- 1Interventional Department of Peripheral Vascular Disease, Gansu Provincial Hospital of Traditional Chinese Medicine (TCM), Lanzhou, China
- 2Department of Radiology, Xuzhou Central Hospital, Xuzhou, China
- 3Department of Human Affairs, Gansu Center for Disease Control and Prevention, Lanzhou, China
- 4Department of Digestive Disease, Xuzhou Central Hospital, Xuzhou, China
- 5Department of Interventional Radiology, Xuzhou Central Hospital, Xuzhou, China
Background: While hilar cholangiocarcinoma (HCCA) patients commonly undergo radioactive stent (RS) insertion treatment, the relative benefits of unilateral versus bilateral RS insertion procedures remain to be established. Accordingly, this study was designed to evaluate the relative safety and efficacy of percutaneous bilateral and unilateral RS insertion for patients with HCCA.
Methods: In total, 126 HCCA patients who underwent unilateral (n=64) or bilateral (n=62) RS insertion from January 2017 - December 2021 were included in this analysis. Treatment efficacy and long-term outcomes were compared between groups. The primary endpoint was stent patency, and the secondary endpoints included technical success rate, clinical success rate, local control rate, overall survival (OS), and complications.
Results: The respective technical success rates in the unilateral and bilateral groups were 90.6% (58/64) and 93.5% (58/62) (P = 0.782). The clinical success rates were 82.8% and 86.2% in unilateral and bilateral groups, respectively (P = 0.608). Both groups exhibited comparable medial post-intervention bilirubin levels (100 vs. 99 μmol/L; P = 0.501), and restenosis occurred in 12 (20.7%) and 15 (25.9%) patients over the follow-up interval (P = 0.510). The stent reintervention rate was significantly higher in the unilateral group than bilateral group (66.7% vs. 0.0%, P < 0.001). The median stent patency in the unilateral and bilateral groups was 189 and 210 days, respectively (P = 0.796), while the median OS interval was 222 and 229 days, respectively (P = 0.969). Comparable cholangitis (17.2% vs. 22.4%, P = 0.485) and cholecystitis (3.4% vs. 3.4%, P = 1.000) rates were also detected in these two groups.
Conclusions: In summary, HCCA patients exhibit comparable efficacy when undergoing unilateral and bilateral radioactive stenting, suggesting that unilateral RS can be routinely performed owing to the simpler nature of this procedure.
Introduction
Hilar cholangiocarcinoma (HCCA) tumors are malignancies of the hepato-biliary system that can only be surgically resected in under 30% of cases (1). HCCA patients often experience obstructive jaundice and a consequent reduction in their quality of life (2, 3). In these inoperable HCCA patients, the insertion of a metal stent is often used as a first-line approach to alleviating jaundice symptoms (4–6). In 2012, Zhu et al. (7) combined a metal biliary stent and 125I seeds to generate a radioactive stent (RS) capable of prolonging stent patency and patient overall survival (OS) owing to the brachytherapeutic effects exerted by persistent 125I seed exposure.
While stenting is well-established as a therapeutic approach, controversy persists as to whether unilateral or bilateral stent insertion should be conducted in HCCA patients. Some studies have determined that endoscopic bilateral stent insertion can result in superior liver drainage and the prolongation of stent patency, with lower perioperative mortality rates (8–10). Others, however, have found that the technical success rates, clinical success rates, OS, stent patency, and complication rates associated with using a percutaneous trans-hepatic approach for unilateral or bilateral stent insertion were comparable (11, 12). All of these prior studies also employed traditional metal stents irrespective of whether a percutaneous or endoscopic insertion approach was used (8–13), and a detailed understanding of the relative benefits of unilateral or bilateral RS insertion is currently lacking.
This study was designed to address this knowledge gap by comparing the safety and efficacy of percutaneous unilateral and bilateral RS insertion in individuals diagnosed with HCCA.
Methods
Study subjects
This retrospective study was conducted by 2 centers (Gansu Provincial Hospital of TCM and Xuzhou Central Hospital) from January 2017 to December 2021. The local Institutional Review Board of Gansu Provincial Hospital of TCM (No. 2023-131-01) and Xuzhou Central Hospital (No. XZXY-LK-20220519-039) approved the present retrospective analysis, for which the requirement for informed consent was waived. In total, this study included 126 HCCA patients who underwent either unilateral (n=64) or bilateral (n=62) RS insertion (Table 1).
Patients eligible for inclusion were individuals with (i) a histology- or cytopathology-confirmed diagnosis of HCCA, (ii) inoperable disease due to tumor invasion or metastasis, (iii) obstructive jaundice; and (d) an ECOG performance status (PS) ≤ 2. Patients were excluded if they (i) had undergone prior hepatectomy, (ii) suffered from uncontrollable intractable ascites, (iii) were Bismuth type I patients, or (iv) had a life expectancy of less than 3 months.
Diagnosis
Intraductal biopsy was used to confirm the pathological diagnosis of HCCA in all patients. Abdominal MRI and CT scans were used to assess the patients’ Bismuth type and the degree of biliary obstruction.
RS production
To prepare an RS, a metal stent (Micro-Tech, Nanjing, China) was combined with an 125I seed strand composed of a 4F medical catheter (PBN MEDICALS Denmark A/S, Stenlose, Denmark) and multiple 125I seeds (Chinese Atomic Energy Science Institution, Beijing, China). These seeds were placed in a line within the catheter, followed by sealing the ends of the catheter. Each of these seeds (0.8 mm × 4.5 mm) emitted low-dose γ-rays (35.5 keV) and soft X-rays (28.6 keV), and each had 0.80 mCi, a 59.6 day half-life, and a 17 mm radius of effective antitumor activity. Each strand included a number of seeds determined based on the length of the stent as follows: N = stent length (mm)/4.5 + 4 (14).
RS insertion
A percutaneous trans-hepatobiliary tract approach was used for unilateral RS insertion under fluoroscopic guidance. After successfully puncturing the intra-hepatic biliary tract and the placement of a catheter sheath, a 4F catheter (Cordis, FL, USA) was introduced into the biliary tract across the site of the obstruction using a 0.035-inch guide-wire (Terumo, Tokyo, Japan). After the catheter was in the duodenum, this guide-wire was exchanged for a 0.035-inch stiff guide-wire (Cook, IN, USA). Then, another guide-wire was used to introduce a 6F catheter sheath (Terumo) across the obstructed site, and the stiff guide-wire was used to deploy the stent in the center of the obstructed site. 125I seeds were placed within this 6F sheath, with the sheat gradually removed so that the seed strand remains positioned between the stent and the biliary wall (Figure 1A).
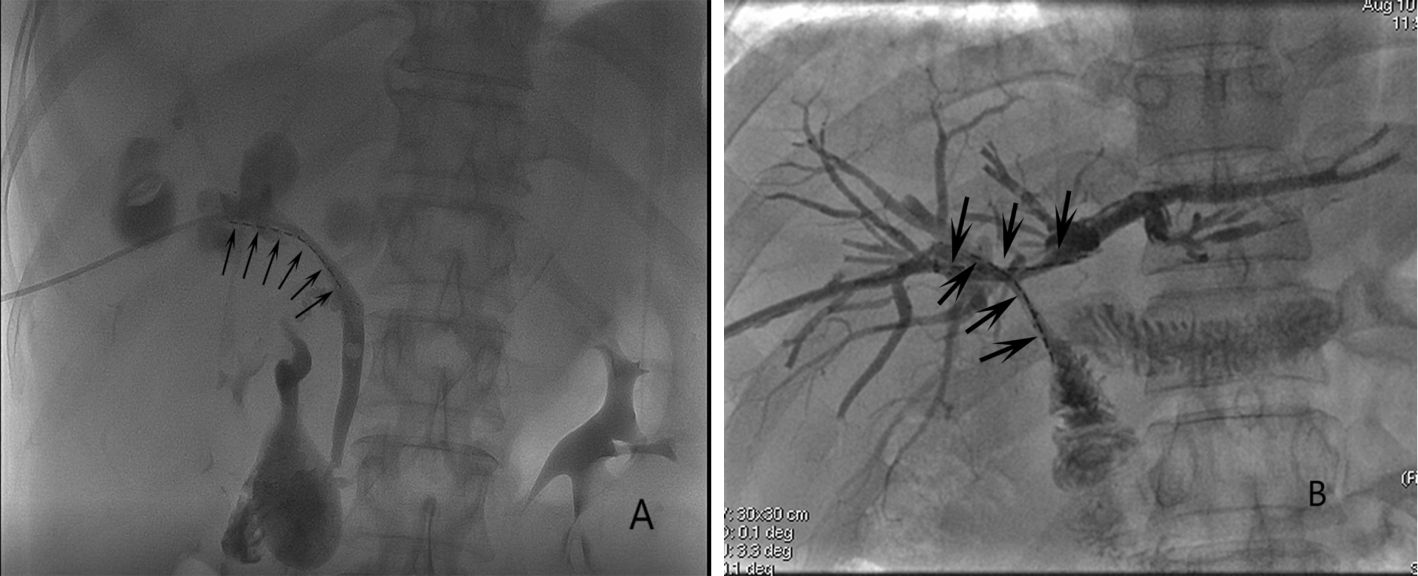
Figure 1. The images of (A) unilateral RS insertion (arrows) and (B) bilateral RS insertion (arrows).
A side-by-side approach was employed for bilateral RS insertion (Figure 1B), where the stents were placed in parallel (4). A Y- or T-configuration was selected for stent insertion based on the extent of disease in each HCCA patient.
An 8.5F temporary biliary drainage catheter (Cook) was placed instantly after stent insertion for internal drainage for 5 days, during which all patients underwent hemostatic and anti-inflammatory drug treatment.
Definitions
Technical success was defined as successful biliary obstruction recanalization without any 125I seed strand migration. Clinical success was defined as defined as a pre-intervention reduction by at least 50% in the level of total bilirubin (TBIL) within 2 weeks after stenting (13). The local tumor response was assessed by the response evaluation criteria in solid tumors (RECIST) criteria (Supplementary Table 1). Local control was calculated based on the number of patients with complete response, partial response and stable disease (1). Stent restenosis was defined as a recurrence of jaundice and/or cholangitis due to any reason (12). The reintervention was defined as any type of endoscopic or percutaneous procedure necessary to improve biliary drainage for jaundice or cholangitis after stent insertion (10). Stent patency was measured from stent insertion to initial jaundice recurrence. OS was measured from stent insertion to all-cause mortality. In cases where patients died without recurrent jaundice, OS and stent patency were the same. The treatment-related adverse events were classified according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon (Supplementary Table 2) (15).
The primary endpoint was stent patency, and the secondary endpoints included technical success rate, clinical success rate, local control rate, OS, and complications.
Follow-up
All patients underwent follow-up at 1 week, 1, 3, and 6 months after stenting, and every 6 months after that. Follow-up analyses included physical examinations, liver function tests, and abdominal CT imaging.
Statistical assessment
Data were analyzed with SPSS 16.0 (SPSS, Inc., IL, USA). Normally distributed data are reported as means with mean ± standard deviation, while they were otherwise presented as medians (Q1; Q3). These results were compared with paired samples t-tests and Wilcoxon tests as appropriate. Categorical variables were compared using χ2 tests or Fisher’s exact test. Stent patency and OS rates were compared using Kaplan-Meier curves and the log-rank test. Parameters independently associated with stent patency and OS were identified with a multivariate Cox regression analysis, while parameters significantly related to cholangitis were identified through a multivariate logistic regression approach. P < 0.05 served as the threshold for significance.
Results
Technical and clinical success
The respective technical success rates for HCCA patients who underwent unilateral and bilateral RS insertion were 90.6% (58/64) and 93.5% (58/62) (P = 0.782). All stents were 8 mm in diameter and 60-80 mm long. In the 10 cases of technical failure, the guide-wire could not pass through the obstruction site. External drainage catheters were placed in these 10 patients, and stent insertion was again attempted after 1 week. However, the guide-wire remained unable to pass the site of the obstruction in all 10 cases. The clinical success rates were 82.8% and 86.2% in unilateral and bilateral groups, respectively (P = 0.608). Among patients in the unilateral group, the median TBIL declined from 190 μmol/L (Q1: 124, Q3: 348) before stenting to 100 μmol/L (Q1: 39, Q3: 121) after stenting (P < 0.01). Similarly, the median TBIL levels in the bilateral group declined from 182 μmol/L (Q1: 125, Q3: 268) to 99 μmol/L (Q1: 58, Q3: 116) after stenting (P < 0.01). No significant differences in post-intervention TBIL levels were noted between groups (P = 0.501). Chemotherapy was administered following stent insertion for 23 patients in each group.
Treatment response
In the unilateral group, no patient achieved complete response, 12 (20.7%) patients achieved partial response, 32 (55.2%) patients achieved stable disease, and 14 (24.1%) patients experienced tumor progression. The local control rate was 75.9% (44/58). In the bilateral group, no patient achieved complete response, 11 (20.0%) patients achieved partial response, 37 (63.8%) patients achieved stable disease, and 10 (17.2%) patients experienced tumor progression. The local control rate was 82.8% (48/58). There was no significant difference between 2 groups in the aspect of local control rate (P = 0.359, Table 2).
Stent patency
Of these patients, 12 (20.7%) and 15 (25.9%) experienced stent restenosis throughout follow-up (P = 0.510, Table 2), with restenosis resulting from tumor ingrowth in all cases. Of the patients in the unilateral group, 8 underwent the insertion of a second metal stent from the contralateral intra-hepatic biliary tract, and 4 patients underwent biliary drainage catheter insertion. In bilateral group, all 15 patients underwent biliary drainage catheter insertion. No additional reintervention procedure was performed in the patients in both study groups. The stent reintervention rate was significantly higher in the unilateral group than bilateral group (66.7% vs. 0.0%, P < 0.001).
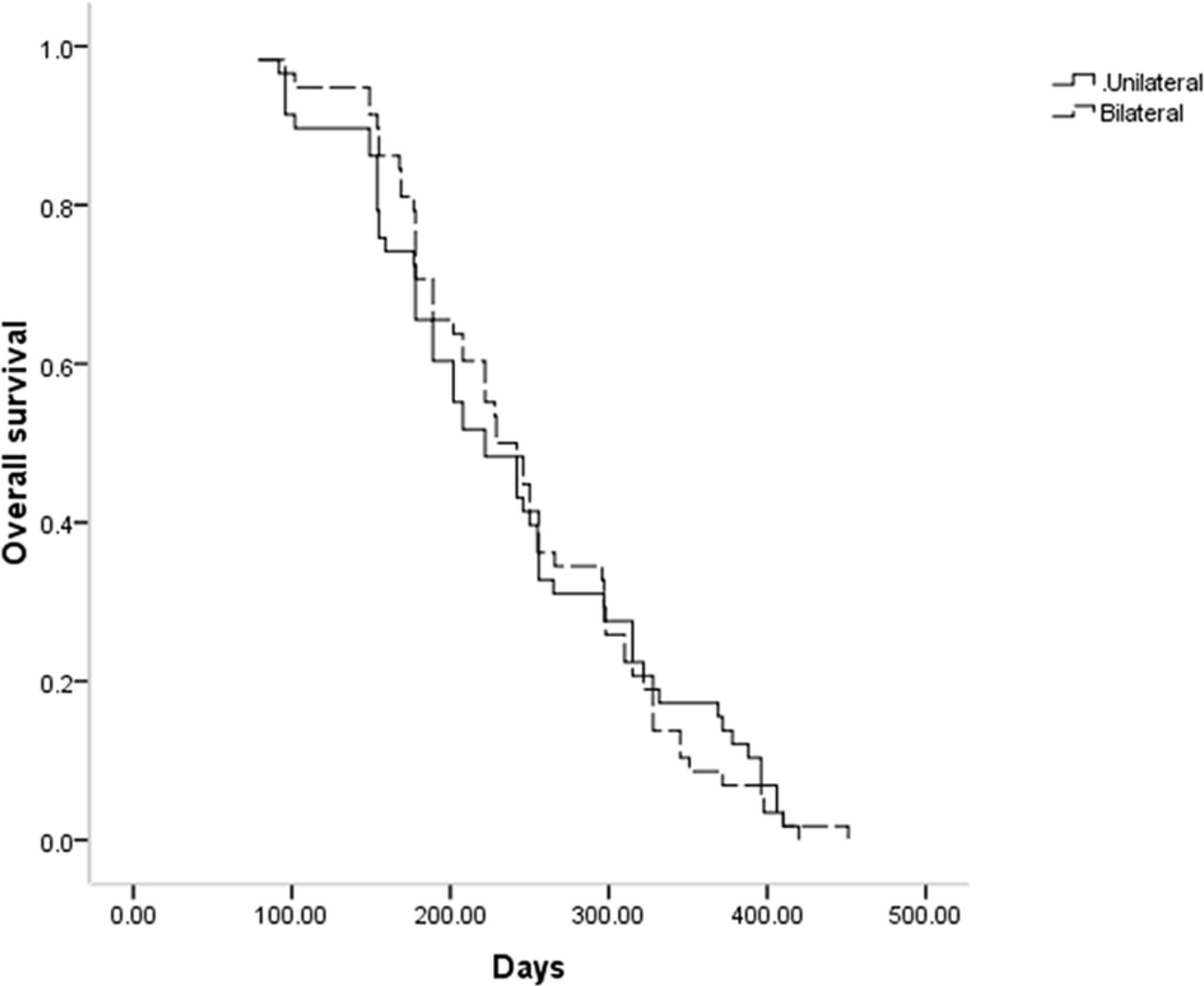
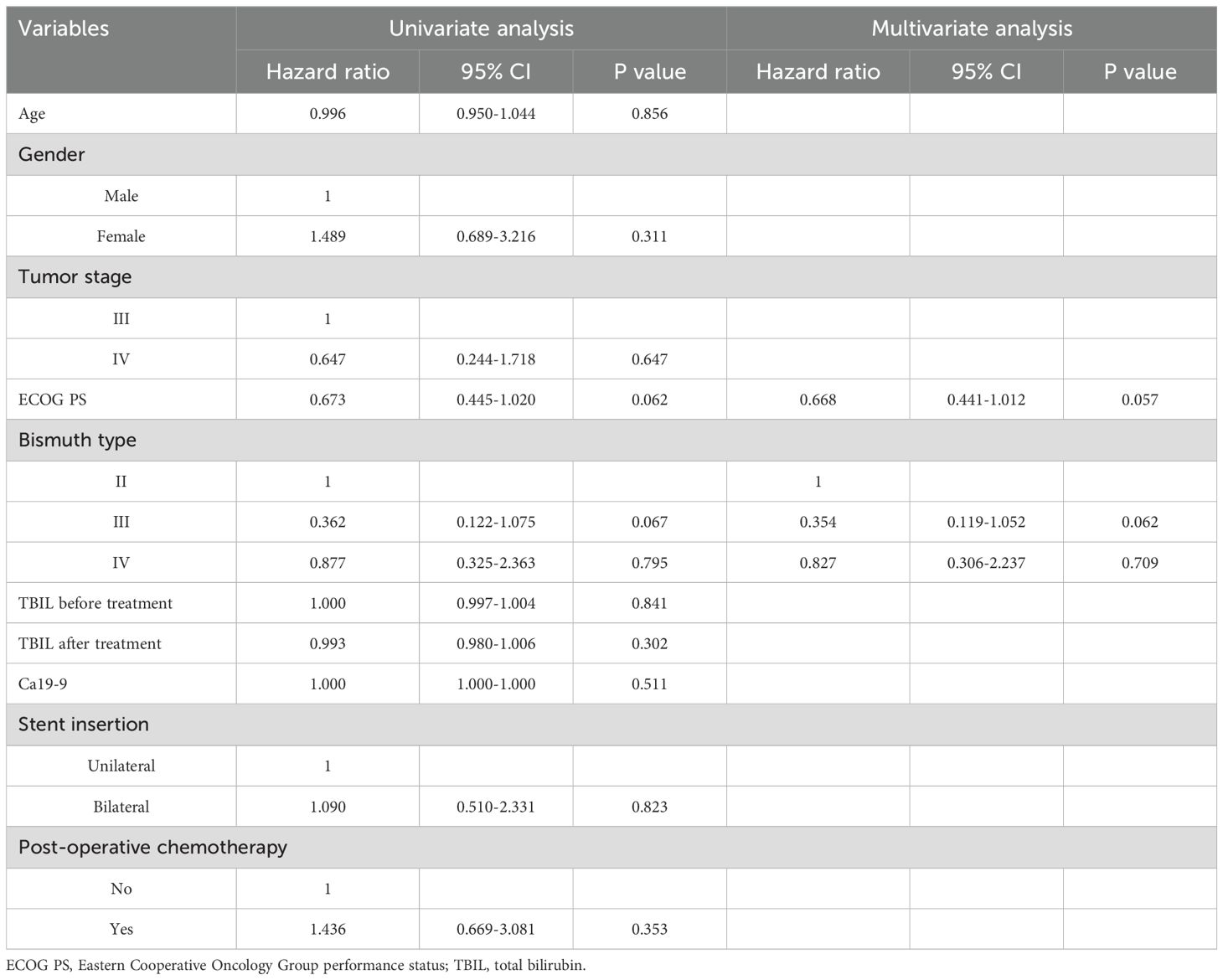
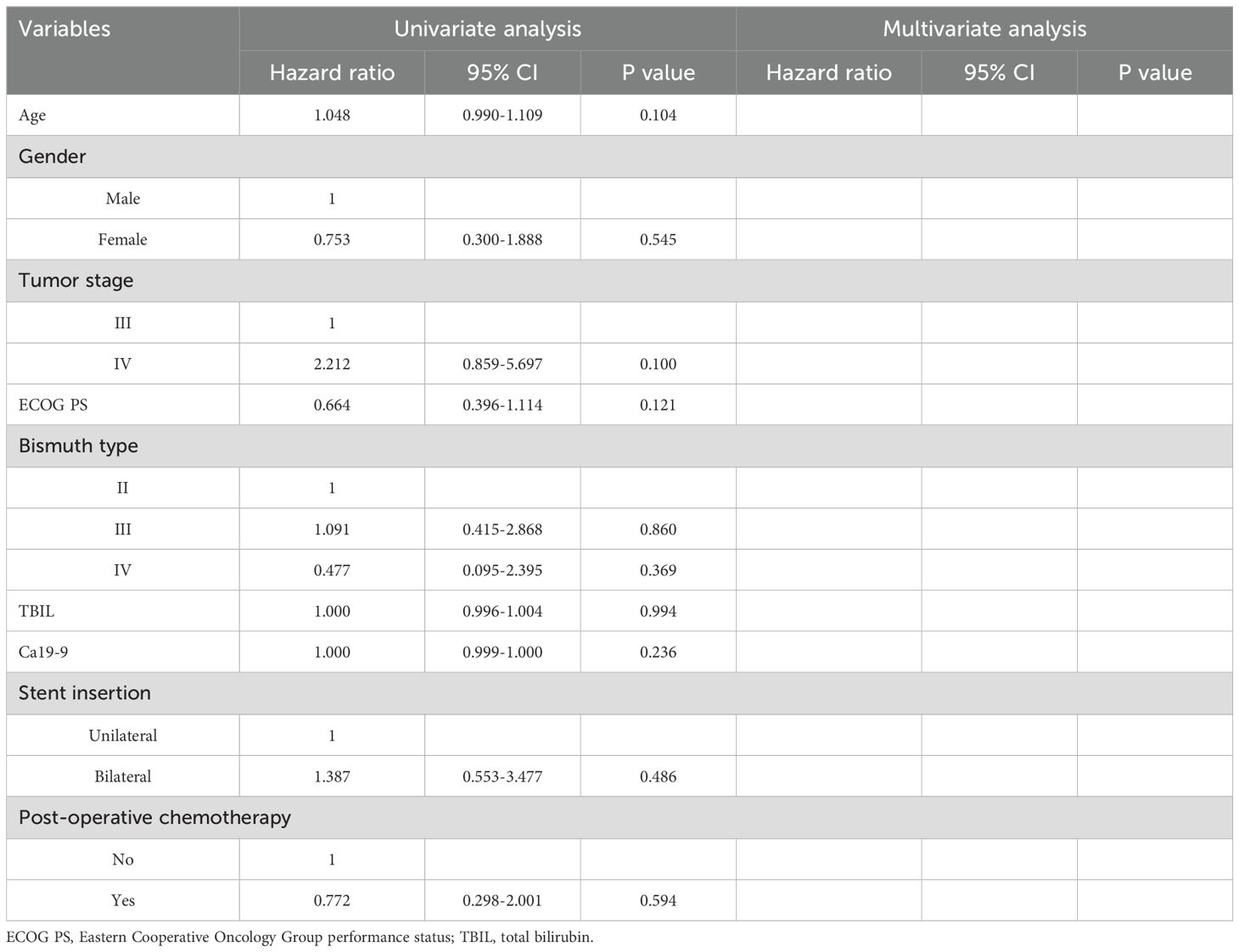
The median stent patency in the unilateral and bilateral groups was 189 and 210 days, respectively (P = 0.796, Figure 2). While univariate analyses revealed that both lower ECOG PS (P = 0.062) and Bismuth type III obstruction (P = 0.067) were related to restenosis, no risk factors associated with stent restenosis were detected through multivariate Cox regression analyses (Table 3).
OS
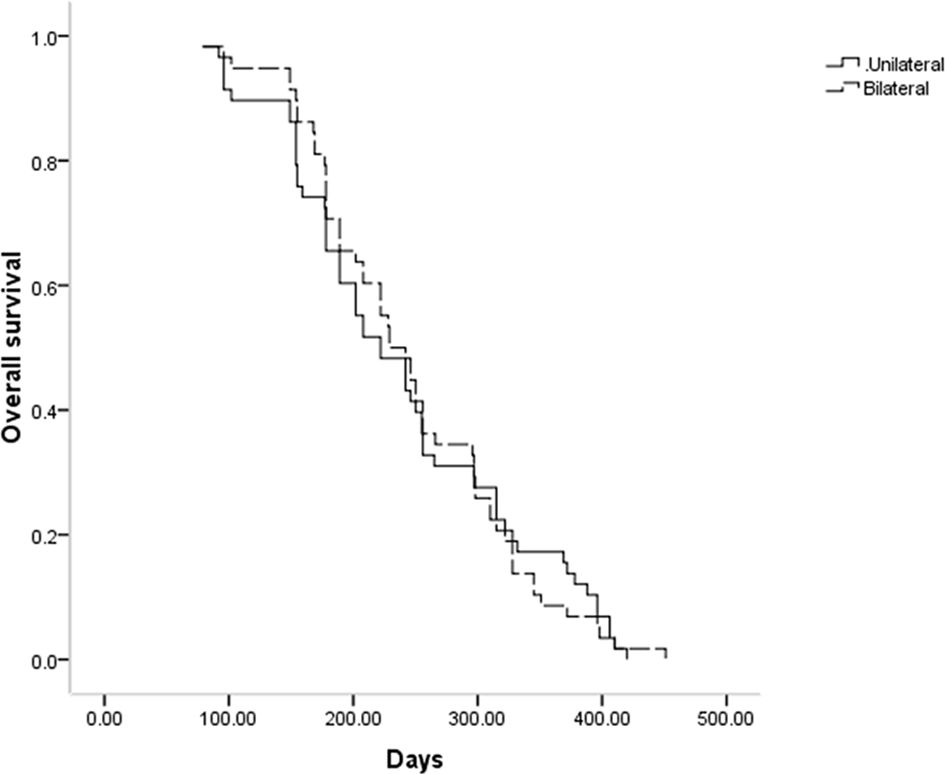
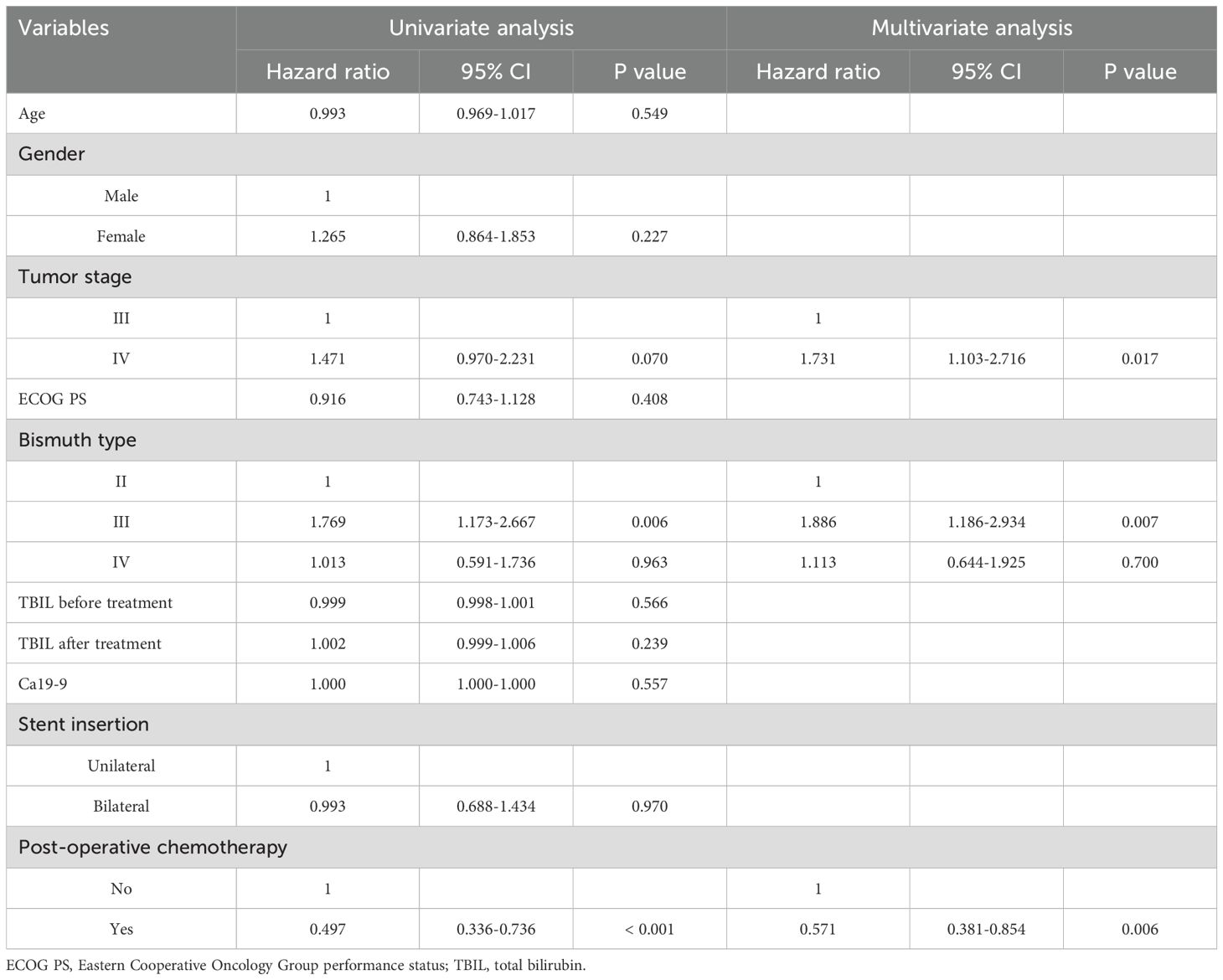
Throughout follow-up, all patients included in this study died as a result of tumor progression, with a respective median OS of 222 and 229 days in the unilateral and bilateral groups (P = 0.969, Figure 3). Univariate Cox regression analyses identified tumor stage IV (P = 0.070), Bismuth type III obstruction (P = 0.006), and postoperative chemotherapy (P < 0.001) as being associated with shorter patient OS, and multivariate analyses confirmed that tumor stage IV (P = 0.017), Bismuth type III obstruction (P = 0.007), and postoperative chemotherapy (P = 0.006) were all independently associated with the risk of shorter OS (Table 4).
Adverse events
Similar rates of cholangitis (17.2% vs. 22.4%, P = 0.485) and cholecystitis (3.4% vs. 3.4%, P = 1.000) were noted in the unilateral and bilateral groups (Table 2). All these adverse events were moderate events according to the ASGE lexicon. Logistic regression analyses failed to identify any cholangitis-related risk factors (Table 5).
Subgroup analyses
There were 24 and 32 Bismuth type II patients who underwent unilateral and bilateral RS insertion in this study. The technical (95.8% vs. 93.8%, P = 1.000), clinical success (78.3% vs. 83.3%, P = 0.910), local control (78.3% vs. 80.0%, P = 1.000), and restenosis (34.8% vs. 33.3%, P = 0.912) rates were all comparable between 2 groups. The median stent patency (222 d vs. 225 d, P = 0.344) and OS (297 d vs. 229 d, P = 0.488) were both comparable between 2 groups. The adverse event rates were comparable between 2 groups (21.7% vs. 20.0%, P = 1.000).
There were 40 and 30 Bismuth type III-IV patients who underwent unilateral and bilateral RS insertion in this study. The technical (87.5% vs. 93.3%, P = 0.690), clinical success 85.7% vs. 89.2%, P = 0.723), local control (74.3% vs. 85.7%, P = 0.265), and restenosis (11.4% vs. 17.9%, P = 0.717) rates were all comparable between 2 groups. The median stent patency (189 d vs. 208 d, P = 0.299) and OS (202 d vs. 222 d, P = 0.588) were both comparable between 2 groups. The adverse event rates were comparable between 2 groups (22.9% vs. 25.0%, P = 0.843).
Discussion
This study was designed to evaluate the safety and efficacy of percutaneous bilateral and unilateral RS insertion approaches in patients diagnosed with HCCA. Both of these procedures exhibited comparably high technical success rates in this analysis (90.6% vs. 93.5%, P = 0.782), confirming the feasibility of both procedures for treating HCCA.
Unlike the previous studies which focused on the bilateral and unilateral conventional metal stent insertion for HCCA (8–12), this present study used the RS instead of the conventional stent. The biliary RS was developed in 2012 with the aim of combining the advantages of rapid relief of the jaundice with stent insertion and brachytherapy with 125I seeds (7). Therefore, compared to the conventional stent, RS can decrease the restenosis rate and prolong the stent patency (14).
In many cases, endoscopic guided biliary stent insertion is preferred because it avoids hepatic puncture and allows for physiological biliary tract drainage into the intestine (16, 17). While the technical and clinical success rates associated with stent insertion under endoscopic guidance and via the percutaneous trans-hepatic approach are comparable in cases of distal biliary obstruction, the complication rate associated with the endoscopic approach is notably lower (16, 17). However, endoscopic biliary stenting is inferior to percutaneous trans-hepatic biliary stenting in cases of malignant hilar obstruction, particularly for Bismuth type III/IV obstructions (18, 19). A percutaneous trans-hepatic approach is also important in cases of RS insertion to allow for the insertion of 125I seeds through a catheter sheath in the intra-hepatic biliary tract (14).
Theoretically, bilateral stent insertion should yield two different passages for biliary drainage that should be able to drain more bile and prolong stent patency (20, 21). Here, TBIL levels were found to decrease significantly following RS insertion irrespective of the approach employed, with comparable postoperative TBIL values and restenosis rates in the unilateral and bilateral groups, suggesting that these two procedures can yield similar levels of clinical efficacy. These findings may be attributed to the following factors: (a) unilateral stenting can effectively alleviate jaundice in HCCA patients via the drainage of > 25% of bile from the liver, thus providing clinical success (20, 21); (b) using RS instead of traditional metal stents may have inhibited restenosis owing to the brachytherapeutic effects of these stents and the associated inhibition of tumor growth, which is the primary driver of restenosis (22). Moreover, unilateral RS insertion may be superior to bilateral RS insertion in the aspect of the higher successful rate of reintervention for stent restenosis (23).
Both patient groups in this study exhibited comparable OS outcomes, likely owing to the similar stent patency observed in these groups. While RS insertion can suppress tumor growth, this is a palliative treatment strategy rather than a curative one. The local nature of RS brachytherapy also limits its efficacy in treating metastases to lymph nodes or distant sites (24). Here, postoperative chemotherapy was identified as a significant predictor of prolonged patient OS. Chemotherapy can enhance 125I seed brachytherapy-related treatment efficacy (25), facilitating the more effective management of lymph nodes and distant metastatic lesions.
Cholangitis is the most important major complication associated with biliary stenting. Deviere et al. (26) reported lower rates of cholangitis following bilateral stent insertion, potentially owing to the more effective drainage of bile than that achieved via the unilateral stent insertion approach. In contrast, Chen et al. (21) posited that the side-by-side insertion of a pair of stents into the common biliary tract can contribute to greater biliary wall compression that may, in turn, exacerbate the cholangitis risk in these patients. No differences in cholangitis were noted between the two RS insertion groups in the present study, nor were any predictors of cholangitis risk successfully identified through logistic analyses. This may be because a temporary biliary drainage catheter was employed following stent insertion, enabling better clearance of the biliary tract in these patients.
There are several limitations to this study. For one, this was a retrospective analysis. Differences in Ca19-9 levels among groups may have also contributed a substantial amount of bias, although no association was noted between Ca19-9 levels and either OS or stent patency. Moreover, the sample size for this study was relatively small, potentially explaining why no risk factors associated with cholangitis or stent patency were identified. Lastly, postoperative chemotherapy was performed in a manner dependent on the condition and consent of each patient, potentially introducing selection bias into these results.
Conclusion
In summary, these data demonstrate that percutaneous unilateral and bilateral RS insertion procedures can provide HCCA patients with comparable clinical efficacy and long-term outcomes. As a simpler approach, the unilateral RS insertion procedure can thus be routinely recommended in the clinic.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Gansu Provincial Hospital of TCM and Xuzhou Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective analysis.
Author contributions
J-LJ: Data curation, Formal analysis, Writing – original draft. WL: Methodology, Writing – original draft. Z-XW: Validation, Writing – review & editing. A-QF: Supervision, Writing – review & editing. HL: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1412933/full#supplementary-material
References
1. Zhang C, Song M, Sun Z, Fang Y, Liu Y, Xu K, et al. Biliary drainage combined with simultaneous 125I seed strand brachytherapy for the treatment of hilar cholangiocarcinoma. BMC Cancer. (2023) 23(1):418. doi: 10.1186/s12885-023-10868-5
2. Moll CF, de Moura DTH, Ribeiro IB, Proença IM, do Monte Junior ES, Sánchez-Luna SA, et al. Endoscopic Biliary Darinage (EBD) versus Percutaneous Transhepatic Biliary Drainage (PTBD) for biliary drainage in patients with Perihilar Cholangiocarcinoma (PCCA): A systematic review and meta-analysis. Clinics (Sao Paulo). (2023) 78:100163. doi: 10.1016/j.clinsp.2022.100163
3. Chen GF, Yu WD, Wang JR, Qi FZ, Qiu YD. The methods of preoperative biliary drainage for resectable hilar cholangiocarcinoma patients: A protocol for systematic review and meta analysis. Med (Baltimore). (2020) 99(21):e20237. doi: 10.1097/MD.0000000000020237
4. Zhou WZ, Liu S, Yang ZQ, Xian YT, Xu HD, Wu JZ, et al. Percutaneous stent placement for Malignant hilar biliary obstruction: side-by-side versus stent-in-stent technique. BMC Gastroenterol. (2020) 20(1):174. doi: 10.1186/s12876-020-01316-w
5. Jiao D, Huang K, Zhu M, Wu G, Ren J, Wang Y, et al. Placement of a newly designed Y-configured bilateral self-expanding metallic stent for hilar biliary obstruction: A pilot study. Dig Dis Sci. (2017) 62(1):253–63. doi: 10.1007/s10620-016-4284-1
6. Lee TH, Moon JH, Park SH. Biliary stenting for hilar Malignant biliary obstruction. Dig Endosc. (2020) 32:275–86. doi: 10.1111/den.13549
7. Zhu HD, Guo JH, Zhu GY, He SC, Fang W, Deng G, et al. A novel biliary stent loaded with (125)I seeds in patients with Malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol. (2012) 56(5):1104–11. doi: 10.1016/j.jhep.2011.12.018
8. Yang F, Wang XM, Xia FF, Han XQ. Endoscopic metal stenting for Malignant hilar biliary obstruction: an update meta-analysis of unilateral versus bilateral stenting. Wideochir Inne Tech Maloinwazyjne. (2021) 16(3):472–81. doi: 10.5114/wiitm.2021.104196
9. Ashat M, Arora S, Klair JS, Childs CA, Murali AR, Johlin FC. Bilateral vs unilateral placement of metal stents for inoperable high-grade hilar biliary strictures: A systemic review and meta-analysis. World J Gastroenterol. (2019) 25(34):5210–9. doi: 10.3748/wjg.v25.i34.5210
10. Fu YF, Xu YS, Shi YB, Zong RL, Cao C. Percutaneous metal stenting for Malignant hilar biliary obstruction: a systematic review and meta-analysis of unilateral versus bilateral stenting. Abdom Radiol (NY). (2021) 46(2):749–56. doi: 10.1007/s00261-020-02643-y
11. Yin X, Li DM, Yang F, Liu TG, Xia FF, Fu YF. Self-expanded metallic stent insertion for hilar cholangiocarcinoma: comparison of unilateral and bilateral stenting. J Laparoendosc Adv Surg Tech A. (2019) 29(12):1501–6. doi: 10.1089/lap.2019.0509
12. Chang G, Xia FF, Li HF, Niu S, Xu YS. Unilateral versus bilateral stent insertion for Malignant hilar biliary obstruction. Abdom Radiol (NY). (2017) 42(11):2745–51. doi: 10.1007/s00261-017-1174-8
13. Samanta J, Sundaram S, Dhar J, Mane K, Gupta P, Gupta V, et al. EUS-guided biliary drainage in patients with moderate-severe cholangitis is safe and effective: a multi-center experience. Surg Endosc. (2023) 37:298–308. doi: 10.1007/s00464-022-09495-1
14. Zhou C, Li H, Huang Q, Wang J, Gao K. Biliary self-expandable metallic stent combined with Iodine-125 seeds strand in the treatment of hilar Malignant biliary obstruction. J Int Med Res. (2020) 48(4):300060519887843. doi: 10.1177/0300060519887843
15. Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. (2010) 71:446–54. doi: 10.1016/j.gie.2009.10.027
16. Giri S, Seth V, Afzalpurkar S, Angadi S, Jearth V, Sundaram S. Endoscopic ultrasound-guided versus percutaneous transhepatic biliary drainage after failed ERCP: A systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. (2023) 33(4):411–9. doi: 10.1097/SLE.0000000000001192
17. Hassan Z, Gadour E. Percutaneous transhepatic cholangiography vs endoscopic ultrasound-guided biliary drainage: A systematic review. World J Gastroenterol. (2022) 28:3514–23. doi: 10.3748/wjg.v28.i27.3514
18. Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. (2009) 69(1):55–62. doi: 10.1016/j.gie.2008.04.005
19. Saluja SS, Gulati M, Garg PK, Pal H, Pal S, Sahni P, et al. Endoscopic or percutaneous biliary drainage for gallbladder cancer: a randomized trial and quality of life assessment. Clin Gastroenterol Hepatol. (2008) 6(8):944–950.e3. doi: 10.1016/j.cgh.2008.03.028
20. Li M, Wu W, Yin Z, Han G. Unilateral versus bilateral biliary drainage for Malignant hilar obstruction: a systematic review and meta-analysis. Zhonghua Gan Zang Bing Za Zhi. (2015) 23(2):118–23. doi: 10.3760/cma.j.issn.1007-3418.2015.02.009
21. Chen ZK, Zhang W, Xu YS, Li Y. Unilateral versus side-by-side metal stenting for Malignant hilar biliary obstruction: A meta-analysis. J Laparoendosc Adv Surg Tech A. (2021) 31(2):203–9. doi: 10.1089/lap.2020.0400
22. Lin LW, Ke K, Chen R, Yang WZ, Huang N, Wu ZZ. Safety and efficacy of biliary stenting combined with iodine-125 seed strand followed by hepatic artery infusion chemotherapy plus lenvatinib with PD-1 inhibitor for the treatment of extrahepatic cholangiocarcinoma with Malignant obstructive jaundice. Front Immunol. (2024) 14:1286771. doi: 10.3389/fimmu.2023.1286771
23. Yasuda I, Mukai T, Moriwaki H. Unilateral versus bilateral endoscopic biliary stenting for Malignant hilar biliary strictures. Dig Endosc. (2013) 25 Suppl 2:81–5. doi: 10.1111/den.12060
24. Bi Y, Li J, Yi M, Yu Z, Han X, Ren J. Self-expanding segmental radioactive metal stents for palliation of Malignant esophageal strictures. Acta Radiol. (2020) 61(7):921–6. doi: 10.1177/0284185119886315
25. Hong J, Shi YB, Fu YF, Yang LL. Iodine-125 seeds insertion with trans-arterial chemical infusion for advanced lung cancer: a meta-analysis. J Contemp Brachytherapy. (2022) 14(4):403–10. doi: 10.5114/jcb.2022.118117
Keywords: radioactive, stent, hilar, cholangiocarcinoma, survival
Citation: Jin J-L, Li W, Wu Z-X, Feng A-Q and Li H (2024) Unilateral and bilateral radioactive stent insertion in patients diagnosed with inoperable hilar cholangiocarcinoma: a comparative analysis. Front. Oncol. 14:1412933. doi: 10.3389/fonc.2024.1412933
Received: 06 April 2024; Accepted: 16 September 2024;
Published: 01 October 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Fengfei Xia, Binzhou People’s Hospital, ChinaXinjian Xu, Jiangyin People’s Hospital, China
Jahnvi Dhar, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2024 Jin, Li, Wu, Feng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: An-Qiang Feng, aW50ZXJuYXRpb25hbDkxMUAxNjMuY29t; Hao Li, bGg3NjEyMjBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jin-Long Jin1†
Jin-Long Jin1† Hao Li
Hao Li