
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 28 June 2024
Sec. Cancer Epidemiology and Prevention
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1413590
 Bo-Guen Kim1†
Bo-Guen Kim1† Hyun Lee1†
Hyun Lee1† Sun-Kyung Lee1,2
Sun-Kyung Lee1,2 Sun Young Paik1
Sun Young Paik1 Seo-Hyoung Yun3
Seo-Hyoung Yun3 Chang-Joo Park3
Chang-Joo Park3 Yoomi Yeo1
Yoomi Yeo1 Tai Sun Park1
Tai Sun Park1 Ji-Yong Moon1
Ji-Yong Moon1 Tae-Hyung Kim1
Tae-Hyung Kim1 Jang Won Sohn1
Jang Won Sohn1 Sang-Heon Kim1
Sang-Heon Kim1 Ho Joo Yoon1‡*
Ho Joo Yoon1‡* Dong Won Park1‡*
Dong Won Park1‡*Background: The impact of long-term chronic periodontal conditions on the risk of lung cancer could not be accurately evaluated. Our aim was to provide more evidence on the connection between chronic periodontitis (CP) and lung cancer using a nationwide dataset.
Methods: This study used data from the Korean National Health Insurance Service National Sample Cohort. We enrolled 72,658 individuals with CP (CP cohort) between 2005 and 2019 and 1:1 age- and sex-matched controls without CP (non-CP cohort).
Results: During the median follow-up period of 5.1 (interquartile range, 2.8–8.0) years, 0.56% (n = 405/72,658) of the CP cohort and 0.29% (n = 212/72,658) of the matched non-CP cohort developed lung cancer, with incidence rates of 8.3 and 4.5 per 10,000 person-years. The risk of incident lung cancer was significantly higher in the CP cohort than in the matched non-CP cohort (adjusted hazard ratio = 2.27, 95% confidence interval = 1.94–2.65). The risk of incident lung cancer was 2.45-fold and 2.10-fold higher in mild and moderate-to-severe CP cohorts than in the matched non-CP control. The risk of incident lung cancer was especially higher in the 40–59 age group, females, and never-smokers than their counterparts.
Conclusion: We demonstrate that the risk of incident lung cancer is higher in individuals with CP than in those without. The risk of lung cancer was especially high in individuals with more severe CP, females, never-smokers, and obese populations.
Periodontal disease stems from the infection and inflammation affecting the supportive and anchoring tissues for teeth. This disease encompasses a range from mild gingivitis to more harmful periodontitis, marked by severe degradation of attachment structures like the alveolar bone and periodontal ligament, often resulting in tooth loss (1). Approximately 10% of the global population suffers from severe periodontitis (2).
Research indicates that periodontal disease, which is highly prevalent and affects approximately 90% of the world’s population (3), is correlated with an increased risk of lung cancer (4–6), even when accounting for smoking (5, 7, 8), which contributes to both periodontal disease and lung cancer. Interestingly, when evaluating this issue, in most previous studies, various types of periodontal disease, including acute periodontitis were included (1, 4–10). However, considering longstanding inflammatory conditions are more likely to be linked to carcinogenesis (11), it might be more plausible to focus on the impact of “chronic” periodontal diseases on lung cancer risk rather than evaluating any types of periodontal diseases as a whole. In addition, since most studies were conducted in Western societies (USA, Sweden, or Greece), little information is available in Asian societies (1, 10).
Accordingly, our objective was to contribute further evidence regarding the association between CP and lung cancer incidence within an Asian population by conducting an extensive analysis of a nationwide large dataset. To examine the impact of chronic inflammation and infection resulting from periodontal diseases on the incidence of lung cancer, we specifically focused on chronic periodontitis (CP) among various periodontal conditions. Additionally, we explored how the severity of CP influences the occurrence of lung cancer.
This study used data from the Korean National Health Insurance Service National Sample Cohort (NHIS-NSC), including a 2.2% representative sample of Korean citizens. In Korea, NHIS, a universal insurance provider managed by the government covers 97% of the Koran population, approximately 50 million citizens (12). The NHIS-NSC dataset includes information on demographic and socioeconomic variables (e.g., age, sex, income status, residential area), healthcare utilization, health screening examination findings, disease diagnosis under International Classification of Diseases-10th Revision (ICD-10) codes, drug prescription, and death. The NHIS-NSC has been widely used in various epidemiologic studies (13).
This study was approved by the Institutional Review Board of Hanyang University Hospital (IRB No. HYUH 2022-09-031). The requirement of informed consent from the participants was waived because the NHIS database was constructed after anonymization.
A total of 1,137,861 patients were identified between January 1, 2002, and December 31, 2019. We excluded 147,520 patients who received a diagnosis of any periodontitis between January 1, 2002, and December 31, 2004. Among the remaining 990,341 patients, 565,835 patients had at least one new CP diagnosis code and 424,506 patients did not have CP diagnosis code. The index date was defined as the date when the patients received the first CP diagnosis code.
To establish the CP cohort, 147,049 patients having CP diagnosis codes twice per year and at least one treatment code within 1 year of the first diagnosis were further selected. Among those, patients who were younger than 20 years (n = 5,185) and those who were diagnosed with any type of cancer before the index date (n = 6,520) were excluded. In addition, patients who were diagnosed with lung cancer within 1 year after the index date (n = 72), those who died within 1 year after the index date (n = 208), and those who had any missing health screening data (n = 50,052) were also excluded from the CP cohort. A total of 85,012 patients were enrolled in the CP cohort.
To establish the non-CP cohort, of the initial pool of 424,506 patients without CP diagnosis code, we excluded the patients who met the same exclusion criteria applied to the CP cohort (i.e., under 20 years of age, any cancer before the index date, death or lung cancer diagnosis within 1 year after the index date, and health screening data unavailable). A total of 138,445 patients were enrolled in the non-CP cohort.
Thereafter, we performed 1:1 matching between the CP and non-CP cohorts based on age and sex and finally enrolled 72,658 patients for the CP and non-CP cohorts (Figure 1). Individuals were followed up for 1 year after study enrolment to the date of lung cancer diagnosis, death, or Dec 2019.
The exposure of this study was periodontitis, of which the definition required 1) diagnosis codes with periodontitis ICD-10 code (K05.3) twice per year and 2) at least one treatment code (U2232, U2233, U2240, U1010, U4411, U4412, U1051, U1052, U1701, U1702, U1081, U1082, and U1083) within 1 year of the first diagnosis.
The CP cohort was divided into 3 groups according to the severity of periodontitis (mild, moderate, and severe CP), and the severity of periodontal disease was classified according to CP-related treatment (14). Patients with periodontitis who underwent scaling (U2232, U2233) and root planning (U2240) were classified into the mild CP group, while patients who only received subgingival curettage (U1010) were classified into the moderate periodontitis group. Patients who underwent tooth extraction and severe dental treatment such as tooth extraction (U4411, U4412), periodontal flap operation (U1051, U1052), bone graft for alveolar bone defects (U1071, U1702), and guided tissue regeneration (U1081, U1082, U1083), were assigned to the severe CP group.
The primary outcome of this study was newly diagnosed lung cancer during the follow-up period. Lung cancer was defined by an ICD-10 code of C34. The study population was followed from 1 year after the index date to the date of lung cancer event, date of death, or until the end of the study period (December 31, 2019), whichever came first.
Household income was categorized into quartiles based on insurance premium levels (in Korea, insurance premiums are determined by income level), with those covered by Medical Aid (poorest 3%) being merged into the lowest income quartile (15–17). The lowest income quartile group was defined as “low income”. Detailed information on the patient’s smoking status was obtained through self-reported questionnaires. Smoking status was categorized into never, former, and current smokers. Body mass index (BMI) was calculated as the participant’s body weight (kg) divided by the square of the participant’s height (m2) and was categorized as low (<18.5), normal (18.5–22.9), overweight (23–24.9), and obese (≥25) according to the Asia-Pacific BMI criteria established by the World Health Organization. The Charlson Comorbidity Index (CCI) was categorized as 0, 1 to 2, or ≥ 3 to assess the overall comorbidity load (18).
The baseline characteristics are presented as mean ± standard deviation (SD) for continuous variables and numbers with percentages for categorical variables. We compared the two groups using the χ2 test for categorical variables, and t-tests for continuous variables. The incidence rates of lung cancer were estimated as the number of events per 10,000 person-years. The cumulative incidence of lung cancer was presented using the Kaplan-Meier curve.
Cox proportional hazards regression analyses were used to evaluate the risk of incident lung cancer in the CP cohort versus the matched non-CP cohort. Model 1 was unadjusted, and Model 2 was adjusted for BMI and smoking status. Model 3 was further adjusted for low income, and CCI in addition to the variables adjusted in Model 2. Stratified analyses were performed based on age, sex, smoking status, BMI, and CCI. A two-sided p-value < 0.05 was considered statistically significant, and all analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and graphs were generated using R software version 4.2.1 (Vienna, Austria).
The baseline characteristics of the study population are summarized in Table 1. The mean age of the study population was 46.5 years (SD, 13.4 years) and 50.8% were males.
In terms of age and sex, both groups were well-balanced (p > 0.999). The proportion of ex-smokers, obesity, and comorbidities except for chronic kidney disease (p = 0.281), was significantly higher in the CP cohort than in the non-CP cohort (p < 0.001 for all). Additionally, the CCI score was higher in the CP cohort than in the non-CP cohort (p < 0.001, p for trend < 0.001).
In the CP cohort, the proportion of mild, moderate, and severe CP was 52.7% (n = 38,292), 35.9% (n = 26,073), and 11.4% (n = 8,293), respectively.
During the median follow-up period of 5.1 (interquartile range, 2.8–8.0) years, 0.56% (405/72,658) of the CP cohort and 0.29% (212/72,658) of the matched non-CP cohort developed lung cancer, with incidence rates of 8.3 and 4.5 per 10,000 person-years, respectively. Even after adjusting for potential confounders, the risk of incident lung cancer was also significantly higher in the CP cohort than in the matched non-CP cohort: unadjusted hazard ratio (HR) = 2.06, 95% confidence interval [CI] = 1.80–2.37; adjusted HR in Model 2 = 2.29, 95% CI = 1.96–2.67; adjusted HR in Model 3 = 2.27, 95% CI 1.94–2.65) (Table 2). Similarly, a cumulative incidence plot depicts a significantly higher incidence of lung cancer in the CP cohort than in the matched non-CP cohort (a log-rank p < 0.001; Figure 2A).
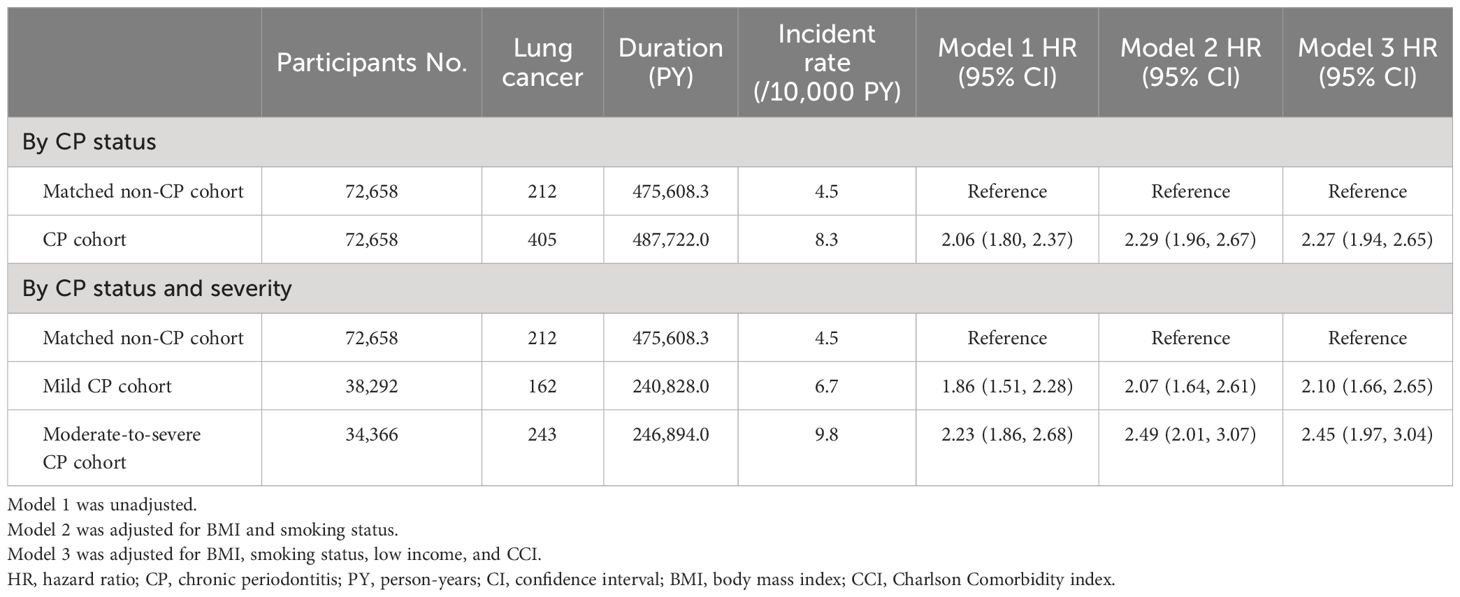
Table 2 Subdistribution incidence and HRs for Incident lung cancer in the CP and matched non-CP cohort.
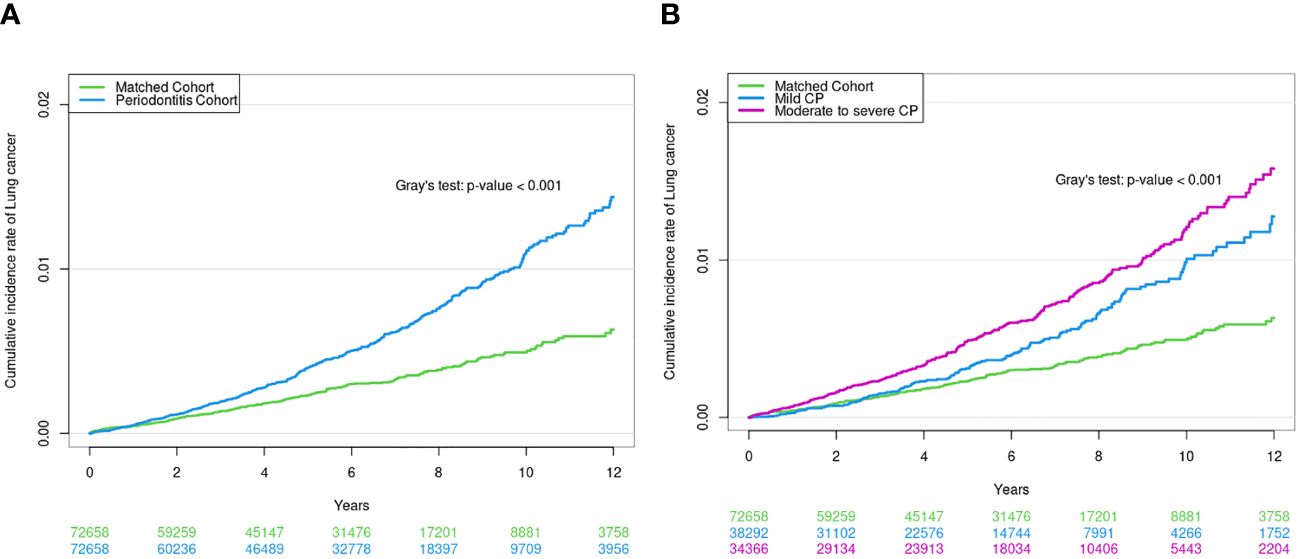
Figure 2 (A) Cumulative incidence of lung cancer in CP cohort and matched non-CP cohort. (B) Cumulative incidence of lung cancer according to CP severity.
When CP was classified according to severity (control vs. mild vs. moderate-to-severe), 0.42% (162/38,292) of the mild CP cohort and 0.71% (243/34,366) of the moderate-to-severe CP cohort developed lung cancer, with incidence rates of 6.7 and 9.8 per 10,000 person-years, respectively. The risk of incident lung cancer was 2.10-fold (95% CI, 1.66–2.65) and 2.45-fold (95% CI, 1.97–3.04) higher in mild and moderate-to-severe CP cohorts than in matched non-CP controls (fully adjusted Model 3), respectively (Table 2). The cumulative incidence plot depicts similar results (a log-rank p < 0.001; Figure 2B).
Stratified analyses regarding the risk of lung cancer among patients with CP compared with subjects without CP are shown in Table 3. Age, sex, and smoking had a significant interaction on the association of CP with lung cancer development (p for interaction < 0.001 for all). The risk of incident lung cancer was higher in the 40–59 age group, females, and never-smokers compared to their counterparts.
This study provides longitudinal evidence from an Asian population, using nationwide data with a median follow-up of 5 years to explore the association between CP and lung cancer risk. We investigated whether CP is a predisposing factor for the development of lung cancer, and which factors are related to an increased risk of lung cancer among individuals with CP. The results showed that the CP cohort had a lung cancer incidence rate of 8.3 per 10,000 person-years, which is 2.3-fold higher compared with the matched non-CP cohort. There was a significant dose-dependent relationship between CP severity and lung cancer risk. The risk of incident lung cancer was 2.10-fold and 2.45-fold higher in mild and moderate-to-severe CP cohorts than in matched non-CP controls. Specifically, certain groups including those aged 40–59, females, and never smokers exhibited a higher susceptibility to lung cancer development.
Previous meta-analyses showed that the risk of lung cancer is 1.24 to 1.40-fold higher in individuals with periodontal disease compared to those without periodontal disease (6, 8, 19). However, in these meta-analyses, various types of periodontal disease were included. Considering longstanding inflammatory conditions are more likely to be linked to carcinogenesis, it might be more plausible to hypothesize chronic periodontal disease may be associated with increased lung cancer risk. As we assumed, the strength of the association between CP and lung cancer risk in our study (aHR = 2.3) was higher than that of previous meta-analyses as well as a previous result that was performed in a similar study population in Korea (1). Besides this issue, we also like to emphasize that there is not enough evidence on the association between periodontal disease and the risk of lung cancer in the Asian population. Since most studies included in the previous meta-analyses were conducted in Western society (4, 5, 8, 9), little information has been available on the Asian population (1, 10), needing supporting data on this population. From this view, our comprehensive analyses that considered numerous confounders (e.g., demographics, smoking status, and comorbid conditions) would be valuable by providing supporting information on this issue in Asian population.
The relationship between CP and lung cancer can be explained by the following hypotheses. First, the oral microbiome may influence lung cancer incidence. With mounting evidence highlighting the connection between human microorganisms and malignant tumors, it has become evident that a unique microbiome exists in the lungs, potentially exerting influence on the development of lung cancers (20). Recent findings from data using three prospective cohort studies in the US reinforce this notion (21). It has been proposed that these microorganisms predominantly originate from the oral microbiome through micro-aspiration of oral fluids (22). The potential mechanisms behind this phenomenon involve an increase in the inflammatory environment, which could promote carcinogenesis, microbial influences on host metabolism, and genotoxicity. Yan et al. discovered significantly elevated counts of Veillonella and Capnocytophaga species in the saliva of lung cancer patients (23). One study in ARIC study included 4,263 cancer-free participants with previously measured antibodies of oral bacteria, and 1,287 participants from whom subgingival plaque was collected (9). They reported positive associations with lung cancer for Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis counts, and significant positive associations were found for the count to antibody ratio for Prevotella intermedia and P. gingivalis (9).
P. gingivalis-stained sections exhibited significantly higher frequency and intensity in cancerous tissues of small cell lung cancer, lung adenocarcinoma, and lung squamous cell carcinoma, when compared to adjacent lung tissues (24). Furthermore, by evaluating immunoglobulin G (IgG) antibodies specific to P. gingivalis in the serum of lung cancer patients, researchers have identified a positive correlation between the antibody levels and the risk of developing lung cancer, as compared to a cohort of healthy controls (25). Similar to the findings related to P. gingivalis, a positive correlation has been identified between serum levels of IgG antibodies specific to F. nucleatum and the incidence of lung cancer (9). This alignment between antibody levels and lung cancer highlights a potential association between F. nucleatum and the pathogenesis of lung cancer.
Second, although the exact carcinogenic mechanisms remain unclear, periodontal disease, along with the release of inflammatory factors into the blood, might impact the incidence of lung cancer. One study investigated the relationship between immune surveillance mechanisms and periodontitis in cancer patients (26). In this study, the peripheral blood concentration of IL-6 was significantly higher in cancer patients than in non-cancer patients, and it was even higher in cancer patients with periodontitis compared to those without. Additionally, the peripheral blood proportion of regulatory T cells was significantly higher in cancer patients with periodontitis than in other groups (including cancer patients without periodontitis, non-cancer patients with periodontitis, and non-cancer patients without periodontitis). These results suggest that the presence of periodontitis might synergistically contribute to cancer development and progression. Other studies have also described a positive relationship between CP and systemic inflammation (27, 28), as well as the association between systemic inflammation and lung cancer development. CP leads to increased level of IL-6 as well as C-reactive protein, IFN-γ, and IL-1β (27–29). These periodontal pathogens and inflammation products enter the bloodstream, triggering a systemic inflammatory response (30). Previous studies have linked elevated levels of C-reactive protein, IL-6, IFN-γ, and IL-1β to an increased risk of lung cancer (31, 32).
In our study, a higher risk of lung cancer in the CP group versus controls was related to females and never-smokers. In females populations, it is known that gastroesophageal reflux disease (GERD) is more common than their counterparts (33); Considering CP-associated changes in oral microbiota might influence lung microbiota, which can influence the occurrence of lung cancer, through micro-aspiration of oral microbiota into the lungs. Interestingly, the risk of lung cancer in subjects with CP versus those without CP was more substantial among never-smokers. Although the reasons are not fully explainable, we carefully suggest that the impact of smoking on the development of lung cancer was more significant than that of CP, and, as a result, the influence of CP might have been relatively obscured among smokers.
We would like to suggest one important clinical implication of our study. Current lung cancer screening strategies do not reflect risk factors other than smoking status and age (34). However, numerous studies have revealed there are various important risk factors for lung cancers, such as air pollution, infectious/inflammatory conditions (e.g., tuberculosis, bronchiectasis, connective tissue diseases, chronic periodontitis, etc.) (35–37), which might be more important for lung cancer not related to smoking. From this view, our study results would be very helpful for building future lung cancer prediction models or strategies, although more studies are performed on which variables need to be included to build a cost-effective prediction model for lung cancer.
Our study has two important limitations. First, since this study was conducted in a Korean population, it may be difficult to generalize the study results to other ethnic and country groups. Second, CP, lung cancer, and comorbidities were determined using ICD-10 codes. Thus, there might be over or underestimation of the diagnoses.
In conclusion, a nationwide longitudinal database demonstrates that the risk of incident lung cancer is higher in individuals with CP than in those without, and the more severe the CP, the higher the risk of lung cancer. Additionally, our results suggest that CP may further influence lung cancer development in females and never-smokers. Providing care and treatment for CP in these populations will be particularly important.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Institutional Review Board of Hanyang University Hospital (IRB No. HYUH 2022-09-031). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin the NHIS database was constructed after anonymization.
B-GK: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Validation. S-KL: Data curation, Formal analysis, Methodology, Visualization, Writing – review & editing, Investigation, Writing – original draft. SP: Data curation, Writing – review & editing, Investigation, Validation, Writing – original draft. S-HY: Data curation, Writing – review & editing, Investigation, Writing – original draft. C-JP: Data curation, Writing – review & editing, Investigation, Writing – original draft. YY: Data curation, Writing – review & editing, Investigation, Writing – original draft. TP: Data curation, Writing – review & editing, Investigation, Writing – original draft. J-YM: Data curation, Writing – review & editing, Investigation, Writing – original draft. T-HK: Data curation, Writing – review & editing, Investigation, Writing – original draft. JS: Data curation, Writing – review & editing, Investigation, Writing – original draft. S-HK: Data curation, Investigation, Writing – review & editing, Writing – original draft. HY: Writing – original draft, Writing – review & editing, Data curation, Investigation, Methodology, Supervision. DP: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT)(No.2019M3E5D1A01069361).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, Body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; CP, Chronic periodontitis; GERD, gastroesophageal reflux disease; HR, hazard ratio; ICD-10, international Classification of Diseases-10th Revision; IgG, immunoglobulin G; NHIS-NSC, National Health Insurance Service National Sample Cohort; SD, standard deviation.
1. Kim EH, Nam S, Park CH, Kim Y, Lee M, Ahn JB, et al. Periodontal disease and cancer risk: A nationwide population-based cohort study. Front Oncol. (2022) 12:901098. doi: 10.3389/fonc.2022.901098
2. Bernabe E, Marcenes W, Hernandez CR, Bailey J, Abreu LG, Alipour V, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. J Dent Res. (2020) 99:362–73. doi: 10.1177/0022034520908533
3. Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. (2019) 394:249–60. doi: 10.1016/S0140-6736(19)31146-8
4. Michaud DS, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. (2017) 39:49–58. doi: 10.1093/epirev/mxx006
5. Michaud DS, Lu J, Peacock-Villada AY, Barber JR, Joshu CE, Prizment AE, et al. Periodontal disease assessed using clinical dental measurements and cancer risk in the ARIC study. J Natl Cancer Inst. (2018) 110:843–54. doi: 10.1093/jnci/djx278
6. Wang J, Yang X, Zou X, Zhang Y, Wang J, Wang Y. Relationship between periodontal disease and lung cancer: A systematic review and meta-analysis. J Periodontal Res. (2020) 55:581–93. doi: 10.1111/jre.12772
7. Mai X, LaMonte MJ, Hovey KM, Nwizu N, Freudenheim JL, Tezal M, et al. History of periodontal disease diagnosis and lung cancer incidence in the Women's Health Initiative Observational Study. Cancer Causes Control. (2014) 25:1045–53. doi: 10.1007/s10552-014-0405-3
8. Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. (2008) 9:550–8. doi: 10.1016/S1470-2045(08)70106-2
9. Zhou B, Lu J, Beck JD, Moss KL, Prizment AE, Demmer RT, et al. Periodontal and other oral bacteria and risk of lung cancer in the atherosclerosis risk in communities (ARIC) study. Cancer Epidemiol Biomarkers Prev. (2023) 32:505–15. doi: 10.1158/1055-9965.EPI-22-0601
10. Wen BW, Tsai CS, Lin CL, Chang YJ, Lee CF, Hsu CH, et al. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. Qjm. (2014) 107:283–90. doi: 10.1093/qjmed/hct248
11. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
12. Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. (2022) 6:9–31. doi: 10.23838/pfm.2021.00135
13. Shin DW, Cho B, Guallar E. Korean national health insurance database. JAMA Intern Med. (2016) 176:138. doi: 10.1001/jamainternmed.2015.7110
14. Kim SJ, Kim K, Choi S, Chang J, Kim SM, Park SM, et al. Chronic periodontitis and community-acquired pneumonia: a population-based cohort study. BMC Pulm Med. (2019) 19:268. doi: 10.1186/s12890-019-1017-1
15. Choi H, Han K, Yang B, Shin DW, Sohn JW, Lee H. Female reproductive factors and incidence of nontuberculous mycobacterial pulmonary disease among postmenopausal women in korea. Clin Infect Dis. (2022) 75:1397–404. doi: 10.1093/cid/ciac134
16. Lee HR, Yoo JE, Choi H, Han K, Jung JH, Park J, et al. Tuberculosis and risk of ischemic stroke: A nationwide cohort study. Stroke. (2022) 53:3401–9. doi: 10.1161/STROKEAHA.122.039484
17. Lee HR, Yoo JE, Choi H, Han K, Lim YH, Lee H, et al. Tuberculosis and the risk of ischemic heart disease: A nationwide cohort study. Clin Infect Dis. (2022) 76:1576–1584. doi: 10.1093/cid/ciac946
18. Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. (2010) 11:1. doi: 10.1186/1471-2296-11-1
19. Zeng XT, Xia LY, Zhang YG, Li S, Leng WD, Kwong JS. Periodontal disease and incident lung cancer risk: A meta-analysis of cohort studies. J Periodontol. (2016) 87:1158–64. doi: 10.1902/jop.2016.150597
20. Maddi A, Sabharwal A, Violante T, Manuballa S, Genco R, Patnaik S, et al. The microbiome and lung cancer. J Thorac Dis. (2019) 11:280–91. doi: 10.21037/jtd
21. Vogtmann E, Hua X, Yu G, Purandare V, Hullings AG, Shao D, et al. The oral microbiome and lung cancer risk: an analysis of 3 prospective cohort studies. J Natl Cancer Inst. (2022) 114:1501–10. doi: 10.1093/jnci/djac149
22. Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. (2016) 1:16031. doi: 10.1038/nmicrobiol.2016.31
23. Yan X, Yang M, Liu J, Gao R, Hu J, Li J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. (2015) 5:3111–22.
24. Liu Y, Yuan X, Chen K, Zhou F, Yang H, Yang H, et al. Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Transl Oncol. (2021) 14:100972. doi: 10.1016/j.tranon.2020.100972
25. Ampomah NK, Teles F, Martin LM, Lu J, Koestler DC, Kelsey KT, et al. Circulating IgG antibodies to periodontal bacteria and lung cancer risk in the CLUE cohorts. JNCI Cancer Spectr. (2023) 7. doi: 10.1093/jncics/pkad029
26. Kajihara R, Sakai H, Han Y, Amari K, Kawamoto M, Hakoyama Y, et al. Presence of periodontitis may synergistically contribute to cancer progression via Treg and IL-6. Sci Rep. (2022) 12:11584. doi: 10.1038/s41598-022-15690-w
27. Joshipura KJ, Wand HC, Merchant AT, Rimm EB. Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res. (2004) 83:151–5. doi: 10.1177/154405910408300213
28. Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. (2006) 1088:251–64. doi: 10.1196/annals.1366.032
29. Salvi GE, Brown CE, Fujihashi K, Kiyono H, Smith FW, Beck JD, et al. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J Periodontal Res. (1998) 33:212–25. doi: 10.1111/j.1600-0765.1998.tb02313.x
30. Hayashi C, Gudino CV, Gibson FC 3rd, Genco CA. Review: Pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol. (2010) 25:305–16. doi: 10.1111/omi.2010.25.issue-5
31. Meaney CL, Mitchell KA, Zingone A, Brown D, Bowman E, Yu Y, et al. Circulating inflammation proteins associated with lung cancer in african americans. J Thorac Oncol. (2019) 14:1192–203. doi: 10.1016/j.jtho.2019.03.014
32. Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. (2013) 105:1871–80. doi: 10.1093/jnci/djt309
33. Fakhre Yaseri H. Gender is a risk factor in patients with gastroesophageal reflux disease. Med J Islam Repub Iran. (2017) 31:58. doi: 10.14196/mjiri.31.58
34. Horeweg N, van der Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J. (2013) 42:1659–67. doi: 10.1183/09031936.00197712
35. Moon SM, Choi H, Kim SH, Kang HK, Park DW, Jung JH, et al. Increased lung cancer risk and associated risk factors in tuberculosis survivors: A Korean population-based study. Clin Infect Dis. (2023) 77:1329–1339. doi: 10.1093/cid/ciad373
36. Choi H, Park HY, Han K, Yoo J, Shin SH, Yang B, et al. Non-cystic fibrosis bronchiectasis increases the risk of lung cancer independent of smoking status. Ann Am Thorac Soc. (2022) 19:1551–60. doi: 10.1513/AnnalsATS.202111-1257OC
Keywords: chronic periodontitis, lung cancer, epidemiology, risk, periodontitis
Citation: Kim B-G, Lee H, Lee S-K, Paik SY, Yun S-H, Park C-J, Yeo Y, Park TS, Moon J-Y, Kim T-H, Sohn JW, Kim S-H, Yoon HJ and Park DW (2024) Chronic periodontitis and risk of lung cancer: a nationwide cohort study. Front. Oncol. 14:1413590. doi: 10.3389/fonc.2024.1413590
Received: 18 April 2024; Accepted: 17 June 2024;
Published: 28 June 2024.
Edited by:
Carlo Eduardo Medina-Solis, Autonomous University of the State of Hidalgo, MexicoReviewed by:
Shimaa Hussein Kotb, Sphinx University, EgyptCopyright © 2024 Kim, Lee, Lee, Paik, Yun, Park, Yeo, Park, Moon, Kim, Sohn, Kim, Yoon and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ho Joo Yoon, aGp5b29uQGhhbnlhbmcuYWMua3I=; Dong Won Park, ZG9uZ3dvbnBhcmtAaGFueWFuZy5hYy5rcg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.