P. Platelets in inflammation and infection. Platelets. (2015) 26:286–92. doi: 10.3109/09537104.2015.1010441
43. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. (2018) 33:965–83. doi: 10.1016/j.ccell.2018.03.002
44. Gu D, Szallasi A. Thrombocytosis portends adverse prognosis in colorectal cancer: A meta-analysis of 5,619 patients in 16 individual studies. Anticancer Res. (2017) 37:4717–26. doi: 10.21873/anticanres.11878
45. Belluco C, Forlin M, Delrio P, Rega D, Degiuli M, Sofia S, et al. Elevated platelet count is a negative predictive and prognostic marker in locally advanced rectal cancer undergoing neoadjuvant chemoradiation: a retrospective multi-institutional study on 965 patients. BMC Cancer. (2018) 18:1094. doi: 10.1186/s12885-018-5022-1
46. Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. (2007) 7.
47. Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. (2017) 16:137. doi: 10.1186/s12943-017-0707-7
48. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. (2017) 3:e172319. doi: 10.1001/jamaoncol.2017.2319
49. Huang XZ, Chen WJ, Zhang X, Wu CC, Zhang CY, Sun SS, et al. An elevated platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with colorectal cancer: A meta-analysis. Dis Markers. (2017) 2017:1053125. doi: 10.1155/2017/1053125
Keywords: rectal cancer, resection, platelet-to-lymphocyte ratio, survival, meta-analysis
Citation: Ma L, Yang F, Guo W, Tang S and Ling Y (2024) Prognostic role of platelet-to-lymphocyte ratio in patients with rectal cancer undergoing resection: a systematic review and meta-analysis. Front. Oncol. 14:1415443. doi: 10.3389/fonc.2024.1415443
Received: 10 April 2024; Accepted: 03 September 2024;
Published: 30 September 2024.
Edited by:
Daniel Reis Waisberg, Hospital das Clinicas da Faculdade de Medicina da USP (HC-FMUSP), BrazilReviewed by:
Beatriz Martin-Perez, University Hospital of Badajoz, SpainJin Tung Liang, National Taiwan University, Taiwan
Copyright © 2024 Ma, Yang, Guo, Tang and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Yang, NzgyMzE1Nzk0QHFxLmNvbQ==
 Lijuan Ma
Lijuan Ma Fei Yang*
Fei Yang*