
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 12 November 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1414492
Background: Fine-needle aspiration (FNA) biopsy is typically used in conjunction with cytopathologic evaluation to differentiate between benign and malignant thyroid nodules. Even so, the cytology results for 20-30% of thyroid nodules are indeterminate. This study sought to evaluate the usefulness of next-generation sequencing (NGS)-based multi-gene panel testing for risk stratification and the differentiation of benign from malignant thyroid nodules.
Methods: Thyroid nodule samples were obtained from a cohort of 359 patients who underwent FNA. An NGS-based multi-gene panel testing was conducted for these samples, in which single-nucleotide variants (SNVs) and small insertion/deletions (InDels) can be detected in 11 genes and fusion events can be identified in 5 genes. Surgical resection was conducted for 113 patients (113/359), and then histopathology results were obtained.
Results: In comparison to cytology alone, the diagnostic sensitivity of NGS combination cytology increased from 0.7245 (95% CI: 0.6289-0.8032) to 0.898 (95% CI: 0.8223-0.9437); the associated AUC was 0.8303 (vs. Cytology AUC: 0.7622, P < 0.001). BRAFV600E was identified in 136 patients, of whom 79 underwent surgery and were diagnosed with papillary thyroid carcinoma (PTC) pathologically. TERT promoter mutations or BRAF/RAS co-mutations with other genes were identified in 5 patients, while 4 patients were diagnosed with malignant thyroid cancer using the pathological method. RAS mutations were identified in 27 patients, while 10 patients underwent surgery, which showed that 3 patients were classified as PTC and 7 cases were benign. In addition, 4 RET fusions, 1 RET activation mutation, and 3 TP53 inactivation mutations were identified in the remaining 8 patients who have not undergone surgery. Negative genetic test results or variants with uncertain significance were identified in 183 patients. Among these patients, 12 malignant thyroid tumors, including 11 PTC and 1 MTC, were diagnosed in 20 patients who received surgery.
Conclusion: Thyroid nodules coupled with BRAFV600E, TERT promoter variants, BRAF/RAS co-mutations with other genes, RET fusions, and RET activating mutations were classified as high-risk. Nodules with RAS mutations (NRAS, KRAS, HRAS) and TP53 inactivating mutations were considered to be in the intermediate-risk group, while those with non-pathogenic mutations (negative and variants of uncertain significance) were placed in the low-risk group. When combined with cytopathology, NGS increases the sensitivity of diagnosing benign and malignant thyroid nodules, and the reference is useful for patient risk stratification.
Thyroid cancer (TC) is an endocrine illness that includes three primary forms: differentiated thyroid cancer (DTC), medullary thyroid carcinoma (MTC), and anaplastic thyroid carcinoma (ATC). According to the latest available data, there were 39,079 new TC diagnoses in 2019 with an age standard incidence rate (ASIR) of 2.05/100,000 and 7,240 deaths with an age standard mortality rate (ASMR) of 0.39/100,000 (1). In China, the ASIR was 3.21/105 in 2005 and increased to 9.61/105 in 2015, whereas the ASMR was 0.30/105 in 2005 and rose to 0.35/105 in 2015 (2). DTC patients had an overall excellent prognosis, with a 5-year survival rate of 98.5% when given appropriate treatment (3). High-resolution ultrasonography was used to detect thyroid nodules, and the results showed a prevalence of 20-76%, in which 10-15% of patients harbored cancerous thyroid nodules (4, 5). Thyroid nodules require medical attention because they have the potential to become malignant.
When combined with cytopathology and taking the nodule’s size and ultrasound appearance into consideration, a fine-needle aspiration (FNA) biopsy is the most widely used and cost-effective diagnostic technique for distinguishing benign from malignant nodules. According to recent research, the benign and malignant nature of thyroid nodules in a sizable fraction of patients was consistent with their FNA cytopathology report, even though a tiny percentage of samples showed cytological indeterminacy. The aforementioned conditions comprise 2-18% of nodules with atypia of undetermined significance/follicular lesion of undetermined significance, 2-25% with follicular neoplasm/suspicious for follicular neoplasm, and 1-6% with suspicious for malignancy (6). Indeterminate thyroid nodules can now be more accurately diagnosed when combining cytologic diagnosis with molecular testing.
In 2014, the Cancer Genome Atlas Research Network (TGCA) conducted a study on the molecular profiles of papillary thyroid cancers (PTCs), which showed two distinct molecular profiles: BRAFV600E-like and RAS-like (7). The widely accepted molecular classifications are as follows: BRAFV600E -like included the BRAFV600E mutation and RET and BRAF fusions; RAS-like included RAS family (KRAS, HRAS, and NRAS) mutations, BRAFK601E mutation, EIF1AX mutations, and PPARG and THADA fusions (8). A study based on 458 Chinese patients with thyroid cancer reported that the prevalence of BRAF driver mutations is 76.0%, followed by RET rearrangements (7.6%), while the prevalence of RAS mutations is only 4.1% (9). Additional molecular profiles have also been thoroughly investigated. These include promoter mutations in TERT, which are independently predictive of DTC-related death and strongly associated with high tumor aggressiveness (10). BRAF/RAS co-mutations with other genes (e.g., TERT, PIK3CA, and TP53) indicate an increasing risk of malignancy in thyroid cancer (6).
According to current guidelines, molecular testing may be employed to supplement the malignancy risk assessment of thyroid nodules. In this study, we applied a molecular test project including 11 genes for thyroid FNAs. Based on the molecular profiles detected in thyroid nodules, we aimed to evaluate the value of our next-generation sequencing (NGS)-based multi-gene testing panel for the differentiation of benign from malignant and the risk stratification of thyroid nodules.
359 thyroid nodule samples from patients who underwent FNA operations at the Second Affiliated Hospital of Jiaxing University between May 2022 and December 2023 were collected both retrospectively and consecutively. NGS-based panel testing is recommended for patients with (1) thyroid nodules with malignant features on conventional ultrasound: solid, hypoechogenicity, microcalcification, unclear margins, vertical growth, or taller-than-wide shape; and (2) thyroid nodules that have grown too rapidly (more than 50% increase in size in one year) or have new malignant ultrasound features during follow-up. Patients for whom NGS-based panel testing is not recommended: (1) pure cystic nodules; and (2) those with coagulation abnormalities.
The FNA biopsy samples underwent both cytology and NGS-based multi-gene panel testing simultaneously. 113 patients received surgical resections, and a histopathology assay was conducted. FNA biopsy samples and surgically resected tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The results were reviewed and reported by two pathologists. The mean time between FNA biopsy and surgical resection in these 113 individuals was 29.6 days, with a median of 19 days. The clinic data, NGS test results, cytological diagnoses, and histological diagnoses were all subjected to a retrospective analysis. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Jiaxing University. All subjects signed informed consent forms.
DNA for next-generation sequencing was extracted using the Quick-DNA/RNA Microprep Plus Kit (D7005, Zymo Research Corp., USA). The libraries were then established with the Thyroid Cancer Mutation Analysis Panel Kit (Dian Diagnostic, Hangzhou, China), which includes SNV/InDel for 10 genes (coding sequence of BRAF, HRAS, KRAS, NRAS, TP53, RET, NTRK1, NTRK3, PAX8, THADA), promote region of the TERT gene, and 5 frequently rearranged genes in thyroid carcinoma (RET, NTRK1, NTRK3, PAX8, and THADA), according to the standard operating procedure. The libraries were then sequenced on an Illumina NextSeq 500 platform (Illumina, San Diego, CA).
FASTQ sequencing files were aligned to the human reference genome (UCSC hg19; Feb 2009 release) with Burrows-Wheeler Aligner Tool (BWA, version 0.7.17-r1188). STAR-Fusion (version 1.12.0) and Arriba software (version 2.1.0) were used to detect gene fusions. The candidate gene fusion transcripts were reviewed and visualized in Integrative Genome Viewer (IGV, Broad Institute, version 2.13.0). Given the unavailability of germline sequencing data, we employed a rigorous filtering strategy to eliminate potential germline variants. Variants present in one or more population databases (ESP, 1000Genome, ExAC, gnomAD) with MAF ≥ 1% were excluded. Variants classified as “benign” or “like benign” in the ClinVar database were also excluded.
We built linear regression to determine if the NGS combined with cytology can predict the benign and malignant thyroid nodules. Sensitivity (Sen), specificity (Spe), positive predictive value (PPV), and negative predictive value (NPV) were analyzed with reference to the receiver operating characteristic curve (ROC). The area under the receiver operating characteristic curve (AUC) was plotted to assess the diagnostic performance. Statistical analyses were performed using VassarStats, an open-source statistical tool. Studies have shown that 95% confidence intervals (CIs) provide good coverage over time. And our research spanned 18 months, so the confidence interval was reported as a two-sided 95%.
359 FNA biopsy samples of thyroid nodules were analyzed in this work, and an NGS-bases multi-gene analysis was ordered to detect genomic alterations. The position and size of the thyroid nodule were determined by ultrasonography, and the thyroid cytopathologic diagnosis was established based on the Bethesda System. The final diagnosis was based on histopathology (if the patient underwent surgery). The clinical data of the patients is shown in Table 1. The age of patients ranged from 19 to 79 years (mean 46 years), and the ratio of females to males was 2.55:1. All samples have previously undergone cytopathology. 33 (9.19%) samples were classified as non-diagnostic (Bethesda I), 149 (41.50%) as benign (Bethesda II), 47 (13.10%) as atypia of indeterminate significance/follicular lesion of indeterminate significance (AUS/FLUS, Bethesda III), 7 (1.95%) as follicular neoplasm/suspicious for follicular neoplasm (FN/SFN, Bethesda IV), 17 (4.74%) as suspicious for malignancy (SUSP, Bethesda V), and 123 (29.53%) as malignant (Bethesda VI). Following an 18-month follow-up, 113 out of 359 patients received surgical resection, in which 98 patients were diagnosed as having malignant thyroid tumors, including 95 PTC, 1 FTC, 1 MTC, and 1 differentiated high-grade thyroid carcinoma (DHGTC).
To assess the diagnostic efficiency of NGS for malignant nodules, we compared the diagnostic sensitivity and specificity of cytology and NGS combined with cytology. The diagnostic sensitivity of NGS combined with cytology increased from 0.7245 (95% CI: 0.6289-0.8032) to 0.898 (95% CI: 0.8223-0.9437), while the positive predictive value (PPV) and negative predictive value (NPV) showed no statistically significant change (Table 2). The patients with RAS mutations enrolled in this study were the reason for the significant decrease in specificity, as thyroid nodules with RAS mutations belong to the intermediate-risk category. However, a ROC curve was calculated with an AUC of 0.8303 (P < 0.001) (Figure 1), indicating an excellent diagnostic performance of the NGS combination cytology.
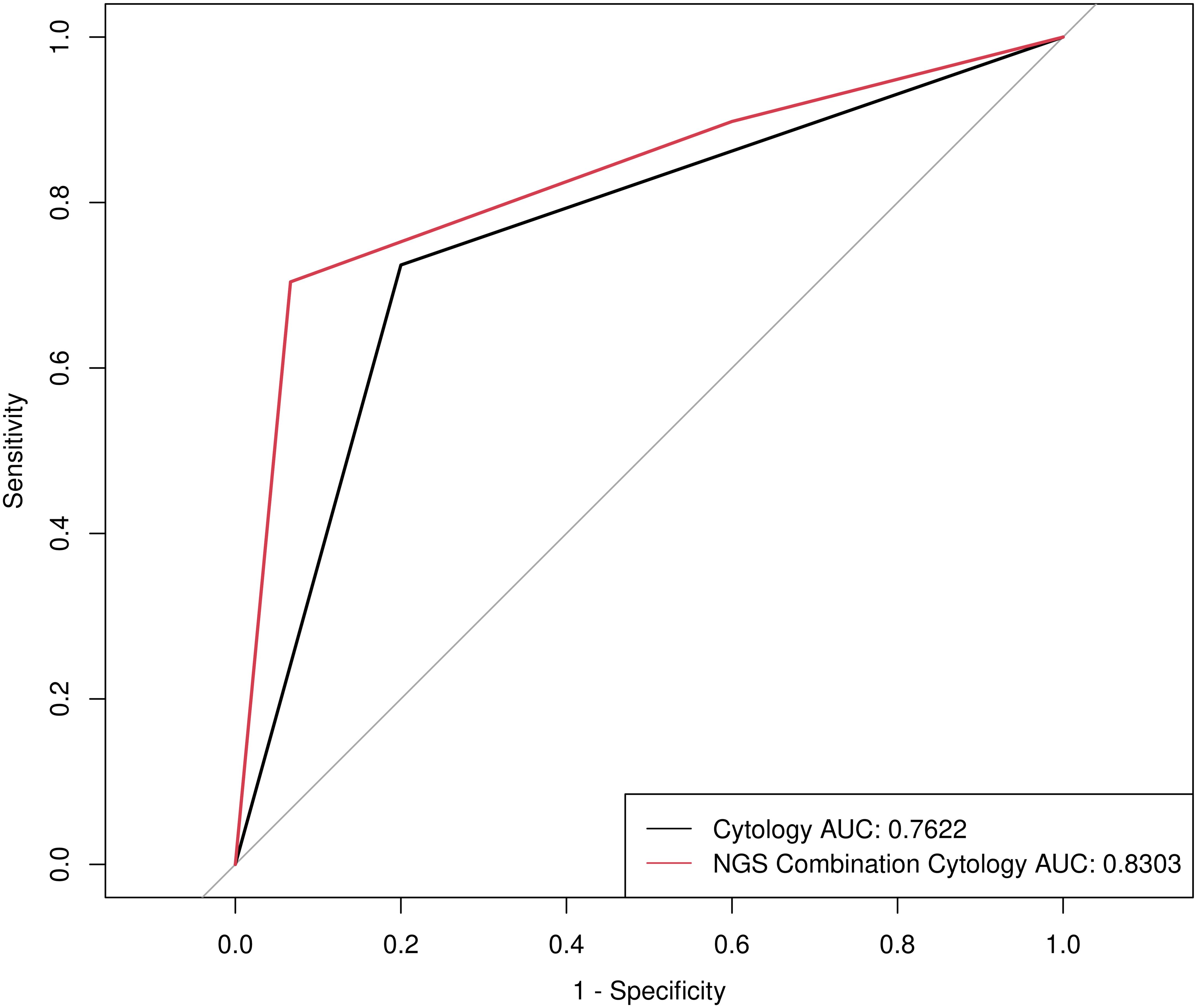
Figure 1. Receiver operating characteristic (ROC) curve for determining benign and malignant thyroid nodules by Cytology and NGS combination Cytology.
In this study, 113 patients underwent surgical resection, 84 of whom had thyroid nodules <10 mm. In comparison to cytology alone, the diagnostic sensitivity of NGS combined with cytology demonstrated an increase from 0.75 (95% CI: 0.6422-0.8337) to 0.9211 (95% CI: 0.8383-0.9633). It is proposed that the combination of NGS and cytology may significantly enhance the precision of preoperative diagnosis of thyroid nodules with a diameter of <10 mm. Consequently, it can be concluded that the NGS can markedly improve the detection rate of malignant thyroid nodules in comparison to that by cytology.
NGS-based multi-gene testing panel was used to analyze the molecular characteristics of 359 FNA biopsy samples of thyroid nodules (Table 3). With regard to the 113 patients who had thyroid nodule resection operations and the 246 patients who did not, all Bethesda classifications were covered.

Table 3. Molecular features of 359 FNA biopsy samples of thyroid nodules by analysis of NGS based multi-gene testing panel.
The most prevalent harmful mutation in those samples was BRAFV600E. 136 patients with thyroid nodules carrying BRAFV600E were identified from 359 FNA biopsy samples. Of these, 79 underwent surgery and were pathologically diagnosed as suffering from papillary thyroid carcinoma (PTC), including two nodules with a size ≤10 mm and FNA cytology classified as non-diagnostic results (Bethesda I). 56 patients in this cohort of patients who did not underwent surgery carried BRAFV600E, of which 71.43% (n = 40) were suspicious for malignancy or malignant thyroid nodules diagnosed by FNA cytopathology and classified as Bethesda V/VI.
5 patients with thyroid nodules carried molecular characteristics such as TERT promoter mutations (n = 1) and BRAF/RAS co-mutations with other genes (BRAFV600E +TP53 n = 1, KRAS+TP53 n = 1, HRAS+TERT n = 1, BRAFV600E +TERT n = 1), which are known to indicate an increased risk of malignancy in thyroid cancer. 4 out of these patients underwent surgery, and surgical pathology results indicate malignant nodules, including 2 PTC, 1 DHGTC, and 1 FTC. A rare type of DHGTC was confirmed histopathologically in a patient with a TERT promoter variation based on a resected nodule exhibiting ≥5 mitoses per 2 mm2 and/or tumor necrosis. Despite the cytology indicating a classification of Bethesda II, a patient with co-mutations in HRAS and TERT was identified as having a widely invasive FTC through the surgical pathology method. The prognosis of FTC is generally worse than that of PTC, and widely invasive FTC is the subtype with the worst prognosis.
RAS variants were detected in 27 FNA biopsy samples. Of these, the NRAS mutations of interest were all exon3 p.Q61X; the HRAS and KRAS variants found were the common activating mutations p.G12X, p.G13X, and p.Q61X. 10 of these patients received surgical treatment (NRAS mutations n = 8, KRAS mutations n = 2). Among these 10 patients, 3 were diagnosed with PTC, and 7 had benign conditions. Interestingly, none of the PTCs had harmful KRAS mutations; all of them only carried deleterious NRAS mutations. It’s probable that harmful NRAS variants are more useful for diagnosis than KRAS variants.
Further pathogenic variations found in patients who did not undergo surgery include RET activating mutations (n = 1), RET fusions (n = 3), and TP53 inactivating mutations (n = 3). Elevated calcitonin in a patient with a RET activating mutation was clinically thought to be MTC.
183 patients harbored variants of uncertain significance or negative results, according to genetic tests. 20 (10.93%) out of the 183 underwent surgery, and 12 were diagnosed as malignant thyroid tumors, including 11 PTC and 1 MTC.
Presently, the application of NGS-based multi-gene testing and molecular markers in conjunction with cytologic diagnosis has shown promising results in improving the preoperative diagnosis of indeterminate thyroid nodules, consequently decreasing the number of needless surgeries. Multi-gene assays with favorable Sen, Spe, PPV, and NPV are becoming more and more common. In an Indian study, NGS-based panel testing including six genes was performed on FNA biopsy samples from 69 thyroid nodules patients, with a sensitivity of 81.5% (11). Additionally, a study conducted in China included 73 FNA biopsy samples, with a sensitivity of 85.5% by NGS-based panel testing, which included sixteen gene detections (12). While the diagnostic sensitivity of NGS combined with cytology in this study is 89.8% (95% CI: 0.8223-0.9437).
We also found that a significant portion of previous studies carried out up to this point have concentrated on thyroid nodules with cytological indeterminacy, possibly omitting portions of nodules that have been cytopathologically classified as Bethesda I and II. Although these nodules are classified as non-diagnostic or benign on cytopathology, there is a possibility that they may be malignant. In the present research, two patients were cytopathologically classified as Bethesda I; however, our NGS-based multi-gene test of the FNA biopsy samples revealed a BRAFV600E mutation. In addition, the resected nodule samples were eventually surgically pathologically classified as PTC. It was known that NGS-based multi-gene panel testing needed fewer samples than that of cytopathology, validating that the NGS-based multi-gene panel would correctly identify some of the samples that cytopathology was unable to detect.
Additionally, all patients with BRAFV600E who underwent surgery were identified as PTC by surgical pathology, which indicates a sensitivity of 1 (95% CI: 0.9531-1). These findings align with previous research; certain mutations, such as BRAFV600E and TERT, exhibit a nearly 100% risk of PTC and are very specific (10, 13). Regardless of the cytopathological Bethesda classification, patients with thyroid nodules who have previously had an FNA biopsy sample tested using an NGS-based multi-gene panel should be alerted that the nodule is PTC if the result is a BRAFV600E mutation.
Single TERT promoter variants, BRAFV600E or RAS mutations and TERT promoter mutations or TP53 inactivating mutations co-mutations will reduce progression-free survival and are highly associated with aggressive tumor cells and a high rate of recurrence (6, 10, 14–16). The following variants were linked to a poor prognosis in our study: 1 TERT promoter, 1 BRAFV600E +TP53, 1 KRAS+TP53, 1 HRAS+TERT, 1 BRAFV600E +TERT. By surgical pathology, 4 of these patients were diagnosed with malignant thyroid nodules, including 2 PTC, 1 FTC, and 1 DHGTC. The surgical pathological diagnosis results confirmed that the aforementioned mutations found by NGS were, in fact, closely associated with malignant thyroid nodules. Patients should be reminded to schedule surgical resection as soon as possible and to think about broadening the scope of surgery when any of the aforementioned high-risk mutations are detected.
RAS is a family of GTP-binding proteins. It is believed that activating mutations in RAS play a crucial role in the initiation of follicular thyroid carcinoma (FTC) (17). RAS mutations have been reported to occur in 28-68% of FTCs and up to 43% of follicular variant PTCs (18), as well as in 20-25% of follicular adenomas (19). The PPV of RAS mutations is approximately 30%, according to numerous studies conducted to date (20, 21). According to the NGS-based multi-gene panel testing, the PPV of RAS mutations was 0.3 (95% CI: 0.1078-0.6032) in our cohort of surgical patients, while the PPV of FNA cytopathology was 0.2 (95% CI: 0.0567-0.5098). Of the patients with RAS mutations in the cohort of non-surgical patients, 82.35% were classified as Bethesda I/II. This highlights the limitations of predicting the prognosis of TC solely based on RAS mutations. RAS mutations are therefore regarded as intermediate-risk variants. Both benign and malignant thyroid diseases can have RAS mutations. The doctors should carefully evaluate whether surgery or additional follow-up is necessary for the patients with RAS mutations according to their other clinical symptoms.
Additionally, RET activating mutations, RET fusions, and TP53 inactivating mutations were found by our NGS-based multi-gene panel testing. However, since the patients did not undergo surgery, the benign and malignant nodules could not be identified. According to the aggressiveness of MTCs carrying RET mutations research has been conducted to divide RET mutations into three risk groups: highest-, high-, and moderate-risk mutations (22, 23). In the aforementioned studies, RETC634R, which was detected in our statistical sample, was categorized as high-risk. And RET fusions are substantially more common than NTRK fusion, BRAFV600E and RAS mutations in aggressive tumor behavior, which includes high rates of lymph node and distant metastases (24). Inactivating mutations in TP53 are the genetic hallmarks of ATC. Recent research discovered that 86% of resected nodules with isolated TP53 mutations were benign, while 82% of nodules carrying TP53 mutations along with other gene alterations were cancerous; although the majority of thyroid nodules containing a single TP53 genetic variant have been shown to be benign adenocarcinomas, there has been speculation that TP53-mutant adenocarcinomas could be precursors to malignancies that have not yet differentiated (25). Thus, we thought thyroid nodules containing TP53 inactivating mutations required special attention, while RET activating mutations and RET fusions are closely linked to malignant thyroid nodules.
Among the 183 patients with negative genetic test results or variants of uncertain significance, 20 patients underwent surgery, and 12 patients were diagnosed with malignant thyroid tumors. Of the 12 malignant tumors, 10 presented negative results from genetic testing. It is reasonable to anticipate that an NGS-based multi-gene panel utilizing FNA biopsy samples will yield a negative result or a variant of uncertain significance. Thus, the potential for deterioration needs to be emphasized even in thyroid nodules identified by NGS-based multi-gene probes as carrying non-pathogenic variants. Furthermore, the DNA from HE-stained sections appears to be a useful source of DNA for routine DNA testing (26). In clinical practice, FNA biopsy samples can be obtained for HE staining and cytopathologic analysis. After that, the HE-stained slides are sent for NGS testing, thereby reducing the results of negative or variants of uncertain significance. Concurrently, through the observation of additional clinical indicators, clinicians can ascertain whether patients with negative or variants of uncertain significance require surgical intervention or regular clinical evaluation. The most prevalent clinical indicators at present are the ultrasound-described nodule size, the nodule boundary’s clarity, the nodule’s calcification content, and the type of blood supply of nodules.
There are a few limitations pertinent to this study, with a limited number of genes included in the panel due to cost. The current research about the incidence, metastasis, and prognosis of thyroid cancer indicated that PIK3CA, PTEN, CDKN1B, TSHR, and EIF1AX were also involved, which should be considered in the multi-gene panel (12, 27). It is important to recognize the limitations of FNA biopsy samples for NGS-based panel testing. The following aspects require particular consideration: tumor heterogeneity, insufficient sampling, false negative risk, sample contamination, and puncture technical issues. In addition, with longer follow-up, more patients’ surgical pathology results may be available (but there was still a reasonable number of cases), making the results more convincing. This study is a single-center clinical trial and showed slightly larger intervention effects than did multi-center trials. However, we were successfully able to identify that the thyroid nodules with BRAFV600E, TERT promoter mutations, BRAF/RAS co-mutation with other genes (e.g., KRAS+TP53, BRAFV600E+TP53, HRAS+TERT, BRAFV600E+TERT), RET activating mutations, and RET fusions belonged to the high-risk group. The intermediate-risk group includes the RAS mutations and TP53 inactivating mutations, while the non-pathogenic (negative and variants of uncertain significance) mutations identified were classified as low-risk. Our study stratifies and assesses the risk of thyroid nodules based on specific gene mutations, which can offer patients more individualized diagnostic reference recommendations. However, in the traditional genetic test category, the results are expressed as pathogenic or negative. A flow chart was constructed, and a summary of the clinical workflow for the management of thyroid nodules based on cytopathology and NGS-based panel test results was produced (Figure 2).
When it comes to risk stratification and the ability to distinguish benign from malignant thyroid nodules, NGS-based multi-gene panel testing has demonstrated diagnostic performance that exceeds expectations (28–30). The current goals of the present research on the molecular characteristics of thyroid nodules are to improve the ability to distinguish between benign and malignant thyroid nodules and to forecast their clinical course. A further step in patient risk stratification is extending the follow-up period for patients with thyroid nodules. Given that the genetic variant map of TC is gradually being shown in greater detail, the diagnostic performance and application of NGS-based multi-gene panel testing are also anticipated.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Institutional Review Board of The Second Affiliated Hospital of Jiaxing University (Grant No. 2021AD30114). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MF: Conceptualization, Writing – original draft. DD: Conceptualization, Data curation, Writing – original draft. XO: Resources, Writing – review & editing. WS: Project administration, Writing – review & editing. FZ: Conceptualization, Data curation, Writing – review & editing. BZ: Resources, Writing – review & editing. LQ: Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Authors DD, FZ, and LQ were employed by Dian Diagnostics Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cheng F, Xiao J, Shao C, Huang F, Wang L, Ju Y, et al. Burden of thyroid cancer from 1990 to 2019 and projections of incidence and mortality until 2039 in China: findings from global burden of disease study. Front Endocrinol. (2021) 12:738213. doi: 10.3389/fendo.2021.738213
2. Wang J, Yu F, Shang Y, Ping Z, Liu LJE. Thyroid cancer: incidence and mortality trends in China, 2005–2015. Endocrine (2020) 68:163–73. doi: 10.1007/s12020-020-02207-6
3. Cancer statistics - surveillance, epidemiology, and end results program. Available online at: https://seer.cancer.gov/statfacts/html/thyro.html (accessed January 23, 2024).
4. 2024 American Cancer Society, Inc. Available online at: https://www.cancer.org/cancer/types/thyroid-cancer/about/what-is-thyroid-cancer.html (accessed January 23, 2024).
5. Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: a review. JAMA (2018) 319(9):914–24. doi: 10.1001/jama.2018.0898
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
7. Agrawal N, Akbani R, Aksoy BA, Ally A, Arachchi H, Asa SL, et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 159(3):676–90. doi: 10.1016/j.cell.2014.09.050
8. Vignali P, Macerola E, Poma AM, Sparavelli R, Basolo FJD. Indeterminate thyroid nodules: from cytology to molecular testing. Diagnostics (2023) 13(18):3008. doi: 10.3390/diagnostics13183008
9. Du Y, Zhang S, Zhang G, Hu J, Zhao L, Xiong Y, et al. Mutational profiling of Chinese patients with thyroid cancer. Front Endocrinol. (2023) 14:1156999. doi: 10.3389/fendo.2023.1156999
10. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. (2014) 32(25):2718. doi: 10.1200/JCO.2014.55.5094
11. Vishwanath D, Shanmugam A, Sundaresh M, Hariharan A, Saraf S, Bahadur U, et al. Development of a low-cost NGS test for the evaluation of thyroid nodules. Indian J Surg Oncol. (2019) 13:17–22. doi: 10.1007/s13193-019-01000-w
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Tan L-C, Liu W-L, Zhu X-L, Yu P-C, Shi X, Han P-Z, et al. Next-generation sequencing enhances the diagnosis efficiency in thyroid nodules. Front Oncol. (2021) 11:677892. doi: 10.3389/fonc.2021.677892
13. Liu R, Xing M-r. Diagnostic and prognostic TERT promoter mutations in thyroid fine needle aspiration biopsy. Endocrine-Related Cancer (2014) 21(5):825. doi: 10.1530/ERC-14-0359
14. Wang JR, Montierth M, Xu L, Goswami M, Zhao X, Cote G, et al. Impact of somatic mutations on survival outcomes in patients with anaplastic thyroid carcinoma. JCO Prec Oncol. (2022) 6. doi: 10.1200/PO.21.00504
15. Alzahrani AS, Alsaadi R, Murugan AK, Sadiq BB. TERT promoter mutations in thyroid cancer. Endocrine-Related Cancer (2016) 7:165–77. doi: 10.1007/s12672-016-0256-3
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Mayson SE, Haugen BRJE, Clinics M. Molecular diagnostic evaluation of thyroid nodules. Endocrinol Metab Clin. (2019) 48(1):85–97. doi: 10.1016/j.ecl.2018.10.004
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
17. Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol. (2020) 11:102. doi: 10.3389/fendo.2020.00102
18. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. (2003) 63(7):1454–7.
19. Xing M. Clinical utility of RAS mutations in thyroid cancer: a blurred picture now emerging clearer. BMC Med. (2016) 14:1–4. doi: 10.1186/s12916-016-0559-9
20. Guan H, Toraldo G, Cerda S, Godley FA, Rao SR, McAneny D, et al. Utilities of RAS mutations in preoperative fine needle biopsies for decision making for thyroid nodule management: results from a single-center prospective cohort. Thyroid (2020) 30(4):536–47. doi: 10.1089/thy.2019.0116
21. Bellevicine C, Migliatico I, Sgariglia R, Nacchio M, Vigliar E, Pisapia P, et al. Evaluation of BRAF, RAS, RET/PTC, and PAX8/PPARg alterations in different Bethesda diagnostic categories: a multicentric prospective study on the validity of the 7heidi panel test in 1172 thyroid FNAs deriving from different hospitals in South Italy. Cancer Cytopathol. (2020) 128(2):107–18. doi: 10.1002/cncy.v128.2
22. Wells SA Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the American Thyroid Association Guidelines Task Force on medullary thyroid carcinoma. Thyroid (2015) 25(6):567–610. doi: 10.1089/thy.2014.0335
23. Milićević S, Bergant D, Žagar T, Perić B. Crude annual incidence rate of medullary thyroid cancer and RET mutation frequency. Croatian Med J. (2021) 62(2):110–9. doi: 10.3325/cmj.2021.62.110
24. Pekova BB, Sykorova V, Mastnikova K, Vaclavikova E, Moravcova J, Vlcek P, et al. RET fusion genes in pediatric and adult thyroid carcinomas: cohort characteristics and prognosis. Endocrine-Related Cancer. (2023) 30(12). doi: 10.1530/ERC-23-0117
25. Nikitski AV, Nikiforova MN, Yip L, Karslioglu-French E, Carty SE, Nikiforov YE. Can TP53-mutant follicular adenoma be a precursor of anaplastic thyroid carcinoma? Endocrine-Related Cancer (2021) 28(9):621–30. doi: 10.1530/ERC-21-0095
26. Morikawa T, Shima K, Kuchiba A, Yamauchi M, Tanaka N, Imamura Y, et al. No evidence for interference of H&E staining in DNA testing. Am J Clin Pathol. (2012) 138:122–9. doi: 10.1309/AJCP28LAOOKSZSVW
27. Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, et al. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid (2015) 25(11):1217–23. doi: 10.1089/thy.2015.0305
28. Xiong Y, Li X, Liang L, Li D, Yan L, Li X, et al. Application of biomarkers in the diagnosis of uncertain samples of core needle biopsy of thyroid nodules. Virchows Archiv (2021) 479:961–74. doi: 10.1007/s00428-021-03161-y
29. Song Y, Zhang B, Ma T. Highly accurate NGS-based multi-gene testing in the diagnosis of thyroid nodules with indeterminate cytology. Am Soc Clin Oncol. (2020) 38(15_suppl). doi: 10.1200/JCO.2020.38.15_suppl.e13579
30. Silaghi CA, Lozovanu V, Georgescu CE, Georgescu RD, Susman S, Năsui BA, et al. Thyroseq v3, Afirma GSC, and microRNA panels versus previous molecular tests in the preoperative diagnosis of indeterminate thyroid nodules: a systematic review and meta-analysis. Front Endocrinol. (2021) 12:649522. doi: 10.3389/fendo.2021.649522
Keywords: thyroid nodules, next-generation sequencing, fine-needle aspiration, molecular diagnostics, risk stratification
Citation: Fei M, Ding D, Ouyang X, Shen W, Zhang F, Zhang B and Qin L (2024) The value of NGS-based multi-gene testing for differentiation of benign from malignant and risk stratification of thyroid nodules. Front. Oncol. 14:1414492. doi: 10.3389/fonc.2024.1414492
Received: 09 April 2024; Accepted: 23 October 2024;
Published: 12 November 2024.
Edited by:
Akira Sugawara, Tohoku University, JapanReviewed by:
Fumihiko Furuya, Fukushima Medical University, JapanCopyright © 2024 Fei, Ding, Ouyang, Shen, Zhang, Zhang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhang, bGFuY2V0MTEzQDEyNi5jb20=; Lan Qin, cWlubGFuQGRhemQuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.