
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 23 July 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1414780
This article is part of the Research Topic Comprehensive Treatment Strategy for Improving Surgical Resection Rate of Retroperitoneal Sarcomas View all 10 articles
 Qisheng Hao1,2†
Qisheng Hao1,2† Lichao Cha1,2†
Lichao Cha1,2† Bin Zhou1,2
Bin Zhou1,2 Xinyu Li1,2
Xinyu Li1,2 Mingkai Gong1
Mingkai Gong1 Qingze Li1,2
Qingze Li1,2 Guofei Dong1
Guofei Dong1 Mengqi Song1
Mengqi Song1 Zehua Wu1
Zehua Wu1 Zhongyi Guo1
Zhongyi Guo1 Fabo Qiu1,2
Fabo Qiu1,2 Xiaowei Wang3*
Xiaowei Wang3* Lantian Tian1,2*
Lantian Tian1,2*Background: The Da Vinci Surgical System (DVSS) has the advantages of minimal invasion, rapid recovery, safety, and reliability. Although the DVSS has been widely used in various abdominal surgeries, descriptions of its use in robot-assisted retroperitoneal tumor resection (RRTR) are limited to case reports; large-sample systematic studies are lacking. The present study was performed to analyze the data of RRTR in our center, summarize our experience, and provide a reference for other retroperitoneal tumor centers.
Methods: We retrospectively analyzed the clinical data of 105 patients who underwent RRTR at the Affiliated Hospital of Qingdao University from January 2015 to December 2022. Logistic univariate and multivariate analyses were performed to identify independent risk factors affecting RRTR. A receiver operating characteristic curve was used to find the cut-off value, which was then included in the logistic multivariate analysis for verification.
Results: Among the 105 patients, 87 successfully underwent RRTR (DVSS group) and 18 underwent conversion to open surgery (conversion group). There was no significant difference in sex, age, body mass index, history of abdominal surgery, or tumor location between the two groups (P > 0.05). The maximum tumor diameter [odds ratio (OR), 1.041; 95% confidence interval (CI), 1.015-1.067; P = 0.002] and pathological property (OR, 8.646; 95% CI, 2.370-31.544; P = 0.001) were independent risk factors for conversion to open surgery. Further analysis confirmed that the success rate of RRTR was higher for tumors with a maximum diameter of ≤64 mm and benign tumors. Based on our experience and statistical results, we believe that retroperitoneal tumors that meet the following criteria have a higher success rate of DVSS resection: maximum tumor diameter of ≤64 mm, benign tumors, the tumor has relatively clear boundary, no obvious invasion of surrounding tissues and organs, and no need for combined organ resection.
Conclusions: RRTR is safe and effective in the treatment of RPT, and the clinical prognosis is similar to that of open surgery. The success rate of RRTR in patients with appropriate surgical indications for this procedure is higher.
A retroperitoneal tumor (RPT) arises from fat, muscle, lymph, nerve, and residual embryonic tissue. These tumors may be located anywhere within the retroperitoneal space, which extends from the plane of the diaphragm to the potential retroperitoneal space above the pelvis (1). Statistical data indicate that the incidence of RPT ranges from 0.5 to 1.0 per 100,000 individuals (2). Malignant retroperitoneal tumors constitute approximately 70% of all RPTs and account for 0.1%–0.2% of all human malignancies (3). Despite their rarity, about one-third of RPTs are sarcomas, which are associated with an extremely poor prognosis and high recurrence rates (4). Surgical intervention remains the primary treatment modality for RPT and is a crucial factor in determining patient outcomes (5, 6). The main surgical techniques for RPT include traditional open surgery, laparoscopic approaches, and robot-assisted procedures [including the use of the Da Vinci Surgical System (DVSS) (Intuitive Surgical, Sunnyvale, CA, USA)]. The challenges presented by the limited surgical space, narrow surgical field, restricted surgical range, deep tumor location, various pathological types, and proximity to blood vessels in the retroperitoneal space have been addressed by increasing numbers of surgeons specializing in retroperitoneal tumors. These surgeons have demonstrated the safety and effectiveness of open surgery for RPT resection through good exposure of the surgical field and the ability to identify tumors by intraoperative palpation. Therefore, traditional open surgery is widely performed for retroperitoneal tumor resection. However, traditional open surgery cannot avoid the need for a large surgical incision, intraoperative manipulation of organs, slow postoperative recovery, and potential complications; thus, clinicians still face many challenges in perioperative management. With the development of minimally invasive techniques in recent years, successful laparoscopic treatment of RPT has been reported, and many retroperitoneal tumor surgeons continue to progress in this field (7, 8). For example, Chatelet et al. (9) laparoscopically removed a large schwannoma measuring 17 × 8 × 6 cm. Laparoscopic RPT resection is feasible even when the tumor is large or attached to adjacent vascular structures, and although several challenges remain (10), laparoscopic surgery is technically safe, improves patients’ prognosis, and is a viable surgical option (11). The development of robot-assisted surgical systems is one of the greatest advances in laparoscopic technology. Several reports have confirmed that the DVSS is safe and effective for RPT resection and that it can significantly reduce surgical trauma and promote patient recovery (12–19). However, no systematic, large-sample studies have been performed to evaluate the application of DVSS robot-assisted RPT resection (RRTR). The present study was performed to analyze the clinical data of patients who underwent RRTR in our hospital, identify the risk factors affecting RRTR, and provide a reference for the application of RRTR in other retroperitoneal tumor centers.
This study involved 105 patients who underwent RRTR at the Affiliated Hospital of Qingdao University from January 2015 to December 2022. Preoperative color Doppler ultrasound, contrast-enhanced computed tomography or magnetic resonance imaging, endoscopic ultrasonography, and three-dimensional imaging were used to comprehensively diagnose and evaluate the resectability of RPT. All patients were evaluated by a multidisciplinary treatment team before surgery, and the surgical plan was formulated. Preoperatively, patients were informed in detail of the surgical plan and the possibility of conversion to open surgery. This study was approved by the Institutional Review Board of the Affiliated Hospital of Qingdao University.
The inclusion criteria for the study were relatively clear tumor boundaries observed during preoperative examinations, indicating that complete resection was feasible based on preoperative assessments, absence of preoperative anesthetic or surgical contraindications, no evidence of metastasis, and no prior exposure to preoperative chemoradiotherapy or targeted therapy. The exclusion criteria were severe uncontrolled infection; tumor recurrence; unsuitability for surgery because of severe cardiovascular or cerebrovascular disease, hematological disease, immune system disease, or diabetes; and pregnancy or lactation.
The basic data, perioperative information, and pathological reports of all patients who had successfully undergone RRTR, recovered, and been discharged were obtained from the electronic medical records. The operation time, estimated blood loss, blood transfusion volume, and postoperative complications were analyzed.
Data on the tumor location, number of tumors, pathological properties, maximum tumor diameter, and adhered to major blood vessels were obtained from imaging and pathology reports. Tumor adhered to major vessels was defined as tumor contact with the great vessels, including the aorta, inferior vena cava, portal vein, renal artery and vein, splenic artery and vein, and superior mesenteric artery and vein. Postoperative complications were graded according to the Clavien–Dindo classification.
All procedures were performed with the DVSS by the same team of surgeons who had received standardized training in robotic surgery and were certified to perform the procedure. The surgical position varied according to the patient’s body size, body mass index, and tumor location. The supine position or the contralateral 70° lateral decubitus position was chosen to establish pneumoperitoneum, insert a trocar, and install a robotic arm. After ensuring that no metastasis was present, the tumor was exposed, carefully separated along the tumor capsule, completely removed, and loaded into a specimen bag. The specimen was then removed for routine examination. All patients were followed up at the outpatient clinic 1 month after discharge and every 3 months thereafter.
SPSS 24 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Continuous variables are expressed as mean ± standard deviation and were compared using the t test. Categorical variables are expressed as count ratio and were compared using the chi-square test. In total, 105 patients who underwent RRTR were divided into the DVSS group and the conversion group according to whether they had undergone conversion to open surgery. Correlations between parameters were analyzed. Logistic univariate and multivariate analyses were performed to identify independent risk factors affecting the need for conversion to open surgery. Logistic multivariate analysis was used to verify the results. A P value of <0.05 was considered statistically significant.
Among the 105 patients, 87 successfully underwent RRTR (DVSS group) and 18 underwent conversion to open surgery (conversion group). The 105 patients comprised 40 men and 65 women. The baseline characteristics of the study population are shown in Table 1.
The pathological results were based on the final histopathology, which revealed 37 malignant tumors and 68 benign tumors. As shown in Figure 1, schwannoma (n = 27) was the most common, followed by paraganglioma (n = 13), cystic lesion (n = 11), pheochromocytoma (n = 11), extragastrointestinal stromal tumor (n = 7), lymphangioma (n = 7), hemangioma (n = 6), ganglioneuroma (n = 5), leiomyosarcoma (n = 5), bronchogenic cyst (n = 4), gangliocytoma (n = 4), neurofibroma (n = 2), ganglioneuroblastoma (n = 1), myelolipoma (n = 1), and aggressive fibromatosis (n = 1).
The patients’ perioperative characteristics are shown in Table 2. No intraoperative complications occurred in the DVSS group. One patient received 4 U red blood cells during the operation, and one patient developed a postoperative complication (unilateral atelectasis). One patient in the conversion group had intraoperative blood loss of 2400 mL, received an intraoperative transfusion of 8 U red blood cells and 840 mL plasma, and received a postoperative transfusion of 570 mL plasma. One patient developed a postoperative complication (pancreatic fistula, which healed after nonsurgical treatment).
There were no significant differences in sex (P = 0.253), age (P = 0.449), body mass index (P = 0.738), history of abdominal surgery (P = 0.555), or tumor adhesion to large vessels (P = 0.824) between the DVSS group and conversion group. However, there were significant differences in the maximum tumor diameter (P = 0.006), pathological property (P = 0.002), blood loss (P = 0.002), operation time (P = 0.037), and postoperative hospital stay (P = 0.026). There was no significant difference in the frequency of blood transfusion between the two groups (1.1% vs. 5.6%, P = 0.281). The operation time in the DVSS group was significantly shorter than that in the conversion group (163.28 ± 47.76 vs. 212.22 ± 88.39 min, P = 0.037). Additionally, the blood loss volume was lower (31.69 ± 32.56 vs. 230.00 ± 556.35 mL, P = 0.002) and the postoperative hospital stay was shorter (3.62 ± 1.11 vs. 6.11 ± 6.16 days, P = 0.026) in the DVSS group. The hospitalization cost was higher in the conversion group (60441.33 ± 7047.89 vs. 68230.22 ± 10168.35 yuan, P = 0.046).
No patient required reoperation or readmission, and the 90-day mortality rate was 0%. The median follow-up time was 19 months (range, 13–36 months) and there was no imaging evidence of tumor recurrence in any patient.
To evaluate the influence of various factors on the need for conversion to open surgery, relevant parameters were included in the univariate logistic regression analysis to screen out risk factors and further included in the multivariate logistic regression analysis. The results showed that the independent risk factors for conversion to open surgery were the pathological property of the tumor [odds ratio (OR), 8.646; 95% confidence interval (CI), 2.370-31.544; P = 0.001] and maximum tumor diameter (OR, 1.041; 95% CI, 1.015-1.067; P = 0.002) (Figure 2, Table 3). These findings indicate that a larger maximum tumor diameter is associated with a higher probability of conversion to open surgery and that the probability of conversion to open surgery is higher for malignant than benign tumors. Figure 3 shows the receiver operating characteristic curve generated according to the maximum tumor diameter, with a determined cut-off value of 64 mm for conversion to open surgery. The classification variable and pathological property were used as covariates, and the decision to convert to open surgery served as the dependent variable for logistic regression analysis. The Hosmer test indicated a good fit for the model (P = 0.787 > 0.05). The pathological property of the tumor (OR, 9.805; 95% CI, 2.403-40.003; P = 0.001) and a maximum tumor diameter of >64 mm (OR, 14.228; 95% CI, 3.504–57.774; P < 0.001) were independent risk factors for conversion to open surgery (Table 4, Figure 4). These findings indicate that RRTR has a higher success rate for retroperitoneal benign tumors with a maximum diameter of ≤64 mm.
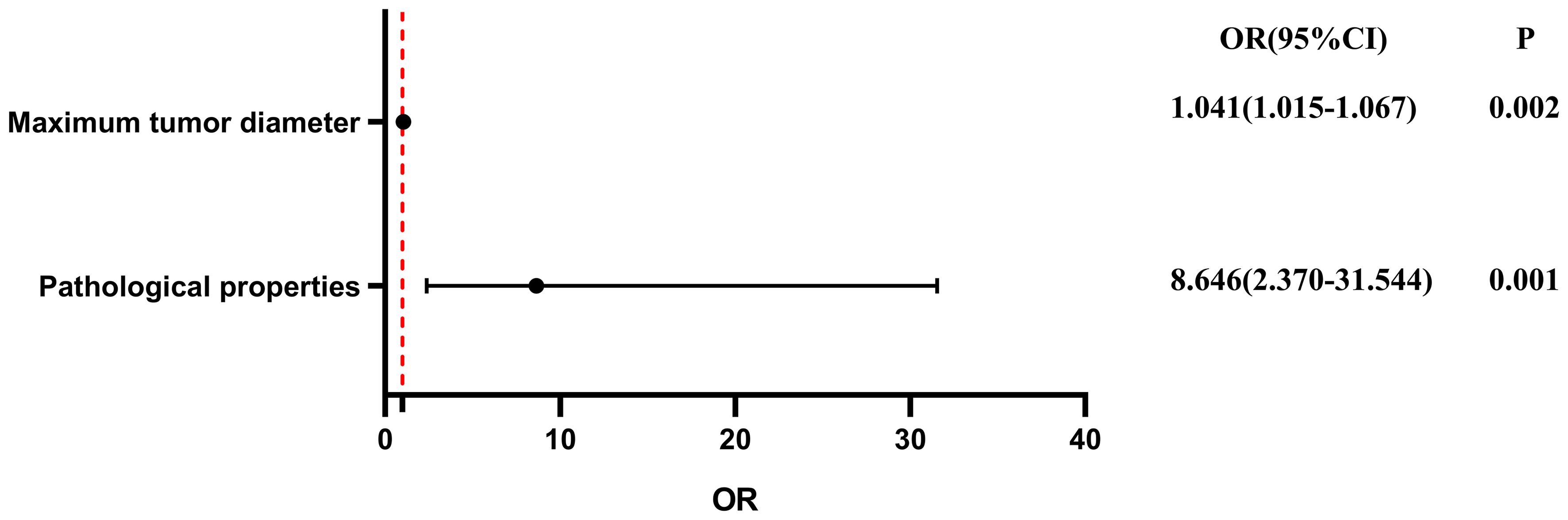
Figure 2 Multivariate logistic regression analysis of conversion from Da Vinci surgery to open surgery. OR, odds ratio; CI, confidence interval.
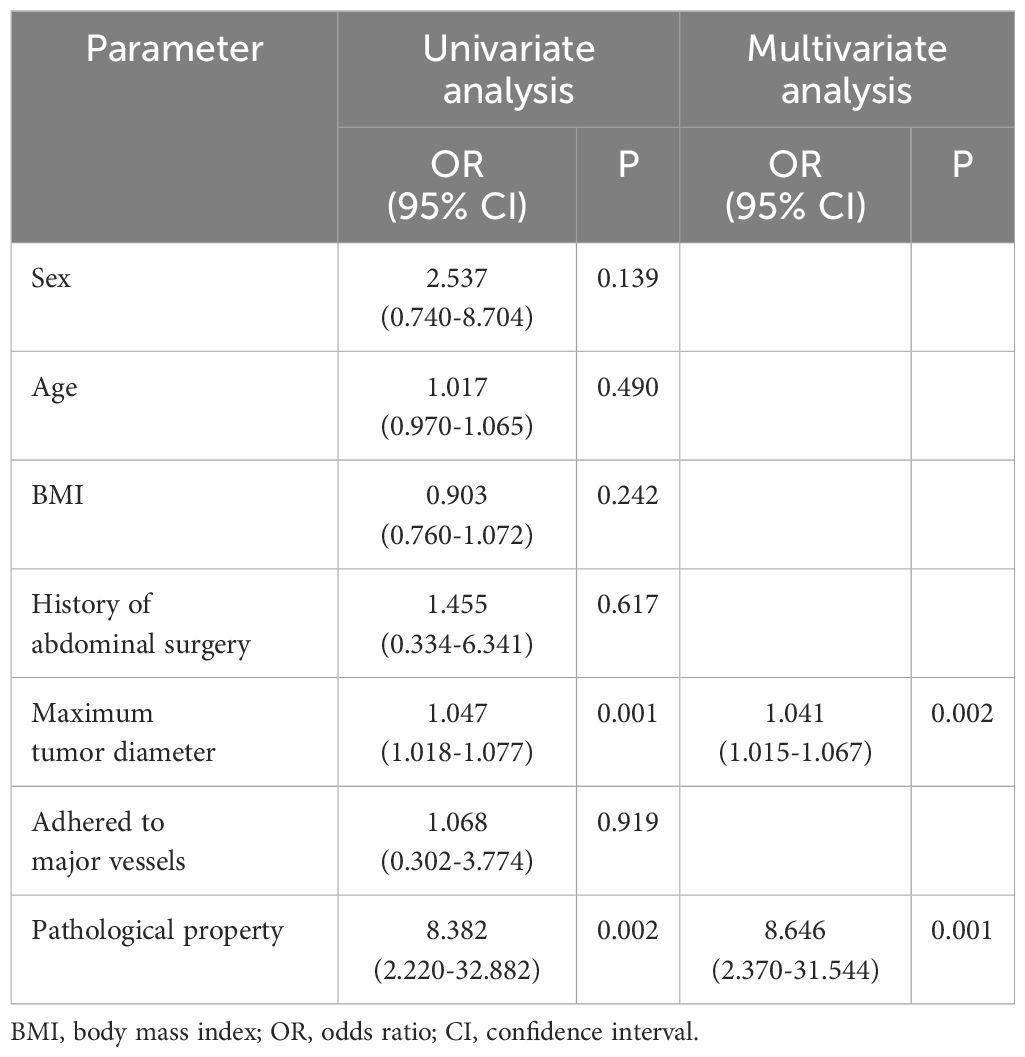
Table 3 Univariate and multivariate logistic regression analysis of conversion from Da Vinci robotic surgery to open surgery.
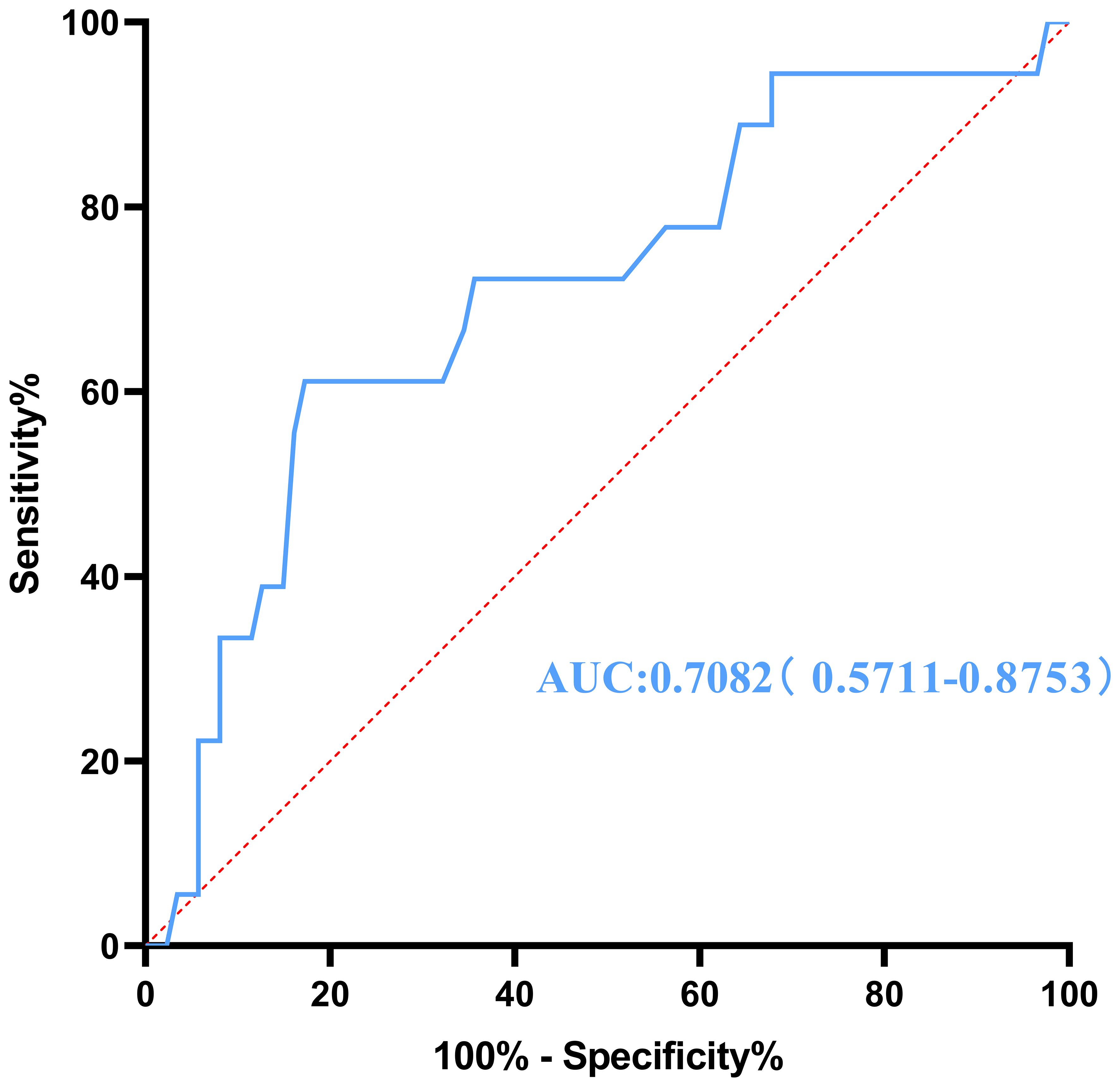
Figure 3 Receiver operating characteristic curve for conversion to open surgery generated according to the maximum tumor diameter. AUC, area under the curve.
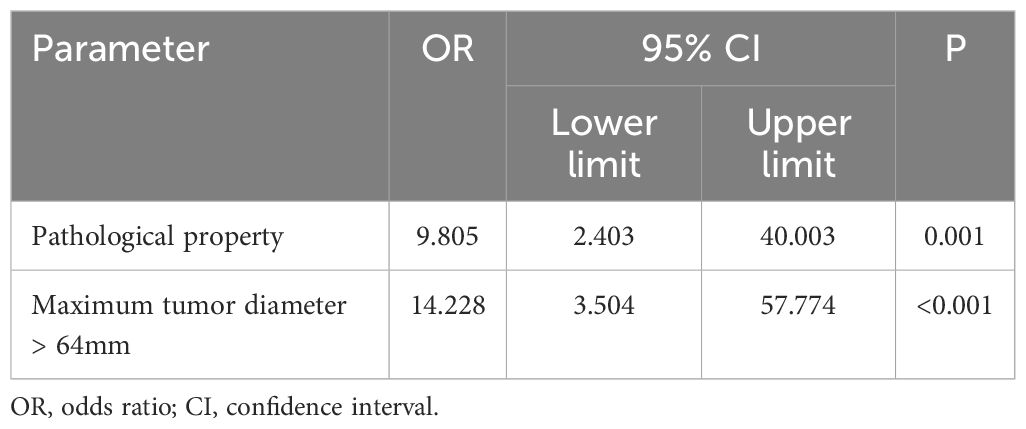
Table 4 Multivariate logistic regression analysis of risk factors for conversion to open surgery after classification by cutoff value.
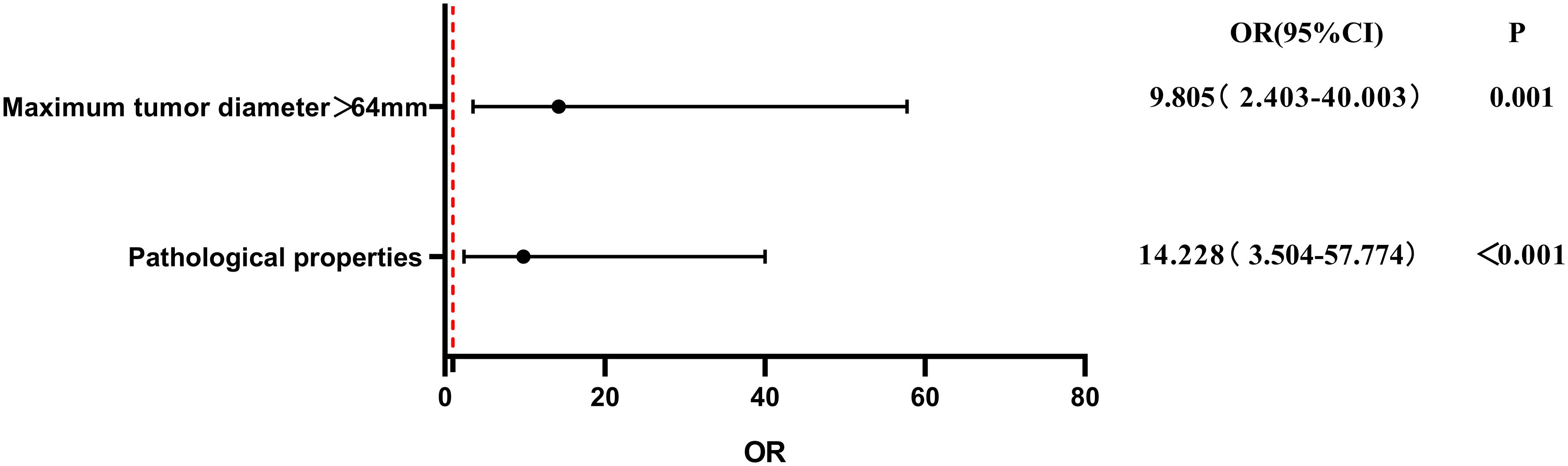
Figure 4 Multivariate logistic regression analysis of risk factors for conversion to open surgery after classification by cut-off value. OR, odds ratio; CI, confidence interval.
In this study, the total conversion rate was 17.14%. As shown in Table 5, the reasons for conversion included poor vision (7 cases), uncontrolled bleeding (4 cases), difficulties in achieving radical resection with DVSS (5 cases), and the occurrence of hypertensive crisis during surgery (2 cases). In the conversion group, there were 8 cases of combined organ resection. Given the proximity to the inferior vena cava (IVC), 2 cases of right upper abdominal RPT were challenging to separate, leading to partial resection and repair of the IVC during surgery. One case of right upper abdominal RPT required partial duodenectomy because of its extensive invasion into the duodenum. Another case of left upper abdominal RPT, situated between the left kidney and pancreas and closely associated with the tail of the pancreatic body and left kidney, necessitated distal pancreatectomy. Additionally, 1 case of left upper abdominal RPT involved the gastric antrum and was managed with partial gastrectomy. One case of RPT in the right lower abdominal mesenteric region required partial small intestine resection and enterostomy. The left lower abdominal RPT in 2 cases was challenging to separate from the left psoas major muscle, resulting in partial resection of this muscle during surgery.
The DVSS is currently the most widely used robotic system. It has the advantages of magnified and stable three-dimensional stereovision, multidimensional robotic arm movement, elimination of hand tremor, flexible instruments to enhance tactile feedback, no need for reverse operation or intensive training of operators and assistants, and the potential for use in remote surgery. It has the same surgical efficacy as laparoscopic techniques in the treatment of various diseases, and it overcomes several limitations of laparoscopic surgery such as the lack of three-dimensional depth perception in two-dimensional imaging, high technical difficulty, and a steep learning curve (20–23). Widespread use of the DVSS can also reduce reliance on surgical assistants, thereby reducing the number of assistants and saving medical resources (24). At the same time, competition between hospitals and increased patient expectations have contributed to the popularity of robot-assisted surgery (25). Surgical resection of RPT is often accompanied by a variety of postoperative complications due to the limited working space and rich, fine structures within the retroperitoneal space, which prompts retroperitoneal tumor surgeons to attempt resection with minimal trauma and the shortest possible operation time. It is quite helpful to use robotic techniques that are finer, safer, and more stable than traditional laparoscopic surgery. Such techniques can significantly reduce damage to the surrounding tissues and the occurrence of complications (19, 26). However, no systematic large-sample case study on RRTR has been performed to date. To further explore the feasibility of RRTR and promote safer use of robotic technology, we analyzed the data of 105 patients who underwent RRTR in our hospital and identified risk factors for conversion to open surgery. With this study, we aim to provide a preliminary basis for preoperative evaluation of surgical indications and guide clinical practice. To the best of our knowledge, this is the first large-sample study of RRTR.
In total, 105 patients who underwent RRTR were included in this study. Among them, 87 patients successfully underwent RRTR (DVSS group) and 18 patients underwent conversion to open surgery (conversion group). There were no significant differences in sex (P = 0.253), age (P = 0.449), body mass index (P = 0.738), history of abdominal surgery (P = 0.555), or tumor adhered to major vessels (P = 0.824) between the DVSS group and conversion group, although patients with a history of abdominal surgery inevitably had different degrees of intestinal adhesion. Changes in the normal anatomical structure of the abdomen and tumor adhesion to large abdominal blood vessels will also increase the difficulty of the operation, but these are not key reasons for conversion of robotic to open surgery. Using the DVSS, the main blood vessels around the tumor can be accurately dissected and bleeding can be controlled. This approach is beneficial in terms of the tumor anatomy and may be more suitable for RRTR. This is consistent with the results reported by Liu (12). In our study, the procedure time was shorter in the DVSS than conversion group (163.28 ± 47.76 vs. 212.22 ± 88.39 min, P = 0.037). Additionally, the blood loss volume was lower (31.69 ± 84.27 vs. 230.00 ± 556.35 mL, P = 0.002) and the postoperative hospital stay was shorter (3.62 ± 1.11 vs. 6.11 ± 6.16 days, P = 0.026) in the DVSS group. The dexterity and precision of the DVSS can reduce surgical trauma (27, 28), and minimally invasive surgery can reduce postoperative pain (29, 30); both of these factors promote faster patient recovery after RRTR. Conversion to open surgery requires a change of surgical instruments, which may have contributed to the increased operation time in the conversion group; however, this longer operation time may not be clinically significant.
This study revealed that the pathological property of the tumor (OR, 9.805; 95% CI, 2.403-40.003; P = 0.001) and maximum tumor diameter of >64 mm (OR, 14.228; 95% CI, 3.504–57.774; P < 0.001) were independent risk factors for conversion to open surgery. Higher success rates are observed in benign retroperitoneal tumors with a maximum diameter of ≤64 mm. Therefore, the pathological property and size of the tumor should be determined according to preoperative imaging examination or biopsy, which is helpful for evaluating the difficulty of the operation and provides a preliminary basis for clinical and surgical decision-making. A study by the Transatlantic Australasian Retroperitoneal Sarcoma Working Group showed that schwannomas increase in size at a rate of 10.5% per year (31). If the tumor size increases by ≥20% per year, surgical resection is recommended regardless of the presence or absence of symptoms, and the success rate of R0/R1 resection for benign RPT is as high as 91.6% (32). Therefore, patients with asymptomatic retroperitoneal tumors detected by physical examination should be actively treated with surgery because of the unpredictability of tumor growth and the possible progression to malignancy. The present study indicates that minimally invasive surgery is preferable for benign tumors measuring ≤64 mm in diameter, with RRTR being the treatment of choice for these lesions.
In the present study, the total conversion rate was 17.14%, with the rate of conversion to open surgery for malignant tumors standing at 32.43%. Moreover, two-thirds of the tumors treated by conversion to open surgery were malignant. The reasons for conversion to open surgery, consistent with findings from other studies (33), included poor visibility (seven cases), uncontrolled bleeding (four cases), difficulty achieving radical resection with DVSS (five cases), and the occurrence of hypertensive crisis during surgery (two cases). In instances where malignant tumors could not be radically cured through RRTR, our team promptly performed conversion to open surgery. Retroperitoneal malignant tumors, noted for their invasiveness, necessitate a wider resection margin to ensure negative margins, thereby increasing the risk of damage to surrounding tissues and the possibility of incomplete resection. Given these considerations and the implications for patient prognosis, open surgery remains the recommended approach because of its safety (34). The need for combined organ resection often arises under several circumstances: 1. suspected tumor invasion; 2. tumor involvement in the peripheral vascular supply of organs; 3. tumors encasing or adhering to organs, making separation difficult; and 4. iatrogenic injuries necessitating organ resection. In our study, the Da Vinci group, consisting of 96 cases, did not report any intraoperative combined organ resections. However, in the conversion group, which included 18 cases, 8 required intraoperative combined organ resections. Of these, five involved tumors in the upper abdomen, where vital organs such as the liver, kidneys, pancreas, spleen, and duodenum are located alongside major vessels like the inferior vena cava (IVC), abdominal aorta, hepatic hilum, and renal hilum, presenting complex anatomical challenges and surgical difficulties. There were three cases of combined organ resection for tumors in the lower abdomen, which includes structures such as the colon, small intestine, mesentery, psoas major, and related vessels. During surgical dissection in these cases, it is crucial to protect the ureter, mesenteric vessels, and iliac vessels. Additionally, in cases where pheochromocytoma is suspected, preparations to convert to open surgery should be made intraoperatively, as approximately 15% of patients may experience hemodynamic instability or crisis despite adequate preoperative preparation (35).
We recommend conversion to open surgery when complications occur during RRTR, the clinician suspects incomplete tumor resection, or the intraoperative pathology suggests malignancy requiring wide resection. Especially for malignant tumors, the following principles of radical tumor treatment must be followed: complete resection of the tumor and surrounding tissue, minimization of direct physical manipulation of the tumor (non-contact principle of tumor surgery), achievement of adequate margins, and complete lymph node dissection (36).
Retroperitoneal tumors with a diameter of >64 mm will have a limited surgical field, narrow surgical space, and increased difficulty of surgery; thus, they are more suitable for open surgery. However, tumor size is not an absolute contraindication for minimally invasive surgery (37). For example, a retroperitoneal tumor with a maximum diameter of 131 mm was removed by RRTR in our center. Thus, even if the tumor is large, RRTR can still be considered based on factors such as whether the tumor is easy to expose. Notably, for huge or malignant tumors, the increased operation time may introduce additional risks such as anesthetic complications, pulmonary infections, and postoperative nursing challenges. From doctors’ perspective, striving for high rates of minimally invasive procedures is valuable but should not be done at the expense of patient safety.
Although the learning curve of RRTR is unknown, it should not be ignored. Mastering RRTR is indeed a challenging undertaking, and surgeons are advised to proceed with great caution even if they are already very familiar with open and laparoscopic RPT procedures.
The DVSS has the disadvantages of a long training time, long setup time, long operation time, and high cost, all of which limit its application. The high costs associated with using the DVSS are mainly related to the purchase and maintenance of the equipment, the high cost of the instruments, and the long operating time. Although the main limitation of using the DVSS is the additional cost to the patient, this may be offset by the benefits of reduced trauma and bleeding, a shorter hospital stay, and an earlier return to work. With the emergence of increasingly more new robotic systems, such as the avatera® robotic system (avateramedical GmbH, Jena, Germany) and the hinotori™ robotic system (Medicaroid, Kobe, Japan), the cost and limitations of robotic surgery will gradually decrease (38, 39). Its wide applicability is likely to facilitate further substantial progress over the next decade. Reasonable selection of surgical methods can improve resource utilization and reduce costs for patients and the medical system. As a complex surgical method, RRTR must be explored in detail to help clinicians make informed decisions and benefit more patients.
This was a retrospective study and has certain inherent limitations. First, this study involved a single-center retrospective analysis. To the best of our knowledge, this is the first large-sample study of RRTR; nevertheless, the number of cases was limited, increasing the risk of statistical bias. Multicenter prospective studies are needed to confirm the conclusions drawn in this study. Second, retroperitoneal tumors are clinically rare, and the sample size of this study is low; this may reduce the reliability of the final results to some extent. Third, there may have been errors in the data obtained from the medical records, such as the anesthetic details, operation time, and blood loss, and such errors may have affected the statistical results. Fourth, younger patients with a higher socioeconomic status or better health status may be more inclined to choose robot-assisted surgery, which may lead to selection bias. Fifth, patients who received neoadjuvant therapy before surgery were not included in this study; therefore, whether neoadjuvant chemotherapy or radiotherapy affects the DVSS procedure remains unclear.
RRTR is safe in experienced centers, and its clinical prognosis is similar to that of open surgery. Patients with retroperitoneal tumors who undergo RRTR have a higher chance of surgical success when the maximum tumor diameter is ≤64 mm, the tumor is benign, the tumor has relatively clear boundary, there is no obvious invasion of surrounding tissues and organs, and there is no need for combined organ resection.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The patients involved in the database have obtained Medical Ethics Committee of The Affiliated Hospital of Qingdao University approval (QYFY WZLL 28271). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
QH: Data curation, Formal analysis, Software, Writing – original draft. LC: Writing – original draft, Writing – review & editing. BZ: Methodology, Resources, Writing – review & editing. XL: Data curation, Writing – original draft. MG: Data curation, Writing – original draft. QL: Data curation, Investigation, Writing – original draft. GD: Validation, Writing – original draft. MS: Investigation, Project administration, Writing – review & editing. ZW: Methodology, Project administration, Validation, Writing – review & editing. ZG: Data curation, Formal analysis, Writing – review & editing. FQ: Funding acquisition, Resources, Supervision, Writing – review & editing. XW: Methodology, Supervision, Writing – review & editing. LT: Funding acquisition, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No.82073078).
We would like to thank our colleagues at the Affiliated Hospital of Qingdao University for their support and assistance in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ye S, Wang P, Zhu L, Jing T, Qin J, Zhu Y, et al. Robot-assisted laparoscopic excision of complicated retroperitoneal tumors with four arms via retroperitoneal way: A unique minimal-invasive approach. J Laparoendosc Adv Surg Tech A. (2020) 30:1110–6. doi: 10.1089/lap.2019.0532
2. Chinese Medical Association, Cancer Society of Chinese Medical Association, Journal of Chinese Medical Association, Anorectal Physicians Branch of Chinese Medical Association, Professional Committee on Retroperitoneal and Pelvic Floor Diseases, Chinese Research Hospital Association. [Expert consensus on treatment of Retroperitoneal tumors in China(Edition 2019)]. Zhonghua Zhong Liu Za Zhi. (2019) 41:728–33. doi: 10.3760/cma.j.issn.0253-3766.2019.10.002.
3. Neville A, Herts BR. CT characteristics of primary retroperitoneal neoplasms. Crit Rev Comput Tomogr. (2004) 45:247–70. doi: 10.3109/10408370490506616
4. Gemici K, Buldu I, Acar T, Alptekin H, Kaynar M, Tekinarslan E, et al. Management of patients with retroperitoneal tumors and a review of the literature. World J Surg Oncol. (2015) 13:143. doi: 10.1186/s12957-015-0548-z
5. Karadayi K, Yildiz C, Karakus S, Kurt A, Bozkurt B, Soylu S, et al. Well-differentiated abdominal liposarcoma: experience of a tertiary care center. World J Surg Oncol. (2015) 13:166. doi: 10.1186/s12957-015-0580-z
6. Hueman MT, Herman JM, Ahuja N. Management of retroperitoneal sarcomas. Surg Clin North Am. (2008) 88:583–97. doi: 10.1016/j.suc.2008.03.002
7. Sato T, Matsuo Y, Shiga K, Saito K, Morimoto M, Miyai H, et al. Laparoscopic resection of retroperitoneal lymphangioma around the pancreas: a case report and review of the literature. J Med Case Rep. (2015) 9:279. doi: 10.1186/s13256-015-0760-z
8. Sakai K, Ohashi T, Hara S, Osawa H, Ide Y, Noro H, et al. [Laparoscopic surgery for synchronous retroperitoneal tumor and ovarian mature teratoma-A case report]. Gan To Kagaku Ryoho. (2020) 47:2329–31.
9. Chatelet F, Bardier-Dupas A, Menegaux F, Chereau N. Laparoscopic resection of a giant retroperitoneal melanotic schwannoma. J Surg Case Rep. (2018) 2018:rjy040. doi: 10.1093/jscr/rjy040
10. Ahn KS, Han HS, Yoon YS, Kim HH, Lee TS, Kang SB, et al. Laparoscopic resection of nonadrenal retroperitoneal tumors. Arch Surg. (2011) 146:162–7. doi: 10.1001/archsurg.2010.342
11. Dalpiaz O, Gidaro S, Lipsky K, Schips L. Case report: Laparoscopic removal of 10-cm retroperitoneal liposarcoma. J Endourol. (2007) 21:83–4. doi: 10.1089/end.2006.0152
12. Liu Q, Zhou R, Zhao Z, Gao Y, Zhao G, Liu R. Robotic versus open resection of benign nonadrenal retroperitoneal tumors: A propensity score-matched study. Int J Surg. (2019) 65:19–24. doi: 10.1016/j.ijsu.2019.02.021
13. Bindal V, Bhatia P, Kalhan S, Khetan M, John S, Ali A, et al. Robot-assisted excision of a large retroperitoneal schwannoma. JSLS. (2014) 18:150–4. doi: 10.4293/108680813X13654754534873
14. Lehrfeld T, Natale R, Sharma S, Mendoza PJ, Schwab IC, Lee DI. Robot-assisted excision of a retroperitoneal mass between the left renal artery and vein. JSLS. (2010) 14:447–9. doi: 10.4293/108680810X12924466006927
15. Chopra S, Dharmaraja A, Satkunasivam R, Gill IS. Robot-assisted laparoscopic resection of a pelvic schwannoma. Urol Case Rep. (2017) 11:63–5. doi: 10.1016/j.eucr.2016.11.027
16. Alguraan Z, Agcaoglu O, El-Hayek K, Hamrahian AH, Siperstein A, Berber E. Retroperitoneal masses mimicking adrenal tumors. Endocr Pract. (2012) 18:335–41. doi: 10.4158/EP11240.OR
17. Wei TC, Chung HJ, Lin AT, Chen KK. Robot-assisted laparoscopic excision of a retroperitoneal paracaval tumor. J Chin Med Assoc. (2013) 76:724–6. doi: 10.1016/j.jcma.2013.08.001
18. Ragu R, Blanchard C, Meurette G. Robotic excision of large retroperitoneal Schwannoma (with video). J Visc Surg. (2017) 154:297–9. doi: 10.1016/j.jviscsurg.2017.05.003
19. Liu Q, Gao Y, Zhao Z, Zhao G, Liu R, Lau WY. Robotic resection of benign nonadrenal retroperitoneal tumors: A consecutive case series. Int J Surg. (2018) 55:188–92. doi: 10.1016/j.ijsu.2018.04.013
20. Boga MS, Ates M. Retroperitoneal robot-assisted laparoscopic partial nephrectomy for posterior located renal tumours: Technique and early term outcomes. Int J Clin Pract. (2021) 75:e13851. doi: 10.1111/ijcp.13851
21. Materazzi G, Rossi L. Robot-assisted adrenalectomy: state of the art. Updates Surg. (2021) 73:1131–46. doi: 10.1007/s13304-020-00915-2
22. Choi JE, You JH, Kim DK, Rha KH, Lee SH. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. (2015) 67:891–901. doi: 10.1016/j.eururo.2014.12.028
23. Zargar H, Allaf ME, Bhayani S, Stifelman M, Rogers C, Ball MW, et al. Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: a multi-institutional study. BJU Int. (2015) 116:407–14. doi: 10.1111/bju.12933
24. Zhan W, Hu M, Han C, Tian H, Jing W, Li X, et al. Safety and effectiveness of the da Vinci robot with the "3+2" mode for distal pancreatectomy. Cancer Med. (2019) 8:4226–34. doi: 10.1002/cam4.2353
25. Moore LJ, Wilson MR, Waine E, Masters RS, McGrath JS, Vine SJ. Robotic technology results in faster and more robust surgical skill acquisition than traditional laparoscopy. J Robot Surg. (2015) 9:67–73. doi: 10.1007/s11701-014-0493-9
26. Autorino R, Zargar H, Kaouk JH. Robotic-assisted laparoscopic surgery: recent advances in urology. Fertil Steril. (2014) 102:939–49. doi: 10.1016/j.fertnstert.2014.05.033
27. Corcione F, Esposito C, Cuccurullo D, Settembre A, Miranda N, Amato F, et al. Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc. (2005) 19:117–9. doi: 10.1007/s00464-004-9004-9
28. Garcia-Ruiz A, Gagner M, Miller JH, Steiner CP, Hahn JF. Manual vs robotically assisted laparoscopic surgery in the performance of basic manipulation and suturing tasks. Arch Surg. (1998) 133:957–61. doi: 10.1001/archsurg.133.9.957
29. Lacroix V, Mosala NZ, Kahn D, Steyaert A, Poncelet A, Pieters T, et al. Pain, quality of life, and clinical outcomes after robotic lobectomy. Thorac Cardiovasc Surg. (2017) 65:344–50. doi: 10.1055/s-0036-1587590
30. Darr C, Cheufou D, Weinreich G, Hachenberg T, Aigner C, Kampe S. Robotic thoracic surgery results in shorter hospital stay and lower postoperative pain compared to open thoracotomy: a matched pairs analysis. Surg Endosc. (2017) 31:4126–30. doi: 10.1007/s00464-017-5464-6
31. Monteiro De Barros J, Hodson J, Glasbey J, Massey R, Rintoul-Hoad O, Chetan M, et al. Intercontinental collaborative experience with abdominal, retroperitoneal and pelvic schwannomas. Br J Surg. (2020) 107:452–63. doi: 10.1002/bjs.11376
32. Sassa N. Retroperitoneal tumors: Review of diagnosis and management. Int J Urol. (2020) 27:1058–70. doi: 10.1111/iju.14361
33. Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. (2010) 24:1646–57. doi: 10.1007/s00464-009-0825-4
34. Morizawa Y, Miyake M, Shimada K, Hori S, Tatsumi Y, Nakai Y, et al. Extended resection including adjacent organs and Ki-67 labeling index are prognostic factors in patients with retroperitoneal soft tissue sarcomas. World J Surg Oncol. (2016) 14:43. doi: 10.1186/s12957-016-0810-z
35. Vorselaars W, Postma EL, Mirallie E, Thiery J, Lustgarten M, Pasternak JD, et al. Hemodynamic instability during surgery for pheochromocytoma: comparing the transperitoneal and retroperitoneal approach in a multicenter analysis of 341 patients. Surgery. (2018) 163:176–82. doi: 10.1016/j.surg.2017.05.029
36. Tomassini F, Scuderi V, Colman R, Vivarelli M, Montalti R, Troisi RI. The single surgeon learning curve of laparoscopic liver resection: A continuous evolving process through stepwise difficulties. Med (Baltimore). (2016) 95:e5138. doi: 10.1097/MD.0000000000005138
37. Di Nuzzo MM, Castiglioni S, Pirozzi N, Di Santo AA, De Luca M, Tedesco A, et al. Laparoscopic adrenalectomy for giant pheochromocytoma. What is the size limit? Ann Ital Chir. (2023) 94:52–5.
38. Hinata N, Yamaguchi R, Kusuhara Y, Kanayama H, Kohjimoto Y, Hara I, et al. Hinotori Surgical Robot System, a novel robot-assisted surgical platform: Preclinical and clinical evaluation. Int J Urol. (2022) 29:1213–20. doi: 10.1111/iju.14973
Keywords: retroperitoneal tumor, robotic surgery, minimally invasive technique, Da Vinci surgery system, robot-assisted retroperitoneal tumor resection
Citation: Hao Q, Cha L, Zhou B, Li X, Gong M, Li Q, Dong G, Song M, Wu Z, Guo Z, Qiu F, Wang X and Tian L (2024) Da Vinci robot-assisted retroperitoneal tumor resection in 105 patients: a single-center experience. Front. Oncol. 14:1414780. doi: 10.3389/fonc.2024.1414780
Received: 09 April 2024; Accepted: 08 July 2024;
Published: 23 July 2024.
Edited by:
Yong Zhang, Fudan University, ChinaCopyright © 2024 Hao, Cha, Zhou, Li, Gong, Li, Dong, Song, Wu, Guo, Qiu, Wang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowei Wang, d2FuZ3h3MjAxMkBxZHUuZWR1LmNu; Lantian Tian, dGlhbmxhbnRpYW5AcWR1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.